Abstract
Background
Early in the coronavirus disease 2019 (COVID-19) pandemic, there was serious concern that the United States would encounter a shortfall of mechanical ventilators. In response, the US government, using the Defense Production Act, ordered the development of 200,000 ventilators from 11 different manufacturers. These ventilators have different capabilities, and whether all are able to support COVID-19 patients is not evident.
Research Question
Evaluate ventilator requirements for affected COVID-19 patients, assess the clinical performance of current US Strategic National Stockpile (SNS) ventilators employed during the pandemic, and finally, compare ordered ventilators’ functionality based on COVID-19 patient needs.
Study Design and Methods
Current published literature, publicly available documents, and lay press articles were reviewed by a diverse team of disaster experts. Data were assembled into tabular format, which formed the basis for analysis and future recommendations.
Results
COVID-19 patients often develop severe hypoxemic acute respiratory failure and adult respiratory defense syndrome (ARDS), requiring high levels of ventilator support. Current SNS ventilators were unable to fully support all COVID-19 patients, and only approximately half of newly ordered ventilators have the capacity to support the most severely affected patients; ventilators with less capacity for providing high-level support are still of significant value in caring for many patients.
Interpretation
Current SNS ventilators and those on order are capable of supporting most but not all COVID-19 patients. Technologic, logistic, and educational challenges encountered from current SNS ventilators are summarized, with potential next-generation SNS ventilator updates offered.
Key Words: ARDS, COVID-19, strategic national stockpile, ventilators
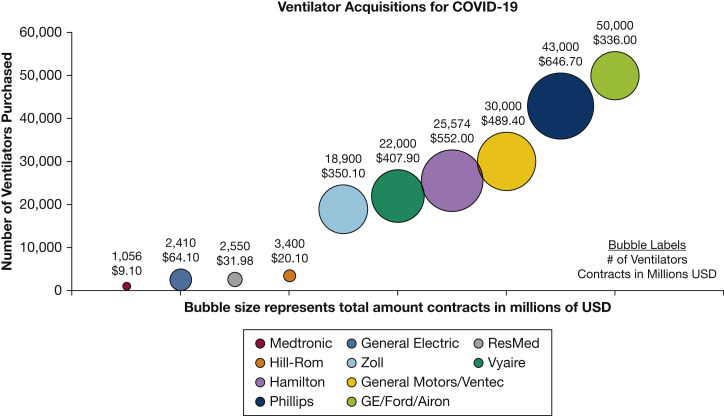
Although the current number of mechanical ventilators in the United States remains unknown, a 2010 survey of US hospitals estimated approximately 62,000 full-feature ventilators, reflecting a ratio of less than 1 ventilator per each US ICU bed.1 , 2 The same assessment also identified nearly 100,000 additional less featured positive-pressure devices. Some of these devices had sufficient capabilities to be useful if a surge in mechanical ventilation were required, and others have limited utility. Until the current coronavirus disease 2019 (COVID-19) pandemic, no event had exhausted modern health-care systems or mechanical ventilation capacity, so the utility of these surge devices remained speculative and untested. In the spring of 2020, a number of these devices were used during the initial response to COVID-19, and despite their use, the need for effective positive-pressure devices were anticipated to exceed demand. In addition to the devices held at US hospitals, thousands of sophisticated transport ventilators were deployed from the US Strategic National Stockpile (SNS) to assist hospitals in need. Because many communities requested additional ventilators simultaneously, it became apparent that the existing stockpile of approximately 20,000 ventilators may not be sufficient to meet national demand. As the event rapidly unfolded, the US federal government used the Defense Production Act to create new ventilators under an Emergency Use Authorization (EUA).3 Through this process, the United States ordered approximately 200,000 ventilatorsdevices with tremendous variation in capabilities (Figs 1 and 2 ).
Figure 1.
The number of ventilators initially procured from each manufacturer and total cost of each initial contract.30, 31, 32, 33 As of August 31, 2020, the initial order of nearly 200,000 ventilators has been decreased to approximately 130,000.34
Figure 2.
Full-function ventilators (in white) vs ventilators for (primarily) less severely ill patients (in yellow), noninvasive ventilation (NIV in red), or transport (in green)50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63 (based on data presented in Table 3). The designation of full-featured requires the measurement of exhaled tidal volume, display of pressure volume and flow waveforms, and precise delivery of a constant Fio2 from 0.21 to 1.0. SNS = Strategic National Stockpile.
This statement has three objectives. First, it is intended to evaluate the ventilator requirements for COVID-19 and the performance of current SNS ventilators employed during this pandemic. Second, this statement will provide a comparison of devices that have been ordered under the EUA and appropriate usage for patient situations. Surplus devices are in consideration for deployment to other countries, and this review will assist those nations to appreciate the features and limitations of the equipment received.4
Finally, the technologic, logistic, and educational challenges of current SNS ventilators are summarized and potential future design updates proposed.
Definition of a Ventilator
A ventilator is a device used to provide oxygenation and respiratory support in settings in which the patient's own pulmonary system is compromised.5 Fundamentally, ventilator mechanics involve a mode of operation that incorporates four basic parameters: pressure or volume, Fio 2, respiratory rate, and end-expiratory pressure. Ventilator modes vary based on patients’ individual physiologic needs and are set often based on lung compliance, gas exchange, and minute ventilation.6 In addition, standard ventilators are expected to sense when a patient attempts to breathe and deliver the appropriate breath accordingly. Full-feature mechanical ventilators used to support critically ill patients have advanced instrumentation that gather and display data on the patient's pulmonary mechanics. These data inform how the ventilator's mode and basic parameters can be adjusted to achieve clinical improvement and liberation from the ventilator.7 , 8 In contrast, positive-pressure ventilation devices (eg, anesthesia machines, noninvasive CPAP and bilevel positive pressure devices, and so forth) have a limited number of operational modes and are of limited utility for the prolonged management of patients with COVID-19-associated respiratory failure.
Ventilator Capabilities for Managing COVID-19
Investigators have described multiple phases of respiratory insufficiency associated with COVID-19.9 Soon after development of respiratory distress, patients often retain high pulmonary compliance despite poor oxygenation. Minute ventilation in these patients is typically high, and they may not demonstrate overt respiratory distress despite significant hypoxemia. These individuals may be managed with tidal volumes (VT) above the typical 4 to 6 mL/kg ideal body weight used for ARDS. Some patients may progress to more classic patterns of hypoxemic respiratory failure with reduced compliance, increased requirements for positive end-expiratory pressure (PEEP), and optimal use of smaller tidal volumes.10, 11, 12, 13 Operationally, COVID-19 patients require a wide spectrum of the four different ventilator support parameters described previously.
