Abstract
Background
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are commonly used inflammatory markers utilized to aid in the diagnosis of periprosthetic infection (PJI). Patients with obesity, however, are known to have elevated baseline levels of these inflammatory markers. Therefore, this retrospective study aimed to determine the relationship between elevated ESR and CRP and body mass index (BMI) in patients undergoing total knee arthroplasty (TKA). In doing so, physicians can better determine whether BMI should be taken into account when evaluating the prognostic value of elevated preoperative ESR and CRP levels for risk of PJI in primary TKA patients.
Methods
This is a retrospective case series of 181 patients who had undergone primary TKA at a single institution. Patients undergoing primary unilateral TKA were eligible unless they had undergone previous TKA, contralateral knee symptoms, or elevated white blood cell (WBC) count. A linear regression model was utilized to demonstrate the relationship between proportions of patients with elevated biomarker values and categories of BMI. Analysis of variance and independent two-sample t-tests were utilized to assess differences in mean ESR, CRP, and WBC levels between the “healthy patients” and “patients with comorbidities” subgroups within each BMI category.
Results
Eligible patients (n = 181) were stratified by BMI category. Elevated ESR was associated significantly with BMI (ESR: r2 = 0.89, P < 0.001) unlike elevated CRP (r2 = 0.82, P = 0.133) and WBC count (r2 = .01; P = .626). No statistically significant differences in ESR values and WBC count between the “healthy patients” versus “patients with comorbidities” were demonstrated within any BMI category. In patients of normal weight (BMI 20–25 kg/m2), “healthy patients” had a statistically significantly higher mean CRP level than “patients with comorbidities” (1.73 mg/L vs. 0.70 mg/L, P < 0.001). There were no other statistically significant differences in mean CRP levels by health status.
Conclusion
Caution is advised when utilizing ESR and CRP to diagnose periprosthetic joint infection without considering BMI given that increasing preoperative levels of ESR and CRP are correlated with higher BMI.
Keywords: Total knee arthroplasty, Body mass index, Erythrocyte sedimentation rate, C-reactive protein, Periprosthetic joint infection
1. Introduction
Between 1993 and 2012, 7.8 million primary total knee arthroplasty (TKA) procedures were performed in United States (US) with the number of surgeries increasing by 224% during this period.1 Researchers using data from the 2000–2014 US National Inpatient Sample and Census Bureau projected that the total annual use of primary TKA in the US would increase by 401% from 2014 to 2040.2 The increased demand for TKA will likely result in a higher volume of complications arising from this procedure, including infections, such as periprosthetic infection (PJI).3, 4, 5
PJI is a potentially fatal major complication that can occur following a technically successful TKA and has a prevalence of approximately 2.0–2.4% in patients undergoing total hip or knee arthroplasty.6, 7, 8 PJI requires either a septic knee washout or, most commonly, a revision procedure to replace the prosthesis, prevent septic complications, and restore functionality.6, 7, 8 PJI is the leading cause for revision TKA, accounting for about 40% of these procedures, and imposes an extremely large financial burden on the healthcare system with a mean cost of $110,000 per patient.9,10 The largest cost of PJI is to the patient as revision TKA compromises patients’ quality of life and exposes them to a procedure with a higher complication rate (including deep infections), extended hospitalization, unsatisfactory functional outcomes, and a relatively shorter survival than primary TKA.10,11
Given the significant clinical and economic burden associated with PJI, preoperative risk stratification for PJI following TKA is important for patient optimization, targeted prevention, and early diagnosis. To date, five studies have examined the prognostic value of elevated preoperative inflammatory biomarkers, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), for developing PJI following TKA.12, 13, 14, 15, 16 Four of the five studies found that preoperative elevated CRP and/or ESR was associated with an increased risk for complications, including PJI following primary TKA, whereas one study found no relationship between inflammatory biomarkers and PJI.12, 13, 14, 15, 16 In the largest and most recent of these studies, among 3376 consecutive unilateral primary TKA patients, 140 (4.1%) had elevated preoperative ESR and/or CRP.12 The rate of PJI was significantly higher in TKA patients with both elevated ESR and CRP (12.5%, 4/32) compared with patients with either elevated ESR or CRP (0.9%, 1/108) and age-, sex-, and BMI-matched controls with both normal biomarkers (1.4%, 2/140). Patients with elevated ESR and CRP were almost 16 times more likely to develop PJI than those with normal inflammatory biomarkers after adjusting for age, sex, BMI, diabetes, smoking, prior joint injection, and anesthesia type.
