Abstract
Legume grains such as field peas and field beans can be produced on a local level, and may be reliable sources of dietary protein and energy apart from common soybean and rapeseed meals. In ruminants, protein, starch, and carbohydrates from peas and field beans are fermented in large part before reaching the small intestine. The objective of this study was to evaluate the effects of a combination of ensiling and hydro-thermic treatment (i.e., toasting at 160 °C for 30 min) of grains of peas and field beans on the concentrations of post-ruminal crude protein (PRCP) and rumen-undegraded protein (RUP). Moreover, 24-h gas production and methane production were measured. For this, an in vitro batch culture system with ruminal fluid from sheep was used. Rumen-undegraded protein was determined using the Streptomyces griseus protease test. Scanning electron micrographs were used to visualize morphological changes of starch granules and their joint matrices in peas and field beans after ensiling, toasting, or a combination of both. Native pea grains contained crude protein (CP) at 199 g/kg DM, PRCP at 155 g/kg DM at a ruminal passage rate of 0.08/h (Kp8), RUP at 33 g/kg DM at Kp8, and starch at 530 g/kg DM. Native field beans contained CP at 296 g/kg DM, PRCP at 212 g/kg DM at Kp8, RUP at 54 g of/kg DM at Kp8, and starch at 450 g/kg DM. The PRCP did not considerably differ among native and treated peas or field beans. Especially in the peas, RUP at Kp8 increased after ensiling by 10 g/kg DM (i.e., 30%; P < 0.05). Toasting increased RUP (Kp8) in ensiled peas by another 28% (P < 0.05). Toasting had no effect on PRCP or RUP when the peas or field beans were not ensiled before. Gas and methane production were not affected by any treatment, and scanning electron micrographs did not reveal structural changes on the starches doubtless of any treatment. Protein seemed to be more affected by treatment with ensiled + toasted peas than with ensiled + toasted field beans, but starches and other carbohydrates from both legumes remained unaffected.
Keywords: Legume grain silage, Toasting, In vitro gas production, Protein evaluation, Streptomyces griseus protease test
1. Introduction
The demand for avoiding use of genetically modified (GM) foods is increasing in societies worldwide. Hence, also feed production without using GM crops is in a particular public and political spotlight. In the livestock sector, soybean meal (SBM) and rapeseed meal are the most intensively used protein feeds, whereas SBM is originating nearly completely from GM sources (ISAAA, 2016). In the European Union (EU-28), about 95% of utilized soybeans are imported (Tillie and Rodríguez-Crezo, 2015). The cultivation of non-GM soybeans in Europe, e.g., in the Danubian region, will not be able to significantly reduce the imports of GM-SBM anytime soon (Weber, 2017). This has led to national programs searching for alternative protein sources for animal feeds, which can be insects (Makkar et al., 2014, Neumann et al., 2017, Cutrignelli et al., 2018), microalgae (Gatrell et al., 2014, Neumann et al., 2017), or, in a ruminants’ nutrition spotlight, indigenous legume grains such as lupines, field peas, and field beans (Valencia et al., 2008, Tufarelli et al., 2012). The use of peas and field beans also supports feed production and nutrient cycles on a local level. Field peas and field beans are currently restricted by availability. In EU-28, only about 2% of arable land is used for the cultivation of dry pulses in general (De Cicco, 2017).
In dairy cows, at least a partial substitution of SBM or rapeseed meal, and cereal grains by peas and field beans is possible without health risks or performance depression (Corbett et al., 1995, Kuhnitzsch et al., 2019a). Field peas and field beans have lower protein but higher starch concentrations than SBM (Masoero et al., 2005). Especially the protein of these legumes is readily degradable in the rumen (Yu et al., 2002, Vaga et al., 2017). Increasing the amounts of rumen-undegraded protein (RUP), while maintaining amino acid availability, and of resistant starch through heat and pressure treatments thus might be a strategy to improve their nutritional value for dairy cows and ruminants per se (Goelema et al., 1998, Yu et al., 2002, Masoero et al., 2005, Vaga et al., 2017). Legume grains may contain relevant quantities of bioactive compounds such as saponins, tannins, vicin, convicin, or trypsin inhibitors, which mostly evolve anti-nutritional properties (Gdala and Buraczewska, 1997, Krupa, 2008, Szumacher-Strabel et al., 2019) and affect microbial communities in the rumen. Ensiling and further thermic processing may decrease such compounds, which has been shown e.g. with trypsin inhibitors or tannins in peas and field beans (Adamidou et al., 2011, Bachmann et al., 2019, Gefrom et al., 2013). However, ensiling may also increase the concentration of anti-nutritional compounds as with saponins, which is associated with reduction of protein concentration and quality losses (Szumacher-Strabel et al., 2019).
We hypothesized that toasting (hydro-thermic treatment without adding of liquids) of native or ensiled peas and field beans 1) increases concentrations of RUP and post-ruminal crude protein (PRCP), which is the sum of RUP from the feed and microbial protein produced in the rumen; and 2) improves resistance of starch to ruminal degradation, which is reflected by increased gas production and morphological changes of starch granules and their joint matrices.