Since 2007, the Centers for Disease Control and Prevention, the American Association for Respiratory Care, the Society of Critical Care Medicine, and the Task Force for Mass Critical Care have advocated a list of standard criteria for ventilators in case of a mass casualty respiratory failure event similar to severe acute respiratory syndrome (SARS) which resulted in ARDS.11 Development of these criteria were not device- or price-based, but rather performance-based.12 As noted previously, infection by COVID-19 can cause progressive hypoxemic respiratory failure characterized by diffuse alveolar damage (clinical ARDS).13, 14, 15 The type of ventilator needed for these critically ill patients must be capable of ventilating patients with severe ARDS, with respiratory system compliance between 25 and 50 mL/cm H2O, for a period of several weeks.12 , 16, 17, 18
In Table 1 , patients treated in the ARDSnet ARMA trial with 6 mL/kg vs 12 mL/kg predicted ideal body weight VT are compared with patients with COVID-19 treated at Massachusetts General Hospital.12 , 18 COVID-19 patients received similar initial PEEP settings but had higher Fio 2 requirements.18 The continuum of care requires lung-protective ventilation, assuring patient-ventilator synchrony, and adequate monitoring to evaluate the patient’s response.
Table 1.
| Settings | Day 1 | MGH COVID-19 |
|---|---|---|
| PEEP (cm H2O) | 9.4 ± 3.6 (6-13) | 10 (8-12) |
| Fio2 (%) | 0.56 ± 0.19 (0.35-0.75) | 0.70 |
| VE (L/min) | 12.9 ± 3.6 (10-18) | 9.1 |
| PIP (cm H2O) | 32 ± 8 (20-40) | 21 (19-26)a |
| Respiratory frequency (b/min) | 29 ± 7 (12-35) | 20.5 |
Plateau pressure.
As of March 2020, the SNS previously supported by the Centers for Disease Control and Prevention, and now centrally by the US Department of Health and Human Services, maintains the following ventilators (those present in the SNS prior to 2020 include the Coviden Puritan Bennett LP10, Vyaire CareFusion LTV 1200, and Zoll Impact Instrumentation Univent 754)19:
| · Covidien (Puritan Bennett) LP10 · GE CARESCAPE R860 Ventilator · GE/Ford pNeuton Model A-E · GM/Ventec V+ Pro · Hamilton C-1 · Hamilton C-3 · Hamilton T-1 |
· Hamilton Military T-1 · Hillrom Life 2000 · Philips EV300 · ResMed Astral 150 · Vyaire (CareFusion) LTV1200 · Zoll EMV+ 731 Ventilator · Zoll (Impact Instrumentation) · Univent 754 |
These ventilators are not commonly used in ICU settings, are designed and packed to withstand delivery by helicopter, and are delivered with an instruction card, video or CD, and a manual (Fig 3 ). The website has extensive links to training; however, because of the rapidity of the surge of COVID-19 patients and the novelty of the organism and its pathogenesis, there was little time for just-in-time education in use of SNS ventilators. Although these ventilators are intended to be used in austere settings by non-intensivists, the physiology of COVID-19 created challenges for the user. These ventilators have been used by the military in transporting critically ill patients, with prior complaints including limited battery life, monitor display limits, and inaudible alarms. During the Spring 2020 deployment to meet ICU surges, additional lessons included reports of battery pack challenges, missing hoses and tubing, and need for equipment repair. However, when implemented in ICUs, the challenges have not been merely technical, as highlighted by the description from New York (Fig 4 , Table 5).20, 21, 22, 23, 24, 25, 26, 27
Figure 3.
The Vyaire LTV 1200 as delivered by the SNS in “kitted” form. See Figure 2 for expansion of abbreviation.
Figure 4.
SNS Ventilator Lessons Learned: New York City and the LTV 1200. See Figure 2 for expansion of abbreviation.
Table 5.
Comparison Between Full Feature ICU Ventilator Compared With a Portable Ventilator Regarding Potential Sources of Decreased Tidal Volume (VT), Which May Help Explain the Differences in Ventilation Seen When Switching Between Devices (From Figure 4)50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63
| Potential Factors Associated With Decreased Tidal Volume | ICU Full Feature Ventilator | Portable Ventilator |
|---|---|---|
| Humidification | ICU ventilators typically have a heated humidifier attached for conditioning inspired gases. | Switching to a portable ventilator using a heat and moisture exchanger (HME) adds dead space to the circuit. The volume of these devices is typically 20-60 mL. At tidal volumes typically used for ARDS, this decreases the effective tidal volume (VT) by 10%-20%. Solution: increase VT on transition to portable ventilator, and verify new set tidal volume is indeed equivalent to ICU ventilator VT. |
| Compressible volume compensation | ICU ventilators typically compensate for the loss of volume in the circuit secondary to tubing compliance. As an example, if set VT is 450 mL and the peak pressure is 30 cm H2O and with normal circuit compliance is 2-3 mL/cm H2O, then 90 mL could potentially be lost in the circuit reducing the volume delivered to the patient to 360 mL. The ICU ventilator would increase the VT by 90 mL (540 mL) to assure the patient receives the full 450 mL. VT |
The transport ventilator does not compensate for compressible volume loss in the circuit due to tubing compliance. In the example, the effective VT on the portable ventilator is then 90 mL less than on the ICU ventilator. At a respiratory frequency of 20 breaths/min, this is a decrease in the minute ventilation of almost 2.0 L. |
| Pressure ventilation | ICU ventilators deliver pressures relative to (above) PEEP. As an example, if PEEP is 10 cm H2O and the set pressure during pressure support or pressure control is 20 cm H2O, then peak pressure would be 30 cm H2O. |
In bilevel devices and some portable ventilators, the pressure is absolute, and not set above PEEP. In the example, with settings of peak pressure of 20, this includes added PEEP and would therefore be only 10 cm above PEEP or expiratory pressure—a pressure difference of 10 cm H2O vs 20 cm H2O compared with the ICU ventilator. |
| Cumulative effect | Set VT = delivered VT. | The combination of the three factors above, which may each individually be only slight, can make a large difference in the delivered minute ventilation, reducing carbon dioxide elimination. |
EUA and Defense Production Act for Ventilators
Since the original purchase of SNS ventilators approximately 10 years ago, the standard of care in ICUs now includes ventilators with graphic display of pressure and volume for evaluation of patient response. Based on the crisis in New York, the Food and Drug Administration (FDA) issued an EUA in late March 2020 permitting the use of respiratory devices not routinely used as ventilators, such as anesthesia machines, to meet the needs of ICU patients.3 A number of devices considered in the EUA are purported to support recovery, whereas ICU full-feature ventilators are triaged to the sickest patients. On April 2, 2020, President Trump invoked the Defense Production Act to increase production of ventilators.28 These federal actions drew well-meaning and skilled individuals from disciplines outside of medicine to develop devices for a projected mechanical ventilator shortage. This marshalling of expertise and goodwill has provided positive results; however, many of these devices provide limited capabilities. Systems such as “bag squeezing” devices rely on automatic compression of a resuscitation bag replacing manual ventilation (a human squeezing the bag) with precision, consistency, and without user fatigue. The first mass respiratory failure event, the 1950s polio epidemic in Denmark, relied on manual ventilation by volunteers.29 These devices previously had been suggested for temporary use in limited-resource environments, but a device incapable of meeting patient requirements for pressure, volume, oxygen delivery, and minute ventilation is not a solution during the COVID-19 pandemic.
