Abstract
Rhododendron sichotense Pojark. and Rhododendron adamsii Rheder have been actively used in ethnomedicine in Mongolia, China and Buryatia (Russia) for centuries, as an antioxidant, immunomodulating, anti-inflammatory, vitality-restoring agent. These plants contain various phenolic compounds and fatty acids with valuable biological activity. Among green and selective extraction methods, supercritical carbon dioxide (SC-CO2) extraction has been shown to be the method of choice for the recovery of these naturally occurring compounds. Operative parameters and working conditions have been optimized by experimenting with different pressures (300–400 bar), temperatures (50–60 °C) and CO2 flow rates (50 mL/min) with 1% ethanol as co-solvent. The extraction time varied from 60 to 70 min. A HPLC-UV-VIS-ESI-MS/MS technique was applied to detect target analytes. A total of 48 different biologically active components have been identified in the Rh. adamsii SC-CO2 extracts. A total of 31 different biologically active components have been identified in the Rh. sichotense SC-CO2 extracts.
Keywords: Rhododendron sichotense, Rhododendron adamsii, supercritical fluid extraction, HPLC–MS/MS, phenolic compounds
1. Introduction
A total of 19 species of rhododendrons are growing in the territory of Russia, the main part of which (13 species) are found only in the flora of the Russian Far East and Eastern Siberia [1]. Russian researchers classify the genus Rhododendron somewhat differently than foreign ones [2]. At present, there is no unified classification scheme for a taxon, since the genus is very large—more than 800 species, as well as the presence of a large number of convergent characters among its representatives, complicating the construction of a natural classification [3].
The genus system, developed and adopted by Russian scientists, divides the genus Rhododendron into subgenera and series. In it, Rhododendron adamsii Rehder and Rhododendron parvifolium Adams are assigned to the subgenus Osmothamnus Maximowicz (Fragrantia E. Busch series and Parvifolia E. Busch series) (Table 1). Species of Rh. dauricum L., Rh. ledebourii Pojarkova, Rh. sichotense Pojarkova and Rh. micronulatum Turczaninowia constitute the series Daurica Pojarkova subgenus Rhodorastrum (Maxim.) Drude [4].
Table 1.
Classification of genus Rhododendron L.
| No. | Species or Variety | Subgenus | Row |
|---|---|---|---|
| 1 | Rh. sichotense Pojark.; Rh. micronulatum Pojark.; Rh. dauricum L.; Rh. ledebourii Pojark. | Rhodorastrum | Daurica Pojark. |
| 2 | Rh. parvifolium Adams [Rh. lapponicum (L.) Wahlenb.] | Osmothamnus Maximowicz | Parvifolia E. Busch |
| 3 | Rh. adamsii Rehd. [Rh. fragrans (Adams) Maxim.] | Fragrantia E. Busch |
Rhododendron sichotense Pojark. is a plant from the genus of rhododendrons [5]. It grows in the Far East (Primorsky Krai) on the eastern slope of the Sikhote Alin ridge. This type of rhododendron is included in the Red Book of the Russian Federation [6]. From its closest relatives, Rhododendron dauricum and Rhododendron micronulatum is distinguished by larger, sometimes more than 7 cm wide, flowers and wider, green leaves from the underside, not falling leaves. Large flowers, lush foliage, winter hardiness suggest that Rh. sichotense is a very promising garden plant for areas with a harsh climate [7].
Rh. adamsii Rheder is a shrub found in Eastern Siberia and Baikal in the alpine and subalpine zones of the mountains, forming a shrub tundra, and at the upper border of the forest at an altitude of 1200–2500 m above sea level. This is a shrub from 1 to 3 m in height with falling leaves. The flowers are pink, large enough, and open before the leaves appear. The leaves are narrow, green above, grayish green below, and very fragrant. Rh. adamsii grows in pine and deciduous forests with grass and moss, on forest edges and especially on rocky mountains [8].
Traditional medicine uses different types of rhododendron to treat a number of diseases of the respiratory system, the gastrointestinal tract, chronic skin diseases, hypertension, rheumatism, helminthiases, etc. [9]. The stupefying smell formed during the burning of leaves and caused by the sharp evaporation of volatile terpenes has long been used by indigenous ethnic groups of Siberia and Far East as a psychoactive, analgesic, and narcotic drug [10,11].
Rh. adamsii Rehder is used as a stimulant and tonic by the populations of Buryatia, Mongolia and China. Decoctions and tinctures of it are used for cold diseases, as a diuretic agent for cardiac edema, as well as an adaptogen [12]. Pharmacological studies have shown that Rh. adamsii has antimicrobial, anti-inflammatory, immunomodulating, antioxidant effects [13,14].
In essential oil of Rh. adamsii, it is possible to isolate the components present both in the leaves and stems of the plant: α- and β-pinenes, β-myrcene, cis-β-ocimene, isoledene, aromadendrene, humulene, β-farnesol, γ-murolene, β-selinene, ledene, α-farnesol, δ-cadinene, trans-nerolidol, spathulenol, β-elemenone, germacrone [15]. The essential oil of the stems of the plant contains germacrene D and germacrene B, which are absent from the essential oil of the leaves. In almost all samples of essential oil of leaves and stems of Rh. adamsii there is found 4-phenyl-2-butanone, the content of which is from 3 to 13%, as well as its related 4-phenyl-2-butanol, the content of which is from 1.9 to 7.4% [16].
This study considers the possibility and effectiveness of supercritical carbon dioxide (SC-CO2) extraction of biologically active substances from stems and leaves of Rh. adamsii. Previously, the authors of this article successfully used SC-CO2 extraction to obtain biologically active substances from plants of the Far Eastern taiga Panax ginseng, Rhodiola rosea, and Schisandra chinensis, which are extremely popular in traditional medicines of Southeast Asia [17,18].
Supercritical fluid extraction (SFE) has been used since the late 1970s to analyze food products, isolate biologically active substances and determine lipid levels in food, as well as levels of toxic substances [19,20,21]. The use of SFE for fractionation and/or enrichment of certain components in products has been reported since the 1980s [22,23,24]. In addition, the products do not have residues of organic solvents, which occur with conventional extraction methods, and solvents can be toxic, for example, in the case of methanol and n-hexane. Easy solvent removal from the final product, high selectivity and the use of moderate temperatures in the extraction process are the main attractive factors of supercritical technology, leading to a significant increase in research for use in the food and pharmaceutical industries [25,26,27].
An alternative to the use of co-solvents in the case of poorly soluble or practically insoluble compounds is to completely change the process scheme using the so-called supercritical solvent extraction (SAE). Industrial-scale devices with technological schemes containing CO2 processing plants have already been developed; therefore, most of the solvent/anti-solvent is recovered. SAE increases are associated with the same process conditions as the pressure, temperature and concentration of solutes in the slurry. However, the main parameter is the molar fraction of CO2. It depends on the relative flow rate of the CO2 and the solvent liquid to set the supercritical precipitant composition for the CO2/solvent mixture used [28].
Popova et al. [29] (2018) investigated the possibility of SC-CO2 extracting chlorophylls and carotenoids of Ledum palustre L. (Rhododendron tomentosum Harmaja) by supercritical fluid extraction using supercritical carbon dioxide and a co-solvent of ethyl alcohol as a solvent. It has been found that by varying the pressure and temperature of the fluid, the duration of processing and the moisture content of the raw material, extracts can be obtained enriched in one or both of the recoverable pigments. Furthermore, in this case, the amount of ethanol used as a co-solvent was 5% and was necessary and sufficient for efficient extraction of pigments with supercritical CO2.
The anti-inflammatory activity of two extracts from the aerial parts of Ledum palustre L. (Rh. tomentosum Harmaja) has been reported by [30]. The volatile oil was obtained by SC-CO2 extraction and the essential oil by hydrodistillation (HD). The anti-inflammatory activity was evaluated by the subcutaneous carrageenan injection-induced hind paw oedema. The results show that L. palustre essential oil enhanced a significant inhibition of oedema (50–73%) for HD oil and (52–80%) for SFE oil.
The results of SC-CO2-extraction of leaves and branches of rhododendrons, in particular, indicated that, when using this technology, the extract contained all biologically active components of the plant, as well as inert mixtures of extracted compositions. This study is devoted to comparative mass spectrometry of extracted biologically active substances from two closely related subgenus of rhododendron: Rh. sichotense Pojark. and Rh. adamsii Rehder.
2. Results and Discussion
In the first instance, the influence of the supercritical parameters (CO2 flow rate, temperature, % co-solvent, and pressure) on extraction yield was investigated. The cumulative quantitative extracts yield are summarized in the Table 2. Several experimental conditions were investigated working in a pressure range of 300–400 bar, with co-solvent EtOH and a temperature range of 50–60 °C.
Table 2.