Interpreting preoperative elevated inflammatory biomarkers in primary TKA patients requires an understanding of how these variables are influenced by patient characteristics. Research has shown that ESR and CRP are influenced by age, sex, and BMI in the general population and in people with certain disease states, but there is a paucity of literature regarding the relationship between these biomarkers and BMI in TKA patients.17, 18, 19, 20, 21 Current recommendations for the use of ESR and CRP in diagnosing PJI may not consider the influence of BMI on these inflammatory biomarkers.22 The current study was designed to examine the relationship between idiopathically elevated ESR and CRP and BMI in patients undergoing primary TKA. Results from this study should help physicians determine whether BMI should be taken into account when evaluating the prognostic value of elevated preoperative ESR and CRP levels for risk of PJI in primary TKA patients.
2. Materials & methods
2.1. Patient cohort selection
224 patients who had undergone TKA, selected by diagnostic codes, at a single institution by a single surgeon between 2009 and 2011 were initially selected. 43 of these patients were excluded due to a previous TKA procedure. This is a retrospective case series of 181 patients who had undergone primary TKA at a single institution. Patients who had undergone previous TKA, presented with contralateral knee symptoms, or had preoperative elevated white blood cell (WBC) count were excluded.
2.2. Baseline patient information and stratification
At the study site, ESR, CRP, and WBC values are routinely obtained for all patients within one month prior to surgery. Patients were stratified by BMI using increments of 5 kg/m2 (seven categories). Two subgroups were defined for subgroup analyses: patients were classified as either “healthy patients” or “patients with comorbidities” based on presence of certain clinical conditions. The “patients with comorbidities” subgroup was defined based on comorbidities known to cause elevated ESR or CRP, which included 1) active leukocytosis; 2) past or current inflammatory conditions, including anemia, infection, kidney disease, malignancy, thyroid disease, vasculitis, autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, rosacea, and sarcoidosis), past or current hepatitis C; and 3) any infection < 1 month prior to surgery (Table 1). All patients not meeting any of the aforementioned criteria defining “patients with comorbidities” were classified as “healthy.”
Table 1.
Conditions defining the “patients with comorbidities” subgroup.
| Comorbiditiesa | N | % |
|---|---|---|
| Anemia | 14 | 7.6 |
| Infection <1 month prior to surgery | 11 | 6.0 |
| Thyroid disease | 10 | 5.4 |
| Kidney disease | 8 | 5.2 |
| Malignancy | 5 | 2.7 |
| Systemic lupus erythematosus | 4 | 2.2 |
| Rheumatoid arthritis | 3 | 1.6 |
| Hepatitis C | 1 | 0.5 |
| Inflammatory bowel disease | 1 | 0.5 |
| Sarcoidosis | 1 | 0.5 |
| Rosacea | 1 | 0.5 |
| Vasculitis | 0 | 0.0 |
| Active leukocytosis | 0 | 0.0 |
Some patients had more than one condition.
Thresholds for defining elevated inflammatory biomarkers were based on widely accepted clinical standards. For men and women of all ages, WBC count >11,000 cells/μL and CRP >10 mg/L were considered elevated. For men <50 years, ESR >15 mm/h was considered elevated. ESR >20 mm/h was considered elevated for men ≥50 years and females younger than 50 years of age. ESR >30 mm/h was considered elevated for females greater than 50 years of age.23, 24, 25, 26
2.3. Statistical analysis
All statistical analyses conducted in this current study were carried out using the IBM® SPSS® Statistics Version 25 software. (IBM Corporation, Armonk, NY). A P value of < .05 was considered significant for this study. The proportions of patients with elevated levels of ESR, CRP, and WBC count were calculated within each of the seven BMI categories. Chi-square tests and Fischer’s exact tests (for expected cell sizes <5) were used to assess differences in the percentage of patients with elevated ESR, CRP, and WBC levels between BMI categories. A linear regression model was utilized to demonstrate the relationship between proportions of patients with elevated biomarker values and categories of BMI. The strength of the correlation between biomarkers and BMI was expressed by R2. Analysis of variance and independent two-sample t-tests were utilized to assess differences in mean ESR, CRP, and WBC levels between the “healthy patients” and “patients with comorbidities” subgroups within each BMI category.