There were 198,890 ventilators initially ordered by the federal government at a cost of just over 2.9 billion dollars.30, 31, 32, 33 Table 2 compares the technical specifications for each ventilator ordered, and Table 3 the indications and potential uses for COVID-19, data also shown graphically in Figures 1 and 2. On analysis of the technical data, ventilators may be divided into those that have the full technical sophistication to support severe COVID-19 patients and those that do not. Ventilators unable to fully support severe COVID-19 patients may still be used on less severely ill patients, for noninvasive ventilation (NIV), or as transport ventilators.
Table 2.
Technical Assessment of Ventilators Ordered in the Current EUA Compared With the Criteria Outlined by the 2014 Mass Critical Care Task Force Panel50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63
| Criterion | Ventilator |
Airon AEa |
Hamilton C3 |
Hamilton C1 |
Hamilton T1 |
CARESCAPE R860a |
Life2000 |
Medtronic 560a |
|
|---|---|---|---|---|---|---|---|---|---|
| Manufacturer | Airon, GE, Ford | Hamilton Medical | General Electric | Hill-Rom | Medtronic | ||||
| Ventilator criteria | Mandatory Features | ||||||||
| Power source | AC power with battery back up | NO | YES | YES | YES | YES | NO | YES | |
| FDA approval | Adult and pediatric use | NO | YES | YES | YES | YES | NO | YES | |
| Minimum tidal volume | 360 mL | YES | YES | YES | YES | 50 mL | YES | ||
| Modes of ventilation | CPAP Volume control Assist control SIMV |
YES YES NO YES |
YES NOa YES YES |
YES NOa YES YES |
YES NOa YES YES |
YES YES YES YES |
NO YES YES NO |
YES YES YES YES |
|
| Control of settings | Respiratory rate PEEP Tidal volume Flow or I:E FIO2 with 50 psig inlet |
YES YES YES NO YES |
YES YES NOa YES YES |
YES YES NOa YES YES |
YES YES NOa YES YES |
YES YES YES YES YES |
YES YES NO YES NO |
YES YES YES YES NO |
|
| Flow range | <10 to > 80 L/min for mandatory breaths | NO | YES | YES | YES | YES | NO | YES | |
| PEEP | PEEP compensation | YES | YES | YES | YES | YES | YES | YES | |
| Fio2 | Fio2 from 0.21-1.0 | NO | YES | YES | YES | YES | NO | NO | |
| Operation | Able to operate with high-pressure or low-pressure oxygen source | NO | YES | YES | YES | NO | NO | NO | |
| Measurements | Measure and display VT Peak inspiratory pressure |
NO YES |
YES YES |
YES YES |
YES YES |
YES YES |
NO YES |
YES YES |
|
| Easy to set up/set ventilation settings/troubleshoot | Ability to read screen at a distance, in sunlight and low light | NO | YES | YES | YES | YES | YES | YES | |
| Gas consumption | Reported bias flow (L/min) | 10 | 5 | 5 | 4 | 2-10 | a | 2-10 | |
| Audible and visible alarms Disconnect Apnea High pressure Low source gas pressure |
YES YES NO YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
||
| Costs | < $13,000 | YES | NO | NO | NO | NO | YES | YES | |
| Criterion | Ventilator |
Astral 150 |
SAVE IIa |
LTV -2 2200a |
LTV-2 2150a |
EVOa |
EV 300 |
V+ Proa |
EMV+ 731 |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Manufacturer | ResMed | Combat Medical Systems | Vyaire | Philips Respironics | Ventec Lifesystems | Zoll | |||||
| Ventilator criteria | Mandatory features | ||||||||||
| Power source | AC power with battery back up | YES | YES | YES | YES | YES | YES | YES | YES | ||
| FDA approval | Adult and pediatric use | YES | NO | YES | YES | YES | YES | YES | YES | ||
| Minimum tidal volume 50 mL | YES | 200 mL | YES | YES | YES | YES | YES | YES | |||
| Modes of ventilation | CPAP Volume control Assist control SIMV |
YES YES YES YES |
NO YES YES NO |
YES YES YES YES |
YES YES YES YES |
YES YES YES YES |
YES YES YES YES |
YES YES YES YES |
YES YES YES YES |
||
| Control of settings | Respiratory rate PEEP Tidal volume Flow or I:E Fio2 with 50 psig inlet |
YES YES YES YES NO |
YES YES YES NO NO |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES NO |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
||
| Flow range | <10 to >80 L/min for mandatory breaths | YES | NO | YES | YES | YES | YES | YES | YES | ||
| PEEP | PEEP compensation | YES | YES | YES | YES | YES | YES | YES | YES | ||
| Fio2 | Fio2 from 0.21-1.0 | NO | NO | YES | YES | YES | YES | YES | YES | ||
| Operation | Able to operate with high-pressure or low-pressure oxygen source | NO | NO | YES | NO | NO | YES | YES | YES | ||
| Measurements | Measure and display VT Peak inspiratory pressure |
YES YES |
NO YES |
YES YES |
YES YES |
YES YES |
YES YES |
YES YES |
NO YES |
||
| Easy to set up/set ventilation settings/troubleshoot | Ability to read screen at a distance, in sunlight and low light | YES | NO | YES | YES | YES | YES | YES | YES | ||
| Gas consumptionb | Recorded bias flow (L/min) | 2-15 | 0 | 0-10 | 0-10 | 0 | |||||
| Audible and visible alarms Disconnect Apnea High pressure Low source gas pressure |
YES YES YES YES YES |
YES YES NO YES NO |
YES YES YES YES YES |
YES YES YES YES NO |
YES YES YES YES NO |
YES YES YES YES YES |
YES YES YES YES YES |
YES YES YES YES YES |
|||
| Costs | <$13,000 | YES | YES | NO | NO | YES | NO | NO | NO | ||
Recalls vendor must disclose all recalls on ventilator and equipment in the past 3 years.
