Abstract
Background
To examine whether the duration of unremitted psychotic symptoms after the onset of a first episode of psychosis (FEP) is associated with cortical thickness and hippocampal volume, as well as structural covariance of these measures.
Method
Longitudinal MRI scans were obtained for 80 FEP patients shortly after entry to FEP clinic (baseline), and then 12 months and 24 months later. The proportion of time patients experienced unremitted positive symptoms for 2 interscan intervals (baseline to 12 mo, 12 mo to 24 mo) was calculated. Changes in cortical thickness and hippocampal volumes were calculated for each interscan interval and associated with duration of unremitted psychotic symptoms. Significant regions were then used in seed-based structural covariance analyses to examine the effect of unremitted psychotic symptoms on brain structural organization. Importantly, analyses controlled for antipsychotic medication.
Results
Cortical thinning within the left medial/orbitofrontal prefrontal cortex and superior temporal gyrus were significantly associated with the duration of unremitted psychotic symptoms during the first interscan interval (ie, baseline to 12 mo). Further, changes in cortical thickness within the left medial/orbitofrontal cortex positively covaried with changes in thickness in the left dorsal and ventrolateral prefrontal cortex during this period. No associations were observed during the second interscan interval, nor with hippocampal volumes.
Conclusions
These results demonstrate that cortical thickness change can be observed shortly after an FEP, and these changes are proportionally related to the percentage of time spent with unremitted psychotic symptoms. Altered structural covariance in the prefrontal cortex suggests that unremitted psychotic symptoms may underlie reorganization in higher-order cortical regions.
Keywords: cortical thickness, hippocampus, prefrontal cortex, relapse, structural covariance
Introduction
Longitudinal brain imaging studies have provided strong evidence for progressive brain changes in schizophrenia.1–3 However, the factors underlying such changes remain actively debated.4,5 Antipsychotic medications are one likely contributor; one meta-analysis in schizophrenia showed that gray matter tends to be inversely correlated with cumulative exposure to antipsychotic treatment.6 However, studies in enduring schizophrenia are more prone to the influence of multiple confounds related to illness chronicity, which can be ameliorated by studying early phases of psychosis.
Interestingly, some have suggested that progressive brain changes are more pronounced in the first few years after a first episode of psychosis (FEP).7–9 In one of the largest longitudinal imaging studies in FEP, Andreasen et al,10 observed that maximal gray matter loss occurred within the first 2 years after an FEP, and subsequently found that relapse duration, and not the number of relapses, was significantly related to brain volume decreases.11 While these represent landmark studies, they were limited by the fact that the majority of follow-up scans were done after 5 or more years, although it is likely that pronounced changes may have been occurring earlier. Most of the patients were also initially treated with a first-generation antipsychotic, which is known to have a greater impact on brain anatomy than second-generation antipsychotics.12 Finally, cannabis use was not systematically controlled for and could represent another important confound.13,14
Recently, Emsley et al,15 examined changes in gray and white matter volume over the first year of treatment following an FEP in initially antipsychotic-naïve patients, and observed a decrease in overall cortical volume relative to healthy controls, which was driven significantly by antipsychotic dose received during the interscan interval. However, it should be noted that a relatively gross measure of gray matter was used, as well as a total Positive and Negative Syndrome Scale (PANSS) score which does not dissociate positive symptoms from other dimensions of psychopathology.
There is a need to examine neuroanatomical changes more thoroughly after the onset of psychosis, alongside a measure of unremitted psychotic symptoms, while controlling for potential confounding factors such as antipsychotic treatment and cannabis use. One relevant study examined brain volume changes and the duration of psychotic symptoms in 48 FEP patients over a 5-year period and observed an overall gray matter volume loss.16 However, this early study included several limitations, including a retrospective assessment of positive symptoms, a crude measure of gray matter (total volume), relatively small sample, and a fairly long follow-up period of 5 years. Finally, none of the studies reviewed earlier examined the hippocampus, a region hypothesized to be critical for the pathophysiology of schizophrenia.17 Its examination in relation to relapse and unremitted psychotic symptoms is warranted.
Here we report on a longitudinal study to examine whether unremitted psychotic symptoms contribute to progressive brain changes over the 2–2.5 years following an FEP while controlling for the potential effect of antipsychotic treatment and cannabis use. Two specific objectives were considered. First, we examined whether the percentage of time spent with clinically significant psychotic symptoms affected the brain longitudinally. We hypothesized that cortical thickness (CT) in frontal and temporal lobe areas and volume of the hippocampus would decrease as a function of the duration of unremitted positive symptoms. Second, we used seed-based structural covariance analyses18 to examine whether (seed) regions showing significant associations with the duration of unremitted psychotic symptoms influence cortico-cortical and cortical-hippocampal relationships.