Extraction yield of Rh. adamsii presented depending on operational parameters (pressure, temperature, CO2 flow rate, % co-solvent).
| No. | Temperature (°C) | Pressure (Bar) | CO2 Flow Rate (mL/min) | % Co-Solvent EtOH | Extraction Yield (mg/g) |
|---|---|---|---|---|---|
| 1 | 50 | 300 | 30 | 1 | 1.23 |
| 2 | 50 | 350 | 50 | 2 | 3.27 |
| 3 | 50 | 370 | 30 | 1 | 3.25 |
| 4 | 50 | 400 | 50 | 1 | 4.15 |
| 5 | 60 | 300 | 30 | 2 | 5.81 |
| 6 | 60 | 350 | 30 | 2 | 7.12 |
| 7 | 60 | 370 | 50 | 1 | 10.86 |
| 8 | 60 | 400 | 30 | 2 | 8.13 |
| 9 | 70 | 300 | 30 | 1 | 7.15 |
| 10 | 70 | 350 | 50 | 1 | 7.28 |
| 11 | 70 | 370 | 30 | 1 | 8.10 |
| 12 | 70 | 400 | 30 | 1 | 7.90 |
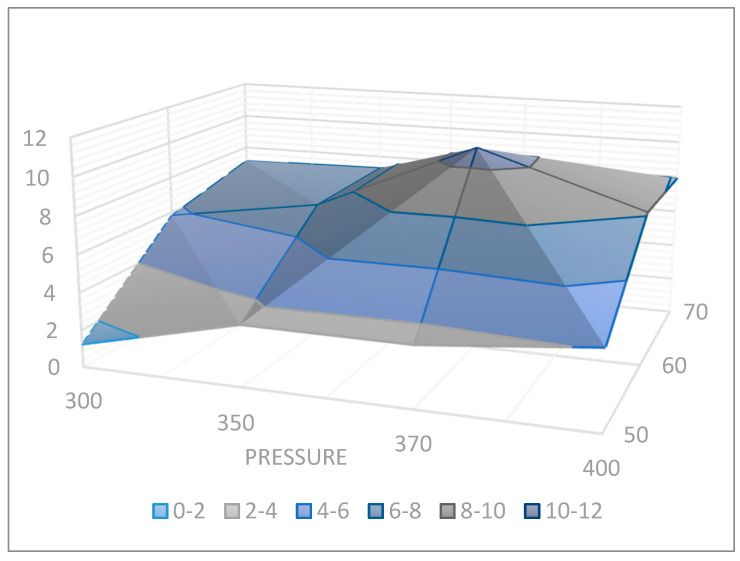
Orthogonal projection representing the extraction yield at 300 to 400 Bar and 50–70 °C is shown in Figure 1. The best results were obtained at 370 Bar and 60 °C. An ion trap amaZon SL BRUKER DALTONIKS equipped with an electron spray ionization (ESI) source in the negative and positive ion modes and analysis of fragmented ions was used in this scientific work.
Figure 1.
Orthogonal projection representing the extraction yield of target analytes at 300 to 400 Bar and 50–70 °C.
A screening of biologically active substances from Rh. adamsii sample and Rh. sichotense sample was obtained using this method. Typical base peak chromatograms (BPC) of analyzed target analytes are shown in the Supplementary Material. Identification of compounds was assigned by comparison of their UV-Vis spectra and mass spectrometric data obtained under both negative and positive electron spray ionization (ESI−/ESI+) conditions and with the scientific literature. Under these conditions a total of 800 peaks were detected in the ion chromatogram.
Series studies by HPLC–MS/MS of both samples of rhododendrons (Rh. sichotense and Rh. adamsii) were carried out, and the results of studies of the target compounds are presented below (Table 3).
Table 3.
Compounds identified from SC-CO2 extracts by two varieties of rhododendron: Rh. sichotense and Rh. adamsii.
| NO. | Identification | Formula | Calculated Mass | Rh. adamsii (104) | Rh. adamsii (105) | Rh. adamsii (109) | Rh. adamsii (110) | Rh. adamsii (108) | Rh. adamsii (112) | Rh. adamsii (116) | Rh. adamsii (117) | Rh. adamsii (118) | Rh. adamsii (122) | Rh. adamsii (123) | Rh. sicho (175) | Rh. sicho (176) | Rh. sicho (177) | Rh. sicho (178) | Rh. sicho (190) | Rh. sicho (192) | Rh. sicho (193) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lepalol [5-(3-Furyl)-2-methyl-1-penten-3-ol | C10H14O2 | 166.217 | [31,45,48] | ||||||||||||||||||
| 2 | Caffeic acid [(2E)-3-(3,4-Dihydroxyphenyl)acrylic acid] | C9H8O4 | 180.1574 | [10,35,42,43,50] | ||||||||||||||||||
| 3 | Azelaic acid (Nonanedioic acid) | C9H16O4 | 188.2209 | [15,16] | ||||||||||||||||||
| 4 | Calamenene [Cis-Calamenene] | C15H22 | 202.3352 | [15,16,38] | ||||||||||||||||||
| 5 | Germacron | C15H22O | 218.3346 | [4,5,10,42,44] | ||||||||||||||||||
| 6 | Myristic acid (Tetradecanoic acid; N-Tetradecanoic acid) | C14H28O2 | 228.3709 | [15,16] | ||||||||||||||||||
| 7 | Pentadecanoic acid (Pentadecylic acid) | C15H30O2 | 242.3975 | [15,16] | ||||||||||||||||||
| 8 | Palmitoleic acid | C16H30O2 | 254.4082 | [15,16] | ||||||||||||||||||
| 9 | Cis-cyclopropan-9,10-hexadecanoic acid | C17H32O2 | 268.4348 | [15,16] | ||||||||||||||||||
| 10 | Linoleic acid (Linolic acid; Telfairic acid) | C18H32O2 | 280.4455 | [15,16,49] | ||||||||||||||||||
| 11 | Stearic acid (Octadecanoic acid; Stearophanic acid) | C18H36O2 | 284.4772 | [15,16] | ||||||||||||||||||
| 12 | Kaempferol | C15H10O6 | 286.2363 | [36,39,40,43,50] | ||||||||||||||||||
| 13 | Cis-cyclopropan-9,10-octadecanoic acid | C19H32O2 | 292.4562 | [15,16] | ||||||||||||||||||
| 14 | Nonadecanoic acid (N-Nonadecanoic acid) | C19H38O2 | 298.5038 | [15,16] | ||||||||||||||||||
| 15 | Kaempferol 5-methyl ether | C16H12O6 | 300.2629 | [39] | ||||||||||||||||||
| 16 | Farrerol | C17H16O5 | 300.3059 | [34,39] | ||||||||||||||||||
| 17 | Quercetin | C15H10O7 | 302.2357 | [34,36,39,40,43,50] | ||||||||||||||||||
| 18 | Dihydroquercetin (Taxifolin; Taxifoliol) | C15H12O7 | 304.2516 | [35,39,40] | ||||||||||||||||||
| 19 | Cannabigerorcinic acid (Cannabigerorcinolic acid; Cannabiorcogerolic acid | C18H24O4 | 304.3808 | [15,16] | ||||||||||||||||||
| 20 | Docosane | C22H46 | 310.6006 | [48] | ||||||||||||||||||
| 21 | 8-Demethyleucalyptin [5-Hydroxy-4′,7-dimetoxy-6-methylflavone; Pabalate; Sodium salicylate] | C18H16O5 | 312.3166 | [33] | ||||||||||||||||||
| 22 | Arachic acid (Arachidic acid; eicosanoic acid) | C20H40O2 | 312.5304 | [15,16] | ||||||||||||||||||
| 23 | Azaleatin [5-O-Methylquercetin] | C16H12O7 | 316.2623 | [10,39,41,42] | ||||||||||||||||||
| 24 | Myricetin | C15H10O8 | 318.2351 | [36,34,39,40,50] | ||||||||||||||||||
| 25 | Gossypetin [Articulatidin; Equisporol] | C15H10O8 | 318.2351 | [37] | ||||||||||||||||||
| 26 | Ampelopsin [Dihydromyricetin; Ampeloptin] | C15H12O8 | 320.251 | [39] | ||||||||||||||||||
| 27 | Heneicosanoic acid (Heneicosylic acid) | C21H42O2 | 326.557 | [15,16] | ||||||||||||||||||
