Abstract
Pathology training programs throughout the United States have endured unprecedented challenges dealing with the ongoing coronavirus disease 2019 pandemic. At Houston Methodist Hospital, the Department of Pathology and Genomic Medicine planned and executed a trainee-oriented, stepwise emergency response. The focus was on optimizing workflows among areas of both clinical and anatomic pathology, maintaining an excellent educational experience, and minimizing trainee exposure to coronavirus disease 2019. During the first phase of the response, trainees were divided into 2 groups: one working on-site and the other working remotely. With the progression of the pandemic, all trainees were called back on-site and further redeployed within our department to meet the significantly increased workload demands of our clinical laboratory services. Adjustments to trainee educational activities included, among others, the organization of a daily coronavirus disease 2019 virtual seminar series. This series served to facilitate communication between faculty, laboratory managers, and trainees. Moreover, it became a forum for trainees to provide updates on individual service workflows and volumes, ongoing projects and research, as well as literature reviews on coronavirus disease 2019–related topics. From our program’s experience, redeploying pathology trainees within our department during the coronavirus disease 2019 pandemic resulted in optimization of patient care while ensuring trainee safety, and importantly, helped to maintain continuous high-quality education through active involvement in unique learning opportunities.
Keywords: pathology trainee redeployment, coronavirus disease 2019 pandemic, pathology education during coronavirus disease 2019, graduate medical education during coronavirus disease 2019, redeployment during coronavirus disease 2019
Introduction
The Texas Medical Center (TMC) is no stranger to disasters. Over the past 20 years, natural disasters such as Tropical Storm Allison in June 2001 have caused catastrophic losses throughout the TMC.1 Hospitals such as Memorial Hermann closed for a significant amount of time in 2001 due to flooding. Similarly, Baylor College of Medicine (BCM) was severely impacted due to the location of the animal quarters below ground; suffering over 495 million dollars in research losses.1 Houston Methodist Hospital (HMH) experienced massive flooding in the basement level, knocking out the hospital power generators. The HMH Department of Pathology rapidly set up temporary operations across the street in a hotel meeting room to provide uninterrupted anatomic pathology (AP) services. The department and hospital functioned under challenging spartan conditions with emergency diesel generators, and it was many months before it was safe to reopen the operating rooms (ORs) due to lack of adequate power and fungal contamination.
Texas Medical Center staff, faculty, and trainees have experienced and survived Hurricanes Katrina (2005) and Ike (2008), as well as the flooding during Memorial Day (2015) and “Tax Day” (2016), and more recently, Hurricane Harvey (2017). We have learned to adapt during these extreme situations, and believe that these past experiences allowed the TMC and HMH to survive the most recent major hurricane (Harvey) with minimal damage and disruption of services.2-5 For example, during Tropical Storm Allison, one lesson learned was that the inability of employees and physicians to access the hospital during the flooding of their homes may significantly impact patient care. Our institution made infrastructure changes and implemented disaster staffing plans that continue today. We have also tried to apply the lessons learned in our medical training programs. During Hurricane Katrina, HMH and BCM accommodated trainees from Tulane University, eventually transferring these residents to both institutions. Additionally, HMH provided residents from the University of Texas Medical Branch in Galveston extended rotations during Hurricane Ike. Moving forward, our aim has continued to be to respond quickly to adverse events and to continue providing a safe and quality education, as well as a unique learning experience for our trainees.
In March 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), was formally designated a pandemic by the World Health Organization.6 The number of cases has quickly escalated both internationally and throughout the United States over the past few months, leading to varied and unprecedented responses to this viral pandemic among governments, health care systems and providers, as well as academic and education institutions. While the ultimate impacts of the COVID-19 pandemic on modern institutions are yet to be determined, the historical events of the 1918 Spanish Influenza pandemic, as well as those of many natural disasters, are vividly remembered today.7,8 Medical residents and other graduate medical education (GME) trainees are actively playing an integral part in the nation’s emergency health care response to COVID-19, and this pandemic undoubtedly will impact the training they receive and their future careers in medicine.
Maintenance of trainee education amid disasters such as the COVID-19 pandemic is crucial. However, in many health care institutions, strict regulations have been implemented which render in-person education nearly impossible. Some programs have reported turning to alternative modalities to meet their workload and educational requirements.9-12 For example, an otolaryngology training program implemented a virtual research curriculum, in which each trainee was assigned a mentor with whom they met and worked closely.13 Similarly, radiology training programs have implemented preparedness plans that included dividing residents into 2 groups: one working on-site and another continuing their education from their homes (trainees in the latter group were available to be called back in if needed).10 This program also opted to perform patient hand-offs and consults primarily over the phone.10 An additional example includes a general surgery program that restructured trainees into teams covering inpatients, operations, and outpatients at their institution (telemedicine, home-call status). Their reorganization allowed them to decrease the number of trainees exposed to COVID-19 inpatients, while also maintaining a reserve pool of trainees without hospital exposure to the virus.11
At the time of the preparation of this article, a review of the English literature yielded a single article addressing Laboratory Medicine during the COVID-19 pandemic, which focused on changes to Transfusion Medicine services.14 An additional article only briefly discusses the potential impact of the current public health crisis on the education of pathology trainees.15 Herein, we describe our program’s trainee-oriented, stepwise emergency response in the setting of the ongoing COVID-19 pandemic which provided seamless transitions based on our goal to maintain the health and safety of faculty, staff, trainees, and our patients.
Materials and Methods
Residents and fellows in the Pathology Residency and Fellowship Programs at HMH, led by their program directors, organized a retrospective review of the intradepartmental pathology trainee redeployment at our institution during the COVID-19 pandemic. The individual trainees involved in this publication include 1 to 3 volunteer representatives from each area (n = 14) assigned to document the ongoing changes within Clinical Pathology (CP) and AP services at our institution during the first 4 weeks of redeployment due to the pandemic. These services included areas of Laboratory Medicine as follows: Molecular Diagnostics, Transfusion Medicine, Chemistry, Diagnostic Immunology, Histocompatibility and Transplant Immunology (HLA), Microbiology, Informatics, Hematopathology, Coagulation, and Laboratory Management; and areas of AP services, to include Surgical Pathology, Cytopathology, Neuropathology, and Autopsy Pathology.
Trainees, including both residents and fellows, involved in this study were asked to communicate with the laboratory medical directors, laboratory managers, and other personnel in their respective areas, to review changes or adjustments implemented to these workflows during the current COVID-19 pandemic, and to assess the responses with documentation of the relevant findings. In addition, trainees were asked to narrate their experiences during the redeployment, with a special focus on trainee learning opportunities during this time. Any adjustments made to the schedules of trainees within our residency and fellowship programs were documented, including both AP and CP. In addition, details of any changes or adaptations in the daily routine of trainees within our program were recorded, emphasizing the unique educational activities and opportunities arising during the redeployment. All assignments were compiled and results were reviewed closely with the directors, then further shared with the faculty in our department.
Epidemiological national statistics were obtained from the COVID-19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University16; the state- and county-level data were obtained from the Texas Department of State Health Services.17A retrospective data review for specimen accessioning and resulting information by date was performed using the management reporting functionality in SoftPathDx (SCC). Briefly, all surgical pathology, cytopathology, and neuropathology (NP) accessioning requests from 2019 to 2020 and autopsy requests from 2017 to 2020 were exported from SCC to a Microsoft Excel 2013 sheet in comma-separated value format. PivotTables were used to perform granular filtering of cases by month and specimen type, focusing on the time frame of January through May. These findings were then organized and graphed using Microsoft Excel 2013. The analysis of the trainee service assignments was processed with Excel Power Query to create source-destination mappings to generate data for an alluvial (Sankey) diagram. Sankey diagrams with hierarchical layouts were created using a JavaScript library for visualizing complex data (D3.js), to represent the individual trajectories of trainees in the different clinical services during the pandemic. Flowcharts were designed using Microsoft Powerpoint 2013. Additional formatting changes were completed using Adobe Illustrator CS6.
Results
Reorganization and Intradepartmental Redeployment
At our institution, the number of trainees within the Department of Pathology and Genomic Medicine is as follows: 19 residents (18 AP/CP, 1 AP only), 12 AP fellows, and 8 CP fellows (n = 39). Our regular scheduling consists of 4-week blocks, for a total of 13 blocks per year. During standard operations, there are approximately 24 to 27 trainees on AP services for any given block, with the remainder of trainees on CP services. From our experience, this distribution of trainees has allowed for satisfactory coverage of all pathology services during routine circumstances, as shown in Figure 1 and Figure 2 (Standard Operations).
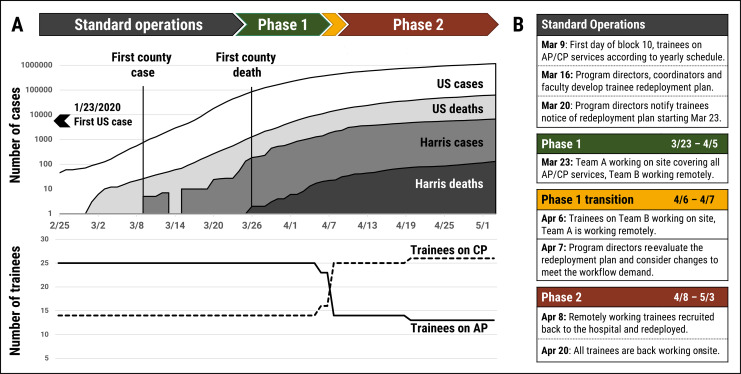
Figure 1.