Methods
Participants
Patients were recruited from the Prevention and Early Intervention Program for Psychoses (PEPP-Montreal), at the Douglas Institute in Montreal, Canada, and were part of a longitudinal naturalistic outcome study. PEPP is a specialized early intervention service for individuals between the ages of 14–35 who have recently experienced an FEP within a local catchment area of Southwest Montreal. Details are outlined in Evans19. Briefly, the program involves a comprehensive approach with intensive medical and psychosocial interventions provided within the context of a modified assertive case management program. Inclusion criteria at PEPP include a diagnosis of affective or non-affective psychosis, an IQ above 70, and no past antipsychotic medication treatment for more than 1 month.
It should be noted that no control group was formally included in this investigation for several reasons. First, the primary aim of this paper is to map the impact of unremitted positive symptoms on the brain, which inherently cannot be assessed in nonclinical controls. Second, while the inclusion of age-matched nonclinical subjects could theoretically provide control for normative brain aging, we do not expect substantive aging effects in our relatively young FEP sample (mean age 24.18) over a 1- to 2-year period. Nonetheless, we are controlling for age in our various analyses as we have done in the past.20 Thirdly, our group has previously compared longitudinal brain changes in patients compared to a healthy control group in both cortical21 and limbic structure (ie, hippocampus).22 In healthy controls specifically (who were age- and sex-matched to our patient group), we did not observe any significant changes in cortical thickness over the same time interval reported in the current study.
Neuroimaging Component
The neuroimaging study began in 2004 and was completed in 2016 on the same MRI scanner. The study design comprised 3 scheduled visits: baseline (Scan1), 12-month follow-up (Scan2), and 24-month follow-up (Scan3). In total, 80 patients with longitudinal data were included in the study. See supplementary material for complete breakdown of the number of patients who were not retained in the study due to attrition/exclusion criteria. Only individuals ages 18 and over were considered for the neuroimaging study. Exclusion criteria included the presence of a major medical disorder, a history of neurological illnesses and head trauma resulting in loss of consciousness that could affect cognition, presence of neurological disorder determined by medical record examination, lifetime diagnosis of substance dependence, and/or any potential contraindication for the MRI scan.
Clinical Assessment and Demographic Data
Diagnosis for each patient was made on the basis of a structured clinical interview for the DSM-IV,23 performed by a trained interviewer, and confirmed by at least one senior research psychiatrist (R.J. or A.M.). Positive and negative symptoms were assessed with the Scale for the Assessment of Positive/Negative Symptoms (SAPS/SANS, respectively).24,25 Depression was assessed with the Calgary Depression Scale for Schizophrenia (CDSS).26 Antipsychotic medication dosages were converted to chlorpromazine equivalents according to the literature,27 and multiplied by percent medication adherence over the longitudinal follow-up period. Parental socioeconomic status (SES) was estimated using the Hollingshead SES Rating Scale,28 and handedness determined with the Edinburgh Handedness Inventory.29
Proxy Measure of Cannabis Use
Frequency of cannabis use was assessed with the Chemical Use Abuse Dependence (CUAD) Scale.30 We calculated the percentage of time a patient used cannabis for each of the 2 interscan intervals (ie, between Scan1-Scan2 and Scan2-Scan3), for direct comparison to the measure of unremitted psychotic symptoms presented below. Specifically, we used the upper bound of cannabis use for each category of cannabis frequency; eg, a score of “0 = No consumption” would be equivalent to 0% of cannabis use per month, a score of “4 = ≤3 times per week” was coded as 3*4 = 12 times per month or 40% of cannabis use per month, a score of “5 = daily” was equivalent to 100%, etc. For the category “1 = <1 time per month,” where a clear upper bound was not available, we coded cannabis use as “0.5 times per month,” which was equivalent to approximately 2% use per month. Percent of cannabis use for each interscan interval thus reflected an average over all clinical timepoints between scans for which data was available.
Measure of Unremitted Psychotic Symptoms
We adapted our definition from Andreasen and colleagues31; however, here we only considered positive symptoms. Unremitted psychotic symptoms were defined as a score of 3 or more on the global items from the SAPS. Data was collected using clinical interviews at multiple timepoints (entry, month 1/2/3/6/9/12/15/18/21/24). For clinical timepoints where data were not available or missing, these data were supplemented by information collected using telephone interviews that were offered to each client on a monthly basis after entering the early intervention program. For each of the 2 interscan intervals, we calculated the duration of unremitted psychotic symptoms, and normalized it by length of the interscan interval, to account for the variation in time of the interscan interval among participants.
