Abstract
Patients with lung cancer are presumed to be at high risk from COVID-19 infection due to underlying malignancy. A total of 31 COVID-19 patients with pre-diagnosed lung cancer and 186 age and sex matched COVID-19 patients without cancer in 6 hospitals in Wuhan, China were identified in our study. There was a significantly higher level of IL-6 in lung cancer group showed by multifactorial analysis. The restricted mean survival time in 10, 20, and 53 days in COVID-19 patients with lung cancer were ealier than non-cancer COVID-19 patients in the same observation time (all P values < 0.05). Our results indicated that pre-diagnosed lung cancer was associated with higher morbidity and mortality in COVID-19 patients.
Keywords: Covid-19, lung cancer, restricted mean survival time
Introduction
Since late December of 2019, the whole world has been hit by 2019 novel coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of June, in 2020, more than 6,000,000 individuals were infected with this new virus causing a fatality rate of about 6.9%. Mounting evidence from recent reports has demonstrated that patients with cancer have the higher morbidity and death rate of COVID-19 compared to non-cancer individuals.1,2 However, the relationship of a certain cancer type with the risk of COVID-19 remains inconclusive. Interestingly, it has been reported that individuals with lung cancer had the greatest infection rate of SARS-CoV-2 among all cancer patients.3,4 Therefore, in this study, We sought to investigate the risk posed by COVID-19 to lung cancer population, and identify factors that placed lung cancer patients at highest risk of fatality from COVID-19.
Materials and Methods
Study Design and Participants
This multi-center study was performed in 6 hospitals designated for COVID-19 patients in Wuhan from January 1, 2020 to March 1, 2020 (listed in the Supplementary Appendix). COVID-19 was diagnosed according to the WHO interim guidance.5 Over all, 31 COVID-19 patients with pre-diagnosed lung cancer were collected in our study. An age and sex-matched cohort of 186 patients at a 6:1 ratio of non-cancer to cancer COVID-19 patients from the same time period and from the same hospital system was also obtained after propensity matching and used as control to estimate the increased risk posed to lung cancer population. The study was approved by the institutional ethics review board and the need for informed consent was waived.
Data Collection
Patient medical records were obtained from the electronic medical records of the above 6 hospitals. Collected medical records included demographic characteristics, symptoms, signs, underlying comorbid conditions, laboratory results and clinical outcomes. Three independent physicians who had been taking care of patients with SARS-CoV-2 infection from each hospital double-checked the medical records to confirm data accuracy. Nasopharyngeal swabs were obtained for nucleic acid test of SARS-CoV-2 infection. Four primary outcomes were analyzed including: death, admission into the intensive care unit (ICU), development of severe symptoms, and utilization of invasive mechanical ventilation. The clinical destination of severe symptoms follow the 5th edition of 2019 Novel Coronavirus Disease (COVID-19) Diagnostic criteria published by the National Health Commission in China (listed in the Supplementary Appendix).
Statistical Analysis
For categorical data, percentages of patients in each category were calculated. The Wilcoxon rank sum test was used to compare continuous data and Fisher’s exact test was used to compare categorical data from different categories without multi-test adjustment. Time from onset of symptoms to severe outcomes was investigated using survival analysis, with follow-up from initial onset of symptoms until the last day of follow-up. Multivariable Cox regression was conducted to estimate the hazard ratios and their corresponding 95% confidence interval. The Kaplan-Meier product-limit estimator was used to conduct survival analysis. All survival analyses were conducted using Lifelines 0.24.0 in Python environment.
Results
Table 1 shows the demographic and clinical characteristics of the 31 COVID-19 patients with lung cancer.The mean age of the overall population was 67.26 years (standard deviation [SD]: 8.40 years), including 25 males and 6 females. Among the lung cancer patients, adenocarcinoma was the most frequent pathological type (14, 45.16%), followed by squamous cell carcinoma (7, 22.58%) and mixed cinoma (2, 6.45%). Ten patients (38.71%) were diagnosed with stage IV cancer. The most common symptoms on admission were fever (21, 67.74%), Cough(8, 25.81%) and Shortness of breath (6, 19.35%). There was a significantly higher level of IL-6 in lung cancer group with an average of 67.43 pg/ml (95% CI: 30.25-104.61 pg/ml) increase showed by multifactorial analysis, compared to non-cancer Covid-19 patients (Supplementary Table2).
