Abstract
We investigated the effects of dietary supplementation with Bacillus subtilis PB6 (B. subtilis PB6) during late gestation and lactation on sow reproductive performance, antioxidant indices, and gut microbiota. A total of 32 healthy Landrace × Yorkshire sows on d 90 of gestation were randomly assigned to 2 groups, with 16 replicates per group, receiving basal diet (CON) or the basal diet + 0.2% B. subtilis PB6, containing 4.0 × 108 CFU/kg of feed (BS). The litter sizes (total born) and numbers of piglets born alive were larger in the BS group (P < 0.01), whereas the weights of piglets born alive and the piglet birth intervals were lower in the BS group (P < 0.05). Although the litter weights and piglet bodyweights (after cross-fostering) were lower after BS treatment (P < 0.05), the litter sizes, litter weights, lactation survival rate, and litter weight gains at weaning were higher in BS group (P < 0.05). The concentrations of malondialdehyde (MDA) in the sow sera at parturition were lower in the BS group (P < 0.01). The serum total antioxidant capacity (T-AOC) at parturition and the serum catalase (CAT) concentrations on d 21 of lactation were higher in the BS group (P < 0.05). Dietary supplementation with B. subtilis PB6 (P < 0.05) reduced the serum endotoxin concentrations in the sows and the serum cortisol concentrations of the piglets at d 14 of lactation. The α-diversity indices of microbial were higher in the CON group (P < 0.05). At the phylum level, B. subtilis PB6 supplementation increased the relative abundances of Gemmatimonadete and Acidobacteria (both P < 0.01) and reduced those of Proteobacteria, and Actinobacteria (both P < 0.05). At the genus level, B. subtilis PB6 supplementation increased the relative abundance of Ruminococcaceae_UCG-013 cc (P < 0.05) and reduced that of Streptococcus (P < 0.05). This study demonstrated that adding 4.0 × 108 CFU/kg B. subtilis PB6 to sows’ feed during late gestation and lactation could shorten piglet birth intervals, enhance the growth performance of suckling piglets, and improve the gut health of sows during late gestation.
Keywords: Bacillus subtilis PB6, Reproductive performance, Gut microbiota, Sow
1. Introduction
During late gestation and lactation, sows undergo stress from environmental and physical changes, including restricted feeding, housing changes and infections, and so forth (Kranendonk et al., 2007; Oliviero et al., 2010). The internal balance of the body is also broken, such as proinflammatory cytokines increase and anti-inflammatory cytokines decline during late gestation (Cheng et al., 2018). Furthermore, intestinal balance changes due to reduced intestinal bacterial diversity and increased inflammatory bacteria as the gestational age of the sows increases (Kong et al., 2017). The stress that sows experience changes dramatically during pregnancy and lactation, and these changes are harmful to the health of the sow. Even a long period of farrowing could reduce the productive performance of sows (Olivier et al., 2013), whereas nutrient absorption and metabolism during gestation and lactation may affect the weights of piglet at birth and weaning (Kranendonk et al., 2007). However, recent studies have indicated that the health and productivity of sows were improved when Bacillus subtilis was added to their feed during gestation and lactation.
B. subtilis is used as a growth promoter, enhancing sow reproductive performance and improving the viability of their progeny. Sow (from d 90 of gestation until postpartum d 21) fed Bacillius-based direct-fed (3.75 × 108 CFU/kg of feed) diets had more piglets and greater weaning weights of piglets (Baker et al., 2013). Hayakawa et al. (2016) demonstrated that compound probiotics containing a Bacillius mesentericus strain (2.0 × 108 CFU/kg of feed) improved the reproductive performance of sows (farrowing) and growth performance of piglets (weaning). However, Rychen et al. (2017) reported that adding B. subtilis PB6 (1.0 × 108 CFU/kg of feed) caused no improvement in the productive performance of sows when adding only 3 weeks before parturition.
B. subtilis PB6 used in this study was a natural strain isolated from the intestines of healthy chickens. It produces antimicrobial substances with broad activity against various strains of Clostridium sp. in necrotic enteritis in poultry and Campylobacter sp. in vitro (Teo and Tan, 2005), and also secretes substances that promote the growth of Lactobacillus. Besides, surfactin produced by B. subtilis PB6 is a cyclic lipopeptide antibiotic and biosurfactant, which has hemolytic, antibacterial properties (Heerklotz and Seelig, 2001; Jayaraman et al., 2013). B. subtilis PB6 has been used in broiler chickens and laying hens, and has improved intestinal health and eggshell quality respectively (Abdelqader et al., 2013; Jayaraman et al., 2013). Based on the effects of B. subtilis in various animals and the obvious effects of B. subtilis PB6 in broiler chickens and laying hens, we undertook to verify the effects of B. subtilis PB6 on sows.
The purpose of this study was to investigate the effects of B. subtilis PB6 supplementation during late gestation and lactation on the reproductive performance, antioxidation indices, and intestinal microbial composition on sows.
2. Materials and methods
The protocol of this study was approved by the Animal Care and Use Committee of Animal Nutrition Institute, Sichuan Agricultural University, and the study was performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
2.1. Experimental design and animals
This experiment was performed at a commercial pig farm in Sichuan Province, China. A total of 32 mixed-parity Landrace × Yorkshire sows with parity of 2.47 ± 0.50 (mean ± SD) and backfat (BF) thickness of 14.72 ± 1.30 mm, which were bred with the semen of a pool of Landrace boars, were selected. On d 90 of gestation, the sows were randomly assigned to 1 of 2 groups according to their parity and BF, with 16 replicates per group. The dietary treatments included a basal gestation and lactation diet (CON; Table 1) and the same basal diet supplemented with 0.2% B. subtilis PB6 (BS; containing B. subtilis 4.0 × 108 CFU/kg of feed; Table 1) from d 90 of gestation to weaning on d 21 of lactation. The B. subtilis PB6 strain was provided by Kemin Industries, Kemin (Zhuhai, China) Technologies Co., Ltd, and contained a B. subtilis concentration of 2.0 × 108 CFU/g of product. It had been isolated from the intestines of healthy chickens and was shown to inhibit Clostridium perfringens. All the sows were fed 2.80 kg of the experimental diet from d 90 of gestation to parturition. During gestation, all the sows were housed in individual stalls and fed the gestation-period diet twice a day (08:00 and 15:00), with access to water ad libitum throughout the study. On d 110 of gestation, the sows were moved to the farrowing room. The day of parturition was defined as d 0 of lactation, and the piglets were weaned on d 21 of lactation.
Table 1.
Ingredients and chemical compositions of basal diets (as-fed basis, %).
| Item | Gestation | Lactation |
|---|---|---|
| Ingredients | ||
| Yellow corn | 33.58 | 40.08 |
| Wheat | 20 | 28 |
| Soybean meal (43% CP) | 14.5 | 18.2 |
| Fish meal (67% CP) | 0 | 2 |
| Expanded soybean | 0 | 5 |
| Wheat bran | 8 | 0 |
| Soybean hulls | 18 | 0 |
| l-Lys HCl (98%) | 0.03 | 0.28 |
| l-Thr (98.5%) | 0 | 0.1 |
| dl-Met (99%) | 0 | 0.03 |
| Limestone | 1.4 | 1.3 |
| Dicalcium phosphate | 1.2 | 1.2 |
| Choline chloride (50%) | 0.15 | 0.15 |
| Sodium chloride | 0.4 | 0.4 |
| Vitamin-mineral premix1 | 2.74 | 3.26 |
| Total | 100 | 100 |
| Nutrient composition | ||
| Digestible energy, MJ/kg | 11.92 | 13.39 |
| Crude protein | 15.03 | 18.76 |
| Crude fiber | 8.75 | 2.48 |
| Calcium | 1.09 | 1.08 |
| Total phosphorus | 0.63 | 0.69 |
| Available phosphorus | 0.38 | 0.43 |
| Total lysine | 0.73 | 1.11 |
The vitamin-mineral premix provided the following per kilogram of basal diet: 8,000 IU vitamin A, 2,000 IU vitamin D3, 12.5 IU vitamin E, 2.5 mg vitamin K, 0.2 mg biotin, 0.25 mg folic acid, 17.5 mg niacin, 12.5 mg pantothenic acid, 8.0 mg riboflavin, 1.0 mg thiamin, 3.00 mg vitamin B6, 15 μg vitamin B12, 16 mg copper, 0.3 mg iodine, 165 mg iron, 30 mg manganese, 0.3 mg selenium, and 165 mg zinc. The sources of the trace elements were CuSO4·5H2O, KI, FeSO4, MnSO4·H2O, Na2SeO3, and ZnSO4.
