Dear editor,
The SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2)-related acute respiratory distress syndrome (ARDS) is associated with an elevated coagulation activation pattern [1] and a high incidence of pulmonary embolism [2]. The diagnosis of pulmonary embolism (PE) may be challenging in these patients because computed tomography pulmonary angiogram (CTPA) requires an intrahospital transport with potential adverse effects and also may increase the risk of acute kidney failure (contrast-induced nephropathy). This is even more the case in up to 10% of SARS-Cov-2 ARDS patients who require venovenous extracorporeal membrane oxygenation (vv-ECMO) as an extracorporeal lung support [1]. In addition to the inherent risks of hospital transport, which are particularly high in these patients [3], extracorporeal circulation is likely to alter the quality of the contrast agent distribution and may reduce the diagnostic performance of the CTPA [4]. Finally, systematic curative antithrombotic therapy is not a safe option as it exposes to a serious risk of bleeding, especially when prolonged vv-ECMO is expected [5]. For all the abovementioned reasons, alternative techniques allowing the diagnosis of PE in these vv-ECMO patients would be of the highest interest.
Here, we describe the feasibility, safety, and diagnostic accuracy of endobronchial ultrasound (EBUS) to detect PE in patients with severe SARS-CoV-2 ARDS requiring vv-ECMO. Between April 15 and May 1, 2020, eleven patients were included. The procedure was performed using a 6.7-mm-outer-diameter, real-time, bronchoscope (EB-530US; FUJIFILM Medical Corporation, Tokyo, Japan) with a 7.5-MHz linear ultrasound transducer (SU-1 H; FUJIFILM Medical Corporation, Tokyo, Japan) equipped with color-Doppler. For each patient, EBUS procedure followed the same roadmap [6]. All EBUS images and videos were reviewed by two independent experts in thoracic radiology (S.B. and D.T.) blind from the CTPA interpretation. The study was approved by the research ethics committee of Sorbonne University (CER-SU N°2020-48) and information was given to the patients or their relatives.
Patients were mostly men (n = 10), 52 [49–55] years old, with a body mass index of 29 [28–31] kg/m2 (Table 1). The time between intubation, vv-ECMO and EBUS was 21 [11–27] and 13 [7–18] days, respectively. At the time of EBUS procedure, three patients were not receiving antithrombotic therapy, two were receiving effective curative unfractionated heparin, and six were receiving prophylactic unfractionated heparin (dose was 18,000 [14,000–20,000] UI/day). Pulmonary embolism was observed on EBUS in five of the eleven patients (45%) (Fig. 1). The duration of the procedure was 15 [13–17] min and no major adverse effect of EBUS (e.g., serious bleeding, arterial oxygen saturation < 85%) was reported. EBUS could explore part of segmental arteries in five (45%) patients.