| 28 | Myricetin 5-Methyl ether [5-O-Methylmyricetin] | C16H12O8 | 332.2617 | [39] | ||||||||||||||||||
| 29 | Esculin [Aesculin; Esculoside; Polichrome] | C15H16O9 | 340.2821 | [33,41] | ||||||||||||||||||
| 30 | Behenic acid (Docosanoic acid) | C22H44O2 | 340.5836 | [15,16] | ||||||||||||||||||
| 31 | Pentacosane (N-Pentacosane) | C25H52 | 352.6854 | [48] | ||||||||||||||||||
| 32 | Chlorogenic acid | C16H18O9 | 354.3087 | [10,35,42] | ||||||||||||||||||
| 33 | Scopolin [Scopoloside; Scopoletin-7-glucoside; Murrayin] | C16H18O9 | 354.3087 | [41] | ||||||||||||||||||
| 34 | Tricosanoic acid (N-Tricosanoic acid) | C23H46O2 | 354.6101 | [15,16] | ||||||||||||||||||
| 35 | Lignoceric acid (Tetracosanoic acid) | C24H48O2 | 368.6367 | [15,16] | ||||||||||||||||||
| 36 | Fraxin (Fraxetin-8-O-glucoside) | C16H18O10 | 370.3081 | [33] | ||||||||||||||||||
| 37 | Daurichromenic acid | C23H30O4 | 370.4819 | [15,16] | ||||||||||||||||||
| 38 | Pentacosanoic acid (N-Pentacosanoic acid) | C25H50O2 | 382.6633 | [15,16] | ||||||||||||||||||
| 39 | Fraxetin-7-O-beta-glucuronide | C16H16O11 | 384.2916 | [41] | ||||||||||||||||||
| 40 | Beta-Sitosterin [Beta-Sitosterol] | C29H50O | 414.7067 | [10,30,42] | ||||||||||||||||||
| 41 | Cyanidin-3-alpfa-l-arabinoside | C20H19O10 | 419.3589 | [10,42] | ||||||||||||||||||
| 42 | Montanic acid (Amyrin; Beta-Amyrenol) | C28H56O2 | 424.743 | [15,16] | ||||||||||||||||||
| 43 | Alpha-Amyrin [Viminalol] | C30H50O | 426.7174 | [30] | ||||||||||||||||||
| 44 | Lupeol [Fagarasterol; Clerodol; Monogynol B; Lupenol] | C30H50O | 426.7174 | [30] | ||||||||||||||||||
| 45 | Dihydroquercetin-3-arabinofuranoside | C20H16O11 | 432.3344 | [10,42] | ||||||||||||||||||
| 46 | Afzelin [ Kaempferol-3-Rhamnoside; Kaempferin] | C21H20O10 | 432.3775 | [39,40] | ||||||||||||||||||
| 47 | Quercetin-3-O-beta-xyloside (Reynoutrin; Quercetin 3-O-Beta-d-Xylopyranoside) | C20H17O11 | 433.3424 | [34] | ||||||||||||||||||
| 48 | Avicularin (Quercetin 3-Alpha-l-Arabinofuranoside; Avicularoside) | C20H18O11 | 434.3503 | [10,33,39,40,42] | ||||||||||||||||||
| 49 | Pentoside dihydroquercetin | 436 | [40] | |||||||||||||||||||
| 50 | Erithrodiol [3-beta-Erytrodiol] | C30H50O2 | 442.7168 | [30] | ||||||||||||||||||
| 51 | Uvaol | C30H50O2 | 442.7168 | [30] | ||||||||||||||||||
| 52 | Quercitrin [Quercetin 3-l- Rhamnoside; Quercetrin] | C21H20O11 | 448.3769 | [33,39,46] | ||||||||||||||||||
| 53 | Catechin-7-O-glucoside | C21H24O11 | 452.4087 | [34] | ||||||||||||||||||
| 54 | Micromeric acid | C30H46O3 | 454.6844 | [30] | ||||||||||||||||||
| 55 | Hyperoside (Quercetin 3-O-galactoside; Hyperin) | C21H20O12 | 464.3763 | [10,33,34,39,40,41,42] | ||||||||||||||||||
| 56 | Quercetin 3-O-glucoside [ Isoquercitrin] | C21H20O12 | 464.3763 | [33,46] | ||||||||||||||||||
| 57 | Alpha.-Tocopherol-Beta-d-Mannoside [Dihydro-2H-Chromen-6-YI Hexofuranoside] | C35H60O7 | 592.8467 | [48] |
Colors are added for readability to avoid confusing columns. Green shades Rh. adamsii. blue Rh. sichotense.
Table 4 summarized all the molecular masses of the target analytes isolated from SC-CO2 of Rh. sichotense and Rh. adamsii. Among them, 57 biologically active substances were authenticated (m/z values and fragment ions) by comparison with the literature data [4,5,10,11,12,13,15,16,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. A total of 48 different biologically active components have been identified in the Rh. adamsii SC-CO2 extracts. A total of 31 different biologically active components have been identified in the Rh. sichotense SC-CO2 extracts.
Table 4.
Components identified from the SC-CO2 extracts of Rh. sichotense and Rh. adamsii.
| No. | Identification | Formula | Calculated Mass | Observed Mass [M − H]− | Observed Mass [M + H]+ | Observed Mass [M + Na]+ | MS/MS Stage 2 Fragmentation | MS/MS Stage 3 Fragmentation | MS/MS Stage 4 Fragmentation | Species of Rhododendron |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lepalol [5-(3-Furyl)-2-methyl-1-penten-3-ol | C10H14O2 | 166.217 | 165.06 | 147.01 | Rh. adamsii | ||||
| 2 | Caffeic acid [(2E)-3-(3,4-Dihydroxyphenyl) acrylic acid] | C9H8O4 | 180.1574 | 181.08 | 163.03; 135.11 | Rh. adamsii | ||||
| 3 | Azelaic acid [Nonanedioic acid] | C9H16O4 | 188.2209 | 210.09 | 192.12 | 175.06; 136.12 | Rh. adamsii | |||
| 4 | Calamenene [Cis-Calamenene] | C15H22 | 202.3352 | 203.09 | 147.05 | 119.06 | Rh. sichotense | |||
| 5 | Germacron | C15H22O | 218.3346 | 219.06 | 201.07; 149.07 | 159.07 | Rh. adamsii | |||
| 6 | Myristic acid (Tetradecanoic acid; N-Tetradecanoic acid) | C14H28O2 | 228.3709 | 251.09 | 150.48 | 149.08 | Rh. adamsii | |||
| 7 | Pentadecanoic acid (Pentadecylic acid) | C15H30O2 | 242.3975 | 243.06 | 201.01; 137.05 | 181.05; 135.04 | Rh. adamsii | |||
| 8 | Palmitoleic acid | C16H30O2 | 254.4082 | 277.09 | 275.04; 207.05 | 256.99 | 157.11 | Rh. adamsii | ||
| 9 | Cis-cyclopropan-9,10-hexadecanoic acid | C17H32O2 | 268.4348 | 269.02 | 185.97; 121.08 | 176.96 | 154.98 | Rh. adamsii | ||
| 10 | Linoleic acid [Linolic acid; Telfairic acid] | C18H32O2 | 280.4455 | 303.06 | 285.05; 163.00 | 180.95; 135.06 | 162.99 | Rh. adamsii | ||
