Abstract
This feeding study investigated the hypothesis that over-processing of meat and bone meal (MBM) would impair the performance, gut health and ileal digestibility of nutrients in birds challenged with necrotic enteritis (NE). The effect of phytase (500 vs. 5,000 FTU/kg) was also examined using manufacturers recommended matrix values for 500 FTU for both levels. Ross 308 male broilers (n = 768) were assigned to 8 diets, with 6 replicate pens per diet and 16 birds per replicate pen using a randomized design with a factorial arrangement of treatments. Factors were NE challenge (no or yes), MBM (as received or over-processed), and phytase level (500 or 5,000 FTU/kg). Half of the birds were challenged with 5,000 oocysts of field strains of Eimeria acervulina and Eimeria brunetti, and 2,500 oocysts of Eimeria maxima on d 9 and 108 CFU/mL of Clostridium perfringens strain EHE-NE18 on d 14 and 15 post-hatch. Challenge × MBM interactions were detected for weight gain (WG), feed conversion ratio (FCR) and feed intake (FI) at d 14, 21 and 28, showing that challenged birds fed over-processed MBM had decreased WG (P < 0.05) and FI (P < 0.05) at d 14, increased FCR (P < 0.05) at d 21 and decreased WG (P < 0.05) and FI (P > 0.05) at d 28. Birds fed low phytase had increased livability (P < 0.05) at d 42. The challenge increased the prevalence and severity of NE induced lesions in the jejunum (P < 0.05) and ileum (P < 0.05). The birds fed over-processed MBM had decreased pH in the jejunum (P < 0.05) and ileum (P < 0.05) at d 16. High phytase increased apparent ileal digestibility (AID) of Ca (P < 0.05) and P (P < 0.05), and over-processed MBM increased AID of carbon (C; P < 0.05) and Ca (P < 0.05) at d 29. The challenge increased the caecal counts of Lactobacillus spp. (P < 0.05) and C. perfringens (P < 0.05) at d 16. The results indicated that supplementation of diets with high phytase reduces the negative impact on performance from over-processed MBM during NE as a result of increased nutrient digestibility.
Keywords: Broilers, Indigestible proteins, Meat and bone meal, Necrotic enteritis, Phytase
1. Introduction
Necrotic enteritis (NE) has received much research attention in the last few years due to its economic significance in the poultry industry. The heightened interest in this area is partly due to the ongoing removal of in-feed antibiotics as the conventional means for preventing NE in poultry (Branton et al., 1997, Barekatain et al., 2013, M'Sadeq et al., 2015, Yang et al., 2016, Kheravii et al., 2017). Digestion inefficiency, presence of viscous cereal grains (barley, rye, oats and wheat) and poorly digested animal protein sources (meat and bone meal [MBM]; fishmeal) are predisposing factors that can increase susceptibility of broilers to NE (Hoerr, 1998, Palliyeguru et al., 2010, Guo et al., 2013, Knight et al., 2016). The poor digestibility leads to a build-up of undigested nutrients in the hindgut that serve as substrates for opportunistic pathogenic bacteria, including Clostridium perfringens.
Over-processing of MBM during heat processing causes crosslinking of the free epsilon amino group in lysine (Fernandez and Parsons, 1996, Fontaine et al., 2007, Almeida et al., 2014). Such crosslinks including isopeptides, disulfide bridges, aldol condensation, malonaldehyde links, polyphenol complexes that reduce the overall digestibility of both lysine and protein (Hammann and Schmid, 2014, Zhang et al., 2018). The amount of available lysine in MBM has been used as an indicator amino acid (AA) digestibility and optimum processing (Pahm et al., 2008, Rutherfurd, 2015). Lipid oxidation (e. g. malonaldehyde) increases with heat and may form enzyme-resistant cross-links resulting in racemization that decreases hydrolysis of peptide bonds involving D- AA (Dai et al., 2019). Keratin proteins present in wool, feathers, and hair are not digestible by pepsin unless the rendering process includes a pressure cycle or enzymes capable of hydrolyzing keratin. Hence, MBM that contains a high proportion of such proteins has low pepsin digestibility (Hegedus et al., 1989). Apajalahti and Vienola (2016) suggested that the indigestible proteins in MBM that escape gastric digestion might be used as a substrate by C. perfringens, meaning that high inclusion of MBM in poultry diets is a potential precursor for the onset of NE. Fermentation of AA in the terminal part of the gut produces metabolites including biogenic amines, total volatile nitrogen (TVN) and ammonia (Qaisrani et al., 2014, Qaisrani et al., 2015a). These metabolites increase the pH of the lower gut, providing a favorable environment for the proliferation of pathogen bacteria leading to changes in microbial population and species diversity (Corrier et al., 1990, Walugembe et al., 2015, Ilhan et al., 2017).
Exogenous phytase has been used over the years to reduce the amount of inorganic phosphorus (P) and calcium (Ca) required in chicken diets and improve feed conversion ratio (FCR) and AA digestibility. The effect of phytase supplementation on improving growth and AA digestibility in broilers fed diets deficient in lysine has been well documented (Ravindran et al., 2001). Therefore, exogenous phytase supplementation, particularly at high inclusion level, might be used as a potential substitute for MBM as a source of protein, Ca and P in chicken diets. It was hypothesized that over-processed MBM, that is, excessive heating beyond what should be commercially practiced in rendering plants in Australia (110 to 115 °C without pressure), would exacerbate the onset of NE, due to the increased presence of undigested protein. It was also hypothesized that this effect may be countered using a super dose of phytase (5,000 FTU), above what is routinely included in most commercial poultry feed globally, to replace MBM. The objective of this study was to determine the effect of over-processed MBM and phytase supplementation on the performance, intestinal lesions and pH, bacterial counts and apparent ileal digestibility of nutrients in broilers under or not a NE challenge protocol.
2. Materials and methods
2.1. Birds and management
All experimental procedures were reviewed and approved by the University of New England's Animal Ethics Committee. A total of 768 of the chicks were weighed and randomly allocated to 48-floor pens (0.85 m2), with softwood shavings (8 cm) as bedding materials, using 6 replicate pens per treatment and 16 birds per pen. The chicks were reared and housed in an environmentally controlled room with ad libitum access to feed and water. Each pen was fitted with a single tube feeder (diameter = 32 cm) and 4 nipple drinkers. The lighting and temperature program for the experimental period followed the Ross 308 recommendations (Aviagen, 2014). Mortality was recorded daily and cumulative pen weight and feed consumption were recorded on d 7, 14, 21, 28, 35 and 42. For brevity, only d 14, 21, 28 and 42 results are reported.
2.2. Dietary treatment
The crude protein (CP), AA, crude fiber (CF), and crude fat component of homogenized samples of MBM, canola meal, soybean, and wheat were analyzed using a near-infrared spectroscopy (NIRS, Evonik AminoProx, Frankfurt, Germany) prior to feed formulation. Diets were then formulated in accordance with Ross 308 standard ileal digestibility AA and energy specifications (Table 1). The diets were mixed and pelleted using a Palmer PP300SW Pellet Press (Palmer Milling Engineers Plt Ltd, Altin Street, Griffith, NSW, Australia) at 65 °C. Treatments were arranged in a 2 × 2 × 2 factorial design. Factors were NE challenge (no or yes), MBM (as received or over-processed), phytase (500 or 5,000 FTU/kg) (Quantum Blue, AB Vista, Malborough, UK). The phytase matrix values for 500 FTU/kg were applied in both 500 and 5,000 FTU/kg phytase groups. The diets were offered ad libitum throughout the starter (d 0 to 14), grower (d 14 to 28) and finisher (d 28 to 42) phases. The starter diets were fed to the birds in crumbled form and the grower and finisher were fed in pelleted form.
Table 1.
Ingredients and nutrient composition of basal diets (%, as-fed).
