Abstract
Background:
Cancer-cachexia is associated with chronic inflammation, impaired muscle metabolism and body mass loss, all of which are classical targets of physical exercise.
Objectives:
This systematic review and meta-analysis aimed to determine the effects of exercise on body and muscle mass in cachectic cancer hosts.
Data Sources:
PubMed/Medline, EMBASE, CINHAL, ISI Web of Science, and Cochrane Library were searched until July 2019.
Study Selection:
Trials had to be randomized controlled trials or controlled trials including cancer patients or animal models with cachexia-inducing tumors. Only sole exercise interventions over at least 7 days performed in a controlled environment were included.
Data Extraction:
Risk of bias was assessed and a random-effects model was used to pool effect sizes by standardized mean differences (SMD).
Results:
All eligible 20 studies were performed in rodents. Studies prescribed aerobic (n = 15), strength (n = 3) or combined training (n = 2). No statistical differences were observed for body mass and muscle weight of the gastrocnemius, soleus, and tibialis muscles between the exercise and control conditions (SMD = ‒0.05, 95%CI-0.64-0.55, P = 0.87). Exercise duration prior to tumor inoculation was a statistical moderator for changes in body mass under tumor presence (P = 0.04).
Limitations:
No human trials were identified. A large study heterogeneity was present, probably due to different exercise modalities and outcome reporting.
Conclusion:
Exercise does not seem to affect cancer-cachexia in rodents. However, the linear regression revealed that exercise duration prior to tumor inoculation led to reduced cachexia-severity, possibly strengthening the rationale for the use of exercise in cancer patients at cachexia risk.
Keywords: muscle wasting, tissue wasting syndrome, cancer cachexia, clinical exercise science, exercise oncology, supportive cancer therapy, exercise training
Introduction
Despite tremendous improvements in cancer treatment, cancer patients are often faced with severe cancer-related and treatment-induced side effects, such as fatigue or chemotherapy-induced peripheral neuropathy.1 Cancer cachexia is among the most severe side effects and is characterized as a multifactorial disturbance of metabolism and the immune system, leading to progressive loss of total body mass and muscle mass.2 According to previous estimates, almost 50% of all cancer patients develop a cachectic condition, while this concerns even 80% of hospitalized or advanced staged cancer patients.3,4
Although cachexia may occur in all types of cancer, especially gastrointestinal and lung cancer patients are disproportionally affected.5 Moreover, chemotherapeutic drugs, such as doxorubicin,6 may further exaggerate cachexia symptoms. In light of this, previous research has provided evidence that cachexia may reduce the patients’ tolerance to the medical treatment.7 Furthermore, cachexia may induce perturbations of hormonal and hemorheological homeostasis and, thus, may lead to insulin resistance, anemia, hypogonadism, or edema as well as asthenia and fatigue, eventually reducing the patients’ quality of life.2,8-10 As a consequence of rapid weight loss, cancer cachexia also dramatically increases morbidity and mortality rates.7
Although most of the pathophysiologic origin of cachexia is still unknown, chronic systemic inflammation is considered a main mediator.9,11 Thus, especially increased levels of tumor necrosis factor-α, interleukin-1 (IL-1), and IL-6 are often observed, all of which promote alterations in the protein metabolism, such as protein degradation signaling and reduced muscular protein synthesis.12,13
Considering the severity of cancer cachexia, it is somewhat surprising that treatment options remain limited, mostly reporting an inconsistent or inadequate efficacy.14 Pharmacological treatments typically aim for reductions of inflammation and concomitant appetite stimulation, whereas nutritional treatment provides energy- and protein-rich supplementation and diet counselling.14 However, from a mechanistic point of view, exercise training also appears to be a promising approach for the treatment of cancer cachexia. For example, aerobic exercise training has been shown to reduce low-grade systemic inflammation, while strength exercise is considered a crucial stimulus of muscle synthesis even under catabolic conditions.15-17 In fact, exercise training is commonly recommended to patients with cachexia of other origins, such as heart failure or rheumatoid arthritis.18,19 However, studies examining the efficacy of exercise training in cachectic cancer patients are still limited.20
Therefore, the purpose of this systematic literature review and meta-analysis was to elucidate the effects of exercise training as a countermeasure for cancer cachexia in both human and animal models. Special consideration was given to the effects of different exercise training interventions on total body mass (BM) as the primary outcome and muscle mass and muscle cross-sectional area (CSA) as secondary outcomes.
Methods
Search Process
The databases PubMed/Medline, EMBASE, CINHAL, ISI Web of Science, and Cochrane Library were systematically searched for relevant literature until July 4, 2019. The search procedure followed the guidelines provided by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). The original protocol was registered with the international database for prospectively registered systematic reviews in health and social care (PROSPERO: CRD42019137964). However, the protocol was later changed in the following domains: (1) the screened electronic databases were extended from PubMed to PubMed/Medline, EMBASE, CINHAL, ISI Web of Science, and Cochrane Library and (2) the systematic review was extended to a meta-analysis. The search was carried out using both medical subject headings as well as keywords adapted according to the requirements of the database (Table 1). The results of the search and medical subject headings terms were gathered, duplicates were removed, and 2 reviewers screened the remaining articles for title and abstract independently. If the title and abstract met the inclusion criteria, the articles were evaluated for eligibility in a subsequent full-text analysis. Furthermore, references and citation reports of the included studies were checked for additional eligible literature. Disagreements between the reviewers were resolved by consensus or further consultation of a third author. Finally, studies eligible for the systematic review were screened for inclusion into the pooled analysis. If data were missing or could not be determined, corresponding authors were contacted to provide the missing data.
Table 1.
MeSH and Search Terms.
| Database | MeSH/search terms |
|---|---|
| PubMed MeSH | Cachexia [MeSH] OR Muscular atrophy [MeSH] AND Neoplasm [MeSH] AND Exercise [MeSH] OR Exercise therapy [MeSH] |
| PubMed Free | Exercise OR Exercise therapy AND Cachexia OR Muscle wasting AND Cancer |
| CINHAL MeSH | Cachexia [MeSH] OR Atrophy [MeSH] AND Neoplasm [MeSH] AND Exercise [MeSH] OR Therapeutic Exercise [MeSH] |
| CINHAL Free | Exercise OR Exercise therapy AND Cachexia OR Muscle wasting AND Cancer |
| EMBASE MeSH | Cachexia [MeSH] OR Muscle atrophy [MeSH] AND Neoplasm [MeSH] AND Exercise [MeSH] OR Kinesiotherapy [MeSH] |
| EMBASE Free | Cachexia OR Muscle wasting AND Cancer AND Exercise OR Exercise therapy |
| COCHRANE MeSH | Cachexia [MeSH] OR Muscular atrophy [MeSH] AND Neoplasm [MeSH] AND Exercise [MeSH] OR Exercise therapy [MeSH] |
| COCHRANE Free | Cachexia OR Muscle wasting AND Cancer AND Exercise OR Exercise therapy |
| Web of Science Free | Cachexia OR Muscle wasting AND Cancer AND Exercise OR Exercise therapy |
Abbreviation: MeSH, medical subject heading.
