Abstract
Frailty, a clinical syndrome characterized by multisystem dysregulation, has been associated with high levels of oxidative stress. We investigated the association between serum total antioxidant capacity (TAC) and frailty in older men. This cross-sectional study included 581 men (age 60–90 years) enrolled in the Geelong Osteoporosis Study. Frailty comprised at least three of unintentional weight loss, exhaustion, low physical activity, slowness, and weakness. Serum TAC was measured by quantitative colorimetric determination and expressed as uric acid equivalents (mM). Relationships between TAC (in SD units) and frailty were explored using multivariable logistic regression models. Sociodemographic, anthropometric, and lifestyle variables were tested as potential confounders and effect modifiers. A sensitivity analysis excluded participants (n = 145) in the upper quartile of TAC, who were likely to have hyperuricemia. Fifty (8.6%) men were frail. There was evidence that higher TAC levels were associated with increased likelihood of frailty (OR 1.34, 95% confidence interval [CI; 0.99, 1.80]), and this was attenuated after adjustment for age and body mass index (BMI; OR 1.26, 95% CI [0.93,1.71]). No effect modifiers or other confounders were identified. The sensitivity analysis revealed a positive association between TAC and frailty, before and after accounting for age and BMI (adjusted OR 1.79, 95% CI [1.01, 3.17] p = .038). These results suggest a positive association between TAC levels and frailty, supporting the hypothesis that this biomarker could be useful in identifying individuals at risk of frailty. We speculate that a milieu of heightened oxidative stress in frailty may elevate the oxidative stress regulatory set point, raising antioxidant activity. This warrants further investigation.
Keywords: Total antioxidant capacity, frailty, oxidative stress
Frailty is a clinical syndrome characterized by multisystem dysregulation and increased vulnerability to minor stressors, and it is associated with adverse health outcomes such as falls, fractures, hospitalization, and institutionalization (Cesari et al., 2016; Dent et al., 2016; Fried et al., 2001). These outcomes are costly and create a substantial burden on the health system (Dent et al., 2016). The pathogenesis of this condition is yet to be fully understood, though there have been proposed mechanisms involving biological factors of inflammation and oxidative stress (Walston et al., 2002). Oxidative stress, a state of imbalance between reactive oxygen and nitrogen species (RONS) and the antioxidants that counteract them (Kregel & Zhang, 2006), has been associated with the process of aging (Liguori et al., 2018). Under physiological conditions, it has been stated that the balance between prooxidants and antioxidant compounds moderately favor prooxidants; thus, the body is at a slight oxidative stress requiring endogenous antioxidant systems (Pisoschi & Pop, 2015). The endogenous antioxidants include enzymes such as superoxide dismutase, catalase, glutathione peroxidase, nonenzymatic compounds like glutathione proteins, low-molecular-weight scavengers like uric acid, coenzyme Q, and lipoic acid. Exogenous antioxidants include vitamins C and E, carotenoids, and phenolics (Pisoschi & Negulescu, 2012). These compounds lower oxidative stress, DNA mutations, malignant transformations, as well as other parameters of cell damage. Antioxidants can prevent the occurrence of reactive oxygen species and block and capture free radicals. Antioxidants are also involved in repair processes, by removing damaged biomolecules before their aggregation enables alteration of cell metabolism (Pisoschi & Pop, 2015).
Data from recent studies indicate that oxidative stress may play a role in the development of frailty (Chen et al., 2014; Iqbal et al., 2013). A recent systematic review by Soysal et al. (Soysal et al., 2017) suggested that higher peripheral oxidative stress biomarkers and lower antioxidant factors were evident in frail compared with non-frail adults, although some studies reported no differences. Another study by Garcia-Equinas et al. (García-Esquinas et al., 2016) reported that levels of serum uric acid (an antioxidant) were positively associated with frailty. In addition, these studies have measured different antioxidants and standardized against different antioxidant equivalence; however, none has measured total antioxidant capacity (TAC) in reference to uric acid equivalents. Thus, we aimed to investigate the association between serum TAC and frailty in older men. We hypothesize that frailty will be associated with lower TAC levels.
Material and Methods
Participants
This cross-sectional study included 581 men (aged 60–90 years) enrolled in the Geelong Osteoporosis Study (GOS; Pasco et al., 2012). The GOS is a population-based cohort study involving 1,494 women (1993–1997) and 1,540 men (2001–2006) at baseline, who were randomly selected from the Barwon Statistical Division in southeastern Australia. For the men, repeat assessments were performed 5, 6, and 15 years later. For these analyses, we utilized men data from the baseline visit, as some data for the women were not available at this visit. Only men aged ≥60 years who provided Fried phenotype and TAC data were included in this study. There were no exclusions for comorbidities or health behaviors.
