Abstract
OBJECTIVES:
To investigate how cardiometabolic-inflammatory risk factors are related to cognition among older adults in India and the United States.
DESIGN:
The Longitudinal Aging Study in India–Diagnostic Assessment of Dementia (LASI-DAD) and the Harmonized Cognitive Assessment Protocol of the Health and Retirement Study (HRS-HCAP) in the United States conducted an in-depth assessment of cognition, using protocols designed for international comparison.
SETTING:
Cognitive tests were conducted in hospital or household settings in India and in household settings in the United States.
PARTICIPANTS:
Respondents aged 60 years and older from LASI-DAD (N = 1,865) and respondents aged 65 years and older from HRS-HCAP (N = 2,111) who provided venous blood specimen.
MEASUREMENTS:
We used total composite scores from the common cognitive tests administered. Cardiovascular risk was indicated by systolic and diastolic blood pressure, pulse rate, pro-B-type natriuretic peptide (proBNP), and homocysteine. Metabolic risk was measured by body mass index, glycosylated hemoglobin (HbA1c), high-density lipoprotein cholesterol, and lipoprotein (a) (only in India). Inflammatory risk was indicted by white blood cell count, C-reactive protein, albumin, and uric acid (only in India).
RESULTS:
The distribution of both total cognition scores and of cardiometabolic risk factors differed significantly between India and the United States. In both countries, lower cognition was associated with older age, lower education, elevated homocysteine, elevated proBNP, and lower albumin levels. The associations between HbA1c levels and cognitive measures were statistically significant in both countries, but in the opposite direction, with a coefficient of 1.5 (P < .001) in India and −2.4 (P < .001) in the United States for one percentage increase in absolute HbA1c value.
CONCLUSION:
Cardiometabolic-inflammatory biomarkers are associated with cognitive functional levels in each country, but the relationships may vary across countries.
Keywords: dementia risk factors, Harmonized Cognitive Assessment Protocol, Longitudinal Aging Study in India, Health and Retirement Study, international comparisons
INTRODUCTION
Dementia has become an important public health and socioeconomic challenge in India as well as in the United States.1,2 Because of population aging, the prevalence of dementia will continue to increase in both countries, leading to higher medical and personal care expenses, decreased functioning and quality of life for affected individuals, and stress and loss of productivity for family caregivers. With ongoing population aging, it is estimated that the number of worldwide dementia cases will triple by 2050.3
Cardiometabolic diseases may increase the risk for cognitive decline and dementia through multiple causal pathways, including direct effects on neurons, hypoperfusion due to reduced cerebral blood flow, and promotion of amyloid cascade.4 Cardiovascular disease may also alter epithelium cells, which dislocate blood-brain barrier, resulting in disturbance in amyloid clearance. India has been undergoing a significant change in disease burden, with swift increase in cardiovascular and metabolic diseases. Data from the Global Burden of Diseases, Injuries, and Risk Factors Study indicated that cardiovascular diseases accounted for 28.1% of the total deaths in India in 2016, compared with 15.2% in 1990.5 However, this epidemiological transition is occurring decades later than that in the United States. Indians appear to have a unique “South Asian phenotype” with high propensity for metabolic syndrome, insulin resistance, greater degree of central obesity, as well as a high prevalence of hypertension at relatively young ages.6 In addition, there seems to be suboptimal management of cardiometabolic diseases in India, with only 10.7% of rural hypertension patients and 20.2% of urban patients having their blood pressure under control.7,8
This study provides an analysis of how risk factors—biological and sociodemographic—relate to cognitive functioning in a representative sample of Indians older than 60 years and compares the results with those in a representative sample of Americans aged 65 years and older. This comparison offers a unique opportunity to compare and contrast how cardiometabolic risk factors are related to cognitive functioning in these two populations. One has virtually complete literacy, and the other has a large nonliterate component. The marked difference in educational levels between older Indians and older Americans leads to significant differences in late-life cognition. In addition, the American older population has had extensive medical care throughout much of its life, whereas care for most Indians remains classified as fairly rudimentary.
METHODS
Study Populations
The study population in India is participants of the Longitudinal Aging Study in India–Diagnostic Assessment of Dementia (LASI-DAD). The details of study design and methods have been described in this special issue.9 LASI-DAD, a subsample from the population-representative LASI, was established to provide a more accurate prevalence estimate of dementia at the national level and study key risk factors associated with cognitive decline and dementia in India. It recruited 3,224 LASI participants aged 60 years and older with oversampling of those at high risk of cognitive impairment. To facilitate cross-country comparisons, LASI-DAD adopted the protocol from the Health and Retirement Study–Harmonized Cognitive Assessment Protocol (HRS-HCAP) to assess cognitive function and modified where necessary, considering India’s higher illiteracy and innumeracy as well as cultural context.
The details about HRS-HCAP have been published previously.10 Briefly, the HRS is an ongoing nationally representative panel study of about 20,000 adults aged 51 years or older in the United States. HRS-HCAP is a substudy within HRS designed to better measure and identify cognitive impairment and dementia in a representative population-based sample of U.S. adults aged 65 years and older. It inter-viewed the target HRS respondents as well as informants nominated by respondents when self-response was not possible. The HRS-HCAP cognitive test battery was designed to measure a range of key cognitive domains affected by cognitive aging and to allow harmonization and comparisons to other studies in the United States and around the world.
