Abstract
Although severe early life stress has been shown to accelerate the development of frontolimbic resting-state functional connectivity (RSFC), less is known about the effects of socioeconomic disadvantage, a prolonged and multifaceted stressor. In a cross-sectional study of 127 participants aged 5–25, we examined whether lower neighborhood socioeconomic status (SES; measured by Area Deprivation Index and neighborhood poverty and educational attainment) was associated with prematurely reduced amygdala-ventromedial prefrontal cortex (vmPFC) RSFC. We further tested whether neighborhood SES was more predictive than household SES and whether SES effects on connectivity were associated with anxiety symptoms. We found reduced basolateral amygdala-vmPFC RSFC at earlier ages in participants from more disadvantaged neighborhoods; this effect was unique to neighborhood SES and absent for household SES. Furthermore, this reduced connectivity in more disadvantaged youth and increased connectivity in more advantaged youth were associated with less anxiety; children who deviated from the connectivity pattern associated with their neighborhood SES had more anxiety. These results demonstrate that neighborhood socioeconomic disadvantage is associated with accelerated maturation of amygdala-vmPFC RSFC and suggest that the pathophysiology of pediatric anxiety depends on a child’s neighborhood socioeconomic characteristics. Our findings also underscore the importance of examining SES effects in studies of brain development.
Keywords: anxiety, brain development, fMRI, functional connectivity, stress acceleration
Introduction
Socioeconomic status (SES) indexes social prestige and financial resources and has profound and lasting mental health effects (Bradley and Corwyn 2002b; Hackman and Farah 2009; Reiss 2013). At the household level, SES is commonly assessed by measures such as occupation, educational attainment, and household income. Importantly, household SES often covaries with factors linked with mental wellbeing, such as access to stable and secure shelter (Gilman et al. 2003), mental healthcare access (Hodgkinson et al. 2017), household stability (Evans et al. 2005), and exposure to stressful childhood experiences (Goldmann et al. 2011). These stressors likely contribute to critical socioeconomic disparities in health outcomes (Baum et al. 1999). At the neighborhood level, SES is often assessed as both the combined SES of a community’s constituent households as well as shared aspects of a community’s material and social infrastructure, including health care resources, healthy food availability, neurotoxic environmental burden, exposure to local violence, and social cohesion (Macintyre et al. 2002; Wilson et al. 2004; Braveman et al. 2005; Xue et al. 2005; Kohen et al. 2008; Meyer et al. 2014; Burt et al. 2016; Chang et al. 2016; DeVylder et al. 2018). Lower household and neighborhood SES are linked to poorer mental and physical health outcomes, including greater risk of anxiety, depression, and substance abuse (Leventhal and Brooks-Gunn 2000; Boardman et al. 2001; Singh et al. 2002; Singh 2003; Cutrona et al. 2006; Menec et al. 2010; Slopen et al. 2010; Spijkers et al. 2012; Ruscio et al. 2017).
Some work points to dissociable effects of household and neighborhood SES. Specifically, observational and experimental data suggest that, relative to household SES, neighborhood SES better explains variance in externalizing behaviors (Brooks-Gunn et al. 1993; Chase-Lansdale and Gordon 1996; Ludwig et al. 2001; Jackson et al. 2009) and internalizing symptoms (Chase-Lansdale and Gordon 1996; Leventhal and Brooks-Gunn 2003; Leventhal and Dupere 2011), particularly in children. The relatively strong effects of neighborhood disadvantage on child development may in fact be mediated by lack of neighborhood cohesion and engagement in peer-encouraged antisocial behavior (Ludwig et al. 2001; Kohen et al. 2008). Despite the known association between household and neighborhood SES on mental health and the dissociable effects described above, few neuroimaging studies have investigated the dissociable neural consequences of household and neighborhood SES (Gianaros et al. 2017; Miller et al. 2018) or how these effects underlie risk for psychopathology (Marshall et al. 2018; Tomlinson et al. 2020).
The human neuroscience of socioeconomic status is an emerging field that builds on a robust literature on early life stress in animals and humans (Hackman et al. 2010; Farah 2018). Importantly, SES and stress are not identical; nevertheless, low SES families have greater perceived (Stronks et al. 1998) and physiological markers of stress (Seeman et al. 2010; Hackman et al. 2012; Schulz et al. 2012; Vliegenthart et al. 2016; Finegood et al. 2017; Theall et al. 2017; Roubinov et al. 2018), and stress has been understood as a primary mechanism by which socioeconomic disadvantage confers its deleterious effects (Baum et al. 1999; Evans and Schamberg 2009; Hackman et al. 2010; Merz et al. 2019). In rodents, early life stress triggers developmental cascades characterized by hypothalamus-pituitary-adrenal (HPA) axis dysregulation (Maniam et al. 2014), altered neural development in psychiatrically important brain regions such as the ventromedial prefrontal cortex, amygdala, hippocampus, and their connections (McEwen 2013; van Bodegom et al. 2017), and aberrant behavioral development (Bolton et al. 2017). Indeed, human studies of severe early life stress as well as studies of household socioeconomic disadvantage have converged in implicating these very same neuroendocrine pathways in the etiology of stress-related psychopathology with disadvantaged individuals exhibiting altered stress physiology (Lupien et al. 2000; Cohen et al. 2006; Raymond et al. 2018), reduced hippocampal volumes (Hanson et al. 2015; Barch et al. 2016; Merz et al. 2019), and increased internalizing (Everson et al. 2002; Heim and Binder 2012; Gee, Gabard-Durnam, et al. 2013a; Ruscio et al. 2017) and externalizing (Kim et al. 2003; Russell et al. 2016) psychopathology.
One consistent finding in rodent and human research of early life stress, but not yet extended to human SES research, is that disadvantage precipitates precocious development of the amygdala-ventromedial prefrontal cortex emotion regulation pathway (more generally termed the “stress acceleration hypothesis”) (Ono et al. 2008; Callaghan and Richardson 2011; Gee, Gabard-Durnam, et al. 2013a; Ishikawa et al. 2015; Callaghan and Tottenham 2016; Thijssen et al. 2017; VanTieghem and Tottenham 2018; Tottenham 2020). Whereas functional connectivity between the amygdala and the ventromedial prefrontal cortex normally decreases (shifts from positive to negative or near zero) over the course of child development to promote emotion regulation (Lee et al. 2012; Gee, Humphreys, et al. 2013b; Motzkin et al. 2015; Jalbrzikowski et al. 2017), this shift is accelerated in individuals exposed to high levels of early life stress (Gee, Gabard-Durnam, et al. 2013a; Wolf and Herringa 2016). Interestingly, although stress itself is associated with increased risk for internalizing psychopathology (Hicks et al. 2009; McLaughlin et al. 2010; Carr et al. 2013), precociously negative amygdala-vmPFC development seems to confer resilience against symptoms, and positive connectivity is associated with more symptoms in stressed individuals (Gee, Gabard-Durnam, et al. 2013a; Wolf and Herringa 2016). In contrast, other studies that do not directly investigate stress effects have found reduced connectivity in children with anxiety (Hamm et al. 2014). One possible explanation for these divergent findings is that the behavioral relevance of amygdala-vmPFC functional connectivity may be context-dependent, such that reduced connectivity is protective in some pediatric contexts but not in others (Tottenham 2020).
It remains unknown whether socioeconomic disadvantage has a similar effect to early life stress on associations between age and amygdala-vmPFC resting-state functional connectivity (RSFC), whether household and neighborhood SES have convergent or distinct effects on connectivity and to what extent effects on connectivity are associated with anxiety symptoms. Here, we sought to investigate whether the stress acceleration hypothesis extends to associations between amygdala-vmPFC resting-state functional connectivity and neighborhood or household SES. Specifically, we hypothesized that participants from high SES neighborhoods would demonstrate a normative pattern of amygdala-vmPFC connectivity, such that RSFC would vary inversely with age. In contrast, we expected that participants from lower SES neighborhoods would demonstrate more adult-like connectivity at younger ages relative to higher SES participants, consistent with findings from individuals with early life stress (Gee, Gabard-Durnam, et al. 2013a). Second, given findings suggesting distinct contributions of neighborhood and household SES to mental health (Brooks-Gunn et al. 1993; Chase-Lansdale and Gordon 1996; Ludwig et al. 2001; Leventhal and Brooks-Gunn 2003; Jackson et al. 2009), we expected neighborhood effects on connectivity to be dissociable from household effects. Third, consistent with prior findings in individuals with severe early life stress (Gee, Gabard-Durnam, et al. 2013a), we hypothesized that more adult-like connectivity in low neighborhood SES youth would be associated with fewer anxiety symptoms.