| Item | Starter |
Grower |
Finisher |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR + low phytase | AR + high phytase | OP + low phytase | OP + high phytase | AR + low phytase | AR + high phytase | OP + low phytase | OP + high phytase | AR + low phytase | AR + high phytase | OP + low phytase | OP + high phytase | |
| Ingredients | ||||||||||||
| Wheat | 64.17 | 64.17 | 64.17 | 64.17 | 70.82 | 70.82 | 70.82 | 70.82 | 71.41 | 71.41 | 71.41 | 71.41 |
| Soybean meal | 26.56 | 26.56 | 26.56 | 26.56 | 18.98 | 18.98 | 18.98 | 18.98 | 17.33 | 17.33 | 17.33 | 17.33 |
| Canola expeller cold | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| MBM (AR) | 4.03 | 4.03 | – | – | 4.00 | 4.00 | – | – | 4.00 | 4.00 | – | – |
| MBM (OP) | – | – | 4.03 | 4.03 | – | – | 4.00 | 4.00 | – | – | 4.00 | 4.00 |
| Canola oil | 0.538 | 0.538 | 0.538 | 0.538 | 1.269 | 1.269 | 1.269 | 1.269 | 2.581 | 2.581 | 2.581 | 2.581 |
| Limestone | 0.529 | 0.529 | 0.529 | 0.529 | 0.423 | 0.423 | 0.423 | 0.423 | 0.223 | 0.223 | 0.223 | 0.223 |
| Phytase 1 | 0.1 | 1.0 | 0.1 | 1.0 | 0.1 | 1.0 | 0.1 | 1.0 | 0.1 | 1.0 | 0.1 | 1.0 |
| Xylanase2 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Salt | 0.104 | 0.104 | 0.104 | 0.104 | 0.076 | 0.076 | 0.076 | 0.076 | 0.077 | 0.077 | 0.077 | 0.077 |
| Na bicarb | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 |
| TiO2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Vitamins3 | 0.075 | 0.075 | 0.075 | 0.075 | 0.060 | 0.060 | 0.060 | 0.060 | 0.060 | 0.060 | 0.060 | 0.060 |
| Trace minerals4 | 0.100 | 0.100 | 0.100 | 0.100 | 0.080 | 0.080 | 0.080 | 0.080 | 0.080 | 0.080 | 0.080 | 0.080 |
| Choline Cl (60%) | 0.061 | 0.061 | 0.061 | 0.061 | 0.062 | 0.062 | 0.062 | 0.062 | 0.058 | 0.058 | 0.058 | 0.058 |
| l-Lysine HCl | 0.268 | 0.268 | 0.268 | 0.268 | 0.250 | 0.250 | 0.250 | 0.250 | 0.226 | 0.226 | 0.226 | 0.226 |
| dl-Methionine | 0.233 | 0.233 | 0.233 | 0.233 | 0.164 | 0.164 | 0.164 | 0.164 | 0.149 | 0.149 | 0.149 | 0.149 |
| l-Threonine | 0.066 | 0.066 | 0.066 | 0.066 | 0.055 | 0.055 | 0.055 | 0.055 | 0.049 | 0.049 | 0.049 | 0.049 |
| Calculated nutrients | ||||||||||||
| ME poultry, kcal/kg | 3,000 | 3,000 | 3,000 | 3,000 | 3,100 | 3,100 | 3,100 | 3,100 | 3,200 | 3,200 | 3,200 | 3,200 |
| Crude protein | 24.46 | 24.46 | 24.46 | 24.46 | 21.57 | 21.57 | 21.57 | 21.57 | 20.82 | 20.82 | 20.82 | 20.82 |
| Ash | 4.68 | 4.68 | 4.68 | 4.68 | 4.75 | 4.75 | 4.75 | 4.75 | 4.66 | 4.66 | 4.66 | 4.66 |
| Standardized ileal digestibility | ||||||||||||
| Arginine | 1.40 | 1.40 | 1.40 | 1.40 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Lysine | 1.24 | 1.24 | 1.24 | 1.24 | 1.05 | 1.05 | 1.05 | 1.05 | 0.99 | 0.99 | 0.99 | 0.99 |
| Methionine | 0.539 | 0.539 | 0.539 | 0.539 | 0.443 | 0.443 | 0.443 | 0.443 | 0.42 | 0.42 | 0.42 | 0.42 |
| Methionine + Cystine | 0.9 | 0.9 | 0.9 | 0.9 | 0.79 | 0.79 | 0.79 | 0.79 | 0.762 | 0.762 | 0.762 | 0.762 |
| Tryptophan | 0.264 | 0.264 | 0.264 | 0.264 | 0.229 | 0.229 | 0.229 | 0.229 | 0.221 | 0.221 | 0.221 | 0.221 |
| Isoleucine | 0.853 | 0.853 | 0.853 | 0.853 | 0.73 | 0.73 | 0.73 | 0.73 | 0.7 | 0.7 | 0.7 | 0.7 |
| Threonine | 0.79 | 0.79 | 0.79 | 0.79 | 0.68 | 0.68 | 0.68 | 0.68 | 0.65 | 0.65 | 0.65 | 0.65 |
| Valine | 0.98 | 0.98 | 0.98 | 0.98 | 0.857 | 0.857 | 0.857 | 0.857 | 0.826 | 0.826 | 0.826 | 0.826 |
| Calcium | 0.9 | 0.9 | 0.9 | 0.9 | 0.84 | 0.84 | 0.84 | 0.84 | 0.76 | 0.76 | 0.76 | 0.76 |
| Available phosphorus | 0.45 | 0.45 | 0.45 | 0.45 | 0.439 | 0.439 | 0.439 | 0.439 | 0.437 | 0.437 | 0.437 | 0.437 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.18 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Choline, mg/kg | 1,699 | 1,699 | 1,699 | 1,699 | 1,600 | 1,600 | 1,600 | 1,600 | 1,550 | 1,550 | 1,550 | 1,550 |
| Analyzed | ||||||||||||
| Crude protein | 25.46 | 26.45 | 25.79 | 26.32 | 23.66 | 24.31 | 23.55 | 23.63 | 22.64 | 23.34 | 21.90 | 21.64 |
| Calcium | 1.36 | 1.26 | 1.31 | 1.37 | 1.21 | 1.15 | 1.22 | 1.24 | 1.21 | 1.18 | 1.09 | 1.18 |
| Total phosphorus | 0.88 | 0.84 | 0.85 | 0.88 | 0.79 | 0.79 | 0.82 | 0.82 | 0.83 | 0.80 | 0.76 | 0.79 |
AR = as-received; OP = over-processed; MBM = meat and bone meal.
Phytase (Quantum Blue 5G, AB Vista feed ingredient, UK). Both 500 (0.1% phytase) and 5,000 (1.0% phytase) FTU/kg phytase used the matrix values for Ca, P, Na, arginine, lysine, methionine, methionine + cystine, tryptophan, isoleucine, threonine and valine at 1.65, 1.50, 0.35, 0.130, 0.170, 0.039, 0.390, 0.190, 0.255, 0.330 and 0.230 g/kg respectively.
Xylanase (Econase XP 25, AB Vista feed ingredient, UK).
Vitamin premix provided the following per kilogram diet: vitamin A, 12 MIU; vitamin D, 5 MIU; vitamin E, 75 mg; vitamin K, 3 mg; nicotinic acid, 55 mg; pantothenic acid, 13 mg; folic acid, 2 mg; riboflavin, 8 mg; cyanocobalamin, 0.016 mg; biotin, 0.25 mg; pyridoxine, 5 mg; thiamine, 3 mg; antioxidant, 50 mg.
Mineral premix provided the following per kilogram diets: Cu, 16 mg as copper sulphate; Mn, 60 mg as manganese sulphate; Mn, 60 mg as manganous oxide; I, 0.125 mg as potassium iodide; Se, 0.3 mg; Fe, 40 mg, as iron sulphate; Zn, 50 mg as zinc oxide; Zn, 50 mg as zinc sulphate.
2.3. Challenge
The NE challenge was performed in accordance with reported procedures (Stanley et al., 2014, Rodgers et al., 2015). Half of the birds (384) were challenged with 5,000 oocysts of field strains of Eimeria acervulina and 5,000 oocysts of Eimeria maxima and 2,500 oocytes of Eimeria brunetti (Eimeria Pty Ltd) on d 9 and 108 CFU/mL of C. perfringens strain EHE-NE18 (known to express NetB toxin, Commonwealth Scientific and Industrial Research Organization, Geelong, Australia) on d 14 and 15 while the remaining were gavaged with sterile buffer or broth.
2.4. Meat and bone meal processing
About 10 kg each of commercial MBM (Northern Cooperative Meat Company Limited, Casino, NSW, Australia; lot number 48855) were either kept as received or autoclaved at a temperature of 128 °C at 2 bars for 90 min in an autoclave (Hirayama manufacturing corporation, Saitama, Japan). The resulting over-processed MBM was crushed and milled through 0.5-mm sieves. The analysis of nutrient composition and pepsin digestibility of both as-received and over-processed MBM were carried out in triplicates and the averages are shown in Table 2. Pepsin digestibility was measured using 0.2% pepsin using the method 971.09 (AOAC, 2006). Amino acids were measured using method 982.30 (AOAC, 2006) and the Carpenter method (Carpenter, 1960) was used to measure available lysine.
Table 2.
Percentage DM composition and pepsin digestibility of meat and bone meal (MBM).
| Item | MBM |
|
|---|---|---|
| AR | OP | |
| Crude protein | 49.9 | 48.9 |
| Ether extract | 8.53 | 8.08 |
| Ash | 33.8 | 33.3 |
| Pepsin digestibility | 86.8 | 85.0 |
| Hydroxyproline | 3.58 | 3.77 |
| Aspartic acid | 3.54 | 3.56 |
| Threonine | 1.51 | 1.52 |
| Serine | 1.83 | 1.93 |
| Glutamic acid | 5.83 | 5.99 |
| Proline | 4.51 | 4.56 |
| Glycine | 7.13 | 7.28 |
| Alanine | 3.92 | 3.99 |
| Cysteine | 0.43 | 0.36 |
| Valine | 1.99 | 2.00 |
| Methionine | 0.57 | 0.57 |
| Isoleucine | 1.33 | 1.33 |
| Leucine | 2.81 | 2.83 |
| Tyrosine | 1.02 | 1.03 |
| Phenylalanine | 1.70 | 1.64 |
| Hydroxylysine | 0.38 | 0.38 |
| Lysine | 2.57 | 2.49 |
| Histidine | 0.78 | 0.76 |
| Arginine | 3.79 | 3.80 |
| Tryptophan | 0.23 | 0.23 |
| Available lysine1 | 2.16 | 2.09 |
AR = as-received; OP = over-processed.
Analyzed using the Carpenter method (Carpenter, 1960).
2.5. Data collection
2.5.1. Performance
The birds and feed were weighed on a pen basis weekly. Feed intake (FI), weight gain (WG) and feed conversion ratio (FCR, feed:gain ratio) were calculated weekly. The pens were monitored for mortality twice daily and post-mortem examinations were conducted on dead birds throughout the study period. The FCR was calculated as the total feed consumed per pen for each period divided by total pen gain (live and dead). This gives the FCR of surviving live birds. Feed intake was calculated as FCR × WG. Livability was calculated weekly as the number of live birds/the number of starting birds × 100.
2.5.2. Gastrointestinal pH
Immediately post euthanasia (d 16 and 29), the crop, gizzard, ileum, and caeca were removed intact from 2 birds per pen. A digital pH meter (Mettler-Toledo, UK) with a spear tip piercing pH electrode (Sensorex, California, USA) was directly inserted into the digesta in the lumen of the proximal gizzard, ileum and caeca of the same bird while ensuring the pH electrode did not touch the walls. The pH was measured and recorded in duplicate. The probe was rinsed with ultra-pure water (ICW 3000 water purifier for ion chromatography, Millipore) between each measurement. The mean of the 2 readings per site of the tract was calculated and recorded. The digesta of the ileum and caeca from the 2 birds per pen used for measuring the pH and an additional bird from each pen totaling 3, were sampled to determine the digestibility of nutrients and bacterial quantification, respectively.
2.5.3. Intestinal lesions
The entire length of the small intestine (duodenum, jejunum, and ileum) of the sampled birds were scored for lesions as described by Keyburn et al. (2006) as follows: 0, no lesions; 1, thin-walled and friable intestines; 2, focal necrosis or ulceration (1 to 5 foci); 3, focal necrosis (6 to15 foci); 4, focal necrosis (16 or more foci); 5, patches of necrosis 2 to 3 cm long; and 6, diffuse necrosis typical of acute field cases. Two trained personnel were involved in the scoring. The average of the scores was computed and the pen was the experimental unit for lesion scoring.