MRI Acquisition
All scanning was carried out at the Montreal Neurological Institute (MNI) on a 1.5 T Siemens Sonata whole-body MRI system. Structural T1 volumes were acquired for each participant using a 3-dimensional gradient echo pulse sequence with sagittal volume excitation (repetition time = 22 ms, echo time = 9.2 ms, flip angle = 30, one hundred eighty 1 mm contiguous sagittal slices). The rectangular field of view (FOV) for the images was 256 mm (AP) 204 mm (SI). Information about quality control (QC) is detailed in supplementary material.
Post-Processing
All raw scans that passed QC were submitted to the CIVET pipeline (Version 2.1.0: http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET; 32,33). CT was estimated using the Laplacian distance between the gray-white matter boundary surface and pial surface34 across 81 924 vertices. Detailed steps have been described by our group elsewhere,35 and are also detailed in supplementary material.
Hippocampal Volume
Hippocampal structures were extracted bilaterally using the Multiple Automatically Generated Templates (MAGeT)-Brain algorithm36 (https://github.com/CobraLab/MAGeTbrain). This technique utilizes a limited number of high-resolution atlases that have been manually segmented (https://github.com/CobraLab/atlases). Extensive validation of MAGeT has been done previously, as shown in several references from our group,36,37 which have also included subsets of the described patient sample here, with data acquired on a 1.5T scanner. The pipeline yields 10 hippocampal labels per subject, corresponding to 5 hippocampal subfields per hemisphere. For the purpose of this analysis, the subfield volumes per hemisphere were summed, such that only total left and right hippocampal volumes were considered. Additional details on processing with MAGeT are included in supplementary material.
Statistical Analyses
Demographic and clinical variables were analyzed with 1-way ANOVAs for continuous variables or Kruskall-Wallis H-tests for nominal variables. For IQ, an ANCOVA was used to covary for test version. Analyses of clinical variables were conducted using PASW Statistics 21 (SPSS Inc., 2009), and were 2-tailed with a critical P-value of .05.
Relationship Between Unremitted Psychotic Symptoms and Cannabis Use Frequency
Partial correlations were applied between the percent of time with unremitted psychotic symptoms and percent of time using cannabis for the 2 interscan intervals, covarying for age and cumulative antipsychotic medication exposure.
Main Effect of Unremitted Psychotic Symptoms on Brain Structure
Relationship With Change in CT.
Statistics were performed across 81,924 vertices of the cortical surface using the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat/). First, we assessed the main effect of duration of unremitted symptoms on changes in CT. Changes in CT were calculated as the difference in cortical thickness between 2 scans for each subject per vertex; only changes between Scan1-Scan2, and between Scan2-Scan3 were considered. The linear model defined below was applied to the change in CT data:
where Y represents changes in CT for each vertex, β 1 represents the regression coefficient of interest, β 2–5 represents regression coefficients that were covaried for in the model, and ε is residual error. β 2 and β 5 were taken from the first scan (ie, Scan1 for the first interscan interval, Scan2 for the second interscan interval). β 4 is cumulative antipsychotic medication exposure expressed as chlorpromazine equivalents, taking percent adherence into account, as reported at the second scan for each of the interscan pairs (ie, Scan2 for the first interscan interval, Scan 3 for the second interscan interval). Mean CT was included in the model as it improved the model fit, as determined by a lower value when applying the Akaike Information Criterion.
Relationship With Change in Hippocampal Volume.
Similar to the CT analysis, hippocampal volume changes were assessed by calculating the difference in volume between the 2 interscan intervals of interest. The same linear model as above was used, except the dependent variable Y was the change in hippocampal volume, and instead of covarying for mean CT, total brain volume was included as a covariate.
Structural Covariance Analyses
Significant cortical/hippocampal regions extracted from analyses above were used as seeds to map structural covariance networks involved with unremitted psychotic symptoms. Thus, the interaction between changes in cortical thickness or hippocampal volume seeds of interest and duration of unremitted symptoms was evaluated against CT changes across 81 924 vertices of the brain surface, for the 2 interscan intervals. The following model was used:
Where variables are defined similarly as above; β 1 reflects the main interaction of interest, and “Change in CT Seed” is the value of CT change for the seed vertex informed by prior regression analysis. A similar model would be applied for potential significant findings with the hippocampus, using total brain volume as a covariate instead of Mean CT. All results were corrected for multiple comparisons using Random Field Theory (RFT),38 using a P-cluster threshold of P = .005.