2. Materials and methods
2.1. Donor animals
The animals used in this experiment were kept and cared for by the Research Centre for Agricultural and Nutritional Sciences, Martin Luther University Halle-Wittenberg, Merbitz, Wettin/Löbejün (Saxony-Anhalt, Germany) and used with approval by Saxony-Anhalt Federal Administration Authority (approval no. 203.k-42502-3-657MLUMerbitz).
For the current batch culture experiment, ruminal fluid was obtained from 4 rumen cannulated Suffolk or Pomeranian coarsewool wethers. The animals were 6 years of age. They had free access to tap water. Meadow haylage (analyzed composition: DM, 807 g/kg; crude ash (CA), 60 g/kg DM, crude protein (CP), 54 g/kg DM; acid ether extract (AEE), 10 g/kg DM; neutral detergent fiber; 630 g/kg DM; acid detergent fiber, 333 g/kg DM) was offered ad libitum and supplemented with 200 g/d per animal of a pelleted concentrate (IBEKA PANTO Schäferstolz, HL Hamburger Leistungsfutter GmbH, Hamburg, Germany; 3-mm pellet size; analyzed composition: 897 g DM/kg, CA, 80 g/kg DM; CP, 177 g/kg DM; AEE, 24 g/kg DM, neutral detergent fiber, 234 g/kg DM; acid detergent fiber, 106 g/kg DM) and 10 g/d per animal of a mineral feed (basu-kraft Top-Mineral, BASU Heimtierspezialitäten GmbH, Bad Sulza, Germany).
2.2. Substrates and treatments
As test substrates, the field pea cultivar Alvesta (KWS SAAT SE, Einbeck, Germany) and the field bean cultivar Taifun (SAATEN-UNION GmbH, Isernhagen, Germany) were used. The cultivars were grown and harvested in 2016. From the native material, model silages (Rostock Model Silages) were prepared according to Hoedtke and Zeyner (2011) after the material was re-moistened to approximately 71% DM. The ground grains were vacuum-sealed in double-layered polyethylene bags with punctual perforation of the inner layer for gas release, and stored at approximately 25 °C for 60 d. A lactic acid bacteria preparation that included Lactobacillus plantarum and Pediococcus acidilactici strains, and enzymes (together 6.8 × 106 colony forming units per gram of fresh matter; Josilac classic; Josera GmbH & Co. KG, Kleinheubach, Germany) was used as an inoculant. It was applied as recommended with 6 × 10−6 g/g, i.e., 6 g/t of fresh matter. The fermentation parameters are summarized in Table 1. Organic acids and ethanol, which were produced during the fermentation, were determined by high performance liquid chromatography (HPLC) and refractive index detection (method no. LKS FMUAA 1662018-05) using a Shimadzu LC-20A Prominence (Shimadzu Corp., Kyoto, Japan) and a Hi-Plex H 8-μm column (300 mm × 7.7 mm; Agilent Technologies Inc., Santa Clara, CA, USA). The method was accredited according to DIN EN ISO/IEC 17025:2005. The aerobic stability was tested following the procedure of Honig (1990). Native and ensiled field pea and field bean grains were subsequently toasted, simulated in a drying oven at 160 °C for 30 min with 1-kg material each (i.e., 2-cm layer height in a 21 cm × 33 cm foil carton). The analyzed chemical composition of field pea and field bean treatments is shown in Table 2, Table 3, Table 4. Additionally, maize starch and a cellulose preparation (Vitacel R200, J. Rettenmaier & Söhne GmbH & Co. KG, Rösenberg, Germany) were used as standardized substrates, which mark high and low borders of gas production capacity and enable classification of gas production from the test substrates.
Table 1.
Fermentation characteristics of the model silages.
| Item | DMR1, % | pH1 | pH2 | LA, g/kg DM | EA, g/kg DM | BA, g/kg DM | Ethanol, g/kg DM | AS3, d |
|---|---|---|---|---|---|---|---|---|
| Ensiled field peas | 2.8 | 4.6 | 4.6 | 18.8 | 1.7 | n.d. | 2.6 | ≥7 |
| Ensiled field beans | 0.0 | 4.3 | 4.4 | 15.3 | 2.2 | n.d. | 1.6 | ≥7 |
DMR = DM reduction; LA = lactic acid; EA = ethanoic acid; BA = butyric acid; AS = aerobic stability; n.d. = not detected.
After ensiling.
After 7 d of aerobic storage.
The AS is given in days until the temperature difference between material and environment exceeds 3 °C.
Table 2.