A (top), This figure depicts several epidemic curves showing the number of coronavirus disease 2019 (COVID-19) cases and related deaths within the United States (white and light gray colors, respectively) and within Harris County, Texas (dark gray and black colors, respectively) over the timeline of trainee redeployment.16,17 A (bottom), Line graph depicting the number of overall trainees on anatomic pathology and clinical pathology services during COVID-19 redeployment in our department. Compared to standard operations, a majority of our trainees were assigned to clinical pathology rotations rather than anatomic pathology. B, Table shows the specific dates and actions occurring before and during the various phases of trainee redeployment at our institution.
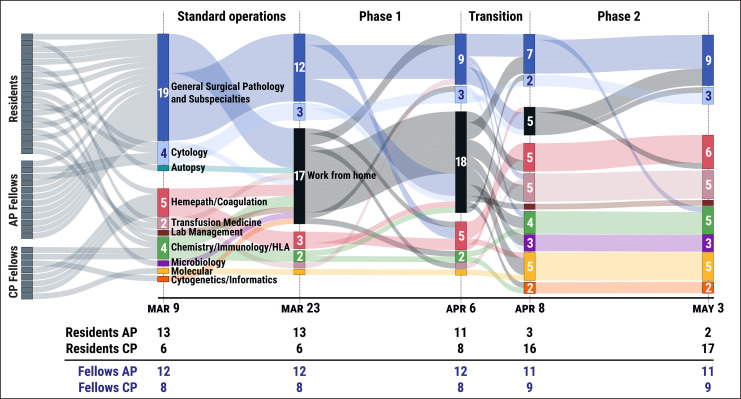
Figure 2.
Sankey plots with hierarchical layouts are shown, highlighting the changes in trainee rotation distribution within both clinical and anatomic pathology (CP/AP) services over the phases of our training program’s redeployment during the coronavirus disease 2019 (COVID-19) pandemic. In summary, the overall reduction in surgical procedures allowed for the intradepartmental redeployment of approximately half of our trainees on AP services to CP services. Specifically, the distribution of residents to AP and CP services shifted from 13 residents on AP services and 6 residents on CP services before and during phase 1, to 3 residents on AP services and 16 residents on CP services during phase 2. Distribution of fellows on AP and CP services remained relatively stable, with only one fellow shifting from AP to CP during this time.
During the initial stages of the COVID-19 pandemic in Texas, our institution’s Designated Institutional Official and staff organized training of all residents and fellows in the use of specialized personal protective equipment (PPE) in the event that a concerted redeployment strategy might be necessary to cover clinical services. In fact, volunteers were requested to serve as scribes on at least 2 occasions. However, discussions between our Residency Program Director and department Chair resulted in communication with GME officials that Pathology trainees have a unique skill set that other physicians do not and that their contribution to the COVID-19 response efforts was best served by continuing to work in the anatomic and clinical laboratories. With the expectation for all surgical procedures to decrease significantly (due to a state mandate to restrict procedures to emergent and critical cases, eliminating all “elective” cases), and with the hope of minimizing trainees’ hospital exposure to COVID-19, our initial redeployment strategy to maintain health and wellness of all trainees and faculty divided the trainees into 2 groups, one working on-site, while the rest worked remotely. This initial strategy allowed for a reserve workforce of Pathology trainees that could be called back on-site if needed, whether due to increased demand or due to illness of members of the on-site group, and would additionally allow for trainees to continue with their planned rotations according to the original yearly schedule. In general, our strategy consisted of subdividing the trainees into 2 teams (A and B). The teams were each composed of approximately half of the trainees from every residency class (postgraduate years 1-4) and half of the fellows from all AP and CP services, allowing adequate coverage of all basic duties for pathology service lines. The selection of trainees for each team was made by the program directors with input from the chief residents. Each team was assigned to alternate between a 2-week period working on-site (beginning with team A), and a subsequent 2-week period working remotely.
Our plan was activated on March 23, 2020, when the number of COVID-19 cases began to escalate in Houston, Texas. A detailed depiction of our trainee redeployment is shown in Figure 2. During the initial phase of this plan, the distribution consisted of 56% working on-site (15 trainees on AP and 7 on CP services) and 44% working from home (17 trainees), as shown in Figure 1 and Figure 2 (designated within our program as: phase 1).
In the third week of redeployment, 2 days after trainees started block 11 (with team B working on site, and team A working remotely), the trainee distribution was reevaluated in light of the changing needs of our department, as depicted in Figure 1 and Figure 2 (designated within our program as: phase 1 transition). With the number of COVID-19 patients steadily increasing in our hospital system, there was a significantly increased demand for SARS-CoV-2 laboratory testing, validation of new assays, and an expansion of needs among the CP services, particularly Molecular Diagnostics, Diagnostic Immunology, Informatics, and Transfusion Medicine/Donor Center areas. The decision was made by our program director and associate directors, with input from laboratory medical directors and managers, to escalate the trainee redeployment plan to a second phase, in which the majority of team A was called back on-site (shown in Figure 1 and Figure 2 designated within our program as: phase 2).
In terms of the new rotation assignments for trainees during phase 2 of redeployment, only AP/CP residents were considered eligible for reassignment to an alternate rotation within our department. The individual reassignments took into consideration any electives required or requested for the upcoming academic year and any pending academic requirements for each trainee, which could include the possibility of coverage of either AP or CP services. Our AP-only resident (n = 1) remained on AP services throughout. Additionally, the vast majority of fellows in our Accreditation Council for Graduate Medical Education (ACGME)-accredited Pathology subspecialties were assigned to cover services within their designated area, either AP or CP, to assure comprehensive training within their specialty and to maintain their board eligibility. One exception to this was a NP fellow who voluntarily switched a planned elective rotation to join the Molecular Diagnostics service, with the goal of acquiring a unique experience that could benefit her prior to her upcoming Molecular Genetic Pathology fellowship (scheduled to begin elsewhere on July 1, 2020). Finally, for a period of 3 days, 2 fellows (1 AP and 1 CP, both graduating in June 2020) were assigned to the Laboratory Management service. These fellows were specifically redeployed to this rotation for a short time, as they were both prior chief residents in our program, and had experience with leadership in other crisis situations, such as Hurricane Harvey. One of these graduating fellows is entering private practice and the other is entering an academic position; the additional management experience was considered preparation for their future leadership activities in practice. During those 3 days, they helped to organize our phase 2 plan and worked hand in hand with the newly assigned chief residents and fellow to ensure a smooth transition. This short reassignment did not affect their subspecialty board qualification. In summary, during phase 2, the trainee distribution consisted of 87% working on-site (9 trainees on AP and 25 on CP services) and 13% (5 trainees) working from home.
Two weeks into our second phase of redeployment, the team A trainees remaining at home were also called back to the hospital. Both initial teams were then consolidated, and our Pathology services continued to be reinforced with trainees predominantly covering CP areas. At the time of writing this article, the distribution of trainees within our department consisted of 100% working on-site, with 12 trainees on AP and 27 on CP services, as shown in detail in Figure 2 (phase 2). Of note, 3 AP fellows and 3 AP/CP residents were rotating, or were scheduled to rotate, at outside institutions within the TMC during phases 1 and 2 of redeployment. These outside rotation experiences were either allowed to continue by the hosting institution or transitioned to a virtual platform. In one case, the trainee was brought back to Houston Methodist (home institution) and acted as a senior fellow on service. Records of these scheduling changes have been maintained by the program, by the residents themselves, and by the current chief residents, so that any “makeup” rotations are accounted for in individual trainee schedules during the upcoming academic year (or future academic years, if necessary).
Maintaining Protected Trainee Education Time
A substantial component of Pathology trainee educational activities at our institution involves daily conferences, including a morning lecture, a noon lecture (where trainees typically gather and have lunch together), numerous tumor boards, as well as several monthly to quarterly interinstitutional conferences within the TMC. First-year residents have an additional daily interactive teaching session around the microscope with one of our senior surgical pathology faculty. These activities are organized by the chief residents and include topics led by invited speakers, faculty, and trainees (overseen by faculty) from all institutions and at all levels. Our conference schedule is available online to all faculty and trainees throughout the TMC and is updated on a daily basis, which facilitates rapid communication regarding any changes or adjustments to all participants.
As part of our institution’s response to COVID-19, Centers for Disease Control and Prevention (CDC)-recommended physical distancing was implemented throughout the hospital system and the TMC. All meetings and gatherings were initially cancelled; however, our program quickly began a gradual reinstatement of the usual daily conference series and tumor boards, conducted virtually using modalities such as Cisco Webex. Specifically, regarding our AP conferences, the content remained as planned prior to the COVID-19 pandemic, with the exception of the cancellation of the daily interactive teaching session around the microscope in which predominantly first-year residents participate. As in pre-pandemic times, trainees continued to have protected time during these didactic sessions. Given the Texas governor’s cancellation of all elective surgeries during the peaks of the pandemic, our institution required all planned cancer surgery to have multidisciplinary approval at one of the multiple weekly specialty tumor boards. These difficult and complex discussions at the now virtual tumor boards have provided our trainees unique clinical insights. During the initial redeployment, with many trainees continuing their education remotely, online pathology-related conferences sponsored by societies such as the College of American Pathologists, American Society of Cytopathology, American Medical Association, American Society of Clinical Pathology, and United States and Canadian Academy of Pathology were made available. Trainees were asked to keep a daily log of all their activities, with weekly review by the program director.