Materials and Methods
Participants
Data were pooled for secondary data analysis from 151 participants ranging from 5 to 25 years old who were all recruited from the New York City area for several case-control studies between 2011 and 2017 as healthy controls (n = 84) or patients with attention deficit hyperactivity disorder (ADHD; n = 67; no other clinical groups were included; see Supplementary Table 1 for recruitment strategies) (Marsh et al. 2011; Tau et al. 2014; Cha et al. 2015; Davis et al. 2018). Healthy participants exhibited no psychiatric diagnosis as determined by diagnostic interview (Kiddie-Schedule for Affective Disorders, Diagnostic Interview Schedule for Children, or Structured Clinical Interview for DSM-IV). Participants with ADHD were included to maximize sample size. Furthermore, participants with ADHD were not expected to differ in amygdala-vmPFC connectivity (Posner et al. 2011; Posner et al. 2013; Castellanos and Aoki 2016). Statistical methods were employed to test whether ADHD diagnosis accounted for any observed effects (see Statistical Analyses). The Institutional Review Board of the New York State Psychiatric Institute approved this study.
Socioeconomic Status
Neighborhood SES was measured using the Area Deprivation Index (ADI) (https://www.neighborhoodatlas.medicine.wisc.edu/) (Singh 2003; Kind et al. 2014; Kind and Buckingham 2018). The ADI is a widely used measure of neighborhood SES derived on the census block level based on a factor analysis of neighborhood characteristics from the 2013 American Community Survey 5-Year Estimates, including poverty prevalence, adult educational attainment, home value, and household crowding (Marshall et al. 2020). Census block groups correspond to areas with populations approximately ranging from 600 to 3000 (census.gov.programs-surveys/geography/about/glossary.html). The ADI reports neighborhood SES as national percentiles with the highest percentiles corresponding to the most disadvantaged neighborhoods in the United States. The ADI was calculated based on participant address at the time of scanning; participants located in crowded neighborhoods (i.e., college campus) were excluded because the ADI is not calculated for those communities.
According to the factor analysis used to develop the ADI, the score is most heavily driven by the proportion of individuals in the census block group with incomes below 150% of the poverty threshold and the proportion of individuals 25 years or older with at least a high school diploma contribute (Kind et al. 2014). We acquired these values for each participant’s neighborhood from the American Community Survey (https://data.census.gov/cedsci/). We then determined whether neighborhood poverty or neighborhood educational characteristics contributed more to any observed neighborhood effects.
Given the bimodal distribution of ADI percentiles in the current sample, the variable was analyzed in three groups: low (90–100), middle (11–89), and high neighborhood SES (1–10; Fig. 1). Participants classified as having low neighborhood SES resided in neighborhoods in which, on average, 54.3% of families had incomes under 150% of the poverty line, while this rate was 25.9% and 20.0% for middle and high neighborhood SES families, respectively (Supplementary Table 2). ADI was also analyzed as a continuous variable.
Figure 1 .
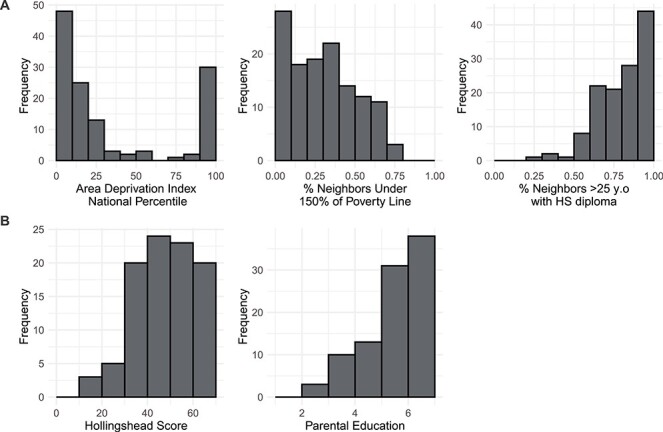
Distributions of (A) neighborhood and (B) household socioeconomic measures. Higher Area Deprivation Index National Percentile corresponded to lower neighborhood SES. Hollingshead scores range from 8 to 66 with lower numbers corresponding to lower household SES. Parental education is an ordinal variable ranging from 1 to 7: 1 = less than middle school; 2 = completed middle school; 3 = some high school; 4 = completed high school/attained GED; 5 = some college/associate’s degree; 6 = bachelor’s degree; 7 = graduate degree.
Household socioeconomic status was measured with the Hollingshead Four-Factor Index of Social Status (Hollingshead 1975). The Hollingshead score is determined as a weighted sum of parental education level (1–7) and parental occupation scores (1–9) with higher numbers representing greater educational attainment, occupational prestige, and income (see Supplemental Methods for coding). For households with two employed parents, the score was calculated as the average of both parents’ scores; for households with one parent, the score was calculated for that one parent. The Hollingshead scores derived from this averaging procedure are highly correlated with scores derived from scores derived from maximal parental education and occupation (r = 0.92); nevertheless, results using this method are presented in the supplement. We acknowledge that single-parent status is a contributor to SES (McLanahan and Sandefur 1994; Bradley and Corwyn 2002a; Conger et al. 2010); however, because only 10 participants were from single-parent households, we were unable to examine its effects in this study. Parental education was also analyzed independently as an ordinal variable corresponding to the parent with the greatest educational attainment. Household income was not collected in these studies as they had originally intended to examine group differences in brain measures between clinical and control samples. The distributions of all measures of socioeconomic status are presented in Figure 1, and how they vary across studies is presented in Supplementary Figure 1.
Anxiety
Given the role of amygdala-vmPFC connectivity in anxiety disorders (Gee, Gabard-Durnam, et al. 2013a; Hamm et al. 2014; Wolf and Herringa 2016), we examined anxiety symptoms as measured by the Anxious/Depressed subscale T-score of the Child Behavior Checklist (CBCL) in a subset of participants with available data (n = 65) (Achenbach 1983). The CBCL was available for participants 6–17 years old (mean = 10.7 years; standard deviation = 3.5 years).
MRI Acquisition
All MRI data were acquired on a General Electric Signa 3 Tesla LX scanner (Milwaukee, WI). Specific acquisition parameters from each study are presented in Supplementary Table 1. To account for slight differences in MRI acquisition (flip angle, TR, and run length), a three-group dummy code was included as a covariate in all imaging analyses.
Preprocessing and Motion Correction
MRI data preprocessing was performed using the CONN Toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012). Steps included functional realignment and unwarping, slice timing correction, scrubbing, and simultaneous segmentation and normalization to the Montreal Neurological Institute (MNI) template. Head motion outliers were identified using the ART tools (>0.5 mm framewise displacement or Z > 3 change in global signal). Frames with head motion outlier were regressed in participant-level models along with anatomical nuisance regressors (aCompCor) from white matter (10 components) and cerebrospinal fluid (10 components) (Behzadi et al. 2007). Participants were included in final analyses if they had more than 5 min of functional data uncontaminated by motion. Functional data were band-pass filtered (0.01–0.1 Hz). Supplementary analyses examined models including global signal regression.
Statistical Analyses
Regions of interest (ROI) included left and right amygdala subregions (basolateral and centromedial; Juelich Histological Atlas with 50% probability threshold) (Amunts et al. 2005) and the ventromedial prefrontal cortex (Harvard-Oxford atlas). The blood oxygen level-dependent (BOLD) time course of each ROI was calculated as the average of the time courses of its constituent voxels. Resting-state functional connectivity between two ROIs was calculated as the Fisher Z-transformed correlation coefficient of their time courses in CONN.
Functional connectivity between the vmPFC and amygdala subregions (4 subregions; 4 connections total) served as dependent variables in multiple linear regression models with age, neighborhood SES group (3 levels), and their interaction as predictors of interest and sex, mean head motion during MRI acquisition, dummy-coded pulse sequence, and ADHD diagnosis as covariates. The high SES group served as the reference group for these analyses as they were expected to show the previously observed negative association between age and connectivity. The specific models used for analyses are detailed in the Supplementary Methods, as well as sample R code (R version 3.5.3) (Core Team 2015). All reported betas are standardized. All model residuals were tested for normality using the Shapiro-Wilk test.