Table 1.
Demographics and Clinical Characteristics of COVID-19 Patients With Lung Cancer.
| Characteristics | N.(%) |
|---|---|
| Age | 67.26 ± 8.40 |
| Gender | |
| Male | 25 (80.6%) |
| Female | 6 (19.4%) |
| Smoking History | |
| Current | 5 (16.13%) |
| Former | 10 (32.26%) |
| Never | 16 (51.61%) |
| Drinking | 5 (16.13%) |
| Comorbidities | |
| Hypertension | 9 (29.03%) |
| Diabetes | 4 (16.13%) |
| Cardiovascular disease | 4 (12.90%) |
| Cerebrovascular disease | 2 (6.45%) |
| COPD | 3 (9.68%) |
| Pathological Type | |
| Squamous cell carcinoma | 7 (22.58%) |
| Adenocarcinoma | 14 (45.16%) |
| Mixed | 2 (6.45%) |
| Pathological Stage | |
| I | 9 (33.33%) |
| II | 2 (6.45%) |
| III | 4 (12.90%) |
| IV | 12 (38.71%) |
| Anti-cancer Treatments | |
| Surgery | 7 (22.58%) |
| Radiotherapy | 8 (25.81%) |
| Chemotherapy | 10 (32.26%) |
| Targeted therapy | 3 (9.68%) |
| Immunotherapy | 4 (12.90%) |
| Signs at oneset | |
| Fever (temperature ≥37·3°C) | 21 (67.74%) |
| Cough | 8 (25.81%) |
| Expectoration | 5 (16.13%) |
| Myalgia | 3 (9.67%) |
| Abdominal pain or Diarrhea | 3 (9.67%) |
| Shortness of breath | 6 (19.35%) |
| Outcomes | |
| Severe illness | 17 (54.8%) |
| ICU | 10 (32.26%) |
| IMV | 5 (16.13%) |
| Death | 8 (25.81%) |
Data are presented as mean (SD) or n (%). Anti-Cancer treatments present treatments within 3 months before the onset of COVID-19 symptoms. Abbreviations: COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; IMV = invasive mechanical ventilation.
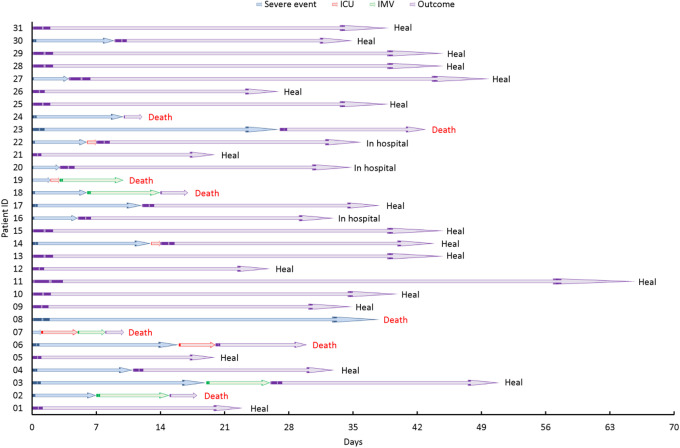
Compared with COVID-19 patients without cancer, patients with lung cancer had higher observed severe events (OR 4.28, 95% CI [1.44, 12.71], p = 0.009), higher rates of ICU admission (HR 4.72, 95% CI [1.19, 18.72], p = 0.027) and higher death rates (HR 3.87, 95% CI [1.02, 14.71, p = 0.047) (Figure 1). To evaluate the time-dependent evolution of the disease, we also conducted survival analysis on occurrence of any severe condition which included death, ICU admission, having severe/critical symptoms and utilization of invasive mechanical ventilation (Supplementary Figure 1). Figure 2 demonstrates the time line of different events for COVID-19 patients with lung cancer with death and other severe events marked in the Figure. In general, patients with lung cancer deteriorated more rapidly than those without cancer.