At farrowing, the numbers of piglets born alive, stillborn, and mummified and the birthweights of the piglets born alive were recorded. Based on the number of effective teats on the sows, the litters were standardized to approximately 12 piglets per sow within 24 h after birth by cross-fostering within the treatment group. The piglets were weighed after the standardization of the litters and at weaning, and underwent routine processing procedures (ear notching, tail docking, castration, and supplemental iron injection) within 3 d of farrowing. The feed allowance was progressively increased stepwise by 1.0 kg/d from 1.0 kg on d 0 of lactation to their maximum feed intake, and then the sows were allowed free access to feed until d 21 of lactation (weaning). The feed allocation and refusals were recorded daily. The sows were fed the lactation period diet 4 times a day (at 08:00, 11:00, 15:00, and 20:00) during lactation. The piglets also had free access to water during the lactation, but had no access to creep feed. The temperature of the environment in the farrowing house was maintained at 20 to 25 °C. The temperature of the insulation boards was maintained at 30 to 32 °C, and was reduced as the neonatal piglet age increased.
2.2. Sample collection
The BF thickness was measured at 65 mm to the left side of the dorsal midline at the last rib level, using ultrasound (Renco Lean-Meatier; Renco Corp., Minneapolis, MN) and recorded on d 89 of gestation and d 1 and 21 of lactation. The total litter sizes were calculated as the sum of the numbers of live-born piglets, stillborn piglets, and mummified piglets. At farrowing, the birth times of the first and last piglets (born alive, stillborn, or mummified) were recorded, and the difference was defined as the duration of farrowing. Piglet birth interval was calculated as the duration of farrowing divided by the total litter size. Fasting blood samples (10 mL) were collected from the sows via the marginal ear vein at farrowing (d 0 of lactation) and on d 14 and 21 of lactation, before the morning meal. Blood samples were collected from the piglets via the anterior vena cava at 14 and 21 d of age. All blood samples were collected into vacuum tubes (5 mL; Jiangsu Yu Li Medical Instrument Co., Ltd, Jiangsu, China). The samples were immediately placed on ice and then centrifuged at 3,000 × g for 10 min at room temperature. The serum was stored at −20 °C.
Colostrum samples (30 mL) were collected from each sow before any piglets had sucked, and milk samples (30 mL) were obtained from each sow on d 14 of lactation. Briefly, the piglets were separated from their dams and the udders were cleaned with water, and then 2 mL of oxytocin was injected into the ear vein of each sow. Each sample was a mixture of milk from the anterior, middle, and posterior functional glands and was collected by hand milking. Six samples were collected in each treatment group. The colostrum and milk samples were centrifuged at 3,000 × g for 15 min at room temperature. All samples were refrigerated at −20 °C before subsequent analysis.
Fresh feces samples from the sows were collected into sterile tubes. Five samples were collected in each treatment group, and immediately frozen in liquid nitrogen, then transferred to a freezer at −80 °C on d 110 of gestation.
2.3. Milk composition analysis
The frozen colostrum and milk samples were thawed at 4 °C, and 15 mL of each sample were used for analyzing the milk fat, protein, and lactose content with an ultrasonic milk analyzer (Milkyway-CP2; Hangzhou Simple Technology Co., Ltd, Hangzhou, China).
2.4. Oxidant and antioxidant content analyses
The content of malondialdehyde (MDA), the total antioxidant capacity (T-AOC), and the activities of glutathione peroxidase (GSH-Px) and catalase (CAT) were assessed in the sera of sows with specific assay kits (Catalog, A003-1-2, A015-2-1, A006-2-1, A007-1-1; Nanjing Institute of Jiancheng Biological Engineering, Nanjing, China). MDA was quantified with thiobarbituric acid reactive substances (TBARS). T-AOC and the CAT and GSH-Px activities were measured according to a previous study (Wang et al., 2016; Mou et al., 2017).
2.5. Cortisol and endotoxin assays
The endotoxin concentrations in the sow sera and the cortisol concentrations in the piglet sera were determined with respective commercial ELISA kits (Catalog, NO.H094, H255; Nanjing Institute of Jiancheng Biological Engineering). The limits for the determination of the cortisol and endotoxin concentrations were 5.0 ng/mL and 3 EU/mL, respectively. The intra- and inter-assay coefficients of variation were all <10% and <12% for cortisol and endotoxin assays, respectively.
2.6. Microbial analyses
The total bacterial DNA in each fecal sample from the CON (n = 5) and BS groups (n = 5) was extracted on d 110 of gestation with the MO BIO Power Fecal DNA Isolation Kit (MO BIO Laboratories, Inc.) according to the manufacturer's protocol. Before sequencing, the concentration and purity of the extracted genomic DNA were measured. The integrity of the extracted genomic DNA was determined by electrophoresis on a 1% (wt/vol) agarose gel. The DNA was diluted to 1 ng/μL with sterile water. The extracted fecal DNA samples were sent to Novogene Bioinformatics Technology (Beijing, China) for amplicon pyrosequencing on the Illumina HiSeq PE250 platforms. The V4 hypervariable region of the 16S rRNA gene was amplified with the 515F and 806R primers (5′-GTGCCAGCMGCCGCGGTAA-3′ and 5′-GGACTACHVGGGTWTCTAAT-3′). The raw paired-end reads obtained with Illumina HiSeq sequencing were spliced. The spliced sequences were called “raw tags.” The raw tags were quality filtered under specific filtering conditions to obtain high-quality clean tags (Bergmark et al., 2012), according to the QIIME (V1.7.0, http://qiime.org/index.html; Caporaso et al., 2010) quality-controlled process. Chimeric filtering was then performed to obtain the effective tags (Fig. 1A). The effective tags were assigned to operational taxonomic units (OTU) using the Uparse software (v7.0.1001 http://drive5.com/uparse/) with 97% sequence similarity. A representative sequence of each OTU was screened for further annotation. The Ribosomal Database Project Classifier version 2.2 used to assign a taxonomic rank to each representative sequence. OTU abundance information was normalized with a standard sequence number corresponding to the sample with the least sequences. Subsequent analysis of α-diversity and β-diversity was based on these normalized output data. The relative abundance of each OTU was examined at different taxonomic levels. At the phylum level, because the sum of the 10 phyla with the greatest relative abundances exceeded 98%, we selected these top 10 phyla for statistical analysis, using the CON group as a reference. At the genus level, we selected those genera with relative abundances ≥0.1% in any samples for statistical analysis.
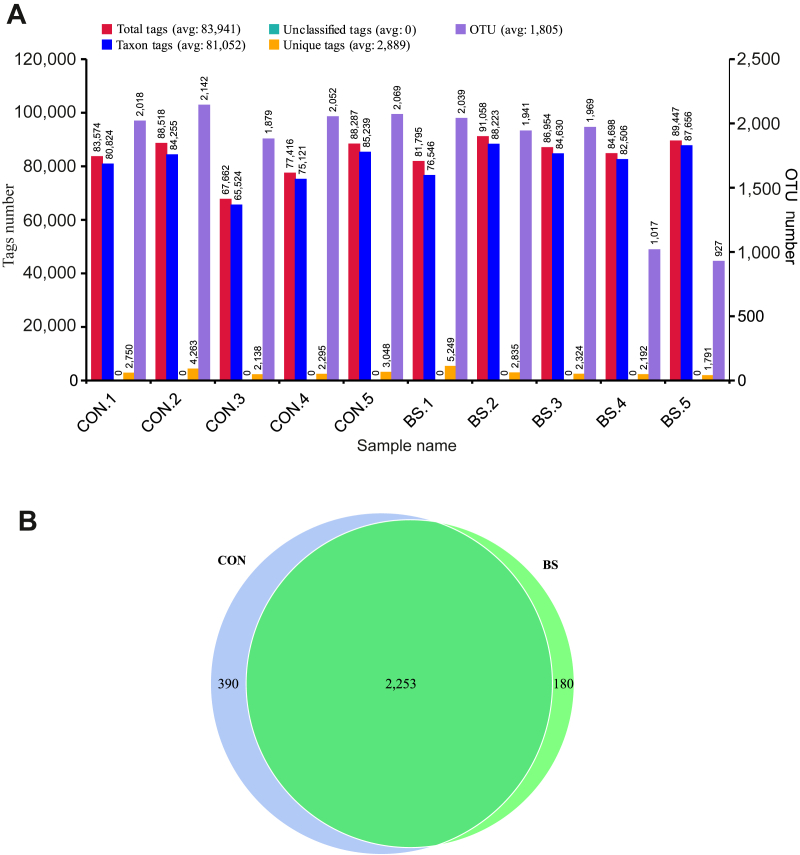
Fig. 1.