Measurements
Participants were assessed using dual-energy x-ray absorptiometry (DXA, Lunar Prodigy-Pro, Madison, WI, USA), anthropometry (including weight, height, waist, and hip circumference), and tests of functional mobility (including gait speed and timed up and go [TUG] test). Information about habitual physical activity, exhaustion, current smoking, and use of medications and supplements were documented by a questionnaire (Pasco et al., 2012). Alcohol consumption was quantified using the Cancer Council Food Frequency questionnaire (Giles, 1996) and considered high if >30 g/d. Vitamins A, C, and E and multivitamins were grouped with thiazides as they increase antioxidant capacity, and the use of xanthine oxidase inhibitors was identified as these limit uric acid production. The Australian Bureau of Statistics (ABS) Index of Relative Socio-Economic Advantage and Disadvantage (IRSAD) was used to determine area socioeconomic status (SES) in quintiles 1 (most disadvantaged) to 5 (most advantaged). These quintiles were collapsed into low (1 and 2), medium (3), and high (4 and 5) due to small numbers.
Frailty Assessment
Frailty was assessed using a modified Fried frailty phenotype (Fried et al., 2001) that considers unintentional weight loss, weakness, low level of physical activity, exhaustion, and slowness. Unintentional weight loss, low level of physical activity, and exhaustion were self-reported using questionnaires. Exhaustion was assessed by a single question that asked, “Do you have a lack of energy?” Low physical activity was ascertained using a single question that asked about the current mobility status of participants. Low physical activity was indicated when participants selected the following responses: “limited, inactive, chair or bedridden, and bedfast.” Weakness was assessed as maximal isometric strength of hip flexors and hip abductors on both legs determined by a handheld dynamometer (the Nicholas Manual Muscle Tester), with the lowest 20% considered as having weakness (Marino et al., 1982; Pasco et al., 2012), as previously described (Pasco et al., 2020). Slowness was measured using the TUG test, the time taken to stand from a chair (with no armrest), walk 3 m, turn around, and return to sit on the chair. TUG exceeding 10 s was considered as slow (Fried et al., 2001; Podsiadlo & Richardson, 1991). Having at least three of the five items in the modified Fried tool was categorized as frail, one to two items as pre-frail, and zero items as robust. Frailty was also dichotomized into frail versus non-frail (robust and pre-frail combined).
Total Antioxidant Measurement
Blood samples were collected after an overnight fast and sera aliquoted and stored at −80°C until analysis; 581 men provided blood samples for this analysis. TAC was measured by quantitative colorimetric determination using Cell Biolabs Inc. (San Diego, CA) Total Antioxidant Capacity (TAC) Assay commercial kits. In this method, copper II (Cu2+) is reduced to copper I (Cu+) by antioxidants present in the sample. The Cu+ in turn forms a colored complex with a dye reagent, which is read using a spectrometer at 490 nm. The absorbance was measured on spectrophotometer (Biorad xMarkTM, Hercules, CA, USA, running Microplate Manager 6 software).The net absorbance values are proportional to the sample’s total reductive capacity. Results are expressed as uric acid equivalents (mM).
Ethical Considerations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Barwon Health Human Research Ethics Committee (Approval number 00/56).
All participants provided written, informed consent.
Statistical Analysis
Normality of TAC values was tested using Anderson–Darling test and a histogram with an overlay of a normal curve was also used to visually inspect the data. TAC values were found to be normally distributed and expressed as SD units for analyses. Correlations between TAC and age or body mass index (BMI) were determined using Pearson’s correlation tests. Multivariable logistic regression models (binary and ordinal) were developed to determine the relationship of TAC with frailty, and age, BMI, smoking, alcohol consumption, SES, and use of supplements and medications were tested as potential confounders and/or effect modifiers. A sensitivity analysis excluded participants in the upper quartile of TAC who were likely to have hyperuricemia (García-Esquinas et al., 2016). Statistical analyses were performed using Minitab (v18, USA); p value <.05 was considered as significant.
Results
Fifty men (8.6%) were frail. Participant characteristics are presented in Table 1 for the whole group and according to frailty status. Frail men were older and tended to have a higher TAC than the non-frail men. TAC was positively correlated with BMI (r = +0.1, p = .01).
Table 1.