End User Training Program: These three criteria are part of our initial specifications but could not be verified for all devices.
Denotes developed under emergency use authorization (EUA).
Gas consumption requires testing; instead we report bias flow (flow during the expiratory time); Hamilton ventilators do not operate in volume control; adaptive pressure is used where the clinician can set the tidal volume and the ventilator increases or decreases the pressure to maintain the desired minimum volume.
Table 3.
Ventilator Indications and Potential Use in COVID-19 Patients, and Approximate Cost per Unit Ventilator Delivered50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63
| Ventilator | Intended Indicationsa | Potential Use in COVID-19 | Estimated Cost per Ventilator (Based on Total Expense of Contract and Total No. of Ventilators Ordered) |
|---|---|---|---|
| GE/Ford/Airon | pNeuton is a pneumatic ventilator. Electrical power is not required for patient ventilation. pNeuton has been specifically designed for patient support during transport and non-critical care unit mechanical ventilation. It may be used during intra and inter-hospital transport, in aircraft, on ambulances, in emergency rooms, MRI, and other radiology suites. |
Use for transport from facility to facility or ED to ICU | $6,700 |
| Hamilton C1 | The HAMILTON-C1 ventilator is intended to provide positive-pressure ventilatory support to adults and pediatrics, and optionally infants and neonates. Intended areas of use: In the intensive care ward, intermediate care ward, emergency ward, long-term acute care hospital, or in the recovery room During transfer of ventilated patients within the hospital |
Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$25,000-$30,000 |
| Hamilton C3 | The HAMILTON-C3 ventilator is intended to provide positive-pressure ventilatory support to adults and pediatrics and optionally infants and neonates. Intended areas of use:
|
Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$25,000-$30,000 |
| Hamilton T1 | The HAMILTON-T1 ventilator is intended to provide positive-pressure ventilatory support to adults and pediatrics, and optionally infants and neonates. Intended areas of use:
|
Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$25,000-$30,000 |
| General Electric CARESCAPE R860 | The CARESCAPE R860 ventilator is designed to provide mechanical ventilation or support to neonatal, pediatric, and adult patients weighing 0.25 kg and above. The CARESCAPE R860 ventilator is a microprocessor-based, electronically controlled, pneumatically driven ventilator that includes integrated monitoring of Fio2, airway pressure, flow, and volume. The system is designed for facility use, including within-facility transport, and should only be used under the orders of a clinician. |
Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$30,000 |
| Hill Rom Life2000 | The Life2000. Ventilator is intended to provide continuous or intermittent ventilatory support for the care of individuals who require mechanical ventilation. The ventilator is intended for use by qualified, trained personnel under the direction of a physician. Specifically, the ventilator is applicable for adult patients who require the following types of ventilatory support:
|
Use to provide NIV before intubation Not for use in critically ill patients |
$6,000 |
| Medtronic 560 | The Puritan Bennett 560 Ventilator is indicated for the continuous or intermittent mechanical ventilatory support of patients weighing at least 11 lb (5 kg) who require mechanical ventilation. The ventilator is a restricted medical device intended for use by qualified, trained personnel under the direction of a doctor. It is essential to read, understand, and follow these instructions before use. The ventilator is suitable for use in institutional, home, and portable settings. It is not intended for use in Emergency Medical Service (EMS), such as an emergency transport. |
Use in hospital for less severely ill or recovering patients | $8,600 |
| ResMed Astral 150 | The Astral 100/150 provides continuous or intermittent ventilatory support for patients weighing more than 11 lb (5 kg) who require mechanical ventilation. The Astral device is intended to be used in home, institution/hospital, and portable applications for both invasive and noninvasive ventilation. |
Use in hospital for less severely ill or recovering patients | $12,500 |
| Combat Medical Systems SAVE II | The SAVe II+ is designed to be used in prehospital, field hospitals, outpatient environments, hospitals, ICUs, transport environments, or any other health-care environment requiring the use of a ventilator. The SAVe II+ can be used in lieu of a bag valve mask (BVM) in the prehospital environment or during interhospital and intrahospital transport. It is a simplified ventilator that is designed to support a wide range of situations and environments. | Transport Not for use in critically ill patients |
$7,500 |
| Vyaire LTV 2 2200 | The LTV2 Series ventilators are intended to provide continuous or intermittent ventilator support for the care of the patients requiring mechanical ventilation. The ventilator is a restricted medical device intended for use by qualified, trained personnel under the direction of a physician. Specifically, the ventilator is suitable for adult and pediatric patients weighing at least 5 kg (11 pounds) who require the following types of ventilator support:
|
Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$18,500 |
| Vyaire LTV 2 2150 | The LTV2 Series ventilators are intended to provide continuous or intermittent ventilator support for the care of the patients requiring mechanical ventilation. The ventilator is a restricted medical device intended for use by qualified, trained personnel under the direction of a physician. Specifically, the ventilator is suitable for adult and pediatric patients weighing at least 5 kg (11 pounds) who require the following types of ventilator support:
|
Limited use for COVID-19 owing to inability to maintain a constant Fio2. Only has a low-flow oxygen inlet (no blender). Fio2 fluctuates with minute ventilation. Transport as required |
$18,500 |
| Phillips EVO | The Trilogy Evo ventilator provides continuous or intermittent positive-pressure ventilation for the care of individuals who require mechanical ventilation. Trilogy Evo is intended for pediatric through adult patients weighing at least 2.5 kg. The ventilator can measure, display, record, and alarm Spo2, Fio2, CO2, and pulse rate data when integrated with the appropriate accessories. The ventilator is suitable for use in institutional, home, and nonemergency transport settings such as wheelchair or personal vehicle. It may be used for both invasive and noninvasive ventilation. | Use in hospital for less severely ill or recovering patients Transport |
$4,000 |
| Phillips EV300 | The Trilogy EV300 ventilator provides continuous or intermittent positive-pressure ventilation for the care of individuals who require mechanical ventilation. Trilogy EV300 is intended for pediatric through adult patients weighing at least 2.5 kg. The ventilator can measure, display, record, and alarm Spo2, Fio2, CO2, and pulse rate data when integrated with the appropriate accessories. The ventilator is suitable for use in institutional, home, and nonemergency transport settings, for example, wheelchair or personal vehicle. It may be used for both invasive and noninvasive ventilation. The Trilogy EV300 ventilator is intended to be used:
|
Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$16,000 |
| General Motors Ventec Life Systems V+ Pro | VOCSN V+ Pro Unified Respiratory System is intended to provide continuous or intermittent ventilatory support for the care of individuals who require mechanical ventilation. It may be used in invasive and noninvasive applications. VOCSN is intended for pediatric through adult patients weighing at least 5 kg. It is intended for use in home, hospital, institutional, and transport settings, including portable applications. | Use in the hospital for definitive care of patients with COVID-19 Transport as required |
$16,000 |
| Zoll 731 | Each model of the ZOLL 731 Series of ventilators is indicated for use in the management of infant through adult patients weighing ≥5 kg with acute or chronic respiratory failure or during resuscitation by providing continuous positive-pressure ventilation. ZOLL Ventilators are appropriate for use in hospitals, outside the hospital, during transport and in severe environments where they may be exposed to rain, dust, rough handling, and extremes in temperature and humidity. | Use in hospital for less severely ill or recovering patients Transport |
$18,000 |
From the manual.