2.4. Institutional review board approval
This study was approved by the institutional review board at the authors’ home institution.
3. Results
3.1. Demographics and BMI
The final cohort of 181 primary TKA patients was predominately female (69.1% n = 125) with a mean (±standard deviation [SD]) range of 64.73 ± 11.90 years (range: 21 years, 95 years). Almost 7 of 10 patients (69.8%) in the overall sample were obese (BMI ≥ 30 kg/m2) and the mean ± SD BMI was 33.6 ± 6.62 kg/m2. The “patients with comorbidities” subgroup consisted of 44 patients (24.31%). Table 1 shows the conditions qualifying patients for classification into this subgroup. The remaining 137 patients were classified as the “healthy patients” cohort, categorically assigned into one of seven BMI groups: 20 to <25 kg/m2 (n = 12; 8.76%), 25 to <30 kg/m2 (n = 33; 24.09%), 30 to <35 kg/m2 (n = 42; 30.66%), 35 to <40 kg/m2 (n = 28; 20.44%), 40 to <45 kg/m2 (n = 13; 9.49%), 45 to <50 kg/m2 (n = 7; 5.11%), and 50 to <55 kg/m2 (n = 2; 1.46%).
3.2. Relationship between inflammatory biomarkers and BMI
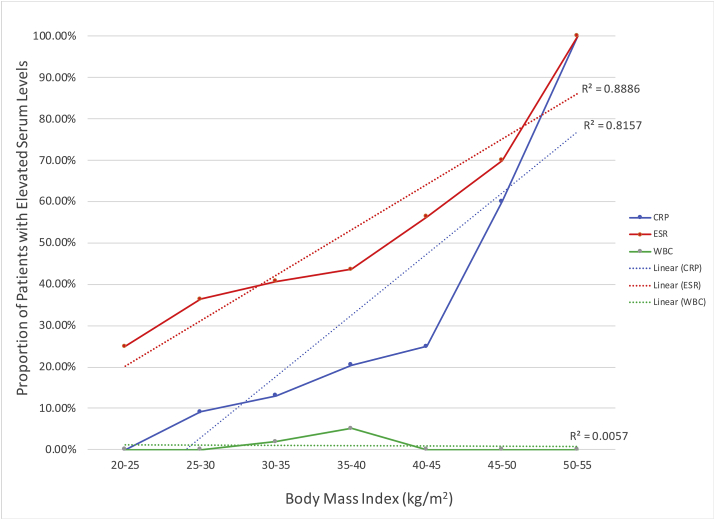
The prevalence of idiopathically elevated ESR within each BMI category was: 25.0% (20 to <25 kg/m2), 36.4% (25 to <30 kg/m2), 40.7% (30 to <35 kg/m2), 43.6% (35 to <40 kg/m2), 56.3% (40 to <45 kg/m2), 70.0% (45 to <50 kg/m2), 100.0% (50 to <55 kg/m2). The prevalence of idiopathically elevated CRP within each BMI category was 0% (20 to <25 kg/m2), 9.1% (25 to <30 kg/m2), 13.0% (30 to <35 kg/m2), 20.5% (35 to <40 kg/m2), 25.0% (40 to <45 kg/m2), 60.6% (45 to <50 kg/m2), and 100.0% (50 to <55 kg/m2). As shown in Table 2 and Fig. 1, elevated ESR and BMI was positively and statistically significantly correlated (r2 = 0.89; P < 0.001) whereas the correlations between elevated CRP (r2 = 0.82, P = 0.133) and WBC count (r2 = 0.01; P = 0.626) were not statistically significant.
Table 2.
ESR, CRP, and WBC levels by body mass index category.
| BMI Category | % Elevated ESR | Mean (SD) ESR | % Elevated CRP | Mean (SD) CRP | % Elevated WBC | Mean (SD) WBC |
|---|---|---|---|---|---|---|
| 20 to <25 kg/m2 | 25.0 | 15.67 (11.06) | 0.0 | 1.73 (0.98) | 0.0 | 6.73 (2.43) |
| 25 to <30 kg/m2 | 36.4 | 19.88 (12.41) | 9.1 | 3.24 (3.36) | 0.0 | 6.67 (1.81) |
| 30 to <35 kg/m2 | 40.7 | 24.57 (17.32) | 13.0 | 3.96 (3.93) | 1.9 | 6.83 (1.60) |
| 35 to <40 kg/m2 | 43.6 | 25.50 (16.23) | 20.5 | 6.11 (8.42) | 5.1 | 6.98 (1.55) |
| 40 to <45 kg/m2 | 56.3 | 36.62 (21.12) | 25.0 | 12.40 (24.02) | 0.0 | 7.56 (1.39) |
| 45 to <50 kg/m2 | 70.0 | 33.14 (22.66) | 60.0 | 18.73 (12.04) | 0.0 | 7.53 (1.85) |
| 50 to <55 kg/m2 | 100.0 | 31.00 (1.41) | 100.0 | 18.65 (7.14) | 0.0 | 8.10 (2.12) |
CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; SD = standard deviation; WBC = white blood cell.