Effect of Bacillus subtilis PB6 supplementation of sows on the fecal microbiota on d 110 of gestation. (A) operational taxonomic units (OTU) clustering and annotation per sample; (B) Venn diagram of OTU. CON, basal diet treatment during gestation; BS, basal diet + 0.2% B. subtilis PB6 treatment during gestation.
2.7. Statistical analysis
The original data were checked with Grubbs’ test. If |Xp - X| > λ (α, n) S, Xp was considered an outlier. The data were tested for homogeneity of variance and a normal distribution with the Shapiro–Wilk method in SAS 9.4 (SAS Institute Inc., Cary, NC) before the parametric analyses. Statistical analyses were performed with the t-test procedure in SAS 9.4. Data on the relative abundances of the gut microbiota were analyzed with the Glimmix procedure in SAS 9.4. Differences between means in all statistical analyses were considered statistically significant at P < 0.05, and tended to be significant at 0.05 ≤ t, P < 0.10.
3. Results
3.1. Reproductive performance of sows at farrowing
The effects of B. subtilis PB6 on the reproductive performance of the sows are presented in Table 2. Litter sizes (total born) and numbers of piglets born alive were highly significant greater in the BS group than those in the CON group (P < 0.01), whereas the weight of per piglet born alive (P < 0.01) and the piglet birth interval (P = 0.022) were lower in the BS group than those in the CON group. The duration of farrowing tended to be shorter after B. subtilis PB6 supplementation (P = 0.092).
Table 2.
Reproductive performance of sows at farrowing1.
| Item | CON2 | BS2 | P-value |
|---|---|---|---|
| Parity | 2.50 ± 0.13 | 2.44 ± 0.13 | 0.734 |
| Litter size (total born) | 13.13 ± 0.53 | 14.94 ± 0.36 | <0.01 |
| Number of piglets born alive | 11.63 ± 0.64 | 14.00 ± 0.32 | <0.01 |
| Born alive rate, % | 88.29 ± 2.89 | 93.97 ± 1.66 | 0.098 |
| Number of stillborn piglets | 0.81 ± 0.29 | 0.63 ± 0.21 | 0.601 |
| Stillborn rate, % | 6.17 ± 8.75 | 4.04 ± 5.13 | 0.409 |
| Number of mummies | 0.69 ± 0.22 | 0.31 ± 0.12 | 0.142 |
| Mummies rate, % | 5.57 ± 1.92 | 2.01 ± 0.78 | 0.101 |
| Litter weight at parturition, kg | 18.16 ± 1.12 | 19.00 ± 0.74 | 0.539 |
| Born alive weight per piglet, kg | 1.56 ± 0.05 | 1.35 ± 0.04 | <0.01 |
| Duration of farrowing, min | 255.60 ± 18.98 | 218.81 ± 8.54 | 0.092 |
| Piglet birth interval, min | 21.65 ± 2.55 | 15.03 ± 0.58 | 0.022 |
Values are means ± SEM.
CON, basal diet treatment; BS, basal diet + 0.2% B. subtilis PB6 treatment.
3.2. Reproductive performance of sows during lactation
As shown in Table 3, whereas litter weights (P = 0.035) and piglet bodyweights (P = 0.026) by cross-fostering were lower in the BS group than those in the CON group (litters were standardized to approximately 12 piglets per sow by cross-fostering within the treatment groups), the litter sizes, litter weights, lactation survival rate, and litter weight gains at weaning were significantly increased by supplementation with B. subtilis PB6 (P < 0.05).
Table 3.
Growth performance of suckling piglets, feed intake, backfat (BF) thickness, and BF loss in sows during lactation1.
| Item | CON2 | BS2 | P-value |
|---|---|---|---|
| Litter size by cross-fostering | 12.31 ± 0.33 | 12.31 ± 0.22 | 1.000 |
| Litter size at weaning | 9.56 ± 0.39 | 10.88 ± 0.20 | <0.01 |
| Lactation survival rate, % | 78.13 ± 3.12 | 88.63 ± 1.99 | <0.01 |
| Litter weight by cross-fostering, kg | 19.03 ± 0.75 | 17.09 ± 0.43 | 0.035 |
| Litter weight at weaning, kg | 57.92 ± 4.07 | 69.04 ± 1.48 | 0.019 |
| Litter weight gain, kg | 38.89 ± 4.17 | 51.95 ± 1.68 | <0.01 |
| Piglet body weight by cross-fostering, kg | 1.55 ± 0.05 | 1.40 ± 0.04 | 0.026 |
| Piglet body weight at weaning, kg | 6.03 ± 0.30 | 6.37 ± 0.15 | 0.331 |
| ADG, g/d | 213.6 ± 15 | 236.8 ± 6.9 | 0.174 |
| Feed intake during lactation, kg/d | 5.77 ± 0.15 | 5.76 ± 0.10 | 0.956 |
| BF thickness at d 89, mm | 15.32 ± 0.37 | 14.63 ± 0.26 | 0.139 |
| BF thickness at farrowing, mm | 16.06 ± 0.47 | 15.44 ± 0.22 | 0.242 |
| BF thickness at weaning, mm | 13.13 ± 0.50 | 12.50 ± 0.20 | 0.260 |
| BF loss during lactation, mm | 2.94 ± 0.23 | 2.94 ± 0.19 | 1.000 |
Values are means ± SEM.
CON, basal diet treatment; BS, basal diet + 0.2% B. subtilis PB6 treatment.
3.3. Composition of colostrum and milk
As shown in Table 4, the fat content of the colostrum tended to be higher (P = 0.090) after B. subtilis PB6 supplementation, whereas the lactose and protein content of the milk did not differ between the 2 groups (both P > 0.05).
Table 4.
Effect of Bacillus subtilis PB6 supplementation during gestation and lactation on compositions of sows’ colostrum and milk1.
| Item | CON2 | BS2 | P-value |
|---|---|---|---|
| Colostrum, g/kg | |||
| Fat | 50.93 ± 2.43 | 66.15 ± 7.15 | 0.090 |
| Protein | 96.58 ± 5.63 | 95.50 ± 5.71 | 0.895 |
| Lactose | 34.98 ± 3.89 | 34.52 ± 3.71 | 0.933 |
| Milk, g/kg | |||
| Fat | 62.83 ± 7.39 | 70.28 ± 6.89 | 0.478 |
| Protein | 53.30 ± 1.76 | 53.07 ± 1.66 | 0.925 |
| Lactose | 49.00 ± 2.82 | 46.72 ± 2.91 | 0.544 |
Values are means ± SEM, n = 6 per treatment.
CON, basal diet treatment; BS, basal diet + 0.2% B. subtilis PB6 treatment.
3.4. Oxidative and antioxidative indicators in the sera of sows
As shown in Table 5, the MDA concentrations of the sow sera were highly significant lower in the BS group than those in the CON group at parturition (P = 0.004).
Table 5.
Effect of Bacillus subtilis PB6 supplementation during late gestation and lactation on MDA concentrations in sera of sows (nmol/mL)1.
| Item | CON2 | BS2 | P-value |
|---|---|---|---|
| At parturition | 7.16 ± 0.77 | 4.07 ± 0.27 | 0.004 |
| Day 14 of lactation | 6.05 ± 0.62 | 4.72 ± 0.50 | 0.128 |
| Day 21 of lactation | 4.66 ± 0.46 | 4.94 ± 0.60 | 0.721 |
Values are means ± SEM, n = 6 per treatment.
CON, basal diet treatment; BS, basal diet + 0.2% B. subtilis PB6 treatment.
As shown in Table 6, T-AOC of the sow sera at parturition (P = 0.044) and the CAT activities in the sow serum on d 21 of lactation (P = 0.014) were higher in the BS group than those in the CON group. The activity of GSH-Px did not differ between the 2 groups (P > 0.05).