In addition, a daily COVID-19 virtual seminar series was implemented with the second phase of trainee redeployment, and temporarily replaced the 1-hour interactive microscope sessions that were cancelled. The COVID-19 virtual seminar was considered protected time for all pathology trainees, and involved the participation of our program directors, laboratory medical directors, and other Pathology faculty. The daily seminar was led by our Associate Program Director for Clinical Pathology, along with the trainee assigned to the Laboratory Management rotation and those trainees who volunteered to present materials. The Laboratory Management trainee’s duties were expanded to include daily rounds through all areas of laboratory medicine. Their main objective was to summarize and discuss salient updates within each laboratory area and CP service for the COVID-19 virtual seminar, with a special emphasis on COVID-19-related developments. Residents rotating on each CP service provided details of the daily workflow and ongoing laboratory assay validations, clinical trials, research projects, quality control and quality assurance, quality management projects, or other ongoing projects. Following the discussion, a volunteer presented literature reviews or other information on COVID-19-related topics. A list of suggested relevant topics was continuously updated and available online for trainees to review. Examples of topics presented during the COVID-19 virtual seminar include SARS-CoV-2 virology, vaccine development, serologic assays, reviews of recently published literature on COVID-19, relevant changes in the workflow of specific laboratories, details regarding new assay development or validations, changes to autopsy procedures and published findings from COVID-19 patients, and drafting an Investigational New Drug (IND) protocol for submission to the Food and Drug Administration (FDA), among many others.
Effective Communication
To maintain adequate communication while following physical distancing guidelines, our chief residents and chief fellow increased the number of trainee meetings with our program director (from once a month to once a week), all held virtually. Multiple daily didactic sessions were also conducted virtually, with efforts made to promote interactive trainee participation through available chat options or through the utilization of smartphones (using applications such as participoll.com and pollev.com) for multiple-choice question and answer session (Q&A) sessions. Of note, our daily COVID-19 seminar series has become a very useful source of communication among medical directors, faculty, laboratory managers, and trainees. Meeting minutes were documented and made accessible to all invited participants. Furthermore, COVID-19 topic presentations with respective references were also made available for review or access at any time. Finally, prior to ending the meetings, we performed daily announcements, reinforced the importance of using PPE and physical distancing, and allowed an open time for any questions. Additional activities within our hospital system included a weekly virtual physician town hall meeting, open to all attendings and trainees, which provided institutional COVID-19-related updates. These meetings included a Q&A session to address any concerns in real time. Also, the GME House Staff Council held a weekly town hall virtual meeting exclusively for trainees.
Training in Anatomic Pathology During the Coronavirus Disease 2019 Pandemic
Surgical pathology
During the initial height of the COVID-19 pandemic in our state, surgical procedures were limited to emergent and critical cases, by mandate of the Governor of Texas. This was a precautionary measure aimed at reducing the number of patients occupying hospital beds, should they become needed to accommodate the growing numbers of critically ill COVID-19 patients.
Specifically, the distribution of residents to AP and CP services shifted from 13 residents on AP services and 6 residents on CP services before and during phase 1 to 3 residents on AP services and 16 residents on CP services during phase 2. Distribution of fellows on AP and CP services remained relatively stable, with only one fellow shifting from AP to CP during this time. There were 12 fellows on AP services and 8 fellows on CP services before and during phase 1, and 11 fellows on AP services and 9 fellows on CP services during phase 2.
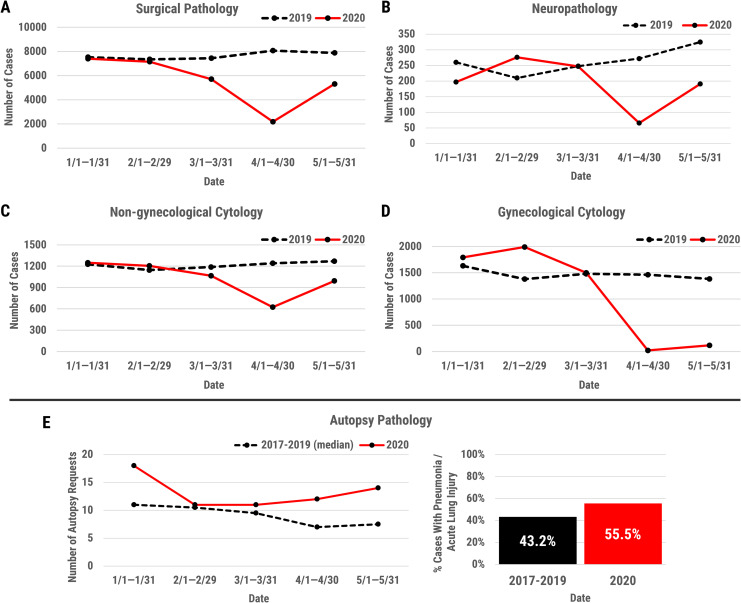
With the implementation of phase 1, the number of trainees on Surgical Pathology continued to be 13 residents on AP services, working either on-site or remotely (Figure 2). This allowed for adequate coverage of all services, including frozen sections, routine and subspecialty cases, biopsy services, and tumor boards. During the phase 1 transition, the state mandate measures and additional precautionary measures taken by our institution led to a further reduction in the surgical pathology workload. Specifically, the volume of cases decreased by up to 75% to 80% during certain weeks of the first height of the pandemic (eg, the week of April 6, 2020) compared to the same time period in 2019 (Figure 3, panel A). The decrease in surgical pathology case volumes and the closure of elective surgical services facilitated our program’s decision to redeploy trainees to CP services. During phase 2, the AP service volumes were 30% to 40% of our usual (eg, the week of April 13, 2020), compared to the same time for the previous year (Figure 3, panel A). At this point, all cases were adequately covered by the AP fellows and our (1) AP-only resident.
Figure 3.
Line graphs depicting the changes in surgical pathology (A), neuropathology (B), nongynecological cytology (C), gynecological cytology (D) case volumes with the onset of the coronavirus disease 2019 (COVID-19) pandemic, including the months of January through May 2020. Case volumes for each service from the same time period in 2019 are also shown. Autopsy cases during the months of January through May 2020 were compared to the median number of cases performed during the years 2017 to 2019 over the same period (E, left panel). The percent of pneumonia and/or acute lung injury (eg, acute, resolving diffuse alveolar damage) findings in autopsy cases performed from January to May 2020, compared to the median number of cases with these findings performed during the same period of the years 2017 to 2019 is shown (E, right panel). For each group, the percentage of cases with pneumonia or acute lung injury findings was determined based on all autopsies performed in that time frame with lung tissue available for examination. Cases with incidental and intravascular pathologies only (eg, small vessel thrombi), but no alveolar pathologies, were not included as positive findings. For January to May 2017 to 2019, the average percentage of positive cases is shown (43.2%), reflecting an expected rate of these pathologies at our institution in the pre-COVID-19 period. For January to May 2020, this rate was 54.2%, emphasizing the need for caution in autopsy performance, even with negative COVID-19 testing.
Overall, the redeployment of trainees to CP services did not diminish patient care. Throughout phases 1 and 2, our pathologist assistants (n = 5) continued their usual rotation schedule, including grossing of routine surgical and biopsy cases, as well as coverage in frozen section areas. With the institutional consolidation of ORs (including temporarily closing down several OR floors in separate buildings), the pathology assistants and their duties were not significantly affected by the trainee redeployment, as they were able to handle the case volume. Although elective surgical procedures were essentially halted during the initial COVID-19 response at our institution, the trainees on AP services continued to have excellent educational opportunities with complex emergent and transplant surgical cases. Interestingly, the usual volume of transplant surgeries increased somewhat at our institution during trainee redeployment, as patients were referred from other centers not performing these procedures during the COVID-19 pandemic.
Neuropathology
Although nonemergent and elective cases were limited during this time, urgent neurosurgical procedures continued throughout for intracranial and spinal tumors in patients with neurological deficits. The total number of NP cases, which are included within the Surgical Pathology volume (Figure 3, panel A), were significantly reduced. Specifically, during certain weeks, our volume was reduced to approximately 25% of our usual during the same period in 2019 (eg, the week of April 1, 2020; Figure 3, panel B). The faculty service was maintained as usual. One of the NP fellows, as mentioned previously, returned to the home institution from an outside rotation during redeployment. Having already completed 8 months of clinical service, this trainee’s return to service allowed for extended graduated responsibility. A rotation at the Harris County Medical Examiner’s office, where our program’s fellow acts as the neuropathologist on-site, was also implemented for the fellow during this period. The NP fellow’s curtailed outside rotation will resume in the first 4 blocks of the new academic year and will be followed by research. All trainee placements during the redeployment were satisfactory and their training was not negatively impacted by the changes.
