Abstract
Introduction
Osteoporosis is defined as a systemic skeletal disease characterized by reduced bone mass and degeneration of bone tissue microarchitecture which leads to bone fragility and fracture risk. Annually, 100 to 200 million people around the world are at risk for osteoporotic fractures. One way to prevent osteoporosis fracture is by using medications such as bisphosphonates. Alendronate is the most prescribed bisphosphonate in the world. The objective of this article is to evaluate the effect of alendronate on bone fracture healing.
Material and methods
15 adult male rats that were 60 days old were used, divided into three groups: A or Control, B (non-osteoporotic bones plus alendronate application) and C (osteoporotic bones plus alendronate application). Osteoporotic bones were compared with non-osteoporotic bones that underwent bone window creation and administration of alendronate sodium. These bones were submitted to radiographic and histological analysis.
Results
All of Group A had complete bone healing, reaching the phase of bone remodeling. While in groups B and C, the rats were in the repair phase.
Conclusions
The drug alendronate interferes with delayed fracture healing and delayed bone remodeling. The article advises that studies in humans are needed in order to assess whether the alendronate interferes with bone healing.
Keywords: Alendronate (MeSH ID: D019386), Bone fracture (MeSH ID: D050723), Corticoid (MeSH ID: 000305), Fracture healing (MeSH ID: D017102), Osteoporosis (MeSH ID: D010024)
1. Introduction
The fracture is the loss of the continuity solution of a bone and the traumatic action that leads to a particular fracture causes damage to the neighboring structures, thus forming a complex of changes that make up the secondary complex1,2. The fracture can occur due to high energy trauma (motor vehicle accidents and falls from great heights) or low energy (falls from the same height). Regarding gender, men have bimodal distribution (accidents in young adults due to work and lifestyle and in the elderly due to bone fragility, osteoporosis), while in women there is a unimodal distribution (in elderly patients due to osteoporosis)3.
The bone healing occurs in three phases1: inflammatory phase, during which the necrotic tissue is removed2; reparative phase, when the rapid synthesis of a new matrix occurs3; remodeling phase, the disorganized matrix of the previous phase undergoes a maturation process, becoming a compact and functionally efficient structure in addition to the formation of the bone marrow tissue.1,2
There are factors that can interfere with the bone healing and are divided into two groups: mechanical and biological. The mechanical factors are: trauma intensity, fracture type, exposed or unexposed fracture and inadequate fracture stability. Biological factors are: vascular diseases, metabolic diseases (such as osteoporosis), deficiencies, medication use (such as corticosteroids), among others.1
Osteoporosis is defined as a systemic skeletal disease characterized by reduced bone mass and bone tissue microarchitecture degeneration which leads to bone fragility and fracture risk. There is negative bone remodeling balance where there is bone absorption increase performed by osteoclasts and bone deposition decrease performed by osteoblasts. Osteoporosis is a common disease in the elderly, 40% of women and 14% of men over 50 years old will suffer fractures related to this disorder. Annually, 100 to 200 million people worldwide risk osteoporotic fractures.4 The most important risk factors related to osteoporosis and postmenopausal fracture are: age, female, white or oriental ethnicity, previous personal and family history of fracture, low bone mineral density in the femoral neck, low body mass index, oral glucocorticoid use (dose greater than or equal to 5 mg/day of prednisone for more than three months), environmental factors, smoking, alcohol consumption, physical inactivity and low dietary calcium intake.5
One way to prevent osteoporosis fracture is by using medications such as bisphosphonates. These inhibit bone remodeling by inhibiting osteoclast function.6 And alendronate is the most prescribed bisphosphonate in the world.7
The objective of this article is to evaluate the effect of alendronate on the bone fracture healing in osteoporotic and non-osteoporotic bones.
2Material and methods
The research was carried out at the Experimental Laboratory for the Study of Pain (LEED) located on the Bacanga campus of the Federal University of Maranhão. The project was approved by the Ethics Committee on the Use of Animals of the Federal University of Maranhão (UFMA) with the number 23115.034,794/2018–51.
Rats from the Rattus norvegicus (albinus variety) species of WISTAR lineage that were approximately 60-day-old male adults, obtained from the UFMA Central Vivarium were used and fed with standard food ad libitum and kept under controlled light and temperature conditions.