For this analysis, we included 1,865 individuals from LASI-DAD and 2,111 individuals from HRS-HCAP. Among 3,224 initial LASI-DAD respondents, 2,254 provided venous blood specimen (VBS) samples. We lost 139 respondents who were missing in various VBS assays, including pro-B-type natriuretic peptide (proBNP), C-reactive protein (CRP), albumin, homocysteine, high-density lipoprotein (HDL) cholesterol, glycosylated hemoglobin (HbA1c), and white blood cell count (WBC). In addition, we lost 250 respondents due to missing body mass index (BMI), pulse rate, or systolic and diastolic blood pressure. The details of venous blood collection and assay protocol are described in Dey et al11 and are available on the project website (www.lasi-dad.org).
A total of 3,347 HCAP participants attempted the cognitive testing. Of these participants, 3,210 had complete data on all the tests used here, and another 124 had nearly complete data for which imputation was done for missing tests using the performance on all other tests. We excluded 13 for incomplete data on cognition and about 340 because they did not have complete data on blood tests from the 2016 whole blood draw. In addition, we dropped about 900 because they did not have data from the HRS 2014 or HRS 2016 home visit, which were used for data on blood pressure, pulse, and BMI. For a total of 229 persons in the remaining sample of 2,111, we imputed a missing value of HbA1c based on their fasting plasma glucose using the relationship between the two observed in the cases with both measures.
Comparing respondents who had missing information with those who had complete data, we found that, in India, missingness was associated with being 75 years or older, having no education, and living in a rural area; in the United States, being missing was associated with being 75 years or older and being female. In each country, these predictors explained only 1% of the variance in missingness.
Measures
Cognitive Function Measure
For cross-country comparisons, we used a harmonized total cognitive score, which provides a comprehensive assessment of various cognitive domains. The total cognitive score is a summary score based on the following tests: 10-word learning, including immediate and delayed recall and recognition 12; logical memory (i.e., Brave Man story only), including immediate and delayed recall and recognition13; Mini-Mental State Examination (MMSE)14 or a validated Hindi version of MMSE summary score15; verbal fluency score,16 which was the number of named animals within 60 seconds; the community screening instrument for dementia score17; and the Raven test,18 a count of the number of correct answers to a series of images that required the respondent to select the missing piece. The range of total cognitive scores was from 0 to 175.
We imputed information for missing values in the cognitive tests. The implemented method used for the Indian data was inspired by the imputations of cognition variables used in the HRS.19 In a nutshell, this imputation method replaces missing values with random draws from a conditional distribution such that the estimated joint distribution from the completed (imputed) data is an unbiased estimator of the true joint distribution of these variables. The conditional distribution for each test score is obtained by regressing the to-be-imputed test score on other, previously imputed test scores, as well as demographic, health, and socioeconomic variables. Further details of imputation strategy are described in another article in this issue.20 Imputation has not yet been done on the available HRS-HCAP data; for this analysis, we imputed data for the 124 cases using a conventional nearest neighbor approach.
Cardiometabolic-Inflammatory Biomarkers
Both LASI-DAD and HRS-HCAP conducted face-to-face computer-assisted personal interviews on respondents and their informants, measured anthropometric and physical parameters, and collected venous blood for laboratory tests, including those related to cardiometabolic-inflammatory diseases.
Cardiovascular System
Biomarkers included high blood pressure, defined as systolic blood pressure of 140 mmHg or higher or diastolic blood pressure of 90 mmHg or higher, pulse rate, proBNP, and homocysteine. At-risk proBNP was defined as a level of 900 pg/mL or greater for those aged 50 to 74 years and 1,800 pg/mL or greater for those aged 75 years or older. These cutoff points are usually used to diagnose congestive heart failure (CHF).21,22
Metabolic System
Biomarkers included BMI based on measured height and weight (self-reported if measured height and weight are not available), HbA1c, HDL cholesterol, and lipoprotein (a) (which was not available in HRS). In HRS-HCAP, we have imputed HbA1c for those who have glucose in the VBS data but no value for HbA1c. Imputation was done separately for fasting and nonfasting samples. Because LASI-DAD had a significant percentage of nonfasting participants at the time of blood collection, we did not include glucose, low-density lipoprotein (LDL) cholesterol, and tri-glycerides in this analysis.
Inflammatory System
Biomarkers included WBC, CRP, albumin (a negative acute-phase reactant), and uric acid.
Covariates
Demographic characteristics included age and sex. Education was included as a categorical variable with three categories for the United States (0–11, 12, and ≥13 years) and four categories for India (0, 1–11, 12, and ≥13 years). The extra category for India is because a large proportion of older population has no formal education, whereas this is not true in the United States. Household wealth was also included as a categorical variable based on quintile distribution.
Statistical Approach
We use multivariate ordinary least squares regression analysis with the cognitive measure as the outcome variable. There are three equations for each country. The first includes cardiometabolic-inflammatory biomarkers only, the second adds age and sex, and the third adds education and household wealth. Comparison of these equations allows us to assess how the biomarkers are associated with cognitive function, independent of sociodemographic characteristics. In addition, we can assess how much of the variability in the outcomes is explained by these variables when we compare the R2 of the three equations.
