Abstract
This study evaluated the effects of micro-encapsulated (protected) organic acids (OA) and essential oils (EO) combination, P(OA + EO), and effects of a regular blend of free acids (FA) on the growth, immune responses, intestinal barrier and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli (ETEC) F4 (K88+). A total of 30 crossbred (Duroc × Landrace × Large White) weaned barrows (7.41 ± 0.06 kg, 28 d old) were assigned randomly to 5 treatments: 1) non-challenged positive control (PC), 2) ETEC F4 (K88+)-challenged negative control (NC), 3) NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + free acidifier (FA) at 5 g/kg, 4) NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + P(OA + EO) at 1 g/kg (P1), 5) NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + P(OA + EO) at 2 g/kg (P2). Each dietary treatment had 6 replicates of one piglet each and the study lasted for 3 wk. On d 7, pigs in NC, FA, P1 and P2 were orally dosed with 10 mL of ETEC F4 (K88+) culture (1 × 109 CFU/mL). From d 7 to 14 after the ETEC F4 (K88+) challenge, P1 increased gain-to-feed ratio (G:F) significantly (P < 0.05) compared with NC and FA groups. From d 14 to 21, P2 increased the average daily gain of pigs (P < 0.05) compared with NC and FA groups. Compared with NC, P2 reduced tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-10 concentrations (P < 0.05) in sera collected at 4 h later after ETEC F4 (K88+) challenge. On d 21, P1 increased occludin and zonula occludens-1 protein expression in ileum compared with NC (P < 0.05). After this 3-wk experiment, alpha diversity of gut microbiota was decreased by P2 compared with PC, and P1 increased the relative abundance of Lactobacillus in ileum, cecum and colon (P < 0.05). In conclusion, dietary P(OA + EO) additive at 2 g/kg combined with antibiotics could improve piglet performance and attenuate inflammation, and P(OA + EO) additive at 1 g/kg combined with antibiotics improved intestinal barrier and increased beneficial microbiota composition after an F4 (K88+) challenge.
Keywords: Essential oil, Organic acid, Enterotoxigenic Escherichia coli F4 (K88+), Gut health, Weaned piglet
1. Introduction
Enterotoxigenic Escherichia coli (ETEC) F4 (K88+) is one of the main factors causing infection, disease and diarrhea in weaned pigs, and is strongly associated with decreased growth performance and increased mortality, causing serious damage in production (Bosi et al., 2004; Fairbrother et al., 2007). It was reported that infection due to ETEC F4 (K88+) could release enterotoxins, therefore stimulate the immune response, impair intestinal barrier function and increase intestinal permeability (Fossum, 1998; Guttman et al., 2006; Guignot et al., 2007). A healthy gut environment has an important role in the development and homeostasis of the immune system and provides a barrier against pathogens (Guarner and Malagelada, 2003; Clemente et al., 2012). Although antibiotics were generally used in the diets to treat pathogen infection and improve gut health, their efficacy was not always optimal in post-weaning piglets. The lack of effect of antibiotics called for other solutions as new additives added on top to help to prevent diarrhea caused by pathogen infection.
Organic acids (OA) have been used in commercial compound feeds for decades, some OA such as formic, propionic, lactic, citric, fumaric and sorbic acids are used for feed preservation (Lückstӓdt, 2014). It has been shown that most of OA has a pKa (the pH at which the acid is half dissociated) between 3 and 5 and possess antimicrobial activities (Khan and Iqbal, 2015). Our previous study showed that pigs fed mixed OA increased Lactobacillus concentration and reduced Escherichia concentration in feces (Li et al., 2019). The main mode of action of OA is the ability to penetrate bacterial cells when they are non-dissociated, which could disrupt the normal physiology of certain types of bacteria (Khan and Iqbal, 2015). Essential oils (EO), natural bioactive compounds derived from plants, were studied recently and considered as an effective additive in improving animal growth, immune system and modulating intestinal health as well as diarrhea (Yang et al., 2019). The antimicrobial activity of EO has been extensively tested in vitro against a wide range of pathogenic bacteria, therefore it could be considered as one of the alternatives in maintaining the balance of microbiota (Kalemba and Kunicka, 2003; Ouwehand et al., 2010). The mechanism by which EO exerts the antimicrobial activity appears to be by participation in the lipid membrane of the bacterial cells and disturbing the structures of pathogens (Burt, 2004; Di Pasqua et al., 2007), and OA could pass into cells and inhibit essential metabolic reactions and increase stress on intracellular pH homeostasis (Brul and Coote, 1999).
Our previous study showed that combined supplementation of dietary OA and EO improved performance by having different positive effects on intestinal health and digestive enzymes of weaned pigs (Xu et al., 2018). However, the effects of a micro-encapsulated OA and EO combination on growth performance, immunological parameters, intestinal barrier function and microbiota of weaned pigs after an ETEC F4 (K88+) challenge have rarely been reported. At the initiation of this trial, it was generally accepted and a common practice in the local industry to use free OA in combination with antibiotics. Therefore, in this study, we used a regular unprotected (free) acidifier product to make a comparison between the free acids and the micro-encapsulated (protected) combination of OA and EO. The micro-encapsulated OA and EO combination in the diet would have a slow and progressive release in the intestine and we hypothesized that it would enhance intestinal health by improving intestinal barrier function and microbiota, alleviate inflammatory response by reducing pro-inflammatory cytokines. In the current study, ETEC F4 (K88+) challenge was supplemented to establish a model of intestinal injury as it is one of the most important causes of postweaning diarrhea in pigs (Daudelin et al., 2011). The aim of the present study was to investigate in a context similar to the reality of industrial use of feed additives (including antibiotics) whether micro-encapsulated (protected) combination of OA and EO has better efficacy to improve animal growth and attenuate intestinal injury caused by ETEC F4 (K88+) challenge compared with free acidifier.
2. Materials and methods
All the procedures used in the animal experiment were approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China).
2.1. Micro-encapsulated organic acids and essential oils
The micro-encapsulated (protected) OA and EO combination, P(OA + EO), is a selected formula of OA (fumaric, citric, malic and sorbic acids) and EO (thymol, vanillin and eugenol) micro-encapsulated in a matrix of triglycerides from hydrogenated vegetable oil (Jefo Nutrition Inc., Canada). Free acidifier (FA) is a regular blend of 35% free acids (lactic acid, citric acid, phosphoric acid) adsorbed on silicon dioxide.