Eligibility Criteria
Study eligibility was assessed using the PICOS (population, intervention, comparison, outcomes, and study design) method (Table 2). Studies identified in the systematic review were eligible for the meta-analysis if they reported mean values and standard deviations of at least one relevant outcome for both exercise and control conditions.
Table 2.
Screening Criteria for Study Inclusion Into the Review and Meta-Analysis.
| PICOS | Description of detail |
|---|---|
| P | Population: Adults (>18 years of age), cancer patients with an identified stage of cachexia (according to Fearon et al,21 2011), or animal models with a cachexia-inducing tumor implanted |
| I | Intervention: Sole, repetitive exercise performed at least for 7 days in a controlled (ie, supervised) exercise protocol (excluding voluntary exercise trials) |
| C | Comparison: Human or animal tumor hosts without structured exercise influence (also excluding studies which performed unilateral exercise and studies using the contralateral body part as control) |
| O | Outcomes: Primary: total body mass; Secondary: muscle mass and muscle cross-sectional area |
| S | Study design: Randomized-controlled trials or controlled trials |
Data Extraction
The following data were extracted: (1) name of authors, (2) year of publication, (3) study design and population, (4) animal and tumor model, and (5) characteristics of the intervention, such as type, duration, intensity, volume, and frequency. Furthermore, objective measures of BM as well as muscle mass and muscle CSA were extracted for both intervention and control groups. Because of inconsistencies in total BM assessment, we summarized the changes in total BM, carcass mass, and BM gain, unless differences were present within individual studies.
Data Synthesis and Analysis
The number of parameters considered for pooled analysis had to be present in at least 3 studies. The analysis was carried out using the standardized mean difference (SMD) as the outcome measure and a random-effects model was used to pool effect sizes using R (3.6.1),22 RStudio (1.2.1335),23 and the metafor package (version 2.2.1).24 The amount of heterogeneity (ie, τ2), was estimated using the restricted maximum-likelihood estimator.25 In addition to the estimate of τ2, the Q test for heterogeneity26 and the I2 statistic27 were reported. Cook’s distances were used to examine whether study results may be influential in the context of the model. Studies with a Cook’s distance larger than the median plus 6 times the interquartile range of the Cook’s distances were considered to be influential.28 Additionally, linear regression to account for heterogeneity using a mixed-effects model were conducted to test the following moderator variables: (1) type of exercise, (2) duration of intervention prior or (3) post tumor inoculation, (4) frequency of training, and (5) frequency × total duration of exercise intervention. A trim-and-fill-contour funnel plot was provided to estimate the number of studies potentially missing from the meta-analysis.29 The rank correlation test30 and the regression test31 using the standard error of the observed outcomes as predictor were used to check for funnel plot asymmetry. The model was initially calculated using reported post-values only. Due to the design of a majority of eligible studies in which training was commenced weeks before tumor injections, pooled effects sizes were additionally calculated for BM, using relative changes from pre-tumor injection to killing.
Risk of Bias Assessment
Risk of bias of the included studies was assessed independently by two reviewers, using the tool provided by the Office of Health Assessment and Translation. The Office of Health Assessment and Translation tool provides an approach to evaluate both human and animal model studies for their risk of bias.32 All studies were screened for the following risk of bias domains (and subdomains): (1) selection bias (randomization and allocation concealment), (2) performance bias (identical experimental conditions and blinding), (3) attrition/exclusion bias (complete data, exposure characterization, and outcome assessment), (4) all measured outcomes reported, and (5) other bias (threats to internal validity). The risk of bias tool rates every domain and subdomain within the range of (1) definitely low risk of bias, (2) probably low risk of bias, (3) probably high risk of bias or not reported, and (4) definitely high risk of bias.
Results
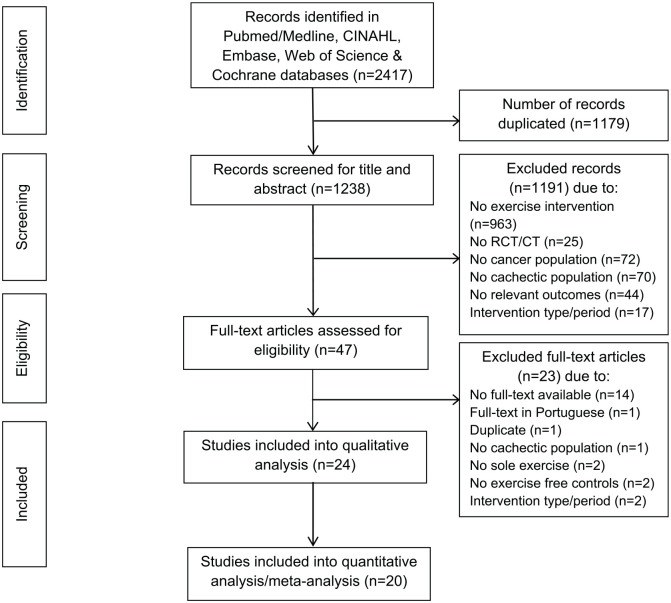
A total of 2417 references were identified during the search process. Out of these hits, 24 studies met the inclusion criteria for the review and thereof 20 studies were included in the meta-analysis (Figure 1). We contacted 14 corresponding authors to provide missing data. Subsequently, six authors provided the missing data,33-38 four authors did not respond but their studies contained partial data to be considered in the analysis,39-42 and an additional four authors did not respond and were excluded because of a lack of considerable data.43-46
Figure 1.
Flowchart of the search process.
All eligible studies were performed with animal models and, thus, no human trials were included. The included studies used the following tumor models: (1) Walker-256 breast carcinoma,3,34,36,42,43,45,47-50 (2) Colon-26 carcinoma,33,38,40,41,51 (3) the MC4-L2 breast cancer,52 (4) the Yoshida sarcoma,39 (5) 4T1-breast tumor,35 (6) the Lewis Lung carcinoma,38 (7) Morris hepatoma 7777,53 (8) ApcMin/+ with IL-6 overexpression for intestinal neoplasia,8,46 and (9) N-methyl-N-nitrosourea–induced breast cancer.37,54 A detailed overview including the study description and individual results of all eligible studies is provided in Table 3.
Table 3.