We examined the intercorrelations among all the biomarkers and found high correlation coefficients only between systolic and diastolic blood pressure values. As a result, we defined high blood pressure as either systolic blood pressure of 140 mmHg or higher or diastolic blood pressure of 90 mmHg or higher, and included this dichotomous variable in the models. To assess possible nonlinear relationships, we categorized four variables in the regression analysis: age (60–64, 65–69, 70–74, and ≥75 years); BMI (<18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2); and education and household wealth as described above. Other cardiometabolic-inflammatory biomarkers were included as continuous variables in the models. Log transformations were done for WBC, CRP, and proBNP due to significant right-skewed distributions. To explore potential effects from residual confounding, we have also conducted a sensitivity analysis among HRS participants to include race/ethnicity as a covariate.
RESULTS
The characteristics of the samples are summarized in Table 1. The two populations differed markedly in age and education. The Americans were much older, with no members of the cohort younger than 65 years, whereas about 28% of the Indians were of this age, and many more Americans were aged 75 years or older, about 41% in the United States and 23% in India (Table 1). Almost half (46%) of the Indian sample had no formal schooling, whereas 84% of the American cohort had at least a high school education, as did only 8% of the Indian cohort. The percentage of the sample with high-risk levels of most of the cardiovascular and metabolic risk factors was higher in India than in the United States. The notable exception was BMI: less than one-third of Indians and more than three-fourths of Americans were overweight. The prevalence of elevated measured blood pressure was significantly higher in India than in the United States: both systolic and diastolic blood pressures were more likely to be elevated in India. Although more Indians had HbA1c equal to or higher than 6.5%, the distribution of HbA1c values was fairly similar in the two countries. The prevalence for high homocysteine levels was three times higher in India than in the United States, and adverse levels of HDL (low levels) were much higher in India than in the United States. As indicated above, we used age-specific cutoff points for CHF to define high proBNP, which are 900 pg/mL or greater for those aged between 60 and 74 years and 1,800 pg/mL or greater for those aged 75 years and older. The prevalence of high-risk proBNP levels was similar between India and the United States. Prevalence of elevated levels of CRP, an indicator of overall systemic inflammation, was fairly similar in two countries.
Table 1.
Comparison of Select Sample Characteristics Between LASI-DAD (N = 1,865) and HRS-HCAP (N = 2,111)
| Variables | LASI-DAD | HRS-HCAP |
|---|---|---|
| Age, y | ||
| 60–64 | 27.9 | |
| 65–69 | 29.8 | 33.9 |
| 70–74 | 18.9 | 25.6 |
| ≥75 | 23.3 | 40.6 |
| Sex (% female) | 52.9 | 54.9 |
| Education, y | ||
| 0 (LASI-DAD) | 46.0 | |
| ≤11a | 46.1 | 16.2 |
| 12 | 3.0 | 33.5 |
| ≥13 | 4.9 | 50.3 |
| Cardiovascular characteristics | ||
| Systolic blood pressure ≥140 mmHg | 38.5 | 26.2 |
| Diastolic blood pressure ≥90 mmHg | 22.5 | 9.8 |
| Systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg | 42.5 | 28.0 |
| Pulse >100 beats/min | 6.5 | 1.2 |
| Homocysteine >15 mol/L | 67.1 | 22.3 |
| At-risk pro-B-type natriuretic peptideb | 4.5 | 5.1 |
| Metabolic characteristics | ||
| Body mass index ≥25 kg/m2 | 29.9 | 77.9 |
| Glycosylated hemoglobin ≥6.5% | 26.8 | 15.6 |
| HDL cholesterol <40 mg/dL | 39.7 | 16.0 |
| Lipoprotein (a) >30 mg/dL | 41.3 | |
| In ammation characteristics | ||
| C-reactive protein >3 mg/L | 36.8 | 35.7 |
| White blood cell count >11,000/mm3 | 5.7 | 2.8 |
| Albumin <3.5 g/dL | 3.2 | 6.1 |
| Uric acid >7 mg/dL | 7.1 |
Note: Data are given as percentage of each group.
Abbreviations: HDL, high-density lipoprotein; HRS-HCAP, Health and Retirement Study–Harmonized Cognitive Assessment Protocol; LASI-DAD, Longitudinal Aging Study in India–Diagnostic Assessment of Dementia.
HRS-HCAP includes the sample that never attended school in this category (N = 12).
Pro-B-type natriuretic peptide is 900 pg/mL or greater for those aged 60 to 74 years and 1,800 pg/mL or greater for those aged 75 years and older.