Fig. 1.
Proportion of Patients with Elevated Serum Levels of CRP, ESR, and WBC as a Function of BMI. Linear regression models of prevalence of elevated inflammatory markers as a function of BMI categories.
3.3. Relationship between inflammatory biomarkers and BMI by healthy/comorbidities status
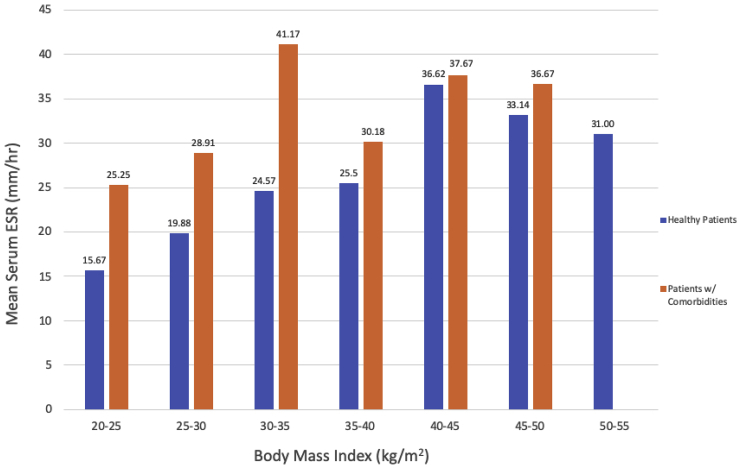
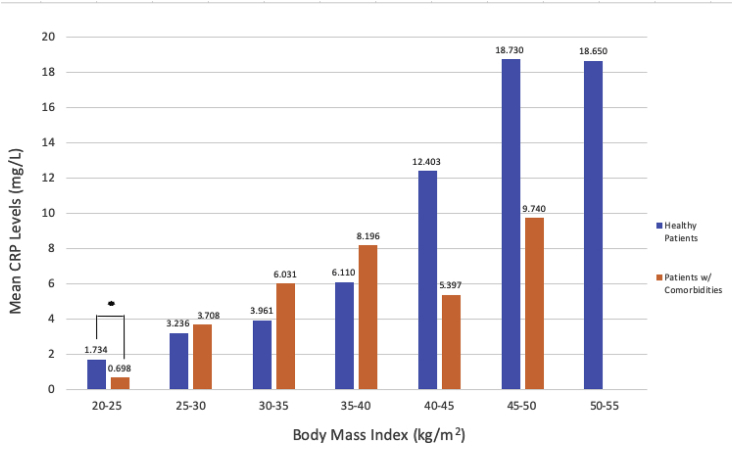
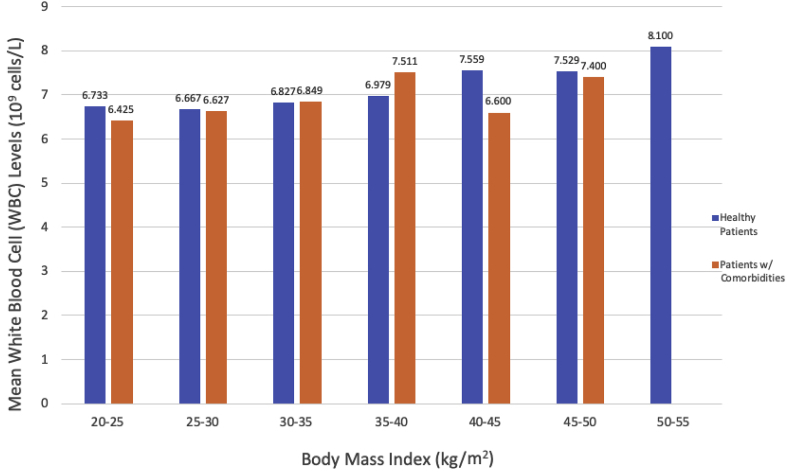
Fig. 2 shows the mean ESR within BMI categories by patient health status. There were no statistically significant differences between ESR values in “healthy patients” versus “patients with comorbidities” within any BMI category. Fig. 3 shows the mean CRP within BMI categories by patient health status. In patients of normal weight (BMI 20–25 kg/m2), “healthy patients” had a statistically significantly higher mean CRP level than “patients with comorbidities” (1.73 mg/L vs. 0.70 mg/L, P < 0.001). There were no other statistically significant differences in mean CRP levels by health status. There were no statistically significant differences in WBC count between “healthy patients” versus “patients with comorbidities” within any BMI category (Fig. 4).