Table 6.
Effect of Bacillus subtilis PB6 supplementation during late gestation and lactation on the antioxidant capacity in the sera of sows1.
| Item | CON2 | BS2 | P-value |
|---|---|---|---|
| T-AOC, active U/mL | |||
| At parturition | 8.65 ± 1.51 | 15.68 ± 2.65 | 0.044 |
| Day 14 of lactation | 10.16 ± 1.55 | 15.39 ± 4.29 | 0.292 |
| Day 21 of lactation | 7.08 ± 1.93 | 10.92 ± 2.38 | 0.238 |
| CAT, active U/mL | |||
| At parturition | 1.95 ± 0.31 | 2.24 ± 0.28 | 0.509 |
| Day 14 of lactation | 2.02 ± 0.29 | 1.96 ± 0.31 | 0.884 |
| Day 21 of lactation | 1.27 ± 0.18 | 2.36 ± 0.31 | 0.014 |
| GSH-Px, active U/mL | |||
| At parturition | 763.67 ± 32.89 | 780.17 ± 49.47 | 0.787 |
| Day 14 of lactation | 1,000.17 ± 33.15 | 924.33 ± 38.68 | 0.168 |
| Day 21 of lactation | 1,003.17 ± 50.00 | 1,024.33 ± 40.81 | 0.750 |
Values are means ± SEM, n = 6 per treatment.
CON, basal diet treatment; BS, basal diet + 0.2% B. subtilis PB6 treatment.
3.5. Endotoxin and cortisol in the sera of sows and piglets
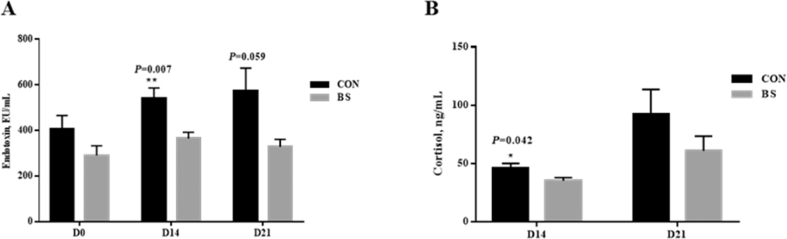
As shown in Fig. 2A, the endotoxin concentrations in the sow sera on d 14 of lactation were highly significant lower in the BS group than those in the CON group (P = 0.007), and the endotoxin concentrations in the sow sera on d 21 of lactation tended to be lower in the BS group (P = 0.059). In Fig. 2B, the cortisol concentrations in the piglet sera were significantly lower at 14 d of age in the BS group than those in the CON group (P = 0.042).
Fig. 2.
Effect of Bacillus subtilis PB6 supplementation during late gestation and lactation on endotoxin concentrations in the sera of sows and the cortisol concentrations in the sera of piglets. (A) Endotoxin concentrations in sow serum. (B) Cortisol concentrations in piglet sera. CON, basal diet treatment during lactation; BS, basal diet + 0.2% B. subtilis PB6 treatment during lactation. D 0 = at parturition; D 14 = d 14 of lactation; D 21 = d 21 of lactation. Data are expressed as means ± SEM; n = 6 for each treatment. ∗, P < 0.05; ∗∗, P < 0.01.
3.6. Fecal microbiota
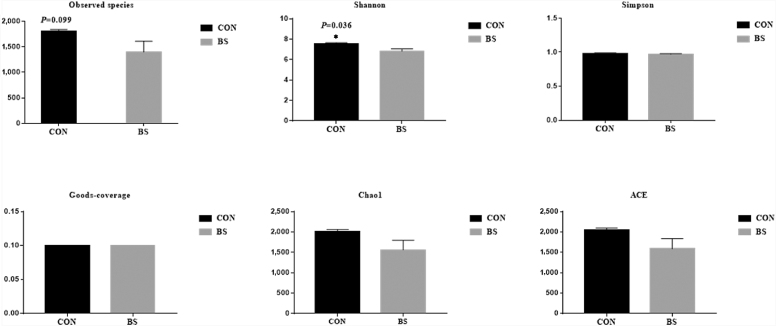
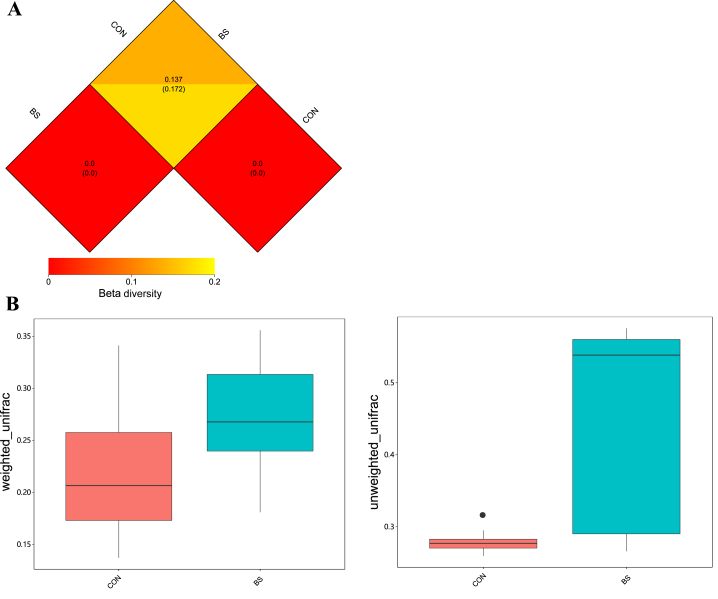
As shown in Fig. 1A, a total of 839,409 effective tags were obtained from all the feces samples, ranging from 67,662 to 91,058 per sample. In total, 810,524 OTU (at the 97% identity level) were detected in all the samples, with an average of 1,805.3 ± 445.6 per sample. A Venn diagram was used for evaluating the distributions of the OTU in the 2 groups. Based on this analysis, a total of 2,253 OTU were shown in both groups (Fig. 1B). The α-diversity and β-diversity of a microbial community reflected its richness and diversity, respectively. The α-diversity indices investigated were the numbers of Observed species, Shannon's index, and the abundance-based coverage estimator (ACE) (Fig. 3). The magnitude of the change in β-diversity was compared with weighted and unweighted UniFrac distances (Fig. 4) (Lozupone et al., 2006).
Fig. 3.
Effect of Bacillus subtilis PB6 supplementation of sows on the α-diversity of their gut microbial communities on d 110 of gestation. CON, basal diet treatment during gestation; BS, basal diet + 0.2% B. subtilis PB6 treatment during gestation. Data are expressed as means ± SEM; n = 5 for each treatment. ∗, P < 0.05.
Fig. 4.
Effect of Bacillus subtilis PB6 supplementation of sows on the β-diversity of their gut microbial communities on d 110 of gestation. (A) Distance matrix heatmap of β-diversity. Upper and lower numbers in the grid represent the weighted UniFrac and unweighted UniFrac distances, respectively. (B) The weighted UniFrac and unweighted UniFrac distances in β-diversity in the 2 groups. Data are expressed as means ± SEM; n = 5 for each treatment. CON, the basal diet treatment during gestation; BS, the basal + 0.2% B. subtilis PB6 treatment during gestation. The dot on the bar of the control in Fig. 4B (right) represents a outlier in control group.
As shown in Fig. 3, Shannon's index was lower in the BS group than in the CON group (P = 0.036). The number of Observed species also tended to be higher in the CON group than in the BS group (P = 0.099).