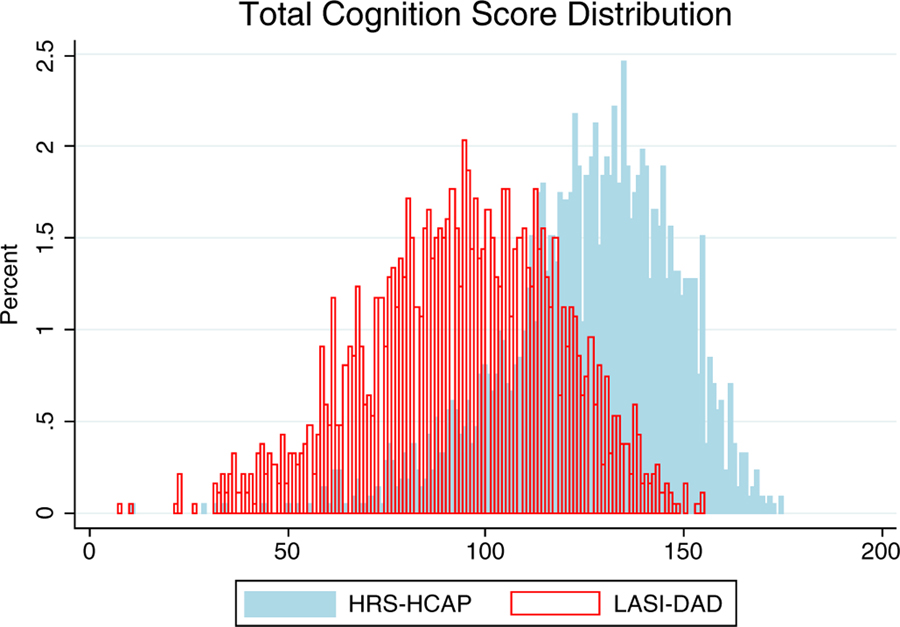
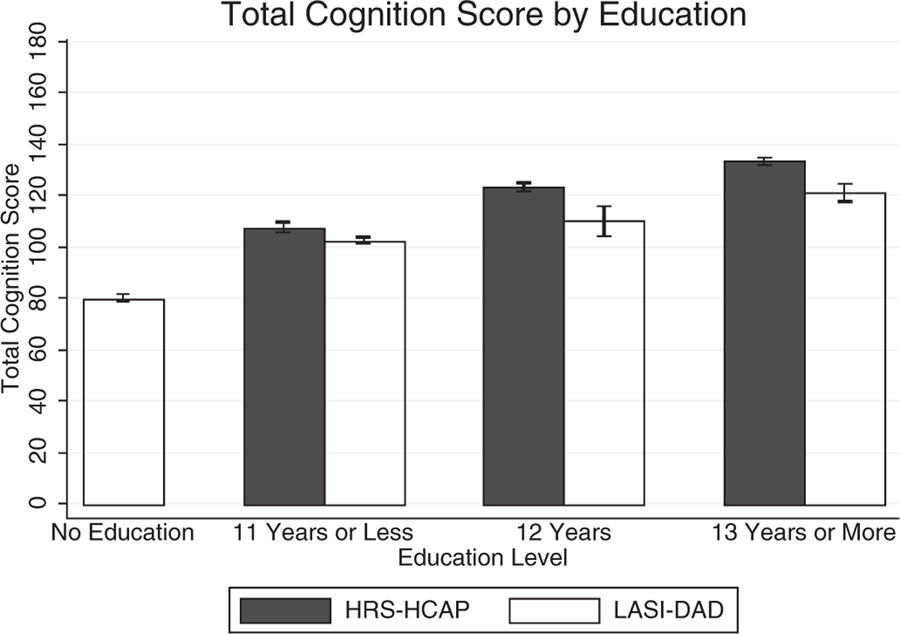
Figure 1 shows distribution of total cognitive scores for the two countries. It is clear that the scores are much higher in the United States than in India. Because educational attainment differs so much in the two populations and has been shown to be so highly related to cognitive functioning, we also examine mean total cognitive scores by education levels in the two countries (Figure 2). The data confirmed that cognitive functioning score increased with more education. However, even though the whole sample of Indians had significantly lower total cognitive scores than Americans, at the same education level these differences were relatively small between the two countries. This pattern of similar education-specific cognitive performance was observed in all individual components of the total cognitive score (Table 2). For example, the mean MMSE score was 27.0 for Indians with 12 years of education and 26.9 for Americans at same education level. For those with 13 years of education and above, the mean MMSE score was identical at 28.1 for both countries. Because HRS-HCAP had few respondents without any formal education, we were not able to do cross-country comparison of cognitive performance among illiterate individuals.
Figure 1.
Comparison of distributions of total cognitive function scores between the Longitudinal Aging Study in India–Diagnostic Assessment of Dementia (LASI-DAD) and the Health and Retirement Study–Harmonized Cognitive Assessment Protocol (HRS-HCAP).
Figure 2.
Comparison of mean total cognitive scores by education levels between the Longitudinal Aging Study in India–Diagnostic Assessment of Dementia (LASI-DAD) and the Health and Retirement Study–Harmonized Cognitive Assessment Protocol (HRS-HCAP).
Table 2.