Results
Sociodemographic and Clinical Results
In total, 80 FEP patients had usable positive symptom remission data and neuroimaging data at Scan1 and Scan2, and 60 FEP patients had sufficient clinical and neuroimaging data at Scan2 and Scan3. In the FEP group, Scan1 was performed on average 4.0 (SD = 1.9) months from entry into PEPP. For the entire group, including controls, interscan intervals were approximately 13.1 (SD = 1.3) months between Scan1 and Scan2, and 12.6 (SD = 1.7) months between Scan2 and Scan3. All FEP patients were on second-generation antipsychotics. Supplementary table 1 presents the socio-demographic and clinical variables for the whole group of FEP participants. Table 1 presents clinical/demographic variables comparing the 2 interscan intervals.
Table 1.
Demographic and Clinical Information for Longitudinal Sample
| Variable | Scan1-Scan2 (N = 80) | Scan2-Scan3 (N = 60) |
|---|---|---|
| Agea | 24.18 (3.99) | 24.97 (4.08) |
| Sex, M (%) | 56 (70%) | 44 (73.3%) |
| Right-handedness (N, %) | 67 (83.8) | 53 (88.3%) |
| SES | 3.12 (1.04) | 3.09 (0.97) |
| IQ | 99.96 (15.73) | 100.88 (16.12) |
| Years education | 11.93 (2.60) | 11.77 (2.60) |
| Interscan interval (mo) | 13.23 (1.29) | 12.64 (1.82) |
| % Non-remission | 59.91 (37.73) | 24.84 (32.47) |
| % Cannabis exposureb | 14.34 (26.52) [52] | 24.21 (38.90) [35] |
| CPZ equivalentsc | 3258.00 (2745.73) | 5090.50 (4776.30) |
| Adherence | 80.1 (24.3) | 75.27 (29.95) |
| DUP (wk) | 75.40 (145.94) | 77.84 (157.87) |
| Diagnosis (N, %) | ||
| Schizophrenia spectrum | 59 (73.8) | 42 (70.0) |
| Affective | 14 (17.5) | 12 (20.0) |
| Delusional disorder | 2 (2.5) | 2 (3.3) |
| Psychosis NOS | 5 (6.3) | 4 (6.7) |
| Other medication (N, %)d | ||
| Antidepressants | 11 (14.3) | 9 (16.7) |
| Benzodiazepines | 3 (3.9) | 3 (5.6) |
| Anticholinergics | 2 (2.6) | 1 (1.9) |
| Mood stabilizers | 8 (10.4) | 7 (13.0) |
Note: CPZ, chlorpromazine;DUP, duration of untreated psychosis;FUP1, follow-up year 1;FUP2, follow-up year 2;NOS, not otherwise specified;SES, socioeconomic status.General Demographics for whole sample are presented as a function of the 2 interscan intervals. Please see supplementary table 1 for clinical data presented separately for each timepoint.
aRefers to value at first scan in the contrast, ie, for Baseline-FUP1, age/SAPS at baseline is reported. For FUP1-FUP2, age/SAPS at FUP1 is reported.
bData missing for several patients. Number in square brackets represents adjusted N.
cCumulative CPZ equivalents at the second timepoint in the contrast are reported.
d3 and 6 patients were missing other medication data for the first and second interscan interval, respectively. Other medication data is reported based on the second timepoint in contrast, and proportions were calculated based on adjusted sample size.
Relationship Between Unremitted Psychotic Symptoms and Cannabis Use Frequency
Fifty-two of 80 patients and 35/60 patients had sufficient data entered in the CUAD scale for assessment of cannabis use frequency for the first and second interscan interval, respectively. Partial correlations, controlling for age and chlorpromazine equivalents, revealed no significant associations between percent of time with unremitted psychotic symptoms and percent of time using cannabis for either of the 2 interscan intervals (first interscan interval: r = −0.092, P = .527, df = 48; second interscan interval: r = −0.064, P = .723, df = 31).
Cortical Thickness Analyses
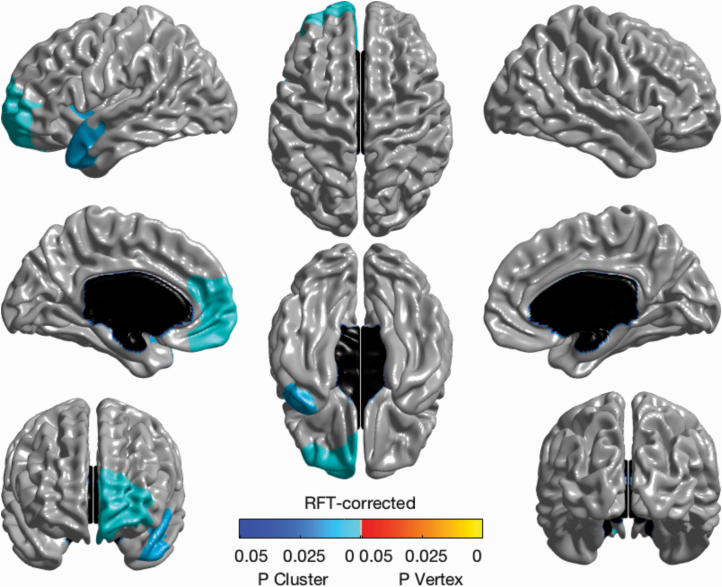
The main effect of unremitted psychotic symptoms on changes in CT for the first interscan interval revealed a significant negative association with 2 clusters (ie, more time spent with unremitted psychotic symptoms associated with cortical thinning) (figure 1). One cluster encompassed medial and orbitofrontal areas of the left medial prefrontal cortex and the second encompassed the left superior temporal gyrus spanning anteriorly to the ventrolateral prefrontal cortex and frontal insula (P < .05, RFT-corrected; df = 74). No significant associations were observed for the second interscan interval (see figure 2).