Eleven ventilator manufacturers were initially contracted to build 15 types of ventilators. Based on their level of technologic sophistication, at least 58,000 of these nearly 200,000 newly procured ventilators would be considered “full feature” ventilators capable of supporting severe COVID-19 patients, and another 75,000 could be used on other patients or circumstances (Table 2). Of note, Philips Corporation was contracted to build 43,000 ventilators, which were split between two models, only one of which was a feature ventilator; and Vyaire Corporation was contracted to build 22,000 ventilators, again split between two models, only one of which is a full-feature ventilator. Public records do not specify the number of each ventilator ordered, thus precluding knowledge of the total number of full-function ventilators ordered.
Since the time of the initial orders, some of these orders have been significantly reduced (August 31, 2020). Vyaire will deliver only 4,000 of an initial order of 22,000; Hamilton up to only 4,518 of 25,574; and Phillips only 12,300 out of 43,000, for a total reduction of 69,576 ventilators.34 , 35 Nonetheless, understanding the functionality of all types of ventilators ordered will inform their best use.
Consensus groups have recommended positive-pressure ventilation surge device capabilities for mass respiratory failure.11 , 36 , 37 Newly purchased stockpile ventilators will remain in storage and on-demand for short notice in critical emergency situations. Ventilators require iterative preventive maintenance, and many require ongoing battery charging to maintain their readiness. Because nearly 200,000 ventilators (now down to 130,000) will not come from a single vendor, most will not have interchangeable parts, and most will require massive stockpiling of spare parts for maintenance, which in and of themselves have a storage lifespan. It is therefore important to buy the correct number and type of ventilators, and to ensure future maintenance and parts fulfillment.38
Ventilators that have full feature support capable of supporting the spectrum of potential COVID-19 patients include Hamilton (C3, C1, and T1), General Electric (Carescape R860), Vyaire (LTV 2200), Phillips (Trilogy EV300), and General Motors/Ventec Life Systems (VOCSN V+ Pro). The EUA has a number of noteworthy provisions (Fig 5 ), but purchasers should be aware of the limited requirements of manufacturers to support these devices over time.
Figure 5.
The Public Readiness and Emergency Preparedness (PREP) Act.39,40
The PREP Act provides liability immunity for manufacturers of ventilators and other devices under the EUA (Fig 5).39 , 40 Evidence for effectiveness includes those devices that “may be effective.” An example is the EUA approval of “splitters” to allow multiple patients to be ventilated using a single ventilator, but these “plumbing solutions” fail to take into account the physiologic issues that create a potentially dangerous technique that should only be attempted by experts under regulatory approval.36 , 41 In the absence of an EUA, likely none of these “splitters” could prove safe and otherwise would not obtain FDA approval. If the manufacturer fails to submit additional safety and procedural document 510K in a timely manner after expiration of the EUA, the devices will become nonsupported and a liability for the recipient.
How Many Ventilators Are Needed
In determining how many additional ventilators are needed to meet the surge capacity demands of COVID-19 across the United States with variable waves of disease, the assumption that the entire country will be simultaneously impacted may be flawed. During the initial 2020 COVID-19 pandemic wave, the United States has not in fact run out of ventilators, although several regions of hot spots have come remarkably close to implementing triage protocols for ventilator allocation, as alluded to in the New York experience.37 For analysis, we may consider an extreme worst-case second-wave scenario with the 1918 influenza pandemic as a model, in which the second wave is five times6 worse than the first wave.38 (No current models predict such a number.) This would bring the number of critically ill patients to a total of approximately 500,000 who could require mechanical ventilation. This vastly exceeds the amount of staff and space available to support these patients and likely would result in crisis triage implementation.
A more realistic model would have the second wave similar to the first, admittedly with a slightly easier course, given the medical community is now familiar with the disease, with well-tested protocols for managing these patients. Because the United States is quite diverse in terms of population density and outbreaks, disease incidence will not be homogenous. To supplement a hot spot with a smaller increase in ventilator inventory, we would require a much smaller number of full-feature ventilators (approximately 10,000-15,000). Importantly, it is not the cumulative number of ventilators required across the duration of the pandemic, but the concurrent number in use that is the relevant goal. The two key variables are not the total number of cases but rather the rate of new ventilator-dependent patients admitted and their length of stay. A surplus, if supplied to regions approaching crisis triage levels, could be supplied by doubling the current US ventilator inventory by an additional 50,000 to 75,000 full-featured ventilators.
All patients afflicted with COVID-19 respiratory failure would not need a ventilator on the same day. The current pandemic has placed demands on local resources in epicenters (New York, Boston, Seattle, New Orleans, and now Phoenix and Miami), whereas other cities had reduced ventilator usage because of public health directives in place regarding elective surgeries and social distancing. Sharing ventilators between health systems from cities minimally impacted and cities under duress should be explored. As New York struggled for ventilators, hospitals in the center of the country had ventilators standing idle; 400 ventilators in Seattle were able to be returned to the SNS.42 Given the generally milder spectrum of COVID-19 infection among children, pediatric hospitals have been relatively spared and could (and have) lend full-feature devices with adult capabilities, along with trained critical care staff, to adult hospitals.