Participant Characteristics for All and Stratified by Frailty Status. Data Are Presented as Median (IQR), Mean ± SD or n (%)
| All |
Frailty |
|||
|---|---|---|---|---|
| n = 581 | Yes (n = 50) | No (n = 531) | p | |
| Age (years) | 74 (67–83) | 78 (72–83) | 74 (67–81) | .004 |
| Weight (kg) | 81.0 ± 13.3 | 82.0 ± 16.6 | 80.8 ± 13.0 | .557 |
| Height (m) | 1.70 ± 0.07 | 1.71 ± 0.06 | 1.72 ± 0.07 | .157 |
| Body mass index (kg/m2) | 27.4 ± 4.0 | 28.1 ± 5.3 | 27.2 ± 3.9 | .158 |
| Alcohol (>3 units/d) | 107 (18%) | 9 (18%) | 98 (18%) | .927 |
| Smoker | 41 (7%) | 5 (10%) | 36 (7%) | .395 |
| Socioeconomic statusa | .319 | |||
| Low | 261 (45%) | 27 (54%) | 234 (44%) | |
| Medium | 103 (18%) | 9 (18%) | 94 (18%) | |
| High | 217 (37%) | 14 (28%) | 203 (38%) | |
| Medication/supplements | ||||
| Antioxidantsb and thiazides | 161 (28%) | 14 (28%) | 147 (28%) | .962 |
| Xanthine oxidase inhibitors | 49 (8%) | 8 (16%) | 41 (8%) | .044 |
| Total antioxidant capacity (mM) | 5.292 ± 0.984 | 5.549 ± 0.929 | 5.268 ± 0.987 | .054 |
Note. aSocioeconomic status grouped into low (most disadvantaged), medium, and high (most advantaged). bAntioxidants included vitamins A, C, and E and multivitamins.
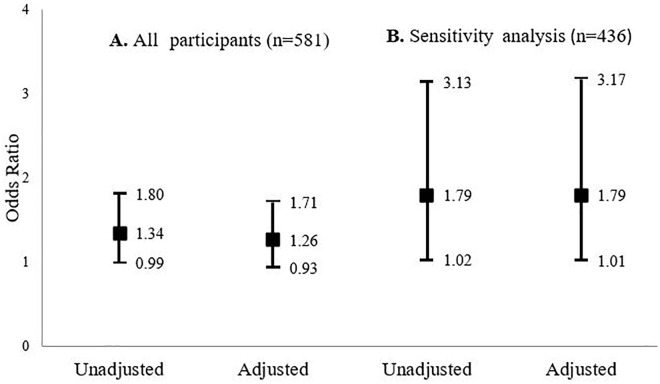
In ordinal logistic regression models that considered frailty as frail, pre-frail, and robust, no statistically significant associations were observed (data not shown). In binary logistic regression models, higher TAC was associated with increased likelihood of frailty with borderline significance (Table 2). For each SD increase in TAC, odds for frailty increased 1.34-fold (OR 1.34, 95% confidence interval [CI; 0.99, 1.80] p = .054; Figure 1). Adjustments for age and BMI attenuated the association (OR 1.26, 95% CI [0.93, 1.71] p = .127). No effect modifiers or other confounders were identified.
Table 2.
Binary Logistic Regression Models of the Association of Total Antioxidant Capacity (TAC) and Frailty in Men. Data Presented as OR (95% confidence interval [CI])
| Models | OR (95% CI) | p value |
|---|---|---|
| Main analysis | ||
| Unadjusted | 1.34 [0.99, 1.80] | .054 |
| Adjusted for age | 1.30 [0.96, 1.74] | .086 |
| Adjusted for age (years) and BMI (kg/m2) | 1.26 [0.93, 1.71] | .127 |
| Sensitivity analysis a | ||
| Unadjusted | 1.79 [1.02, 3.13] | .033 |
| Adjusted for age | 1.78 [1.01–3.15] | .037 |
| Adjusted for age (years) and BMI (kg/m2) | 1.79 [1.01, 3.17] | .038 |
Note. BMI = body mass index.
The sensitivity analysis excluded participants (n =145) who were likely to have hyperuricemia.
Figure 1.
ORs (95% confidence intervals) for the unadjusted and age- and BMI-adjusted binary logistic regression models regarding the associations between serum TAC and frailty for (A) the whole sample and (B) the sensitivity analysis, which excluded participants who were likely to have hyperuricemia. BMI = body mass index; TAC = total antioxidant capacity.
Sensitivity Analysis
After excluding 145 (25%) men because of likely hyperuricemia, our sensitivity analysis revealed a positive association between TAC and frailty, which was significant before (OR 1.79, 95% CI [1.02, 3.13] p = .033) and after adjustment for age and BMI (OR 1.79, 95% CI [1.01, 3.17] p = .038; Figure 1).