Comparison of Scores of Individual Cognitive Domains by Education Levels Between the LASI-DAD and the HRS-HCAP
| Variable | Range | LASI-DADa |
HRS-HCAPa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–11 | 12 | ≥13 | All | 0–11 | 12 | ≥13 | All | ||
| 10Word list, immediate recall | 0–30 | 10.0 | 13.7 | 14.3 | 15.9 | 11.9 | 14.8 | 17.7 | 19.2 | 18.0 |
| 10-Word list, delayed recall | 0–10 | 2.6 | 4.0 | 3.8 | 5.0 | 3.3 | 4.0 | 5.2 | 5.9 | 5.4 |
| 10Word list, recognition | 0–20 | 15.0 | 17.4 | 17.5 | 18.3 | 15.2 | 17.6 | 18.7 | 19.1 | 18.7 |
| Logical memory recognition score | 0–15 | 6.9 | 8.3 | 8.8 | 10.5 | 7.7 | 8.8 | 10.4 | 11.2 | 10.6 |
| Brave Man immediate recall score | 0–12 | 4.8 | 6.3 | 6.7 | 7.5 | 5.6 | 5.9 | 7.2 | 7.8 | 7.3 |
| Brave Man delayed recall score | 0–12 | 1.8 | 4.1 | 5.1 | 5.4 | 3.0 | 3.6 | 5.1 | 6.1 | 5.3 |
| MMSE summary score | 0–30 | 20.7 | 25.8 | 27.0 | 28.1 | 23.3 | 24.3 | 26.9 | 28.1 | 27.1 |
| Verbal fluency score | 0–43b | 10.6 | 12.9 | 13.7 | 15.9 | 11.9 | 12.6 | 16.2 | 19.2 | 17.1 |
| CSID score | 0–4 | 3.3 | 3.6 | 3.8 | 3.8 | 3.5 | 3.6 | 3.7 | 3.8 | 3.8 |
| Raven test | 0–17 | 6.6 | 8.8 | 10.1 | 11.4 | 7.8 | 9.8 | 12.6 | 14.2 | 13.0 |
| Total cognition score | 0–175 | 82.3 | 104.9 | 110.7 | 121.8 | 94.2 | 104.9 | 123.8 | 134.5 | 126.1 |
Abbreviations: CSID, community screening instrument for dementia; HRS-HCAP, Health and Retirement Study–Harmonized Cognitive Assessment Protocol; LASI-DAD, Longitudinal Aging Study in India–Diagnostic Assessment of Dementia; MMSE, Mini-Mental State Examination.
Data are given according to years of education.
The upper limit is set at maximum number of correct answers provided by respondents.
Multivariate regression analysis indicates that the cardiovascular biomarkers most consistently associated with cognitive function in both countries were homocysteine and proBNP. The associations were negative for both measures. They were also fairly similar in coefficient size, with proBNP of −3.3 in India and −2.8 in the United States; and for homocysteine, the coefficients were −0.1 and −0.6, respectively (Table 3, model 1). The associations between HbA1c and the cognitive measure were also significant, but in the opposite direction between India and the United States, with a regression coefficient of 1.5 in India and −2.4 in the United States for one percentage increase in absolute HbA1c value. This pattern did not change after including quadratic and cubic terms of HbA1c in the regression equation or categorizing HbA1c values into less than 5.7%, 5.7% to 6.4%, and 6.5% or greater. BMI was associated with cognitive function in India, with people with higher BMI performing better, including those in the range of obesity. On the other hand, low BMI (<18.5 kg/m2) was not significantly associated with poor cognitive functioning, but obesity (BMI ≥30.0 kg/m2) was related to better cognitive functioning among Americans. Among inflammatory markers, albumin was consistently and positively associated with cognitive function in both countries.
Table 3.
Comparison of the Relationship Between Biological and Sociodemographic Characteristics and Total Cognitive Function Scores Between LASI-DAD (N = 1,865) and HRS-HCAP (N = 2,111)
| Variable | LASI-DAD |
HRS-HCAP |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 (biomarkers) | Model 2 (biomarkers + age + sex) | Model 3 (biomarkers + age + sex + education, wealth) | Model 1 (biomarkers) | Model 2 (biomarkers + age + sex) | Model 3 (biomarkers + age + sex + education, wealth) | |||||||
| Coefficient (SE) | P value | Coefficient (SE) | P value | Coefficient (SE) | P value | Coefficient (SE) | P value | Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Cardiovascular characteristics | ||||||||||||
| Hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) | −0.1 (1.0) | .905 | 0.1 (1.0) | .926 | 0.2 (0.9) | .842 | −4.7 (1.0) | <.0001 | −3.5 (1.0) | .0005 | −2.1 (0.9) | .0201 |
| Pulse | −0.2 (0.0) | <.001 | −0.2 (0.0) | <.001 | −0.1 (0.0) | .007 | −0.0 (0.0) | .4446 | −0.1 (0.0) | .1521 | −0.0 (0.0) | .8939 |
| Homocysteine | −0.1 (0.0) | .048 | −0.1 (0.0) | .012 | −0.1 (0.0) | .019 | −0.6 (0.1) | <.0001 | −0.5 (0.1) | <.0001 | −0.3 (0.1) | .0012 |
| ProBNP (log) | −3.3 (0.5) | <.001 | −1.9 (0.5) | <.001 | −1.4 (0.4) | .002 | −2.8 (0.4) | <.0001 | −1.6 (0.4) | <.0001 | −1.4 (0.4) | .0001 |
| Metabolic characteristics | ||||||||||||
| Body mass index (reference: 18.5–24.9), kg/m2 | ||||||||||||
| <18.5 | −9.3 (1.4) | <.001 | −9.5 (1.4) | <.001 | −6.7 (1.2) | <.001 | −8.0 (5.6) | .1525 | −8.7 (5.4) | .1059 | −7.0 (4.9) | .1477 |