Cytopathology
In the Cytopathology Laboratory, there are typically 1 to 2 cytopathology fellows and 1 to 2 additional pathology trainees on service during standard operations. A wide spectrum of services is provided, including a superficial fine needle aspiration (FNA) clinic, adequacy assessment for image-guided biopsies performed by radiologists, and rapid on-site evaluation (ROSE) for endobronchial ultrasound (EBUS)–guided FNA and endoscopic ultrasound (EUS)–guided FNA procedures utilizing a combination of on-site evaluation and Telepathology. Additionally, the Cytopathology Laboratory provides evaluation of nongynecological cytopathology specimens, such as bronchioalveolar lavages, brushings, washings, and fluids from various sites, and gynecological cytopathology specimens for the entire 8-hospital system.
During the implementation of phase 1, there was an overall decrease in nongynecological cytopathology case volume, as well as surgical and diagnostic services in the hospital system. However, patients continued to be scheduled for the superficial FNA clinic. Although there was an overall decrease in image-guided biopsies performed by radiologists, a portion of biopsies performed continued to have adequacy assessments by cytopathologists. Rapid on-site evaluation services were still requested for many of the EBUS and EUS-guided FNAs that were performed. During the phase 1 transition and beginning of phase 2 of our trainee redeployment, the volume of cases had decreased to approximately 50% of the usual for specific weeks compared to the same period on 2019 (eg, the week of April 1, 2020; Figure 3, panel C). At this point, specific procedure and operating rooms (ORs) were designated for these types of procedures and required fewer people in order to observe physical distancing measures in a limited space. In EBUS- and EUS-guided FNA cases where ROSE was not requested, due to the desire to decrease potential health care worker exposure to COVID-19 infection, the obtained specimens were handled with increased precautions in the Cytopathology Laboratory (additional safety measures are discussed more in detail under: “Safety, well-being, and resilience”).
Regarding gynecological cytopathology, at the time of our phase 1 transition during trainee redeployment, only infrequent Papanicolaou (Pap) tests from pregnant patients or inpatients were being received for evaluation. Starting on March 30, essentially no Pap tests were received for the outpatient gynecological cytopathology service, due to the closures of physician clinics. Rare exceptions included Pap tests from pregnant patients and inpatient Pap tests or biopsies (Figure 3, panel D). The decrease in Pap test volumes to approximately 1.3% of the usual volume during the same period on 2019 (eg, the week of April 1, 2020) allowed for residents rotating on the gynecological cytopathology service to be redeployed to CP services, with the expectation that trainees would complete their cytopathology training in subsequent months or academic years when the volumes of Pap cases return to baseline. Anatomic pathology fellows covered the cytopathology service during the phase 1 transition and phase 2 (Figure 2). At the time of writing this article, the gynecological cytopathology service has returned to near normal volumes.
Autopsy pathology
Severe acute respiratory syndrome coronavirus 2, as with SARS-CoV and other infectious agents, may carry a risk for personnel involved in autopsy, especially during the use of aerosolizing procedures (eg, use of an oscillating saw). During the SARS-CoV pandemic, for instance, the use of BSL-3 operating principles were employed by some groups for the safety of personnel.18 Accordingly, in February 2020, the early period of COVID-19 spread in the United States, the CDC provided guidance on biosafety and infection control during the collection of postmortem specimens, including at autopsy.19 The emphasis of these guidelines included avoidance of aerosol generating procedures, including use of an oscillating saw, recommendations for engineering controls, and recommendations for PPE use. In particular, the engineering controls recommended by the CDC included (a) use of negative pressure spaces (eg, airborne isolation room for autopsy), (b) a minimum of 6 air changes per hour for older structures and 12 per hour for new structures, and (c) air exhausted directly to the outside of the facility of through a high-efficiency particulate aerosol filter.
Provided these recommendations, early in March 2020, our morgue was evaluated by the heating, ventilation, and air conditioning (HVAC) team at our facility. Testing by HVAC personnel revealed our morgue, which is over 30 years old, did not meet the controls recommended by the CDC. We subsequently confirmed with the Harris County Medical Examiner’s office that they would assist, as needed, in COVID-19 positive cases meeting their typical jurisdictional requirements per the Texas Code of Criminal Procedure Section 49.25. Given these constraints, it became necessary to develop a temporary approach to continue the autopsy service, provide for the safety of personnel, avoid inadvertent performance of SARS-CoV-2 autopsies (provided the relatively unknown safety profile for personnel), and also provide residents with ongoing experience in autopsy.
The autopsy service remained open throughout the pandemic, but our approach was modified based on the needs and concerns outlined above. First, the number of personnel involved in autopsy in our morgue was limited to the minimum number necessary (one autopsy technician and the Autopsy Director). Second, once available in-house and, in coordination with the Molecular Diagnostics medical directors, all decedents had a postmortem nasopharyngeal swab collected for SARS-CoV-2 molecular testing, and cases proceeded in our facility only if the postmortem swab result was negative. Despite these restrictions, the number of autopsies performed to date in 2020, particularly in March to May 2020, exceeded the expected number based on median case numbers per month from 2017 to 2019 (Figure 3, panel E, left). Of the 66 requests for autopsies received during this period, only 6 cases were declined in confirmed or suspected COVID-19 patients, or when there was insufficient clinical information available to make this determination. Despite the performance of only swab-negative autopsies, the rate of pneumonias and acute and resolving lung injury was greater than seen in prior years (Figure 3, panel E, right). Third, N95 masks were utilized in the morgue for swab-negative patients with chest imaging findings of pneumonia, acute respiratory distress syndrome, or other clinical and imaging features of pulmonary disease. Fourth, in these same cases, oscillating saws were not used, to comply with CDC recommendations and in line with studies showing that their use generates fine, respirable particles which may serve to deliver virus to the respiratory tract of personnel.20 Fifth, in persons under investigation (swab-negative), the autopsy was targeted to structures of particular interest to avoid dispersion of potentially hazardous fluids around the morgue in the case of a false-negative result. Sixth, chest blocks, and lungs in particular, were perfused with 10% formalin prior to further examination (lung being further perfused via bronchi after removal with formalin in a steel pan).
An additional major challenge for the autopsy service has been continuing trainee education in autopsy during the pandemic. To this end, from the beginning of our modified workflow, 2 trainees per case assisted the faculty with reviewing all available slide materials (including special stains when indicated), reviewing the patient’s history, reviewing the gross photographs, and crafting the report. These cases were signed out in virtual meetings to review the report and slide findings, while maintaining physical distancing guidelines.
At the time of publication, we have resumed a regular rotation of autopsy faculty and one resident dissecting per case, while applying the procedural modifications described above. This includes performing COVID-19 molecular testing prior to autopsy and applying additional limitations on the extent of the procedure when indicated. The resident assigned to dissection is determined by the complexity of the case, with more senior residents involved in higher complexity cases. The resident involved in the prosection shares their findings with an additional resident assigned to the case, and some cases will include incoming first-year trainees. For routine cases where COVID-19 is not a concern, new trainees will also work side-by-side with the designated autopsy faculty. In this way, during the course of an ongoing pandemic with unique risks to autopsy personnel, incoming Pathology residents will receive autopsy education both firsthand and via work with faculty and their senior colleagues.
Training in Laboratory Medicine During the Coronavirus Disease 2019 Pandemic
Molecular diagnostics
The COVID-19 pandemic has created an unprecedented singular need for SARS-CoV-2 molecular testing. At our institution, the Molecular Diagnostics Laboratory has played a critical role in validating and performing these tests on multiple platforms for the diagnosis and management of COVID-19 patients. One of the earliest adaptations made to accommodate the high demands for COVID-19 testing during the ongoing pandemic was to substantially restructure the workflow of our Molecular Diagnostics Laboratory. These adjustments were and continue to be implemented in a stepwise manner, with close involvement by our residents and fellows.
Restructuring of the Molecular Diagnostics Laboratory began with the send out of all non-SARS-CoV-2 requests for molecular testing to reference laboratories, including oncology-related and other infectious disease testing. At the time of this article, our Molecular Diagnostics Laboratory remains solely dedicated to performing SARS-CoV-2 molecular diagnostic assays, in contrast to the large number of molecular assays routinely performed before the onset of the pandemic (78 items on the College of American Pathology activity menu). The switch to sending out oncology-related molecular tests provided the Molecular Pathology Genetic (MGP) Fellow an opportunity to be involved, along with the Medical Directors of Molecular Pathology and Anatomic Pathology, in selecting which molecular tests and panels at specific reference laboratories would best serve our patients. Working with these Medical Directors, and with a designated member of our Laboratory Information System (LIS) information technology (IT) team, the MGP fellow also prepared physician-friendly reporting templates for molecular diagnostics results from reference laboratories to be integrated into surgical and cytopathology reports. The focus was on standardizing the comprehensive reporting of send out oncology-related molecular testing as well as providing the MGP fellow a unique educational opportunity that would be of future value in practice.