| 11 | Stearic acid [Octadecanoic acid; Stearophanic acid] | C18H36O2 | 284.4772 | 285.07 | 284.18; 229.07; 163.02 | 180.90; 135.05 | 163.03 | Rh. adamsii; Rh. sichotense | ||
| 12 | Kaempferol [3,5,7-Trihydroxy-2-(4-hydro- xyphenyl)-4-H-chromen-4-one] | C15H10O6 | 286.2363 | 287.00 | 286.24; 204.96; 163.02 | 181.02 | 162.88 | Rh. adamsii | ||
| 13 | Cis-cyclopropan-9,10-octadecanoic acid | C19H32O2 | 292.4562 | 293.05 | 274.98 | 256.99; 162.98 | 201.03 | Rh. adamsii | ||
| 14 | Nonadecanoic acid [N-Nonadecanoic acid] | C19H38O2 | 298.5038 | 300.09 | 243.04 | 201.02 | Rh. adamsii; Rh. sichotense | |||
| 15 | Kaempferol 5-methyl ether | C16H12O6 | 300.2629 | 300.98 | 283.01; 177.01 | 264.98 | 200.98 | Rh. adamsii; Rh. sichotense | ||
| 16 | Farrerol [5,7-Dihydroxy-2-(4-hydroxyphenyl)-6,8-dimethylchroman-4-one] | C17H16O5 | 300.3059 | 301.05 | 283.04 | 241.01; 162.96 | Rh. adamsii; Rh. sichotense | |||
| 17 | Quercetin [2-(3,4-Dihydroxyphenyl)-3,5,7-trihy- droxy-4-H-chromen-4-one] | C15H12O7 | 302.2357 | 301.09 | 303.08 | 285.01; 163.02 | 180.97; 145.00 | 162.98 | Rh. adamsii; Rh. sichotense | |
| 18 | Dihydroquercetin [Taxifolin; Taxifoliol] | C15H12O7 | 304.2516 | 303.09 | 285.04 | 266.96; 241.09; 215.05; 135.05 | 171.02 | Rh. adamsii | ||
| 19 | Cannabigerorcinic acid [Cannabigerorcinolic acid; Cannabiorcogerolic acid] | C18H24O4 | 304.3808 | 303.08 | 285.05 | 241.07; 159.07 | 159.01 | Rh. adamsii | ||
| 20 | Docosane | C22H46 | 310.6006 | 311.10 | 293.06; 167.01 | 259.03 | 240.97; 162.96 | Rh. adamsii | ||
| 21 | 8-Demethyleucalyptin [5-Hydroxy-4′,7-dimetoxy-6-methylflavone; Pabalate; Sodium salicylate] | C18H16O5 | 312.3166 | 311.14 | 311.10; 182.99 | Rh. adamsii | ||||
| 22 | Arachic acid [Arachidic acid; eicosanoic acid] | C20H40O2 | 312.5304 | 311.14 | 335.04 | 303.06; 195.01 | 284.99; 238.14; 163.00 | 180.89; 135.14 | Rh. adamsii | |
| 23 | Azaleatin [5-O-Methylquercetin] | C16H12O7 | 316.2623 | 315.08 | 297.01; 167.04 | 235.04; 149.00 | Rh. adamsii | |||
| 24 | Myricetin [3,5,7-Trihydroxy-2-(3,4,5-Trihydroxyphenyl)-4H-Chromen-4-One] | C15H10O8 | 318.2351 | 317.08 | 299.01; 241.01 | 240.06; 197.09 | 238.99; 197.04 | Rh. adamsii; Rh. sichotense | ||
| 25 | Gossypetin [Articulatidin; Equisporol] | C15H10O8 | 318.2351 | 319.07 | 287.09; 176.98 | 146.99 | Rh. adamsii | |||
| 26 | Ampelopsin [Dihydromyricetin; Ampeloptin] | C15H12O8 | 320.251 | 319.08 | 317.01; 275.09 | 257.12; 217.11 | Rh. adamsii | |||
| 27 | Heneicosanoic acid [Heneicosylic acid] | C21H42O2 | 326.557 | 325.11 | 327.08 | 271.01; 217.03; 177.06 | 149.10 | Rh. adamsii; Rh. sichotense | ||
| 28 | Myricetin 5-Methyl ether [5-O-Methylmyricetin] | C16H12O8 | 332.2617 | 331.03 | 168.94 | 149.96 | Rh. sichotense | |||
| 29 | Esculin [Aesculin; Esculoside; Polichrome] | C15H16O9 | 340.2821 | 341.09 | 281.01; 217.11; 151.06 | 174.96 | Rh. adamsii; Rh. sichotense | |||
| 30 | Behenic acid [Docosanoic acid] | C22H44O2 | 340.5836 | 341.05 | 323.10; 243.11; 177.04 | 159.05 | Rh. adamsii; Rh. sichotense | |||
| 31 | Pentacosane (N-Pentacosane) | C25H52 | 352.6854 | 353.12 | 270.97; 162.97 | 180.93 | 162.96 | Rh. sichotense | ||
| 32 | Chlorogenic acid | C16H18O9 | 354.3087 | 355.09 | 287.05; 164.02 | 180.95 | 163.03 | Rh. adamsii; Rh. sichotense | ||
| 33 | Scopolin [Scopoloside; Scopoletin-7-glucoside; Murrayin] | C16H18O9 | 354.3087 | 355.02 | 323.00 | 303.96; 184.89 | 162.86 | Rh. adamsii; Rh. sichotense | ||
| 34 | Tricosanoic acid [N-Tricosanoic acid] | C23H46O2 | 354.6101 | 355.08 | 322.96; 163.00 | 180.96 | 162.96 | Rh. adamsii | ||
| 35 | Lignoceric acid [Tetracosanoic acid] | C24H48O2 | 368.6367 | 367.12 | 369.08 | 351.08; 285.02; 218.92; 162.98 | 163.02 | 144.97 | Rh. adamsii; Rh. sichotense | |
| 36 | Fraxin (Fraxetin-8-O-glucoside) | C16H18O10 | 370.3081 | 371.08 | 338.99 | 320.96; 177.03 | 224.96 | Rh. adamsii; Rh. sichotense | ||
| 37 | Daurichromenic acid | C23H30O4 | 370.4819 | 371.09 | 352.98; 287.08; 235.08; 179.02 | 231.04; 205.05; 162.99 | 180.93; 144.97 | Rh. adamsii; Rh. sichotense | ||
| 38 | Pentacosanoic acid [N-Pentacosanoic acid] | C25H50O2 | 382.6633 | 383.07 | 405.08 | 351.04; 287.99 | 229.04 | 211.03 | Rh. adamsii; Rh. sichotense | |
| 39 | Fraxetin-7-O-beta-glucuronide | C16H16O11 | 384.2916 | 383.09 | 365.09; 190.96 | 266.97; 215.02 | 170.97 | Rh. adamsii; Rh. sichotense | ||
| 40 | Beta-Sitosterin [Beta-Sitosterol] | C29H50O | 414.7067 | 415.04 | 384.02 | 369.01 | 338.00 | Rh. adamsii; Rh. sichotense | ||
| 41 | Cyanidin-3-alpfa-l-arabinoside | C20H19O10 | 419.3589 | 418.51 | 399.05; 319.02; 194.99 | 381.068 162.02 | 337.02; 253.08 | Rh. adamsii | ||
| 42 | Montanic acid [Octacosanoic acid] | C28H56O2 | 424.743 | 425.02 | 407.00 | 389.00; 348.98; 298.99; 240.97 | 333.00; 280.97; 173.02 | Rh. adamsii; Rh. sichotense | ||
| 43 | Alpha-Amyrin [Viminalol] | C30H50O | 426.7174 | 427.05 | 408.27; 308.99; 202.91 | 389.02; 309.01 | 373.08; 229.10; 142.80 | Rh. adamsii; Rh. sichotense | ||
| 44 | Lupeol [Fagarasterol; Clerodol; Monogynol B; Lupenol] | C30H50O | 426.7174 | 427.04 | 409.01; 202.99 | 389.02; 247.99 | 370.96; 264.80 | Rh. adamsii | ||