Fifteen rats were used, distributed in three groups with five rats in each. This N of 5 animals was found using the formula n = 1 + [2C∗(s/d)2].8 All rats were submitted to bone window creation (what we consider as day 1). In group A (or control group), they underwent only the surgical procedure for making bone window and served as a comparison as to the stage of bone healing. In group B, rats were given alendronate sodium on the same day as the surgical procedure while group C rats were first submitted to corticoid-induced osteoporosis, and then they were submitted to the surgical procedure and administration of alendronate sodium.
Alendronate sodium was administered subcutaneously at a dose of 0.7 mg/kg dissolved in saline once a week.9 The initial dose was administered on the same day as the bone window was made for groups B and C.
Osteoporosis was induced in group C by applying an intramuscular injection of 7 mg/kg of dexamethasone once a week for five weeks 10,11.
Alendronate and dexamethasone were manipulated at these doses for application in rats.
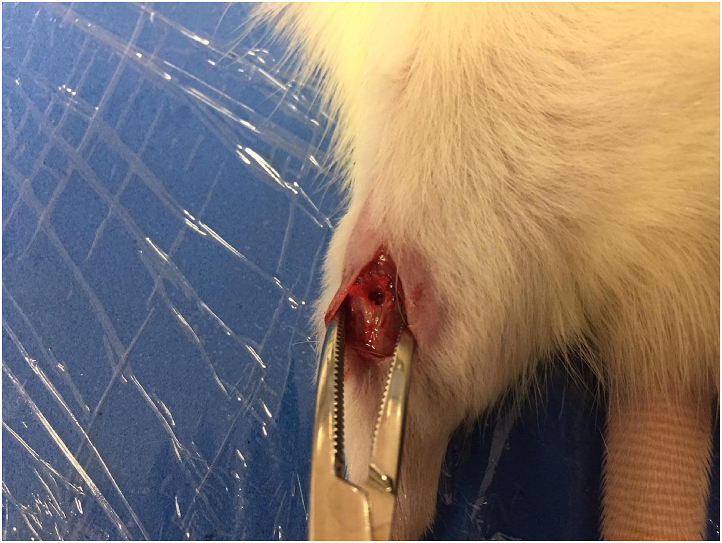
For the surgical procedure creating the bone window, animals were subcutaneously (SC) submitted to pre-anesthetic morphine (Dimorf® 10 mg/ml, Cristália) at a dose of 2 mg/kg 30 min before anesthesia. Anesthesia was performed with ketamine (Dopalen® 100 mg/ml, CEVA) at a dose of 60–90 mg/kg plus xilasine (Anasedan®, 20 mg/ml, CEVA) 6–10 mg/kg, both intraperitoneal (IP).12 With the anesthetized rats, they were submitted to trichotomy of the right hind leg and the procedure was carried out respecting asepsis and antisepsis techniques. The incision was made in the medial region of the leg plus dissection by planes up to the tibia. With bone exposure, a bone window was made on the medial face of the diaphysis (looking for it to be halfway between the joints) using a 2.0 mm Kirschner wire under manual pressure (Fig. 1). After creating the bone window, the wound was mechanically washed with 0.9% saline and sutured. For postoperative analgesia, anti-inflammatory Meloxicam® (15mg/1,5 ml, Europharma) 1 mg/kg/day SC was applied for 72 h.12
Fig. 1.
Visualization of bone window in rat tibia.
As directed by the National Council for the Control of Animal Experimentation (CONCEA), rats were evaluated by facial expressions regarding pain assessment.12 All rats evolved well after making the bone window, so that they were able to sustain the load with the leg that underwent surgery and did not lose weight.
Euthanasia was performed after a 2-week period (day 14) after the bone window was made under high-dose IP with the anesthetics ketamine and xilazine.12 After death and leg removal in which the procedure was performed, the animals were discarded in hospital waste.
Radiographs of the animals’ legs were obtained with a CR Philips radiography device. Radiographic images were taken with the following parameters: 85 cm focus-film distance, 0.8s exposure time, 45 kV potential difference, amperage of 50 mA. Radiographs were developed using the scanning technique. This test was performed on the day the rat was subjected to making the bone window (day 1) and on the day the animal was euthanized (day 14). For radiographic analysis, the scores of Oryan et al. (2015) were used for the Bone Healing criterion.2 Table 1. Right after the surgical procedure, the rats (still under the effect of anesthesia) were taken to the radiography room and subjected to the examination.
Table 1.