To address our first hypothesis, we first tested if connectivity was inversely associated with age in the high SES group by inspecting the “age” term in each of our models. Next, we inspected the age×SES interaction terms to determine if SES (low or middle vs. high) moderated the association between age and resting-state functional connectivity. In the absence of interaction effects, the effects of dummy-coded SES groups were then inspected to determine whether mean connectivity varied between groups. All 20 significance tests for these analyses (age, age × middle SES, age × low SES, middle SES, and low SES terms for 4 connections) were two-sided, and multiple comparisons correction was performed using false discovery rate (FDR; “P.adjust” function from stats package). We also investigated whether neighborhood poverty rates or neighborhood educational attainment (the two variables that contribute most to the ADI score) better explained observed effects. To confirm our main findings, post hoc analyses (detailed in Supplementary Methods and Supplementary Results) examined effects of global signal regression, treating neighborhood SES as a continuous variable, quadratic age effects (Shaw et al. 2008), and whether our results might have been driven by the inclusion of participants with ADHD.
To test our second hypothesis that neighborhood SES and household SES would have dissociable effects on the association between age and amygdala-vmPFC RSFC, we included interactions between age and both neighborhood SES and household SES (parental education or Hollingshead score) terms in the same model. We also performed this analysis with only household SES and its interaction with age in the model.
To test our third hypothesis that adult-like connectivity in low rather than high neighborhood SES youth would be associated with reduced anxiety symptoms in the subset of participants with available data, we tested the three-way interaction between age, neighborhood SES (continuous given reduced sample size and power), and amygdala-vmPFC RSFC on anxiety/depression symptoms, controlling for age, sex, motion, and pulse sequence. We note that these results are preliminary given reduced sample size and power.
Results
Participants
Of the 151 participants with neuroimaging data and home addresses available, 15 were excluded for having less than 5 min of useable data after motion correction, leaving 136 with useable MRI data. Because 9 participants reported a college campus as their residence, 127 participants had useable imaging data and addresses that could be converted into an ADI score. Sample characteristics are presented in Table 1. Briefly, 127 participants, 5–25 years old, were included in main analyses; 73 were recruited as healthy controls and 54 had ADHD. Forty-eight participants were from high SES neighborhoods, 48 were from middle, and 31 were from low SES neighborhoods. Additional details about neighborhood SES and details about our behavioral subsample are presented in Supplementary Results.
Table 1.
Sample characteristics
| N = 127 | Mean (SD)/N (%) | Range |
|---|---|---|
| Sex (female) | 61 (48.0%) | Female/male |
| Age (years) | 14.7 (5.3) | 5–25 |
| Low neighborhood SES | 15.0 (5.9) | 6–25 |
| Middle neighborhood SES | 14.9 (5.0) | 5–23 |
| High neighborhood SES | 13.8 (4.9) | 6–24 |
| ADHD diagnosis | 54 (42.5%) | ADHD/healthy |
| Neighborhood disadvantage | ||
| Area deprivation index (ADI) | 35.4 (38.2) | 1–100 |
| Low SES (ADI: 90–100) | 31 (24.4%) | |
| Middle SES (ADI: 11–89) | 48 (37.8%) | |
| High SES (ADI: 1–10) | 48 (37.8%) | |
| Ethnicity | ||
| White | 44 (34.6%) | |
| Hispanic | 35 (27.6%) | |
| Black | 25 (19.7%) | |
| Asian | 11 (8.7%) | |
| Multiracial | 4 (3.1%) | |
| Other/unknown | 8 (6.3%) | |
| Parental education | ||
| Some middle school | 0 (0%) | |
| Completed middle school | 0 (0%) | |
| Some high school | 3 (2.4%) | |
| High school/GED | 12 (9.4%) | |
| Some college/associates | 15 (11.8%) | |
| Bachelor’s degree | 32 (25.2%) | |
| Graduate degree | 40 (31.5%) | |
| Hollingshead | 47.5 (12.5) | 14–66 |
| Mean head motion (mm) | 0.19 (0.17) | 0.05–1.15 |
| % Frames useable | 90.3 (0.09) | 58.2–100 |
Neighborhood SES and Amygdala-vmPFC Functional Connectivity
The neighborhood SES × age interaction predicted resting-state functional connectivity (RSFC) between right basolateral amygdala (RBLA) and ventromedial prefrontal cortex (vmPFC). Among participants from high but not low or middle SES neighborhoods, age was inversely associated with connectivity, such that connectivity shifted from positive in younger participants to negative connectivity in older participants (Fig. 2A; Table 2). The proportion of young participants (age < mean age of 14.7 years) with positive connectivity varied with neighborhood SES such that 90%, 53%, and 63% of young participants had positive connectivity in the high, middle, and low neighborhood SES groups, respectively (χ2 = 7.07, P = 0.029).
Figure 2 .
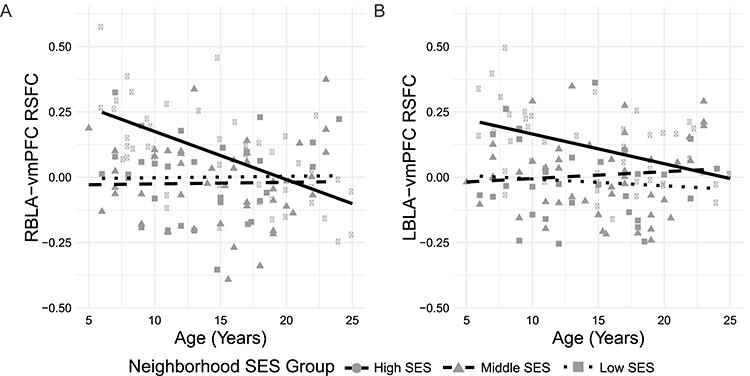
The association between age and basolateral right amygdala (RBLA)-ventromedial prefrontal cortex (vmPFC) resting-state functional connectivity (RSFC) depends on neighborhood socioeconomic status (SES). RBLA-vmPFC connectivity shifts from positive to negative with increasing age among participants with high neighborhood SES. LBLA-vmPFC connectivity is near zero among participants with lower neighborhood SES. Fit lines represent slopes within each neighborhood SES group.
Table 2.
Effects of age, neighborhood SES, and their interaction on amygdala-vmPFC connectivity
| N = 127 | Age (in high neighborhood SES participants) |
Age × neighborhood SES | Neighborhood SES | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Middle SES > High SES | Low SES > High SES | Middle SES > High SES | Low SES > High SES | |||||||
| β | P | β | P | β | P | β | P | β | P | |
| RBLA | −0.56 | 0.00009* | 0.61 | 0.002* | 0.58 | 0.01* | −0.65 | 0.001* | −0.52 | 0.02 |
| LBLA | −0.24 | 0.092 | 0.43 | 0.028 | 0.20 | 0.37 | −0.56 | 0.005* | −0.76 | 0.0009* |
| RCMA | −0.32 | 0.037 | 0.26 | .22 | 0.35 | 0.16 | −0.42 | 0.05 | −0.32 | 0.18 |
| LCMA | −0.19 | 0.21 | 0.06 | .78 | 0.39 | 0.11 | −0.28 | 0.18 | −0.31 | 0.19 |
Notes:
1. In all analyses, the high socioeconomic status (SES) group served as the reference group, meaning that the age effect beta refers to the effect of age within the high SES group, moderation betas refer to the difference between age effects in middle or low SES groups and the high SES group, and main SES effects betas refer to group differences in covariate-adjusted mean connectivity; R/LBLA = right/left basolateral amygdala; R/LCMA = right/left centromedial amygdala
2. All models control for sex, head motion, pulse sequence, and ADHD diagnosis; reported betas are standardized; *P < 0.05 with FDR-correction across all 20 regression coefficient significance tests
The interaction between age and neighborhood SES did not predict left BLA-vmPFC RSFC. However, compared with high SES individuals, individuals from middle and low SES neighborhoods had significantly lower connectivity between left BLA and vmPFC, such that mean connectivity was 0.11, 0.01, and −0.02 in the high, middle, and low SES groups, respectively (Fig. 2B; Table 2). The proportion of participants with positive connectivity varied with neighborhood SES such that 73%, 59%, and 36% of participants had positive connectivity in the high, middle, and low neighborhood SES groups (χ2 = 7.97, P = 0.018). No age, SES, or interaction effects survived multiple comparisons correction for connectivity between right or left centromedial amygdala and vmPFC (Table 2). Analyses that included global signal regression or treated neighborhood SES as a continuous variable yielded similar results (Supplementary Tables 3 and 4).