Fig. 2.
Mean ESR in “Healthy Patients” Versus “Patients with Comorbidities” Within BMI Categories. Within each BMI category, mean ESR values for “healthy patients” versus “patients with comorbidities” were compared using two-sample t-tests or Fisher’s exact test (for cell sizes <5).
Fig. 3.
Mean CRP in “Healthy Patients’ Versus “Patients with Comorbidities” in “Healthy Patients” Versus “Patients with Comorbidities” Within BMI Categories. Within each BMI category, mean CRP values for “healthy patients” versus “patients with comorbidities” were compared using two-sample t-tests or Fisher’s exact test (for cell sizes <5).
Fig. 4.
Mean WBC Count in “Healthy Patients” Versus “Patients with Comorbidities” Within BMI Categories. Within each BMI category, mean CRP values for “healthy patients” versus “patients with comorbidities” were compared using two-sample t-tests or Fisher’s exact test (for cell sizes <5).
4. Discussion
Although there is currently no consensus on the use of CRP and ESR for preoperative evaluation of orthopedic patients, a number of studies suggest a link between elevated preoperative ESR and CRP and postoperative complications.12, 13, 14, 15 Risk stratification of TKA patients based on preoperative inflammatory biomarker levels may help reduce the risk of developing PJI and facilitate earlier diagnosis. In the present study, elevated ESR was most strongly associated with BMI after adjusting for age and sex. Elevated CRP was also positively but non-significantly correlated with BMI. These correlations were not mitigated by patients’ health status, suggesting that the relationship between ESR/CRP and BMI is independent of comorbidities known to increase these biomarkers. A recent study showed that obesity but not age or sex was a significant risk factor for PJI following TKA.27 Our data suggest that a potential mechanism by which obesity increases risk of infection following TKA is by increasing levels of circulating inflammatory biomarkers.
In the present study, the prevalence of elevated CRP and ESR was positively and linearly related to increasing BMI with the peak prevalence of elevated ESR/CRP in the highest BMI group (50 to <55 kg/m2). These results are consistent with the literature showing that excess adipose tissue and obesity fosters a pro-inflammatory state whereby activated macrophages and T-cells in visceral adipose tissue produce adipokines and pro-inflammatory cytokines.28, 29, 30, 31, 32, 33 As these chemical messengers activate inflammatory pathways, acute phase proteins, such as ESR and CRP, are expected to rise. The relationship between elevated inflammatory biomarkers and BMI did not appear to be affected by the presence of comorbidities known to increase levels of inflammatory biomarkers, suggesting that this is a strong and independent association.
Serum WBC count did not correlate with increasing BMI in this study. As the bone marrow’s response to a multitude of hematopoietic signals is a multifactorial process, it is possible that the study sample size failed to capture the underlying relationship between elevated WBC levels and higher BMI categories. It is also possible that the wide variation in basal leukocyte count in the human population reflects individual homeostatic states. Future studies analyzing the effect of increasing BMI on leukocytosis in individuals over time may show the same trends seen in this current study between increasing ESR/CRP and BMI. We also note that serum WBC count may not be the best biomarker to assess risk for PJI as synovial fluid rather than serum WBC count has been shown to the diagnostic accuracy of PJI.34,35
Weight is a modifiable risk factor that could be addressed prior to TKA to reduce the risk of PJI. The risk for PJI increases gradually throughout the full range of BMI and there is a 10% increased risk for PJI for each BMI unit above normal (25 kg/m2).36,37 It is not clear whether there is a distinct BMI threshold above which the risk for infection increases substantially.38 Aside from reducing the levels of inflammatory biomarkers through weight loss, patients with elevated biomarkers prior to surgery should be identified and carefully monitored to detect PJI early. Previous literature suggests that early identification of PJI in orthopedic procedures and intervention with aggressive antibiotic administration and debridement can lead to favorable results.39,40 Given the association between elevated preoperative inflammatory biomarkers and BMI found in this study, physicians should be cautious about interpreting postoperative elevated levels of inflammatory biomarkers in patients with a high BMI and take preoperative values into account to avoid false positive PJI diagnoses.