Summary of Relevant Outcomes in all 24 Included Studiesa.
| References | Year | Study design | Study population | Animal and tumor model | Intervention | Duration | Frequency | Intergroup comparison |
|---|---|---|---|---|---|---|---|---|
| Ballaro et al33 | 2019 | RCT | N = 16 8 TCs and 8 TEs |
BALB/c mice, C26 tumor | TE: treadmill exercise (11 m/min; moderate intensity, 45 minutes) | Pre tumor: 5 days Post tumor: 12 days |
5×/week | TE versus TC: Total body mass ↔ Tibialis muscle mass ↑ GSN muscle mass ↔ |
| Bover et al51 | 2019 | RCT | N = 12 6 TCs and 6 TEs |
BALB/c mice, C26 tumor | TE: Combined exercise Strength exercise: ladder climbs with additional weight (maximum 50% of bodyweight, 85° incline, 3 sets of 2 repetitions) Aerobic exercise: treadmill (5-9 m/min, moderate intensity, 25 minutes) |
Pre tumor: 4 weeks Post tumor: 11 days |
4×/week | TE versus TC: Total body mass ↔ Tibialis muscle mass ↑ GSN muscle mass ↔ |
| Moreira et al34 | 2018 | RCT | N = 10-18 5-9 TCs and 5-9 TEs |
Male Wistar rats, Walker-256 tumor | TE: treadmill exercise (50% to 65% of maximal running speed, 44 minutes) | Pre tumor: 8 weeks Post tumor: 2 weeks |
3×/week | TE versus TC: Total body mass ↓ |
| Amani-Shalamzari et al52 | 2018 | RCT | N = 20 10 TCs and 10 TEs |
BALB/c mice, MC4-L2 tumor | TE: treadmill exercise (16-18 m/min, 0% incline, 10-14 minutes) | Post tumor: 6 weeks | 5×/week | TE versus TC: Total body mass ↔ GSN muscle mass ↑ |
| Tanaka et al39 | 2018 | RCT | N = 12 6 TCs and 6 TEs |
Wistar rats, AH130 Yoshida tumor | TE: treadmill exercise (15 m/min, 30 minutes) | Post tumor: 10 days | 8×/10days | TE versus TC: Total body mass ↔ Soleus muscle mass ↑ |
| Shamsi et al35 | 2017 | RCT | N = 16 8 TCs and 8 TEs |
Female Balb/c mice, 4T1-tumor | TE: treadmill exercise (10 minutes Warmup + intervals at 2 minutes 70% VO2 maximum and 2 minutes 50% VO2 maximum for 10 minutes) | Pre tumor: 6 weeks Post tumor: 6 weeks |
5×/week | TE versus TC: Total body mass ↔ GSN muscle mass ↑ |
| Padilha et al36 | 2017 | RCT | N = 18 9 TCs and 9 TEs |
Male Wistar rats, Walker-256 tumor | TE: strength exercise (ladder climbing, 4 to 8 climbs with 50% to 100% of maximal carrying capacity) | Pre tumor: 6 weeks Post tumor: 12 days |
3×/week | TE versus TC: Total body mass ↑ Soleus muscle mass ↔ Soleus muscle CSA ↑ |
| Padrao et al37 | 2017 | RCT | N = 25 10 TCs and 15 TEs |
Female Sprague-Dawley rats, MNU | TE: treadmill exercise (moderate, 20 m/min, 60 minutes) | Post tumor: 35 weeks | 5×/week | TE versus TC: Total body mass ↔ GSN muscle mass ↔ GSN muscle CSA ↑ |
| Khamoui et al40 | 2016 | RCT | N = 25 9 TCs, 8 TSEs, and 8 TAEs |
Balb/c mice, C26 tumor | TSE: ladder climbs with additional 50% of body weight, increasing 10% every 2 weeks (5 sets of 3 repetitions) TAE: progressively increasing treadmill exercise (5-7 m/min, 60 minutes) |
Pre tumor: 8 weeks Post tumor: 3 weeks |
TSE: 3×/week TAE: 5×/week |
TAE40 versus TC: Total body mass ↔ GSN muscle mass ↔ GSN muscle CSA ↔ TSE40 versus TC: Total body mass ↔ GSN muscle mass ↔ GSN muscle CSA ↔ |
| Jee et al41 | 2016 | RCT | N = 30 10 TCs, 10 TMEs, and 10 TIEs |
CDF1 mice, C26 tumor | TME: treadmill exercise (70% max HR, moderate 45 minutes) TIE: treadmill exercise (90% max HR, intense 45 minutes) |
Post tumor: 4 weeks | Every second day | TME41 versus TC: Total body mass ↔ GSN muscle mass ↑ Soleus muscle mass ↔ TIE41 versus TC: Total body mass ↑ GSN muscle mass ↑ Soleus muscle mass ↑ TIE41 versus TME41: Total body mass ↑ GSN muscle mass ↑ Soleus muscle mass ↑ |
| Pin et al38 | 2015 | RCT | N = 45 C26 2 weeks: 7 TCs and 7 TEs C26 8 weeks: 7 TCs and 8 TEs LLC 4 weeks: 8 TCs and 8 TEs |
Balb/C or C57BL/6 mice, C26, or LLC tumor | TE: treadmill exercise (14 m/min, 60% to 70% VO2 maximum, 45 minutes) | Post tumor: C26 2 weeks, LLC 4 weeks, subset of C26 (8 weeks) Pre tumor: 6 weeks and post tumor: 2 weeks |
5×/week | C26 2 weeks38 TE versus TC: Total body mass ↓ GSN muscle mass ↔ Tibialis muscle mass ↔ C26 8 weeks38 TE versus TC: Total body mass ↔ GSN muscle mass ↔ Tibialis muscle mass ↔ LLC 4 weeks38 TE versus TC: GSN muscle mass ↔ Tibialis muscle mass ↔ |
| Kryczyk et al47 | 2014 | RCT | N = 20 10 TCs and 10 TEs |
Wistar rats, Walker-256 tumor | TE: combined exercise Strength exercise: jumping in water (6 sets of 30 seconds with 50% body weight load attached) + aerobic exercise: swimming (30 minutes, 6% body weight load attached) |
Pre tumor: 6 weeks Post tumor: 2 weeks |
4×/week | TE versus TC: Total body mass ↔ Mass change (body mass − tumor mass) ↑ |
| Donatto et al3 | 2013 | RCT | N = 14 7 TCs and 7 TEs |
Wistar rats, Walker-256 tumor | TE: strength exercise (3-5 ladder climbs with additional load 75% to 100% of the animal’s maximal carrying capacity) | Pre tumor: 6 weeks Post tumor: 2 weeks |
Every third day | TE versus TC: Total body mass ↑ GSN muscle mass ↑ |
| Faustino-Rocha et al54 | 2013 | RCT | N = 21 11 TCs and 10 TEs |
Sprague-Dawley rats, MNU | TE: treadmill exercise (20 m/min, 60 minutes) | Post tumor: 34 weeks | 5×/week | TE versus TC: Total body mass ↔ GSN muscle mass ↔ |
| Lira et al48 | 2012 | RCT | N = 14 8 TCs and 6 TEs |
Wistar rats, Walker-256 tumor | TE: treadmill exercise (60% to 65% VO2 max, 60 minutes) | Pre tumor: 6 weeks Post tumor: 2 weeks |
5×/week | TE versus TC: Total body mass ↓ |
| Puppa et al8 | 2011 | RCT | N = 27 15 TCs and 12 TEs |
Apc Min/+ with IL-6 overexpression (to induce cachexia) | TE: treadmill exercise (moderate, 18 m/min, 5% incline, 60 minutes). | Pre IL-6 overexpression: 7 weeks Post IL-6 overexpression: 2 weeks |
6×/week | TE versus TC: Total body mass ↔ |
| Baracos53 | 1989 | RCT | N = 20 10 TCs and 10 TEs |
Sprague-Dawley rats, Morris hepatoma 7777 | TE: swimming exercise (5 minutes on the first day, increasing 5 minutes each session, maximum 120 minutes per session) | Pre tumor: 3 weeks Post tumor: 3 weeks |