| 25.0–29.9 | 5.6 (1.3) | <.001 | 6.1 (1.3) | <.001 | 2.9 (1.2) | .013 | −0.3 (1.3) | .8061 | −0.4 (1.3) | .7297 | −0.1 (1.1) | .9498 |
| ≥30.0 | 8.4 (2.0) | <.001 | 9.1 (1.9) | <.001 | 4.9 (1.7) | .004 | 4.0 (1.4) | .0031 | 2.8 (1.3) | .0326 | 2.8 (1.2) | .0198 |
| Glycosylated hemoglobin | 1.5 (0.3) | <.001 | 1.3 (0.3) | <.001 | 0.6 (0.3) | .033 | −2.4 (0.5) | <.0001 | −2.4 (0.5) | <.0001 | −1.0 (0.5) | .0318 |
| HDL cholesterol | −0.1 (0.1) | .068 | −0.1 (0.1) | .282 | −0.1 (0.0) | .139 | 0.1 (0.0) | .0233 | 0.0 (0.0) | .4689 | −0.0 (0.0) | .0649 |
| Lipoprotein (a) | −0.0 (0.0) | .669 | −0.0 (0.0) | .707 | 0.0 (0.0) | .842 | ||||||
| Inflammation characteristics | ||||||||||||
| C-reactive protein (log) | −0.4 (0.4) | .336 | −0.4 (0.4) | .379 | 0.0 (0.4) | .921 | 0.8 (0.5) | .1059 | 0.4 (0.5) | .4562 | 0.4 (0.4) | .3657 |
| White blood cell count (log) | −0.9 (1.8) | .628 | −1.0 (1.8) | .563 | −0.1 (1.6) | .977 | 0.4 (1.7) | .8285 | 0.5 (1.6) | .7512 | 0.9 (1.5) | .5584 |
| Albumin | 9.6 (1.7) | <.001 | 7.4 (1.7) | <.001 | 4.4 (1.5) | .003 | 5.7 (1.6) | .0004 | 4.9 (1.6) | .0020 | 2.9 (1.4) | .0429 |
| Uric acid | 2.1 (0.4) | <.001 | 1.6 (0.4) | <.001 | 0.8 (0.4) | .028 | ||||||
| Age (reference: 65–69), y | ||||||||||||
| 60–64 | 3.3 (1.3) | .011 | 2.3 (1.1) | .041 | ||||||||
| 70–74 | −4.4 (1.4) | .002 | −4.6 (1.3) | <.001 | −3.3 (1.3) | .0109 | −3.2 (1.2) | .0060 | ||||
| ≥75 | −10.4 (1.4) | <.001 | −11.4 (1.3) | <.001 | −11.7 (1.1) | <.0001 | −11.2 (1.0) | <.0001 | ||||
| Sex (reference: male) | ||||||||||||
| Female | −6.4 (1.1) | <.001 | 0.1 (1.0) | .926 | 3.9 (1.0) | <.0001 | 6.5 (0.9) | <.0001 | ||||
| Education (reference: 1–11 y for LASI-DAD and 0–11 y for HRS-HCAP), y | ||||||||||||
| 0 (LASI) | −19.3 (1.0) | <.001 | ||||||||||
| 12 | 4.5 (2.7) | .093 | 13.0 (1.2) | <.0001 | ||||||||
| ≥13 | 13.2 (2.0) | <.001 | 20.1 (1.2) | <.0001 | ||||||||
| Household wealth (reference: third quintile) | ||||||||||||
| First quintile | −0.4 (1.4) | .771 | −6.4 (1.3) | <.0001 | ||||||||
| Second quintile | −2.5 (1.4) | .075 | −1.5 (1.3) | .2514 | ||||||||
| Fourth quintile | 2.1 (1.3) | .108 | 1.4 (1.3) | .2804 | ||||||||
| Fifth quintile | 1.9 (1.4) | .170 | 4.5 (1.3) | .0005 | ||||||||
| R2 | 0.18 | 0.23 | 0.41 | 0.10 | 0.16 | 0.32 | ||||||
Abbreviations: HDL, high-density lipoprotein; HRS-HCAP, Health and Retirement Study–Harmonized Cognitive Assessment Protocol; LASI-DAD, Longitudinal Aging Study in India –Diagnostic Assessment of Dementia; ProBNP, pro-B-type natriuretic peptide; SE, standard error.
Older age and lower educational attainment were independently associated with lower total cognitive scores in both Indians and Americans (Table 3, models 2 and 3). Compared with those with less than 12 years of education, the mean total cognitive scores were 19.3 points lower among Indians who were illiterate, 4.5 points higher among Indians who completed high school, and 13.2 points higher among those who had at least some college. Among Americans, the differences in total cognitive scores among those with higher education levels were 13.0 and 20.1 points, respectively. Compared with males, females scored lower in India but higher in the United States, although this sex difference in India was not statistically significant after controlling for education and wealth.
After sociodemographic variables were added to the models, the associations of total cognitive scores with proBNP, homocysteine, BMI, and HbA1c remained statistically significant (Table 3, model 3), although the strength of associations decreased. For example, the coefficients for higher proBNP decreased from −3.3 to −1.4 in India and from −2.8 to −1.4 in the United States.
R2 values from the analysis indicate that a greater proportion of the variance in total cognitive scores was explained in India than in the United States (0.41 vs 0.32). R2 values for cardiometabolic-inflammatory biomarkers alone were 0.18 in India and 0.10 in the United States. Addition of education levels and household wealth significantly improved our ability to predict cognitive function in both countries.
To assess the effects of other potential confounding variables, we conducted a sensitivity analysis among HRS participants to include race/ethnicity, an important correlate of cognitive functioning in the United States. Adding race/ethnicity only increases the variance explained by 1%. It reduces the magnitude of effects for HbA1c and albumin, so that they are no longer significantly associated with cognitive functioning.