Pediatric Capability
Children constitute approximately 25% of the US population, and infants and younger children cannot be supported by many mechanical ventilators designed for adults. In recognition of this, the 2014 CHEST Mass Critical Care Guidelines11 recommended stockpiled equipment for mass disasters, and pandemics should be adaptable to the needs of critically ill children. Most importantly, in recognition of the central role of ventilator support in the provision of critical care, the guidelines recommended that US FDA approval be obtained for stockpiled mechanical ventilators intended for use in infants and children. Typically, these ventilators will have adjustable (1) tidal volumes (that can be decreased to 30-50 mL total) and (2) flow triggers that can be adjusted to be more sensitive so as to detect and trigger an infant’s breath. Pediatric considerations for stockpiled mechanical ventilators were detailed by the Pediatric Task Force for Emergency Mass Critical Care in 2011 and are consistent with the above recommendations.43
Of ventilators currently considered for (or already receiving) federal funding, multiple ventilators are not FDA-approved for pediatric use (Table 2) because their tidal volumes cannot be lowered sufficiently to ventilate the much smaller lungs of a child. These ventilators would be entirely unusable for most pediatric patients.
Pediatric critical care practitioners historically use significantly more noninvasive mechanical ventilation in comparison with adult critical care medicine, in part to avoid the higher risks associated with pediatric intubation and mechanical ventilation. In initial reports, some adults with COVID-19 also fare better with the use of high-flow nasal oxygenation and noninvasive ventilation strategies, thus avoiding the risks associated with intubation and invasive mechanical ventilation.18 Therefore, it is likely important for both children and adults to consider mechanical ventilators that may be adapted to noninvasive mechanical ventilation modes (such as CPAP and bilevel pressure ventilation) or high-flow nasal cannula.
Fortunately, acute COVID-19 critical illness appears to be relatively rare and less severe in the pediatric population.44, 45, 46 However, we are beginning to discover a new inflammatory condition in children associated with prior asymptomatic COVID-19 infection: Multisystem Inflammatory Syndrome in Children (MIS-C). These patients typically present with some element of septic and cardiogenic shock and often require mechanical ventilation.47 How many of these children we should expect as time passes and pediatric infection rates rise remains unclear. If we were to have a large enough surge of MIS-C patients, we would require pediatric-adaptable mechanical ventilators to support them. Regardless of whether MIS-C becomes a problem that surpasses current pediatric ventilator resources, we do have a duty to steward our limited resources and prepare wisely for future mass disasters and pandemics that may have a very different pediatric risk profile. Saving the lives of children must be part of our strategic national vision.
Surplus Ventilators to Other Countries
Low- and middle-income countries (LMICs), including the countries to the south of the United States, are facing multiple challenges associated with COVID-19. Mexico, for example, lacks sufficient quantity of ventilators and has limited access to purchase ventilators from the global marketplace, mainly because of two reasons: (1) Mexican institutions arrived late to place orders from different companies to purchase ventilators and therefore were behind the queue of wealthier countries that anticipated their needs earlier; and (2) The ventilators accessible to Mexico are quite different compared with those purchased by the United States. Mexico can only purchase small transport ventilators or those originally designed to be noninvasive ventilators that have been modified to use as invasive ventilation.
According to recent social media from the acting administrator for the United States Agency for International Development,48 ventilators are currently being sent to countries such as Ecuador, El Salvador, Vietnam, Honduras, and the Caribbean. LMICs routinely struggle with shortages of oxygen supplies and funding for nurses and physicians. Sophisticated or difficult-to-use ventilators will further challenge the recipients of donated equipment who may have less experience with the devices offered.49 Gaps in knowledge, use, training, tech support, and re-supply of tubing should be considered before donation of surplus ventilators to LMICs, because they may not meet the needs of COVID-19 patients in foreign countries.
Conclusions
COVID-19 induces a wide spectrum of acute lung disease, including ARDS physiology with varying degrees of hypoxemia and abnormal compliance. The current SNS ventilators (especially the LTV 1200) were able to support most, though perhaps not all, patients with severe COVID-19 ARDS lung disease. As described in Table 4 , there were many technologic, logistic, and educational challenges encountered, with potential next-generation SNS ventilator updates listed alongside.
Table 4.
Technologic, Logistic, and Educational Challenges Encountered With Current SNS Ventilators, and Potential Next-Generation SNS Ventilator Updates
| Challenges Encountered With Current SNS Ventilators During COVID-19 Pandemic | Potential Design Updates for Next-Generation SNS Ventilators Based on COVID-19 Pandemic Current Challenges Encountered |
|---|---|
| COVID-19 patients develop severe ARDS and were at times not able to be fully supported by an SNS ventilator | SNS ventilators should have a full suite of features and compatible supplies to closely match the likely expected deployment and implementation of their use, including severe ARDS |
| Logistic difficulties with SNS ventilators included short or absent gas hoses, insufficient amount of compatible disposable supplies, and some ventilators arrived damaged or requiring maintenance before they could be used. | SNS ventilators should include power cords and gas hoses long enough for use in most environments, standardized size, disposable elements, and easy maintainability; and standard reliable maintenance and upkeep while housed in the SNS.a |
| Health-care professionals were not familiar with SNS ventilators, had difficulty setting them up, and did not have time to use the training materials. |
|
| SNS ventilators were more difficult to use because they did not have graphics display information, could not connect to medical records, and had difficult-to-hear alarms | SNS ventilators should incorporate:
|
| New SNS ventilators are not all able to be used with pediatric patients | All future SNS ventilators must be designed to support pediatric patients |
| When sharing ventilators with international partners, compatibility with their current infrastructure is not always taken into account | The United States should publish manuals and educational materials in the language of each international partner, with assured compatibility with their existing supplies and capacity for maintenance and repairs |
After purchase of the LTV-1200 devices from Vyaire, the company was purchased by Becton-Dickson, and the contract for maintenance was transferred to a third party. This transition resulted in litigation, during which time maintenance tasks were in limbo.
Determining the “right” number of SNS ventilators was also a challenge. The Federal Emergency Management Agency has currently issued orders for 15 different ventilators and devices that together with current SNS ventilators should meet US current and projected ventilator needs even with a severe second pandemic phase. In addition, all geographic regions are unlikely to be affected simultaneously, and the potential for a national ventilator-sharing strategy should continue to be further evaluated. However, stockpiling, maintenance, and training of health-care staff for this many devices in the long term is untenable; the pre-pandemic SNS had three different ventilator types, which seems a reasonable standard.
Our current COVID-19 pandemic data and experience along with described SNS ventilator challenges should inform next-generation SNS ventilator development, with potential updates noted in Table 4. New ventilator models should be easy to set up and initiate use; include “cutting edge” technologies such as user friendly, intuitive interfaces that are easily and quickly understood; offer graphic displays of pressure, volume, and flow waveforms for rapid pulmonary mechanics interpretation; interface with electronic health records; include easily audible alarm systems; and be operable remotely wirelessly to obviate the constant need for donning personal protective equipment to enter a patient’s room. Next-generation SNS ventilators must all be compatible for use on pediatric patients.