Score for radiographic evaluation of healing evolution of bone healing in bone defects.2
| Bone healing | Grade |
|---|---|
| No healing | 1 |
| Possible healing | 2 |
| Complete healing | 3 |
After the animal was euthanized, the tibia was extracted and set in 10% formalin for 24 h and immersed in a descaling solution for 15 days, followed by routine method processing until inclusion in paraffin blocks. The compartments were separated longitudinally and sent for routine histological preparation using Hematoxylin-Eosin and Safranin. Tissue sections were placed in rectangular molds, forming blocks that were subsequently sectioned by 4 μm microtome steel knives. For analysis of the bone healing stage, the histological classification proposed by Allen et al. (1980)13,14 was used. Table 2.
Table 2.
Histological classification proposed by Allen et al. (1980) for analysis of bone healing stage.
| Grade | Characteristic |
|---|---|
| 4 | Complete bone healing |
| 3 | Incomplete bone union due to the presence of a small amount of callus cartilage |
| 2 | Visibility of a well-formed hyaline cartilage bridge, joining the main fragments (complete cartilaginous union) |
| 1 | Incomplete cartilaginous union, with fibrous element retention in the chondral plate |
| 0 | Absence or delay in fracture repair, characterized by the presence of cartilage between fragments and remains of hematoma or other fluid (pseudoarthrosis) |
The comparison of means from different experimental groups was performed using Student’s t-test or univariate analysis of variance (One-way ANOVA), followed by the Tukey test. In the evaluation of two variability sources, bivariate analysis of variance (Two-way ANOVA) will be used. The value of P < 0.05 was considered as indicative of significance and the data obtained was analyzed using the software “Graph pad Instat® (GraphPad software, San Diego, CA).
3. Results
3.1. Radiographic analysis
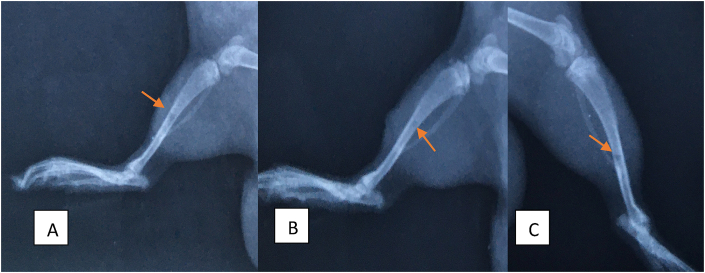
A group C, one rat had complications during osteoporosis induction and was euthanized before the end of the study. The 14 rats underwent radiography shortly after the surgical procedure and after 14 days. Radiography on day 1 was important to ensure that all rats had a bone defect of a similar pattern. Fig. 2 shows the radiographs taken in D1 with a representative from each group.
Fig. 2.
Shows the radiographs taken in D1 with a representative from each group. The arrows point to the locations of the bone defect.
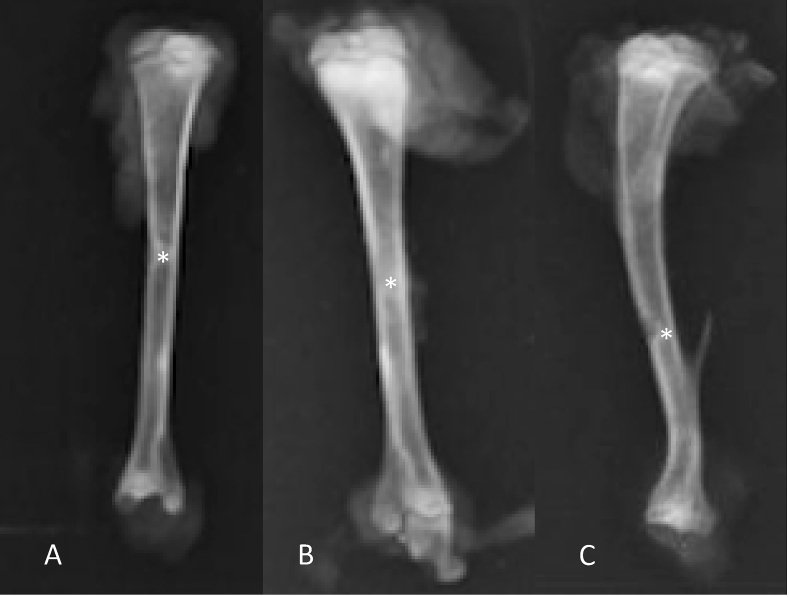
In Group A, 14 days are necessary for complete bone healing, showing a small area of reactional sclerosis (increased density) around the healed area. In accordance with scores of Oryan et al. (2015), all cases had complete bone healing. Fig. 3.