2.2. Experimental design and diets
A total of 30 crossbred (Duroc × Landrace × Large White) weaned barrows (initial BW = 7.41 ± 0.06 kg, 28 d old) were housed in individual metabolic cages (1.2 m × 0.4 m × 0.5 m) with plastic slatted flooring throughout the experiment. Pigs were assigned randomly to 1 of 5 treatments. Each dietary treatment had 6 repetitions of one piglet each. There were 5 treatments: 1) non-challenged positive control (PC), 2) ETEC F4 (K88+)-challenged (on d 7) negative control (NC), 3) NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + free acidifier (FA) at 5 g/kg, 4) NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + P(OA + EO) at 1 g/kg (P1), 5) NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + P(OA + EO) at 2 g/kg (P2). We chose the antibiotics kitasamycin and olaquindox as they are commonly used as a growth promoter for piglets in commercial farms in China in order to ensure a healthy transition around weaning. A corn-soybean meal basal diet was formulated to meet the nutrient requirements of pigs according to NRC (2012) (Table 1). Pigs in the experimental treatments had free access to feed and water. Temperatures were controlled by air conditioners at 25 to 28 °C. The piglets were in a healthy condition and the living environment was provided in accordance with animal welfare standards in the whole experimental period. The experimental period lasted for 3 wk.
Table 1.
Composition and nutrient levels of the basal diet (as-fed basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Corn (8.0% CP) | 52.96 |
| Extruded soybean (35.5% CP) | 15.30 |
| Soybean meal (45% CP) | 8.00 |
| Whey power (7.0% CP) | 10.00 |
| Fish meal (64.6% CP) | 6.00 |
| Spray-dried plasma protein (75% CP) | 3.00 |
| Glucose | 2.00 |
| Soybean oil | 0.70 |
| Dicalcium phosphate | 0.02 |
| Limestone | 0.80 |
| Salt | 0.30 |
| l-Lysine·hydrochloride (98%) | 0.09 |
| dl-Methionine (98%) | 0.03 |
| Zinc oxide (85%) | 0.30 |
| Vitamin-mineral premix1 | 0.50 |
| Total | 100 |
| Nutrient levels | |
| Digestible energy, Mcal/kg | 3.49 |
| Crude protein | 20.22 |
| Calcium | 0.70 |
| Available phosphorus | 0.35 |
| Digestible lysine | 1.22 |
| Digestible methionine | 0.36 |
| Digestible threonine | 0.75 |
| Digestible tryptophan | 0.21 |
Vitamin-mineral premix provided the following per kilogram of diet: vitamin A, 12,000 IU as vitamin A acetate; vitamin D, 2,500 IU as vitamin D3; vitamin E, 30 IU as DL-α-tocopheryl acetate; 12 μg of vitamin B12; vitamin K, 3 mg as menadione sodium bisulfate; d-pantothenic acid, 15 mg as calcium pantothenate; 40 mg of nicotinic acid; choline, 400 mg as choline chloride; Mn, 40 mg as manganous oxide; Zn, totally 2,250 mg of Zn2+ concentration from zinc oxide added additionally and zinc sulfate from premix; Fe, 90 mg as iron sulfate; Cu, 8.8 mg as copper oxide; I, 0.35 mg as ethylenediamine dihydroiodide; and Se, 0.3 mg as sodium selenite.
2.3. Enterotoxigenic ETEC F4 (K88+) challenge and fecal score
The enterotoxigenic ETEC F4 (K88+) (serotype O139:K88) was obtained from the China Institute of Veterinary Drug Control (Beijing, China), and grown in Luria Broth. The expanded culture of ETEC F4 (K88+), approximately 1 × 109 CFU/mL, was further prepared for oral dosing as previously described by Pan et al. (2017). At 08:00 on d 7, pigs in group NC, treatments FA, P1 and P2 were orally dosed with 10 mL of ETEC F4 (K88+) culture (1 × 109 CFU/mL) to induce diarrhea as described whereas pigs in group PC were orally administered with an equal amount of sterile physiological saline. The diarrhea scores were recorded at 09:00 from d 7 to 14 by a single operator unaware of the distribution of dietary treatments. Diarrhea score was monitored according to the previously described system (Pierce et al., 2005): 1, hard firm feces; 2, slightly soft feces; 3, soft, partially formed feces; 4, loose, semi-liquid feces (diarrhea); 5, watery, mucus-like feces (severe diarrhea).
2.4. Sample collection and measurements
The piglet's weight and feed intake were recorded and average daily gain (ADG), average daily feed intake (ADFI), gain-to-feed ratio (G:F) were calculated from d 0 to 7, d 7 to 14, and d 14 to 21, respectively. Four hours after the ETEC challenge, blood samples were obtained from an anterior vena cava puncture using vacuum container tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). Blood samples were clot at room temperature for 20 min and centrifuged at 4,000 × g for 10 min at 4 °C to obtain serum samples and were kept at −80 °C until measurement. On d 21 (2 wk after ETEC F4 [K88+] challenge), 4 piglets per treatment, close to the average body weight (BW) were selected and sacrificed by exsanguination after electrical stunning to obtain intestinal contents and tissues. Briefly, 20 cm of medial jejunum and ileal segments were opened longitudinally and the digesta was flushed with ice-cold PBS, then a sterile glass microscope slide was used to obtain 1-g mucosa of jejunum and ileum from 3 pigs per treatment. Digesta samples (2 to 3 g) in the midterm ileum, cecum and colon were collected in sterile bags for intestinal microbiota analysis. The mucosa and digesta samples were immediately frozen in liquid nitrogen and stored at −80 °C for further analysis.
2.5. Chemical analysis
2.5.1. Immune and intestinal permeability parameters
Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10) were analyzed using commercially available swine enzyme-linked immune sorbent assay (ELISA) kits (R&D Systems Inc., Minneapolis, MN, and Biosource International Inc., Camarillo, CA). Serum concentrations of endotoxin were determined using a commercial chromogenic endpoint Tachypleus kit (Xiamen Limulus Amebocyte Lysate Company, Xiamen, China), according to the manufacturer's instructions. Diamine oxidase (DAO) was determined using a commercial ELISA kit purchased from the Beijing Luyuan Byrd Biological Technology Company (Beijing, China), following the standard procedure described by the manufacturer. The immune globulins (IgG, IgM and IgA) in serum were measured by ELISA kits (IgG, IgM and IgA quantitation kits; Bethyl Laboratories, Inc., Texas, USA).
2.5.2. Western blot
The method for protein extraction of the intestinal mucosa was in accordance with the procedures of Pan et al. (2017). Briefly, the protein contained from the mucosa of jejunum and ileum was extracted according to the ProteoJET Total Protein Extraction Kit (Fermentas, Hanover, MD). After determining the protein concentration, protein extracts (20 μg) were fractionated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Skim milk (5%) solution was used to block the membranes at room temperature for 2 h and then incubated with diluted antibodies. Specific primary antibodies included rabbit anti-zonula occludens-1 (ZO-1) (1:200) and rabbit anti-occludin (1:200) from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and rabbit anti-β-actin (1:1,000) from Sigma Aldrich (Sigma Aldrich Inc., St. Louis, MO, USA). After incubation with goat anti-rabbit IgG secondary antibody, signals were visualized by the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) after incubation. Blot analysis was performed on 4 replicates per treatment using Quantity One Software (BioRad Laboratories, Hercules, CA). The relative abundance of target proteins including occludin and ZO-1was expressed as the target protein-to-β-actin protein ratio.