Chemical composition of the test substrates (g/kg DM).
| Item | DM, g/kg | CA | CP | AEE | Starch | DSB, % | Sugars | CF | aNDFom | ADFom | ADL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Native field peas | 873 | 32 | 199 | 14 | 530 | <5 | 45 | 80 | 195 | 109 | 7 |
| Ensiled field peas | 705 | 35 | 211 | 14 | 572 | <5 | 47 | 62 | 290 | 82 | 5 |
| Toasted field peas | 901 | 34 | 241 | 16 | 513 | <5 | 45 | 60 | 125 | 93 | 11 |
| Ensiled + toasted field peas | 723 | 37 | 214 | 15 | 565 | <5 | 46 | 60 | 96 | 85 | 5 |
| Native field beans | 914 | 40 | 296 | 19 | 450 | <5 | 21 | 77 | 156 | 106 | 9 |
| Ensiled field beans | 713 | 44 | 291 | 17 | 461 | <5 | 17 | 95 | 138 | 124 | 10 |
| Toasted field beans | 911 | 42 | 295 | 16 | 445 | <5 | 28 | 71 | 192 | 99 | 10 |
| Ensiled + toasted field beans | 731 | 42 | 287 | 15 | 449 | <5 | 10 | 92 | 154 | 129 | 9 |
CA = crude ash; CP = crude protein; AEE = acid ether extract; DSB = degree of starch breakdown; CF = crude fiber; aNDFom = neutral detergent fiber assayed with heat stable amylase and expressed exclusive of residual ash; ADFom = acid detergent fiber expressed exclusive of residual ash; ADL = acid detergent lignin.
Table 3.
Crude protein composition of the test substrates.
| Item | CP, g/kg DM | TP, g/kg DM | Soluble protein1, % of CP |
Fractions2, % of CP |
CPip, % of CP | NH3–N, % of total N | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B1 | B2 | B3 | C | ||||||
| Native field peas | 199 | 184 | 77 | 7.4 | 69.7 | 20.6 | 1.6 | 0.7 | 5.0 | 0.47 |
| Ensiled field peas | 211 | 188 | 64 | 11.0 | 53.1 | 27.1 | 8.5 | 0.3 | 4.4 | 0.60 |
| Toasted field peas | 241 | 191 | 79 | 20.9 | 57.6 | 19.6 | 0.9 | 0.9 | 4.3 | 0.11 |
| Ensiled + toasted field peas | 214 | 168 | 61 | 21.4 | 40.0 | 37.0 | 1.2 | 0.5 | 4.7 | 0.65 |
| Native field beans | 296 | 242 | 67 | 18.3 | 49.0 | 30.8 | 1.0 | 0.9 | 5.9 | 0.49 |
| Ensiled field beans | 291 | 239 | 68 | 18.0 | 50.0 | 31.0 | 1.0 | 0.8 | 6.9 | 0.73 |
| Toasted field beans | 295 | 251 | 74 | 14.8 | 59.1 | 24.1 | 0.6 | 1.4 | 4.5 | 0.27 |
| Ensiled + toasted field beans | 287 | 236 | 65 | 17.8 | 47.5 | 31.3 | 2.6 | 0.8 | 5.9 | 0.66 |
CP = crude protein; TP = true protein; CPip = crude protein insoluble in pepsin.
Soluble protein = Fraction A + Fraction B1.
Fraction A, non-protein nitrogen; fraction B1, buffer-soluble TP; fraction B2, buffer-insoluble TP minus TP insoluble in neutral detergent; fraction B3, TP insoluble in neutral detergent, but soluble in acid detergent; fraction C, TP insoluble in acid detergent.
Table 4.
Amino acid composition of the test substrates (g/kg DM).
| Item | Essential amino acids |
Non-essential amino acids |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg | His | Ile | Leu | Lys | Met | Phe | Thr | Trp | Val | Ala | Asp | Cys | Glu | Gly | Pro | Ser | Tyr | |
| Native field peas | 14.7 | 5.0 | 8.4 | 14.4 | 15.0 | 1.8 | 9.8 | 7.7 | 1.9 | 9.3 | 8.7 | 23.0 | 2.9 | 33.7 | 8.7 | 7.5 | 9.6 | 7.2 |
| Ensiled field peas | 14.4 | 5.2 | 8.9 | 15.1 | 14.5 | 1.8 | 10.2 | 7.8 | 1.9 | 9.7 | 9.4 | 23.6 | 2.7 | 33.5 | 9.1 | 8.3 | 9.8 | 7.8 |
| Toasted field peas | 15.9 | 5.3 | 9.1 | 15.3 | 16.0 | 1.9 | 10.5 | 8.1 | 2.0 | 10.0 | 9.3 | 23.6 | 3.0 | 36.2 | 9.2 | 8.3 | 10.0 | 7.7 |
| Ensiled + toasted field peas | 14.5 | 5.2 | 9.2 | 15.3 | 14.4 | 1.9 | 10.4 | 7.8 | 1.9 | 10.0 | 9.5 | 23.9 | 2.7 | 33.8 | 9.2 | 8.3 | 9.7 | 7.6 |
| Native field beans | 24.6 | 7.0 | 10.6 | 19.0 | 18.9 | 1.9 | 11.0 | 9.5 | 2.6 | 11.9 | 11.0 | 28.5 | 3.2 | 45.0 | 11.3 | 10.6 | 12.6 | 9.3 |
| Ensiled field beans | 24.2 | 7.1 | 10.5 | 19.1 | 15.6 | 1.9 | 11.1 | 9.6 | 2.5 | 11.8 | 11.2 | 28.1 | 3.0 | 44.7 | 11.6 | 10.8 | 12.8 | 9.5 |
| Toasted field beans | 24.7 | 7.3 | 11.0 | 19.3 | 17.3 | 2.0 | 11.3 | 9.7 | 2.7 | 12.4 | 11.4 | 29.0 | 3.3 | 45.9 | 11.6 | 11.2 | 12.7 | 9.7 |
| Ensiled + toasted field beans | 23.9 | 7.0 | 10.5 | 18.9 | 15.6 | 1.9 | 11.0 | 9.5 | 2.5 | 11.7 | 11.1 | 28.0 | 2.9 | 44.0 | 11.5 | 10.8 | 12.6 | 9.3 |
Arg = arginine; His = histidine; Ile = isoleucine; Leu = leucine; Lys = lysine; Met = methionine; Phe = phenylalanine; Thr = threonine; Trp = tryptophan; Val = valine; Ala = alanine; Asp = aspartic acid; Cys = cysteine; Glu = glutamic acid; Gly = glycine; Pro = proline; Ser = serine; Tyr = tyrosine.