To sustain continuous technologic advancement from events such as the COVID-19 pandemic, SNS ventilators should continually be reevaluated through multiyear cycles with new, updated ventilators added yearly, older ventilators either repurposed or sold, and all ventilators undergoing routine scheduled maintenance to optimize readiness for deployment. Sufficient stockpiling of spare ventilator parts needs to be contracted at the time of purchase to ensure that stockpile ventilators remain usable over time.
Finally, as the body of knowledge and experience grows from the COVID-19 pandemic, assimilating it should combine input from the best experts from the critical care scientific community along with skilled ventilator engineers and incorporate an ongoing iterative and dynamic quality improvement process for SNS ventilators. Input from SNS ventilator end-user communities is also necessary, including the US military and national disaster management systems, among others.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. B. reports financial interest in Ventec Life Sytems, and has worked as a consultant for Zoll Medical and Mallinckrodt. None declared (J. R. D., H. F., A. D., D. D., J. B., T. H., M. G., M. K., M. B., M. D. C., G. D.-C., K. H., A. M. O. M., M. H., D. O., J. P., D. R., R. C. M., N. K., L. R.).
Other contributions: Some of the authors (Mr Rodriquez and Dr Maves) are US government employees or military service members. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Air Force, the Department of Defense, nor the U.S. Government.
References
- 1.Rubinson L., Vaughn F., Nelson S., et al. Mechanical ventilators in US acute care hospitals. Disaster Med Public Health Prep. 2010;4(3):199–206. doi: 10.1001/dmp.2010.18. [DOI] [PubMed] [Google Scholar]
- 2.Niska R.W., Burt C.W. Emergency response planning in hospitals, United States: 2003-2004. Adv Data. 2007;(391):1–13. [PubMed] [Google Scholar]
- 3.Emergency Use Authorization for Ventilators 2020; Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-2019) Public Health Emergency: Guidance for Industry and Food and Drug Administration Staff. https://www.fda.gov/media/136318/download
- 4.Becoming ‘King of Ventilators’ may result in unexpected glut. Modern Healthcare. 2020 https://www.modernhealthcare.com/government/becoming-king-ventilators-may-result-unexpected-glut [Google Scholar]
- 5.Tobin M.J. Advances in mechanical ventilation. N Engl J Med. 2001;344(26):1986–1996. doi: 10.1056/NEJM200106283442606. [DOI] [PubMed] [Google Scholar]
- 6.Singer B.D., Corbridge T.C. Basic invasive mechanical ventilation. South Med J. 2009;102(12):1238–1245. doi: 10.1097/SMJ.0b013e3181bfac4f. [DOI] [PubMed] [Google Scholar]
- 7.Walter J.M., Corbridge T.C., Singer B.D. Invasive mechanical ventilation. South Med J. 2018;111(12):746–753. doi: 10.14423/SMJ.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobin M.J. Physiologic basis of mechanical ventilation. Ann Am Thorac Soc. 2018;15(Suppl 1):S49–S52. doi: 10.1513/AnnalsATS.201705-417KV. [DOI] [PubMed] [Google Scholar]
- 9.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L., Chiumello D., Caironi P., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einav S., Hick J.L., Hanfling D., et al. Surge capacity logistics: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 Suppl):e17S–e43S. doi: 10.1378/chest.14-0734. [DOI] [PubMed] [Google Scholar]
- 12.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 13.Adachi T., Chong J.M., Nakajima N., et al. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Emerg Infect Dis. 2020;26(9):2157–2161. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter T., Haslbauer J.D., Nienhold R., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziehr D.R., Alladina J., Petri C.R., et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ASPR—Public Health Emergency. SNS-held ventilator resources. 2020. https://www.phe.gov/emergency/events/COVID19/SNS/ventilators/Pages/training.aspx Accessed August 22, 2020.
- 20.Elliott J., Waldman A., Kaplan J. How New York City’s emergency ventilator stockpile ended up on the auction block. 2020. https://www.propublica.org/article/how-new-york-city-emergency-ventilator-stockpile-ended-up-on-the-auction-block
- 21.Rahhal N. New York is putting two coronavirus patients on one ventilator and making them out of anesthesia machines as Cuomo warns patients could be on them for 21 days. 2020. https://www.dailymail.co.uk/health/article-8157185/New-York-splitting-ventilators-converting-anesthesia-machines-coronavirus-patients.html
- 22.Norton A. How U.S. hospitals cope with ventilator shortages. 2020. https://www.webmd.com/lung/news/20200330/how-us-hospitals-cope-with-ventilator-shortages#1
- 23.Haina K.M.K.J. Use of anesthesia machines in a critical care setting during the coronavirus disease 2019 pandemic. A & A Practice. 2020;14(7):e01243. doi: 10.1213/XAA.0000000000001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.New York State Task Force on Life and the Law New York State Department of Health Ventilator Allocation Guidelines 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf
- 25.Dwyer C. “This Is A Big Deal”: New York Hails Ventilator Deliveries From China and Oregon. 2020. https://www.npr.org/sections/coronavirus-live-updates/2020/04/04/827314791/this-is-a-big-deal-new-york-hails-ventilator-deliveries-from-china-and-oregon
- 26.The Official Website of the City of New York Mayor de Blasio announces James O’Neill as COVID-19 Senior Advisor. 2020. https://www1.nyc.gov/office-of-the-mayor/news/215-20/mayor-de-blasio-james-o-neill-covid-19-senior-advisor#/0
- 27.Sanger D.E., Zolan K.-Y., Kulish N. A ventilator stockpile, with one hitch: thousands do not work. 2020. https://www.nytimes.com/2020/04/01/us/politics/coronavirus-ventilators.html
- 28.Defense Production Act authorization for Ventilators. 2020. https://www.whitehouse.gov/presidential-actions/memorandum-order-defense-production-act-regarding-purchase-ventilators/ Accessed March 2020.