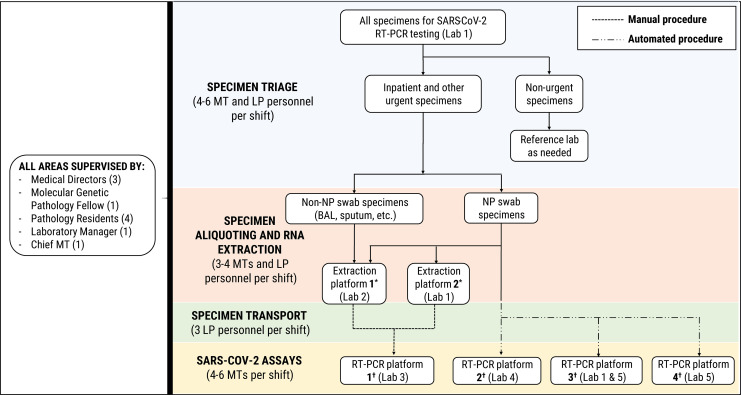
The first SARS-CoV-2 in-house molecular testing at our institution began in March 2020, with an average daily testing volume of 93.7 tests. Over April and May 2020, our SARS-CoV-2 molecular diagnostic test volumes increased to daily averages of 361.6 and 442.9 tests per day, respectively. In order to expand the capacity for COVID-19 molecular testing, the Molecular Diagnostics Laboratory repurposed instruments for RNA extraction and amplification now used exclusively for SARS-CoV-2 testing, as well as actively acquiring and validating new instruments for automated, high-throughput molecular testing. This required moving certain testing operations into new laboratory spaces and the development of an internal courier system for specimens (Figure 4). Additional staff, including medical technologists, were hired and trained. Laboratory personnel from other laboratory areas, as well as personnel from an institutional labor pool, were also recruited and trained to assist with specimen triage, accessioning, transportation, and preparation for testing, as shown in Figure 4. Labor pool personnel included registered nurses and other clinical staff who typically work in the ORs at our institution.
Figure 4.
Specimen triage and distribution of the analytic workflow for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) molecular diagnostic testing performed on various RT-PCR platforms in the molecular diagnostics laboratory at Houston Methodist Hospital. Locations of various instruments within 5 separate laboratory areas are denoted by (Lab #). (*) RNA extraction platforms include EZ1 advanced XL (Qiagen) and qiasymphony SP (Qiagen). (†) SARS-CoV-2 RT-PCR assay platforms include (1) ABI 7500 fastdx (Applied Biosystems), (2) panther fusion (Hologic, Inc), (3) genexpert infinity (Cepheid) and genexpert Dx GX-IV (Cepheid), and (4) filmarray 2.0 (BioFire Defense, LLC). BAL indicates bronchoalveolar lavage; LP, labor pool personnel; MT, medical technologist; NP, nasopharyngeal; RT-PCR, reverse transcription polymerase chain reaction.
During redeployment, 4 additional Pathology trainees joined the Molecular Pathology service, along with the MGP fellow. This is in contrast to the fellow and occasional Pathology resident typically rotating on this service. The molecular team, including additional assigned trainees, the MGP fellow, and molecular medical director, met several times daily while observing physical distancing. This included receiving daily assignments to specific areas of the laboratory or testing platforms, as well as an evening debrief to provide updates on the day, ask and answer questions, and discuss ongoing assay validations or COVID-19-related issues and their potential impact on molecular-based testing. Additional trainee duties included working closely with the medical directors and laboratory manager to oversee and track the workflow and testing reagents, participating in the interpretation and sign out of SARS-CoV-2 reverse transcription polymerase chain reaction molecular test results, and troubleshooting technical and interpretive issues with these assays. In addition, trainees were involved with writing verification and validation plans for new SARS-CoV-2 molecular testing platforms acquired by the laboratory, performing literature reviews, and investigating other potential protocols for nucleic acid extraction and amplification.
Transfusion medicine
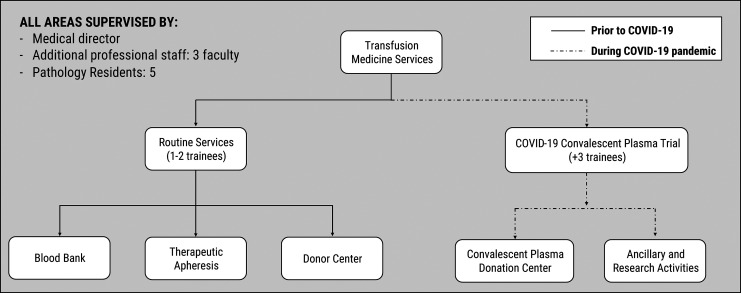
During standard operations at our institution, there are typically 1 to 2 trainees assigned to the Transfusion Medicine service. Their duties include covering the apheresis clinic and the blood donor center, as well as conducting reviews of blood product utilization, reviewing antibody panels, and managing clinical consults. The COVID-19 pandemic led to significant restructuring of the transfusion medicine workflow, including the addition of new clinical service areas. This required the redeployment of 5 trainees to the Transfusion Medicine service, as shown in Figure 5.
Figure 5.
Flowchart depicting changes in the transfusion medicine services and workflow during the coronavirus disease 2019 (COVID-19) pandemic. Routine services including blood bank, therapeutic apheresis, and donor center continued operations and were staffed by 1 to 2 trainees involved in prospective blood utilization reviews and supervision/management of apheresis patients and blood donors. With the initiation of an in-house COVID-19 convalescent plasma trial, our services required a significant diversion of transfusion medicine personnel and resources, with 3 additional trainees redeployed to assist. Their duties included triaging, screening, and collecting clinical information from plasma donors, donor recruitment, and research activities such as aggregation and analysis of data.
Apheresis clinic
Our apheresis clinic is visited by 12 to 20 patients per day in routine circumstances. During the pandemic, patient volumes decreased enough to require only one trainee to cover this clinic. A significant challenge encountered was the clinical presentation of apheresis patients with symptoms mimicking those of COVID-19, including shortness of breath, arthralgias, and even fever. Similar symptoms may be seen in patients with myasthenia gravis, sickle cell disease with acute chest crisis, and lung transplant rejection, for whom therapeutic apheresis is indicated. Regarding inpatients, close communication and coordination with the clinical teams was required. For those patients with a clinical suspicion of SARS-CoV-2 infection, molecular testing was performed or imaging modalities were reviewed to rule it out. Overall, very strict precautions were taken by trainees and other clinic personnel at all times.
Blood bank laboratory and donor center
An early event in the ongoing pandemic was the cancellation of elective surgeries and standard preoperative testing, which decreased the workflow in our blood bank laboratory. The decrease was not as significant, because transplants in our high-volume multi-organ transplant services continued to occur daily. Regarding the donor center, blood drive volumes also decreased, largely due to the physical distancing restrictions of sponsoring institutions. Nonetheless, in-hospital blood drives continued, and a slightly increased number of donors was observed overall. Trainee duties, supported by the attendings on service, focused on remaining vigilant for possible blood product shortages by virtually monitoring our inventory in real time through a recently implemented custom online inventory tracker, and performing both retrospective and prospective daily reviews of blood product utilization. Trainees were also actively involved in laboratory management decisions, including communication with blood supply centers regarding available products, staffing issues, and equipment maintenance during a pandemic. Reports on these areas were given daily during the COVID-19 virtual seminar series.
Convalescent plasma patient donor clinic
Our institution was the first academic medical center in the United States to obtain an emergency IND approval by the FDA for a protocol to transfuse COVID-19 patients with convalescent plasma from recovered patients.21 Trainees redeployed to the Transfusion Medicine service reviewed and discussed the FDA-approved IND protocol and the consent forms with their attendings. In addition, they delivered virtual presentations in our daily COVID-19 seminar series, highlighting crucial elements of the process and protocol. One example is a discussion of the differences in the process of obtaining patient consent within a trial, compared with our usual consenting procedure in the apheresis clinic.
Along with several research assistants, the residents on the Transfusion Medicine service were trained in convalescent plasma donor recruitment and scheduling. They became an integral part of the recruitment team, explaining the procedure and process to potential donors and addressing their questions. Under supervision, the trainees were actively involved in triaging donors for their physical examination and predonation COVID-19 testing, determining if donors met the designated eligibility requirements of the study, assisting with obtaining donor consent, and collecting clinical and demographic information from the donors for further data analysis. Additionally, the trainees collaborated in the creation of a website to facilitate the process of donor recruitment for the convalescent plasma protocol. This website has served to expedite the recruitment and scheduling process for donors and the collection of patient information for enrollment.
With the increased number of donors recruited for the study, new equipment for plasma collection was acquired in the donor clinic. Trainees were actively involved in the validation of these instruments, as well as the development of precision assays required to determine antibody titer concentrations of donated units. In addition, the trainees worked daily on data collection and analysis, including tracking the clinical course and the evaluation of various clinical and laboratory parameters of patients who had received convalescent plasma transfusions. The data generated was relayed to the faculty for consideration of any important decisions regarding the ongoing study.
Histocompatibility and transplant immunology/diagnostic immunology/chemistry
At our institution, the HLA, Diagnostic Immunology, and Chemistry laboratories typically have 2 to 4 trainees per block during standard operation. As shown in Figures 1 and 2 (phase 1 transition), during the COVID-19 pandemic, 5 to 6 trainees were redeployed to help cover these CP services. The trainees in these 3 laboratories worked very closely together and met on a daily basis to discuss ongoing tasks, with regular discussions and updates to the group via our daily COVID-19 virtual seminar.