Fig. 1.
Main effect of unremitted psychotic symptoms on change in cortical thickness. Significant negative effect of unremitted psychotic symptoms on change in cortical thickness over the first interscan interval using a general linear model, covarying for centered age, sex, chlorpromazine equivalents, and medication adherence. Brain map shows RFT-corrected results (P < .05).
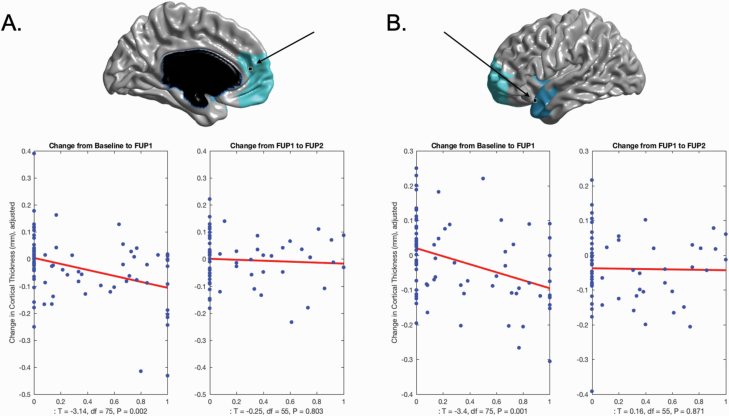
Fig. 2.
Plots of main effect of unremitted psychotic symptoms on change in cortical thickness. (A) Significant cortical thinning as a function of percentage of time spent with unremitted psychotic symptoms was observed in left medial prefrontal region (x = −9, y = 47, z = 11). As can be seen in the plots, the effect was more pronounced during the first interscan interval (baseline to FUP1) relative to the second interscan interval (FUP1 to FUP2). (B) Significant cortical thinning as a function of percentage of time spent with unremitted psychotic symptoms was observed in left temporal superior gyrus (x = −50, y = 17, z = −22). Similarly to Plot A, the effect was more pronounced during the first interscan interval (Scan1 to Scan2) relative to the second interscan interval (Scan2 to Scan3).Abbreviations: FUP1, follow-up year 1; FUP2, follow-up year 2.
Hippocampal Volumes
No significant associations between unremitted psychotic symptoms with change in left or right hippocampal volume were observed for either interscan interval.
Structural Covariance Analyses: Interaction With Unremitted Psychotic Symptoms
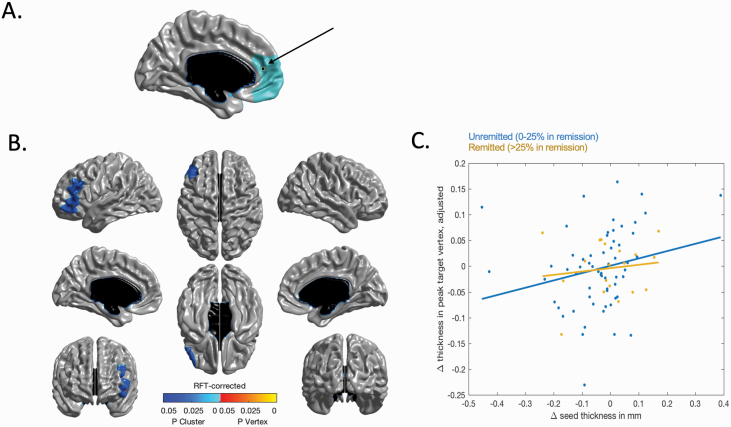
The next set of analyses were limited to changes in CT over the first interscan interval. We first extracted the vertices at the peak t-statistic for each of the 2 significant clusters discovered above: left medial prefrontal cortex (x = −9; y = 47; z = 11; peak t-statistic = 3.47) and the left superior temporal gyrus (x = −50; y = 17; z = −22; peak t-statistic = 3.64), and correlated changes in CT between each of these vertices and their interaction with unremitted psychotic symptoms on CT changes of the remaining 81 923 vertices of the brain. A significant interaction between unremitted psychotic symptoms and change in CT within the left medial prefrontal seed emerged, on changes in a large cluster of the left lateral frontal cortex, including both dorsolateral and ventrolateral prefrontal cortex (P < .05, RFT-corrected; df = 72). To better visualize the direction of the effect of the interaction between 2 continuous variables, patients were split into 2 categories: “Remitted” (0%–25% of time spent with unremitted psychotic symptoms) and “Unremitted” (>25% of time spent with unremitted psychotic symptoms), as can be seen in figure 3. This plot suggests that the “Unremitted” group drove this significant association, where more positive covariation was found between changes in CT of the left medial prefrontal seed and left lateral cortex. The same structural covariance analysis using the superior temporal gyrus seed did not reveal any significant association with change in other cortical regions over the first interscan interval.