Fig. 3.
Radiographs of the tibiae of rats, with a representative from group A, one from group B and one from group C. Figure A: complete consolidation with little area of reaction sclerosis (∗). Figure B: complete bone consolidation with a large area of reactional sclerosis (∗). Figure C: absence of bone healing (∗).
In Group B, all rats were classified as grade 3 according to scores of Oryan et al. (2015), however in three cases an area of major sclerosis was found around the consolidation site. Fig. 3.
In Group C, in accordance with Oryan et al. (2015), two rats had grade 3 and two rats had grade 1. Fig. 3.
When statistical analysis was applied in the comparison of three groups, it was possible to observe that groups A and B were statistically different (p < 0.001) from the group that was induced by osteoporosis (group C).
3.2. Histological analysis
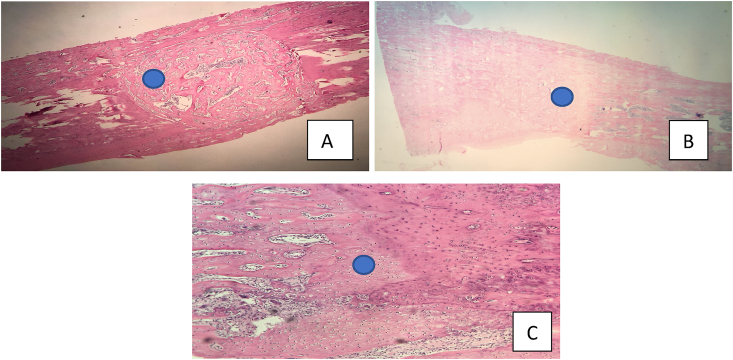
All of Group A were classified as grade 4 by Allen et al. (1980) and also shows signs of complete bone remodeling, as it presents bone marrow tissue. Fig. 4.
Fig. 4.
Histological sections in hematoxylin-eosin with a representative of groups A, B and C. Figure A: histological section (10x) of group A with emphasis on bone marrow. Figure B: histological section (10x) of group B highlighting the absence of bone marrow. Figure C: histological section (20x) of group C with evidence of hyaline cartilage bridge.
Regarding Group B, three of the five rats were classified as grade 3 by Allen et al. (1980) due to the presence of a small amount of cartilage in the bone callus and the other two rats were classified as grade 4. None of Group B presented bone remodeling, that is, the bones were in the repair phase of bone healing. Fig. 4.
In Group C, two rats were classified as grade 4 and two rats as grade 2 by Allen et al. (1980), that is, visualization of a well-formed hyaline cartilage bridge joining the main fragments (complete cartilaginous union). And in grade 4 cases, no signs of bone remodeling were found. That is, all the bones in this group were in the repair phase of bone healing. Fig. 4.
In this analysis, it was possible to detect a statistically significant difference when comparing group C to the control group (group A) (p = 0.0003).
Regarding the consolidation phase, the result was statistically significant when the control group (group A) was compared with the rats that underwent alendronate application (groups B and C).
Table 3 the statistical analyzes comparing the three groups in relation to postoperative pain, consolidation by radiographic analyzes, consolidation by histological analyzes and bone healing phase.
Table 3.
Statistical analysis comparing groups by the Tukey test.