2.5.4. Chemical analyses
The diets (in duplicate) and pooled ileal digesta samples (from 3 birds on d 16 and 29) were analyzed for nitrogen, carbon (C), Ca and P contents. Data on chemical analyses are presented in Table 2. The nitrogen and C content of the diets and digesta samples were determined on a 0.25-g sample with a combustion analyzer (Leco model FP- 2000 N Analyzer, Leco Corp., St. Joseph, MI) using ethylene diamine tetraacetic acid (EDTA) as a calibration standard, with CP calculated by multiplying percentage nitrogen by a correction factor (6.25). Mineral contents were determined using an inductively coupled plasma–optical emission spectroscopy following digestion in concentrated HNO3.
2.5.5. Titanium dioxide analysis
The spectrophotometric method described by Short et al. (1996) was followed to measure TiO2 concentration in the diet and ileal digesta samples. The TiO2 concentrations were determined in triplicate and duplicate for diets and digesta samples, respectively. Approximately 0.1 g of the freeze-dried digesta and 0.2 g of diet samples were weighed in porcelain crucibles and ashed at 580 °C for 13 h. Upon cooling, 5 mL of H2SO4 (7.4 mol/L) was added to the samples, and then the samples were boiled on a hotplate at 200 °C for 30 min and another 30 min at 250 °C to dissolve completely. The solutions were cooled at room temperature, and 5 mL of Milli-Q H2O was added before filtering (Whatman 541, hardened, ashless, 90 mm, Whatman International Ltd Maidstone, UK) into 50-mL volumetric flasks. Then 10-mL H2O2 (30% vol/vol) was added to each flask and the mixture adjusted to 50 mL with Milli-Q H2O and mixed thoroughly. The absorbance of the solutions and of prepared standards were determined at 410 nm using a Hitachi 150-20 UV spectrophotometer (Hitachi Science Systems Ltd., Ibaraki, Japan). The TiO2 content was calculated from a standard curve.
2.5.6. Digestibility calculation
The apparent ileal digestibility (d 16 and 29) of CP, C and minerals (Ca, P, and Mg) were calculated using the indigestible marker using the following formula:
Apparent ileal digestibility (%) = {1 − [TiO2diet (%)/TiO2digesta (%)] × [Digesta nutrient (%)/Diet nutrient (%)} × 100 .
2.5.7. Extraction of caecal bacterial DNA
The DNA of pooled caecal content collected on d 16 was extracted using PowerFecal QIAcube HT Kit, (Qiagen, Inc., Doncaster, VIC, Australia), with slight modification. In brief, approximately 100 mg of frozen caecal contents were weighed in a 2-mL Eppendorf tube containing 300 mg of glass beads (0.1 mm). Then 500-μL pre-warmed PW1 was pipetted to samples prior to disrupting the cells by Tissuelyser II for 5 min at frequency 30 times/s. The samples were then incubated at 90 °C for 15 min prior to centrifugation at 20,000 × g for 1 min. An aliquot of 400-μL supernatant was mixed with 150 μL of Buffer C3. The mixture was incubated for 5 min at 4 °C prior to centrifuge at 20,000 × g for 1 min. The supernatant was transferred into a loading block (S-block) containing 20-μL Proteinase K and incubated for 10 min at room temperature and the extraction followed the manufacture's instruction using QIACube HT instrument. The extracted caecal DNA was diluted 20 times with nuclease-free water and stored at −20 °C until required.
2.5.8. Quantification of caecal bacteria
The quantitative real-time polymerase chain reaction (PCR) of Bifidobacterium spp., Lactobacillus spp., Bacillus spp., Ruminococcus spp., Bacteroides spp., total anaerobic bacteria, and C. perfringens was achieved by using quantitative PCR (qPCR) assay. The Rotorgene 6000 real-time PCR machine (Corbett, Sydney, Australia) was employed for qPCR assay of the desired bacteria from the extracted caecal DNA. The PCR was performed in duplicate for each sample in 10 μL of reaction where PCR was repeated when the difference between the threshold cycle (CT) values of the duplicates was >0.5. For PCR, a SYBR Green-containing Mix (SensiMix SYBR No-Rox, Bioline, Sydney, Australia) was applied. The reaction in a volume of 10 μL contained 5 μL of 2 × SensiMix, 300 mmol/L of each primer, and 2 μL of diluted DNA template. Table 3 shows the specific 16 S rRNA primers used for the quantification of different groups of bacteria. The PCR was performed in a Rotorgene 6500 real-time PCR machine and a threshold cycle average from the duplicate samples was used for data analysis. Serial dilutions of linearized plasmid DNA (pCR 4-TOPO Vector, Life Technologies, Carlsbad, CA) inserted with respective bacterial amplicons were used to construct a standard curve. The concentrations of the plasmid DNA were measured using NanoDrop ND-8000 (Thermo Fisher Scientific, Waltham, MA) prior to the serial dilutions. The number of target DNA copies was calculated from the mass of DNA taking into account the size of the amplicon insert in the plasmid. Bacteria numbers were expressed as log10 (genomic DNA copy number)/g digesta.
Table 3.
The sequence of primers used for the quantitative PCR analysis of selected microbial populations in caecal digesta samples, d 16.
| Target group or organism | Primer sequence (5ʹ-3ʹ) | Annealing temperature, °C | Reference |
|---|---|---|---|
| Bacillus spp. | F-GCA ACG AGC GCA ACC CTTGA | 63 | Zhang et al. (2015) |
| R-TCA TCC CCA CCT TCC TCC GGT | |||
| Bacteroides spp. | F-GAG AGG AAG GTC CCC CAC | 63 | Layton et al. (2006) |
| R-CGC TAC TTG GCT GGT TCA G | |||
| Bifidobacterium spp. | F-GCG TCC GCT GTG GGC | 63 | Requena et al. (2002) |
| R-CTT CTC CGG CAT GGT GTT G | |||
| Lactobacillus spp. | F-CAC CGC TAC ACA TGG AG R-AGC AGT AGG GAA TCT TCC A |
63 | Wise and Siragusa (2007) |
| Ruminococcus spp. | F-GGC GGC YTR CTG GGC TTT R- CCA GGT GGA TWA CTT ATT GTG TTA A |
63 | Ramirez-Farias et al. (2008) |
| Total bacteria | F- CGG YCC AGA CTC CTA CGG G R- TTA CCG CGG CTG CTG GCA C |
63 | Lee et al. (1996) |
| Clostridium perfringens | F- ATG CAA GTC GAG CGA KG R- TAT GCG GTA TTA ATC TYC CTT T Probe-FAM-TCA TCA TTC AAC CAA AGG AGC AAT CC-TAMRA |
58 | Rinttila et al. (2004) |
2.6. Statistical analyses
The data were analyzed as a 2 × 2 × 2 factorial arrangement of treatments using the Minitab 19 statistical software to assess the main effects and 2- or 3-way interactions, with the factors NE challenge (no or yes), MBM (as-received or over-processed) and Phytase (500 or 5,000 FTU/kg). Tukey's mean separation test was used to make pairwise comparisons between treatment means (P < 0.05). The Box-Cos transformation of the Minitab 19 statistical software was used test for normality of data. Data deemed to be not-normally distributed (including percentages) and were tested for significance using this non-parametric procedure.
3. Results
3.1. Meat and bone meal processing
The analytical results for MBM processing are shown in Table 2. The results show that total lysine and available lysine were both reduced as a result of autoclaving for 90 min. The ratio of available lysine to lysine was unchanged. Cysteine was reduced as a result of autoclaving. Pepsin digestibility in 0.2% pepsin was lowered from 86.8 to 85.0 as a result of autoclaving. The other essential AA expressed on a DM bases were largely unchanged.
3.2. Performance
The results on growth performance are shown in Table 4, Table 5, Table 6. No treatment or main effect was detected for any production indices at d 7 (pre-NE challenge; data not shown). Challenge (with Eimeria but not C. perfringens) × MBM interactions were detected on d 14 (Table 4) indicating over-processing of MBM reduced FI (P < 0.05), WG (P < 0.05) and increased FCR (P > 0.05) only in challenged birds. Challenge × phytase interactions were detected for WG (P < 0.05) and FI (P < 0.05) on d 21 (Table 5), indicating the super dose of phytase increased WG and FI in unchallenged birds but decreased in challenged birds. Additionally, challenge × MBM interactions were detected for WG (P < 0.05) and FCR (P < 0.05) on d 21, where only in the NE challenged birds did over-processed MBM reduce WG and increase FCR relative to counterparts fed as-received MBM. The challenge decreased livability at d 21 (P < 0.05). A challenge × MBM interaction was detected for WG (P < 0.05) and FI (P < 0.05) on d 28 (Table 5). In the challenged birds, over-processed MBM decreased WG (P < 0.05) and FI (P < 0.05) compared to birds fed as-received MBM on d 28. The challenge increased FCR (P < 0.05) on d 28. The over-processed MBM increased FCR (P < 0.05) at d 28. The challenge as a main effect decreased WG (P < 0.05), increased FCR (P < 0.05) and decreased FI (P < 0.05) at d 35 (not shown). Similarly, feeding over-processed MBM decreased WG (P < 0.05), increased FCR (P < 0.05) and decreased FI (P < 0.05) at d 35. Birds fed the low level of phytase tended (P = 0.053) to have increased livability and over-processed MBM tended (P = 0.089) to increase livability at d 35. The challenge decreased WG (P < 0.05), increased FCR (P < 0.05) and decreased FI (P < 0.05) at d 42 (Table 6). Livability was increased in birds fed the low level of phytase (P < 0.05) and over-processed MBM (P < 0.05) on d 42.
Table 4.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on the performance of broilers, d 14.
| Item | Factors |
WG, g | FCR | FI, g | Livability, % | ||
|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | |||||
| Two-way interaction | |||||||
| NE × MBM1 | – | AR | 453a | 1.154b | 480ab | 97 | |
| – | OP | 460a | 1.146b | 484a | 99 | ||
| + | AR | 400b | 1.268a | 459b | 97 | ||
| + | OP | 374c | 1.299a | 435c | 95 | ||
| P-value | |||||||
| NE | 0.001 | 0.001 | 0.001 | 0.182 | |||
| Phy | 0.787 | 0.450 | 0.932 | 0.182 | |||
| MBM | 0.135 | 0.247 | 0.132 | 0.847 | |||
| NE × Phy | 0.760 | 0.445 | 0.423 | 0.182 | |||
| NE × MBM | 0.011 | 0.048 | 0.028 | 0.089 | |||
| Phy × MBM | 0.678 | 0.154 | 0.845 | 0.338 | |||
| NE × Phy × MBM | 0.646 | 0.083 | 0.684 | 0.338 | |||
WG = weight gain; FCR = feed conversion ratio; FI = feed intake; AR = as-received; OP = over-processed.
a, b, c In the same column within 2-way interaction, means with different superscripts are different (P < 0.05).