| 45 | Dihydroquercetin 3-arabinofuranoside | C20H16O11 | 432.3344 | 433.97 | 352.95 | 323.53; 271.96 | 241.95; 181.87 | Rh. adamsii | ||
| 46 | Afzelin [ Kaempferol-3-Rhamnoside; Kaempferin] | C21H20O10 | 432.3775 | 431.04 | 413.00; 372.98; 216.94 | 354.95; 167.01 | 336.98; 148.91 | Rh. sichotense | ||
| 47 | Quercetin-3-O-beta-xyloside (Reynoutrin; Quercetin 3-O-Beta-d-Xylopyranoside) | C20H17O11 | 433.3424 | 434.90 | 302.94 | 256.92; 164.96 | 228.91; 159.11 | Rh. sichotense | ||
| 48 | Avicularin (Quercetin 3-Alpha-l-Arabinofuranoside; Avicularoside) | C20H18O11 | 434.3503 | 433.09 | 415.07; 335.01; 176.98 | 397.06; 190.99 | 353.07; 253.99 | Rh. adamsii; Rh. sichotense | ||
| 49 | Pentoside dihydroquercetin | 436 | 435.16 | 416.54; 300.99; 231.01 | 397.02; 205.96 | 361.11; 283.02; 188.80 | Rh. adamsii; Rh. sichotense | |||
| 50 | Erithrodiol [3beta-Erytrodiol] | C30H50O2 | 442.7168 | 441.12 | 425.06; 381.05; 300.03; 217.04 | 363.06; 246.02 | 319.08; 201.02 | Rh. adamsii | ||
| 51 | Uvaol | C30H50O2 | 442.7168 | 443.22 | 425.01; 233.07 | 407.02; 325.01 | 388.99; 231.11 | Rh. adamsii; Rh. sichotense | ||
| 52 | Quercitrin [Quercetin 3-l-Rhamnoside; Quercetrin] | C21H20O11 | 448.3769 | 448.89 | 370.95; 282.93 | 352.95; 176.98 | 334.90; 222.92; 176.97 | Rh. adamsii | ||
| 53 | Catechin-7-O-glucoside | C21H24O11 | 452.4087 | 453.17 | 435.15; 336.07; 209.06 | 417.16; 336.11; 226.12 | 209.09 | Rh. sichotense | ||
| 54 | Micromeric acid | C30H46O3 | 454.6844 | 455.05 | 408.98; 246.98 | 391.05; 287.96 | 250.96 | Rh. adamsii | ||
| 55 | Hyperoside (Quercetin 3-O- galactoside; Hyperin) | C21H20O12 | 464.3763 | 465.02 | 302.91 | 256.94; 190.87 | 228.96; 172.75 | Rh. sichotense | ||
| 56 | Quercetin 3-O-glucoside [Isoquercitrin] | C21H20O12 | 464.3763 | 465.08 | 447.00 | 386.96 | 369.12; 172 | Rh. sichotense | ||
| 57 | Alpha.-Tocopherol-Beta-d-Mannoside [Dihydro-2H-Chromen-6-YI Hexofuranoside] | C35H60O7 | 592.8467 | 593.11 | 533.08 | 461.10 | 433.11 | Rh. sichotense |
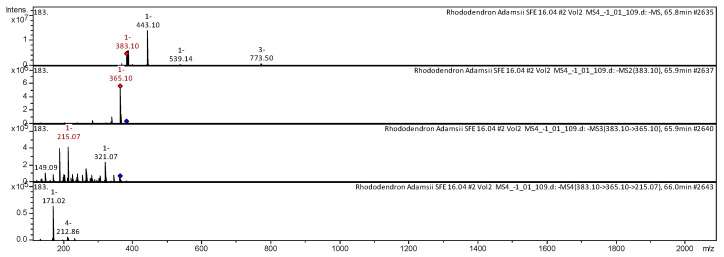
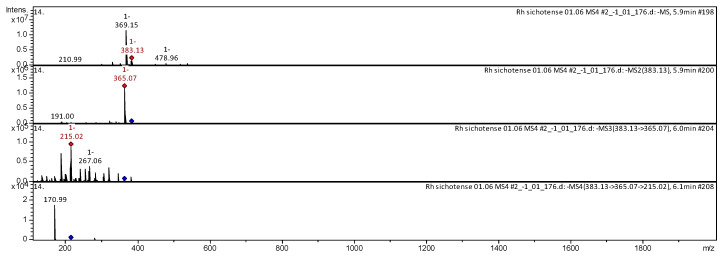
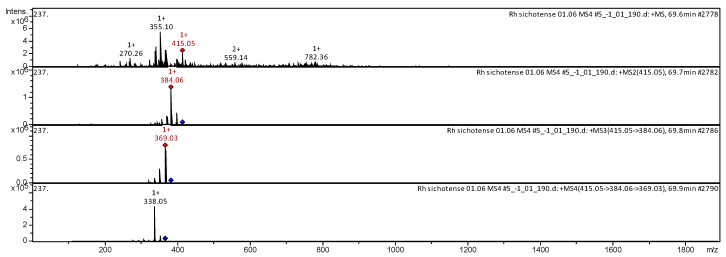
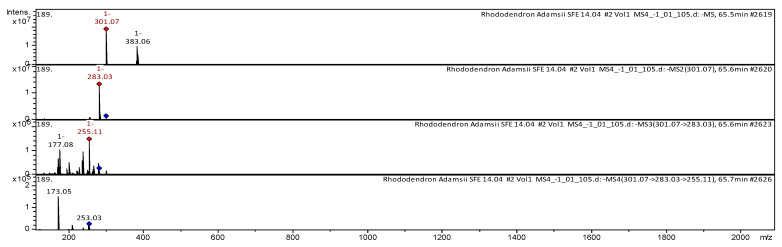
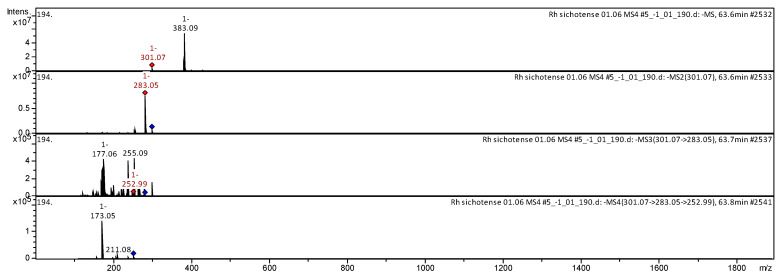
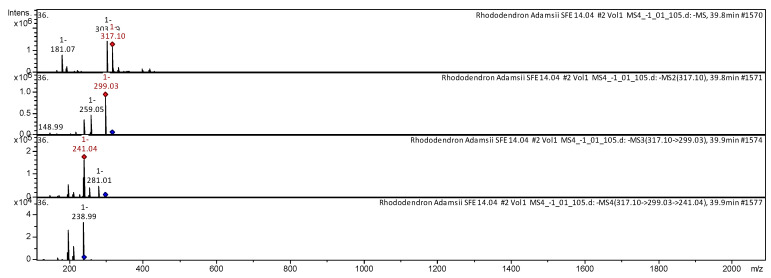
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11 shows examples of the decoding spectra (collision-induced dissociation (CID) spectrum) of the ion chromatogram obtained using tandem mass spectrometry. The CID spectrum in negative ion modes of fraxetin-7-O-beta-glucoronide from Rh. adamsii and Rh. sichotense are shown in Figure 2 and Figure 3.
Figure 2.
Collision-induced dissociation (CID) spectrum of fraxetin-7-O-beta-glucoronide from Rh. adamsii, m/z 383.10.
Figure 3.
CID spectrum of fraxetin-7-O-beta-glucoronide from Rh. sichotense, m/z 383.13.
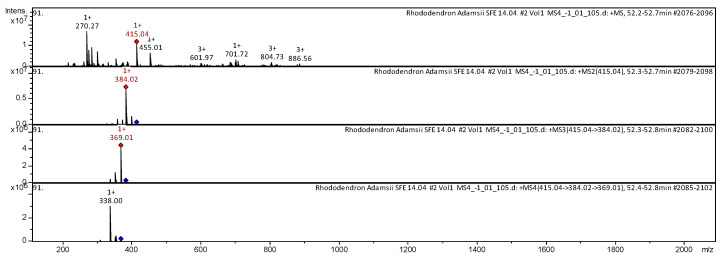
Figure 4.
CID spectrum of Beta-sitosterin from Rh. adamsii, m/z 415.04.
Figure 5.
CID spectrum of Beta-sitosterin from Rh. sichotense, m/z 415.05.
Figure 6.
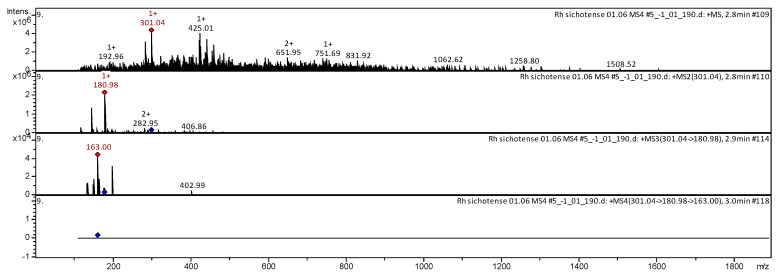
CID spectrum of quercetin from Rh. adamsii, m/z 301.07.
Figure 7.
CID spectrum of quercetin from Rh. sichotense, m/z 301.07.
Figure 8.
CID spectrum of myricetin from Rh. adamsii, m/z 317.10.
Figure 9.
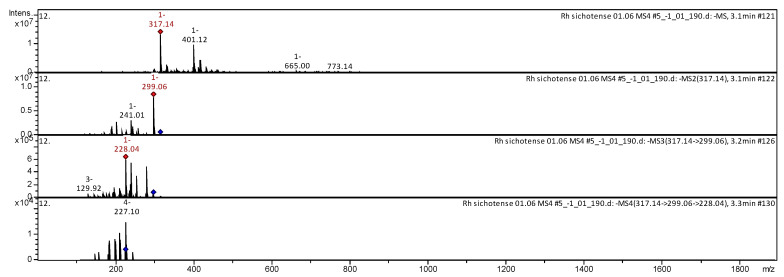
CID spectrum of myricetin from Rh. sichotense, m/z 317.14.
Figure 10.
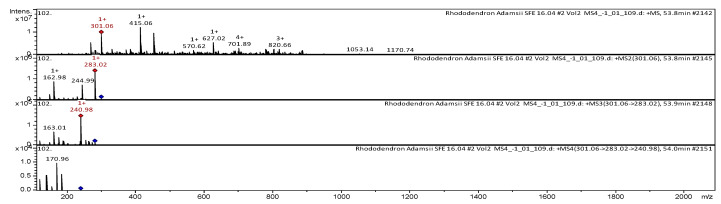
CID spectrum of farrerol from Rh. adamsii, m/z 301.06.
Figure 11.
CID spectrum of farrerol from Rh. sichotense, m/z 301.06.
The [M − H]− ion produced fragment ion with m/z 383.10 (Figure 2). The fragment ion with m/z 383.10 produced characteristic daughter ion with m/z 321.07, m/z 215.07 and m/z 149.09. The fragment ion with m/z 215.07 formed two daughter ions with m/z 171.02, m/z 212.86. It was identified in the bibliography in extract from rhododendron L. palustre [13,30,31,32,33,45].
The [M − H]− ion produced fragment ion with m/z 383.13 (Figure 3).The fragment ion with m/z 383.13 produced two fragments with m/z 365.07, m/z 191.00. The fragment ion with m/z 365.07 produced two characteristic daughter ions with m/z 267.06 and m/z 215.02. The fragment ion with m/z 215.02 formed a daughter ion with m/z 170.99. It was identified in the bibliography of the methanolic extract from rhododendron L. palustre [30,31,32,33,44].
The CID spectrum in positive ion modes of Beta-sitosterin from Rh. adamsii and Rh. sichotense is shown in Figure 4 and Figure 5.
The [M + H]+ ion produced one fragment with m/z 415.04 (Figure 4). The fragment ion with m/z 384.02 produced one daughter ion with m/z 369.01. The fragment ion with m/z 369.01 formed a daughter ion with m/z 338.00. It was identified in the bibliography in the extract from rhododendrons L. palustre [30,31,32,33,41,44,45] and Rh. adamsii [10,12,15,16,42,52].
The [M + H]+ ion produced one fragment with m/z 384.06 (Figure 5). The fragment ion with m/z 384.06 produced one daughter ion with m/z 369.03. The fragment ion with m/z 369.03 formed a daughter ion with m/z 338.05. It was identified in the bibliography in the extract from rhododendrons L. palustre [30,31,32,33,41,44,45] and Rh. adamsii [10,12,15,16,42,52].