The proportion of neighbors below 150% of the poverty threshold interacted with age to predict RBLA-vmPFC functional connectivity (Fig. 3; Table 3); the proportion of neighbors 25 years or older with a high school (HS) diploma also interacted with age to predict connectivity, but did not pass multiple comparisons correction. Post hoc analysis confirmed that age only predicted connectivity among advantaged participants (% neighbors under 150% poverty line <24% and % neighbors 25 years or older with HS diploma >90%; Supplementary Fig. 2). When we included both of these neighborhood socioeconomic measures and their interactions with age in the same model, poverty remained a significant moderator while HS graduation did not [P(age × poverty) = 0.029; P(age × education) = 0.989]. Neither neighborhood poverty nor educational attainment was significantly associated with LBLA-vmPFC RSFC (Table 3).
Figure 3 .
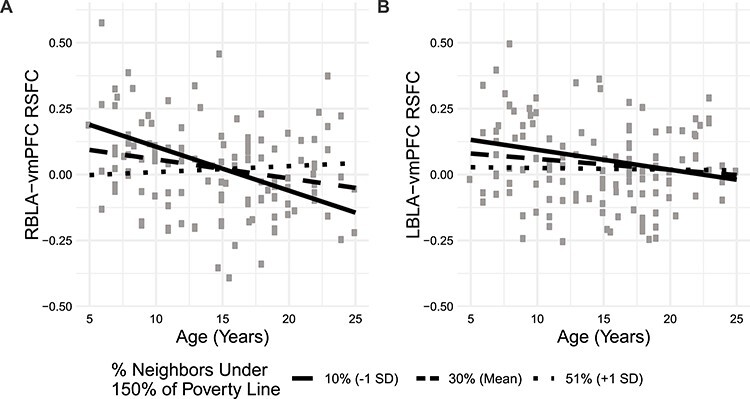
The association between age and right basolateral amygdala (RBLA)-ventromedial prefrontal cortex (vmPFC) resting-state functional connectivity (RSFC) depends on neighborhood poverty rates. RBLA-vmPFC connectivity shifts from positive to negative with increasing age among participants with less neighborhood poverty. Simple slope fit lines are displayed.
Table 3.
Effects of age, neighborhood socioeconomic measures, and their interaction on amygdala-vmPFC connectivity
| N = 127 | Age (at mean neighborhood SES) | Age × % of neighbors < 150% poverty threshold | % of neighbors < 150% poverty threshold | |||
|---|---|---|---|---|---|---|
| β | P | β | P | Β | P | |
| RBLA | −0.17 | 0.088 | 0.27 | 0.003* | 0.002 | 0.98 |
| LBLA | 0.005 | 0.96 | 0.06 | 0.54 | −0.05 | 0.57 |
| RCMA | −0.12 | 0.27 | 0.16 | 0.10 | 0.01 | 0.94 |
| LCMA | −0.05 | 0.63 | .22 | 0.019 | 0.01 | 0.90 |
| N = 127 | Age (at mean neighborhood SES) | Age × % of neighbors ≥ age 25 with a HS Diploma | % of Neighbors ≥ age 25 with a HS Diploma | |||
| β | P | β | P | Β | P | |
| RBLA | −0.17 | 0.089 | −0.24 | 0.023 | 0.07 | 0.41 |
| LBLA | 0.002 | 0.98 | −0.09 | 0.36 | 0.13 | 0.15 |
| RCMA | −0.12 | 0.26 | −0.17 | 0.10 | 0.09 | 0.33 |
| LCMA | −0.05 | 0.65 | −0.24 | 0.025 | −0.003 | 0.97 |
Notes:
1. All models control for sex, head motion, pulse sequence, and ADHD diagnosis; reported betas are standardized; *P < 0.05 with FDR-correction across 12 regression coefficient significance tests per neighborhood socioeconomic measure; SES = socioeconomic status; R/LBLA = right/left basolateral amygdala; R/LCMA = right/left centromedial amygdala.
We detected a significant interaction between age2 and neighborhood SES such that low SES children had a parabolic (in addition to linear) trajectory of RBLA-vmPFC connectivity (Supplementary Table 5).
ADHD had no effect on connectivity in main analyses (Ps > 0.27). Post hoc analyses yielded neither any significant three-way interactions (SES group × age × ADHD diagnosis; Supplementary Table 6), nor any age × ADHD interactions (Supplementary Table 7), indicating that ADHD diagnosis did not underlie observed interactions between age and neighborhood SES.
Neighborhood SES, Household SES, and Amygdala-vmPFC Functional Connectivity
Area deprivation index (ADI) was correlated with both parental education and Hollingshead (r = −0.37, P = 0.0001; r = −0.36, P = 0.0003, respectively). Neighborhood SES × age and neighborhood SES terms remained significant upon the addition of household SES to our first model; household SES and its interaction with age did not predict RSFC (Table 4). Results were nearly identical when using Hollingshead score as determined by maximal household occupation/education (Supplementary Table 8) and when using 3-group formulations of the Hollingshead and parental education (Supplementary Table 9). We also tested whether neighborhood poverty was a stronger moderator than household SES and found convergent results (Supplementary Table 10). Furthermore, neither household SES nor the household SES × age interaction was associated with RSFC in models without neighborhood SES (Supplementary Table 11). All models reported had normally distributed residuals (Shapiro-Wilk test P-values >0.12).
Table 4.
The effects of age, neighborhood SES, and their interaction on basolateral amygdala-vmPFC functional connectivity, controlling for the interaction between household SES and age
| N = 95 | Neighborhood SES × age | Neighborhood SES | Household SES × age | Household SES | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Middle SES > high SES | Low SES > high SES | Middle SES > high SES | Low SES > high SES | ||||||||||
| β | P | β | P | β | P | β | P | β | P | β | P | ||
| Parental education | RBLA | −0.70 | 0.001* | 0.48 | 0.048 | −0.74 | 0.001* | −0.32 | 0.20 | −0.04 | 0.70 | 0.06 | 0.60 |
| LBLA | 0.20 | 0.049 | 0.20 | 0.42 | −0.62 | 0.009* | −0.62 | 0.020* | −0.03 | 0.81 | −0.02 | 0.90 | |
| Hollingshead | RBLA | 0.69 | 0.001* | 0.53 | 0.026* | −0.73 | 0.002* | −0.37 | 0.14 | 0.04 | 0.68 | −0.02 | 0.86 |
| LBLA | 0.43 | 0.050 | 0.21 | 0.40 | −0.63 | 0.008* | −0.59 | 0.023* | −0.01 | 0.93 | 0.03 | 0.78 | |
Notes:
1. All models control for sex, head motion, pulse sequence, and ADHD diagnosis; reported betas are standardized; *P < 0.05 with FDR-correction across 8 regression coefficient significance tests per household socioeconomic measure; SES = socioeconomic status; R/LBLA = right/left basolateral amygdala
Symptom Associations
A three-way interaction between neighborhood SES, age, and RBLA-vmPFC resting-state connectivity predicted CBCL anxiety symptoms, such that low neighborhood SES children (participants at the lower end of the age range) with characteristically more negative connectivity had fewer symptoms (Table 5, Fig. 4A, P = 0.006), as did high SES children with characteristically more positive connectivity. Conversely, low SES children with positive connectivity and high SES children with low connectivity had more anxious/depressed symptoms. In adolescents (participants at the higher end of the age range), connectivity did not predict symptoms regardless of SES (Fig. 4). This three-way interaction was also significant when using neighborhood poverty and neighborhood high school completion (Fig. 4B,C, Table 5), but not when using household SES measures (Supplementary Table 12). No associations were found between symptoms and LBLA-vmPFC functional connectivity (Table 5).
Table 5.