5×/week | TE versus TC: Total body mass ↔ Epitochlearis muscle mass ↔ |
| Deuster et al42 | 1985 | RCT | N = 14 8 TCs and 6 TEs |
Sprague-Dawley rats, Walker-256 carcinoma | TE: treadmill exercise (20 m/min, 13% incline, 100 minutes) | Pre tumor: 2 weeks Post tumor: 29 days |
3×/week | TE versus TC: Total body mass ↔ GSN muscle mass ↑ |
| Salomão et al49 | 2010 | CT | N = 17 9 TCs and 8 TEs |
Wistar rats, Walker-256 tumor | TE: swimming exercise (light aerobic exercise, 45 minutes) | Pre tumor: 60 days Post tumor: 21 days |
5×/week | TE versus TC: Total body mass ↑ GSN muscle mass ↑ |
| Lima et al50 | 2008 | CT | N = 36 18 TCs and 18 TEs |
Wistar rats, Walker-256 tumor | TE: strength exercise, jumping in water (10 sets of 30 seconds with 50% body weight load attached) | Pre tumor: 6 weeks Post tumor: 2 weeks |
4×/week | TE versus TC: Total body mass ↑ |
| Below: included in the systematic review but not in the meta-analysis | ||||||||
| das Neves et al43 | 2016 | RCT | N = 16 8 TCs and 8 TEs |
Wistar rats, Walker-256 tumor | TE: Pre tumor: daily EMS sessions (progressively, 1 to 2 sets with 12 to 15 repetitions, overload 0 to 200 g) Post tumor: strength exercise for the hind limb (“squat-like” movement, 65% of 1 RM, 3 sets with 10 repetitions) |
Pre tumor: 8 days in a row Post tumor: total of 8 sessions in 12 days |
Pre tumor: every day Post tumor: 2 days TE and 1 day rest |
TE versus TC: Plantaris muscle mass ↔ EDL muscle mass ↔ EDL muscle CSA ↔ |
| Lira et al44 | 2008 | RCT | N = 12 7 TCs and 5 TEs |
Wistar rats, Walker-256 tumor | TE: treadmill exercise (60% to 65% VO2 maximum, 60 minutes). | Pre tumor: 6 weeks Post tumor: 2 weeks |
5×/week | TE versus TC: Total body mass ↓ |
| Bacurau et al45 | 2007 | RCT | N = 48 24 TCs and 24 TEs |
Wistar rats, Walker-256 tumor | TE: treadmill exercise (85% VO2 maximum, 30 minutes) | Pre tumor: 8 weeks Post tumor: 2 weeks |
5×/week | TE versus TC: Total body mass ↑ |
| White et al46 | 2012 | CT | N = 36 20 TCs and 16 TEs |
ApcMin/+ with IL-6 overexpression (to induce cachexia) | TE: treadmill exercise (18 m/min, 5% incline, 60 minutes) | Pre IL-6 overexpression: 7 weeks Post IL-6 overexpression: 2 weeks |
6×/week | TE versus TC: GSN muscle mass ↔ |
Abbreviations: 1 RM, one repetition maximum; C26, colon-26; CSA, cross-sectional area; CT, controlled trial; EDL, extensor digitorum longus; EMS, electrical muscle stimulation; GSN, gastrocnemius; IL, interleukin; LLC, Lewis lung carcinoma; max HR, maximum heart rate; MNU, N-methyl-N-nitrosourea; N, statistical population; RCT, randomized-controlled trial; TAE, tumor aerobic exercise; TC, tumor control; TE, tumor exercise; TIE, tumor intense-aerobic exercise; TME, tumor moderate-aerobic exercise; TSE, tumor strength exercise, VO2 max, maximum oxygen uptake; ↑, statistical increase compared with controls; ↔, no effects; ↓, statistical reduction compared to controls.
Trials are sorted by (1) inclusion in meta-analysis or review, (2) study design, (3) year of publication, and (4) alphabetical order.
Risk of Bias
All studies showed a probably high risk of bias within the domain of blinding, which is known to be a persistent difficulty of exercise interventions (Table 4). In addition, several studies reported incomplete data due to missing reports of results.3,8,33-35,38,39,44,46,47,50,52-54 Allocation concealment appeared to be a frequent risk of bias.33,35,39,46,47,49-51,53 Furthermore, particularly the studies of White et al46 and Lima et al50 were rated with a probably high risk of bias in the categories randomization and all measured outcome reported. While we acknowledge that deviations may have not been thoroughly reported in the included studies, we were not able to find other sources of bias (i.e. threats of intervanl validity).
Table 4.
Risk of Bias Scoring of Included Studies Following the OHAT Risk of Bias Assessment Tool.
| OHAT Risk of Bias Tool | Randomization | Allocation concealment | Identical experimental conditions | Blinded | Complete data | Exposure characterization | Outcome assessment | All measured outcome reported | Threat to internal validity |
|---|---|---|---|---|---|---|---|---|---|
| Ballarò et al33/2019 | − | − | + | − | − | + | + | ++ | + |
| Bover et al51/2019 | + | − | ++ | − | − | ++ | + | ++ | + |
| Moreira et al34/2018 | + | + | ++ | − | − | ++ | + | ++ | + |
| Amani-Shalamzari et al52/2018 | + | + | + | −− | − | ++ | + | ++ | + |
| Tanaka et al39/2018 | + | − | ++ | − | − | ++ | + | ++ | + |
| Shamsi et al35/2017 | + | − | ++ | − | − | ++ | + | + | + |
| Padilha et al36/2017 | + | + | ++ | − | ++ | ++ | + | ++ | |
| Padrao et al37/2017 | + | + | ++ | − | ++ | ++ | + | ++ | + |
| das Neves et al43/2016 | + | + | ++ | − | + | ++ | + | ++ | + |
| Khamoui et al40/2016 | + | + | ++ | − | + | ++ | + | − | |
| Jee et al41/2016 | + | + | ++ | − | + | ++ | + | ++ | + |
| Pin et al38/2015 | + | + | ++ | − | − | ++ | + | − | + |
| Kryczyk et al47/2014 | + | − | ++ | − | − | ++ | + | ++ | |
| Donatto et al3/2013 | + | + | ++ | − | − | ++ | + | ++ | + |
| Faustino-Rocha et al54/2013 | + | ++ | ++ | ++ | − | ++ | + | ++ | + |
| Lira et al48/2012 | + | + | ++ | − | ++ | ++ | + | − | |
| Puppa et al8/2011 | − | + | ++ | − | − | ++ | + | ++ | + |
| Lira et al44/2008 | + | + | ++ | − | − | ++ | + | − | + |
| Bacurau et al45/2007 | + | + | ++ | − | ++ | ++ | + | ++ | + |
| Baracos53/1989 | − | − | + | − | − | ++ | + | ++ | + |
| Deuster et al42/1985 | + | + | + | − | ++ | ++ | + | ++ | + |
| White et al46/2012 | − | − | ++ | − | − | ++ | + | − | + |
| Salomão et al49/2010 | − | − | + | − | ++ | ++ | + | ++ | + |
| Lima et al50/2008 | − | − | ++ | − | − | ++ | + | − | + |
Abbreviation: OHAT, Office of Health Assessment and Translation.