Fig. 3.
Structural covariance with left medial frontal seed. (A) Localization of the left medial prefrontal seed (x = −9, y = 47, z = 11) used for the structural covariance analysis. (B) interactions between the degree of structural covariance of changes in cortical thickness in the left medial prefrontal seed and percentage of time spent with positive symptoms during the first interscan interval, on changes in cortical thickness across all other vertices of the cortex. Positive interactions between changes in cortical thickness within the seed region and unremitted psychotic symptoms were found within a large cluster encompassing left dorsolateral and ventrolateral prefrontal cortex. (C) To illustrate the direction of the interaction effect in (B) between 2 continuous variables, patients were split into 2 subgroups (unremitted n = 61 and remitted n = 19). As can be seen in the figure, the unremitted group seems to be driving the positive association in structural covariance between cortical thinning in the left medial prefrontal seed and individual differences in the thinning of the cluster within left lateral prefrontal cortex depicted.
Discussion
In this study, we examined the influence of unremitted psychotic symptoms on progressive brain changes while controlling for exposure to antipsychotics in a longitudinal cohort of FEP patients. We observed a significant association between the duration of unremitted psychotic symptoms and cortical thinning within left medial and orbitofrontal cortex and in left superior anterior temporal gyrus over the first year after an FEP. This was not observed over the second interscan interval, indicating that progressive brain changes in relation to unremitted psychotic symptoms occur shortly after an FEP, and plateau thereafter. Intriguingly, hippocampal volume was not modulated by the duration of unremitted positive symptoms. Using the 2 identified cortical regions as seeds for a structural covariance analysis and limiting our exploration to the first interscan interval, we observed a significant association between cortical thinning in the left medial frontal seed and changes in CT within areas of left dorsolateral and ventrolateral prefrontal cortex as a function of unremitted psychotic symptoms. This finding shows that localized CT changes in FEP co-occur with changes in other cortical regions, suggesting spatially distributed cortical reorganization with more exposure to unremitted psychotic symptoms. Importantly, we also found no significant association between our measure of unremitted psychotic symptoms and frequency of cannabis use, a potentially strong confounder, increasing the confidence of the observed associations.
Evidence of Progressive Changes Related to Unremitted Psychotic Symptoms
Several studies have shown an association in early psychosis between the persistence of symptoms (psychotic and/or negative) and brain structure.39,40 For instance, Bodnar et al, observed cross-sectionally that patients who did not show early symptoms remission had significantly smaller posterior hippocampal39 and parahippocampal40 volumes. Several longitudinal studies have similarly observed that FEP patients with a non-remitting symptom course exhibited a greater reduction in gray matter in the frontal lobes11 and left superior temporal gyrus,41 consistent with our findings. The idea that unremitted psychotic symptoms may impact the brain is not specific to psychosis. Of relevance, several studies in depression have demonstrated greater volume reduction in unremitted patients relative to patients in remission.42,43
Structural Covariance Changes and Cortical Reorganization
MRI-based structural covariance analysis is thought to map structural networks that are affected by common factors including neurodevelopmental (ie, coordinated maturation) and/or environmental factors.18 In the context of psychosis, few studies have examined structural covariance and how it can relate to psychotic symptoms. One study44 found that the severity of auditory hallucinations modulated cortico-cortical interactions of language-related areas (ie, left superior temporal and inferior frontal gyri) in patients with schizophrenia. Similarly, we have found altered structural covariance in such language-related regions in the first 1–1.5 years after an FEP, in relation to unremitted psychotic symptoms.