2.3. In vitro incubation procedure
In vitro incubations were carried out using the ANKOM RF Gas Production System (ANKOM Technology, Macedon, NY, USA) and the Streptomyces griseus protease test, respectively. The study was conducted in January 2018 and was constructed as follows: 6 consecutive runs for ANKOM RF incubations; 4 consecutive runs for the S. griseus protease test; 2 measuring times within each run (at 8 and 24 h of incubation); 2 legume species (field peas and field beans); 4 treatments per legume species (native, ensiled, toasted, ensiled + toasted); 1 fermentation vessel per substrate/treatment and measuring time within each run; 3 blanks per measuring time (i.e., 6 blanks within each run; only with ANKOM RF incubations); 2 starch, 2 cellulose, and 2 yeast standards within each run (only with ANKOM RF incubations).
This led to maximal 6 real replicates per substrate/treatment and measuring time with the ANKOM RF incubations and maximal 4 real replicates with the S. griseus protease test.
The in vitro batch culture experiment followed the protocols given by Menke et al., 1979, Menke and Steingass, 1988, and the Association of German Agricultural Analytic and Research Institutes (VDLUFA, 2012; method no. 25.1). The aim was to reduce the variation of measurements through the standardization of donor animal feeding, time of sampling of ruminal fluid, preparation of buffer and inoculum, and the basic conditions for batch culture fermentation. Ruminal fluid was taken from 2 of the 4 wethers immediately before feeding and at about 1.5 h prior to each trial. The animals were randomly selected for taking rumen fluid. The mixed fluid was filtered through 2 layers of cheesecloth and stored in a thermos bottle during transport to the laboratory. To the buffer, NH4HCO3 was added by 2 g/L and NaHCO3 was reduced by 2 g/L to avoid that nitrogen availability will limit microbial biomass production (Edmunds et al., 2012a). The ruminal fluid had a pH of 6.8 ± 0.093, a redox potential of −327 ± 13.2 mV, and a temperature of 30 ± 1.5 °C when arriving the laboratory. The inoculum was prepared by mixing 2 parts of the buffer (nutrient solution) and one part of ruminal fluid under stirring and continuous CO2 flush to ensure an anaerobic environment. The inoculum had a pH of 6.8 ± 0.018 and a redox potential of −294 ± 42.3 mV. Totally, 0.2 g of each substrate, pulverized using a ball mill (Retsch MM 400, Retsch GmbH, Haan, Germany), was weighed into the fermentation vessels and 30 mL of inoculum were added, respectively, using an automated pump. For in vitro incubation in the ANKOM RF Gas Production System, glass bottles with an actual volume capacity of 136 ± 2.68 mL (i.e., approximately 106 mL headspace volume) were used, and each was capped with a gas pressure measuring module. The bottles were randomly distributed to 2 identical shaking water baths having 80 r/min agitation and consistently 39 °C. To purge out oxygen, each bottle was vented with argon through the module's Luer port until the inner pressure exceeded 55 kPa. Then, all gases were automatically released from the bottles.
2.3.1. Post-ruminal crude protein
After 8 and 24 h, samples from the non-gaseous phase (sample solution) of the fermenter vessels were taken for ammonia nitrogen (NH3–N) analysis, immediately placed in ice water (approximately 0 °C) to stop microbial activity, and then the entire contents were rinsed into distillation tubes (with 2 × 30 mL of distilled water). To lightly alkalize the sampled solution (pH 7 to 10), 4 mL of NaOH (1 mol/L) were added immediately prior to NH3–N analysis. This led to a transfer of ammonium ions into ammonia (NH3), but prevented a surplus of lye, which would have forced protein hydrolysis. NH3–N distillation and titration were performed using a FOSS 2300 Kjeltec Analyser Unit (FOSS GmbH, Rellingen, Germany) without any further addition of lye and water.