, definitely low risk of bias; + probably low risk of bias; − probably high risk of bias; −− definitely high risk of bias.
Risk of Bias domains: (1) selection bias (randomization and allocation concealment), (2) performance bias (identical experimental conditions and blinding), (3) attrition/exclusion bias (complete data, exposure characterization, and outcome assessment), (4) all measured outcomes reported, and (5) other bias (threats to internal validity).
Pooled Analysis
In the meta-analysis, 18 RCTs3,8,33-42,47,48,51-54 and two CTs49,50 were included. In the study of Pin et al,38 three different exercise experiments with rodents were performed, all of which were deemed eligible and consequently included in the pooled analysis. The overall count of included rodents into the meta-analysis was n = 416, out of which 215 rodents were exercised and 201 rodents served as controls. One study provided only a range for the included population and, thus, the median of the range was used for analysis.34
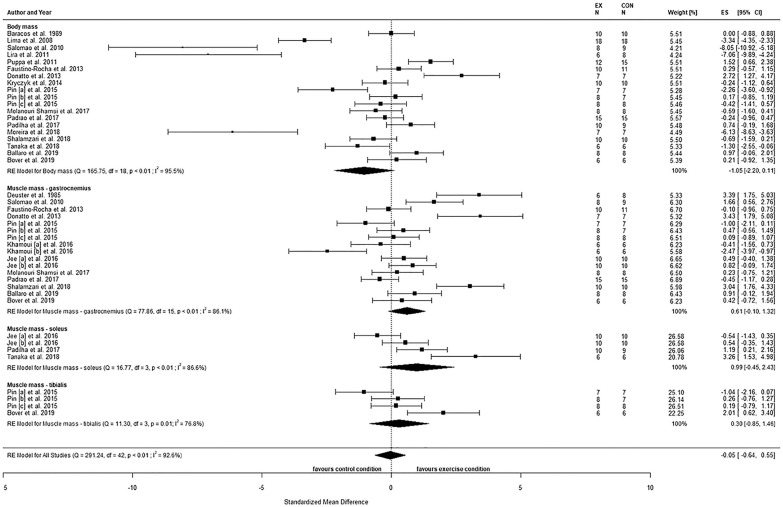
The observed effects of postintervention comparisons for BM (SMD = −1.05, 95% confidence interval [CI] = −2.20 to 0.11, P = .08) showed no statistical difference between the conditions (Figure 2). A large heterogeneity was observed (Q(18) = 165.8, P < .01, τ2 = 6.1, I2 = 95.5%), with two studies being highly influential.48,49 None of the moderators explained any heterogeneity (all P > .05; Table 5).
Figure 2.
Body mass and mass of gastrocnemius, soleus, and tibialis muscles comparing tumor-bearing exercise training interventions (EX) and tumor-bearing control (CON), using absolute values of endpoint comparisons.
Abbreviations: CI, confidence intervals; ES, effects size Cohen’s d (corrected for small samples); df, degrees of freedom; I2 and Q (Cochran’s Q) describe heterogeneity; RE, random effects model. Pin [a] = C26 2 weeks, Pin [b] = C26 8 weeks, Pin [c] = LLC 4 weeks, Khamoui [a] = aerobic exercise, Khamoui [b] = strength exercise, Jee [a] = moderate aerobic exercise, Jee [b] = intense aerobic exercise.
Table 5.
Linear Regression Analysis Using a Mixed-Effect Model.
| Moderators | BM |
ΔBM |
GSN |
SOL |
TIB |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | R2 (%) | P | R2 (%) | P | R2 (%) | P | R2 (%) | P | R2 (%) | |
| Type of exercise | .58 | 0 | .97 | 0 | .99 | 0 | .97 | 0 | .02 | 80.3 |
| Duration pre | .16 | 1.4 | .04 | 48.9 | .86 | 0 | .92 | 0 | .38 | 0 |
| Duration post | .53 | 0 | .71 | 0 | .40 | 0 | .04 | 64.1 | .73 | 0 |
| Frequency | .77 | 0 | .46 | 0 | .27 | 0.6 | .02 | 70.8 | .02 | 80.3 |
| Frequency × Durationtotal | .83 | 0 | .73 | 0 | .30 | 0 | .97 | 0 | .60 | 0 |
Abbreviations: BM, body mass; ΔBM, change in body mass from pre- to postintervention; GSN, gastrocnemius muscle; SOL, soleus muscle; TIB, tibialis muscle.
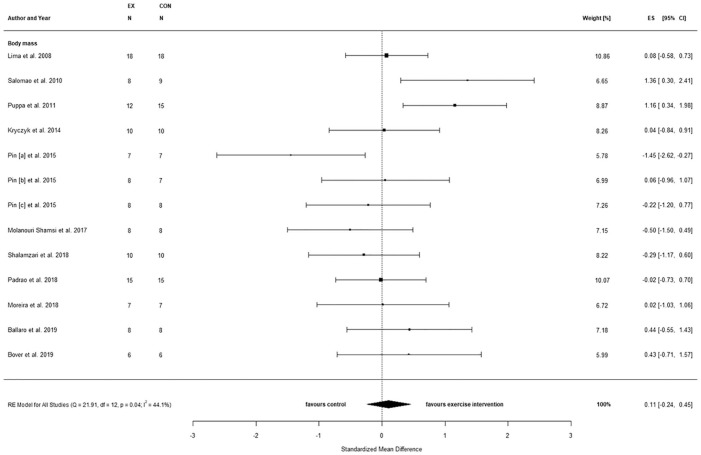
When considering only the training period with tumor presence (Δ), also no statistical between-condition effects were observed for BM (SMD = 0.11, 95% CI = −0.24 to 0.45, P = .11; Figure 3), but study heterogeneity was reduced (Q(12) = 21.9, P = .04, τ2 = 0.2, I2 = 44.1%). Testing for moderators indicated that the duration of exercise training prior to tumor inoculation accounted for 48.9% of the heterogeneity (P = .04), while no effect was observed for the remaining moderators (Table 5).
Figure 3.
Changes in body mass comparing tumor-bearing exercise training interventions and tumor-bearing control.
Abbreviations: CI, confidence interval; ES, effects size Cohen’s d (corrected for small samples); df, degrees of freedom; I2 and Q (Cochran’s Q) describe heterogeneity; RE, random effects model. Pin [a] = C26 2 weeks, Pin [b] = C26 8 weeks, Pin [c] = LLC 4 weeks.