Table 1.
Patients’ characteristics and diagnostic correspondence between Endobronchial Ultrasound (EBUS) and Computed tomography pulmonary angiography (CTPA)
| Patient age (years old) | Ventilator settings, PEEP (cmH2O) FiO2 | EBUS duration (min) | Lowest SpO2 during EBUS | Vt before EBUS (mL) | Vt during EBUS (mL) | CTPA performed before or after EBUS | Time between EBUS-CTPA (days) | Location of PE on EBUS | Location of PE on CTPA | Agreement EBUS-CTPA |
|---|---|---|---|---|---|---|---|---|---|---|
|
Patient 1 54 |
PEEP: 14 FiO2: 100% |
17 | 90 | 80 | 60 | After | 17 | SBRPA | SBRPA | Yes |
|
Patient 2 55 |
PEEP: 12 FiO2: 80% |
15 | 94 | 72 | 45 | Before | 8 | – |
Ap-SBLPA Distal P-SBRPA |
No |
|
Patient 3 61 |
PEEP: 8 FiO2: 70% |
17 | 94 | 480 | 480 | Before | 6 | SBLPA |
SBLPA Segmental IBRPA |
Yes |
|
Patient 4 46 |
PEEP: 12 FiO2: 30% |
21 | 95 | 71 | 35 | Before | 7 | A-SBRPA | – | No |
|
Patient 5 51 |
PEEP: 12 FiO2: 30% |
13 | 98 | 90 | 37 | Before | 10 | – | – | Yes |
|
Patient 6 57 |
PEEP: 12 FiO2: 70% |
11 | 96 | 30 | 25 | After | 5 | Ap-SBRPA | Ap-SBRPA | Yes |
|
Patient 7 35 |
PEEP: 12 FiO2: 50% |
14 | 94 | 430 | 250 | Before | 6 | – | – | Yes |
|
Patient 8 35 |
PEEP: 12 FiO2: 50% |
15 | 100 | 67 | 32 | Before | 7 | – | Yes | |
|
Patient 9 68 |
PEEP: 10 FiO2: 50% |
18 | 100 | 380 | 350 |
Before After |
7 5 |
– IBRPA |
– IBRPA |
Yes Yes |
|
Patient 10 68 |
PEEP: 12 FiO2: 50% |
13 | 92 | 220 | 150 | After | 9 | – | Yes | |
|
Patient 11 39 |
PEEP: 12 FiO2: 60% |
11 | 88 | 50 | 35 | After | 1 | – | Yes |
PEEP positive end-expiratory pressure, Vt tidal volume, PE pulmonary embolism, SBRAP superior bronchial right pulmonary artery, A-SBRPA anterior segment of the SBRPA, P-SBRPA posterior segment of the SBRPA, Ap-SBRPA apical segment of the SBRPA, LPA left pulmonary artery, IBLPA inferior bronchial left pulmonary artery, SBLPA superior bronchial left pulmonary artery, T trachea
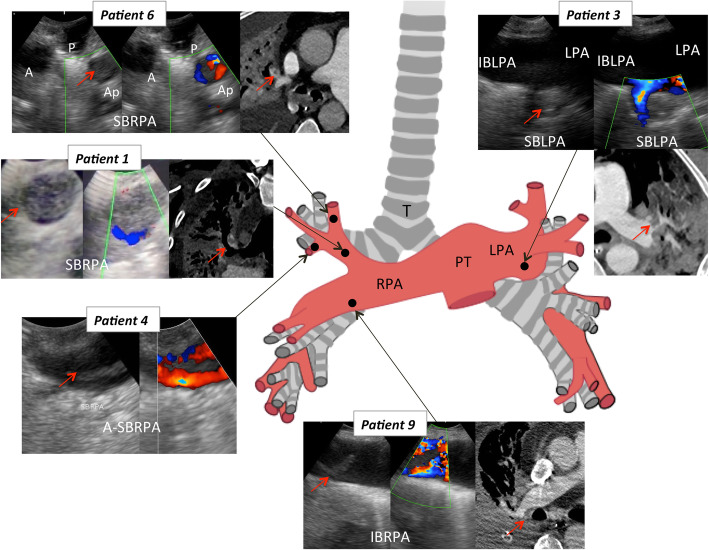
Fig. 1.
Endobronchial ultrasound and computed tomography pulmonary angiography correspondence in the five patients with pulmonary embolism. Red arrows indicate the presence of thrombus in pulmonary arteries. SBRPA, superior bronchial right pulmonary artery; A-SBRPA, anterior segment of the SBRPA; P-SBRPA, posterior segment of the SBRPA; A-SBRPA, apical segment of the SBRPA; LPA, left pulmonary artery; IBLPA, inferior bronchial left pulmonary artery; SBLPA, superior bronchial left pulmonary artery; T, trachea
Diagnostic correspondence between EBUS and CTPA is depicted in Table 1. Excluding patient 4, in which PE may have developed during the 7 days that separated EBUS and CTPA, overall agreement was obtained in 9/10 (90%) patients. The patient (patient 2) without PE on the EBUS had left segmental pulmonary embolism on CTPA, which was not accessible to the EBUS.