Our institution is one of the most comprehensive transplant centers in the United States, and as such, our HLA laboratory is high volume and high complexity. With the onset of the COVID-19 pandemic, transplant procedures continued; however, there was a marked decrease in routine outpatient pre- and posttransplant evaluations. This reduction of routine specimen volume resulted in a shift in the laboratory workflow, allowing the staff and the trainees to focus on optimizing and streamlining existing assays, developing new assays, and collaborating on the convalescent plasma study to test for the presence of HLA antibodies in the donor units.
During the first stage of the COVID-19 pandemic, the Diagnostic Immunology and Chemistry laboratories also adjusted their workflows by maintaining the usual number of trainees, decreasing personnel exposure through purposeful scheduling, and sending out tests with low volume to reference laboratories. As the demand for COVID-19 serology testing increased, new assays were developed and validated, resulting in an increased workload for faculty, trainees, and remaining staff. Changes in the workflow were put in place, but were considered flexible. For example, serum and urine protein electrophoresis and immunofixation assays were initially batched to twice a week; however, when these tests became prerequisites for convalescent plasma donors participating in our FDA-issued IND protocol, the frequency was again changed to 3 times a week. Redeployed trainees on the chemistry and immunology services updated their colleagues on a daily basis via the COVID-19 virtual seminar series.
A very important task assigned to our clinical chemistry fellow and other redeployed trainees in the laboratory was the investigation of new COVID-19-related assays. The assignments included sharing literature with managers and directors, writing validation plans, and resolving various technical and interpretive issues that arose during the course of assay validation/verification. Some examples of these tests include point-of-care serology testing for detection of COVID-19-specific immunoglobulin G and immunoglobulin M antibodies, interleukin-6 measurement, and an additional test that evaluates broad cytokine activity (12 cytokines).
As in many health care institutions, the demand for COVID-19 antibody testing became an important consideration for our hospital system. The trainees redeployed to the HLA, Diagnostic Immunology, and Chemistry services also became actively involved in the development of an in-house COVID-19-specific enzyme-linked immunosorbent assay (ELISA)-based antibody test. They reviewed previously published protocols, as well as other testing modalities approved by the FDA for emergency use. They also assisted with the acquisition of instruments, tests, and reagents required to perform these protocols, quickly learning the difficulties of this task amid the COVID-19 pandemic. The trainees helped find alternate solutions for assay reagents when needed and the validated serology assay is currently being performed in-house, with the goal to have high-throughput capabilities in the very near future. Updates on the work regarding various tests under development were provided to all trainees during our daily COVID-19 virtual seminar series.
Hematology/flow cytometry/coagulation
During the COVID-19 pandemic, testing volumes and regular trainee duties in the Hematology, Flow Cytometry services underwent no significant changes. While regular specimen volumes slightly decreased in the Flow Cytometry laboratory, the overall Hematopathology service, including bone marrow aspirates and biopsies, fluid analyses, and cell counts, continued as usual. The exception to this was the management of bronchoalveolar lavage (BAL) specimens. Due to the lack of an appropriate biological safety cabinet in the Hematology section of the Core lab, cell counts for BAL specimens were instead performed in the Cytopathology Laboratory which has the necessary equipment to safely handle these specimens.
Our Coagulation laboratory also largely continued operations as usual, with a slight decrease in routine coagulation tests for outpatient workups. The trainee on Coagulation service performed a literature review centered on coagulation derangements in COVID-19 patients for the daily virtual seminar series. The results of this research highlighted that increases in D-dimer and fibrinogen levels in COVID-19 patients were closely associated with increased risks of thrombotic events and even death.22-27 This led to a retrospective review within the laboratory, which identified an increase in the number of orders for D-dimer testing (3.3-fold compared to the previous year), while orders for fibrinogen levels remained essentially unchanged (data not shown). Based on the trainee’s efforts, laboratory managers preemptively increased inventory supplies, allowing continued capacity for the increased testing demand.
Informatics
In response to our department’s preparedness plan, virtual private network access was enabled for the bioinformaticians, as well as for trainees who did not previously have access. They were able to obtain secure remote access to the hospital network from home for routine tasks, in addition to any possible COVID-19-related assignments. All conferences and meetings requiring social gathering were converted to virtual events using different web conferencing and videoconferencing applications (eg, Cisco Webex, Zoom). From our experience, Microsoft Teams has proven useful for coordinating many activities, as well as providing a chat interface for communication.
All COVID-19-related IT requests were given the highest priority within our institution and were tracked closely. The 2 Clinical Informatics fellows actively assisted in building new COVID-19-related tests in our LIS and also created an analytics dashboard to visualize and track all testing. This dashboard has been essential to hospital leadership in following the COVID-19 pandemic locally. In addition, the Informatics fellows have been extensively involved in data queries and analytics related to COVID-19 clinical practice as well as research. During redeployment, 2 additional trainees were assigned to support departmental COVID-19 informatics and research efforts, assisting with data gathering from the electronic health record and LIS. Trainees also designed and developed informatics-based projects to solve turnaround time issues and improve overall efficiency within our clinical laboratories, due to a redirection of medical technologists’ time and efforts amid the COVID-19 pandemic. One example is an informatics project developed for the Diagnostic Immunology Laboratory, focused on the digitization of a card database for immunofixation results used by the protein electrophoresis service. Leveraging recent advances in machine learning–based optical character recognition,28 trainees redeployed to Informatics constructed an electronic digital database system with robust searching options to address the outdated, manual card system. The ultimate goal being to entirely replace the paper-based index card workflow, while allowing rapid review of past immunofixation results.
Microbiology
With the onset of the COVID-19 global pandemic, the Microbiology laboratory experienced an unprecedented decrease in the routine volume of specimens. During redeployment, 2 trainees were assigned to the Microbiology service. They were actively involved with the writing and implementation of new assay validation plans (such as extended Mycobacterium tuberculosis testing on nontypical specimens), a quality management project involving antimicrobial susceptibility testing, and a process improvement project. Trainees also participated in key infection control and laboratory management meetings, allowing for them to identify and help troubleshoot day-to-day issues specific to Microbiology. For example, the trainees have been involved in discussions that included maintaining appropriate staffing (during a pandemic and during “normal” times) in a laboratory that runs operations 24/7 and analyzing reimbursements for tests performed, among others. They designed plans to evaluate these issues thoroughly within the laboratory, and to come up with solutions. At the time of this article, the trainees redeployed to Microbiology have been working on an evaluation of testing volumes and staffing for each laboratory section. An additional trainee project during the redeployment included an evaluation of the significance of antibiotic susceptibility testing for morphologically distinct microorganisms of the same species, present in a single patient specimen. The findings resulted in a change to the standard operating procedure for this test, an improvement in turnaround times, and a higher quality of patient care.
Safety, Well-Being, and Resilience
Throughout our institutional response and interdepartmental trainee redeployment, a focus on trainee safety and well-being was of utmost importance. Early on in the pandemic, our institution implemented restricted access and screening measures across the system to protect both patients and employees. Our institutional policy was that employees who felt ill should call Employee Health Services (EHS) to get COVID-19 molecular testing; if negative, they were allowed to return to work after being asymptomatic for at least 3 days. If positive, employees could return to work after testing negative and remaining asymptomatic. Furthermore, within our department, any trainee who felt ill was required to send an email to the program director, the chiefs, and the faculty with whom they were working, and to follow the same EHS requirements. At the time of this article, at least a handful of trainees have reported that they were tested for SARS-CoV-2, none with a positive result. In addition, employee surveillance testing was made available for all employees on a voluntary basis. Of note, employees who needed to quarantine were offered the opportunity to request for 1 to 4 weeks of furnished dormitory housing (at no cost, and with cleaning service included) at Rice University through May 31. Similarly, the University of St. Thomas offered free dormitory housing. Finally, the use of rooms near the TMC was offered to employees from a local hotel at no charge.
In addition to other measures, our hospital policy required that all employees wear face masks at all times. These were provided to each department and distributed on a daily basis. Disinfectant wipes were available to clean designated working areas and all microscopes. Hand sanitizer stations were distributed throughout all hospital areas, with physical distancing and appropriate hand hygiene reinforced in daily practices. Importantly, training on how to use power air purifying respirator suits was provided and required for all current trainees during the pandemic. Given the nationwide PPE shortage, our institution quickly established strict criteria for responsible usage of equipment such as N95 masks and face shields. The details specifying these new policies were distributed to all employees, and allowed our institution to save equipment for critical use during the crisis. At the time of this article, we have not experienced a lack of required PPE.
Within our clinical laboratories, all specimens received were handled using standard precautions, inside an appropriate biological safety cabinet, and with the use of required PPE. In the AP areas, all individuals, including pathology assistants and trainees, working on surgical specimens positive for or with the suspicion for COVID-19 infection followed the protocol of maintaining specimens in formalin for 24 hours prior to gross dissection and the use of N95 masks and face shields. During frozen sections, the procedures our institution has in place were strictly followed, including the use of N95 masks and face shields. These specimens were also only handled within the ventilation system of AP grossing stations, which may decrease any aerosol propagation effect. Details of the safety measures followed during autopsy procedures were previously discussed in “Training in anatomic pathology during the COVID-19 pandemic.”