Figure 1.
Associations of severe conditions in COVID-19 patients with lung cancer and without cancer. Hazard ratio, 95% CI and p values denoted the comparison between COVID-19 patients with lung cancer and age and sex-matched non-cancer COVID-19 patients. Abbreviations: ICU = intensive care unit, IMV = invasive mechanical ventilation.
Figure 2.
Timeline of events for COVID-19 patients with lung cancer. Severe event, ICU admission, IMVand death are marked in the Figure. Abbreviations: ICU = intensive care unit, IMV = invasive mechanical ventilation.
Restricted mean survival time (RMST) is a well-established measure that can be interpreted as the average event-free survival time up to a pre-specified, clinically important time point.6-8 We conducted the analysis of RMST between Covid-19 patients with lung cancer and without cancer. The observation time points were 10, 20, and 53 days from admission day (Table 2). Our data showed that in the 10 days observation time, COVID-19 patients with lung cancer developed severe conditions 1.6 days earlier, required ICU admission 0.9 days earliern than non-cancer COVID-19 patients (all P values < 0.05). COVID-19 patients with lung cancer developed severe conditions 3.7 days earlier, required ICU admission 3.1 days earlier, use invasive mechanical ventilation and death 1.1 days ealier ealier than non-cancer COVID-19 patients in the 20 days of observation time (all P values < 0.05). Furthermore, in the 53 days observation time, lung cancer patients had 17.3 days earlier of developing severe conditions, 11.6 days earlier of admission into ICU and 7.8 days of death than non-cancer patients (all P values < 0.05).
Table 2.
Restricted Mean Survival Time (RMST) Between COVID-19 Patients With Lung Cancer and Without Cancer.
| Variables | Restricted days | Lung cancer | Non cancer | P value |
|---|---|---|---|---|
| Severe event | 10 | 8.4 (7.5,9.4) | 10.0 (10.0,10.0) | 0.002 |
| 20 | 14.1 (11.7,16.6) | 19.7 (19.3,20.2) | <0.001 | |
| 53 | 29.5 (21.5,37.4) | 46.8 (41.3,52.4) | <0.001 | |
| ICU | 10 | 9.1 (8.5,9.8) | 10.0 (10.0,10.0) | 0.013 |
| 20 | 16.9 (14.9,19.0) | 20.0 (20.0,20.0) | 0.003 | |
| 53 | 38.7 (31.5,45.8) | 50.3 (46.2,54.5) | 0.006 | |
| IMV | 10 | 9.9 (9.8,10.1) | 10.0 (10.0,10.0) | 0.309 |
| 20 | 18.9 (17.9,20.0) | 20.0 (20.0,20.0) | 0.043 | |
| 53 | 46.5 (41.4,51.7) | 49.4 (45.5,53.3) | 0.386 | |
| Death | 10 | NA | NA | NA |
| 20 | 18.9 (17.9,19.9) | 20.0 (20.0,20.0) | 0.034 | |
| 53 | 43.8 (38.2,49.5) | 51.6 (49.3,53.9) | 0.013 |
Notes: RMST is a kind of survial time measure method which can be estimated under a restricted period; ICU = intensive care unit; IMV = invasive mechanical ventilation.; N. A = not available.
Discussion
The morbidity and mortality of lung cancer ranks the first among all malignant tumors worldwide. Over 3.8 million were diagnosed as lung cancer in 2014 in China.9,10 Furthermore, patients with lung cancer had a more severe outcome when co-infected influenza or respiratory diseases such as bronchopneumonia or pneumonia.11 Therefore, we believe that lung cancer is a risk factor for COVID-19-related pneumonia.12 Jacobo Rogado et al investigated 17 COVID-19 patients with lung cancer among 1878 COVID-19 patients and found that lung cancer patients have a higher mortality rate than general population. Furthermore, univariate and multivariate logistic regression revealed that combined treatment with hydroxychloroquine and azithromycin significantly improved the outcome of Covid-19 in lung cancer patients.13 However, limited information is known about the clinical characteristics and outcomes of lung cancer patients with COVID-19. Our study indicated that there was a significantly higher level of IL-6 in lung cancer group and that the restricted mean survival time in COVID-19 patients with lung cancer were earlier than non-cancer patients.