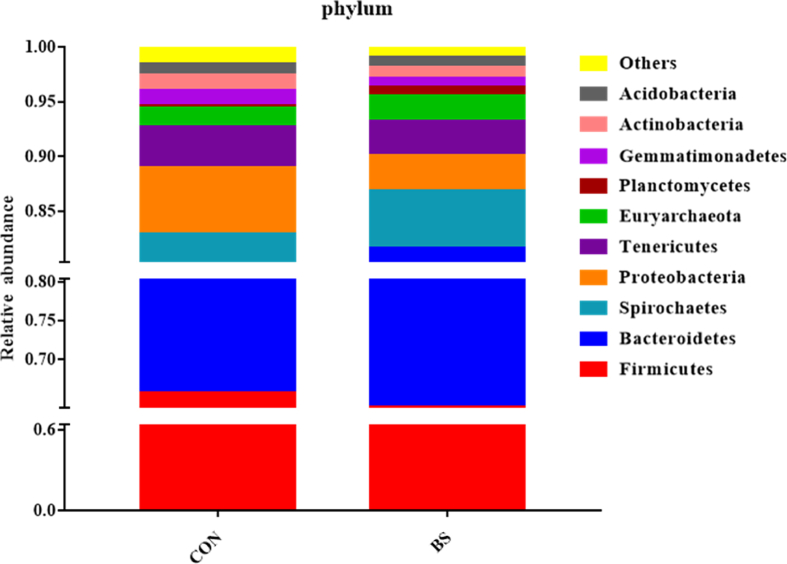
The relative abundances of the 10 most abundant phyla are shown in Fig. 5. These results suggested that at the phylum level, the major proportion of sequences attributable to the phyla were Firmicutes (64.74%) and Bacteroidetes (16.22%), followed by Proteobacteria (4.64%), Spirochaetes (3.89%), and Tenericutes (3.41%). The relative abundances of Gemmatimonadete (P = 0.001) and Acidobacteria (P = 0.003) were significantly increased in the BS group, whereas those of Proteobacteria (P = 0.017) and Actinobacteria (P = 0.004) were significantly reduced. At the genus level, the relative abundances of 32 genera were ≥0.1% in any samples. B. subtilis PB6 increased the relative abundance of Ruminococcaceae_UCG-013 cc (P = 0.040) and reduced that of Streptococcus (P = 0.030) (Table 7).
Fig. 5.
Relative abundances of the fecal microbiota at the phylum level. This figure shows the relative abundances of the top 10 phyla in the fecal microbiota. Data are expressed as means ± SEM; n = 5 for each treatment. CON, the basal diet treatment during gestation; BS, the basal + 0.2% B. subtilis PB6 treatment during gestation.
Table 7.
Effect of Bacillus subtilis PB6 supplementation of sows on the relative abundances in their microbial communities1 at the genus levels (≥0.1% in any samples; the raw data have been changed to log10 values) on d 110 of gestation.
| Item | CON2 | BS2 | P-value |
|---|---|---|---|
| Lactobacillus | 0.59 ± 0.18 | 0.86 ± 0.19 | 0.342 |
| Clostridium_sensu_stricto_1 | 0.93 ± 0.10 | 0.80 ± 0.07 | 0.309 |
| Treponema_2 | 0.29 ± 0.15 | 0.57 ± 0.19 | 0.259 |
| Terrisporobacter | 0.80 ± 0.07 | 0.88 ± 0.06 | 0.372 |
| Lachnospiraceae_XPB1014_group | 0.59 ± 0.08 | 0.56 ± 0.09 | 0.802 |
| Streptococcus | 0.14 ± 0.23 | −0.52 ± 0.11 | 0.030 |
| Romboutsia | 0.29 ± 0.07 | 0.44 ± 0.09 | 0.189 |
| Turicibacter | 0.32 ± 0.07 | 0.30 ± 0.10 | 0.882 |
| Ruminococcaceae_UCG-005 | 0.42 ± 0.07 | 0.38 ± 0.06 | 0.707 |
| Ruminococcaceae_UCG-002 | 0.47 ± 0.06 | 0.43 ± 0.08 | 0.729 |
| Methanobrevibacter | 0.00 ± 0.25 | 0.29 ± 0.17 | 0.367 |
| Ruminococcaceae_NK4A214_group | 0.50 ± 0.04 | 0.42 ± 0.05 | 0.256 |
| Sarcina | −0.28 ± 0.16 | −0.15 ± 0.22 | 0.660 |
| Prevotellaceae_NK3B31_group | 0.02 ± 0.20 | 0.29 ± 0.14 | 0.303 |
| Rikenellaceae_RC9_gut_group | 0.21 ± 0.10 | 0.25 ± 0.06 | 0.715 |
| Christensenellaceae_R-7_group | 0.22 ± 0.03 | 0.26 ± 0.04 | 0.448 |
| Ruminococcaceae_UCG-014 | 0.06 ± 0.06 | −0.01 ± 0.11 | 0.560 |
| Lachnospiraceae_AC2044_group | −0.15 ± 0.09 | −0.20 ± 0.14 | 0.813 |
| [Eubacterium]_coprostanoligenes_group | 0.09 ± 0.08 | −0.02 ± 0.02 | 0.230 |
| Prevotellaceae_UCG-003 | −0.44 ± 0.14 | −0.28 ± 0.13 | 0.421 |
| Desulfovibrio | −0.13 ± 0.07 | −0.36 ± 0.08 | 0.058 |
| Ruminococcus_1 | −0.18 ± 0.05 | −0.37 ± 0.11 | 0.161 |
| Phascolarctobacterium | −0.39 ± 0.11 | −0.63 ± 0.02 | 0.053 |
| Ruminococcaceae_UCG-013 cc | −0.24 ± 0.07 | −0.48 ± 0.07 | 0.040 |
| Parabacteroides | −0.54 ± 0.11 | −0.44 ± 0.14 | 0.600 |
| Family_XIII_AD3011_group | −0.25 ± 0.06 | −0.35 ± 0.09 | 0.433 |
| Oscillospira | −0.39 ± 0.07 | −0.58 ± 0.12 | 0.198 |
| Anaerotruncus | −0.56 ± 0.08 | −0.54 ± 0.12 | 0.926 |
| Ruminococcaceae_UCG-009 | −0.53 ± 0.11 | −0.59 ± 0.07 | 0.622 |
| Papillibacter | −0.46 ± 0.10 | −0.70 ± 0.06 | 0.086 |
| dgA-11_gut_group | −0.52 ± 0.11 | −0.63 ± 0.08 | 0.441 |
| Ruminococcaceae_UCG-010 | −0.41 ± 0.04 | −0.46 ± 0.03 | 0.366 |
Values are means ± SEM, n = 5 per treatment.
CON, basal diet treatment; BS, basal diet + 0.2% B. subtilis PB6 treatment.
4. Discussion
In this study, we concentrated on the effects of B. subtilis PB6 supplementation to sow diets in late gestation and lactation on their reproductive performance.
4.1. Piglets status at farrowing
Although the litter sizes (total born) were larger in the BS group, this difference was not attributable to B. subtilis PB6 supplementation, since litter sizes were determined by conception rate in early pregnancy that occurred before treatment because the B. subtilis PB6 started adding from d 90 of gestation (Böhmer et al., 2006; Baker et al., 2013). It was likely that random errors caused such a result when we selected the sows.
The weight of each piglet born alive in the BS group was smaller than in the CON group, but the litter sizes in the BS group were higher than those in the CON group. Previous study showed that there was a negative linear correlation between litter sizes and piglet weights due to a uterine constraint on prenatal piglet growth when more embryos competed for uterine resources (Kerr and Cameron, 1995; Wolf et al., 2008). Hence, the smaller weight of each piglet born alive could be explained by the larger litter sizes in BS group, although there could be a little effect when B. subtilis PB6 supplied in late gestation owing to restricted feed intake (Wu et al., 2006; Campos et al., 2012). The same result also showed in previous studies. Rychen et al. (2017) reported that the piglet weight at birth in the B. subtilis group was smaller than that in the control group when sows were fed 1.0 × 108 CFU/kg B. subtilis PB6 in their feed from d 90 of gestation until weaning. The number of born alive was larger in BS group than in CON group, which could be explained that supplementation of B. subtilis PB6 declined the transformation of prenatal piglet to mummy in late gestation. This result was consistent with a previous study that supplementation of B. subtilis to sows increased the numbers of live births at farrowing (Baker et al., 2013).
4.2. Duration of farrowing and piglet birth interval
An ever-increasing number of studies have shown that a long period of farrowing could affect the health of the sow until early lactation (Martineau et al., 1992; Herpin et al., 1996; Dijk et al., 2005). Previous studies have also indicated that large litters, large numbers of live-born piglets, and high birthweights could increase the duration of farrowing (Rens and Lende, 2004), and that high birthweights could extend the piglet birth interval (Motsi et al., 2006). In this study, the piglet birth interval was reduced by B. subtilis PB6 supplementation, and the duration of farrowing tended to be shorter in the BS group than in the CON group, whereas the total piglets born and the numbers born alive were larger in the BS group than in the CON group. This differed, in part, from the results of Van Rens and Van der Lende (2004). B. subtilis PB6 may have played an important role. That study showed that the antioxidant capacity of sows was improved by the addition of B. subtilis PB6. Moreover, our results (Table 3) indicated that B. subtilis PB6 fed to sows increased their capacity for breastfeeding. We inferred that the sows and piglets had better physical strengths at farrowing due to the improvement digestibility of sow nutrients when the sows were supplied with B. subtilis PB6, although we did not measure nutrient digestibility. We made this inference based on the study of Patarapree et al. (2018), who showed that B. subtilis improved the digestion and utilization of nutrition in grower period of piglets.