Effects of the interaction between age, neighborhood socioeconomic status, and basolateral amygdala-vmPFC resting-state functional connectivity on the Anxious/Depressed subscale of the CBCL.
| N = 65 | RBLA-vmPFC RSFC × Neighborhood SES × Age | LBLA-vmPFC RSFC × Neighborhood SES × Age | ||
|---|---|---|---|---|
| Β | P | β | P | |
| Area Deprivation Index | −3.29 | .007 | −1.53 | .24 |
| % of Neighbors < 150% Poverty Threshold | −2.85 | .028 | −2.13 | .11 |
| % of Neighbors ≥ age 25 with a HS Diploma | 2.96 | .035 | 1.43 | .39 |
Notes:
1. All models control for sex, head motion, pulse sequence, and ADHD diagnosis; reported betas are standardized. SES = socioeconomic status; R/LBLA = right/left basolateral amygdala; RSFC = resting-state functional connectivity.
Figure 4 .
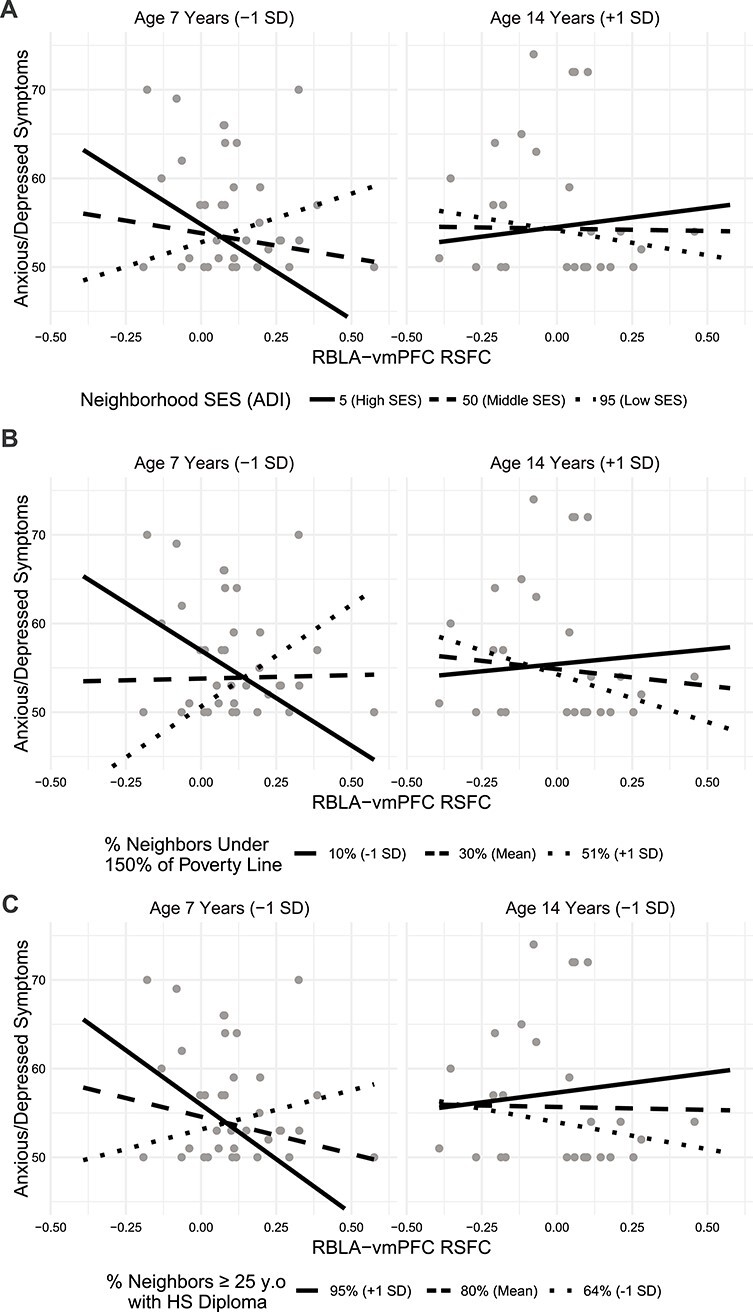
The association between RBLA-vmPFC resting-state connectivity and anxious/depressed symptoms depends on neighborhood socioeconomic status (SES) and age. Younger participants from low SES communities have a positive association between connectivity and anxiety; younger participants from high SES communities have an inverse relationship. Simple slope fit lines are displayed.
Discussion
Summary of Results
Here, we have shown that amygdala subregional-vmPFC RSFC depends on neighborhood socioeconomic status. First, we found that RBLA-vmPFC RSFC was inversely correlated with age in individuals from the most socioeconomically advantaged neighborhoods, while no relationship between age and RSFC was detected in those from less advantaged communities. Further, LBLA-vmPFC RSFC was more negative in participants from less advantaged communities. Importantly, we show this effect using three measures of neighborhood SES and provide evidence that neighborhood economic (rather than educational) factors more precisely explain the observed results. Second, these effects were uniquely explained by neighborhood characteristics and not household socioeconomic measures of education and Hollingshead score. Finally, we provide preliminary evidence that among younger individuals from more disadvantaged communities, negative connectivity may protect against anxiety and depression symptoms, while positive connectivity may protect against these symptoms in younger individuals from more advantaged communities.
Potential Mechanisms Linking Neighborhood Disadvantage to Amygdala-vmPFC RSFC
Our findings are supported by the rich literature on associations among neighborhood disadvantage and stressful life experiences and stress physiology, which in turn affect amygdala function. We showed that neighborhood disadvantage affects amygdala connectivity and propose that stressful experiences inherent to disadvantaged communities may mediate these effects. Despite the considerable heterogeneity across disadvantaged communities, findings suggest that such communities tend to have reduced social cohesion, lower accessibility to healthy food options and higher rates of food insecurity, higher rates of violent crime, lower educational attainment, and increased exposure to environmental neurotoxicants (Duncan 1996; Macintyre et al. 2002; Wilson et al. 2004; Braveman et al. 2005; Xue et al. 2005; Kohen et al. 2008; Meyer et al. 2014; Burt et al. 2016; Chang et al. 2016; DeVylder et al. 2018). The developing brain may be particularly vulnerable to the effects of this onslaught of neighborhood stressors by several mechanisms at different time points including prenatal transplacental stress hormone, inflammatory, and neurotoxicant insult and postnatal hypothalamic-pituitary-adrenal (HPA) axis dysregulation following early life stress. Indeed, children from more disadvantaged neighborhoods have higher basal cortisol levels (Finegood et al. 2017; Gianaros et al. 2017; Roubinov et al. 2018) and more cortisol dysregulation following a laboratory stressor (Hackman et al. 2012; Theall et al. 2017).
Cortisol mediates some effects of stress on brain development, particularly in the amygdala. For example, maternal prenatal cortisol predicts amygdala functional connectivity in neonates (Graham et al. 2019), and both prenatal maternal cortisol (Buss et al. 2012) and postnatal child cortisol (Pagliaccio et al. 2014) predict amygdala structure. Furthermore, the amygdala and its connectivity with the ventromedial prefrontal cortex contribute to HPA axis function (Urry et al. 2006; Vaisvaser et al. 2013; Hakamata et al. 2017). Some studies have begun to investigate the influences of neighborhood disadvantage on inflammatory processes (Broyles et al. 2012; Smith et al. 2017), and it is known that environmental toxicants are distributed in a socioeconomically stratified manner (Cureton 2011; Vivier et al. 2011; Young et al. 2012; Morelli et al. 2017). Furthermore, there is evidence that inflammatory processes affect amygdala connectivity (Harrison et al. 2009; Graham et al. 2018; Mehta et al. 2018). These previous findings provide multiple biologically plausible pathways to explain our findings that neighborhood disadvantage has a measurable impact on child amygdala functional connectivity.