This study has several important limitations. The inherent limitations of a retrospective design preclude determinations of causality; prospective studies will be useful in further elucidating the relationship between preoperative BMI, elevated ESR and CRP, and risk for PJI in primary TKA patients. The “patients with comorbidities” subgroup was defined by patients’ history of conditions known to increase ESR and CRP; however, data were not available to assess the severity of these comorbidities. Because the study was conducted at a single center, results may not be widely generalizable; however, the proportion of females and prevalence of obesity in the study sample was consistent with previously reported literature.38 Patient race/ethnicity was not available for inclusion in the analyses. To our knowledge, race is not a known risk factor for PJI but is certainly associated with BMI.39 Although use of immunosuppressive and disease-modifying agents was not available for inclusion in the analyses, the presence of comorbidities often associated with these medications known to increase ESR and CRP were included in the “patients with comorbidities” subgroup. Finally, the number of BMI categories (7) resulted in small cell sizes for some comparisons and may have reduced our ability to detect statistically significant differences between subgroups within some BMI categories as well as differences in levels of inflammatory biomarkers between BMI categories.
In conclusion, the study finding that elevated preoperative inflammatory biomarkers are positively and linearly associated with BMI, while adjusting for age and sex, suggests that care should be taken when utilizing these postoperative biomarkers to diagnose PJI in TKA patients with high BMI. Postoperative biomarker levels should be interpreted in the context of both preoperative values and patient characteristics known to elevate ESR and CRP.
Funding
This research did not receive any specific grant funding agencies in the public, commercial, or not-for-profit sectors.
The research data analyzed in this manuscript was obtained after Institutional Review Board (IRB) approval.
Ethics committee author statement
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
CRediT authorship contribution statement
William V. Probasco: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Project administration. Charles Cefalu: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Project administration. Ryan Lee: Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Danny Lee: Investigation, Writing - original draft, Writing - review & editing. Alex Gu: Writing - original draft, Writing - review & editing. Vinod Dasa: Investigation, Writing - original draft, Writing - review & editing, Project administration, Supervision.
Declaration of competing interest
The authors report no conflict of interest or funding/payment of any kind for this study.
Contributor Information
William V. Probasco, Email: probasco24@gmail.com.
Danny Lee, Email: dannylee@gwu.edu.
References
- 1.Inacio M.C.S., Paxton E.W., Graves S.E., Namba R.S., Nemes S. Projected increase in total knee arthroplasty in the United States-an alternative projection model. Osteoarthritis Cartilage. 2017;25:1797–1803. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Singh J.A., Yu S., Chen L., Cleveland J.D. Rates of total joint replacement in the United States: future projections to 2020-2040 using the National Inpatient Sample. J Rheumatol. 2019 Apr 15 doi: 10.3899/jrheum.170990. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Belmont P.J., Jr., Goodman G.P., Waterman B.R., Bader J.O., Schoenfeld A.J. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patient. J Bone Joint Surg Am. 2014;96:20–26. doi: 10.2106/JBJS.M.00018. [DOI] [PubMed] [Google Scholar]
- 4.Redfern R.E., Cameron-Ruetz C., O’Drobinak S.K., Chen J.T., Beer K.J. Closed incision negative pressure therapy effects on postoperative infection and surgical site complication after total hip and knee arthroplasty. J Arthroplasty. 2017;32:3333–3339. doi: 10.1016/j.arth.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Bohl D.D., Shershon R.A., Fillingham Y.A., Della Valle C.J. Incidence, risk factors, and sources of sepsis following total joint arthroplasty. J Arthroplasty. 2016;31:2875–2879. doi: 10.1016/j.arth.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz S.M., Lau E., Schmier J., Ong K.L., Zhao K., Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Backe H., Wolff D., Windsor R. Total knee replacement infection alter 2-stage reimplantation. Clin Orthop Surg. 1996:125–131. doi: 10.1097/00003086-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Peel T.N., Buising K.L., Choong P.F. Prosthetic joint infection: challenges of diagnosis and treatment. ANZ J Surg. 2011;81:32–39. doi: 10.1111/j.1445-2197.2010.05541.x. [DOI] [PubMed] [Google Scholar]