4.3. Growth performance of piglets after cross-fostering
There were initial differences in litter weights and average piglet bodyweights by cross-fostering. This could be explained by the following factors. First, based on the number of effective teats on the sows, the litters were standardized to approximately 12 piglets per sow within 24 h after birth (cross-fostering) within the treatment groups; Second, there were no differences in bodyweight per litter within each group, but the bodyweights were smaller in BS group than in the CON group. Finally, we tried to keep the piglets with their maternal sows. Although cross-fostering caused such a different beginning, we still wanted to continue the experiment because we wanted to see if the addition of B. subtilis PB6 improved the growth performance of the piglets on the premise.
The weaning weights of the piglet litters and the litter weight gains were higher when sows were supplemented with B. subtilis PB6, which was consistent with previous studies (Alexopoulos et al., 2004; Stamati et al., 2006; Jeong et al., 2015; Rychen et al., 2017). Kritas et al. (2006) reported higher fat and protein percentages in the milk of ewes treated with B. subtilis. The differences between 2 groups may be associated with the higher fat content of the milk from the sows fed B. subtilis PB6 in this study. Previous studies have also shown that piglets consumed better-quality milk when sows were fed B. subtilis during lactation, which may partly explain the higher weaning weights observed in the present study (Kyriakis et al., 1992; Alexopoulos et al., 2004; Stamati et al., 2006; Zhu et al., 2012; Sun et al., 2013; Kritas et al., 2015). Another reason for these weaning weights and weight gains may be the higher milk yield of the sows supplied with B. subtilis PB6. Inatomi et al., (2017) reported that when sows were fed mixed probiotics (15 g/d) containing B. subtilis, the milk yield of the sow and the litter weights at farrowing improved. Although we did not measure the milk yield, it has been demonstrated in recent studies that sows and ewes supplemented with a compound probiotic containing B. subtilis produced more milk than those without supplementation (Kritas et al., 2006; Inatomi et al., 2017).
Previous studies have shown that B. subtilis or mixed probiotics improved the lactation survival rate. The present study also indicated that the lactation survival rate during suckling was higher in the BS group than in the CON group, as in previous studies (Böhmer et al., 2006; Stamati et al., 2006; Liu et al., 2017). The improved lactation survival rate of piglets when sows were supplied with B. subtilis PB6 may be partly attributable to the transfer of the Bacillus strain from the sows to the piglets or to the reduction of the amount of Clostridium shed into the environment by the sows (ME et al., 2008; Baker et al., 2013). Either way, the difference in the numbers of weaned piglets differed significantly between the BS and CON groups.
4.4. Feed intake and BF loss of sows during lactation
Although the growth performance of the offspring in the BS group was significantly better than that in the CON group during lactation, there were no differences in the feed intake or BF loss by sows during lactation between the 2 groups. The most likely explanation was that B. subtilis PB6 increased the digestive enzyme activity of sows, improving their digestion and their absorption of the nutrients in their feed during lactation. Hayakawa et al. (2016) reported that compound probiotics containing B. subtilis improved the ileal digesta of broilers. Previous studies have shown that B. subtilis could secrete exoenzymes, including proteases and amylases (Zokaeifar et al., 2012), and simultaneously improve the activities of host lipases and proteases (Zokaeifar et al., 2012; Li et al., 2012; Liu et al., 2017). Therefore, better nutrient utilization during lactation would result in higher milk quality when B. subtilis PB6 was added to sows’ feed.
4.5. Antioxidant capacity and endotoxin in sow serum
This study demonstrated that B. subtilis PB6 improved the antioxidant capacity of sows. The gestation, parturition, and lactation of sows are associated with oxidative stress, and the excessive free radicals produced by oxidative stress disrupted the balance between the pro-oxidant and antioxidant systems (Castillo et al., 2005; Berchierironchi et al., 2011). CAT, GSH-Px (antioxidative enzymes) and T-AOC play key roles in the self-defense of an organism (Rajput et al., 2013a), removing excess free radicals and preventing lipid peroxidation. Another important index of the body's antioxidant capacity is MDA (Wills, 1966; Coskun et al., 2005; Nawito et al., 2016), a product of lipid peroxidation. In this study, B. subtilis PB6 reduced the MDA concentrations and increased the T-AOC at parturition and increased the CAT activities on d 21 of lactation in the sow sera. These results were consistent with the research of Wei-fen, 2015, who demonstrated that B. subtilis B10 could protect against oxidative stress by increasing the rate of free radical scavenging by enhancing the enzymatic defense system. However, the average value of MDA concentrations in CON group seemed to decline from parturition to d 21 of lactation but it was stable in BS group, which can be explained by B. subtilis PB6 protected lipid from being oxidized during lactation. Several studies have shown that the application of certain B. subtilis strains could improve the antioxidant capacity of poultry (Rajput et al., 2013a, 2013b; Zhang et al., 2017). Although farrowing lead to oxidant stress (Szczubia et al., 2013), supplementation with B. subtilis PB6 improved the antioxidant capacities of the sows.
The bacterial endotoxin lipopolysaccharide (LPS) causes inflammation (Kauf, 2004). B. subtilis PB6 may ease the inflammation of sows during lactation, because in this study, we demonstrated that the endotoxin concentrations in the sow sera (on postnatal d 14 and 21) were reduced by BS supplementation. This reduction in endotoxin was important in accelerating the physical recovery of the sows. This finding confirmed that the sows in the BS group were more capable of breastfeeding than those in the CON group.
4.6. Cortisol in piglet serum
This study indicated that B. subtilis PB6 could ease the stress that piglets faced during the suckling period. Bacillius subtilis PB6 reduced the cortisol concentrations in the piglet sera on postnatal d 14. Cortisol is a marker of stress in an organism (Roth, 1985; Limberaki et al., 2011), and there is a positive correlation between endotoxin-induced mastitis, neonatal diarrhea, and the plasma cortisol concentrations in cattle (Paape et al., 1974; Gwazdauskas et al., 1978; Massip, 1979). The reduction in the cortisol concentrations in the piglet sera in the BS group indicated that the piglets may have suffered less diarrhea, which was conducive to growth.
4.7. Intestinal microbes of sow at d 110 of gestation
The gut microbiota plays a key role in maintaining health and regulating pathogenesis in the host (Chassard et al., 2012; Ghoshal et al., 2012; Hayakawa et al., 2016). Pregnancy is associated with immunological and metabolic changes that may be related to the compositional dynamics of the microbiota (Koren et al., 2012; Kong et al., 2017). The composition of the intestinal microbiota is affected by multiple factors (Penders et al., 2006; Wu et al., 2011). This study showed that supplementation with B. subtilis PB6 increased the relative abundances of the phyla Gemmatimonadete and Acidobacteria and reduced relative abundances of Actinobacteria, Proteobacteria, and Streptococcus. According to the numbers of Observed species and Shannon's index, the α-diversity of the gut microbial community decreased when sows fed B. subtilis PB6. Kong et al., (2017) reported that the gut microorganismal diversity decreased and the abundances of Proteobacteria and Actinobacteria increased in late gestation in sows. Despite the reduction in microbial diversity, beneficial microbes, such as Gemmatimonadete and Acidobacteria, were more numerous when B. subtilis PB6 was added to the sows' feed. Proteobacteria actively participates in inflammatory bowel disease (Hansen et al., 2012; Koren et al., 2012; Mukhopadhya et al., 2012; Morgan et al., 2012), and a high proportion of Actinobacteria is associated with inflammatory bowel disease and colon cancer (Frank et al., 2007; Brim et al., 2017). Streptococcus is always a pathogenic bacterium (Yong et al., 2008). Bacillus species have been detected that could colonize the intestinal tract (Barbosa et al., 2005; Guo et al., 2006), and display the features for such colonization, including survival and germination in the gut, biofilm formation, and the secretion of antimicrobial compounds. Therefore, B. subtilis PB6 may inhibit the reproduction of harmful bacteria in the intestine in the study. The increase of relative abundance of Ruminococcaceae_UCG-013 cc in BS group may increase the carbohydrate fermentation in the sow gut during lactation (Gosalbes et al., 2011). These results suggested that B. subtilis PB6 may inhibit the proliferation of harmful bacteria and promote beneficial microbial growth, facilitating gut health.