2.5.3. Intestinal microbiota
Total bacterial genomic DNA extraction was performed from digesta samples of ileum, cecum and colon by use of the E.Z.N.A. Stool DNA Kits (Omega Bio-Tek, Norcross, US). The microbial 16S rRNA gene was amplified with indexes and adaptors-linked universal primers (515F: GTGYCAGCMGCCGCGGTAA) 806R: GGACTACHVGGGTWTCTAAT) targeting the V4 region, purified with QIAquick PCR Purification Kit (QIAGEN), and quantified by Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, US) to pool into even concentration. Amplicon libraries were sequenced on Illumina HiSeq PE250 platform (Illumina, San Diego, US) for paired-end reads of 300 bp. The paired-end reads were assembled into longer tags and quality-filtered to remove tags with a length of < 220 bp, an average quality score of < 20, and tags containing > 3 ambiguous bases by PANDAseq. After discarding the singletons, the high-quality tags were clustered into operational taxonomic units (OTU) using the Usearch in Quantitative Insights Into Microbial Ecology (QIIME) pipeline software (version 1.8.0) with a similarity threshold of 0.97 and the OTU were further subjected to the taxonomy-based analysis with the RDP database (https://rdp.cme.msu.edu/), using the RDP classifier at an 0.80 confidence level (Cole et al., 2013). Alpha and beta diversity were analyzed using QIIME (Mahnert et al., 2015; jin et al., 2016). Linear discriminant analysis (LDA) effect size (LEfSe) analyses were performed with the LEfSe tool (Segata et al., 2011). The relative abundance of bacteria was expressed as a percentage.
2.6. Statistical analysis
All data were analysed in a randomized complete block design using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC) with each individual pig considered as an experimental unit, dietary treatment as a fixed effect and block as a random effect. Statistical differences among treatments were separated by Student-Neuman-Keul's multiple range tests. Differences in gut bacterial abundance were analyzed by LDA LEfSe. LEfSe analysis used the Kruskal-Wallis rank sum test to detect significantly different abundances and perform LDA scores to estimate the effect size (threshold: ≥2). Values were presented as least square means with a standard error of the mean (SEM). Significant differences were declared at P ≤ 0.05 and trends were noted when 0.05 < P ≤ 0.10.
3. Results
3.1. Growth performance and fecal score
There were no adverse events during the whole experiment period. Table 2 shows the growth performance of weaned piglets. From d 0 to 7, no difference was observed on piglet performance. For the first week after the ETEC F4 (K88+) challenge, treatment P1 had an improved G:F (P < 0.01) compared with NC and treatment FA and diet effects were shown in ADG and ADFI (P = 0.04 and P = 0.05, respectively). However, statistically significant differences among the treatments were not observed in ADG or ADFI when pairwise comparisons were performed using Student-Neuman-Keul's multiple range tests. For the second week after the ETEC F4 (K88+) challenge, higher ADG of the weaned piglets was observed (P < 0.05) only in P2 treatment compared with NC group and FA treatment. On d 1 after the ETEC F4 (K88+) challenge, the fecal score of the piglets was higher (P < 0.05) in treatment P1 compared with PC. On d 2 after the ETEC F4 (K88+) challenge, the fecal score of piglets was not significantly different after Student-Neuman-Keul's multiple range tests and no treatment effect was observed over the next 6 d (Table 3).
Table 2.
Effects of a regular free acidifier or a micro-encapsulated combination of organic acids and essential oils on performance of weaned pigs pre- and post-challenged with enterotoxigenic Escherichia coli F4 (K88+).
| Item | Treatments1 |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | FA | P1 | P2 | |||
| BW at d 0, kg | 7.42 | 7.41 | 7.41 | 7.42 | 7.41 | 0.06 | 0.99 |
| Day 0 to 7 | |||||||
| ADG, g | 449 | 482 | 478 | 457 | 527 | 30.3 | 0.44 |
| ADFI, g | 697 | 710 | 725 | 666 | 772 | 36.7 | 0.41 |
| G:F | 0.65 | 0.68 | 0.67 | 0.69 | 0.68 | 0.03 | 0.86 |
| Day 7 to 14 | |||||||
| ADG, g | 488 | 356 | 396 | 478 | 504 | 34.2 | 0.04 |
| ADFI, g | 800 | 623 | 713 | 710 | 799 | 42.5 | 0.05 |
| G:F | 0.61ab | 0.56b | 0.55b | 0.68a | 0.63ab | 0.02 | <0.01 |
| Day 14 to 21 | |||||||
| ADG, g | 477ab | 394b | 421b | 499ab | 545a | 31.8 | 0.02 |
| ADFI, g | 856 | 760 | 808 | 851 | 917 | 42.5 | 0.16 |
| G:F | 0.56 | 0.53 | 0.52 | 0.58 | 0.60 | 0.03 | 0.17 |
| Day 7 to 21 | |||||||
| ADG, g | 483 | 390 | 424 | 486 | 521 | 27.1 | 0.08 |
| ADFI, g | 828 | 696 | 785 | 781 | 858 | 42.1 | 0.13 |
| G:F | 0.58 | 0.56 | 0.54 | 0.63 | 0.62 | 0.02 | 0.07 |
| Day 0 to 21 | |||||||
| ADG, g | 471 | 421 | 446 | 476 | 512 | 39.9 | 0.40 |
| ADFI, g | 784 | 707 | 789 | 732 | 830 | 39.9 | 0.22 |
| G:F | 0.60 | 0.59 | 0.56 | 0.65 | 0.62 | 0.02 | 0.09 |
BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; G:F = gain-to-feed ratio.
a,b Different superscripts within a row indicate a significant difference (P < 0.05).
PC, non-challenged positive control; NC, enterotoxigenic Escherichia coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
SEM means standard error of the means (n = 6).
Table 3.
Effects of a regular free acidifier or a micro-encapsulated combination of organic acids and essential oils combination on fecal score in the first week after enterotoxigenic Escherichia coli F4 (K88+) challenge in weaned pigs.
| Item | Treatments1 |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | FA | P1 | P2 | |||
| Day 1 | 2.00b | 3.00ab | 3.00ab | 3.17a | 2.17ab | 0.34 | <0.01 |
| Day 2 | 2.00 | 3.33 | 3.33 | 2.50 | 2.83 | 0.36 | 0.04 |
| Day 3 | 2.00 | 2.67 | 2.00 | 2.17 | 1.67 | 0.40 | 0.59 |
| Day 4 | 2.17 | 2.67 | 2.50 | 1.83 | 2.33 | 0.32 | 0.49 |
| Day 5 | 1.83 | 2.17 | 2.33 | 2.33 | 2.33 | 0.28 | 0.64 |
| Day 6 | 1.83 | 2.50 | 2.00 | 2.33 | 2.50 | 0.27 | 0.41 |
| Day 7 | 1.83 | 2.67 | 2.33 | 2.50 | 2.33 | 0.26 | 0.26 |
a,b Different superscripts within a row indicate a significant difference (P < 0.05).