Precocious Development and the Stress Acceleration Hypothesis
Our study provides evidence supporting the “stress acceleration hypothesis” that stressful early life experiences promote precocious brain development in children in order to better cope with adverse environments (Callaghan and Tottenham 2016). Notably, findings suggest that the maturation of amygdala-vmPFC functional connectivity is characterized by shifts from positive to negative connectivity during an emotional face task (Gee, Humphreys, et al. 2013b) and near zero at rest (Jalbrzikowski et al. 2017). In our study, we show that children from less advantaged neighborhoods had, on average, near-zero amygdala-vmPFC RSFC and a greater likelihood of having negative connectivity than advantaged children, perhaps indicating earlier maturation. Importantly, this finding is in line with a previous study showing reduced amygdala-vmPFC connectivity during an emotional faces task in children who experienced early life caregiver adversity relative to typically developing children (Gee, Gabard-Durnam, et al. 2013a). Furthermore, we provide preliminary evidence that the effects of neighborhood characteristics on connectivity protect against anxiety symptoms. Such negative connectivity may confer resilience against symptoms in disadvantaged contexts, whereas positive connectivity may be protective in advantaged contexts. Importantly, although context-specific brain development following socioeconomic disadvantage appeared to confer benefits in the short term in our study, evidence also suggests that socioeconomic disadvantage does increase risk for anxiety over time (Ruscio et al. 2017). Similar to the disadvantaged participants in our study, anxiety and amygdala-vmPFC connectivity are inversely associated in adults (Etkin et al. 2009; Hahn et al. 2011; Kim et al. 2011). Longitudinal studies are required to understand the factors and developmental sequelae that potentially render these adaptations against anxiety insufficient in the long term. We note that these behavioral results are preliminary given reduced sample size and power. Nevertheless, in addition to supporting the stress acceleration hypothesis, they suggest that neighborhood SES may explain the inconsistent direction in associations between amygdala-vmPFC RSFC and anxiety symptoms in children (Gee, Gabard-Durnam, et al. 2013a; Hamm et al. 2014; Qin et al. 2014; Wolf and Herringa 2016).
Neighborhood and Household SES
Previous work has shown the profound effects of neighborhood characteristics on children’s behavior, particularly in experimental designs that control for household SES (Brooks-Gunn et al. 1993; Chase-Lansdale and Gordon 1996; Ludwig et al. 2001; Leventhal and Brooks-Gunn 2003; Jackson et al. 2009; Leventhal and Dupere 2011). Our brain findings add to emerging literature on the effects of neighborhood SES on neural development (Tooley et al. 2019; Ramphal et al. 2020) that may be dissociable from household effects (Gianaros et al. 2017; Marshall et al. 2018; Miller et al. 2018; Tomlinson et al. 2020). Indeed, although household and neighborhood disadvantage are often correlated (r = 0.36–0.37 in our study), the aforementioned domains of disadvantage that may contribute to neighborhood-brain associations (e.g., neighborhood social cohesion, neighborhood violence, environmental neurotoxicant burden, and food accessibility) are spatially distributed. That is, since they operate at a neighborhood level, measuring them at the neighborhood level offers the best operationalization of how these domains are distributed (Garner and Raudenbush 1991; Sampson et al. 1997; Browning and Cagney 2003; Brulle and Pellow 2006; Walker et al. 2010). Thus, neighborhood disadvantage likely captures the nuances of their distribution more precisely than household disadvantage, which can vary widely within a single neighborhood. For example, a highly educated individual can live in a polluted neighborhood. A poor family can live in a cohesive neighborhood (Farah 2017). As such, neighborhood and household processes are meaningfully distinguishable and future studies of SES should continue to treat them as distinct, albeit related measures.
Although other studies have identified associations between household SES and brain structure, function, and connectivity (Hackman and Farah 2009), we did not. We analyzed household disadvantage in two ways: first, by parental education and second, by Hollingshead score, a measure of household SES that includes consideration of both occupation and education. A more complete examination of household SES would include a measure of household income, which we did not have because our sample consisted of pooled data from several case-control neuroimaging studies. One study examining amygdala-vmPFC connectivity at a single age found that lower income was associated with more negative connectivity (Hanson et al. 2019). Our failure to detect an association between household SES and functional connectivity could be due to the absence of strictly economic household characteristics in our study. Notably this study did not include a measure of neighborhood SES. Another possibility is that neighborhood and household SES interact to contribute to brain development and behavior. Additional work designed to test these interactive effects is necessary to understand the unique and shared contributions of various scales of SES to child brain and emotional development.
What is Normative?
Prior studies have posited that amygdala-vmPFC connectivity normally shifts from positive to negative or near-zero with increasing age, both with cross-sectional (Gee, Humphreys, et al. 2013b) and longitudinal (Jalbrzikowski et al. 2017) designs. Although the cross-sectional nature of our data limits our ability to interpret our findings as strictly developmental, we provide evidence that this protracted reduction in connectivity with increasing age may be limited to participants residing in highly advantaged communities characterized by low poverty rates. Interestingly, age was unassociated with connectivity among the middle SES group which had similar high school graduation and poverty rates as the high SES group. This could be because these specific components of the ADI may not represent the neighborhood characteristics that best distinguish highly advantaged neighborhoods. For example, neighborhoods categorized in our sample as being high, middle, and low SES group had bachelor’s degree attainment rates of 56%, 39%, and 15%, respectively. Highly advantaged communities are also characterized by consistent improvement in the domains of safety, public space, and school quality (Solari 2012), which might further differentiate the effects of high and middle SES neighborhoods.
Among individuals from less advantaged neighborhoods, the absence of age effects and the presence of near-zero connectivity suggest that this pattern of mature connectivity developed prior to the youngest age in our sample. Our findings are consistent with previous work demonstrating that age-brain associations can be strongly biased by sample demographic composition (LeWinn et al. 2017). Altogether, these findings underscore the necessity of examining and reporting the effects of neighborhood socioeconomic status in studies of brain development (Farah 2017).
Our findings add to an emerging literature documenting that socioeconomic deprivation affects the developing brain (Hackman and Farah 2009; Farah 2018). Though our findings point to a mechanism of short-term resilience, other social factors and biological mechanisms likely contribute to known associations between disadvantage and mental illness and additional work should investigate these (Aneshensel and Sucoff 1996; Ross 2000; Browning and Cagney 2003; Luby et al. 2013). Furthermore, our study should not dissuade continued efforts to improve the environments in which children develop. Both neighborhood- (Ludwig et al. 2012; South et al. 2018) and household-level (Costello et al. 2003) socioeconomic interventions have successfully improved mental health. Future studies should also further parse the effects of neighborhood- and household-level intervention to better understand the most efficacious ways of improving mental health disparities.
Supplementary Material
Contributor Information
Bruce Ramphal, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Mariah DeSerisy, Department of Psychology, Fordham University, Bronx, NY 10458, USA.
David Pagliaccio, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Elizabeth Raffanello, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Virginia Rauh, Department of Population and Family Health, Mailman School of Public Health, Columbia University, New York, NY 10032, USA.
Gregory Tau, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Jonathan Posner, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Rachel Marsh, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Amy E Margolis, New York State Psychiatric Institute and Department of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Funding
National Institutes of Health (K23ES026239); Promise Project at Columbia University; The NVLD Project.
References
- Achenbach TM, Edelbrock CS. 1983. Manual for the child behavior checklist and revised child behavior profile. Burlington, VT: University of Vermont, Department of Psychiatry.