- 9.Paxton E.W., Furnes O., Namba R.S., Inacio M.C., Fenstad A.M., Havelin L.I. Comparison of the Norwegian knee arthroplasty register and a United States arthroplasty registry. J Bone Joint Surg Am. 2011;93:20–30. doi: 10.2106/JBJS.K.01045. [DOI] [PubMed] [Google Scholar]
- 10.Lavernia C., Lee D.J., Hernandez V.H. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446:221–226. doi: 10.1097/01.blo.0000214424.67453.9a. [DOI] [PubMed] [Google Scholar]
- 11.Staats K., Merle C., Schmidt-Braekling T., Boettner F., Windhager R., Waldstein W. Is the revision of a primary TKA really as easy and safe as the revision of a primary UKA? Ann Transl Med. 2016;4(24):532. doi: 10.21037/atm.2016.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu C., Guo H., Qu P., Fu J., Kuo F.C., Chen J.Y. Preoperatively elevated serum inflammatory markers increase the risk of periprosthetic joint infection following total knee arthroplasty in patients with osteoarthritis. Therapeut Clin Risk Manag. 2018;14:1719–1724. doi: 10.2147/TCRM.S175854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S., Paul S., Bhattacharjee D.P., Ghosh P., Chatterjee N. Prognostic value of baseline high-sensitivity C-reactive protein in patients undergoing replacement arthroplasty. JNMA J Nepal Med Assoc. 2009;48(174):144–148. [PubMed] [Google Scholar]
- 14.Ackland G.L., Scollay J.M., Parks R.W., de Beaux I., Mythen M.G. Pre-operative high sensitivity C-reactive protein and postoperative outcome in patients undergoing elective orthopaedic surgery. Anaesthesia. 2007;62(9):888–894. doi: 10.1111/j.1365-2044.2007.05176.x. [DOI] [PubMed] [Google Scholar]
- 15.Pfitzner T., Krocker D., Perka C., Matziolis G. [C-reactive protein. An independent risk factor for the development of infection after primary arthroplasty] Orthopä. 2008;37(11):1116–1120. doi: 10.1007/s00132-008-1342-1. [DOI] [PubMed] [Google Scholar]
- 16.Godoy G., Sumarriva G., Ochsner J.L., Jr. Preoperative acute inflammatory markers as predictors for postoperative complications in primary total knee arthroplasty. Ochsner J. 2016;16(4):481–485. [PMC free article] [PubMed] [Google Scholar]
- 17.Siemons L., Ten Klooster P.M., Vonkeman H.E., van Riel P.L., Glas C.A., van de Laar M.A. How age and sex affect the erythrocyte sedimentation rate and C-reactive protein in early rheumatoid arthritis. BMC Muscoskel Disord. 2014;15:368. doi: 10.1186/1471-2474-15-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels L.M., Tosh P.K., Fiala J.A., Schleck C.D., Mandrekar J.N., Beckman T.J. Extremely elevated erythrocyte sedimentation rates: associations with patients’ diagnoses, demographic characteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636–1643. doi: 10.1016/j.mayocp.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Timpson N.J., Nordestgaard B.G., Harbord R.M. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes. 2011;35(2):300–308. doi: 10.1038/ijo.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piéroni L., Bastard J.P., Piton A., Khalil L., Hainque B., Jardel C. Interpretation of circulating C-reactive protein levels in adults: body mass index and gender are a must. Diabetes Metab. 2003;29(2 Pt 1):133–138. doi: 10.1016/s1262-3636(07)70019-8. [DOI] [PubMed] [Google Scholar]
- 21.Alende-Castro V., Alonso-Sampedro M., Vazquez-Temprano N. Factors influencing erythrocyte sedimentation rate in adults: new evidence for an old test. Medicine (Baltim) 2019 Aug;98(34) doi: 10.1097/MD.0000000000016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assasi N., Blackhouse G., Campbell K. CADTH Health Technology Assessments; 2015 Nov. Comparative Value of Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) Testing in Combination versus Individually for the Diagnosis of Undifferentiated Patients with Suspected Inflammatory Disease or Serious Infection: A Systematic Review and Economic Analysis. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. [PubMed] [Google Scholar]
- 23.Haldeman-Englert C., Taylor W. “Erythrocyte sedimentation rate.” erythrocyte sedimentation rate- health encyclopedia - university of rochester medical center. https://www.urmc.rochester.edu/encyclopedia/erythrocyte_sedimentation_rate Last Accessed.