PC, non-challenged positive control; NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
SEM means standard error of the means (n = 6).
3.2. Immune and intestinal permeability parameters of serum
As shown in Table 4, some inflammatory parameters in serum collected at 4 h after ETEC F4 (K88+) challenge were determined. A reduced TNF-α concentration (P < 0.05) was observed in the P2 treatment compared with NC. Moreover, IL-6 and IL-10 levels decreased (P < 0.05) by FA and P2 treatments compared with NC. The concentrations of endotoxin, DAO, IgA, IgG and IgM in serum were not significantly changed by the treatments.
Table 4.
Effects of a regular free acidifier or a micro-encapsulated combination of organic acids and essential oils combination on immunological index after 4 h of the enterotoxigenic Escherichia coli F4 (K88+) challenge in weaned pigs.
| Item | Treatments1 |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | FA | P1 | P2 | |||
| TNF-α, pg/mL | 86.2ab | 105a | 83.0ab | 90.2ab | 69.0b | 6.29 | 0.01 |
| IL-6, pg/mL | 117ab | 127a | 110bc | 122a | 106c | 2.97 | <0.01 |
| IL-10, pg/mL | 37.5ab | 50.5a | 31.5b | 37.6ab | 34.7b | 3.69 | 0.02 |
| Endotoxin, EU/L | 200 | 166 | 146 | 192 | 154 | 19.2 | 0.34 |
| DAO, U/L | 19.5 | 19.2 | 19.5 | 18.4 | 21.3 | 1.31 | 0.50 |
| IgA, g/L | 1.06 | 1.05 | 1.40 | 0.79 | 1.11 | 0.16 | 0.16 |
| IgG, g/L | 6.88 | 7.62 | 6.64 | 7.54 | 8.20 | 0.49 | 0.16 |
| IgM, g/L | 0.68 | 1.03 | 0.85 | 0.63 | 0.71 | 0.10 | 0.07 |
TNF-α = tumor necrosis factor-α; IL = interleukin; EU = endotoxin units; DAO = diamine oxidase; Ig = immunoglobulin.
a,b,c Different superscripts within a row indicate a significant difference (P < 0.05).
PC, non-challenged positive control; NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
SEM means standard error of the means (n = 6).
3.3. Protein expression of tight junction proteins
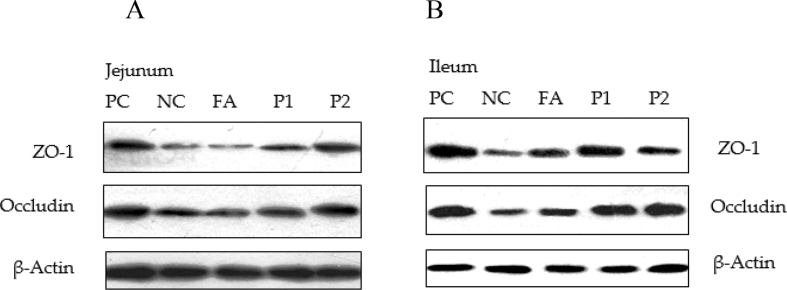
In the jejunum, ETEC F4 (K88+) challenge caused a lower abundance of occludin and ZO-1 proteins in the NC group (P < 0.05) and FA supplementation also decreased ZO-1 protein abundance compared with the PC group. Higher ileal occludin and ZO-1 protein expression was observed in treatment P1 (P < 0.05) compared with the NC group (Table 5 and Fig. 1).
Table 5.
Effects of a regular free acidifier or a micro-encapsulated combination of organic acids and essential oils combination on concentrations of tight junction proteins after enterotoxigenic Escherichia coli F4 (K88+) challenge in weaned pigs.
| Item | Treatments1 |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | FA | P1 | P2 | |||
| Jejunum | |||||||
| Occludin-to-β-actin ratio | 0.69a | 0.21b | 0.28ab | 0.45ab | 0.56ab | 0.09 | 0.04 |
| ZO-1-to-β-actin ratio | 0.62a | 0.22b | 0.27b | 0.38ab | 0.43ab | 0.06 | 0.01 |
| Ileum | |||||||
| Occludin-to-β-actin ratio | 0.69a | 0.18d | 0.37c | 0.52b | 0.31cd | 0.04 | <0.01 |
| ZO-1-to-β-actin ratio | 0.66a | 0.18b | 0.42ab | 0.53a | 0.44ab | 0.07 | 0.01 |
ZO-1 = zonula occludens-1.
a,b,c,dDifferent superscripts within a row indicate a significant difference (P < 0.05).
PC, non-challenged positive control; NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
SEM means standard error of the means (n = 3).
Fig. 1.