The PRCP was calculated (considering a sample weight of 200 mg per fermenter) as follows:
where NH3-Nblank is NH3-N measured in blanks (mg); Nfeed is the amount of nitrogen in 200 mg of sample (feed), i.e., 200 mg × DM of sample (%) × CP of sample (%)/6.25/10,000; NH3-Nsample is NH3‐N measured in the sampled solution (containing nitrogen from feed and microbial nitrogen) after 8 or 24 h of incubation (mg); and DMfeed is the DM content of the substrate (%).
2.3.2. Rumen-undegraded protein
Contents of RUP were estimated using the enzymatic in vitro test proposed by Licitra et al. (1998). In brief, 0.5 g of a certain substrate (i.e., treatments of peas and field beans) was weighed into 136-mL glass bottles, 40 mL of borate-phosphate buffer (i.e., NaH2PO4·H2O at 12.20 g/L + Na2B4O7·10H2O at 8.91 g/L; pH 6.7 to 6.8) were added, and the solution was incubated 1 h at 39 °C in a shaking water bath (80 r/min). A protease solution with an activity of 0.58 U/mL was made following Licitra et al. (1999), for which we used the nonspecific type XIV S. griseus protease (Sigma–Aldrich Chemie GmbH, Munich, Germany; ≥ 3.5 U/mg). This preparation involves caseinolytic activities as well as an aminopeptidase activity (Jurášek et al., 1971). According to the manufacturer, one unit is defined to hydrolyze casein producing color equivalent to 1.0 μmol (i.e., 181 μg) of tyrosine per min at pH 7.5 and 37 °C (color by Folin-Ciocalteu reagent). After 1 h, the protease solution was added to the bottles. The required amount of protease solution was calculated on the basis of true protein (TP) contents for each substrate relative to a soybean standard with 49.3% TP that requires 10 mL of the solution (Licitra et al., 1998). This equals to a ratio of 24 U to 1 g of TP. The TP contents were calculated on the basis of protein fractions, i.e., TP = CP − Non-protein nitrogen (A) (Licitra et al., 1996). After 8 and 24 h, the incubation was stopped, the bottles’ contents were filtered through Whatman #41 filter circles, and each was washed with 250 mL of distilled water. The filters were air-dried, and nitrogen was determined in residues and blank filters using the FOSS 2300 Kjeltec Analyser. The proper incubation times were evaluated in a pre-test, in which the test substrate, a pea grain silage (DM = 705 g/kg, CP = 211 g/kg DM, and TP = 188 g/kg DM) was incubated in triplicate (using 3.81 mL of protease solution with an activity of 0.58 U/mL), and samples were taken after 0 (before enzyme was added), 0.5, 1, 2, 4, 6, 8 and 24 h of the incubation. Enzymatic protein digestion and nitrogen dissolution was plotted over time (Fig. 1), and the differences between RUP contents at minimal (0.5, 1, 2, 4, 6, and 8 h) and maximal incubation times (24 h) on a log time (ln[t]) scale were analyzed using linear regression (Fig. 2). The 24 h maximal incubation time was set following Edmunds et al. (2012b). After 1 h, already, the CP content (211 g/kg DM) was reduced by approximately 70% as soluble parts were readily dissolved in the buffer. Then, ongoing dissolution catalyzed by added protease led to another 13% reduction (RUP = 37 g/kg DM after 24 h) in a curvilinear manner (Fig. 1). Although most protease impact was until 4 h after addition, the 8 to 24 h slope was the steepest in linear regression analysis when plotted against an ln time scale (Fig. 2). However, this procedure did not consider probable curvilinear protein degradation by S. griseus protease.
Fig. 1.
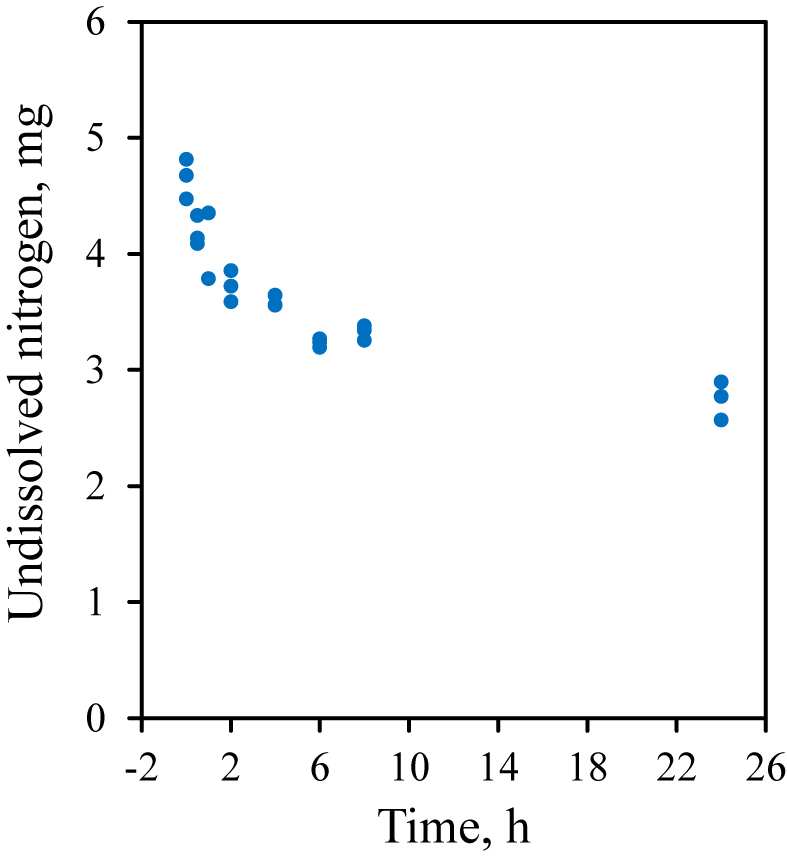
Raw data plot of protein degradation/nitrogen dissolution kinetics of Streptomyces griseus protease (0.58 U/mL) on a field pea grain silage (DM = 705 g/kg, crude protein = 211 g/kg DM, true protein = 188 g/kg DM) within 24 h of incubation.