| Análysis | Group | 95% difference ci | Significant | Adjusted p-value |
|---|---|---|---|---|
| Pain | A x B | −0.6655 a 0.6655 | No | >0.9999 |
| A x C | −0.6655 a 0.6655 | No | >0.9999 | |
| B x C | −0.6655 a 0.6655 | No | >0.9999 | |
| Healing to the radiography | A x B | −0.6655 a 0.6655 | No | >0.9999 |
| A x C | 0.7345 a 2.065 | Yes | <0.0001 | |
| B x C | 0.7345 a 2.065 | Yes | <0.0001 | |
| Histological analysis histological | A x B | −0.06546 a 1.265 | No | 0.0837 |
| A x C | 0.5345 a 1.865 | Yes | 0.0003 | |
| B x C | −0.06546 a 1.265 | No | 0.0837 | |
| Healing phase | A x B | 0.3345 a 1.665 | Yes | 0.0023 |
| A x C | 0.3345 a 1.665 | Yes | 0.0023 | |
| B x C | −0.6655 a 0.6655 | No | >0.9999 |
4. Discussion
Osteoporosis is defined as a systemic skeletal disease characterized by low bone mass and deterioration of bone tissue microarchitecture which consequently increases bone fragility and fracture risk due to increased osteoclast activity, leading to a negative balance in remodeling bone.15 Treatment can be done with anti-absorptive drugs (such as alendronate sodium) which inhibits osteoclast recruitment and activity, thus decreasing the bone remodeling rate, however displaying suppression of bone formation in women and rats as a side effect.6,7,15
In the study by Shimizu et al. (2012), alendronate affects osteoblast differentiation and mineralization.16 For diaphyseal fractures to be healed, alendronate leads large bone callus formation, but with delayed bone remodeling and of irregular bone tissue formation.17
In the study by Nobre et al. (2008), the bone window of 2,5 mm diameter was made in the diaphysis of the femur in rats and local application of alendronate. They observed that the animals submitted to the medication presented less bone neoformation when compared to the control group.18
Cardoso et al. (2011) evaluated the effectiveness of alendronate administration in patients undergoing surgical treatment for transtrochanteric fractures. They described that the patients who used the medication had a consolidation, assessed by radiography, in less time than the control group (7,6 weeks × 11 weeks). However, it was not a paired study comparing patients with and without osteoporosis.19
Regarding the effect of alendronate on bone healing in fractures in oophorectomized rats, the study by Cao et al. (2002) reported that this drug does not prevent the onset of fracture healing and callus formation, however, continued use prevents the bone remodeling process for a long period.7
Systematic review in humans carried out by Molvik and Khan (2015) reports that for distal radius fractures bisphosphonate use was associated with a significantly longer time to healing. In femur fractures showed a difference in union time, but this was not statistically significant. This may be explained by a smaller number in the studies (28 cases and 24 controls).20
In the systematic review carried out by Jin-Hean et al. (2014), the use of bisphosphonate after fracture was evaluated and showed approximate doubling of the risk of non-union with proximal humerus fractures. The increased risk associated with bisphosphonate use persisted in the subgroup of patients without a history of osteoporosis or prior fractures, albeit limited by small sample sizes.21
However, the systematic review in humans by Li et al. (2015) suggests that early administration of bisphosphonates after surgery will not delay fracture healing time, either radiologically or clinically.22
The research compared osteoporotic (induced by corticoid) and non-osteoporotic bones of male rats that underwent alendronate treatment. A control group was to serve as a healing reference without drug interference. For this, the study used bone window in the medial and diaphyseal region of the right tibia as a fracture model. Thus, synthesis was not necessary to maintain fragment reduction and osteotomy size allowed for the sustained load of the operated leg from the immediate postoperative period (which helped to eliminate confusion biases). With the Control group analysis, 14 days were sufficient for complete fracture healing and complete bone remodeling.
The results showed that alendronate delayed bone healing in booth osteoporotic and non-osteoporotic bones. A statistically significant difference was found between non-osteoporotic bones and osteoporotic bones in relation to degree of consolidation visualized by radiography and between the control group and group C in the histological analysis. They also showed that the medication influenced the bone healing phase, since all cases submitted to alendronate did not reach the bone remodeling phase (statistically significant result). This data is in accordance with the studies by Shimizu et al. (2012), Nobre et al. (2008) and Cao et al. (2002).
Recent systematic reviews in humans have shown different results in relation to the use of bisphosphonates after fracture. One possible explanation is the scarcity of clinical trial studies and the small number of patients evaluated.
This article presented the sample size and study time as negative points since it would be possible to assess the time required for complete bone remodeling in bones submitted to alendronate sodium treatment.
5. Conclusions
The results of this study showed that the drug alendronate sodium interferes with delayed fracture healing and delayed bone remodeling in rats. Thus, the article advises that studies in humans are needed in order to assess whether the alendronate interferes with bone healing.