Western blot analysis of occludin and zonula occludens-1 (ZO-1) protein abundance in jejunum and ileum of 2 wk after enterotoxigenic Escherichia coli F4 (K88+) challenge in weaned pigs. (A) The bands were the representative Western blot images of occludin, ZO-1 and β-actin from jejunum; (B) The bands were the representative Western blot images of occludin, ZO-1 and β-actin from the ileum. PC, non-challenged positive control. NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
3.4. The 16S rRNA gene sequence
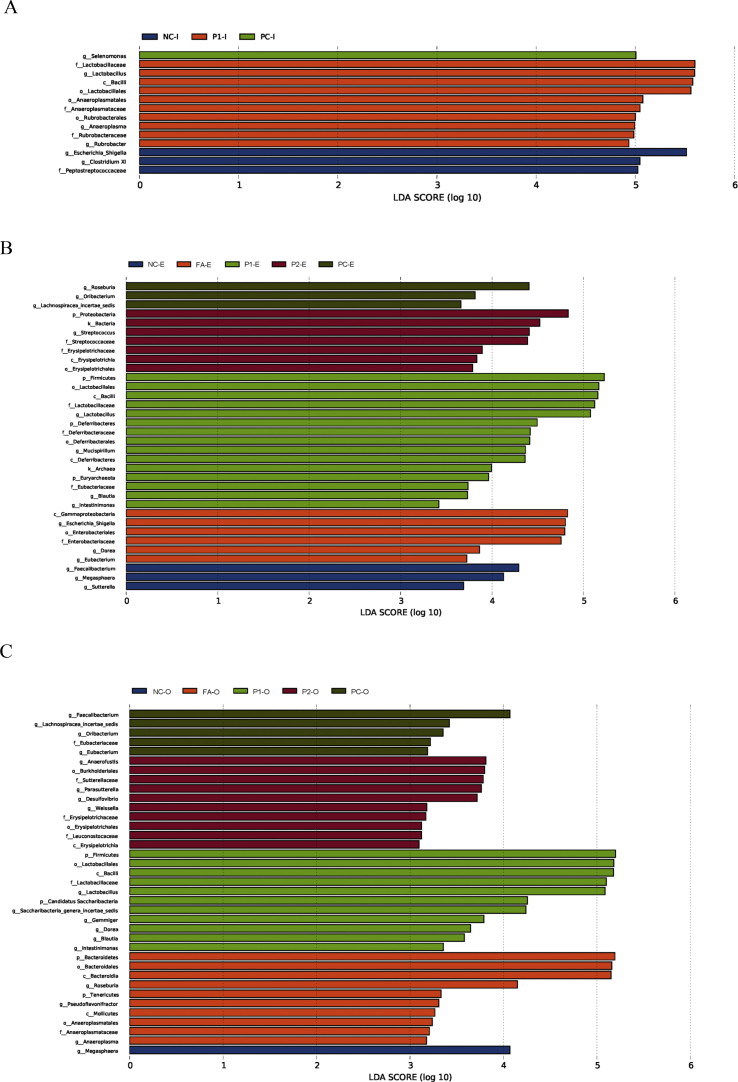
There were 19,768 to 38,939 valid reads obtained from each sample (data not shown). Using a 97% sequence similarity cut-off, 889 OTU were identified from 60 samples (n = 4 per treatment). Table 6 shows alpha diversity analysis including Chao, observed species, Shannon and Simpson indices. In the ileum, no significant difference was observed in alpha diversity among the 5 treatments. Chao and observed species in the cecum were lower in treatment P2 than in the PC group and treatment P1 (P < 0.05), whereas treatments FA and P2 had reduced Shannon index in the colon than in the PC and NC groups (P < 0.05). However, we did not observe any difference in beta diversity in the 5 treatments (data not shown). The microbiota relative abundances at the genus level of all samples suggested that the top 2 genues were Lactobacillus and Escherichia-Shigella in the ileum, Prevotella and Lactobacillus in the cecum and colon (Table 7). In the ileum, the relative abundance of Lactobacillus was significantly increased by the P1 treatment compared with the NC group (P < 0.05), treatments FA and P2, and no difference in the relative abundance of Escherichia-Shigella. In the cecum and colon, the treatment P1 increased the relative abundance of Lactobacillus compared with the other 4 groups (P < 0.05). LEfSe analysis showed dominant microorganisms among the treatments with LDA values of taxa higher than 2.0 (Fig. 2A–C). Fig. 2A shows the NC group had high Escherichia-Shigella composition while treatment P1 had a high Lactobacillaceae family in the ileum. In the cecum, the NC group had abundant Faecalibacterium, the PC group was abundant in Roseburia, treatment FA had high Escherichia-Shigella, treatment P1 still had a high Lactobacillaceae family and treatment P2 had high Proteobacteria, Streptococcaceae and Erysipelotrichaceae families (Fig. 2B). In the colon, the PC group induced high Faecalibacterium, the dominant microbiota in the FA treatment was Bacteroidia, and the P1 treatment resulted in elevated Firmicutes composition, Bacilli and Lactobacillaceae genera (Fig. 2C).
Table 6.
Estimation of diversity within different gut segments after enterotoxigenic Escherichia coli F4 (K88+) challenge in weaned pigs.
| Item | Treatments1 |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | FA | P1 | P2 | |||
| Ileum | |||||||
| Chao | 182 | 210 | 203 | 216 | 253 | 44.8 | 0.85 |
| Observed species | 119 | 144 | 154 | 144 | 199 | 43.5 | 0.77 |
| Shannon | 2.04 | 1.14 | 2.29 | 1.60 | 2.55 | 0.58 | 0.48 |
| Simpson | 0.55 | 0.29 | 0.48 | 0.42 | 0.78 | 0.12 | 0.12 |
| Cecum | |||||||
| Chao | 342a | 310a | 324a | 335a | 253b | 19.4 | 0.04 |
| Observed species | 277a | 256ab | 257ab | 271a | 199b | 16.6 | 0.04 |
| Shannon | 5.47 | 5.38 | 4.84 | 5.11 | 4.60 | 0.30 | 0.25 |
| Simpson | 0.96 | 0.95 | 0.89 | 0.92 | 0.89 | 0.03 | 0.28 |
| Colon | |||||||
| Chao | 393a | 396a | 329b | 341ab | 311b | 22.1 | 0.06 |
| Observed species | 323a | 310ab | 270bc | 277abc | 257c | 17.0 | 0.08 |
| Shannon | 5.89a | 5.89a | 4.95b | 5.13ab | 4.98b | 0.27 | 0.05 |
| Simpson | 0.96 | 0.97 | 0.89 | 0.92 | 0.90 | 0.02 | 0.20 |
a,b,cDifferent superscripts within a row indicate a significant difference (P < 0.05).
PC, non-challenged positive control; NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
SEM means standard error of the means (n = 4).
Table 7.
Microbiota relative abundance in different gut segments at genus level after enterotoxigenic Escherichia coli F4 (K88+) challenge in weaned pigs (%).
| Item | Treatments1 |
SEM2 | P-value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | FA | P1 | P2 | |||
| Ileum | |||||||
| Lactobacillus | 63.9ab | 10.1b | 35.6bc | 85.6a | 5.41c | 12.9 | <0.01 |
| Escherichia-Shigella | 0.16 | 62.5 | 51.1 | 7.04 | 36.2 | 16.8 | 0.09 |
| Cecum | |||||||
| Prevotella | 48.3 | 50.6 | 44.9 | 21.9 | 50.9 | 9.5 | 0.22 |
| Lactobacillus | 8.85b | 1.91b | 0.66b | 30.2a | 3.18b | 2.8 | <0.01 |
| Colon | |||||||
| Prevotella | 45.8 | 52.2 | 55.4 | 25.0 | 36.8 | 6.8 | 0.05 |
| Lactobacillus | 11.8b | 2.83b | 0.30b | 29.7a | 8.82b | 4.2 | <0.01 |
a,b,cDifferent superscripts within a row indicate a significant difference (P < 0.05).
PC, non-challenged positive control; NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oilsat 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg.
SEM means standard error of the means (n = 4).
Fig. 2.
Key phylotypes of gut microbiota in the ileum of pigs after 2 wk of enterotoxigenic Escherichia coli F4 (K88+) challenge were identified using linear discriminant analysis effect size (LEfSe) analyses (n = 4). Taxonomic differences among the 3 treatments are represented by the color of the most abundant class. Linear discriminant analysis (LDA) score was obtained by LDA for significantly functional microbiota with different taxonomic levels (phylum to genus: p, phylum; c, class; o, order; f, family; g, genus). (A) LEfSe analysis from ileum; (B) LEfSe analysis from cecum; (C) LEfSe analysis from colon. PC, non-challenged positive control; NC, enterotoxigenic E. coli F4 (K88+)-challenged (on d 7) negative control; FA, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + regular free acids at 5 g/kg; P1, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 1 g/kg; P2, NC + kitasamycin at 50 mg/kg + olaquindox at 100 mg/kg + micro-encapsulated combination of organic acids and essential oils at 2 g/kg. I = ileum, E = cecum, and O = colon.