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl). 210(5–6):343–352. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Sucoff CA. 1996. The neighborhood context of adolescent mental health. Journal of Health and Social Behavior. 37(4):293–310. [PubMed] [Google Scholar]
- Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, Tillman R, Luby J. 2016. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am J Psychiatry. 173(6):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM. 1999. Socioeconomic status and chronic stress. Does stress account for ses effects on health? Ann N Y Acad Sci. 896:131–144. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (compcor) for bold and perfusion based fmri. Neuroimage. 37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Finch BK, Ellison CG, Williams DR, Jackson JS. 2001. Neighborhood disadvantage, stress, and drug use among adults. J Health Soc Behav. 42(2):151–165. [PubMed] [Google Scholar]
- Bolton JL, Molet J, Ivy A, Baram TZ. 2017. New insights into early-life stress and behavioral outcomes. Curr Opin Behav Sci. 14:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. 2002a. Socioeconomic status and child development. Annu Rev Psychol. 53(1):371–399. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. 2002b. Socioeconomic status and child development. Annu Rev Psychol. 53:371–399. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. 2005. Socioeconomic status in health research: one size does not fit all. JAMA. 294(22):2879–2888. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ, Klebanov PK, Sealand N. 1993. Do neighborhoods influence child and adolescent development? Am J Sociol. 99(2):353–395. [Google Scholar]
- Browning CR, Cagney KA. 2003. Moving beyond poverty: Neighborhood structure, social processes, and health. J Health Soc Behav. 44(4):552–571. [PubMed] [Google Scholar]
- Broyles ST, Staiano AE, Drazba KT, Gupta AK, Sothern M, Katzmarzyk PT. 2012. Elevated c-reactive protein in children from risky neighborhoods: evidence for a stress pathway linking neighborhoods and inflammation in children. PLoS One. 7(9):e45419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulle RJ, Pellow DN. 2006. Environmental justice: human health and environmental inequalities. Annu Rev Public Health. 27(1):103–124. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL, Gorman-Smith D, Neiderhiser JM. 2016. Neighborhood disadvantage alters the origins of children's nonaggressive conduct problems. Clin Psychol Sci. 4(3):511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. 2012. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 109(20):E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. 2011. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 125(1):20–28. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. 2016. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. 2013. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 201(12):1007–1020. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Aoki Y. 2016. Intrinsic functional connectivity in attention-deficit/hyperactivity disorder: a science in development. Biol Psychiatry Cogn Neurosci Neuroimaging. 1(3):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Fekete T, Siciliano F, Biezonski D, Greenhill L, Pliszka SR, Blader JC, Roy AK, Leibenluft E, Posner J. 2015. Neural correlates of aggression in medication-naive children with adhd: multivariate analysis of morphometry and tractography. Neuropsychopharmacology. 40(7):1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LY, Wang MY, Tsai PS. 2016. Neighborhood disadvantage and physical aggression in children and adolescents: a systematic review and meta-analysis of multilevel studies. Aggress Behav. 42(5):441–454. [DOI] [PubMed] [Google Scholar]
- Chase-Lansdale PL, Gordon RA. 1996. Economic hardship and the development of five- and six-year-olds: Neighborhood and regional perspectives. Child Development. 67(6):3338–3367. [Google Scholar]
- Cohen S, Doyle WJ, Baum A. 2006. Socioeconomic status is associated with stress hormones. Psychosom Med. 68(3):414–420. [DOI] [PubMed] [Google Scholar]
- Conger RD, Conger KJ, Martin MJ. 2010. Socioeconomic status, family processes, and individual development. J Marriage Fam. 72(3):685–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R . 2015. R: A language and environment for statistical computing. Vienna, austria: R Foundation for Statistical Computing. [Google Scholar]
- Costello EJ, Compton SN, Keeler G, Angold A. 2003. Relationships between poverty and psychopathology: a natural experiment. JAMA. 290(15):2023–2029. [DOI] [PubMed] [Google Scholar]
- Cureton S. 2011. Environmental victims: environmental injustice issues that threaten the health of children living in poverty. Rev Environ Health. 26(3):141–147. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, Wallace G, Wesner KA. 2006. Neighborhood characteristics and depression: an examination of stress processes. Curr Dir Psychol Sci. 15(4):188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K, Margolis AE, Thomas L, Huo Z, Marsh R. 2018. Amygdala sub-regional functional connectivity predicts anxiety in children with reading disorder. Dev Sci. 21(5):e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVylder JE, Jun HJ, Fedina L, Coleman D, Anglin D, Cogburn C, Link B, Barth RP. 2018. Association of exposure to police violence with prevalence of mental health symptoms among urban residents in the United States. JAMA Netw Open. 1(7):e184945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan DF. 1996. Growing up under the gun: children and adolescents coping with violent neighborhoods. J Prim Prev. 16(4):343–356. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. 2009. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 66(12):1361–1372. [DOI] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Marcynyszyn LA, Gentile L, Salpekar N. 2005. The role of chaos in poverty and children's socioemotional adjustment. Psychol Sci. 16(7):560–565. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. 2009. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci U S A. 106(16):6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson SA, Maty SC, Lynch JW, Kaplan GA. 2002. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 53(4):891–895. [DOI] [PubMed] [Google Scholar]
- Farah MJ. 2017. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 96(1):56–71. [DOI] [PubMed] [Google Scholar]
- Farah MJ. 2018. Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat Rev Neurosci. 19(7):428–438. [DOI] [PubMed] [Google Scholar]
- Finegood ED, JRD R, Blair C, Family Life Project I . 2017. Exploring longitudinal associations between neighborhood disadvantage and cortisol levels in early childhood. Dev Psychopathol. 29(5):1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CL, Raudenbush SW. 1991. Neighborhood effects on educational attainment: a multilevel analysis. Sociol Educ. 64(4):251–262. [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. 2013a. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 110(39):15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. 2013b. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 33(10):4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Kuan DC, Marsland AL, Sheu LK, Hackman DA, Miller KG, Manuck SB. 2017. Community socioeconomic disadvantage in midlife relates to cortical morphology via neuroendocrine and cardiometabolic pathways. Cereb Cortex. 27(1):460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Kawachi I, Fitzmaurice GM, Buka L. 2003. Socio-economic status, family disruption and residential stability in childhood: relation to onset, recurrence and remission of major depression. Psychol Med. 33(8):1341–1355. [DOI] [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, Galea S. 2011. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly african american urban community: the Detroit neighborhood health study. J Trauma Stress. 24(6):747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Entringer S, Ben Ward E, Rudolph MD, Gilmore JH, Styner M, Wadhwa PD, Fair DA, Buss C. 2019. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry. 85(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA, et al. 2018. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry. 83(2):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. 2012. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 6:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. 2009. Socioeconomic status and the developing brain. Trends Cogn Sci. 13(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. 2010. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 11(9):651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. 2011. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 56(3):881–889. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Komi S, Moriguchi Y, Izawa S, Motomura Y, Sato E, Mizukami S, Kim Y, Hanakawa T, Inoue Y, et al. 2017. Amygdala-centred functional connectivity affects daily cortisol concentrations: a putative link with anxiety. Sci Rep. 7(1):8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, Monk CS, Phan KL. 2014. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Albert WD, Skinner AT, Shen SH, Dodge KA, Lansford JE. 2019. Resting state coupling between the amygdala and ventromedial prefrontal cortex is related to household income in childhood and indexes future psychological vulnerability to stress. Dev Psychopathol. 31(3):1053–1066. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. 2015. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 77(4):314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. 2009. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 66(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. 2012. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 233(1):102–111. [DOI] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, McGue M. 2009. Gene-environment interplay in internalizing disorders: consistent findings across six environmental risk factors. J Child Psychol Psychiatry. 50(10):1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson S, Godoy L, Beers LS, Lewin A. 2017. Improving mental health access for low-income children and families in the primary care setting. Pediatrics. 139(1):e20151175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. 1975. Four-factor index of social status. New Haven (CT): Yale University Press. [Google Scholar]
- Ishikawa J, Nishimura R, Ishikawa A. 2015. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur J Neurosci. 41(4):442–453. [DOI] [PubMed] [Google Scholar]
- Jackson L, Langille L, Lyons R, Hughes J, Martin D, Winstanley V. 2009. Does moving from a high-poverty to lower-poverty neighborhood improve mental health? A realist review of 'moving to opportunity'. Health Place. 15(4):961–970. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. 2017. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 82(7):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Conger RD, Elder GH Jr, Lorenz FO. 2003. Reciprocal influences between stressful life events and adolescent internalizing and externalizing problems. Child Dev. 74(1):127–143. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. 2011. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 21(7):1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. 2014. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 161(11):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind AJH, Buckingham WR. 2018. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med. 378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen DE, Leventhal T, Dahinten VS, McIntosh CN. 2008. Neighborhood disadvantage: pathways of effects for young children. Child Dev. 79(1):156–169. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, Reekum CM, Nelson B, Davidson RJ. 2012. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 62(3):1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. 2000. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull. 126(2):309–337. [DOI] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. 2003. Moving to opportunity: an experimental study of neighborhood effects on mental health. Am J Public Health. 93(9):1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal T, Dupere V. 2011. Moving to opportunity: does long-term exposure to 'low-poverty' neighborhoods make a difference for adolescents? Soc Sci Med. 73(5):737–743. [DOI] [PubMed] [Google Scholar]
- LeWinn KZ, Sheridan MA, Keyes KM, Hamilton A, McLaughlin KA. 2017. Sample composition alters associations between age and brain structure. Nat Commun. 8(1):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. 2013. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 167(12):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Duncan GJ, Gennetian LA, Katz LF, Kessler RC, Kling JR, Sanbonmatsu L. 2012. Neighborhood effects on the long-term well-being of low-income adults. Science. 337(6101):1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Duncan GJ, Hirschfield P. 2001. Urban poverty and juvenile crime: evidence from a randomized housing-mobility experiment*. Q J Econ. 116(2):655–679. [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. 2000. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol Psychiatry. 48(10):976–980. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A, Cummins S. 2002. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 55(1):125–139. [DOI] [PubMed] [Google Scholar]
- Maniam J, Antoniadis C, Morris MJ. 2014. Early-life stress, hpa axis adaptation, and mechanisms contributing to later health outcomes. Front Endocrinol (Lausanne). 5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, Walsh BT, Peterson BS. 2011. An fmri study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 168(11):1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER. 2020. Association of lead-exposure risk and family income with childhood brain outcomes. Nature Medicine. 26(1):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NA, Marusak HA, Sala-Hamrick KJ, Crespo LM, Rabinak CA, Thomason ME. 2018. Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Hum Brain Mapp. 39(5):1982–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 2013. The brain on stress: toward an integrative approach to brain, body, and behavior. Perspect Psychol Sci. 8(6):673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLanahan S, Sandefur G. 1994. Growing up with a single parent. What hurts, what helps. Cambridge, Mass: Harvard University Press.