5. Conclusions
The present research suggested that dietary supplementation with 4 × 108 CFU/kg B. subtilis PB6 in late gestation and lactation periods could reduce the piglet birth interval, and improve the growth performance of the suckling piglets (after cross-fostering) by enhancing their antioxidation capacity and reducing the endotoxin concentrations in the sow sera and the cortisol concentrations in the piglet sera. B. subtilis PB6 also could improve the gut health of the sows during late gestation.
Author contributions
De Wu designed the study, Yan Li and Meng Cao performed the research, Yan Li collected the data, Meng Cao and Jian Li analyzed the data, and Qianqian Zhang and Jian LI wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The work was supported by the National Key R&D Program of China (grant no. 2018YFD0501000) and Sichuan Province “135” Breeding Tackle Project (grant no. 2016NYZ0052).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdelqader A., Al-Fataftah A.-R., Da G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim Feed Sci Technol. 2013;179:103–111. [Google Scholar]
- Alexopoulos C., Georgoulakis I.E., Tzivara A., Kritas S.K., Siochu A., Kyriakis S.C. Field evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J Anim Physiol An N. 2004;88:381–392. doi: 10.1111/j.1439-0396.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- Baker A.A., Davis E., Spencer J.D., Moser R., Rehberger T. The effect of a Bacillus-based direct-fed microbial supplemented to sows on the gastrointestinal microbiota of their neonatal piglets. J Anim Sci. 2013;91:3390–3399. doi: 10.2527/jas.2012-5821. [DOI] [PubMed] [Google Scholar]
- Barbosa T.M., Serra C.R., La Ragione R.M., Woodward M.J., Henriques A.O. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol. 2005;71(2):968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchierironchi C.B., Kim S.W., Zhao Y., Correa C.R., Yeum K.J., Ferreira A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Anim Int J Anim Biosci. 2011;5:1774–1779. doi: 10.1017/S1751731111000772. [DOI] [PubMed] [Google Scholar]
- Bergmark L., Poulsen P.H.B., Al-Soud W.A., Norman A., Hansen L.H., Rensen S.J. Lasse Assessment of the specificity of Burkholderia and Pseudomonas qPCR assays for detection of these genera in soil using 454 pyrosequencing. FEMS Microbiol Lett. 2012;333(1):77–84. doi: 10.1111/j.1574-6968.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- Böhmer B.M., Kramer W., Roth-Maier D.A. Dietary probiotic supplementation and resulting effects on performance, health status, and microbial characteristics of primiparous sows. J Anim Physiol Anim Nutr. 2006;90(7–8):309–315. doi: 10.1111/j.1439-0396.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- Brim H., Yooseph S., Lee E., Sherif Z.A., Abbas M., Laiyemo A., Varma S., Torralba M., Dowd S., Nelson K. A microbiomic analysis in african americans with colonic lesions reveals streptococcus sp: vt162 as a marker of neoplastic transformation. Genes. 2017;8:314. doi: 10.3390/genes8110314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos P., Silva B., Donzele J., Oliveira R., Knol E. Effects of sow nutrition during gestation on within-litter birth weight variation: a review. Animal. 2012;6:797–806. doi: 10.1017/S1751731111002242. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C., Hernandez J., Bravo A., Lopezalonso M., Pereira V., Benedito J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet J. 2005;169:286–292. doi: 10.1016/j.tvjl.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Chassard C., Dapoigny M., Scott K.P., Crouzet L., Del'homme C., Marquet P., Martin J.C., Pickering G., Ardid D., Eschalier A. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Jiang S., Peng J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front Microbiol. 2018;9:1989. doi: 10.3389/fmicb.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun O., Kanter M., Korkmaz A., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Dijk A.J.V., Rens B.T.T.M.V., Lende T.V.D., Taverne M.A.M. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology. 2005;64:1573–1590. doi: 10.1016/j.theriogenology.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Frank D.N., Amand A.L.S., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PANS (Pest Artic News Summ) 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal U.C., Shukla R., Ghoshal U., Gwee K.A., Ng S.C., Quigley E.M. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflamm. 2012;2012(3):151085. doi: 10.1155/2012/151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalbes M.J., Durbán A., Pignatelli M., Abellan J.J., Jiménez-Hernández N., Pérez-Cobas A.E. Metatranscriptomic approach to analyze the functional human gut microbiota. PloS One. 2011;6(3):e17447. doi: 10.1371/journal.pone.0017447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Li D., Lu W., Piao X., Chen X. Screening ofbacillusstrains as potential probiotics and subsequent confirmation of the in vivo effectiveness ofbacillus subtilisma139 in pigs. Antonie Leeuwenhoek. 2006;90(2):139–146. doi: 10.1007/s10482-006-9067-9. [DOI] [PubMed] [Google Scholar]
- Gwazdauskas F.C., Gross W.B., Bibb T.L., Mcgilliard M.L. Antibody titers and plasma glucocorticoid concentrations near weaning in steer and heifer calves. Can Vet. 1978;19:150–154. [PMC free article] [PubMed] [Google Scholar]
- Hansen R., Russell R.K., Reiff C., Louis P., McIntosh F., Berry S.H., Mukhopadhya I., Bisset W.M., Barclay A.R., Bishop J. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn's but not in ulcerative colitis. Am J Gastroenterol. 2012;107:1913. doi: 10.1038/ajg.2012.335. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Masuda T., Kurosawa D., Tsukahara T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim Sci J. 2016;87:1501–1510. doi: 10.1111/asj.12565. [DOI] [PubMed] [Google Scholar]
- Heerklotz H., Seelig J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys J. 2001;81:1547–1554. doi: 10.1016/S0006-3495(01)75808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin P., Berthon D., Duchamp C., Dauncey M., Le D.J. Effect of thyroid status in the perinatal period on oxidative capacities and mitochondrial respiration in porcine liver and skeletal muscle. Reprod Fertil Dev. 1996;8:147–155. doi: 10.1071/rd9960147. [DOI] [PubMed] [Google Scholar]
- Inatomi T., Amatatsu M., Romero-Pérez G.A., Inoue R., Tsukahara T. Dietary probiotic compound improves reproductive performance of porcine epidemic diarrhea virus-infected sows reared in a Japanese commercial swine farm under vaccine control condition. Front Immunol. 2017;8:1877. doi: 10.3389/fimmu.2017.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poultry Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Jeong J., Kim J., Lee S., Kim I. Evaluation of Bacillus subtilis and Lactobacillus acidophilus probiotic supplementation on reproductive performance and noxious gas emission in sows. Ann Anim Sci. 2015;15:699–710. [Google Scholar]
- Kauf A.C. 2004. Sow and piglet responses to endotoxin-induced mastitis. [J] [Google Scholar]
- Kerr J.C., Cameron N.D. Reproductive performance of pigs selected for components of efficient lean growth. Anim Sci. 1995;60:281–290. [Google Scholar]
- Kong X., Ji Y., Li H., Zhu Q., Blachier F., Geng M., Chen W., Yin Y. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: effects of food composition at different times of pregnancy. Sci Rep. 2017;6:37224. doi: 10.1038/srep37224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Bäckhed H.K., Gonzalez A., Werner J.J., Angenent L.T., Knight R. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranendonk G., Van der Mheen H., Fillerup M., Hopster H. Social rank of pregnant sows affects their body weight gain and behavior and performance of the offspring. J Anim Sci. 2007;85:420–429. doi: 10.2527/jas.2006-074. [DOI] [PubMed] [Google Scholar]
- Kritas S.K., Govaris A., Christodoulopoulos G., Burriel A. Effect of Bacillus licheniformis and Bacillus subtilis supplementation of Ewe's feed on sheep milk production and young lamb mortality. J Vet Med Ser A. 2006;53:170–173. doi: 10.1111/j.1439-0442.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- Kritas S.K., Marubashi T., Filioussis G., Petridou E., Christodoulopoulos G., Burriel A.R., Tzivara A., Theodoridis A., Pískoriková M. Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (C-3102) J Anim Sci. 2015;93(1):405. doi: 10.2527/jas.2014-7651. [DOI] [PubMed] [Google Scholar]