From the results of multicenter cohort study, patients with pre-existing lung cancer had the severe outcomes including ICU admission (32.3%), invasive mechanical ventilation (16.1%), and death (25.8%), which may be caused by low lung function and immunosuppression.
Recently, restricted mean survival time (RMST) has been proposed as an alternative measure of treatment effect that offers some advantages in design, analysis, and interpretation over the conventional measures, especially in studies among small number of patients.14,15 In our study, we found in the observation time of 10, 20 and 53 days, COVID 19 patients with lung cancer patients developed severe conditions and death faster than non-cancer COVID 19 patients, which indicated lung cancer was associated with increased mortality among COVID-19 patients.
The main endogenous cytokine IL-6, has been suggested to be a prediction factor for severe condition of Covid-19 patients.16,17 Lung cancer patients had a significant increase in IL-6 level compared to non-cancer patients when infected SARS-CoV-2.18 This line of evidence may explain lung cancer patients develop more severe conditions than non-cancer patients during COVID-19 outbreak. According to pathological studies on the COVID-19 patients, there were desquamation of pneumocytes and hyaline membrane formation, implying that these patients had ARDS. ARDS induced by cytokine storm indicated that patients without lung cancer had stronger immune response.19 Moreover, it is possible that certain anti-cancer treatments such as immunotherapy and chemotherapy can induce the release of a large amount of cytokines, which can be toxic to normal cells, including lung epithelial cells, and therefore exacerbates the severity of illness.20 However, due to the small number of patients in current study, further study with a large case population is required. In summary, our findings suggest that lung cancer is the risk factor associated with the prognosis of Covid-19 patients.
Supplemental Material
Supplemental Material, Revised_Supplement_Appendix for Patients With Lung Cancer Have High Susceptibility of COVID-19: A Retrospective Study in Wuhan, China by Meng-Yuan Dai, Zhen Chen, Yan Leng, Meng Wu, Yu Liu, Fuxiang Zhou, Chen Ming, Ningyi Shao, Miao Liu and Hongbing Cai in Cancer Control
Footnotes
Author Contribution: Mengyuan Dai, Zhen Chen, Yan Leng and Meng Wu contributed equally to the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded by grants [National Natural Science Foundation of China 8197103302/H16 (Hong-Bing Cai); Editorial support was provided by the institutional ethics board of Zhongnan Hospital of Wuhan University (No. 2020029).
ORCID iD: Miao Liu  https://orcid.org/0000-0003-3365-0213
https://orcid.org/0000-0003-3365-0213
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu J, Ouyang W, Chua ML, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;25:e200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerableto SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robilotti EV, Esther Babady N, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi:10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020 World Health Organization; 2020. [Google Scholar]
- 6. Uno H, Wittes J, Fu H, et al. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med. 2015;163(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 10. Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–473. [DOI] [PubMed] [Google Scholar]
- 11. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 12. Cooksley CD, Avritscher EB, Bekele BN, Rolston KV, Geraci JM, Elting LS. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer. 2005;104(3):618–628. [DOI] [PubMed] [Google Scholar]
- 13. Rogado J, Pangua C, Serrano-Montero G, et al. Covid-19 and lung cancer: a greater fatality rate?. Lung cancer. 2020;146:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. [DOI] [PubMed] [Google Scholar]
- 16. Song R, Han B, Song M, et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing, China. J Infect. 2020;81(2):e26–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):1. [DOI] [PubMed] [Google Scholar]
- 18. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Revised_Supplement_Appendix for Patients With Lung Cancer Have High Susceptibility of COVID-19: A Retrospective Study in Wuhan, China by Meng-Yuan Dai, Zhen Chen, Yan Leng, Meng Wu, Yu Liu, Fuxiang Zhou, Chen Ming, Ningyi Shao, Miao Liu and Hongbing Cai in Cancer Control