Two-way interaction was separated by Tukey's mean separation test.
Table 5.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on the performance of broilers.
| Item | Factors |
Day 21 |
Day 28 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | WG, g | FCR | FI, g | Livability, % | WG, g | FCR | FI, g | Livability, % | |
| Two-way interactions1 | |||||||||||
| NE × Phy | – | 500 | 904a | 1.273 | 1,102a | 98 | 1,551 | 1.325 | 2,004 | 97 | |
| – | 5,000 | 924a | 1.267 | 1,122a | 98 | 1,548 | 1.333 | 2,013 | 96 | ||
| + | 500 | 680b | 1.476 | 945b | 95 | 1,210 | 1.512 | 1,744 | 95 | ||
| + | 5,000 | 656b | 1.466 | 904b | 93 | 1,253 | 1.409 | 1,700 | 92 | ||
| NE × MBM | – | AR | 925a | 1.256c | 1,114 | 97 | 1,557a | 1.323 | 2,009a | 95 | |
| – | OP | 903a | 1.285c | 1,111 | 99 | 1,542a | 1.335 | 2,009a | 97 | ||
| + | AR | 700b | 1.431b | 948 | 95 | 1,318b | 1.399 | 1,776b | 93 | ||
| + | OP | 635c | 1.511a | 901 | 93 | 1,145c | 1.523 | 1,668c | 93 | ||
| Main effect | |||||||||||
| NE | – | 913 | 1.270 | 1,112 | 98a | 1,549 | 1.329b | 2,009 | 96 | ||
| + | 668 | 1.471 | 924 | 94b | 1,231 | 1.461a | 1,721 | 93 | |||
| MBM | AR | 813 | 1343 | 1031 | 96 | 1,437 | 1.361b | 1,892 | 94 | ||
| OP | 769 | 1.398 | 1006 | 96 | 1,344 | 1.429a | 1,838 | 95 | |||
| P-value | |||||||||||
| NE | 0.001 | 0.001 | 0.001 | 0.015 | 0.001 | 0.001 | 0.001 | 0.078 | |||
| Phy | 0.839 | 0.357 | 0.445 | 0.613 | 0.510 | 0.122 | 0.405 | 0.235 | |||
| MBM | 0.001 | 0.001 | 0.082 | 0.866 | 0.003 | 0.030 | 0.015 | 0.550 | |||
| NE × Phy | 0.048 | 0.827 | 0.034 | 0.400 | 0.450 | 0.075 | 0.212 | 0.550 | |||
| NE × MBM | 0.050 | 0.007 | 0.119 | 0.134 | 0.012 | 0.073 | 0.015 | 0.550 | |||
| Phy × MBM | 0.992 | 0.138 | 0.530 | 0.400 | 0.518 | 0.196 | 0.649 | 0.550 | |||
| NE × Phy × MBM | 0.534 | 0.239 | 0.911 | 0.241 | 0.815 | 0.634 | 0.739 | 0.550 | |||
WG = weight gain; FCR = feed conversion ratio; FI = feed intake; AR = as-received; OP = over-processed.
a, b, c In the same column within the main effect or 2-way interaction, means with different superscripts are different (P < 0.05).
Two-way interaction was separated by Tukey's mean separation test.
Table 6.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on the performance of broilers, d 42.
| Item | Factors |
WG, g | FCR | FI, g | Livability, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | |||||||
| Main effects | |||||||||
| NE | – | 3,134a | 1.437b | 4,446a | 91 | ||||
| + | 2,779b | 1.474a | 4,041b | 90 | |||||
| Phy | 500 | 2,960 | 1.461 | 4,263 | 93a | ||||
| 5,000 | 2,953 | 1.450 | 4,224 | 88b | |||||
| MBM | AR | 2,998 | 1.453 | 4,295 | 88b | ||||
| OP | 2,916 | 1.458 | 4,192 | 93a | |||||
| P-value | |||||||||
| NE | 0.001 | 0.004 | 0.001 | 0.490 | |||||
| Phy | 0.885 | 0.402 | 0.569 | 0.025 | |||||
| MBM | 0.095 | 0.653 | 0.139 | 0.043 | |||||
| NE × Phy | 0.702 | 0.930 | 0.672 | 1.000 | |||||
| NE × MBM | 0.211 | 0.749 | 0.131 | 0.817 | |||||
| Phy × MBM | 0.543 | 0.182 | 0.196 | 1.000 | |||||
| NE × Phy × MBM | 0.734 | 0.678 | 0.532 | 0.644 | |||||
WG = weight gain; FCR = feed conversion ratio; FI = feed intake; AR = as-received; OP = over-processed.
a, b In the same column within the main effect, means with different superscripts are different (P < 0.05).
3.3. Intestinal lesions, d 16
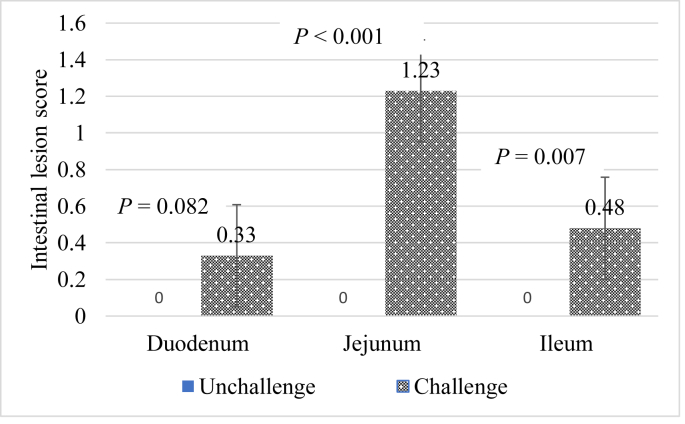
The challenge increased the severity of NE lesions in the jejunum (P < 0.05) and ileum (P < 0.05) as shown in Fig. 1. The challenge tended to increase lesions in the duodenum (P = 0.082).
Fig. 1.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on lesion score, d 16.
3.4. Intestinal pH, d 16
A phytase × MBM interaction was detected for pH in the caeca where, in birds fed high phytase (Table 7), over-processed MBM reduced the pH (P < 0.05). Challenged birds had lower pH in the crop (P < 0.05), jejunum (P < 0.05), ileum (P < 0.05) and caeca (P < 0.05), but higher pH in the gizzard (P < 0.05). The over-processed MBM decreased the pH in the jejunum (P < 0.05) and ileum (P < 0.05).
Table 7.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on intestinal pH response of broilers, d 16.
| Item | Factors |
Crop | Gizzard | Duodenum | Jejunum | Ileum | Caeca | ||
|---|---|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | |||||||
| Two-way interaction1 | |||||||||
| Phy × MBM | 500 | AR | 5.46 | 3.05 | 5.90 | 5.83 | 5.77 | 5.93b | |
| 500 | OP | 5.39 | 2.72 | 5.86 | 5.67 | 5.56 | 6.14ab | ||
| 5,000 | AR | 5.47 | 2.64 | 5.86 | 5.87 | 5.91 | 6.43a | ||
| 5,000 | OP | 5.23 | 2.84 | 6.02 | 5.69 | 5.66 | 6.03b | ||
| Main effects | |||||||||
| NE | – | 5.60a | 2.51b | 5.91 | 5.87a | 5.88a | 6.26a | ||
| + | 5.18b | 3.11a | 5.91 | 5.65b | 5.56b | 6.01b | |||
| MBM | AR | 5.46 | 2.84 | 5.88 | 5.85a | 5.84a | 6.18 | ||
| OP | 5.31 | 2.78 | 5.94 | 5.68b | 5.61b | 6.09 | |||
| P-value | |||||||||
| NE | 0.001 | 0.001 | 0.840 | 0.002 | 0.001 | 0.018 | |||
| Phy | 0.466 | 0.305 | 0.331 | 0.643 | 0.177 | 0.061 | |||
| MBM | 0.137 | 0.656 | 0.360 | 0.018 | 0.010 | 0.356 | |||
| NE × Phy | 0.533 | 0.200 | 0.814 | 0.073 | 0.785 | 0.168 | |||
| NE × MBM | 0.159 | 0.859 | 0.487 | 0.098 | 0.056 | 0.269 | |||
| Phy × MBM | 0.384 | 0.074 | 0.114 | 0.893 | 0.860 | 0.005 | |||
| NE × Phy × MBM | 0.469 | 0.838 | 0.876 | 0.272 | 0.383 | 0.592 | |||
AR = as-received; OP = over-processed.
a, b In the same column within the main effect or 2-way interaction, means with different superscripts are different (P < 0.05).
Two-way interaction was separated by Tukey's mean separation test.
3.5. Intestinal pH, d 29
The challenge decreased pH in the crop (P < 0.05), jejunum (P < 0.05) and ileum (P < 0.05) (Table 8).
Table 8.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on intestinal pH response of broilers, d 29.
| Item | Factors |
Crop | Gizzard | Duodenum | Jejunum | Ileum | Caeca | ||
|---|---|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | |||||||
| Main effects | |||||||||
| NE | – | 5.07a | 2.60 | 5.70 | 5.81a | 5.88a | 5.89 | ||
| + | 4.46b | 2.66 | 5.69 | 5.67b | 5.33b | 5.89 | |||
| P-value | |||||||||
| NE | 0.001 | 0.563 | 0.932 | 0.042 | 0.001 | 0.974 | |||
| Phy | 0.327 | 0.184 | 0.874 | 0.176 | 0.857 | 0.103 | |||
| MBM | 0.949 | 0.683 | 0.454 | 0.458 | 0.930 | 0.309 | |||
| NE × Phy | 0.338 | 0.137 | 0.755 | 0.537 | 0.312 | 0.236 | |||
| NE × MBM | 0.995 | 0.133 | 0.122 | 0.957 | 0.434 | 0.506 | |||
| Phy × MBM | 0.194 | 0.686 | 0.689 | 0.769 | 0.787 | 0.847 | |||
| NE × Phy × MBM | 0.227 | 0.363 | 0.770 | 0.383 | 0.420 | 0.192 | |||
a, b In the same column within the main effect, means with different superscripts are different (P < 0.05).