DISCUSSION
In this analysis of the associations between cognitive function and cardiometabolic-inflammatory risk factors, our data showed that several cardiometabolic-inflammatory risk factors were independently associated with cognitive functional levels. Cognitive functioning was lower among those with high homocysteine and proBNP levels, and among those with low albumin levels, in both countries. The association between HbA1c and cognitive measure was consistently significant, but in the opposite direction between the two countries, negative in the United States and positive in India. The link between weight and cognition seems strong in India, with a clear pattern of higher weight and better cognition; in the United States, only those with obesity have better cognition than those in the normal range.
Studies of older adults in many countries have shown that lower educational levels predict worse global cognitive functioning.23–26 Education attainment is a well-established indicator of cognitive reserve capacity in older people and may have a direct effect on cognition through more developed and effective use of brain networks or cognitive paradigms.27 Our analysis extends previous findings by demonstrating that, across two countries with different distributions of education attainment, the difference in cognitive functioning is relatively small when we compare individuals with similar education levels. This observation seems true for both overall cognitive functioning as well as many of the individual domains (Table 2 and Figure 2).
In addition to the direct effect of education on cognition, years of formal education may also be a surrogate for other factors that might influence cognition, including development and management of cardiovascular and metabolic diseases. The proposed causal pathways through which cardiometabolic diseases lead to cognitive decline and dementia include direct damage of neurons, cerebral blood hypoperfusion, and promotion of amyloid cascade.4 Our finding of an inverse association between cognitive function and homocysteine and proBNP levels is consistent with previous research. In a prospective cohort of individuals 75 years and older, Blasko et al reported that increased homocysteine levels were independently associated with decline of cognitive performance in normal older subjects and patients with Alzheimer’s disease.28 It has been postulated that homocysteine plays a role in promoting oxidative stress, inflammation, thrombosis, endothelial dysfunction, and cell proliferation.29 Earlier studies in developed countries also showed that both cross-sectionally measured proBNP levels and increase in proBNP levels are related to subsequent risk of cognitive decline and dementia, mostly because of the association between proBNP levels and macroischemic and microischemic changes of brain.30–32 Our study confirmed the same relationship between adverse cardiovascular biomarkers and cognitive decline in India. The inverse association with homocysteine might be particularly relevant, because two-thirds (67.1%) of the Indian study population had at-risk homocysteine levels (Table 1).
HbA1c level is a measure of average glucose metabolism during the previous 3 months, and has been used clinically to diagnose diabetes mellitus, type II, and monitor glucose control. A recent meta-analysis indicates that poorer glycemic control is related to cognitive dysfunction.33 However, we found that, unlike Americans, Indians with higher HbA1c levels actually had higher total cognitive scores. This may reflect different stages of epidemiological transition for these two countries. With more recent economic development, Indians with higher socioeconomic status are more likely to adopt a Western diet and have decreased level of physical activities, leading to a higher prevalence of noncommunicable diseases. Previous analysis has shown that obesity and hypertension are more common among LASI participants who have higher education level, whereas these medical conditions are less common among Chinese older adults with better education.34 Therefore, the positive association between HbA1c levels and cognitive function in India highlights the complexity of how cardiometabolic-inflammatory risk factors may influence cognitive decline in countries that are undergoing rapid economic and societal transitions.
This study has some important strengths. LASI-DAD and HRS-HCAP are nationally representative samples of older adults in the two countries. Both studies generated high-quality cognitive functioning and biomarker data. More important, cognitive assessment protocols were harmonized conceptually to allow more accurate cross-country comparisons. Several limitations should also be noted. First, even though LASI-DAD and HRS-HCAP are designed as longitudinal studies, our current analysis is cross-sectional. It does not allow us to examine the temporal relationship between cognitive function and cardiometabolic-inflammatory risk factors. It is possible that the association works in both directions, as severe cognitive decline may affect management of cardiometabolic diseases, causing more pathophysiological dysfunction. Second, only 32% of LASI-DAD respondents provided fasting blood specimens, limiting our ability to examine biomarkers that typically require fasting specimens (e.g., LDL cholesterol) or to formally define metabolic syndrome. Third, some of the cardiometabolic-inflammatory biomarkers may be indicators for more than one system. For example, homocysteine is associated with cardiovascular disease, but its level also goes up in the setting of folate or vitamin B12 deficiency. Last, even though we adjusted for categories of age, sex, education, and wealth, residual confounding remains a possibility.
Despite these limitations, this cross-country study sheds a new insight to the relationships between cognition and cardiometabolic-inflammatory risk factors in two countries, India and the United States. Lower cognitive function is associated with older age, lower educational attainment, elevated homocysteine, elevated proBNP, lower albumin levels, and lower BMI in both countries. The associations between HbA1c and cognitive measure are consistently significant, but in opposite direction between India and the United States. Longitudinal data from the future waves of LASI-DAD and HRS-HCAP will enable us to further investigate how changes in biomarkers are related to changes in cognitive function. These longitudinal analyses could provide more insight on biological pathways for cognitive decline and allow us to explore potential interventions aimed at reducing cognitive decline and dementia.
ACKNOWLEDGMENTS
We thank A. B. Dey, Joyita Banerjee, Pranali Yogirj Khobragade, Bas Weerman, Sandy Chien, and all collaborators at the partner hospitals and laboratories in India.
Financial Disclosure: This project is funded by the National Institute on Aging (R01 AG051125 and RF1 AG055273).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Sponsor’s Role: Sponsor had no role in data analysis or manuscript preparation.