Fig. 2.
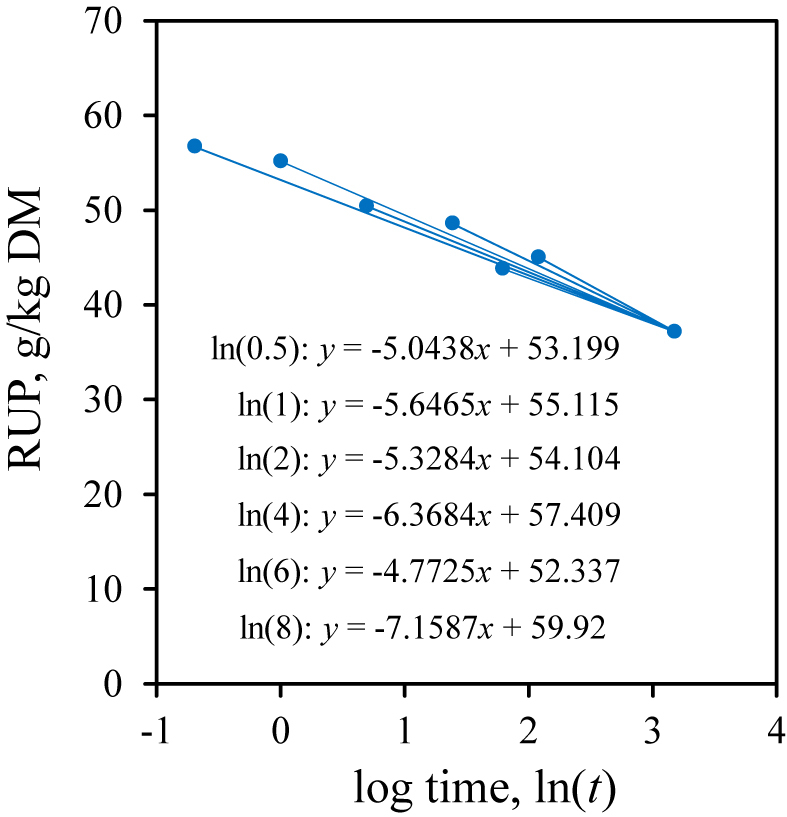
Linear regression between minimal (0.5, 1, 2, 4, 6, and 8 h) and maximal incubation times (24 h) on a log time scale (ln[t]; t = incubation time) to determine rumen-undegraded protein (RUP) in vitro using Streptomyces griseus protease (0.58 U/mL). A field pea grain silage was used as example substrate (DM = 705 g/kg, crude protein = 211 g/kg DM, true protein = 188 g/kg DM).
The RUP content of the field pea and field bean treatments was calculated as follows (considering a sample weight of 0.5 g of each treatment):
where Nresidue is nitrogen measured in the filter residues (mg); Nblank is mean nitrogen measured in the blank filters (mg); and DMfeed is the DM content of substrate (%).
2.3.3. Effective PRCP and effective RUP
Effective PRCP and effective RUP were estimated according to Edmunds et al. (2012a). In brief, for each trial, PRCP and RUP were plotted against an ln(t) scale (t = 8 and 24 h). Intercept (y) and slope (a) of the resulting regression equations were used to calculate effective PRCP and effective RUP for assumed rumen passage rates (Kp) of 0.02, 0.04, 0.06, 0.08, and 0.12/h as follows:
2.3.4. Total gas production and gas production kinetics
The settings for gas production measurements were as follows: 1-min recording interval; a threshold of 10 kPa for the automatic release of accumulated gases to prevent supersaturation in the medium at high gas pressures (Tagliapietra et al., 2010); a 150-ms valve open time; and an agitation interval of 80 r/min in the water baths. The cumulative gas pressures were automatically recorded in real-time. Afterwards, the cumulative gas pressures from 0 to 24 h of incubation were applied to a blank correction (using the mean gas production in blanks) and converted first to moles of gas produced using the Ideal Gas Law and then to milliliters of gas produced using Avogadro's Law. The correct function of the system was verified according to the manufacturer's instructions using a yeast standard (Saccharomyces cerevisiae) that was run in replicate during each trial. The recorded gas pressure kinetics of each yeast unit was compared to that given by the manufacturer and no salient differences were observed.