4. Discussion
Many studies have reported that ETEC infection could induce diarrhea in nursery pigs typically lasting 1 to 5 d after ETEC infection as well as reduce growth performance, especially in the first 1 or 2 wk post-weaning (Verdonck et al., 2007; Kim et al., 2012). Therefore, the ETEC F4 (K88+) challenge was carried out after the first week of weaning as the pigs have a high diarrhea incidence over that time, and piglet performance was recorded for 2 wk after the ETEC F4 (K88+) challenge to see a further effect of the ETEC infection. Although few studies about effects of protected combination of OA and essential oils [P(OA + EO)] on pigs after an ETEC challenge was retrieved in literature, some research have reported that a blend of OA combined with EO showed an increase in digestive enzyme activity and growth performance (Jang et al., 2004; Xu et al., 2018). In the first week of the experimental period, there were no differences in the growth performance of piglets fed with or without antibiotics and P(OA + EO) or FA before the ETEC F4 (K88+) challenge. However, after the ETEC F4 (K88+) challenge, antibiotics and P(OA + EO) supplementation improved either the ratio of gain to feed or ADG of weaning piglets. Treatments P1, P2 and FA were all composed with the same antibiotics mix but were different from each other by the additive combination. As by comparison to treatment FA, treatment P1 achieved a higher G:F from d 7 to 14 and treatment P2 improved the ADG of piglets from d 14 to 21, we observed that combined with the same antibiotics mix, additive P(OA + EO) had a better ability to alleviate the negative effects on performance caused by an ETEC F4 (K88+) challenge than FA additive. Prior to the bacterial challenge, there was no indication of diarrhea or loose stools among any of the pigs. Compared with the no-challenge treatment, although treatment P1 had a higher fecal score on the first day after the ETEC F4 (K88+) challenge, all treatments had a low fecal score. Additionally, except for the first day of post–challenge, no difference was observed in the fecal score among dietary treatments. However, Lei et al. (2017) found a higher diarrhea incidence in 28-d weaning piglets challenged with 5 mL × 109 CFU/mL ETEC K88+ at d 8, 9 and 10 post-weaning, while Pan et al. (2017) observed increased diarrhea incidence in 21-d weaning piglets challenged with 10 mL × 109 CFU/mL ETEC K88+ at d 9 compared with unchallenged groups. Therefore, the inconsistent change of diarrhea in the current study could be explained by the low dose of ETEC F4 (K88+) and different ages of piglets, which caused low fecal score and pigs could recover from the ETEC F4 (K88+) challenge in a short time.
In response to an ETEC F4 (K88+) challenge, lower pro-inflammatory cytokine concentrations, like TNF-α, IL-6 and IL-10 were observed in treatment P2 in this study. A previous study found serum cytokines could be used as biomarkers of mucosal inflammation as they have high correlations, which could be used for intestinal disease assessment (Ljuca et al., 2010). The serum levels of TNF-α are associated with the gut inflammatory disease, and a previous study has found that EO (a mixture of carvacrol and thymol) could reduce mRNA expression of TNF-α in the intestine of weaned piglets and led to the improvement of gut health and growth performance due to reduced inflammation (Wei et al., 2017). It has been shown that the EO, thymol possess anti-inflammatory properties and lowered the production of IL-6 and TNF-α in lipopolysaccharide-induced mouse mammary epithelial cells (Huang and Lee, 2018). However, the studies on the effects of OA on the inflammation response of the weaned pigs after the ETEC challenge were very limited, while Li et al. (2008) did not observe a change of serum IL-6 after the oral ETEC challenge in pigs fed OA. Appropriate amounts of pro-inflammatory cytokines are important in order to alleviate inflammation as over-stimulation of the immune system would induce detrimental effects (Li et al., 2015). Therefore, the improved cytokine profile in serum induced by P2 treatment may indicate the alleviation of inflammatory and could contribute to an improvement in growth performance (Li et al., 2006).
Tight junction proteins such as occludin and claudins can bound to zonula occludens proteins, which are very important in preventing intestinal bacteria from crossing the epithelium by sealing the paracellular space between epithelial cells (Ulluwishewa et al., 2011; Suzuki, 2013). Many previous studies have shown that an ETEC infection would increase intestinal permeability through occludin dephosphorylation and ZO-1 redistribution, which negatively influenced the tight junction protein expression in pigs (Berkes et al., 2003; Roselli et al., 2007; Che et al., 2017). In the current study, we also observed lower occludin and ZO-1 protein expression in ETEC F4 (K88+) challenge group (NC) both in jejunum and ileum while the beneficial effects of treatment P1 on pig performance appeared to be achieved by an improvement of tight junction protein expression in ileum. Zou et al. (2016) found EO promoted intestinal barrier integrity probably through modulating intestinal bacteria and immune status in pigs. Some pro-inflammatory cytokines, such as TNF-α and IFN-γ, are known to cause increased permeability by inducing the endocytosis of the tight junction proteins (Capaldo and Nusrat, 2009). Likewise, the increased pro-inflammatory cytokines in the serum were observed in NC after the ETEC F4 (K88+) challenge, which could be linked with lower tight junction protein expression. Although few studies investigated the effects of OA or EO on gut barrier after an ETEC F4 (K88+) challenge in piglets, the present study demonstrated that P(OA + EO) exerted a better protective effect on inflammatory and barrier function after ETEC F4 (K88+) challenge than treatment FA.