The observed effects of postintervention comparisons for gastrocnemius (GSN) muscle mass (SMD = 0.61, 95% CI = −0.10 to 1.32, P = .09) showed no statistical difference between conditions (Figure 2). A large heterogeneity was observed (Q(15) = 77.9, P < .01, τ2 = 1.8, I2 = 86.1%), but no study was identified as influential. None of the moderators explained any heterogeneity (all P > .05; Table 5).
Similarly, no statistical between-group effect was observed for postintervention comparisons of soleus (SOL) muscle mass (SMD = 0.99, 95% CI = −0.45 to 2.43, P = .18; Figure 2). A large heterogeneity was observed (Q(3) = 16.8, P < .01, τ2 = 1.8, I2 = 86.6%), but no study was identified as being influential. Testing for moderators revealed that the duration of the exercise intervention following tumor inoculation as well as the training frequency accounted for 64.1% (P = .04) and 70.8% (P = .02) of the heterogeneity, respectively (Table 5).
For postintervention comparisons of tibialis (TIB) muscle mass, no statistical between-group difference was observed (SMD = 0.30, 95% CI = −0.85 to 1.46, P = .61; Figure 2). A large heterogeneity was observed (Q(3) = 11.3, P = .01, τ2 = 1.1, I2 = 76.8%), but no study was identified as influential. Both the type of exercise and the training frequency each accounted for 80.3% (P = .02) of heterogeneity, respectively (Table 5).
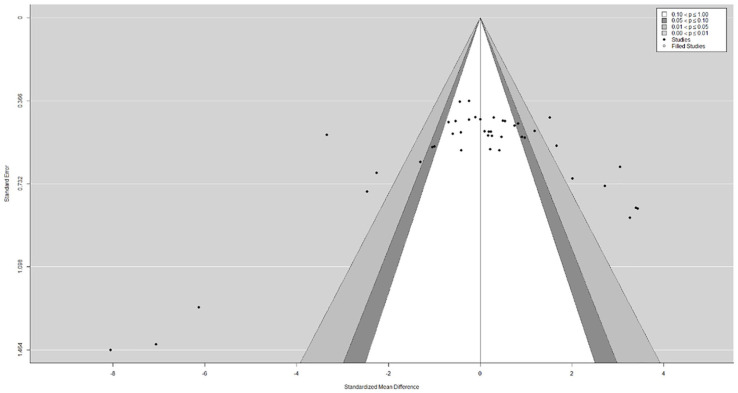
Publication Bias
The funnel plot did not show a clear funnel-shape across all assessed and pooled effect sizes (Figure 4). The regression test indicated funnel plot asymmetry (P < .01) but not the rank correlation test (P = .93). The visual observation provided by the trim-and-fill function confirmed study heterogeneity, while potential publication bias and methodological heterogeneity are likely, as indicated by a large cluster in the top-center of the plot with no values in the bottom right and left corners, respectively.
Figure 4.
Funnel plot for publication bias assessment including the trim-and-fill function to plot potentially missing publications as well as the contour function to visualize a significance threshold.
Discussion
The purpose of this systematic literature review and meta-analysis was to evaluate the current evidence of the effects of exercise interventions on cancer cachexia. A total of 24 animal models were eligible for the systematic review, while thereof 20 studies were included in the meta-analysis. No statistical differences were observed for BM and muscle mass between the control and the exercise conditions. However, a large study heterogeneity was observed for all outcomes. Moreover, exercise duration prior to tumor inoculation was identified as a significant moderator on the BM under tumor presence.
Considering the high prevalence and clinical relevance of cancer cachexia, it was surprising that at the time of screening no human RCT or CT that specifically screened for cachectic symptoms has been published. In fact, this lack of human trials was previously identified by a Cochrane review that was published six years ago20 and appears not having improved ever since. The reasons for this paucity may be related to the criteria of cancer cachexia, which have been established as late as 2012 and, thus, a framework of precise classification and treatment was missing.21 Another major reason might be related to the pathogenesis of cancer cachexia, often developing only in the late stages of the disease, sometimes shortly before demise.3 Therefore, the late but rapid progression of cachexia makes it difficult to conduct well-designed and controlled studies as well as to recruit eligible patients and to complete comprehensive exercise interventions.
All included animal-based studies were conducted with rodents using tumor models well known for the development of cancer cachexia.37,55-59 Our pooled analysis revealed no statistical effects of sole exercise on characteristics of cancer cachexia. However, also a large study heterogeneity was observed for all outcomes. This was attributed to the number of different animal and tumor models used as well as to the profound differences in characteristics of the exercise interventions, such as exercise type, duration, frequency, and intensity. In addition, the assessment methods, the timing of measurements, and eventually the final data reporting varied across the included studies. For example, several studies included in our postintervention comparison commenced exercise prior to tumor inoculation, while BM was assessed or at least reported only immediately prior to the start of the exercise period and after completion.3,36,48,53 These tumor-free exercise periods may strongly affect BM, as was, for example, shown in the study by Salomão et al.49 When calculating relative changes for BM from pretumor injection to killing, it was shown that 60 days of training prior to tumor inoculation led to a much smaller weight gain when compared with inactive controls (~220 g vs ~330 g). Consequently, also BM at killing significantly differed between trained and nontrained rats (~293 g vs ~401 g), but was dramatically affected by the pretumor training rather than the exercise training after tumor inoculation. In fact, this phenomenon was also observed in other studies,34,48,50 indicating potential limitations of a sole comparison based on reported postintervention values. Indeed, this might be one explanation for the observed discrepancies in our calculated effect sizes for exercise training when comparing the pooled analysis based on postintervention values and those retrieved from the relative changes.
The duration of exercise prior to tumor inoculation was identified as the only statistical moderator, explaining study heterogeneity for changes in BM. Our findings, therefore, indicate that a greater level of fitness prior to tumor injection could reduce the severity of cancer cachexia symptoms. Exercise has previously been shown to condition and prime the immune system for the tumor burden and may, therefore, reduce the cancer cachexia impact in rodents, as, for example, discussed in the study of Pedersen et al.60 Therefore, we suggest that an increased overall fitness may provide a preventive measure to reduce cancer-induced BM loss, at least in rodents.