- Kyriakis S.C., Vassilopoulos V., Demade I., Kissels W., Polizopoulou Z., Milner C.K. The effect of virginiamycin on sow and litter performance. Anim Sci. 1992;55:431–436. [Google Scholar]
- Li W., Ya-Li L.I., Qin Y., Dong-You Y.U. Effects of Bacillus subtilis on digestive enzyme activity, intestinal mucosal architecture and gut microflora composition in Broilers. Chin J Vet Sci. 2012;32(5):666–669. [Google Scholar]
- Limberaki E., Eleftheriou P., Gasparis G., Karalekos E., Kostoglou V., Petrou C. Cortisol levels and serum antioxidant status following chemotherapy. Health. 2011;3:512. [Google Scholar]
- Liu H., Wang S., Cai Y., Guo X., Cao Z., Zhang Y., Liu S., Yuan W., Zhu W., Zheng Y. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017;60:326–333. doi: 10.1016/j.fsi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Lozupone C., Hamady M., Knight R. UniFrac – an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinf. 2006;7 doi: 10.1186/1471-2105-7-371. 371-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau G.P., Smith B.B., Béatrice Doizé. Pathogenesis, prevention, and treatment of lactational insufficiency in sows. Vet Clin Food Anim Pract. 1992;8:661–684. doi: 10.1016/s0749-0720(15)30710-6. [DOI] [PubMed] [Google Scholar]
- Massip A. Haematocrit, biochemical and plasma cortisol changes associated with diarrhoea in the calf. Br Vet J. 1979;135:600–605. doi: 10.1016/s0007-1935(17)30014-3. [DOI] [PubMed] [Google Scholar]
- ME D., T P., DC B., BZ dR., ZB J., CV M., T R. Effect of a Bacillus-based direct-fed microbial feed supplement on growth performance and pen cleaning characteristics of growing-finishing pigs. 2008;86:1459–1467. doi: 10.2527/jas.2007-0603. [DOI] [PubMed] [Google Scholar]
- Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motsi P., Sakuhuni C., Halimani T.E., Bhebhe E., Ndiweni P.N.B., Chimonyo M. Influence of parity, birth order, litter size and birth weight on duration of farrowing and birth intervals in commercial exotic sows in Zimbabwe. Anim Sci. 2006;82:6. [Google Scholar]
- Mou D., Wang J., Liu H., Chen Y., Che L., Fang Z., Xu S., Lin Y., Feng B., Li J. Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrition. 2017;45:76–84. doi: 10.1016/j.nut.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Mukhopadhya I., Hansen R., El-Omar E.M., Hold G.L. IBD—what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- Nawito M.F., Amal R.A., El Hameed A., Sosa S.A., Mahmoud Karima GhM. Impact of pregnancy and nutrition on oxidant⁄antioxidant balance in sheep and goats reared in South Sinai. Vet World. 2016;9:801–805. doi: 10.14202/vetworld.2016.801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier C., Kothe S., Heinonen M., Valros A., Peltoniemi O. Prolonged duration of farrowing is associated with subsequent decreased fertility in sows. Theriogenology. 2013;79:1095–1099. doi: 10.1016/j.theriogenology.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Oliviero C., Heinonen M., Valros A., Peltoniemi O. Environmental and sow-related factors affecting the duration of farrowing. Anim Reprod Sci. 2010;119:85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Paape M.J., Schultze W.D., Desjardins C., Miller R.H. Plasma corticosteroid, circulating leukocyte and milk somatic cell responses to Escherichia coli endotoxin-induced mastitis. Proc Soc Exp Biol Med. 1974;145:553–559. doi: 10.3181/00379727-145-37850. [DOI] [PubMed] [Google Scholar]
- Patarapree P., Jaikan W., Juangsaman A. Effects of dietary Bacillus subtilis supplementation as probiotics on growth performance and nutrients digestibility in fattening pigs [J] Pakistan J Nutr. 2018;17(12):634–640. [Google Scholar]
- Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., van den Brandt P.A., Stobberingh E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Rajput I.R., Li Y.L., Xu X., Huang Y., Zhi W.C., Yu D.Y. Supplementary effects of Saccharomyces boulardii and Bacillus subtilis B10 on digestive enzyme activities, antioxidation capacity and blood homeostasis in broiler. Int J Agric Biol. 2013;15:1560–8530. [Google Scholar]
- Rajput I.R., Li Wf, Li Yl, Lei J., Wang Mq. Application of probiotic (Bacillus subtilis) to enhance immunity, antioxidation, digestive enzymes activity and hematological profile of broiler. Pak Vet J. 2013;33:69–72. [Google Scholar]
- Rens B.T.V., Lende T.V.D. Parturition in gilts: duration of farrowing, birth intervals and placenta expulsion in relation to maternal, piglet and placental traits. Theriogenology. 2004;61(1–2):331–352. doi: 10.1016/j.theriogenology.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Roth J.A. Springer; New York, NY: 1985. Cortisol as mediator of stress-associated immunosuppression in cattle. Animal stress; pp. 225–243. [Google Scholar]
- Rychen G., Aquilina G., Azimonti G., Bampidis V., Bastos M.D.L., Bories G., Chesson A., Cocconcelli P.S., Flachowsky G., Gropp J. Safety and efficacy of Bacillus subtilis PB6 (Bacillus subtilis ATCC PTA-6737) as a feed additive for sows. Efsa J. 2017;15 doi: 10.2903/j.efsa.2017.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamati S., Alexooulos C., Siochu A., Saoulidis K., Kyriakis S.C. Probiosis in sows by administration of Bacillius toyoi spores during late pregnancy and lactation: effect on their health status/performance and on litter characteristics. Int J Probiotics Prebiotics. 2006;1:33. [Google Scholar]
- Sun P., Wang J.Q., Deng L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal. 2013;7:216–222. doi: 10.1017/S1751731112001188. [DOI] [PubMed] [Google Scholar]
- Szczubia M., D'Browski R., Bochniarz M., Komar M. The influence of the duration of the expulsive stage of parturition on the occurrence of postpartum oxidative stress in sows with uncomplicated, spontaneous farrowings. Theriogenology. 2013;80:706–711. doi: 10.1016/j.theriogenology.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Teo A.Y.-L., Tan H.-M. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl Environ Microbiol. 2005;71:4185–4190. doi: 10.1128/AEM.71.8.4185-4190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou P., Liu H., Li S., Zhao Y., Deng K., Cao D., Che L., Fang Z., Xu S. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod Domest Anim. 2016;51:492–500. doi: 10.1111/rda.12707. [DOI] [PubMed] [Google Scholar]
- Li, Wei-fen Effect of dietary supplementation of Bacillus subtilisB10 on biochemical and molecular parameters in the serum and liver of high-fat diet-induced obese mice. J Zhejiang Univ - Sci B. 2015;16:487–495. doi: 10.1631/jzus.B1400342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E.D. Mechanisms of lipid peroxide formation in tissues Role of metals and haematin proteins in the catalysis of the oxidation of unsaturated fatty acids. Biochim Biophys Acta Lipids Lipid Metabol. 1966;98:238–251. doi: 10.1016/0005-2760(65)90118-9. [DOI] [PubMed] [Google Scholar]
- Wolf J., Žáková E., Groeneveld E. Within-litter variation of birth weight in hyperprolific Czech Large White sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest Sci. 2008;115:195–205. [Google Scholar]
- Wu G., Bazer F., Wallace J., Spencer T. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.-Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S., Wen Y., Perez-Gutierrez O.N. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. J FEMS (Fed Eur Microbiol Soc) Microbiol Ecol. 2008;(3):3. doi: 10.1111/j.1574-6941.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ma Q., Ma S., Zhang J., Jia R., Ji C. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins. 2017;9(1):1. doi: 10.3390/toxins9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Zhao K., Chen X., Xu J. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]
- Zokaeifar H., Balcázar J.L., Saad C.R., Kamarudin M.S., Sijam K., Arshad A., Nejat N. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012;33:683–689. doi: 10.1016/j.fsi.2012.05.027. [DOI] [PubMed] [Google Scholar]