Alpha diversity is defined as the diversity in a specific area or ecosystem in terms of species richness (Zhang et al., 2017). The Chao or observed species calculation is an estimator of phylotype richness and Shannon or Simpson index of diversity reflects both the richness and community evenness (Zhang et al., 2017). A decreased alpha diversity was shown in treatment P2, which could be explained by the antibacterial function of a high dose of EO and OA. However, the reduction in microbial richness and diversity is associated with the concepts of ecosystem instability and reduced resilience (Mes, 2008), which was also indicated by lower Lactobacillus in the cecum and colon in the FA and P2 treatments. However, only the treatment P1 resulted in a high abundance of Lactobacillus compared with other ETEC F4 (K88+) challenge groups and it could be due to an optimal dose of EO and OA supplementation. To explore the specific abundant bacterial taxa in each treatment, a LEfSe analysis of the gut microbiota was performed. A less abundant microbiota in upper intestine was observed, which was also indicated by a lower alpha diversity index in the ileum compared with the cecum and colon. NC still had abundant Escherichia-Shigella compared with other groups and this may be a consequence of ETEC F4 (K88+) inoculation, whilst abundant Firmicutes, Bacilli and Lactobacillus were found in the cecum and colon of the P1 treatment, which was corresponding with the improvement of tight junction protein of P1 treatment. A study showed that Bacilli could maintain intestinal homeostasis and host health by participating in the metabolism of dietary components, xenobiotics and drugs (Jandhyala et al., 2015; Rowland et al., 2018). Moreover, several in vivo studies indicated that EO and OA increased the Lactobacillus and decreased ETEC or total coliforms in the piglets (Castillo et al., 2006; Zeng et al., 2015; Wei et al., 2017). Ilinskyaya et al. (2017) also concluded that Bacilli and Lactobacillus both have an antimicrobial effect and could produce short-chain fatty acids to improve gut health, also could maintain gut barrier function through the stimulation of tight junction integrity and mucin production. In the present study, the main active OA ingredients in protected OA and EO additive and FA are different, however, their mode of action, which is to penetrate the bacterial cell wall and disrupt the normal physiology of some bacteria, is similar (Khan and Iqbal, 2015). Nevertheless, the micro-encapsulated OA and EO additive was aimed to address its combination effect in the distal part of the intestine to effectively modulate gut health, notably through action of EO changing the structure of bacteria cells and allowing OA to easily enter the membrane, leading to the death of some pathogenic bacteria (Omonijo et al., 2018). As a result, the synergistic effect of EO and OA reduced intestinal pathogens and enrich beneficial microbiota especially Bacilli and Lactobacillus. Lactobacillus can be used as probiotic bacteria, and it has been found effective in inhibiting the inflammatory response in the intestine by mediating inflammatory cytokines (Manichanh et al., 2012), and has been reported to improve intestinal barrier function by modulating the tight junction signaling (Anderson et al., 2010). Therefore, the improvement of microbiota composition may have played an important role in the mechanism of health promoting effects of treatment P1, achieved in the present study by modulating inflammatory status and intestinal tight junction protein, to eventually benefit on animal growth performance.
Many countries have banned or are banning the use of antibiotics in livestock feeds as a routine means of growth promotion whilst the withdrawal of antibiotics are associated with enteric infections. Enzymes, probiotics, oligosaccharides, OA and EO have been widely used as potential alternatives for animal growth promoters in feeds (Thacker, 2013; Omonijo et al., 2018) whereas the effects of OA and EO with use of antibiotics on the intestinal challenge of pigs were fewly studied. Compared with other antibiotics alternatives, the OA and EO combination have a variety of beneficial properties such as stimulation of enzyme secretion, antioxidant and antimicrobial activities, and therefore could effectively improve animal growth performance and reduce intestinal pathogen population (Zhai et al., 2018; Li et al., 2019). In the current study, protected OA and EO additive combined with antibiotics showed a better effect on gut health and animal growth of ETEC challenged piglets compared with FA also combined with the same antibiotics treatment. This results suggested that association of protected OA and EO and antibiotic could help to control the consequences of intestinal challenge in piglets.
5. Conclusions
In conclusion, P(OA + EO) additive at 2 g/kg combined with antibiotics improved the growth and had immunomodulatory effect on the piglet post ETEC F4 (K88+) challenge, while supplementation of P(OA + EO) additive at 1 g/kg combined with antibiotics could increase feed efficiency, protect gut barrier by increasing tight junction protein expression and increase Lactobacillus and Bacilli abundances after ETEC F4 (K88+) challenge. Compared to protected (micro-encapsulated) OA and EO additive, conventional free (unprotected) acidifiers combined with the same antibiotics showed a weaker effect on animal growth and gut health.
Author contributions
Yetong Xu: investigation, data curation, writing - original draft. Ludovic Lahaye: conceptualization, methodology, writing - review & editing. Zhengxiao He: investigation. Jinxiao Zhang: conceptualization, methodology. Chengbo Yang: conceptualization, methodology, writing - review & editing. Xiangshu Piao: conceptualization, methodology, writing - review & editing, Supervision.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The authors thank the grants from the National Natural Science Foundation of China (31772612), Beijing Municipal Natural Science Foundation (6202019) and the products from JEFO Technology (Jefo Nutrition Inc., Canada). We thank Shenfei Long, Qianqian Wang, Xiao Xu and Yang Wu for the assistance of feed production and sampling. We thank Zhikai Zeng for the assistance of data consultation.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Anderson R.C., Cookson A.L., McNabb W.C., Park Z., McCann M.J., Kelly W.J. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10(1):316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes J., Viswanathan V.K., Savkovic S.D., Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52(3):439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi P., Casini L., Finamore A., Cremokolini C., Merialdi G., Trevisi P. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. 2004;82:1764–1772. doi: 10.2527/2004.8261764x. [DOI] [PubMed] [Google Scholar]
- Brul S., Coote P. Preservative agents in foods Mode of action and microbial resistance mechanisms. Int J Food Microbiol. 1999;50:1–17. doi: 10.1016/s0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods–a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Capaldo C.T., Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M., Martín-Orúe S.M., Roca M., Manzanilla E.G., Badiola I., Perez J.F. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J Anim Sci. 2006;84(10):2725–2734. doi: 10.2527/jas.2004-556. [DOI] [PubMed] [Google Scholar]
- Che L., Xu Q., Wu C., Luo Y., Huang X., Zhang B. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br J Nutr. 2017;118(11):949–958. doi: 10.1017/S0007114517003051. [DOI] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.R., Wang Q., Fish J.A., Chai B., McGarrell D.M., Sun Y. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2013;42(D1):D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudelin J.F., Lessard M., Beaudoin F., Nadeau E., Bissonnette N., Boutin Y. Administration of probiotics influences F4 (K88+) enterotoxigenic Escherichia coli attachment and intestinal cytokine expression in weaned pigs. Vet Res. 2011;42:69. doi: 10.1186/1297-9716-42-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasqua R., Betts G., Hoskins N., Edwards M., Ercolini D., Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agr Food Chem. 2007;55(12):4863–4870. doi: 10.1021/jf0636465. [DOI] [PubMed] [Google Scholar]