The CID spectrum in negative ion modes of quercetin from Rh. adamsii and Rh. sichotense is shown in Figure 6 and Figure 7.
The [M − H]− ion produced one fragment ion with m/z 283.03 (Figure 6). The fragment ion with m/z 283.03 produced two daughter ions with m/z 255.11 and m/z 177.08. The fragment ion with m/z 255.11 formed two daughter ions with m/z 253.03 and m/z 173.05. It was identified in the bibliography in extracts from rhododendrons Rh. sichotense, Rh. micronulatum [38,39,40]; Rh. ungernii [34]; Rhodiola crenulata [36]; Rh. adamsii [10,12,15,16,42,52]; Rh. parvifolium [10]; Ocimum [43].
The [M − H]− ion produced one fragment ion with m/z 283.03 (Figure 7). The fragment ion with m/z 283.03 produced three daughter ions with m/z 255.09, m/z 177.06 and m/z 252.99. The fragment ion with m/z 252.99 formed two daughter ions with m/z 211.08 and m/z 173.05. It was identified in the bibliography in extracts from rhododendrons Rh. sichotense, Rh. micronulatum [38,39,40]; Rh. ungernii [34]; Rhodiola crenulata [36]; Rh. adamsii [10,12,15,16,42,52]; Rh. parvifolium [10]; Ocimum [43].
The CID spectrum in negative ion modes of myricetin from Rh. adamsii and Rh. sichotense is shown in Figure 8 and Figure 9.
The [M − H]− ion produced three fragment ions with m/z 299.03, m/z 259.05 and m/z 148.99 (Figure 8). The fragment ion with m/z 299.03 produced two daughter ions with m/z 241.04 and m/z 281.01. The fragment ion with m/z 241.04 formed a daughter ion with m/z 238.99. It was identified in the bibliography in extracts from rhododendrons Rh. sichotense, Rh. micronulatum [39,40,41]; Rh. ungernii [35]; Rhodiola crenulata [37]; Rh. adamsii [16,17,43,53]; Rh. parvifolium [41]; Ocimum [44].
The [M − H]− ion produced two fragment ions with m/z 299.06, m/z 241.01 (Figure 9). The fragment ion with m/z 299.06 produced two daughter ions with m/z 228.04 and m/z 129.92. The fragment ion with m/z 228.04 formed a daughter ion with m/z 227.10. It was identified in the bibliography in extracts from rhododendrons Rh. sichotense [4,5]; Rh. ungernii [34]; Rhodiola crenulata [36]; Rh. adamsii [10,15,16,42,52]; Rh. parvifolium [10,40].
The CID spectrum in positive ion modes of farrerol from Rh. adamsii and Rh. sichotense is shown in Figure 10 and Figure 11. The [M + H]+ ion produced three fragment ions with m/z 283.02, m/z 244.99 and m/z 162.98 (Figure 10). The fragment ion with m/z 283.02 produced two daughter ions with m/z 240.98 and m/z 163.01. The fragment ion with m/z 240.98 formed a daughter ion with m/z 170.96. It was identified in the bibliography in extracts from rhododendrons Rh. dauricum [38,39,40,41]; Rh. ungernii [34].
The [M + H]+ ion produced two fragment ions with m/z 282.95 and m/z 180.98 (Figure 11).
The fragment ion with m/z 180.98 formed a daughter ion with m/z 163.00. It was identified in the bibliography in extracts from rhododendrons Rh. dauricum [38,39,40]; Rh. ungernii [34].
3. Materials and Methods
3.1. Materials
The objects of study were purchased samples of Rh. sichotense (leaves and stems) from Primorsky Krai (the eastern slope of the Sikhote Alin ridge) and Rh. adamsii (leaves and stems) from the area near lake Baykal, Russia. All samples were morphologically authenticated according to the current standard of Russian Pharmacopeia [54]. All samples were immediately washed weighed by 10 g aliquot then frozen and kept until extraction.
3.2. Chemicals and Reagents
HPLC-grade acetonitrile was purchased from Fisher Scientific (Southborough, UK), MS-grade formic acid was from Sigma-Aldrich (Steinheim, Germany). Ultra-pure water was prepared from a SIEMENS ULTRA clear (SIEMENS water technologies, Munich, Germany), and all other chemicals were analytical grade.
3.3. Liquid Chromatography
HPLC was performed using a Shimadzu LC-20 Prominence HPLC (Kanda-Nishikicho 1-chrome, Shimadzu, Chiyoda-ku, Tokyo, Japan), equipped with a UV-sensor and a Shodex ODP-40 4E (250 × 4.6 mm, particle size: 4 μm) reverse phase C18 column to perform the separation of multicomponent mixtures. The gradient elution program was as follows: 0.01–4 min, 100% A; 4–60 min, 100–25% A; 60–75 min, 25–0% A; control washing 75–120 min 0% A. The entire HPLC analysis was performed using a UV-VIS detector SPD-20A (Kanda-Nishikicho 1-chrome, Shimadzu, Chiyoda-ku, Tokyo, Japan) at wavelengths of 230 and 330 nm, at 17 °C provided with a column oven CTO-20A (Kanda-Nishikicho 1-chrome, Shimadzu, Chiyoda-ku, Tokyo, Japan) with an injection volume of 20 μL.
3.4. SC-CO2 Extraction
SC-CO2 extraction was performed using the Supercritical fluid system -500 (Thar SCF Waters, Milford, MA, USA) supercritical pressure extraction apparatus. System options include: Co-solvent pump (Thar Waters P-50 High Pressure Pump), for extracting polar samples. CO2 flow meter (Siemens, Munich, Germany), to measure the amount of CO2 being supplied to the system, multiple extraction vessels, to extract different sample sizes or to increase the throughput of the system. Flow rate was 50 mL/min for liquid CO2 and 1.00 mL/min for EtOH. Samples for extraction of 10 g of frozen Rh. sichotense and Rh. adamsii pre-cut into pieces no more than 1 cm were used. Several experimental conditions were investigated, working in a pressure range of 300–400 bar, with 1% of C2H5OH as co-solvent and a temperature range of 50–60 °C. The extraction time was counted after reaching the working pressure and equilibrium flow, and it was 60–70 min for each sample.
3.5. Mass Spectrometry
MS analysis was performed on an ion trap amaZon SL (BRUKER DALTONIKS, Bremen, Germany) equipped with an ESI source in negative ion mode. The optimized parameters were obtained as follows: ionization source temperature: 70 °C, gas flow: 4 L/min, nebulizer gas (atomizer): 7.3 psi, capillary voltage: 4500 V, end plate bend voltage: 1500 V, fragmentary: 280 V, collision energy: 60 eV MS/MS MS4 (four stages of separation). An ion trap was used in the scan range m/z 100–1700 for MS and the capture rate was one spectrum/s for MS and two spectrum/s for MS/MS. All experiments were repeated three times. A four-stage ion separation mode (MS/MS mode) was implemented.
4. Conclusions
Aiming to optimize the extraction of target analytes from the Rh. sichotense and Rh. adamsii leaves and stems, several experimental conditions were investigated working in a pressure range of 300–400 bar, with 1% of C2H5OH as co-solvent and a temperature range of 50–60 °C. Although this approach is not quantitative for evaluation of each analyte, it is semiquantitative when comparing a series of extractions and allows better comparison of the yield without loss of individual analytes during fractionation and sample preparation. The best results were obtained at 370 bar and 60 °C.
High-accuracy mass spectrometric data were recorded on an ion trap amaZon SL BRUKER DALTONIKS equipped with an ESI source in the negative ion mode. The four-stage ion separation mode was implemented to perform correct identification. Under these conditions a total of 800 peaks were detected in the ion chromatogram. An optimized extraction process with SC-CO2 (co-solvent 1% ethanol) provided the samples for an accurate analytical study by HPLC–MS/MS technique. A total of 50 different biologically active components were identified in the Rh. adamsii SC-CO2 extracts. A total of 30 different biologically active components were identified in the Rh. sichotense SC-CO2 extracts.
An analysis of the similarity of the composition of biologically active components of Rh. sichotense and Rh. adamsii revealed their significant relationship. An analysis of morphometric parameters and composition of Rh. sichotense and Rh. adamsii flavonoids from populations of the Far East and Siberia indicated the presence of ecological and geographical variability. Inter-population differences in morphometric indicators are more significant than in chemical ones. Moreover, the former is more associated with climatic factors, while the latter are more associated with edaphic growth factors. The Rh. adamsii extract was more diverse in chemical composition than Rh. sichotense. A particularly large difference was observed in acidic components such as caffeic acid, azelaic acid, myristic acid, pentadecanoic acid, palmitoleic acid, linoleic acid. The extract of Rh. sichotense contained mainly stearic acid, nonadecanoic acid, montanic acid, behenic acid, tetracosanoic acid and chlorogenic acid. The flavonoid content of both rhododendrons was mainly the same.