These assumptions are in line with current perspectives of cancer cachexia prevention.61 While specific evidence in humans is still lacking, first results of human trials with cancer cachexia-relevant outcomes indicated that multimodal approaches including especially strength exercise might contribute to BM and muscle mass maintenance in patients susceptible for cachexia.62-64 However, our meta-analysis did not identify the type of exercise as a significant moderator, but at the same time it also revealed a dramatic underrepresentation of trials including strength exercise. Indeed, sole strength exercise was deployed in only 5 studies, reporting either a prevention of BM loss,36,50 a mitigated muscle mass loss,3,36 or even an increases in BM.3 This was surprising, considering that strength training is well known as an anabolic stimulus, promoting muscle hypertrophy. In fact, the trials incorporating strength training reported reduced tumor-induced muscle-catabolic factors such as proteolysis-inducing factor (PIF),49 increased testosterone levels,3 and improved muscle CSA,36 all of which suggest promising mechanisms of muscle mass maintenance in cachectic cancer hosts. However, whether the effects of strength exercise for cancer cachexia are superior to that of aerobic training remains unclear. In fact, only one study has directly compared both types of training but failed to show reductions in tumor-induced BM loss in either of the conditions, while providing some evidence for different mechanistic-pathways to counteract cancer cachexia.40
In line with body mass, our pooled analysis did not show a statistically significant benefit of exercise interventions for changes of muscle mass in GSN, TIB, or SOL. However, our analysis was limited by the insufficient reporting of outcome variables, combined with missing response of the contacted authors.40-42 Thus, the results of these analyses need to be interpreted with caution, due to the heterogeneity and potential publication bias as well as the individual risk of bias in some studies. However, our moderator analysis identified exercise variables such as training duration, frequency, and type of exercise as statistical moderators for SOL and TIB muscle mass. In fact, the effects of these training variables may be related to the mechanisms by which exercise may attenuate the loss of muscle mass. Especially aerobic exercise is well known as a potential anti-inflammatory stimulus and previous research has shown that these effects are highly related to the exercise intensity, duration, and muscle mass involvement.65,66 Interestingly, the only 2 trials using high-intensity aerobic exercise and, therefore, a higher metabolic rate found positive effects on total BM41,45 and muscle mass41 compared with both controls and moderate aerobic exercise. However, due to insufficient reporting of outcome values, these studies could not be included in the pooled analysis of BM.41,45 Therefore, the appropriate dosage of exercise remains an additional important factor of exercise planning and should be considered in future studies.
When interpreting the present findings, one has to bear in mind that our interpretations are solely based on animal models and, thus, the translation of the findings into cancer care is currently limited. Nonetheless, our findings can provide preliminary but relevant data and future directions in the conception of human trials incorporating exercise training. Therefore, well-designed and controlled trials assessing not only the safety and feasibility but also the underlying pathophysiology and potential exercise-dependent dose-response relationships in patients with manifested cancer cachexia are warranted. Currently, first evidence is emerging that exercise appears to be safe and feasible in pancreatic cancer patients with cachexia.67 In fact, the 6 months progressive strength training led to significant increases in muscle mass, while total BM remained unchanged. In addition, first clinical trials deploying multimodal interventions including exercise with defined cachectic cancer patients are currently planned68 or even recruiting patients,69 suggesting more insights of into the effects of exercise in cachectic cancer patients in the near future.
Conclusions
Our systematic review revealed a clear lack of human exercise trials including cancer patients specifically screened for cachexia. Moreover, since our meta-analysis of 20 animal models did not reveal statistically significant effects of exercise interventions on total BM or muscle mass, the role of exercise in the treatment of cancer cachexia remains questionable. However, the duration of exercise prior to tumor inoculation was associated with an attenuated loss of BM, suggesting that overall fitness of rodents may affect the cancer cachexia progression. Furthermore, the results of our analyses were affected by a large heterogeneity, somewhat hindering the interpretation of the pooled data. Based on this, we encourage the implementation of human trials in order to develop dose-response relationships of different types of exercise with related targeted cellular pathways. In these trials, a universal cachexia sensitive outcome, such the cachexia index,34,36 could be a useful assessment to standardize clinical outcomes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Timo Niels  https://orcid.org/0000-0002-7309-0161
https://orcid.org/0000-0002-7309-0161
References
- 1. Greten H, Rinninger F, Greten TF, Amling M, eds. Innere Medizin. 13th ed. Thieme; 2000. [Google Scholar]
- 2. Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda). 2005;20:340-348. [DOI] [PubMed] [Google Scholar]
- 3. Donatto FF, Neves RX, Rosa FO, et al. Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour-bearing rats. Cytokine. 2013;61: 426-432. [DOI] [PubMed] [Google Scholar]
- 4. Tisdale MJ. The “cancer cachectic factor.” Support Care Cancer. 2003;11:73-78. [DOI] [PubMed] [Google Scholar]
- 5. Gould DW, Lahart I, Carmichael AR, Koutedakis Y, Metsios GS. Cancer cachexia prevention via physical exercise: molecular mechanisms. J Cachexia Sarcopenia Muscle. 2013;4: 111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lira FS, Neto JCR, Seelaender M. Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metab. 2014;39:679-686. [DOI] [PubMed] [Google Scholar]
- 7. Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr. 2010;29:154-159. [DOI] [PubMed] [Google Scholar]
- 8. Puppa MJ, White JP, Velázquez KT, et al. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle. 2012;3:117-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793-799. [DOI] [PubMed] [Google Scholar]
- 10. Penna F, Busquets S, Pin F, et al. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle. 2011;2:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Argilés JM, López-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999;19:223-248. [DOI] [PubMed] [Google Scholar]
- 12. Fearon KCH, Moses AGW. Cancer cachexia. Int J Cardiol. 2002;85:73-81. [DOI] [PubMed] [Google Scholar]
- 13. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381-410. [DOI] [PubMed] [Google Scholar]
- 14. Sadeghi M, Keshavarz-Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91-104. [DOI] [PubMed] [Google Scholar]
- 15. Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884-886. [DOI] [PubMed] [Google Scholar]
- 16. Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98:1154-1162. [DOI] [PubMed] [Google Scholar]
- 17. Al-Majid S, Waters H. The biological mechanisms of cancer-related skeletal muscle wasting: the role of progressive resistance exercise. Biol Res Nurs. 2008;10:7-20. [DOI] [PubMed] [Google Scholar]
- 18. Alves CRR, da Cunha TF, da Paixão NA, Brum PC. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 2015;125:9-14. [DOI] [PubMed] [Google Scholar]
- 19. Perniconi B, Albertini MC, Teodori L, Belli L, Rocci M. A meta-analysis on a therapeutic dilemma: to exercise or not to exercise in cachexia. Bas Appl Myol. 2008;18:115-120. [Google Scholar]
- 20. Grande AJ, Silva V, Riera R, et al. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev. 2014;(11):CD010804. [DOI] [PubMed] [Google Scholar]