A non-linear regression analysis was performed upon gas production kinetics in SAS MODEL using the Gompertz function (France et al., 2000):
where y(t) is gas production (mL/200 mg DM) at time t; a is the asymptotic maximal gas production; b is the time t (h) until which approximately one-third of a is produced; and c is the time t (h) until which another one-third of a is produced, i.e., b + c = 70% of a.
2.3.5. Methane production
After 8 and 24 h, gas from the bottles' headspace was sampled through the modules’ vent valve using an adapter connected to a gas-proof syringe (SGE Analytical Science, Trajan Scientific and Medical, Ringwood, Australia; 2.5 mL). Briefly, a vacuum was created in the syringe, the module was activated manually, and gas flowed into the syringe. At least 2-mL gas was collected per sample, and methane was analyzed by gas chromatography. A Shimadzu GC 2010 Plus (Shimadzu Corp., Kyoto, Japan) was used, fitted with a 250-μL upstream gas loop, a ShinCarbon micropacked column (Restek Corp., Bellefonte, PA, USA; 2 m × 0.53 mm [inner diameter], 80/100 mesh size), and a flame ionization detector. Nitrogen was used as carrier gas and makeup gas (20.39 mL/min [column flow]; 300 kPa [pressure at the column]). The injection and column temperature was 45 °C and a split of 13.6 was used. Detection was performed at 150 °C. The resulting peak areas were corrected for those detected for blank samples using a mean of 2 blanks per run.
2.4. Scanning electron microscopic imaging
Scanning electron micrographs (SEM) of starch granules and their embedding, covering, or surrounding matrix structures were recorded under vacuum using a JEOL 640 SEM (JEOL Ltd., Tokyo, Japan) to visualize morphological changes that may follow ensiling and toasting of field peas and field beans. Prior to SEM, the pea and field bean grains of the different treatments were oven-dried at 40 °C, ground to an approximately 1 mm sieve size, spread out on a microscope slide, and sputter coated with gold.
2.5. Additional chemical analyses
The DM, CA, CP, AEE, crude fiber, sugar, and the Van Soest detergent fiber contents of the test substrates were analyzed according to the official German key book for feed analysis (VDLUFA, 2012; methods no. 3.1, 4.1.1, 5.1.1 B, 6.1.1, 6.5.1, 6.5.2, 6.5.3, 7.1.1 and 8.1). Neutral detergent fiber was determined after 1 h treatment with a heat stable amylase, added to the neutral detergent solution. Neutral detergent fiber and acid detergent fiber were expressed excluding residual ash. Starch was determined using the amyloglucosidase method (method no. 7.2.5) and the degree of starch breakdown (DSB) was determined according to method no. 7.2.6 (VDLUFA, 2012). The DSB describes the concentration of hydrolyzed starch relative to the crude starch concentration of a feed. The former is determined from the concentration of glucose, which is present after starch degradation with amyloglucosidase. The protein fractions A (i.e., non-protein nitrogen), B1 (i.e., TP, which is soluble in borate-phosphate buffer at pH 6.7 to 6.8, but precipitable), B2 (i.e., TP, which is insoluble in the borate-phosphate buffer minus TP, which is insoluble in neutral detergent), B3 (i.e., TP, which is insoluble in neutral detergent but soluble in acid detergent), and C (i.e., TP, which is insoluble in acid detergent) were determined according to Licitra et al. (1996). For each fraction, residual nitrogen was determined according to the Kjeldahl method. The protein fractions were used to calculate TP content (i.e., B1 + B2 + B3 + C) and soluble protein (i.e., A + B1). The NH3–N contents in the substrates were determined according to method no. 4.8.1 (VDLUFA, 2012). The protein that is insoluble in pepsin was analyzed using the Kjeldahl method after 48 h of incubation in a pepsin-hydrochloric acid solution (Weissbach et al., 1985). In the substrates, the proteins were hydrolyzed with hydrochloric acid and amino acids were analyzed using a Biochrom 30 Amino Acid Analyser with PEEK-Sodium Prewash Column, 100 mm × 4.6 mm, and PEEK-Oxidised Feedstuff Column, 200 mm × 4.6 mm; Biochrom Ltd., Cambridge, UK) according to VDLUFA (2012; method no. 4.11.1), both with preceding oxidation for cysteine and methionine detection, but without oxidation for tyrosine and histidine detection. For detection of tryptophan, proteins were hydrolyzed with phosphoric acid and hydrochloric acid. Tryptophan was analyzed using HPLC (Agilent 1100 Series with ZORBAX Eclipse XDB-C8; 150 mm × 4.6 mm, 5 μm, Agilent Technologies Inc., Santa Clara, CA, USA) according to Fontaine et al. (1998).