These data could support future research for the production of a variety of pharmaceutical products containing ultra-pure SC-CO2 extracts of Rh. sichotense and Rh. adamsii. The richness of various biologically active compounds, including flavonoids: quercetin, kaempferol, dihydroquercetin, farrerol, myricetin, etc. provides great opportunities for the design of new drugs based on extracts from this species of rhododendron.
Supplementary Materials
The following are available online, Figure S1: Chemical profiles of the Rh. adamsii sample represented ion chromatogram from SC-CO2 extract; Figure S2: Chemical profiles of the Rh. sichotense sample represented ion chromatogram from SC-CO2 extract.
Author Contributions
Conceptualization, A.Z. and K.G.; methodology, A.Z., M.R.; software, M.R.; validation, M.R., K.G.; formal analysis, M.R., A.Z., V.G.; investigation, M.R. and A.Z.; resources, K.G. and A.Z.; data curation, K.G.; writing—original draft preparation—M.R., A.Z., S.E.; writing—review and editing A.Z., V.G., S.E. and K.G.; visualization, M.R., A.Z., S.E. and V.G.; supervision, K.G.; project administration, A.Z., K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Council on Grants of the President of the Russian Federation (CΠ3156.2019.4).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Pojarkova A.I. Genus Ericaceae, D.K.-vacciniaceous. [(accessed on 29 June 2020)];Flora USSR. 1952 18:26–93. Available online: https://docviewer.yandex.ru/view/0/?page=1&*=G3rUdUDjrwwhelX3asXdHS%2Fw9897InVybCI6InlhLWRpc2stcHVibGljOi8vN0x0R0lZV1VYaEhFcmRwdTFtTmprVnJoNGl4dmhDMDhsbVBpcjROcjVnaz0iLCJ0aXRsZSI6ItCk0JvQntCg0JBf0KHQodCh0KBf0KIxOF8xOTUyLmRqdnUiLCJub2lmcmFtZSI6ZmFsc2UsInVpZCI6IjAiLCJ0cyI6MTU5NzU4Mjc3MzcwNywieXUiOiI4OTgyMDE4MDYxNTU5MDQ4Mzk5In0%3D. (In Russian) [Google Scholar]
- 2.Aleksandrova M.S. Rhododendrons of Natural Flora of the USSR. Nauka; Moscow, Russia: 1975. [(accessed on 29 June 2020)]. p. 112. Available online: https://docplayer.ru/42008573-Rododendrony-prirodnoy-flory-sssr.html. (In Russian) [Google Scholar]
- 3.Zaytseva G.Y., Ambros E.V., Karakulov A.V., Novikova T.I. Flow cytometric determination of genome size and ploidy level of some frost-resistant cultivars and species of Rhododendron L. native to Asian Russia. Botanica Pacifica. Bot Pac. 2018;7:97–100. doi: 10.17581/bp.2018.07110. [DOI] [Google Scholar]
- 4.Belousova N.I., Khan V.A., Tkachev A.V. The chemical composition of essential oil of Rhododendron. [(accessed on 29 June 2020)];Khimiya Rastitel’nogo Syr′iya (Chem. Plant Raw Mater.) 1999 3:5–38. Available online: https://elibrary.ru/item.asp?id=9444189. (In Russian) [Google Scholar]
- 5.Belousov M.V., Komissarenko N.F., Berezovskaya T.P., Tochkova T.V. Content of flavonoids and coumarins in the Siberian—Far Eastern species of the Ericaceae family. [(accessed on 29 June 2020)];Rastit. Resur. 1994 4:44–47. Available online: https://www.elibrary.ru/item.asp?id=20162300. (In Russian) [Google Scholar]
- 6.Varlygina T.I., Kamelin R.V., Kiseleva K.V. Red Data Book of Russian Federation. KMK; Moskow, Russia: 2008. p. 855. [Google Scholar]
- 7.Firsov G.A., Egorov A.A., Byalt V.V., Neverovsky V.J., Orlova L.V., Volchanskaya A.V., Lavrentyev N.V. Arboreal plants of the Red Data Book of Russia in collection of Saint Petersburg Forest-Technical Academy. [(accessed on 29 June 2020)];Hortus Botanicus. 2010 5:1–16. doi: 10.15393/j4.art.2010.1621. Available online: https://www.elibrary.ru/item.asp?id=21827419. [DOI] [Google Scholar]
- 8.Khokhryakov A.P., Mazurenko M.T. Vascular plants of the Soviet Far East. [(accessed on 29 June 2020)];Science. 1991 5:119. Available online: https://www.elibrary.ru/author_items.asp. (In Russian) [Google Scholar]
- 9.Hubich A.I., Puchkova K.V., Zalesskaya N.A., Kryuchkova N.V. The investigation of the adaptogenic properties of Rhododendron adamsii Rehder. on experimental models in vivo. J. Belarus. State Univ. Biol. 2018;1:60–68. (In Russian) [Google Scholar]
- 10.Mirovich V.M., Konenkina T.A., Fedoseeva G.M. Qualitative structure od essential oil of Rhododendron adamsii and parvifolium, growing in East Siberia. [(accessed on 29 June 2020)];Siberian Med. J. 2008 76:79–82. Available online: https://www.elibrary.ru/item.asp?id=17844859. (In Russian) [Google Scholar]
- 11.Belousova N.I., Khan V.A. Bicyclic monoterpenoids of the essential oil of Ledum palustre. Chem. Nat. Compd. 1990;5:627–629. [Google Scholar]
- 12.Kurshakova G.V., Fedorov A.A., Yakimov P.A. Some data on the chemical composition and pharmacological effect of rhododendron Adams–Rhododendron adamsii Rend. Trudy Botanicheskogo instituta im. V. L. Komarova AN SSSR. 1961;V:216–220. (In Russian) [Google Scholar]
- 13.Belousov M.V., Berezovskaya T.P., Komissarenko N.F., Tikhonova L.A. Flavonoids of Siberian and Far-Eastern species of rhododendrons of the subsgenus Rhodorastrum. Chem. Nat. Compd. 1998;34:510–511. doi: 10.1007/BF02329610. [DOI] [Google Scholar]
- 14.Fini A., Brunetti C., Di Ferdinando M., Ferrini F., Tattini M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant. Signal. Behav. 2011;6:709–711. doi: 10.4161/psb.6.5.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogachev A.D. Ph.D.’s Thesis. Novosibirsk University; Novosibirsk, Russia: 2009. Phytochemical study of Rhododendron Adamsii Rheder. (In Russian) [Google Scholar]
- 16.Rogachev A.D., Fomenko V.V., Sal’nikova O.I., Pokrovskii L.M., Salakhutdinov N.F. Comparative analysis of essential oil compositions from leaves and stems of Rhododendron adamsii, R. aureum, and R. dauricum. Chem. Nat. Compd. 2006;42:426–430. doi: 10.1007/s10600-006-0172-9. [DOI] [Google Scholar]
- 17.Razgonova M.P., Zacharenko A.M., Kalenik T.K., Nosyrev A.E., Stratidakis A.K., Mezhuev Y.O., Burykina T.I., Nicolae A.C., Arsene A.L., Tsatsakis A.M., et al. Supercritical fluid technology and supercritical fluid chromatography for application in ginseng extracts. Farmacia. 2019;67:202–212. doi: 10.31925/farmacia.2019.2.2. [DOI] [Google Scholar]
- 18.Razgonova M., Zakharenko A., Shin T.-S., Chung G., Golokhvast K. Supercritical CO2 Extraction and Identification of Ginsenosides in Russian and North Korean Ginseng by HPLC with Tandem Mass Spectrometry. Molecules. 2020;25:1407. doi: 10.3390/molecules25061407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morozov Y.A., Pupykina K.A., Blagorazumnaya N.V., Aliev A.M., Morozova E.V. Comparative analysis of carbon dioxide extracts from plant material of Schisandra chinensis: Leaves, woody stems, rhizomes with roots. Med. Bull. Bashkortostan. 2018;13:46–51. [Google Scholar]
- 20.Aliev A.M., Radjabov G.K., Musaev A.M. Dynamics of supercritical extraction of biological active substances from the Juniperus communis var. saxatillis. J. Supercrit. Fluids. 2015;102:66–72. doi: 10.1016/j.supflu.2015.04.009. [DOI] [Google Scholar]
- 21.Rovetto L.J., Aieta N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids. 2017;129:16–27. doi: 10.1016/j.supflu.2017.03.014. [DOI] [Google Scholar]
- 22.Baldino L., Della Porta G., Sesti Osseo L., Reverchon E., Adami R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids. 2018;133:65–69. doi: 10.1016/j.supflu.2017.09.026. [DOI] [Google Scholar]
- 23.Mehariya S., Iovine A., Di Sanzo G., Larocca V., Martino M., Leone G.P., Casella P., Karatza D., Marino T., Musmarra D., et al. Supercritical fluid extraction of lutein from Scenedesmus almeriensis. Molecules. 2019;24:1324. doi: 10.3390/molecules24071324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leone G.P., Balducchi R., Mehariya S., Martino M., Larocca V., Di Sanzo G., Iovine A., Casella P., Marino T., Karatza D., et al. Selective Extraction of ω-3 Fatty Acids from Nannochloropsis sp. Using Supercritical CO2 extraction. Molecules. 2019;24:2406. doi: 10.3390/molecules24132406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senica M., Stampar F., Miculic-Petkovsek M. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk. J. Agric. For. 2019;43:576–585. doi: 10.3906/tar-1907-48. [DOI] [Google Scholar]