REFERENCES
- 1.Alzheimer’s & Related Disorders Society of India (ARDSI). The Dementia India Report: Prevalence, Impact, Costs, and Services for Dementia. In: Shaji KS, Jotheeswaran AT, Girish N, et al. , eds. New Delhi: Alzheimer’s & Related Disorders Society of India (ARDSI), 2010. [Google Scholar]
- 2.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80: 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince MJ, Wimo A, Ali G, Wu Y, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia. London, UK: Alzheimer’s Disease International; 2015. https://www.alz.co.uk/research/world-report-2015 Accessed January 9, 2020. [Google Scholar]
- 4.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement (Amst). 2017;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.India State-Level Disease Burden Initiative CVD Collaborators. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the global burden of disease study. Lancet. 2017;390: 2437–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jadhav UM. Cardio-metabolic disease in India-the up-coming tsunami. Ann Transl Med. 2018;6:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anchala R, Kannuri NK, Pant H, et al. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prenissl J, Manne-Goehler J, Jaacks LM, et al. Hypertension screening, awareness, treatment, and control in India: a nationally representative cross-sectional study among individuals aged 15 to 49 years. PLoS Med. 2019;16 (5):e1002801 10.1371/journal.pmed.1002801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Khobragade PY, Banerjee J, et al. Design and Methodology of the Longitudinal Aging Study in India-Diagnostic Assessment of Dementia (LASI-DAD). J Am Geriatr Soc;68:S5–S10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langa KM, Ryan LH, McCammon RJ, et al. The health and retirement study harmonized cognitive assessment protocol project: study design and methods. Neuroepidemiology. 2020;54:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey AB, Banerjee J, Khobragade P et al. Diagnostic assessment of dementia for LASI documentation – 2019 wave 1, early release version A, venous blood collection and assay protocol, All India Institute of Medical Sciences and University of Southern California, 2019. Available at: https://lasi-dad.org/. Accessed January 9, 2020.
- 12.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D Wechsler Memory Scale Fourth Edition (WMS IV) Technical and Interpretive Manual: San Antonio, TX: Pearson, 2009. [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Ganguli M, Ratcliff G, Chandra V, et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry. 1995;10:367–377. [Google Scholar]
- 16.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 17.Hall K, Hendrie HC, Brittain HM, et al. The development of a dementia screening interview in two distinct languages. Int J Methods Psychiatr Res. 1993;3:1–28. [Google Scholar]
- 18.Raven J The Raven’s progressive matrices: change and stability over culture and time. Cogn Psychol. 2000;41:1–48. [DOI] [PubMed] [Google Scholar]
- 19.Fisher GG, Hassan H, Faul JD, Rodgers WL, Weir DR. Health and Retirement Study: Imputation of Cognitive Functioning Measures: 1992–2014 (Final Release Version): Data Description. Ann Arbor: University of Michigan, Survey Research Center; 2017. [Google Scholar]
- 20.Gross AL, Khobragade PY, Meijer E, and Saxton JA. Measurement and Structure of Cognition in the Longitudinal Aging Study in India–Diagnostic Assessment of Dementia. J Am Geriatr Soc;68:S11–S19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildebrandt P, Collinson PO, Doughty RN, et al. Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care. Eur Heart J. 2010;31:1881–1889. [DOI] [PubMed] [Google Scholar]
- 22.Quest Diagnostics. NT-proBNP: reference ranges. 2019. https://testdirectory.questdiagnostics.com/test/test-detail/11188/?cc=MASTER Accessed January 9, 2020.
- 23.Evans DA, Beckett LA, Albert MS, et al. Level of education and change in cognitive function in a community population of older persons. Ann Epidemiol. 1993;3:71–77. [DOI] [PubMed] [Google Scholar]
- 24.Lei X, Hu Y, McArdle JJ, Smith JP, Zhao Y. Gender differences in cognition among older adults in China. J Hum Resour. 2012;47:951–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahodne LB, Glymour MM, Sparks C, et al. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. J Int Neuropsychol Soc. 2011;17:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crimmins EM. Physiological differences across ageing populations reflecting early life and later life nutritional status and later life risk for chronic disease. J Popul Ageing. 2015;8:51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasko I, Jellinger K, Kemmler G, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging. 2008;29:1–11. [DOI] [PubMed] [Google Scholar]
- 29.Ansari R, Mahta A, Mallack E, Luo J. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014;10:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folsom AR, Nambi V, Bell EJ, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostovaneh MR, Moazzami K, Yoneyama K, et al. Change in NT-proBNP (N-terminal pro-B-type natriuretic peptide) level and risk of dementia in multi-ethnic study of atherosclerosis (MESA). Hypertension. 2020;75: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zonneveld HI, Ikram MA, Hofman A, et al. N-terminal pro-B-type natriuretic peptide and subclinical brain damage in the general population. Radiology. 2017;283(1):205–214. [DOI] [PubMed] [Google Scholar]
- 33.Mansur RB, Lee Y, Zhou AJ, et al. Determinants of cognitive function in individuals with type 2 diabetes mellitus: a meta-analysis. Ann Clin Psychiatry. 2018;30:38–50. [PubMed] [Google Scholar]
- 34.Hu P, Wang S, Lee J. Socioeconomic gradients of cardiovascular risk factors in China and India: results from the China health and retirement longitudinal study and longitudinal aging study in India. Int J Public Health. 2017; 62:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]