References
- 1.Pedro Henrique B Mendes, Junior Scofano, Romeu Antônio. Consolidação da fratura após o uso prolongado de corticóide : estudo experimental em ratos ∗. Rev Bras Ortop. 2001;36:345–351. [Google Scholar]
- 2.Oryan A., Monazzah S., Bigham-sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci. 2015;28(1):57–71. doi: 10.3967/bes2015.006. [DOI] [PubMed] [Google Scholar]
- 3.Bucholz R.W., Heckman J.D., Court-Brown C.M., Tornetta P., McQueen M.M., Ricci W.M. 7 ed. vol. 1. Manole, organizador; 2013. pp. 54–55. (Fraturas Em Adultos). [Google Scholar]
- 4.Ruedi T.P., Buckley R.E., Moran C.G. 2 ed. ARTMED, organizador; 2009. Princípios AO do tratamento de fraturas; p. 493. [Google Scholar]
- 5.Cezar S., Bernardo W., Patrícia A. Artigo original Diretrizes brasileiras para o diagnóstico e tratamento da osteoporose em mulheres na pós-menopausa. 2017;7(S 2):452–466. [Google Scholar]
- 6.Barrett J.G., Sample S.J., Mccarthy J., Kalscheur V.L., Muir P., Prokuski L. Effect of short-term treatment with alendronate on ulnar bone adaptation to cyclic fatigue loading in rats. J Orthop Res. 2015;(August 2007):1070–1077. doi: 10.1002/jor.20395. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y., Mori S., Mashiba T. Raloxifene , estrogen , and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17 doi: 10.1359/jbmr.2002.17.12.2237. [DOI] [PubMed] [Google Scholar]
- 8.Weyne GR. de S. Determinação do tamanho da amostra em pesquisas e xperimentais na área de saúde. Arq Med ABC. 2004;29:87–90. [Google Scholar]
- 9.Vicente Neto P., Peccaro C.A., Baulducci E.Z. Análise histomorfométrica do reparo ósseo em tíbias de ratos castrados submetidos ao efeito do alendronato sódico. Rev Gaucha Odontol. 2010;58(4):491–496. [Google Scholar]
- 10.de Sousa L.H.T. Universidade Federal do Ceará; 2016. Influencia da Osteoporose induzida por glicocorticóide na perda óssea alveolar em ratos com periodontite experimental e o efeito do tratamento com Atorvastatina. [Google Scholar]
- 11.Santos C. de L., Rafacho A., Bosqueiro J.R. Efeitos da dministração de Dexametasona in vivo sobre glicemia, insulinemia e substratos circulantes são dependentes do tempo de tratamento. Biosci J. 2001;23:101–110. [Google Scholar]
- 12.Unifesp Uf de Sp . vol. 1. 2017. (Guia anestesia e analgesia em animais de laboratório). [Google Scholar]
- 13.Allen H.L., Wase A., Bear W.T. Indomethacin and Aspirin : effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51 doi: 10.3109/17453678008990848. [DOI] [PubMed] [Google Scholar]
- 14.Vasconcelos J.W., Milanez L., Vasconcelos G.D.A., Marcelo I., Maior S. Avaliação da sinvastatina no processo de consolidação de fraturas em tíbias de ratos. Rev Bras Ortop. 2013;48(2):191–195. [Google Scholar]
- 15.Compston J.E., Mcclung M.R., Leslie W.D. Osteoporosis. Lancet. 2019;393:364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu E., Tamasi J., Partridge N.C. Alendronate affects osteoblast functions by crosstalk through. J Dent Res. 2012;91:268–274. doi: 10.1177/0022034511432170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolios L., Kristin A., Stephan H. Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis Prophylaxis ? Calcif Tissue Int. 2010;86:23–32. doi: 10.1007/s00223-009-9318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobre M.D.P., Fernandes R.G., Chin C.M., Faig-Leite H. Ação local do alendronato sódico na reparação óssea de ratos espontaneamente hipertensos (SHR) Arq Bras Cardiol. 2008;90(4):261–268. doi: 10.1590/s0066-782x2008000400005. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso F.J.N., Nakano A.S., Frisene M., Hereda ME. de F., Batista B. de F., Kanaji P.R.C. Fraturas transtrocanterianas: uso de alendronato no pós-operatório. Acta Ortopédica Bras. 2011;19(1):45–48. [Google Scholar]
- 20.Molvik H., Khan W. Bisphosphonates and their influence on fracture healing: a systematic review. Osteoporos Int. 2015;26(4):1251–1260. doi: 10.1007/s00198-014-3007-8. [DOI] [PubMed] [Google Scholar]
- 21.Jin-Hean A., Yue B., Joseph S., Richardson M. Delayed/non-union of upper limb fractures with bisphosphonates: systematic review and recommendations. ANZ J Surg. 2014;84(4):218–224. doi: 10.1111/ans.12536. [DOI] [PubMed] [Google Scholar]
- 22.Li Y.T., Cai H.F., Zhang Z.L. Timing of the initiation of bisphosphonates after surgery for fracture healing: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2015;26(2):431–441. doi: 10.1007/s00198-014-2903-2. [DOI] [PubMed] [Google Scholar]