- 26.Colak A.M., Okatan V., Polat M., Guclu S.F. Different harvest times affect market quality of Lycium barbarum L. berries. Turk. J. Agric. For. 2019;43:326–333. doi: 10.3906/tar-1808-17. [DOI] [Google Scholar]
- 27.Baldino L., Reverchon E. Challenges in the production of pharmaceutical and food related compounds by SC-CO2 processing of vegetable matter. J. Supercrit. Fluids. 2018;134:269–273. doi: 10.1016/j.supflu.2017.11.034. [DOI] [Google Scholar]
- 28.Popova A.S., Ivahnov A.D., Skrebets T.E., Bogolitsyn K.G. Supercritical fluid extraction of carotenoids and chlorophyll from Ledum palustre. Khimiya Rastitel’nogo Syr’iya (Chem. Veg. Raw Mater.) 2018;1:61–66. (In Russian) [Google Scholar]
- 29.Baananou S., Bagdonaite E., Marongiu B., Piras A., Porcedda S., Falconieri D., Boughattas N.A. Supercritical CO2 extract and essential oil of aerial part of Ledum palustre L.—Chemical composition and anti-inflammatory activity. Nat. Prod. Res. 2015;29:999–1005. doi: 10.1080/14786419.2014.965167. [DOI] [PubMed] [Google Scholar]
- 30.Bukreyeva T.V., Shavarda A.L., Matusevich O.V., Morozov M.A. Ursane, oleanane, lupine triterpenoids from leaves of Ledum palustre (Ericaceae) from North-West Russia. Rastit. Resur. 2013;49:395–403. (In Russian) [Google Scholar]
- 31.Butkiene R., Sakociute V., Latvenaite D., Mockute D. Composition of young and aged shoot essential oils of the wild Ledum palustre L. Chemija. 2008;19:19–24. [Google Scholar]
- 32.Buzuk A.G., Buzuk G.N. The study of chemical variability of essential oil composition of Ledum palustre L., growing on the territory of the republic of Belarus. Vestnik Farmacii. 2016;4:18–25. (In Russian) [Google Scholar]
- 33.Dampc A., Luczkiewicz M. Rhododendron tomentosum (Ledum palustre). A Review of traditional use based on current research. Fitoterapia. 2013;85:130–143. doi: 10.1016/j.fitote.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Dede E., Genc N., Elmastas M., Aksit H., Erenler R. Chemical constituents Isolated from Rhododendron ungernii with Antioxidant Profile. Nat. Prod. J. 2019;9:238–243. doi: 10.2174/2210315508666181024114812. [DOI] [Google Scholar]
- 35.Ganina M.M. and Popova, O.I. Content of phenolic compounds in shoots of Ledum procumbent (Ledum decumbens Lodd. ex Steud) growing on the territory of the Yamalo-nenets autonomous district. Khim.-Farm. Zh. 2015;49:33–35. [Google Scholar]
- 36.Han F., Li Y., Ma L., Liu T., Wu Y., Hu R., Song A., Yin R. A rapid and sensitive UHPLC-FT-ICR MS/MS method for identification of chemical constituents in Rhodiola crenulata extract, rat plasma and rat brain after oral administration. Talanta. 2016;160:183–193. doi: 10.1016/j.talanta.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Harborne J.B., Williams C.A. Leaf survey of flavonoids and simple phenols in the genus Rhododendron. Phytochemistry. 1971;10:2727–2744. doi: 10.1016/S0031-9422(00)97273-X. [DOI] [Google Scholar]
- 38.Izotov D.V., Tagiltsev Y.G., Kolesnikova R.D., Tsyupko V.A. Biologically active substances of Far-Eastern Labrador tea. [(accessed on 29 June 2020)];Lesnoy J. 2010 2:24–30. Available online: https://www.elibrary.ru/item.asp?id=15198739. (In Russian) [Google Scholar]
- 39.Karpova E.A., Karakulov A.V. Flavonoids of some Rhododendron species of flora of Siberia and the Far East. [(accessed on 29 June 2020)];Khimiya Rastitel’nogo Syr’ya (Chem. Plant Raw Mater.) 2013 2:119–126. Available online: https://www.elibrary.ru/item.asp?id=20332638. (In Russian) [Google Scholar]
- 40.Karakulov A.V., Karpova E.A., Vasiliev V.G. Ecological and geographical variation of morphometric parameters and flavonoid composition of Rhododendron parvifolium. Turczaninowia. 2018;21:133–144. [Google Scholar]
- 41.Korotaeva M.S., Belousov M.V., Fursa N.S. Flavonoids and hydroxycinnamic acids content in Ledum palustre (Ericaceae) above-ground part. [(accessed on 29 June 2020)];Rastit. Resur. 2008 44:66–75. Available online: https://www.elibrary.ru/item.asp?id=9940517. (In Russian) [Google Scholar]
- 42.Mirovich V.M., Fedoseeva G.M., Zjubr T.P., Fedoseev A.P., Paisova O.I., Kuklina L.B. Elaboration of the method of receipt of the dry extract from sprouts of Rhododendron adamsii, having actoprotective and antimicrobic activity. Sibirskii medicinskii Zhurnal. 2006;9:96–98. (In Russian) [Google Scholar]
- 43.Pandey R., Kumar B. HPLC-OTOF-MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and heir interspecies variation. J. Liq. Chromatogr. Relat. Technol. 2016;39:225–238. doi: 10.1080/10826076.2016.1148048. [DOI] [Google Scholar]
- 44.Plyashechnik M.A. Chemical composition of Ledum palustre L. essential oil under increasing nitrogen availability in soils of cryolitzone (Central Evenkia) Khimiya Rastitel’nogo Syr’iya (Chem. Plant Raw Mater.) 2012;2:139–144. (In Russian) [Google Scholar]
- 45.Raal A., Orav A., Gretchushnikova T. Composition of the essential oil of the Rhododendron tomentosum Harmaja from Estonia. Nat. Prod. Res. 2014;28:1091–1098. doi: 10.1080/14786419.2014.907287. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H., Sasaki R., Ogata Y., Nakamura Y., Sakurai N., Kitajima M., Takayama H., Kanaya S., Aoki K., Shibata D., et al. Metabolic profiling of flavonoids in Lotus japonicus using liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. Phytochemistry. 2008;69:99–111. doi: 10.1016/j.phytochem.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Taamalli A., Arráez-Román D., Abaza L., Iswaldi I., Fernández-Gutiérrez A., Zarrouk M., Segura-Carretero A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015;26:320–330. doi: 10.1002/pca.2566. [DOI] [PubMed] [Google Scholar]
- 48.Ul’yanovskii N.V., Kosyakov D.S., Pokryshkin S.A., Bogolitsyn K.G., Ul’yanovskaya O.S. Study of volatile compounds composition of Ledum palustre L. using the method of thermodesorption gas chromatography -mass spectrometry. Khimiya Rastitel’nogo Syr’iya (Chem. Plant Raw Mater.) 2014;4:153–161. doi: 10.14258/jcprm.201404201. (In Russian) [DOI] [Google Scholar]
- 49.Yang S.T., Wu X., Rui W., Guo J., Feng Y.F. UPLC/Q-TOF-MS analysis for identification of hydrophilic phenolics and lipophilic diterpenoids from Radix Salviae Miltiorrhizae. Acta Pharm. 2015;27:711–728. doi: 10.1556/AChrom.27.2015.4.9. [DOI] [Google Scholar]
- 50.Zaytseva N.V., Pogulyaeva I.A. Chromatographic Analysis of Chemical Composition of the Genus Rhododendron Plants Growing on the Mountain of Evota (South Yakutia) J. Chem. Chem. Eng. 2014;8:516–523. [Google Scholar]
- 51.Jin C., Strembiski W., Kulchytska Y., Micetich R.G., Daneshtalab M. Flavonoid glycosides from Ledum palustre L. subsp. decumbens (Ait.) Hulton. DARU. J. Pharm. Sci. 1999;7:4. [Google Scholar]
- 52.Komarova N.I., Rogachev A.D., Chernyak E.I., Morozov S.V., Fomenko V.V., Salakhutdinov N.F. Quantitative HPLC determination of main flavonoid content of Rhododendron adamsii leaves and stems. Chem. Nat. Compd. 2009;45:1. doi: 10.1007/s10600-009-9215-3. [DOI] [Google Scholar]
- 53.Okhlopkova Z.M., Chirikova N.K. Component composition analysis of essential oil of the Ledum palustre L., growing in Yakutia. Fundam. Res. 2012;11:1334–1336. [Google Scholar]
- 54.Russian State Pharmacopeia XIII. [(accessed on 29 June 2020)];2016 Available online: http://pharmacopoeia.ru/en/gosudarstvennaya-farmakopeya-xiii-online-gf-13-online/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.