- Fairbrother J.M., Nadeau É., Gyles C.L. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2007;6(1):17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- Fossum C. Cytokines as markers for infections and their effect on growth performance and well-being in the pig. Domest Anim Endocrinol. 1998;15:439–444. doi: 10.1016/s0739-7240(98)80001-5. [DOI] [PubMed] [Google Scholar]
- Guarner F., Malagelada J.R. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Guignot J., Chaplais C., Coconnier-Polter M.H., Servin A.L. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell Microbiol. 2007;9(1):204–221. doi: 10.1111/j.1462-5822.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- Guttman J.A., Li Y., Wickham M.E., Deng W., Vogl A.W., Finlay B.B. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell Microbiol. 2006;8(4):634–645. doi: 10.1111/j.1462-5822.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- Huang C.M., Lee T.T. Immunomodulatory effects of phytogenics in chickens and pigs - a review. Asian-Australas J Anim Sci. 2018;31(5):617–627. doi: 10.5713/ajas.17.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinskaya O.N., Ulyanova V.V., Yarullina D.R., Gataullin I.G. Secretome of intestinal Bacilli: a natural guard against pathologies. Front Microbiol. 2017;8:1666. doi: 10.3389/fmicb.2017.01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar R.D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.S., Ko Y.H., Yang H.Y., Ha J.S., Kim J.Y., Kim J.Y. Influence of essential oil components on growth performance and the functional activity of the pancreas and small intestine in broiler chickens. Asian-Australas J Anim Sci. 2004;17(3):394–400. [Google Scholar]
- Jin D., Zhao S., Wang P., Zheng N., Bu D., Beckers Y. Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front Microbiol. 2016;7:1006. doi: 10.3389/fmicb.2016.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10(10):813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J Appl Anim Res. 2015;44(1):359–369. [Google Scholar]
- Kim J.C., Hansen C.F., Mullan B.P., Pluske J.R. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 2012;173(1):3–16. [Google Scholar]
- Lei X.J., Park J.H., Baek D.H., Kim J.K., Kim I.C.H. Feeding the blend of organic acids and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim Feed Sci Technol. 2017;224:45–51. [Google Scholar]
- Li J., Li D.F., Xing J.J., Cheng Z.B., Lai C.H. Effects of β-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide1. J Anim Sci. 2006;84:2374–2381. doi: 10.2527/jas.2004-541. [DOI] [PubMed] [Google Scholar]
- Li Z.J., Yi G.Y., Yin J.D., Sun P., Li D.F., Knight C. Effects of organic acids on growth performance, gastrointestinal pH, intestinal microbial populations and immune responses of weaned Pigs. Asian-Aust J Anim Sci. 2008;21:252–261. [Google Scholar]
- Li H., Zhao P., Lei Y., Li T., Kim I. Response to an Escherichia coli K88 oral challenge and productivity of weanling pigs receiving a dietary nucleotides supplement. J Anim Sci Biotechnol. 2015;6:49. doi: 10.1186/s40104-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Long S., Wang Q., Zhang L., Hu J., Yang J. Mixed organic acids improve nutrients digestibility, volatile fatty acids composition and intestinal microbiota in growing-finishing pigs fed high-fiber diet. Asian-Australas J Anim Sci. 2019;32(6):856–864. doi: 10.5713/ajas.18.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljuca F., Gegic A., Salkic N.N., Pavlovic-Calic N. Circulating cytokines reflect mucosal inflammatory status in patients with Crohn's disease. Dig Dis Sci. 2010;55(8):2316–2326. doi: 10.1007/s10620-009-1016-9. [DOI] [PubMed] [Google Scholar]
- Lückstädt C. Conference on International Research on Food Security, Natural Resource Management and Rural Development organized by the Czech University of Life Sciences Prague. 2014 Sept. Effects of dietary potassium diformate on growth and gastrointestinal health in weaned piglets in Vietnam; pp. 17–19. [Google Scholar]
- Mahnert A., Moissl-Eichinger C., Berg G. Microbiome interplay: plants alter microbial abundance and diversity within the built environment. Front Microbiol. 2015;6:887. doi: 10.3389/fmicb.2015.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichanh C., Borruel N., Casellas F., Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- Mes T.H.M. Microbial diversity – insights from population genetics. Environ Microbiol. 2008;10:251–264. doi: 10.1111/j.1462-2920.2007.01449.x. [DOI] [PubMed] [Google Scholar]
- Omonijo F.A., Ni L., Gong J., Wang Q., Lahaye L., Yang C. Essential oils as alternatives to antibiotics in swine production. Anim Nutr. 2018;4(2):126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand A.C., Kettunen K.T., Peuranen H.S., Schulze H., Rautonen N. In vitro effects of essential oils on potential pathogens and beneficial members of the normal microbiota. Vet Med. 2010;55:71–78. [Google Scholar]
- Pan L., Zhao P.F., Ma X.K., Shang Q.H., Xu Y.T., Long S.F. Probiotic supplementation protects weaned pigs against enterotoxigenic K88 challenge and improves performance similar to antibiotics. J Anim Sci. 2017;95(6):2627–2639. doi: 10.2527/jas.2016.1243. [DOI] [PubMed] [Google Scholar]
- Pierce K.M., Callan J.J., McCarthy P., O'Doherty J.V. Performance of weanling pigs offered low or high lactose diets supplemented with avilamycin or inulin. Anim Sci. 2005;80(3):313–318. [Google Scholar]
- Roselli M., Finamore A., Britti M.S., Konstantinov S.R., Smidt H., de Vos W.M. The novel porcine Lactobacillus sobrius strain protects intestinal cells from Enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J Nutr. 2007;137(12):2709–2716. doi: 10.1093/jn/137.12.2709. [DOI] [PubMed] [Google Scholar]
- Rowland I., Glenn G., Heinken A., Scott K., Swann J., Thiele I. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker P.A. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol. 2013;4(35):1–12. doi: 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141(5):769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Verdonck F., Tiels P., van Gog K., Goddeeris B.M., Lycke N., Clements J. Mucosal immunization of piglets with purified F18 fimbriae does not protect against F18+ Escherichia coli infection. Vet Immunol Immunopathol. 2007;120(3):69–79. doi: 10.1016/j.vetimm.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Wei H.K., Xue H.X., Zhou Z.X., Peng J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal. 2017;11(2):193–201. doi: 10.1017/S1751731116001397. [DOI] [PubMed] [Google Scholar]
- Xu Y.T., Liu L., Long S.F., Pan L., Piao X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim Feed Sci Technol. 2018;235:110–119. [Google Scholar]
- Yang C., Zhang L., Cao G., Feng J., Yue M., Xu Y. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J Anim Sci. 2019;97(1):133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.K., Xu X.T., Zhang Q., Li P., Zhao P.F., Li Q. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim Sci J. 2015;86(3):279–285. doi: 10.1111/asj.12277. [DOI] [PubMed] [Google Scholar]
- Zhai H., Liu H., Wang S., Wu J., Kluenter A.M. Potential of essential oils for poultry and pigs. Anim Nutr. 2018;4(2):179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Chen Y., Xiang L., Wang Z., Xiao G.G., Hu J. Effect of Curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients. 2017;9(10):1146. doi: 10.3390/nu9101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Xiang Q., Wang J., Peng J., Wei H. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. BioMed Res Int. 2016;2016 doi: 10.1155/2016/5436738. 5436738. [DOI] [PMC free article] [PubMed] [Google Scholar]