3.6. Apparent ileal digestibility, d 16
The challenge decreased AID of C (P < 0.05) but it increased the AID of Ca (P < 0.05) and P (P < 0.05) (Table 9). High phytase as a main effect increased AID of P (P < 0.05). Birds fed over-processed MBM had lower AID of CP (P < 0.05) and C (P < 0.05) but higher AID of Ca (P < 0.05).
Table 9.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on apparent ileal digestibility coefficients of CP, C, Ca and P of broilers, d 16 and 29.
| Item | Factors |
CP |
C |
Ca |
P |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | Day 16 | Day 29 | Day 16 | Day 29 | Day 16 | Day 29 | Day 16 | Day 29 | |
| Treatments1 | |||||||||||
| 1 | – | 500 | AR | 0.76 | 0.74bc | 0.63 | 0.46 | 0.47 | 0.25 | 0.62 | 0.39 |
| 2 | – | 5,000 | AR | 0.80 | 0.80a | 0.66 | 0.62 | 0.43 | 0.32 | 0.71 | 0.56 |
| 3 | – | 500 | OP | 0.74 | 0.76b | 0.61 | 0.62 | 0.40 | 0.21 | 0.55 | 0.37 |
| 4 | – | 5,000 | OP | 0.72 | 0.77ab | 0.57 | 0.60 | 0.51 | 0.31 | 0.73 | 0.54 |
| 5 | + | 500 | AR | 0.75 | 0.72c | 0.46 | 0.56 | 0.52 | 0.30 | 0.67 | 0.47 |
| 6 | + | 5,000 | AR | 0.76 | 0.74bc | 0.50 | 0.59 | 0.59 | 0.44 | 0.76 | 0.66 |
| 7 | + | 500 | OP | 0.72 | 0.71c | 0.41 | 0.58 | 0.66 | 0.32 | 0.71 | 0.47 |
| 8 | + | 5,000 | OP | 0.72 | 0.74bc | 0.44 | 0.62 | 0.67 | 0.43 | 0.76 | 0.65 |
| Main effects | |||||||||||
| NE | – | 0.76 | 0.77 | 0.61a | 0.58 | 0.45b | 0.27b | 0.65b | 0.46b | ||
| + | 0.74 | 0.73 | 0.45b | 0.59 | 0.61a | 0.37a | 0.73a | 0.56a | |||
| Phy | 500 | 0.74 | 0.73 | 0.52 | 0.55b | 0.51 | 0.27b | 0.64b | 0.43b | ||
| 5,000 | 0.75 | 0.76 | 0.54 | 0.61a | 0.55 | 0.37a | 0.74a | 0.60a | |||
| MBM | AR | 0.77a | 0.75 | 0.56a | 0.56 | 0.50b | 0.33 | 0.69 | 0.52 | ||
| OP | 0.73b | 0.75 | 0.50b | 0.60 | 0.56a | 0.32 | 0.69 | 0.51 | |||
| P-value | |||||||||||
| NE | 0.096 | 0.001 | 0.001 | 0.673 | 0.001 | 0.001 | 0.010 | 0.001 | |||
| Phy | 0.654 | 0.001 | 0.423 | 0.025 | 2.000 | 0.001 | 0.001 | 0.001 | |||
| MBM | 0.001 | 0.390 | 0.012 | 0.056 | 0.049 | 0.761 | 0.950 | 0.571 | |||
| NE × Phy | 0.852 | 0.835 | 0.358 | 0.502 | 0.916 | 0.438 | 0.256 | 0.741 | |||
| NE × MBM | 0.556 | 0.963 | 0.905 | 0.357 | 0.083 | 0.534 | 0.429 | 0.807 | |||
| Phy × MBM | 0.231 | 0.059 | 0.341 | 0.082 | 0.468 | 0.986 | 0.664 | 0.872 | |||
| NE × Phy × MBM | 0.262 | 0.005 | 0.437 | 0.053 | 0.080 | 0.583 | 0.215 | 0.933 | |||
CP = crude protein; C = carbon; Ca = calcium; P = phosphorus; AR = as-received; OP = over-processed.
a, b, c In the same column within the main effect or treatment, means with different superscripts are different (P < 0.05).
Three-way interaction was separated by Tukey's mean separation test.
3.7. Apparent ileal digestibility, d 29
A 3-way challenge × phytase × MBM was detected for AID of CP (P < 0.05) indicating that the AID was highest in the unchallenged birds fed high phytase and AR MBM compared with their counterparts fed the same diet in the challenged group (Table 9). A higher AID of CP (P < 0.05) was observed in the unchallenged birds fed low phytase and over-processed MBM compared to those in the challenged group fed the same diet. High phytase as a main effect increased C (P < 0.05), Ca (P < 0.05) and P (P < 0.05). The over-processed MBM as a main effect tended to increase the AID of C (P = 0.056).
3.8. Caecal bacterial count, d 16
The challenge increased the counts of Lactobacillus spp. (P < 0.05), Bacteroides spp. (P < 0.05) and Bifidobacteria spp. (P < 0.05) and C. perfringens (P < 0.05) with no interactions (Table 10). High phytase tended to increase the count of Bifidobacteria spp. (P = 0.065).
Table 10.
Effect of necrotic enteritis (NE), phytase (Phy) and meat and bone meal (MBM) on bacterial quantification (log10 [genomic DNA copies/g of caecal contents]) from broilers, d 16.
| Item | Factors |
Lactobacillus spp. | Ruminicoccus spp. | Bacteroides spp. | Bacillus spp. | Bifidobacteria spp. | Total bacteria | Clostridium perfringens | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NE | Phy | MBM | ||||||||
| Main effects | ||||||||||
| NE | – | 9.35b | 8.86 | 7.52b | 8.15 | 9.51b | 10.47 | 0.00b | ||
| + | 9.72a | 8.67 | 7.84a | 8.32 | 9.76a | 10.60 | 8.92a | |||
| Phy | 500 | 9.54 | 8.74 | 7.64 | 8.18 | 9.54 | 10.53 | 4.34 | ||
| 5,000 | 9.53 | 8.80 | 7.72 | 8.29 | 9.72 | 10.55 | 4.58 | |||
| P-value | ||||||||||
| NE | 0.002 | 0.189 | 0.001 | 0.133 | 0.015 | 0.366 | 0.001 | |||
| Phy | 0.932 | 0.675 | 0.375 | 0.357 | 0.065 | 0.880 | 0.241 | |||
| MBM | 0.155 | 0.383 | 0.783 | 0.408 | 0.603 | 0.913 | 0.769 | |||
| NE × Phy | 0.723 | 0.954 | 0.905 | 0.860 | 0.608 | 0.685 | 0.241 | |||
| NE × MBM | 0.862 | 0.566 | 0.786 | 0.314 | 0.637 | 0.664 | 0.769 | |||
| Phy × MBM | 0.311 | 0.549 | 0.597 | 0.132 | 0.926 | 0.286 | 0.920 | |||
| NE × Phy ×MBM | 0.890 | 0.731 | 0.543 | 0.969 | 0.457 | 0.584 | 0.920 | |||
a, b In the same column within the main effect, means with different superscripts are different (P < 0.05).
4. Discussion
It was established that MBM that predisposes chickens to NE reduces the performance of broilers while the presence of a high phytase dose and antibiotics in diets containing MBM improves performance (Zanu et al., 2020). The present study was designed to test the hypothesis that over-processed MBM by heating under pressure (autoclaving) would decrease the digestibility of the protein and further exacerbate the incidence of NE. A high level of phytase was examined to potentially compensate for nutrient damage likely to result from over-processed MBM.
The concentration and digestibility of AA in feed ingredients are reduced by exposure to excessive heat. Autoclave processing of MBM for 90 min reduced the levels of heat-labile AA cysteine, lysine and available lysine however the ratio of available to total lysine was unchanged. This indicates complete irreversible destruction of a portion of the lysine. Non-essential AA including hydroxyproline, serine, glutamic acid and glycine were increased as a result of autoclaving. The depressed WG, FCR, and FI in challenged birds due to over-processed MBM agree with the hypothesis of this study. The mechanism behind the poor performance only in challenged birds fed over-processed MBM is herein discussed. Foremost, heat processing under pressure caused nutritional damage to MBM and reduced its lysine availability and quality due to conformational changes (Hendriks et al., 1999, Buckley et al., 2012). Lysine has a free amino group; therefore, it is often damaged following exposure to heat (Moughan and Rutherfurd, 1996, Mavromichalis and Baker, 2000, Brestenský et al., 2014). In addition, Shirley and Parsons (2000) determined the effect of pressure processing on AA digestibility of MBM and found that the true digestibility of most AA, especially cysteine and lysine significantly decreased with increasing pressure. The reduction in AA due to pressure heating might have been as a result to the racemization of AA or cross-linking between AA. Further, over-processing might have resulted in the isomerization of l-AA into d-AA and mesoisomers which are poorly digested and unavailable for absorption (Finot, 2005). Cross-linking of peptide bonds between a carbonyl group and amino group (Mallard reaction) during such severe heating processing is another possibility (Heck et al., 2013, Bellagamba et al., 2015).
The implications of poor AA digestibility in over-processed MBM on gut microbiota are clear, in that, bacteria in the gut depend on nutrients from dietary sources which have escaped digestion and absorption in the upper gut or on endogenous secretions (Apajalahti and Vienola, 2016). For instance, the metabolism of bypassed proteins produces total volatile nitrogen consisting of trimethylamine (TMA) and ammonia (NH3) (He et al., 2015) due to nitrogenous putrefactive fermentation. Many caecal bacteria such as Bacteroides, Bifidobacteria, and Clostridia possess putrefactive activity. Ammonia increases the pH of the intestinal contents and favors the growth of C. perfringens (Allison and Macfarlane, 1989, Paiva and McElroy, 2014, Qaisrani et al., 2015b). C. perfringens is reported to be mainly responsible for the build-up of NH3 and other odorous metabolites in digesta of NE-challenged chickens (Sharma et al., 2017). The cascading effects of feeding over-processed MBM might explain the poor performance in the current study.
At d 42, high dietary phytase decreased livability. Though reports on the impact of high phytase on livability are rare, the mild decrease in WG and FI in birds fed 5,000 FTU/kg in the challenge group may indicate phytase promoting NE, perhaps through the release of Ca into the hindgut to fuel C. perfringens activity or a higher AA release due to the higher phytase activity reaching a maximum absorption and then making these AA more available to the bacteria in the hindgut.