An emerging theory of cortical reorganization in response to a pathological process7 could help explain these alterations in structural organization. It has been suggested that several factors affecting neural activity (including sensory loss, new learning, etc.) can trigger this cortical reorganization.7 In line with this view, it could be suggested that the duration of unremitted psychotic symptoms is one of these factors that can influence structural (co)variance of cortical regions. Indeed, it is possible that psychosis or schizophrenia is not necessarily a disorder with a neurodegenerative disease course, wherein disease propagates in a predictable structural neuronal sequence. Rather, our data and that of others shed light on a hypothesis of the distributed impact of symptom-specific localized changes in cortical thickness early in the course of psychosis, independent of a neurodegenerative biological cascade. It is encouraged that future studies test this hypothesis more thoroughly.
Limitations
This study has a number of limitations that need to be taken into consideration. It is possible that our lack of significant results for the second interscan interval may have been a consequence of decreased statistical power to detect differences. Second, we could not use the frequency of cannabis use as a covariate in our imaging analyses due to missing data. Cannabis use could potentially affect progressive brain changes as shown before.13,14 We did, however, examine the association between the frequency of cannabis use and the duration of unremitted psychotic symptoms and found no significant association. A third limitation resides in the timing of MRI scans in relation to entry to the clinic. As mentioned, baseline scans were collected approximately 4 months after PEPP entry; thus the brain changes found over the first interscan interval in relation to unremitted psychotic symptoms were not occurring at identical times in the clinical course of psychosis for each patient; however, we can conclude that the associations detected are indeed happening early in the disease process (approximately 1–1.5 y after psychosis onset). Fourth, we did not account for the duration of untreated psychosis in the current study. However, the primary aim of this study was to use a measure of unremitted psychotic symptoms that directly mapped onto concurrently timed measures of changes in brain structure, without introducing the effects of what many years of untreated psychosis may have had on brain structure at baseline. Lastly, it is worth discussing the null findings with hippocampal volumes. It is possible that more fine-grained analyses of hippocampal subfields, which our 1.5T MRI data did not allow us to resolve may have been more fruitful. Hippocampal volume alone may not be sensitive in detecting relationships with symptoms; perhaps hippocampal shape could be a more sensitive marker to be investigated in the future.
Conclusions
Our study demonstrates a significant association between cortical thinning and altered structural covariance within left prefrontal and superior temporal regions and unremitted psychotic symptoms in relation to duration of unremitted symptoms. Notably, these findings are specific to approximately the first year after an FEP. Our results suggest that psychotic symptoms may promote cortical reorganization early in the disease course of psychosis, and highlights the need for integration of more brain network-based perspectives when examining clinical trajectories in FEP patients.
Supplementary Material
Acknowledgments
The authors would like to thank Lepage Lab research staff for help with recruitment and data collection. The authors would also like to thank patients and their families for their participation in the study.
M.B. reports additional support from Janssen, Otsuka, and CIHR. M.L. reports grants from Otsuka Lundbeck Alliance, personal fees from Otsuka Canada, personal fees from Lundbeck Canada, grants and personal fees from Janssen, and personal fees from MedAvante-Prophase, outside the submitted work. R.J. reports receipt of grants, speaker’s and consultant’s honoraria from Janssen, Lundbeck, Otsuka, Pfizer, Shire, Perdue, HLS, and Myelin and royalties from Henry Stewart Talks. A.M. reports research funding for an investigator-initiated project from BMS Canada and honoraria for lectures and consulting activities (eg, advisory board participation) with Otsuka and Lundbeck, all unrelated to the present article. C.M. and M.M.C. report no competing interests.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR, 68961, MCT-94189); the Fonds de Recherche du Quebec – Sante (FRSQ); Sackler Foundation; and Lobeer Foundation. Salary awards include FRSQ to J.L.S., R.J., M.B., M.M.C., and M.L.; Canadian Institutes of Health Research (CIHR) to C.M. and M.M.C.; Healthy Brains for Healthy Lives (HBHL) to C.M., the Canada Research Chairs to A.M.; and the James McGill Professorship to M.L.