To support trainees, the GME division at our institution organized seminars focused on techniques and tips to promote well-being. Resilience and mindfulness workshops have been in place for sometime, but reminders, including a daily video communication termed “Mindfulness Pause” at noon on weekdays were also instituted. A continuous education course focused on mindfulness has been available for 2 years and continues to be open to trainees interested in participating. Finally, information and advice regarding the financial impact of COVID-19 through open forums was made available, as well as financial reimbursement for trainees in need of childcare services.
On a daily basis, all employees received (and continue to receive) an email series titled “Taking Care of You,” geared to helping them to employ self-care during times of stress. These emails consisted of daily prayers, or drawings sent from schools, organizations, businesses, churches, or individuals from the community, to inspire health care workers. Lastly, our institution’s Department of Spiritual Care and Education performed live streaming events for patients and employees to tune in, including morning prayers, Roman Catholic Mass, and live music. The hospital chapel has remained open at all times, with strict guidelines that included physical distancing.
Unexpected Negative Implications for Pathology Trainees During Coronavirus Disease 2019
The ongoing pandemic has impacted and will continue to directly impact all graduate medical trainees. From our experience within AP, the decrease in surgical and cytopathology case volumes temporarily resulted in fewer specimens being reviewed and managed by pathology trainees. Overall, this has led to fewer specimen grossing opportunities, fewer sign out experiences, fewer on-site adequacy evaluations and FNA procedures, and fewer overall routine cases for all trainees in our program during the first surge of the pandemic. Similarly, Pathology trainee participation in autopsy procedures was significantly reduced to decrease the possibility of their exposure to COVID-19, as discussed above. Our trainee redeployment strategy has been quite successful, as it allowed trainees to be involved in a most unique learning opportunity on very active CP services. We are confident that our trainees’ redeployment experience during the pandemic has not negatively impacted their overall education; we have recently experienced increased numbers of cases in AP due to the rescheduling of delayed surgical procedures. Additionally, as operations have begun to return to a more normal state, a decision was made to reduce the frequency of our COVID-19 virtual conference to twice a week, in order to allow for trainee coverage of resumed AP services and duties.
Within our CP laboratories, trainees have been assigned to cover both routine broad-based services as well as those which were focused on COVID-19-related testing and projects. For example, the trainees assigned to Transfusion Medicine as a group self-selected to various duties including the convalescent plasma trial, the donor center (recruiting convalescent plasma donors as well as others), the apheresis clinic, and within our blood bank laboratory. Trainees assigned to the molecular service during redeployment were limited to coverage of COVID-19-related testing, as all other usual molecular oncology-related and infectious disease testing at our institution has been sent to reference laboratories. While the absence of oncologic and other infectious disease molecular testing may be considered an educational disadvantage during the pandemic, our trainees will have the option for an elective beyond the required rotation in the molecular laboratory under more usual circumstances. Additional lectures for trainees on these molecular topics are also planned to compensate for the decreased on-site live experience. At the time, there have not been any major concerns from our faculty about these issues, but this remains to be addressed in the near future.
Finally, it is worth mentioning that while our institution and the trainees in our program have worked very hard to adapt to physical distancing measures, the negative implications of this on trainee education, well-being, and mental health are unknown and are worth further investigation. Overall, the trainees in our programs have reported that they greatly miss participating in face-to-face regular microscope sessions, noon conferences with lunches, and other usual social and educational gatherings. Although the long-term effects of these necessary physical distancing measures may not be obvious yet, extra efforts are being considered within our program to ensure that new incoming Pathology trainees have opportunities to meet and regularly interact with their peers and all members of our department.
Discussion
The challenges faced by academic medical centers during the ongoing COVID-19 pandemic are numerous. Our hospital system, GME division, and department have remained committed to providing an excellent training environment for resident and fellow physicians by promoting comprehensive learning opportunities, even during difficult times. Lessons learned in previous catastrophic events by our institution have demonstrated the impact unprecedented adverse situations may have on practicing physicians, as well as on Pathology trainees.3-5 The most recent adverse situation, Hurricane Harvey, was experienced by approximately half of our current trainees and by the majority of our faculty and staff. During that event, the faculty and administrative staff quickly established chains of communication among department members, which proved vital to maintain standard operations. In addition, special emphasis was placed on supporting trainee education, as well as their safety and well-being, which included a daily check-in procedure of all trainees with the chief residents and fellow to determine the continuing safety of everyone. Similar priorities were taken into account during the planning and implementation of our departmental strategy for trainee redeployment during the COVID-19 pandemic. Our Pathology training program’s response included the organization and execution of an intradepartmental stepwise redeployment plan for Pathology trainees. By initially dividing our trainees into 2 teams working on-site or remotely from home, we were able to maintain physical distancing and rely on a healthy workforce to cover basic Pathology services if any trainee became ill, as reported by programs of other subspecialties.11,29 In our experience, close communication between our program directors, trainees, medical directors, and laboratory managers was crucial to assess the quickly changing needs, making swift adoption of virtual meeting platforms a key requirement to our response. The flexibility to implement changes in the schedules and distribution of Pathology trainees was also critical to our program’s response, and proved beneficial to our trainees, to our faculty, and most importantly, to our patients. While we did encounter some resistance from a few trainees in the initial stages of our redeployment, this was largely due to the uncertainty of an unprecedented situation. There were concerns regarding what their new duties would entail and how this might affect their training schedules and eligibility for future board certification. However, our trainees were aware that the program directors had carefully evaluated their schedules and were reassured that they would continue to meet all training requirements, even during this difficult time.
Medical education while maintaining physical distancing has proven to be a difficult and unexpected barrier to overcome during the COVID-19 pandemic. 30 In our Pathology residency and fellowship training programs, the trainees routinely convene for daily interactive didactic conferences, microscope sessions, and tumor boards. All of these activities are also attended and supported by our departmental faculty and are considered protected educational time for trainees. From our experience, the transformation of educational activities from in-person to virtual conferences was relatively straightforward and seamless. Similar to the experiences reported by other nonpathology residency programs, we found that utilization of videoconferencing modalities allowed us to maintain a focus on academics and educational opportunities during the COVID-19 pandemic.10,29-33 For our Pathology trainees, these virtual meeting tools have become crucial for maintaining communication and enhancing educational activities.
As others have noted, a lesson learned from our program’s response to the COVID-19 pandemic is that flexibility and an openness to trying new strategies within a training program may be quite valuable in promoting significant educational experiences for trainees.13 For example, during redeployment, the trainee rotating on Laboratory Management was assigned the task of conducting “daily rounds” through all areas of Laboratory Medicine. The trainee had the opportunity to engage daily with each of the laboratory medical directors and managers, to understand the challenges associated with altered workflows, specimen volumes, and reagent inventory throughout our laboratories, and to facilitate involvement of trainees in management decisions, strategies, and troubleshooting in all areas. These COVID-19 redeployment duties differed markedly from those normally performed during this resident rotation, which were previously centered around our core lab and one-on-one teaching with that medical director. After this experience, and through resident feedback sessions, trainees have requested permanent implementation of this change as part of our program’s Laboratory Management rotation. There was also tremendous educational benefit from this activity for all trainees, as they received daily updates of service work, projects, research, and other activities taking place throughout all laboratory areas and CP services via the COVID-19 virtual seminar series. At the request of the trainees, our program plans to transition this into weekly Laboratory Medicine rounds as part of our program’s regular conference schedule following resolution of the COVID-19 pandemic.
In our experience, the redeployment of trainees into various laboratories and CP services allowed them to work very closely with CP faculty and laboratory managers, and to take on invaluable educational opportunities during our department’s emergency response. Some of these unique opportunities included actively participating in the development, validation, verification, interpretation, and continued quality improvement of testing modalities for COVID-19. Many trainees were able to draft validation and verification plans and become involved in LIS test builds and IT validations for new tests. As these occurred at an accelerated rate during the redeployment compared to standard operations, trainees were able to participate in the process of assay development and validation from start to finish. Multiple trainees also became actively involved in the convalescent plasma IND protocol and process,21 while redeployed to the Transfusion Medicine service within our department, leading to additional unique learning and research opportunities. It is worth mentioning that the projects and activities taken on by trainees redeployed to different laboratories during the COVID-19 pandemic have led to multiple planned manuscripts and abstracts, as well as inspiring many trainees to develop a variety of short- and long-term research projects.