- 29.Lassen H., editor. Management of Life-Threatening Poliomyelitis. Edinburgh and London: E.& S. Livingstone Ltd.; Copenhagen, Denmark: 1956. [Google Scholar]
- 30.Release HP HHS Announces New Ventilator Contracts, Orders Now Totaling Over 130,000 Ventilators. 2020. https://www.hhs.gov/about/news/2020/04/13/hhs-announces-new-ventilator-contracts-orders-now-totaling-over-130000-ventilators.html
- 31.Release HP HHS Announces Ventilator Contract with GE Under Defense Production Act. 2020. https://www.hhs.gov/about/news/2020/04/16/hhs-announces-ventilator-contract-with-ge-under-defense-production-act.html
- 32.Release HP HHS Announces Ventilator Contract with GM under Defense Production Act. 2020. https://www.hhs.gov/about/news/2020/04/08/hhs-announces-ventilator-contract-with-gm-under-defense-production-act.html
- 33.Release HP HHS Announces Ventilator Contract with Philips under Defense Production Act. 2020. https://www.hhs.gov/about/news/2020/04/08/hhs-announces-ventilator-contract-with-philips-under-defense-production-act.html
- 34.Stein S. U.S. Prematurely Cancels Ventilator Contracts with Three Makers. Technology. 2020 https://www.bloomberg.com/news/articles/2020-08-31/philips-cuts-outlook-after-u-s-slashes-ventilator-contract [Google Scholar]
- 35.Siddiqui F. The U.S. forced major manufacturers to build ventilators. Now they’re piling up unused in a strategic reserve. 2020. https://www.washingtonpost.com/business/2020/08/18/ventilators-coronavirus-stockpile/
- 36.Beitler J.R., Kallet R., Kacmarek R.P., et al. Ventilator sharing protocol: dual-patient ventilation with a single mechanical ventilator for use during critical ventilator shortages. 2020. https://www.gnyha.org/wp-content/uploads/2020/03/Ventilator-Sharing-Protocol-Dual-Patient-Ventilation-with-a-Single-Mechanical-Ventilator-for-Use-during-Critical-Ventilator-Shortages.pdf
- 37.Hospital experiences responding to the COVID-19 pandemic: results of a national pulse survey March 23-27, 2020. 2020. https://psnet.ahrq.gov/issue/hospital-experiences-responding-covid-19-pandemic-results-national-pulse-survey-march-23-27
- 38.Centers for Disease Control and Prevention 1918 Pandemic (H1N1 virus) Pandemic Influenza. 2019 https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html [Google Scholar]
- 39.Azar A.M.I. Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19; A Notice by the Health and Human Services Department on 03/17/2020. 2020. https://www.federalregister.gov/documents/2020/03/17/2020-05484/declaration-under-the-public-readiness-and-emergency-preparedness-act-for-medical-countermeasures
- 40.Public Readiness and Emergency Preparedness Act. 2020. https://www.phe.gov/Preparedness/legal/prepact/Pages/default.aspx
- 41.Beitler J.R., Mittel A.M., Kallet R., et al. Ventilator sharing during an acute shortage caused by the COVID-19 pandemic. Am J Respir Crit Care Med. 2020;202(4):600–604. doi: 10.1164/rccm.202005-1586LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutman D. Washington sending over 400 ventilators for non-coronavirus patients to New York, harder hit states. 2020. https://www.seattletimes.com/seattle-news/health/washington-sending-over-400-ventilators-for-non-coronavirus-patients-to-new-york-harder-hit-states/
- 43.Kissoon N., Task Force for Pediatric Emergency Mass Critical C Deliberations and recommendations of the Pediatric Emergency Mass Critical Care Task Force: executive summary. Pediatr Crit Care Med. 2011;12(6 Suppl):S103–S108. doi: 10.1097/PCC.0b013e318234a612. [DOI] [PubMed] [Google Scholar]
- 44.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parri N., Lenge M., Buonsenso D. Coronavirus Infection in Pediatric Emergency Departments Research Group. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):1–6. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barsa J. 2020. https://twitter.com/search?q=https%3A%2F%2Ftwitter.com%2FJBarsaUSAID%2Fstatus%2F1283049100808781826%22%20&src=typed_query. Accessed August 22, 2020.
- 49.Torbati Y. US sending ventilators to countries that don't need or can’t use them. 2020. https://www.medscape.com/viewarticle/934094
- 50.CareFusion LTV 2 2200/2150 Ventilator Operators Manual. 2020. https://www.vyaire.com/products/ltv2tm-series-ventilators
- 51.Carescape R860 Ventilator Users Reference Manual 2013. https://www.gehealthcare.com/-/jssmedia/f96ef53464d6430d93c22fcf643aec9f.pdf?la=en-us
- 52.Hamilton-C3 Ventilator Manual. 2017. https://www.hamilton-medical.com/dam/jcr:5687919f-6926-4268-aa7c-f935b513fc5b/HAMILTON-C3-ops-manual-SW2.0.x-en-624446.03.pdf
- 53.Hamilton T1 Ventilator Operators Manual. 2020. https://www.hamilton-medical.com/dam/jcr:bef584f1-2fad-448c-81de-e83921f412da/HAMILTON-T1-ops-manual-SW2.2.x-en-USA-10078282.00.pdf
- 54.Hamilton-C1 Ventilator Operators manual. 2019. https://www.hamilton-medical.com/dam/jcr:fb63acb2-87c1-477d-add9-21fe559bf9e9/HAMILTON-C1-ops-manual-SW2.2.x-en-USA-10078281.00.pdf
- 55.Trilogy Evo Universal Instructions for Use. Phillips Respironics. 2018 https://usermanual.wiki/Respironics/1127941-3851091.pdf [Google Scholar]
- 56.pNeuton transport ventilator Model A Manual (Airon). 2011. https://aironusa.com/wp-content/uploads/2017/10/CD-A-005-Rev-I-pNeuton-Users-Manual-Model-A-English.pdf
- 57.Puritan Bennet 560 Ventilator Manual. http://www.industrie.gov.dz/IMG/pdf/Manual_-_User_PB560_-_English.pdf
- 58.ResMed ventilator manual Astral series. 2020. https://www.resmed.com/us/dam/documents/products/machine/astral-series/user-guide/astral-100-150_user-guide_amer_eng.pdf
- 59.SAVe II+ ventilator operators guide. http://ehyadarman.com/files/upload/product-brochure/admin-Operator_Manual_SAVe_II-eng-1543759244.pdf
- 60.VOCSN V+ PRO Ventilator clinical and technical manual (ventec). 2020. https://www.venteclife.com/assets/Resources/VOCSN_Clinical_Manual.pdf
- 61.Koninkijke Philips N.V. Phillips Trilogy EV 300: ventilator instructions for use. 2019. Murraysville, PA: Phillips Respironics; 2019.
- 62.Zoll Ventilator operators guide, model EMV+, AEV, and Eagle II. https://www.zoll.com/-/media/public-site/products/ventilators/906-0731-01-05-sf_b.ashx/
- 63.Hill Rom Life2000 ventilator clinician instructions for use. https://www.breathetechnologies.com/wp-content/uploads/2020/04/PL-20-0049-A.pdf