Poor growth and impaired FCR in challenged birds were observed in the current study and this finding corroborates with those of other challenge studies (Perez et al., 2011, Rochell et al., 2016a, Rochell et al., 2016b, Wang et al., 2018, Leung et al., 2019). Eimeria infection as part of the challenge model in this study has been reported to damage intestinal epithelial cells (Amerah and Ravindran, 2015, Kraieski et al., 2016). A compromised epithelial lining leads to plasma proteins leaking into the intestinal lumen and serve as substrates for C. perfringens (Collier et al., 2008, Amerah and Ravindran, 2015). Further, inflammation due to either Eimeria or C. perfringens potentiates T-cell-mediated inflammatory response that results in mucin production providing nutrients for C. perfringens (Kleessen et al., 2003, Kim et al., 2013). In addition, malabsorption of nutrients due to damaged intestinal lining might have added to the substrates for C. perfringens to proliferate hence the growth depression observed in the challenge birds (Guo et al., 2013, Amerah and Ravindran, 2015, Rochell et al., 2016c).
The effect of over-processed MBM in reducing WG, FCR, and FI on d 35 and 42 is in line with a report of a recent unchallenged study by Bryan et al. (2018). In that study, the indigestibility of protein decreased WG and FCR of broilers at d 32. Previous reports have also suggested a positive correlation between protein digestibility and broiler performance (Cowieson and Bedford, 2009, Cowieson and Roos, 2013), in that, when the diet is deficient in essential AA due to poor protein digestibility WG is reduced.
Gross examination of the duodenum, jejunum, and ileum revealed lesions of NE. The lesions were more severe in the jejunum. The duodenal lesion recorded in the present study was the lowest. It is probable that more severe lesions in the jejunum might be because this region has a median pH of 6.2 (Ravindran, 2013) and allowed C. perfringens to proliferate more freely. But the jejunal pH recorded in the study due to challenge was lower. Alternatively, the toxins of C. perfringens might have been inactivated by proteolytic enzymes (trypsinogen and chymotrypsinogen) from the pancreatic duct entering the duodenum (Talukdar et al., 2016). Before getting to the jejunum, these enzymes might have been inactivated thus leaving the toxins free to exert their debilitating effect in the jejunum. The jejunum has been reported to be the main site for the absorption of digested products like fat, starch, and protein with the ileum being the site for water and mineral absorption, although some uptake of fat, protein and starch takes place in the ileum (Svihus, 2014). Therefore, a dysfunctional small intestine would impair the digestibility and absorption of these nutrients.
The pH of the gut is important for nutrient bioavailability and intestinal microbiota. In this study, birds fed over-processed MBM and challenged with NE recorded the lowest pH in the jejunum and ileum at d 16 post-challenge. This observation is contrary to what was detected in the preceding trial in the same facility in which normal MBM (5% inclusion) increased the pH in the ileum and the caeca in challenged birds (Zanu et al., 2020). It suggests that MBM as a Ca source has the potential to increase gut pH (Angel et al., 2002) and that over-processing of the MBM was most likely to have reduced the CaCO3 content through the process of calcination (Barros et al., 2009, Yang et al., 2005, Silva et al., 2019). Again, it is most probable that overheating might have decreased the Ca solubility of over-processed MBM agreeing with report by Kim et al. (2018) that highly soluble Ca sources increase gut pH.
The higher caecal pH in birds fed high phytase and as-received MBM might also suggest that both phytase and as-received MBM released Ca to a certain concentration that elevated the pH. A similar elevation of pH in the ileum and caeca was observed in birds fed MBM (as-received) (5%) and high phytase (1,500 FTU/kg) (Zanu et al., 2020). Therefore, is it not surprising that a possible decrease in CaCO3 as explained above would have led to a reduction in pH.
The challenge decreased the pH in the crop, jejunum, ileum, and caeca except for the gizzard where it increased the pH. A low gut pH was also reported in a previous NE challenge study (M'Sadeq et al., 2015). It has been argued that C. perfringens will grow over a wide pH range, varying from 5.5 to 8.5, though optimum pH for growth 6 to 7 (Setlow and Johnson, 2013). It is important to note that the gut environment, composition, and function of the bacterial community may vary from study to study due to differences in age of the birds, dietary components and facilities used (Kers et al., 2018). Feed withdrawal, for instance, had been reported to cause a significant increase in the pH of the crop contents of the broilers after 12 h of feed withdrawal (Hinton et al., 2000). The reason for the increased crop pH in that study was attributed to a reduction in the population of lactic acid bacteria. Conversely, in this study, the low FI due to the challenge led to an increased pH in the gizzard. The low pH in the jejunum and ileum by over-processing might be due to possible degradation of CaCO3 and reduction in solubility (Seiquer et al., 2010, Paiva et al., 2013).
The reduction in nutrient digestibility in the current study as a result of the challenge agrees with previous reports (Timbermont et al., 2011, Amerah and Ravindran, 2015). However, the higher digestibility observed for the minerals (Ca and P) at d 16 and 29 in challenged chickens was rather unexpected. Nonetheless, 2 other studies that preceded the current study had made a similar observation especially for high Ca digestibility in challenged birds. In one of the studies, feeding MBM (5% inclusion rate) led to a higher Ca digestibility in challenged birds (Zanu et al., 2020). Few studies have reported the digestibility of minerals during NE. The only report that was sighted for Ca digestibility in a NE challenged trial in chickens (naturally induced) did not show any trend (Paiva et al., 2014).
The increase in Ca and P digestibility in birds fed high phytase was consistent with previous reports (Walk et al., 2012, Farhadi et al., 2017, Kim et al., 2018). About 61% of the total P found in soybean meal and 72.5% in canola meal are in the form of phytate (Hanna et al., 2017). Therefore, exogenous phytases are routinely added to chicken diets to hydrolyze phytate to liberate Ca and P for absorption, reduce the antinutritional effects of phytate and reduce the cost of inorganic phosphate addition (Liu et al., 2008, Manangi and Coon, 2008, Cowieson et al., 2008, dos Santos et al., 2014, Hamdi et al., 2015).
The lower CP digestibility in the current study on d 16 confirmed the hypothesis of this study, that is, overheating of MBM would damage the AA and reduce their digestibility as also as confirmed in the lower lysine level of over-processed MBM in the present study. The lower AID of CP observed in the challenged birds fed high phytase and as-received MBM compared to those unchallenged but fed similar diets could have been more likely due to the damage incurred on the absorptive surface such that absorption is compromised (and hence AID reduced) but also endogenous losses increased which will result in further reductions in AID of CP. A corresponding lower AID of C was also observed in birds fed over-processed MBM perhaps because of the lower AID of CP since CP forms a significant fraction of the organic component of the diets. The higher digestibility of Ca recorded at d 16 in birds fed over-processed MBM might be due to a decrease in overall solubility of Ca from heating in the autoclave. Paiva et al. (2013) had observed increased AID of Ca in birds fed lower solubility limestone compared to those fed highly soluble calcified seaweed (HSC) at a standard industry level of Ca (0.9%). They explained that because HSC was more soluble than limestone, there was more Ca in the solution that bound to P and precipitated.
The higher counts of C. perfringens in the caeca in the present study were expected as the birds were challenged with the same bacteria. This confirmed that the challenge was successful. However, an increase in the count of Bacteroides in the challenged birds appeared to confirm a positive correlation between C. perfringens and Bacteroides spp. counts in previous studies (Zanu et al., 2019b). Bacteroides are normal intestinal flora but can evolve into a pathogenic form, and the amounts could increase when the gut is pathologically changed or impaired (Phong et al., 2010). Rather, the increased Lactobacillus spp. and Bifidobacteria spp. counts due to the challenge was unexpected. This observation contradicts consensus that favorable acid condition in the caeca leads to an increased population of Lactobacillus spp. and Bifidobacteria spp. thereby outnumbering the population of C. perfringens (Olnood et al., 2015, Li et al., 2018). The rather low pH in the challenge group, suggesting a possible lack of putrifaction of protein from endogenous sources entering the caeca, might have favored the growth of Lactobacillus spp. and Bifidobacteria spp. Lactobacillus spp. and Bifidobacteria spp. are short-chain fatty acid-producing bacteria that secrete bacteriocins at an acidic pH to suppress the growth of pathogens in the gastrointestinal tract (La Ragione et al., 2004, Belenguer et al., 2007, Dec et al., 2014).