Discussion
Here we present data to suggest that frail men have higher serum TAC than their non-frail peers. The association between serum TAC and frailty became stronger after excluding individuals with the high TAC, suggesting that individuals with likely high levels of serum uric acid obscured the apparent association between serum TAC and frailty.
Oxidative stress is a process associated with aging (Sánchez-Flores et al., 2017). It promotes an increase in free radicals and proinflammatory markers, which could result in the depletion of antioxidant reserves (Liguori et al., 2018). This is of great concern for individuals who are vulnerable to minor stressors such as frail people (Soysal et al., 2017). It might be expected, therefore, that frailty could be concordant with low antioxidant capacity. However, our findings did not support this hypothesis.
In accord with our results, a cohort study of 2,198 noninstitutionalized men and women in Spain reported that serum high uric acid concentrations were associated with incident frailty (García-Esquinas et al., 2016). By contrast, data from the Randomly recruited Age-Stratified Individuals from the General population (RASIG) group within the bioMARKers of human AGEing (MARK-AGE) study in Europe (n = 2,220 participants, aged 35–74 years, observed lower levels of antioxidants including β-cryptoxanthin and zeaxanthin among individuals identified as frail based on the physical, cognitive, and psychological domains of frailty (Rietman et al., 2018). A cross-sectional multicenter study in Japan, involving a multigenerational cohort of 2,121 grandmothers (65 years and older), investigated the association between dietary TAC and prevalence of frailty (Kobayashi et al., 2014). In that study, dietary TAC was calculated from a self-reported questionnaire that had been validated using four assays that included ferric reducing ability of plasma, oxygen radical absorbance capacity, Trolox equivalent antioxidant capacity, and total radical-trapping antioxidant parameter. Higher levels of dietary TAC were associated with lower odds of frailty suggesting a strong inverse association between the two.
Inconsistencies in the literature could be related to different criteria used to define frailty and/or the age range of the population studied.
We speculate the existence of a positive feedback loop, whereby frail individuals may overcompensate for a high oxidative stress environment by producing more antioxidants in efforts to restore physiological homeostasis. As such, the elevated total antioxidant levels seen in our study may be due to a perpetual oxidative stress state experienced by these individuals.
The strengths of our study include the randomly selected sample of men from the general population, a wide range of ages across adulthood, and the use of objective measures of serum TAC, muscle strength, and slowness. Our study has some limitations, which include the use of a modified Fried phenotype tool and some self-reported data, which might have led to misclassification of frailty. In addition, given that our participants were men, and mainly Caucasian, we acknowledge that the findings may not be generalizable to other populations including women.
In conclusion, our results suggest a positive association between serum levels of TAC and frailty, which supports the notion that this biomarker could be useful in identifying individuals at risk of frailty. We speculate that a milieu of heightened oxidative stress in frailty may elevate the oxidative stress regulatory set point, thus raising antioxidant activity. However, as the association between serum TAC and frailty was of borderline significance, our findings should be interpreted with caution and warrant further investigation in larger samples and in other populations.
Acknowledgments
The authors acknowledge the men who participated in the study. The authors thank Professor Graham Giles of the Cancer Epidemiology Centre of The Cancer Council Victoria for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: The Cancer Council Victoria, 1996.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Health and Medical Research Council (NHMRC) Australia (projects 299831, 628582). The funding organization played no role in the design or conduct of the study, in the collection, management, analysis, and interpretation of the data, nor in the preparation, review, and approval of the manuscript. MCT and SXS were supported by Deakin Postgraduate Scholarships, KLH-K and CCB were supported by Alfred Deakin Postdoctoral Research Fellowship; SLB-O was supported by NHMRC Career Development Fellowships (1107510) and LJW is supported by an NHMRC Career Development Fellowship (1064272) and a NHMRC Investigator grant (1174060).