- 21. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2014. http://www.R-project.org/ [Google Scholar]
- 23. RStudio Team. RStudio connect 1.8.4. Accessed March 25, 2020 http://www.rstudio.com/
- 24. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2000;36(3). doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 25. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30:261-293. [Google Scholar]
- 26. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101-129. [Google Scholar]
- 27. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [DOI] [PubMed] [Google Scholar]
- 28. Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112-125. [DOI] [PubMed] [Google Scholar]
- 29. Duval S, Tweedie R. A Nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc, 2000;95:89-98. [Google Scholar]
- 30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [Google Scholar]
- 31. Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Wiley; 2006. [Google Scholar]
- 32. Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122:711-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ballarò R, Penna F, Pin F, Gómez-Cabrera MC, Viña J, Costelli P. Moderate exercise improves experimental cancer cachexia by modulating the redox homeostasis. Cancers (Basel). 2019;11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moreira VM, da Silva Franco CC, Prates KV, et al. Aerobic exercise training attenuates tumor growth and reduces insulin secretion in walker 256 tumor-bearing rats. Front Physiol. 2018;9:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shamsi MM, Chekachak S, Soudi S, et al. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine. 2017;90:100-108. [DOI] [PubMed] [Google Scholar]
- 36. Padilha CS, Borges FH, da Silva LECM, et al. Resistance exercise attenuates skeletal muscle oxidative stress, systemic pro-inflammatory state, and cachexia in Walker-256 tumor-bearing rats. Appl Physiol Nutr Metab. 2017;42:916-923. [DOI] [PubMed] [Google Scholar]
- 37. Padrão AI, Figueira ACC, Faustino-Rocha AI, et al. Long-term exercise training prevents mammary tumorigenesis-induced muscle wasting in rats through the regulation of TWEAK signalling. Acta Physiol (Oxf). 2017;219:803-813. [DOI] [PubMed] [Google Scholar]
- 38. Pin F, Busquets S, Toledo M, et al. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotarget. 2015;6:43202-43215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka M, Sugimoto K, Fujimoto T, et al. Preventive effects of low-intensity exercise on cancer cachexia-induced muscle atrophy. FASEB J. 2019;33:7852-7862. [DOI] [PubMed] [Google Scholar]
- 40. Khamoui AV, Park BS, Kim DH, et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism. 2016;65:685-698. [DOI] [PubMed] [Google Scholar]
- 41. Jee H, Chang JE, Yang EJ. Positive prehabilitative effect of intense treadmill exercise for ameliorating cancer cachexia symptoms in a mouse model. J Cancer. 2016;7:2378-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deuster PA, Morrison SD, Ahrens RA. Endurance exercise modifies cachexia of tumor growth in rats. Med Sci Sports Exerc. 1985;17:385-392. [PubMed] [Google Scholar]
- 43. das Neves W, Alves CRR, de Almeida NR, et al. Loss of strength capacity is associated with mortality, but resistance exercise training promotes only modest effects during cachexia progression. Life Sci. 2016;163:11-22. [DOI] [PubMed] [Google Scholar]
- 44. Lira FS, Tavares FL, Yamashita AS, et al. Effect of endurance training upon lipid metabolism in the liver of cachectic tumour-bearing rats. Cell Biochem Funct. 2008;26:701-708. [DOI] [PubMed] [Google Scholar]
- 45. Bacurau AVN, Belmonte MA, Navarro F, et al. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of walker 256 tumor bearing rats. Exp Biol Med (Maywood). 2007;232:1289-1299. [DOI] [PubMed] [Google Scholar]
- 46. White JP, Puppa MJ, Sato S, et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle. 2012;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kryczyk M, Bordignon J, Iagher F, et al. Exercise and shark liver oil supplementation reduce tumor growth and cancer cachexia in Walker 256 tumor bearing rats. J Cancer Sci Ther. 2014;6:87-93. [Google Scholar]
- 48. Lira FS, Yamashita AS, Rosa JC, et al. Exercise training decreases adipose tissue inflammation in cachectic rats. Horm Metab Res. 2012;44:911-998. [DOI] [PubMed] [Google Scholar]
- 49. Salomão EM, Toneto AT, Silva GO, Gomes-Marcondes MCC. Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr Cancer. 2010;62:1095-1104. [DOI] [PubMed] [Google Scholar]
- 50. Lima C, de Alves LE, Iagher F, et al. Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur J Appl Physiol. 2008;104:957-964. [DOI] [PubMed] [Google Scholar]
- 51. Bover Q, Ranjbar K, Ballarò R, et al. Combined exercise training positively affects muscle wasting in tumor-bearing mice. Med Sci Sport Exerc. 2019;51:1387-1395. [DOI] [PubMed] [Google Scholar]
- 52. Amani-Shalamzari S, Daneshfar A, Sablouei MH, Singh MAF, Kazemi A. The effect of aerobic training on tumor growth, adiponectin, leptin and ghrelin in mice models of breast cancer. Iran Red Crescent Med J. 2018;20:e13305. [Google Scholar]
- 53. Baracos VE. Exercise inhibits progressive growth of the Morris hepatoma 7777 in male and female rats. Can J Phisiol Pharmacol. 1989;67:864-870. [DOI] [PubMed] [Google Scholar]
- 54. Faustino-Rocha AI, Oliveira PA, Duarte JA, Ferreira R, Ginja M. Ultrasonographic evaluation of gastrocnemius muscle in a rat model of N-methyl-N-nitrosourea-induced mammary tumor. In Vivo. 2013;27:803-807. [PubMed] [Google Scholar]
- 55. Tayek JA, Istfan NW, Jones CT, Hamawy KJ, Bistrian BR, Blackburn GL. Influence of the Walker 256 carcinosarcoma on muscle, tumor, and whole-body protein synthesis and growth rate in the cancer-bearing rat. Cancer Res. 1986;46:5649-5654. [PubMed] [Google Scholar]
- 56. Al-Majid S, McCarthy DO. Resistance exercise training attenuates wasting of the extensor digitorum longus muscle in mice bearing the colon-26 adenocarcinoma. Biol Res Nurs. 2001;2:155-166. [DOI] [PubMed] [Google Scholar]
- 57. Baltgalvis KA, Berger FG, Pena MMO, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393-R401. [DOI] [PubMed] [Google Scholar]
- 58. Brown JL, Rosa-Caldwell ME, Lee DE, et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2017;8:926-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Regan JN, Mikesell C, Reiken S, et al. Osteolytic breast cancer causes skeletal muscle weakness in an immunocompetent syngeneic mouse model. Front Endocrinol (Lausanne). 2017;8:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554-562. [DOI] [PubMed] [Google Scholar]
- 61. Hardee JP, Counts BR, Carson JA. Understanding the role of exercise in cancer cachexia therapy. Am J Lifestyle Med. 2019;13:46-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uster A, Ruehlin M, Mey S, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. 2018;37:1202-1209. [DOI] [PubMed] [Google Scholar]
- 63. Solheim TS, Laird BJA, Balstad TR. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:778-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schink K, Herrmann HJ, Schwappacher R, et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: a controlled pilot trial. BMC Cancer. 2018,18:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536: 329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cullen T, Thomas AW, Webb R, Hughes MG. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab. 2016;41:803-808. [DOI] [PubMed] [Google Scholar]
- 67. Wiskemann J, Clauss D, Tjaden C, et al. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: a randomized controlled trial. Pancreas. 2019;48:257-266. [DOI] [PubMed] [Google Scholar]
- 68. US National Library of Medicine, ClinicalTrials.gov. Evaluating the combined intervention of nutritional supplementation (Remune) and exercise in patients with cancer cachexia. Published October 18, 2019. Accessed May 5, 2020 https://clinicaltrials.gov/ct2/show/NCT04131426
- 69. US National Library of Medicine, ClinicalTrials.gov. Psycho-educational and rehabilitative intervention for cancer cachexia (PRICC). Published November 6, 2019. Accessed May 5, 2020 https://clinicaltrials.gov/ct2/show/NCT04153019