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. 2010. Childhood adversities and adult psychopathology in the national comorbidity survey replication (ncs-r) iii: associations with functional impairment related to dsm-iv disorders. Psychol Med. 40(5):847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC. 2018. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav Immun. 73:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menec VH, Shooshtari S, Nowicki S, Fournier S. 2010. Does the relationship between neighborhood socioeconomic status and health outcomes persist into very old age? A population-based study. J Aging Health. 22(1):27–47. [DOI] [PubMed] [Google Scholar]
- Merz EC, Desai PM, Maskus EA, Melvin SA, Rehman R, Torres SD, Meyer J, He X, Noble KG. 2019. Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biol Psychiatry. 86(12):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer OL, Castro-Schilo L, Aguilar-Gaxiola S. 2014. Determinants of mental health and self-rated health: a model of socioeconomic status, neighborhood safety, and physical activity. Am J Public Health. 104(9):1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Armstrong CC, Carroll AL, Ozturk S, Rydland KJ, Brody GH, Parrish TB, Nusslock R. 2018. Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proc Natl Acad Sci U S A. 115(47):12063–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli V, Ziegler C, Fawibe O. 2017. Environmental justice and underserved communities. Prim Care. 44(1):155–170. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. 2015. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 77(3):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. 2008. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male balb/c mice. Neuroscience. 156(4):1103–1110. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. 2014. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 39(5):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, Peterson BS. 2011. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 50(8):828–837e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. 2013. Dissociable attentional and affective circuits in medication-naive children with attention-deficit/hyperactivity disorder. Psychiatry Res. 213(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. 2014. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 75(11):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal B, Whalen DJ, Kenley JK, Yu Q, Smyser CD, Rogers CE, Sylvester CM. 2020. Brain connectivity and socioeconomic status at birth and externalizing symptoms at age 2 years. Dev Cogn Neurosci. 45C:100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C, Marin MF, Majeur D, Lupien S. 2018. Early child adversity and psychopathology in adulthood: Hpa axis and cognitive dysregulations as potential mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 85:152–160. [DOI] [PubMed] [Google Scholar]
- Reiss F. 2013. Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc Sci Med. 90:24–31. [DOI] [PubMed] [Google Scholar]
- Ross CE. 2000. Neighborhood disadvantage and adult depression. J Health Soc Behav. 41(2):177–187. [Google Scholar]
- Roubinov DS, Hagan MJ, Boyce WT, Adler NE, Bush NR. 2018. Family socioeconomic status, cortisol, and physical health in early childhood: the role of advantageous neighborhood characteristics. Psychosom Med. 80(5):492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Hallion LS, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, Andrade LH, Borges G, Bromet EJ, Bunting B, et al. 2017. Cross-sectional comparison of the epidemiology of dsm-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 74(5):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AE, Ford T, Williams R, Russell G. 2016. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (adhd): a systematic review. Child Psychiatry Hum Dev. 47(3):440–458. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. 1997. Neighborhoods and violent crime: a multilevel study of collective efficacy. science. 277(5328):918–924. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Mentz G, Lachance L, Johnson J, Gaines C, Israel BA. 2012. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health. 102(9):1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. 2010. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 1186:223–239. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 28(14):3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK. 2003. Area deprivation and widening inequalities in us mortality, 1969-1998. Am J Public Health. 93(7):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Miller BA, Hankey BF. 2002. Changing area socioeconomic patterns in u.S. Cancer mortality, 1950-1998: part ii--lung and colorectal cancers. J Natl Cancer Inst. 94(12):916–925. [DOI] [PubMed] [Google Scholar]
- Slopen N, Fitzmaurice G, Williams DR, Gilman SE. 2010. Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. J Am Acad Child Adolesc Psychiatry. 49(5):444–452. [DOI] [PubMed] [Google Scholar]
- Smith JA, Zhao W, Wang X, Ratliff SM, Mukherjee B, Kardia SLR, Liu Y, Roux AVD, Needham BL. 2017. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics. 12(8):662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari CD. 2012. Affluent neighborhood persistence and change in U.S. cities. City & Community. 11(4):370–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South EC, Hohl BC, Kondo MC, MacDonald JM, Branas CC. 2018. Effect of greening vacant land on mental health of community-dwelling adults: a cluster randomized trial. JAMA Netw Open. 1(3):e180298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijkers W, Jansen DE, Reijneveld SA. 2012. The impact of area deprivation on parenting stress. Eur J Public Health. 22(6):760–765. [DOI] [PubMed] [Google Scholar]
- Stronks K, Mheen H, Looman CW, Mackenbach JP. 1998. The importance of psychosocial stressors for socio-economic inequalities in perceived health. Soc Sci Med. 46(4–5):611–623. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Marsh R, Wang Z, Torres-Sanchez T, Graniello B, Hao X, Xu D, Packard MG, Duan Y, Kangarlu A, et al. 2014. Neural correlates of reward-based spatial learning in persons with cocaine dependence. Neuropsychopharmacology. 39(3):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall KP, Shirtcliff EA, Dismukes AR, Wallace M, Drury SS. 2017. Association between neighborhood violence and biological stress in children. JAMA Pediatr. 171(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S, Muetzel RL, Bakermans-Kranenburg MJ, Jaddoe VW, Tiemeier H, Verhulst FC, White T, Van Ijzendoorn MH. 2017. Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Dev Psychopathol. 29(2):505–518. [DOI] [PubMed] [Google Scholar]
- Tomlinson RC, Burt SA, Waller R, Jonides J, Miller AL, Gearhardt AN, Peltier SJ, Klump KL, Lumeng JC, Hyde LW. 2020. Neighborhood poverty predicts altered neural and behavioral response inhibition. Neuroimage. 209:116536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley UA, Mackey AP, Ciric R, Ruparel K, Moore TM, Gur RC, Gur RE, Satterthwaite TD, Bassett DS. 2019. Associations between neighborhood ses and functional brain network development. Cerebral Cortex. 30(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. 2020. Early adversity and the neotenous human brain. Biol Psychiatry. 87(4):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. 2006. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 26(16):4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, Fruchter E, Wald I, Pine DS, Tarrasch R, et al. 2013. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 7:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodegom M, Homberg JR, Henckens M. 2017. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem MR, Tottenham N. 2018. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr Top Behav Neurosci. 38:117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier PM, Hauptman M, Weitzen SH, Bell S, Quilliam DN, Logan JR. 2011. The important health impact of where a child lives: Neighborhood characteristics and the burden of lead poisoning. Matern Child Health J. 15(8):1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, Rossum EF, Koper JW, Raat H, Akker EL. 2016. Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology. 65:9–14. [DOI] [PubMed] [Google Scholar]
- Walker RE, Keane CR, Burke JG. 2010. Disparities and access to healthy food in the United States: a review of food deserts literature. Health Place. 16(5):876–884. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2(3):125–141. [DOI] [PubMed] [Google Scholar]
- Wilson DK, Kirtland KA, Ainsworth BE, Addy CL. 2004. Socioeconomic status and perceptions of access and safety for physical activity. Ann Behav Med. 28(1):20–28. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ. 2016. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 41(3):822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Leventhal T, Brooks-Gunn J, Earls FJ. 2005. Neighborhood residence and mental health problems of 5- to 11-year-olds. Arch Gen Psychiatry. 62(5):554–563. [DOI] [PubMed] [Google Scholar]
- Young GS, Fox MA, Trush M, Kanarek N, Glass TA, Curriero FC. 2012. Differential exposure to hazardous air pollution in the United States: a multilevel analysis of urbanization and neighborhood socioeconomic deprivation. Int J Environ Res Public Health. 9(6):2204–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