References
- 1. DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34(2):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88–96. [DOI] [PubMed] [Google Scholar]
- 3. van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS. Schizophrenia as a progressive brain disease. Eur Psychiatry. 2008;23(4):245–254. [DOI] [PubMed] [Google Scholar]
- 4. Murray RM. Mistakes I have made in my research career. Schizophr Bull. 2017;43(2):253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39(6):1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37(8):1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palaniyappan L. Progressive cortical reorganisation: a framework for investigating structural changes in schizophrenia. Neurosci Biobehav Rev. 2017;79:1–13. [DOI] [PubMed] [Google Scholar]
- 8. van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS. The course of brain abnormalities in schizophrenia: can we slow the progression? J Psychopharmacol. 2012;26(5 Suppl):8–14. [DOI] [PubMed] [Google Scholar]
- 9. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(7):672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 2015;78(6):403–412. [DOI] [PubMed] [Google Scholar]
- 13. Rais M, Cahn W, Van Haren N, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165(4):490–496. [DOI] [PubMed] [Google Scholar]
- 14. Rais M, van Haren NE, Cahn W, et al. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2010;20(12):855–865. [DOI] [PubMed] [Google Scholar]
- 15. Emsley R, Asmal L, du Plessis S, Chiliza B, Phahladira L, Kilian S. Brain volume changes over the first year of treatment in schizophrenia: relationships to antipsychotic treatment. Psychol Med. 2017;47(12):2187–2196. [DOI] [PubMed] [Google Scholar]
- 16. Cahn W, Rais M, Stigter FP, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19(2):147–151. [DOI] [PubMed] [Google Scholar]
- 17. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. [DOI] [PubMed] [Google Scholar]
- 18. Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. [DOI] [PubMed] [Google Scholar]
- 19. Iyer S, Jordan G, MacDonald K, Joober R, Malla A. Early intervention for psychosis: a Canadian perspective. J Nerv Ment Dis. 2015;203(5):356–364. [DOI] [PubMed] [Google Scholar]
- 20. Makowski C, Lewis JD, Lepage C, et al. Structural associations of cortical contrast and thickness in first episode psychosis. Cereb Cortex. 2019;29(12):5009–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makowski C, Bodnar M, Malla AK, Joober R, Lepage M. Age-related cortical thickness trajectories in first episode psychosis patients presenting with early persistent negative symptoms. npj Schizophr. 2016;2:16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makowski C, Bodnar M, Shenker JJ, et al. Linking persistent negative symptoms to amygdala-hippocampus structure in first-episode psychosis. Transl Psychiatry. 2017;7(8):e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Patient Edition (SCID-I/P V and SCID-I/NP Version 2.0). New York, NY: Biometric Research Department; 1998. [Google Scholar]
- 24. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 25. Andreasen NC. Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 26. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247–251. [DOI] [PubMed] [Google Scholar]
- 27. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller DC. Handbook for Research Design and Social Measurement. 5th ed. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 29. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 30. McGovern MP, Morrison DH. The Chemical Use, Abuse, and Dependence Scale (CUAD). Rationale, reliability, and validity. J Subst Abuse Treat. 1992;9(1):27–38. [DOI] [PubMed] [Google Scholar]
- 31. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449. [DOI] [PubMed] [Google Scholar]
- 32. Ad-Dab’bagh Y, Einarson D, Lyttelton O, et al. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. Paper presented at: The 12th Annual Meeting of the Organization for Human Brain Mapping (OHBM), 2006; Florence, Italy. [Google Scholar]
- 33. Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21(10):1280–1291. [DOI] [PubMed] [Google Scholar]
- 34. Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. [DOI] [PubMed] [Google Scholar]
- 35. Bodnar M, Hovington CL, Buchy L, Malla AK, Joober R, Lepage M. Cortical thinning in temporo-parietal junction (TPJ) in non-affective first-episode of psychosis patients with persistent negative symptoms. PLoS One. 2014;9(6):e101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pipitone J, Park MT, Winterburn J, et al. ; Alzheimer’s Disease Neuroimaging Initiative Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage. 2014;101:494–512. [DOI] [PubMed] [Google Scholar]
- 37. Makowski C, Béland S, Kostopoulos P, et al. Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: comparing automated approaches to manual delineation. Neuroimage. 2018;170:182–198. [DOI] [PubMed] [Google Scholar]
- 38. Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage. 2004;23(Suppl 1):S189–S195. [DOI] [PubMed] [Google Scholar]
- 39. Bodnar M, Malla AK, Czechowska Y, et al. Neural markers of remission in first-episode schizophrenia: a volumetric neuroimaging study of the hippocampus and amygdala. Schizophr Res. 2010;122(1-3):72–80. [DOI] [PubMed] [Google Scholar]
- 40. Bodnar M, Malla AK, Joober R, et al. Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry Res. 2012;201(1):40–47. [DOI] [PubMed] [Google Scholar]
- 41. Gutiérrez-Galve L, Chu EM, Leeson VC, et al. A longitudinal study of cortical changes and their cognitive correlates in patients followed up after first-episode psychosis. Psychol Med. 2015;45(1):205–216. [DOI] [PubMed] [Google Scholar]
- 42. Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22(12):1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaremba D, Dohm K, Redlich R, et al. Association of brain cortical changes with relapse in patients with major depressive disorder. JAMA Psychiatry. 2018;75(5):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A. Structural covariance in the hallucinating brain: a voxel-based morphometry study. J Psychiatry Neurosci. 2009;34(6):465–469. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.