5. Conclusion
Concluding, this study demonstrated and confirmed the widely held theory that reduced protein digestibility as a result of over-processing could predispose chickens to NE and decrease production performance. Therefore, heat treatment of MBM in commercial rendering plants must be monitored so as not to prompt NE. Additionally, the use of the full matrix value of phytase is recommended when formulating diets when incidence of NE is expected so as not increase nutrients to promote the activity of C. perfringens and reduce growth performance.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The authors hereby acknowledge AB Vista Feed Ingredient, Malborough, UK for funding this research. We also do acknowledge Brett Ruth of Ruth Consolidated Industries Pty. Ltd for providing the phytase enzyme we used in this study. Also, the University of New England (UNE), Armidale (Australia) is acknowledged for providing the international postgraduate research award (IPRA) to the lead author.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Fontaine J., Zimmer U., Moughan P.J., Rutherfurd S.M. Effect of heat damage in an autoclave on the reactive lysine contents of soy products and corn distillers dried grains with solubles. Use of the results to check on lysine damage in common qualities of these ingredients. J Agric Food Chem. 2007;55:10737–10743. doi: 10.1021/jf071747c. [DOI] [PubMed] [Google Scholar]
- Allison C., Macfarlane G.T. Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl Environ Microbiol. 1989;55:2894–2898. doi: 10.1128/aem.55.11.2894-2898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida F.N., Htoo J.K., Thomson J., Stein H.H. Effects of heat treatment on the apparent and standardized ileal digestibility of amino acids in canola meal fed to growing pigs. Anim Feed Sci Technol. 2014;187:44–52. [Google Scholar]
- Amerah A.M., Ravindran V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poultry Sci. 2015;94:673–680. doi: 10.3382/ps/pev022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel R., Tamim N.M., Applegate T.J., Dhandu A.S., Ellestad L.E. Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J Appl Poultry Res. 2002;11:471–480. [Google Scholar]
- AOAC . 18th ed. Association of Official Analytical Chemists; Gaithersburgs, MD: 2006. Official Methods of analysis. [Google Scholar]
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim Feed Sci Technol. 2016;221:323–330. [Google Scholar]
- Aviagen Ross 308 broiler: performance objectives. 2014. http://en.aviagen.com/assets/Tech.Center/Ross.Broiler/Ross-308-BroilerPO-2014-EN.pdf Available from:
- Barekatain M.R., Antipatis C., Rodgers N., Walkden-Brown S.W., Iji P.A., Choct M. Evaluation of high dietary inclusion of distillers dried grains with solubles and supplementation of protease and xylanase in the diets of broiler chickens under necrotic enteritis challenge. Poultry Sci. 2013;92:1579–1594. doi: 10.3382/ps.2012-02786. [DOI] [PubMed] [Google Scholar]
- Barros M.C., Bello P.M., Bao M., Torrado J.J. From waste to commodity: transforming shells into high purity calcium carbonate. J Clean Prod. 2009;17:400–407. [Google Scholar]
- Belenguer A., Duncan S.H., Holtrop G., Anderson S.E., Lobley G.E., Flint H.J. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl Environ Microbiol. 2007;73:6526–6533. doi: 10.1128/AEM.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellagamba F., Caprino F., Mentasti T., Vasconi M., Moretti V.M. The impact of processing on amino acid racemization and protein quality in processed animal proteins of poultry origin. Ital J Anim Sci. 2015;14:3770. [Google Scholar]
- Branton S.L., Lott B.D., Deaton J.W., Maslin W.R., Austin F.W., Pote L.M., Keirs R.W., Latour M.A., Day E.J. The effect of added complex carbohydrates or added dietary fiber on necrotic enteritis lesions in broiler chickens. Poultry Sci. 1997;76:24–28. doi: 10.1093/ps/76.1.24. [DOI] [PubMed] [Google Scholar]
- Brestenský M., Nitrayová S., Heger J., Patráš P., Rafay J., Sirotkin A. Methods for determination reactive lysine in heat-treated foods and feeds. J Microbiol Biotechnol Food Sci. 2014;4:13–15. [Google Scholar]
- Bryan D.D.S.L., Abbott D.A., Classen H.L. Development of an in vitro protein digestibility assay mimicking the chicken digestive tract. Anim Nutr. 2018;4:401–409. doi: 10.1016/j.aninu.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M., Penkman K.E.H., Wess T.J., Reaney S., Collins M.J. Protein and mineral characterization of rendered meat and bone meal. Food Chem. 2012;134:1267–1278. doi: 10.1016/j.foodchem.2012.02.167. [DOI] [PubMed] [Google Scholar]
- Carpenter K.J. The estimation of the available lysine in animal protein foods. Biochem J. 1960;77:604–610. doi: 10.1042/bj0770604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Corrier D.E., Hinton A., Ziprin R.L., Beier R.C., DeLoach J.R. Effect of dietary lactose on caecal pH, bacteriostatic volatile fatty acids, and Salmonella typhimurium colonization of broiler chicks. Avian Dis. 1990;34:617–625. [PubMed] [Google Scholar]
- Cowieson A.J., Bedford M.R. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? World’s Poult Sci J. 2009;65:609–624. [Google Scholar]
- Cowieson A.J., Roos F.F. Bioefficacy of a mono-component protease in the diets of pigs and poultry: a meta-analysis of effect on ileal amino acid digestibility. J Appl Anim Nutr. 2013;2 [Google Scholar]
- Cowieson A.J., Ravindran V., Selle P.H. Influence of dietary phytic acid and source of microbial phytase on ileal endogenous amino acid flows in broiler chickens. Poultry Sci. 2008;87:2287–2299. doi: 10.3382/ps.2008-00096. [DOI] [PubMed] [Google Scholar]
- Dai C., Ma J., Li M., Wu W., Xia X., Zhang J. Diversity-oriented submonomer synthesis of azapeptides mediated by Mitsunobu reaction. Org Chem Front. 2019;6:2529–2533. doi: 10.1039/C9QO00296K. [DOI] [Google Scholar]
- Dec M., Puchalski A., Urban-Chmiel R., Wernicki A. Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poultry Sci. 2014;93:2464–2472. doi: 10.3382/ps.2014-04025. [DOI] [PubMed] [Google Scholar]
- dos Santos T.T., Walk C.L., Srinongkote S. Influence of phytate level on broiler performance and the efficacy of 2 microbial phytases from 0 to 21 days of age. J Appl Poultry Res. 2014;23:181–187. [Google Scholar]
- Farhadi D., Karimi A., Sadeghi G., Rostamzadeh J., Bedford M.R. Effects of a high dose of microbial phytase and myo-inositol supplementation on growth performance, tibia mineralization, nutrient digestibility, litter moisture content, and foot problems in broiler chickens fed phosphorus-deficient diets. Poultry Sci. 2017;96:3664–3675. doi: 10.3382/ps/pex186. [DOI] [PubMed] [Google Scholar]
- Fernandez S.R., Parsons C.M. Bioavailability of digestible lysine in heat-damaged soybean meal for chick growth. Poultry Sci. 1996;75:224–231. doi: 10.3382/ps.0750224. [DOI] [PubMed] [Google Scholar]
- Finot P.-A. The absorption and metabolism of modified amino acids in processed foods. J AOAC Int. 2005;88:894–903. [PubMed] [Google Scholar]
- Guo S., Liu D., Zhao X., Li C., Guo Y. Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poultry Sci. 2013;93:94–103. doi: 10.3382/ps.2013-03188. [DOI] [PubMed] [Google Scholar]
- Hamdi M., Sola-Oriol D., Davin R., Perez J.F. Calcium sources and their interaction with the different levels of non-phytate phosphorus affect performance and bone mineralization in broiler chickens. Poultry Sci. 2015;94:2136–2143. doi: 10.3382/ps/peu061. [DOI] [PubMed] [Google Scholar]
- Hammann F., Schmid M. Determination and quantification of molecular interactions in protein films: a review. Materials. 2014;7:7975–7996. doi: 10.3390/ma7127975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna C.D., Foran C.K., Utterback P.L., Stein H.H., Parsons C.M. Phosphorus bioavailability in increased-protein, reduced-fiber canola meal, conventional canola meal, and soybean meal fed to crossbred chicks. Poultry Sci. 2017;97:188–195. doi: 10.3382/ps/pex287. [DOI] [PubMed] [Google Scholar]
- He L.W., Meng Q.X., Li D.Y., Zhang Y.W., Ren L.P. Influence of feeding alternative fiber sources on the gastrointestinal fermentation, digestive enzyme activities and mucosa morphology of growing greylag geese. Poultry Sci. 2015;94:2464–2471. doi: 10.3382/ps/pev237. [DOI] [PubMed] [Google Scholar]
- Heck T., Faccio G., Richter M., Thöny-Meyer L. Enzyme-catalyzed protein crosslinking. Appl Microbiol Biotechnol. 2013;97:461–475. doi: 10.1007/s00253-012-4569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus M., Bokori J., Andrasofszky E. The value of crude protein content and in vitro pepsin digestibility of abattoir by-product meals in the prediction of their available protein content. Acta Vet Hung. 1989;37:27–33. [PubMed] [Google Scholar]
- Hendriks W.H., Emmens M.M.A., Trass B., Pluske J.R. Heat processing changes the protein quality of canned cat foods as measured with a rat bioassay. J Anim Sci. 1999;77:669–676. doi: 10.2527/1999.773669x. [DOI] [PubMed] [Google Scholar]
- Hinton J.A., Buhr R.J., Ingram K.D. Physical, chemical, and microbiological changes in the crop of broiler chickens subjected to incremental feed withdrawal. Poultry Sci. 2000;79:212–218. doi: 10.1093/ps/79.2.212. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J. Pathogenesis of enteric diseases. Poultry Sci. 1998;77:1150–1155. doi: 10.1093/ps/77.8.1150. [DOI] [PubMed] [Google Scholar]
- Ilhan Z.E., Marcus A.K., Kang D.-W., Rittmann B.E., Krajmalnik-Brown R. pH-mediated microbial and metabolic interactions in fecal enrichment cultures. mSphere. 2017;2 doi: 10.1128/mSphere.00047-17. e00047-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Moore R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poultry Sci. 2017;96:3272–3281. doi: 10.3382/ps/pex123. [DOI] [PubMed] [Google Scholar]
- Kim D.K., Lillehoj H.S., Lee S.H., Jang S.I., Lillehoj E.P., Bravo D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poultry Sci. 2013;92:2635–2643. doi: 10.3382/ps.2013-03095. [DOI] [PubMed] [Google Scholar]
- Kim S.-W., Li W., Angel R., Proszkowiec-Weglarz M. Effects of limestone particle size and dietary Ca concentration on apparent P and Ca digestibility in the presence or absence of phytase. Poultry Sci. 2018;97:4306–4314. doi: 10.3382/ps/pey304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleessen B., Hartmann L., Blaut M. Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa-associated Bifidobacteria in gnotobiotic rats. Br J Nutr. 2003;89:597–606. doi: 10.1079/BJN2002827. [DOI] [PubMed] [Google Scholar]
- Knight C.D., Dibner J.J., Vazquez-Anon M., Yan F. Effect of carbohydrase and protease on growth performance and gut health of young broilers fed diets containing rye, wheat, and feather meal. Poultry Sci. 2016;96:817–828. doi: 10.3382/ps/pew300. [DOI] [PubMed] [Google Scholar]