This case series of EBUS to diagnose PE in severe SARS-CoV-2 ARDS patients requiring vv-ECMO suggests that the EBUS procedure is safe and reliable to detect lobar and even segmental PE at bedside. Given the high risk of pulmonary embolism in patients with severe ARDS due to COVID-19, this minimally invasive diagnostic approach seems to be a useful and appropriate diagnostic tool to avoid the multiple adverse effects of CTPA in these severe and often unstable patients. The diagnostic performance of this innovative and promising technique needs now to be confronted to CTPA in larger prospective study and other clinical situations, especially for the analysis of segmental arteries.
Acknowledgements
The authors thank Mrs. Pia Chevalier for her help in the production of Fig. 1.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- Ap-SBRPA
Apical segment of the SBRPA
- A-SBRPA
Anterior segment of the SBRPA
- CT
Computed tomography
- EBUS
Endobronchial ultrasound
- IBLPA
Inferior bronchial left pulmonary artery
- LPA
Left pulmonary artery
- PE
Pulmonary embolism
- PEEP
Positive end-expiratory pressure
- P-SBRPA
Posterior segment of the SBRPA
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SBLPA
Superior bronchial left pulmonary artery
- SBRPA
Superior bronchial right pulmonary artery
- T
Trachea
- vv-ECMO
Venovenous extracorporeal membrane oxygenation
Authors’ contributions
Conception and design: MD, VTD, SB, AD, MD. Data acquisition: MD, VTD, JM, MD. Analysis and interpretation: MD, VTD, SB, MD, AD. Drafting the manuscript: MD, VTD, AD, MD. Final approval: all the authors.
Funding
No funding.
Availability of data and materials
Our data are available to ensure transparency.
Ethics approval and consent to participate
The study was approved by the research ethics committee of Sorbonne University (CER-SU N°2020-48) and information was given to the patients or their relatives.
Consent for publication
Not applicable
Competing interests
Dr. SIMILOWSKI reports personal fees from ADEP Assistance, AstraZeneca France, Boerhinger Ingelheim France, Chiesi France, GSK France, Lungpacer Inc., Novartis France, and TEVA France, outside the submitted work. In addition, Dr. Similowski has a patent titled “brain-ventilator interface” licensed to Air Liquide Medical Systems and MyBrainTechnology, a patent for a “protection device for intubation” pending, and a patent for a “non-contact thoracic movement imaging system” pending.
Alexandre Demoule reports personal fees from Medtronic, grants, personal fees and non-financial support from Philips, personal fees from Baxter, personal fees from Hamilton, personal fees and non-financial support from Fisher & Paykel, grants from French Ministry of Health, personal fees from Getinge, grants and personal fees from Respinor, and grants and non-financial support from Lungpacer, outside the submitted work.
Martin Dres received personal fees and travel expenses from Lungpacer outside the submitted work.
The other authors had no conflict of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F, CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieira J, Frakes M, Cohen J, Wilcox S. Extracorporeal membrane oxygenation in transport part 2: complications and troubleshooting. Air Med J. 2020;39:124–132. doi: 10.1016/j.amj.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Lambert L, Grus T, Balik M, Fichtl J, Kavan J, Belohlavek J. Hemodynamic changes in patients with extracorporeal membrane oxygenation (ECMO) demonstrated by contrast-enhanced CT examinations - implications for image acquisition technique. Perfusion. 2017;32:220–225. doi: 10.1177/0267659116677308. [DOI] [PubMed] [Google Scholar]
- 5.Mazzeffi M, Greenwood J, Tanaka K, Menaker J, Rector R, Herr D, Kon Z, Lee J, Griffith B, Rajagopal K, Pham S. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis. Ann Thorac Surg. 2016;101:682–689. doi: 10.1016/j.athoracsur.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Wu C, Zheng W, Zhao L. Pathway and application value of exploration of the pulmonary artery by endobronchial ultrasound. J Thorac Dis. 2017;9:5345–5351. doi: 10.21037/jtd.2017.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data are available to ensure transparency.