ORCID iD: Monica C. Tembo  https://orcid.org/0000-0003-1210-2437
https://orcid.org/0000-0003-1210-2437
References
- Cesari M., Prince M., Thiyagarajan J. A., De Carvalho I. A., Bernabei R., Chan P., Gutierrez-Robledo L. M., Michel J.-P., Morley J. E., Ong P., Rodriguez Manas L., Sinclair A., Won C. W., Beard J., Vellas B. (2016). Frailty: An emerging public health priority. Journal of the American Medical Directors Association, 17(3), 188–192. 10.1016/j.jamda.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Chen X., Mao G., Leng S. X. (2014). Frailty syndrome: An overview. Clinical Interventions in Aging, 9, 433–441. 10.2147/CIA.S45300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E., Hoon E., Karnon J., Newbury J., Kitson A., Beilby J. (2016). Frailty and health service use in rural South Australia. Archives of Gerontology and Geriatrics, 62, 53–58. 10.1016/j.archger.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W. J., Burke G., McBurnie M. A., & Cardiovascular Health Study Collaborative Research Group. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology: Series A, 56(3), M146–M156. [DOI] [PubMed] [Google Scholar]
- García-Esquinas E., Guallar-Castillón P., Carnicero J. A., Buño A., García-García F. J., Rodríguez-Mañas L., Rodríguez-Artalejo F. (2016). Serum uric acid concentrations and risk of frailty in older adults. Experimental Gerontology, 82, 160–165. 10.1016/j.exger.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Giles G. (1996). Dietary questionnaire for epidemiological studies (Version 2). Melbourne: The Cancer Council Victoria. [Google Scholar]
- Iqbal J., Denvir M., Gunn J., Clegg A., Young J., Iliffe S., Rockwood K. (2013). Frailty in elderly people. The Lancet, 381(9868), 752–762. 10.1016/S0140-6736(13)61203-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Asakura K., Suga H., Sasaki S. (2014). Inverse association between dietary habits with high total antioxidant capacity and prevalence of frailty among elderly Japanese women: A multicenter cross-sectional study. Journal of Nutrition, Health and Aging, 18(9), 827–836. 10.1007/s12603-014-0556-7 [DOI] [PubMed] [Google Scholar]
- Kregel K. C., Zhang H. J. (2006). An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 292(1), R18–36. [DOI] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. (2018). Oxidative stress, aging, and diseases. Clinical Interventions in Aging, 13, 757–772. 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M., Nicholas J. A., Gleim G. W., Rosenthal P., Nicholas S. J. (1982). The efficacy of manual assessment of muscle strength using a new device. The American Journal of Sports Medicine, 10(6), 360–364. 10.1177/036354658201000608 [DOI] [PubMed] [Google Scholar]
- Pasco J. A., Nicholson G. C., Kotowicz M. A. (2012). Cohort profile: Geelong osteoporosis study. International Journal of Epidemiology, 41(6), 1565–1575. 10.1093/ije/dyr148 [DOI] [PubMed] [Google Scholar]
- Pasco J. A., Stuart A. L., Holloway-Kew K. L., Tembo M. C., Sui S. X., Anderson K. B., Hyde N. K., Williams L. J., Kotowicz M. A. (2020). Lower-limb muscle strength: Normative data from an observational population-based study. BMC Musculoskeletal Disorders, 21(1), 89 10.1186/s12891-020-3098-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoschi A. M., Negulescu G. P. (2012). Methods for total antioxidant activity determination: A review. Biochemistry & Analytical Biochemistry, 01(01), 1–10. 10.4172/2161-1009.1000106 [DOI] [Google Scholar]
- Pisoschi A. M., Pop A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry, 97, 55–74. 10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Podsiadlo D., Richardson S. (1991). The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39(2), 142–148. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- Rietman M. L., Spijkerman A. M. W., Wong A., vanSteeg H., Bürkle A., Moreno-Villanueva M., Sindlinger T., Franceschi C., Grubeck-Loebenstein B., Bernhardt J., Slagboom P. E. (2018, January). Antioxidants linked with physical, cognitive and psychological frailty: Analysis of candidate biomarkers and markers derived from the MARK-AGE study. Mechanisms of Ageing and Development, 1–9. 10.1016/j.mad.2018.04.007 [DOI] [PubMed]
- Sánchez-Flores M., Marcos-Pérez D., Costa S., Teixeira J. P., Bonassi S., Pásaro E., Laffon B., Valdiglesias V. (2017). Oxidative stress, genomic features and DNA repair in frail elderly: A systematic review. Ageing Research Reviews, 37, 1–15. 10.1016/j.arr.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Soysal P., Isik A. T., Carvalho A. F., Fernandes B. S., Solmi M., Schofield P., Veronese N., Stubbs B. (2017). Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas, 99, 66–72. 10.1016/j.maturitas.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Walston J., McBurnie M. A., Newman A., Tracy R. P., Kop W. J., Hirsch C. H., Gottdiener J., Fried L. P., & Cardiovascular Health Study. (2002). Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Archives of Internal Medicine, 162(20), 2333–2341. 10.1001/archinte.162.20.2333 [DOI] [PubMed] [Google Scholar]