- Kraieski A.L., Hayashi R.M., Sanches A., Almeida G.C., Santin E. Effect of aflatoxin experimental ingestion and Eimeria vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an intestinal health index. Poultry Sci. 2016;96:1078–1087. doi: 10.3382/ps/pew397. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Narbad A., Gasson M.J., Woodward M.J. In vivo characterization of Lactobacillus johnsonii fi9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett Appl Microbiol. 2004;38:197–205. doi: 10.1111/j.1472-765x.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- Layton A., McKay L., Williams D., Garrett V., Gentry R., Sayler G. Development of Bacteroides 16s rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol. 2006;72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.H., Zo Y.G., Kim S.J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl Environ Microbiol. 1996;62:3112–3120. doi: 10.1128/aem.62.9.3112-3120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H., Patterson R., Barta J.R., Karrow N., Kiarie E. Nucleotide-rich yeast extract fed to broiler chickens challenged with Eimeria: impact on growth performance, jejunal histomorphology, immune system, and apparent retention of dietary components and caloric efficiency. Poultry Sci. 2019 doi: 10.3382/ps/pez213. In press. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Ru Y.J., Cowieson A.J., Li F.D., Cheng X. Effects of phytate and phytase on the performance and immune function of broilers fed nutritionally marginal diets. Poultry Sci. 2008;87:1105–1111. doi: 10.3382/ps.2007-00517. [DOI] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S.-B., Swick R.A., Choct M. Dietary acylated starch improves performance and gut health in necrotic enteritis challenged broilers. Poultry Sci. 2015;94:2434–2444. doi: 10.3382/ps/pev219. [DOI] [PubMed] [Google Scholar]
- Manangi M.K., Coon C.N. Phytate phosphorus hydrolysis in broilers in response to dietary phytase, calcium, and phosphorus concentrations. Poultry Sci. 2008;87:1577–1586. doi: 10.3382/ps.2007-00336. [DOI] [PubMed] [Google Scholar]
- Mavromichalis I., Baker D.H. Effects of pelleting and storage of a complex nursery pig diet on lysine bioavailability. J Anim Sci. 2000;78:341–347. doi: 10.2527/2000.782341x. [DOI] [PubMed] [Google Scholar]
- Moughan P.J., Rutherfurd S.M. A new method for determining digestible reactive lysine in foods. J Agric Food Chem. 1996;44:2202–2209. [Google Scholar]
- Olnood C.G., Beski S.S.M., Choct M., Iji P.A. Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim Nutr. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahm A.A., Pedersen C., Stein H.H. Application of the reactive lysine procedure to estimate lysine digestibility in distillers dried grains with solubles fed to growing pigs. J Agric Food Chem. 2008;56:9441–9446. doi: 10.1021/jf801618g. [DOI] [PubMed] [Google Scholar]
- Paiva D., McElroy A. Necrotic enteritis: applications for the poultry industry. J Appl Poultry Res. 2014;23:557–566. [Google Scholar]
- Paiva D.M., Walk C.L., McElroy A.P. Influence of dietary calcium level, calcium source, and phytase on bird performance and mineral digestibility during a natural necrotic enteritis episode. Poultry Sci. 2013;92:3125–3133. doi: 10.3382/ps.2013-03298. [DOI] [PubMed] [Google Scholar]
- Paiva D., Walk C., McElroy A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poultry Sci. 2014;93:2752–2762. doi: 10.3382/ps.2014-04148. [DOI] [PubMed] [Google Scholar]
- Palliyeguru M.W.C.D., Rose S.P., Mackenzie A.M. Effect of dietary protein concentrates on the incidence of subclinical necrotic enteritis and growth performance of broiler chickens. Poultry Sci. 2010;89:34–43. doi: 10.3382/ps.2009-00105. [DOI] [PubMed] [Google Scholar]
- Perez V.G., Jacobs C.M., Barnes J., Jenkins M.C., Kuhlenschmidt M.S., Fahey G.C., Jr., Parsons C.M., Pettigrew J.E. Effect of corn distillers dried grains with solubles and Eimeria acervulina infection on growth performance and the intestinal microbiota of young chicks. Poultry Sci. 2011;90:958–964. doi: 10.3382/ps.2010-01066. [DOI] [PubMed] [Google Scholar]
- Phong S., Shanmugavelu S., Thayalini K., Noraini S., Wong H. Detection of Lactobacillus, Bacteroides and Clostridium perfringens in the gastrointestinal contents of chicken fed different diets by real-time PCR. J Trop Agric Food Sci. 2010;38:81–87. [Google Scholar]
- Qaisrani S.N., Moquet P.C.A., van Krimpen M.M., Kwakkel R.P., Verstegen M.W.A., Hendriks W.H. Protein source and dietary structure influence growth performance, gut morphology, and hindgut fermentation characteristics in broilers. Poultry Sci. 2014;93:3053–3064. doi: 10.3382/ps.2014-04091. [DOI] [PubMed] [Google Scholar]
- Qaisrani S.N., van Krimpen M.M., Kwakkel R.P., Verstegen M.W.A., Hendriks W.H. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poultry Sci. 2015;94:2152–2164. doi: 10.3382/ps/pev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaisrani S.N., Van Krimpen M.M., Kwakkel R.P., Verstegen M.W.A., Hendriks W.H. Dietary factors affecting hindgut protein fermentation in broilers: a review. World’s Poult Sci J. 2015;71:139–160. [Google Scholar]
- Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2008;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities 1. J Appl Poultry Res. 2013;22:628–636. [Google Scholar]
- Ravindran V., Selle P.H., Ravindran G., Morel P.C.H., Kies A.K., Bryden W.L. Microbial phytase improves performance, apparent metabolizable energy, and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poultry Sci. 2001;80:338–344. doi: 10.1093/ps/80.3.338. [DOI] [PubMed] [Google Scholar]
- Requena T., Burton J., Matsuki T., Munro K., Simon M.A., Tanaka R., Watanabe K., Tannock G.W. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl Environ Microbiol. 2002;68:2420–2427. doi: 10.1128/AEM.68.5.2420-2427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttila T., Kassinen A., Malinen E., Krogius L., Palva A. Development of an extensive set of 16s rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Helmbrecht A., Parsons C.M., Dilger R.N. Interactive effects of dietary arginine and Eimeria acervulina infection on broiler growth performance and metabolism. Poultry Sci. 2016;96:659–666. doi: 10.3382/ps/pew295. [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Parsons C.M., Dilger R.N. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and α1-acid glycoprotein in broilers. Poultry Sci. 2016;95:1573–1581. doi: 10.3382/ps/pew035. [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Usry J.L., Parr T.M., Parsons C.M., Dilger R.N. Effects of dietary copper and amino acid density on growth performance, apparent metabolizable energy, and nutrient digestibility in Eimeria acervulina-challenged broilers. Poultry Sci. 2016;96:602–610. doi: 10.3382/ps/pew276. [DOI] [PubMed] [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S.M. Use of the guanidination reaction for determining reactive lysine, bioavailable lysine and gut endogenous lysine. Amino Acids. 2015;47:1805–1815. doi: 10.1007/s00726-015-2007-0. [DOI] [PubMed] [Google Scholar]
- Seiquer I., Delgado-Andrade C., Haro A., Navarro M.P. Assessing the effects of severe heat treatment of milk on calcium bioavailability: in vitro and in vivo studies. J Dairy Sci. 2010;93:5635–5643. doi: 10.3168/jds.2010-3469. [DOI] [PubMed] [Google Scholar]
- Setlow P., Johnson E.A. Spores and their significance. Food Microbiol Am Soc Microbiol. 2013:45–79. [Google Scholar]
- Sharma N.K., Keerqin C., Wu S.-B., Choct M., Swick R.A. Emissions of volatile odorous metabolites by Clostridium perfringens - in vitro study using 2 broth cultures. Poultry Sci. 2017;96:3291–3297. doi: 10.3382/ps/pex129. [DOI] [PubMed] [Google Scholar]
- Shirley R.B., Parsons C.M. Effect of pressure processing on amino acid digestibility of meat and bone meal for poultry. Poultry Sci. 2000;79:1775–1781. doi: 10.1093/ps/79.12.1775. [DOI] [PubMed] [Google Scholar]
- Short F., Gorton P., Wiseman J., Boorman K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 1996;59:215–221. [Google Scholar]
- Silva T.H., Mesquita-Guimarães J., Henriques B., Silva F., Fredel M. The potential use of oyster shell waste in new value-added by-product. Resources. 2019;8:13. [Google Scholar]
- Stanley D., Wu S.-B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PloS One. 2014;9 doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system1. J Appl Poultry Res. 2014;23:306–314. [Google Scholar]
- Talukdar P.K., Udompijitkul P., Hossain A., Sarker M.R. Inactivation strategies for Clostridium perfringens spores and vegetative cells. Appl Environ Microbiol. 2016;83 doi: 10.1128/AEM.02731-16. e02731-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., McElroy A.P. Influence of limestone and phytase on broiler performance, gastrointestinal pH, and apparent ileal nutrient digestibility. Poultry Sci. 2012;91:1371–1378. doi: 10.3382/ps.2011-01928. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Hsieh J.C.F., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poultry Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Wang X., Peebles E.D., Kiess A.S., Wamsley K.G.S., Zhai W. Effects of coccidial vaccination and dietary antimicrobial alternatives on the growth performance, internal organ development, and intestinal morphology of Eimeria-challenged male broilers. Poultry Sci. 2018;98:2054–2065. doi: 10.3382/ps/pey552. [DOI] [PubMed] [Google Scholar]
- Wise M.G., Siragusa G.R. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J Appl Microbiol. 2007;102:1138–1149. doi: 10.1111/j.1365-2672.2006.03153.x. [DOI] [PubMed] [Google Scholar]
- Yang E.-I., Yi S.-T., Leem Y.-M. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part 1. Fundamental properties. Cement Concr Res. 2005;35:2175–2182. [Google Scholar]
- Yang Y., Hua Y., Yu H., Gong J., Diarra M.S., Wang Q. Functional assessment of encapsulated citral for controlling necrotic enteritis in broiler chickens. Poultry Sci. 2016;95:780–789. doi: 10.3382/ps/pev375. [DOI] [PubMed] [Google Scholar]
- Zanu H.K., Kheravii S.K., Morgan N.K., Wu S.B., Bedford M.R., Swick R.A. Influence of meat and bone meal, phytase and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 1. Growth performance, intestinal pH, apparent ileal digestibility, cecal microbiota and tibial mineralization. Poultry Sci. 2020;99(3):1540–1550. doi: 10.1016/j.psj.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanu H.K., Kheravii S.K., Morgan N.K., Wu S.B., Bedford M.R., Swick R.A. Interactive effect of 2 dietary calcium and phytase levels on broilers challenged with subclinical necrotic enteritis: Part 1. Broiler performance, gut lesions and pH, bacterial counts and apparent ileal digestibility. Poultry Sci. 2019 doi: 10.1016/j.psj.2020.05.033. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen D.W., Yu B., He J., Yu J., Mao X.B., Wang J.X., Luo J.Q., Huang Z.Q., Cheng G.X., Zheng P. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J Anim Sci. 2015;93:2967–2976. doi: 10.2527/jas.2014-8820. [DOI] [PubMed] [Google Scholar]
- Zhang B.H., Fan B., Li M., Zhang Y.H., Gao Z.H. Effects of thermal treatment on the properties of defatted soya bean flour and its adhesion to plywood. R Soc Open Sci. 2018;5:180015. doi: 10.1098/rsos.180015. [DOI] [PMC free article] [PubMed] [Google Scholar]