One critical area of research interest is the issue of trainee burnout and psychological well-being during the COVID-19 pandemic.34 An in-depth discussion about this topic is beyond the scope of this article; however, it may be beneficial to briefly address key points gleaned from our institutional experience to stimulate further investigation in this field. Trainee burnout may be exacerbated by the general sense of fear and anxiety engendered by the severity of the COVID-19 pandemic, and further complicated by the strain that it has imposed on all facets of our health care system. The significant disruption to trainee rotation schedules and standard education modalities can also lead to a sense of apprehension and demoralization regarding professional development.9 Moreover, the impact of social media and various forms of news media have made it nearly impossible for trainees to escape the hysteria and controversies surrounding the pandemic, which may contribute to burnout and affect the well-being of trainees.35 Previously published studies on this topic have shown that highly actionable tools requiring minimal time to learn and implement (so-called “micro-practices”) may be effective to combat stress and burnout. These techniques could potentially prove useful tools for trainees, and some of these practices have already been implemented in our institution. For example, the daily COVID-19 virtual meeting series was a recurrent departmental event that served as a cue for a wellness self-check among colleagues.36 This series became a mechanism of reassurance and motivation among trainees of all levels in our program, and we did not alter the frequency until operations returned to a relatively normal state. Additionally, a previous study at our institution identified that volunteerism during Hurricane Harvey was a method used by trainees to combat stress and burnout and to boost resilience.37
The wealth of unique educational experiences, laboratory service learning, and research opportunities spawned from this exceptional situation for Pathology trainees at our institution has been unprecedented and profound. Other Pathology training programs around the United States, whether at large academic institutions such as ours or at smaller community-based locations, can potentially adopt a strategy similar to the one we have described, providing trainees with continuing education, service responsibilities, safety, and adequate supervision even during adverse situations such as the COVID-19 pandemic. This is beneficial to CP laboratories and their workflows, as well as the overall resident and fellow training experience. In addition, we believe that the approaches we have outlined to mitigate potential obstacles and pitfalls of this redeployment process can be utilized by other training programs to facilitate the execution of a similar response at their institutions, including the reassurance to trainees that any changes implemented would not affect their ACGME requirements for completion of training, the promotion of trainee education by reestablishment of regularly scheduled didactic conferences when possible, and the practicing of physical distancing and other measures to support overall trainee health and well-being. At the time of this article, many cities and states across the United States have begun the process of reopening businesses and resuming operations; however, the stark reality of continuing COVID-19 spread, along with the need for physical distancing and other measures, still remains a significant concern. It is our hope that publication of our recent experiences regarding restructuring and trainee redeployment during this pandemic may help guide other residency and fellowship training programs to craft emergency responses that ensure the health and well-being of trainees, while also enhancing their learning experience through the diverse array of unprecedented CP and Laboratory Management opportunities that have arisen from this pandemic.
Acknowledgments
The authors would like to acknowledge the trainees, faculty, and staff in the Department of Pathology and Genomic Medicine at Houston Methodist Hospital for their support of and participation in our training program’s COVID-19 trainee redeployment plan. The authors would also like to recognize Bonnie Phillips for contributing with the collection of data from the coagulation laboratory; and pathology assistant Angela M. Schniederjan for contributing details of her experience with the trainees during the pandemic.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ahmed N. Shehabeldin  https://orcid.org/0000-0002-4878-3898
https://orcid.org/0000-0002-4878-3898
Dina R. Mody  https://orcid.org/0000-0002-5465-0311
https://orcid.org/0000-0002-5465-0311
Jessica S. Thomas  https://orcid.org/0000-0003-0053-3467
https://orcid.org/0000-0003-0053-3467
References
- 1. RMS Event Report. Tropical storm Allison, June 2001 Risk Management Solutions Website. 2001. Accessed June 6, 2020 https://forms2.rms.com/rs/729-DJX-565/images/tc_2001_tropical_storm_allison.pdf
- 2. Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267–1269. doi:10.1097/ACM.000000000000.2696 [DOI] [PubMed] [Google Scholar]
- 3. Delaughter G. Texas Medical Center makes preparations ahead of possible flooding from Harvey Houston Public Media Website. 2017. Accessed June 3, 2020 https://www.houstonpublicmedia .org/articles/news/2017/08/25/232835/texas-medical-center-makes-preparations-ahead-of-possible-flooding-from-harvey/
- 4. Duhon DJ, Cook J, Bernard DW, Schwartz MR. Diagnosing Harvey: an anatomic pathology department’s response to a disaster situation [abstract]. Arch Pathol Lab Med. 2018;142:e2–e202. doi:10.5858/arpa.2018-0293-AB 30207482 [Google Scholar]
- 5. Duhon D, Cook J, Schwartz MR, Bernard DW. Diagnosing Harvey: a laboratory medicine department’s response to a disaster situation. Am J Clin Pathol. 2018;150:S166–S167. doi:10.1093/ajcp/aqy112.384 [Google Scholar]
- 6. Coronavirus disease 2019 (COVID-19) situation report—51. World Health Organization Website. 2020. Accessed May 6, 2020 https://www.who.int/emergencies/diseased/novel-coronavirus-2019/situation-reports. (Situation report-51).
- 7. The prevailing pandemic of influenza. JAMA. 2020;323:1414–1415. doi:10.1001/jama.2020.4357 [DOI] [PubMed] [Google Scholar]
- 8. Morens DM, Daszak P, Taubenberger JK. Escaping Pandora’s box—another novel coronavirus. N Engl J Med. 2020;382:1293–1295. doi:10.1056/NEJMp2002106 [DOI] [PubMed] [Google Scholar]
- 9. Alvin MD, George E, Deng F, Warhadpande S, Lee SI. The impact of COVID-19 on radiology trainees. Radiology. 2020;296:246–248. doi:10.1148/radiol.202001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chong A, Kagetsu NJ, Yen A, Cooke EA. Radiology residency preparedness and response to the COVID-19 pandemic. Acad Radiol. 2020;27:856–861. doi:10.1016/j.acra.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nassar AH, Zern NK, McIntyre LK, et al. Emergency restructuring of a general surgery residency program during the coronavirus disease 2019 pandemic: the University of Washington experience [published online April 6, 2020] JAMA Surg. 2020. doi:10.1001/jamasurg.2020.1219 [DOI] [PubMed] [Google Scholar]
- 12. Stoj VJ, Grant-Kels JM. Dermatology residents and the care of patients with coronavirus disease 2019 (COVID-19). J Am Acad Dermatol. 2020;82:1572–1573. doi:10.1016/j/jaad.2020.03.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crosby DL, Sharma A. Insights on otolaryngology residency training during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020;163:38–41. doi:10.1177/0194599820922502 [DOI] [PubMed] [Google Scholar]
- 14. Gehrie E, Tormey CA, Sanford KW. Transfusion service response to the COVID-19 pandemic [published online June 25, 2020] Am J Clin Pathol. 2020:aqaa111 doi:10.1093/ajcp/aqaa111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pambuccian SE. The COVID-19 pandemic: implications for the cytology laboratory. J Am Soc Cytopathol. 2020;9:202–211. doi:10.1016/j.jasc.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi:10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Texas Department of State Health Services COVID-19 Dashboard. Texas Department of State Health Services Website. 2020. Updated daily Accessed May 4, 2020 http://dshs.texas.gov/coronavirus/additionaldata/
- 18. Li L, Gu J, Shi X, et al. Biosafety level 3 laboratory for autopsies of patients with severe acute respiratory syndrome: principles, practices, and prospects. Clin Infect Dis. 2005;41:815–821. doi:10.1086/432720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19 interim guidance U.S. Centers for Disease Control and Prevention Website. 2020. Updated June 4, 2020 Accessed June 6, 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html
- 20. Pluim JME, Bou LJ, Gerretsen RRR, Loeve AJ. Aerosol production during autopsies: the risk of sawing in bone. Forensic Sci Int. 2018;289:260–267. doi:10.1016/j.forsciint.2018.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–1690. doi:10.1016/j.ajpath.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi:10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi:10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi:10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi:10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020;2:1379–1382. doi:10.1016/j.jaccas.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi:10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tesseract-OCR [computer program] Version 4.1.1 Google; 2006. [Google Scholar]
- 29. Bray DP, Stricsek GP, Malcolm J, et al. Letter: Maintaining neurosurgical resident education and safety during the COVID-19 pandemic. Neurosurgery. 2020;87:E189–E191. doi:10.1093/neuros/nyaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JSE, Chan JJI, Ithnin F, Goy RWL, Sng BL. Resilience of the restructured obstetric anaesthesia training program during the COVID-19 outbreak in Singapore. Int J Obstet Anesth. 2020;43:89–90. doi:10.1016/j.ijoa.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng A, Wang JJ, Tsui BCH. Keeping trainees safe in a pandemic: the evolving role of medical simulation training [published online April 20, 2020] Can J Anaesth. 2020. doi:10.1007/s12630-020-01662-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vargo E, Ali M, Henry F, et al. Cleveland clinic Akron general urology residency program’s COVID-19 experience. Urology. 2020;140:1–3. doi:10.1016/j.urology.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95:1152–1154. doi:10.1097/ACM.0000000000003419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah K, Chaudhari G, Kamrai D, Lail A, Patel RS. How essential is to focus on physician’s health and burnout in coronavirus (COVID-19) pandemic? Cureus. 2020;12:e7538 doi:10.7759/cureus.7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw SCK. Hopelessness, helplessness and resilience: the importance of safeguarding our trainees’ mental wellbeing during the COVID-19 pandemic. Nurse Educ Pract. 2020;44:102780 doi:10.1016/j.nepr.2020.102780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fessell D, Cherniss C. Coronavirus disease 2019 (COVID-19) and beyond: micropractices for burnout prevention and emotional wellness. J Am Coll Radiol. 2020;17:746–748. doi:10.1016/j.jacr.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeo CJJ, Roman GC, Kusnerik D, et al. Trainee responses to hurricane Harvey: correlating volunteerism with burnout. Front Public Health. 2018;6:224 doi:10.3389/fpubh.2018.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]