- 24.Chernecky C.C., Berger B.J. Differential leukocyte count (Diff) - peripheral blood. In: Chernecky C.C., Berger B.J., editors. Laboratory Tests and Diagnostic Procedures. sixth ed. Elsevier Saunders; St Louis, MO: 2013. pp. 441–450. [Google Scholar]
- 25.Vajpayee N., Graham S.S., Bem S. Basic examination of blood and bone marrow. In: McPherson R.A., Pincus M.R., editors. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 23rd ed. Elsevier; St Louis, MO: 2017. chap. 30. [Google Scholar]
- 26.C-reactive Protein, High Sensitivity, Serum https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/82047 Mayo Medical Laboratories.
- 27.Blanco J.F., Díaz A., Melchor F.R., da Casa C., Pescador D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(2):239–245. doi: 10.1007/s00402-019-03304-6. [DOI] [PubMed] [Google Scholar]
- 28.Westberg M., Grogaard B., Snorrason F. Early prosthetic joint infections treated with debridement and implant retention. Acta Orthop. 2012;83:227–232. doi: 10.3109/17453674.2012.678801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flegal K.M., Carroll M.D., Ogden C.L., Johnson C.L. Prevalence and trends in obesity among US adults, 1999-2000. J Am Med Assoc. 2002;288:1723. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira-Santos J., Santos R., Moreira C. Ability of measures of adiposity in identifying adverse levels of inflammatory and metabolic markers in adolescents. Child Obes. 2016;12:135–143. doi: 10.1089/chi.2015.0124. [DOI] [PubMed] [Google Scholar]
- 31.Kang Y.E., Kim J.M., Joung K.H. The role of adipokines, proinflammatory cytokines, and adipose tissue machrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PloS One. 2016;11 doi: 10.1371/journal.pone.0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt F.M., Weschenfelder J., Sander C., Minkwitz J. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PloS One. 2015;10 doi: 10.1371/journal.pone.0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro R., Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat Inflamm. 2010;2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon Y.M., Antoci V., Jr., Leone W.A., Tsai T.Y., Dimitriou D., Liow M.H. Utility of serum inflammatory and synovial fluid counts in the diagnosis of infection in taper corrosion of dual taper modular stems. J Arthroplasty. 2016;31(9):1997–2003. doi: 10.1016/j.arth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Zmistowski B., Restrepo C., Huang R., Hozack W.J., Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589–1593. doi: 10.1016/j.arth.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 36.Keeney B, Austin D, Jevsevar DS. How Much Preoperative Weight Do Morbidly Obese Patients Undergoing Total Knee Arthroplasty Need to Lose to Meaningfully Improve Outcomes? (Poster P0090) Presented at the 2019 Annual Meeting of the American Academy of Orthopaedic Surgeons, March 12-16, Las Vegas, Nevada.
- 37.Electricwala A.J., Jethanandani R.G., Narkbunnam R. Elevated body mass index is associated with early total knee revision for infection. J Arthroplasty. 2017;32(1):252–255. doi: 10.1016/j.arth.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 38.Herishanu Y., Rogowski O., Polliack A., Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76:516–520. doi: 10.1111/j.1600-0609.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 39.a) Heymsfield S.B., Peterson C.M., Thomas D.M., Heo M., Schuna J.M., Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17:262–275. doi: 10.1111/obr.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Issa K., Banerjee S., Kester M.A., Khanuja H.S., Delanois R.E., Mont M.A. The effect of timing of manipulation under anesthesia to improve range of motion and functional outcomes following total knee arthroplasty. J Bone Joint Surg Am. 2014;96:1349–1357. doi: 10.2106/JBJS.M.00899. [DOI] [PubMed] [Google Scholar]
- 40.American Academy of Orthopedic Surgeons The diagnosis of periprosthetic joint infections of the hip and knee guideline and evidence report. https://www.aaos.org/research/guidelines/PJIguideline.pdf Available at: [DOI] [PubMed]