2.6. Statistical analysis
Single units showing gas leakage, defects, or measuring errors were excluded from the analysis of gas production, methane, and NH3–N (used for PRCP and RUP estimation), respectively. Using boxplots, outliers were identified and removed from the datasets. Outliers were defined as observations that lie above or below the upper or lower fences of the box (i.e., ±1.5 interquartile ranges), respectively. The finally used number of replicates is stated at the respective points below. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Using the MIXED procedure, least squares means (LSM) were estimated for PRCP, RUP, effective PRCP, effective RUP, total gas production, gas production during single stages of the incubation period, and methane production. Differences of the LSM were tested for significance, where differences with P < 0.05 were considered to be significant. The studentized residuals were confirmed for Gaussian distribution according to the Shapiro–Wilk and/or Kolmogorov–Smirnov test.
The following models were used:
| yij = μ + αi + tj + eij , |
where yij is the 24 h gas production including starch and cellulose; μ is the general mean; αi is the combined fixed effect of substrate and treatment i (i = 1, …, 10, where 1 = starch, 2 = cellulose, 3 = native field peas, 4 = ensiled field peas, 5 = toasted field peas, 6 = ensiled + toasted field peas, 7 = native field beans, 8 = ensiled field beans, 9 = toasted field beans, and 10 = ensiled + toasted field beans); tj is the random effect of trial j (j = 1, …, 6), with consideration of repeated measurements per substrate or treatment, respectively; and eij is the random residual effect. For total gas production analysis, 6 replicates were used per substrate.
| yijk = μ + αi+ βj + αβij+tk+eijk , |
where yijk is the 24 h gas production (excluding starch and cellulose), gas production during incubation time stages, or methane production; μ is the general mean; αi is, for 24 h gas production excluding starch and cellulose, the fixed effect of legume species i (i = 1, 2, where 1 = field peas, and 2 = field beans), or, for gas production in time stages and methane production, the fixed effect of substrate i (i = 1, …, 10, where 1 = starch, 2 = cellulose, 3 = native field peas, 4 = ensiled field peas, 5 = toasted field peas, 6 = ensiled + toasted field peas, 7 = native field beans, 8 = ensiled field beans, 9 = toasted field beans, and 10 = ensiled + toasted field beans); βj is, for 24-h gas production excluding starch and cellulose, the fixed effect of treatment j (j = 1, …, 4, where 1 = native, 2 = ensiled, 3 = toasted, and 4 = ensiled + toasted), for 24-h gas production in time stages, the fixed effect of time stage j (j = 1, …, 6, where 1 = 0 to 2 h, 2 = 3 to 4 h, 3 = 5 to 6 h, 4 = 7 to 8 h, 5 = 9 to 12 h, and 6 = 13 to 24 h), or, for methane production, the fixed effect of measuring time j (j = 1, 2, where 1 = 8 h, and 2 = 24 h); αβij is the interaction of fixed effects αi and βj; tk is the random effect of trial k (k = 1, …, 6), with consideration of repeated measurements per substrate or treatment, respectively; and eijk is the random residual effect. For the analysis of total gas production excluding starch and cellulose, 6 replicates were used per substrate or treatment, respectively. For methane analysis, a minimum of 5 replicates were used per treatment and measuring time (6 replicates were removed). Heterogeneous trial variances and residual variances were considered according to the substrate/treatment for the analysis of gas production during time stages. Heterogeneous residual variances were considered according to the measuring time for the analysis of methane production.
| yijkl = μ + αi + βj + γk + αβγijk+tl +eijkl, |
where yijkl is PRCP, RUP, effective PRCP, or effective RUP; μ is the general mean; αi is the fixed effect of legume species i (i = 1, 2, where 1 = field peas, and 2 = field beans); βj is the fixed effect of treatment j (j = 1, …, 4, where 1 = native, 2 = ensiled, 3 = toasted, and 4 = ensiled + toasted); γk is, for PRCP and RUP, the fixed effect of measuring time k (k = 1, 2, where 1 = 8 h, and 2 = 24 h), or, for effective PRCP and effective RUP, the fixed effect of Kp k (k = 1, …, 5, where 1 = Kp2, 2 = Kp4, 3 = Kp6, 4 = Kp8, and 5 = Kp12); αβγijk is the interaction of fixed effects αi, βj, and γk; tl is the random effect of trial l (l = 1, …, 6), with consideration of repeated measurements per treatment; and eijkl is the random residual effect. For the analysis of PRCP, a minimum of 4 replicates were used per legume species, treatment, and measuring time (10 replicates were removed). For the analysis of RUP, a minimum of 2 replicates were used per legume species, treatment, and measuring time (16 replicates were removed). Heterogeneous trial variances and residual variances were considered according to Kp for the analysis of effective PRCP.
3. Results
3.1. PRCP, RUP, effective PRCP, and effective RUP
The results for PRCP and effective PRCP are given in Table 5, and those for RUP and effective RUP are given in Table 6. Post-ruminal CP and RUP were generally higher after 8 h than after 24 h (P < 0.05). Effective PRCP and effective RUP increased with increasing Kp (P < 0.05). The treatments of field beans had more PRCP, RUP, effective PRCP, and effective RUP than the field pea counterparts (P < 0.05). After 8 h, PRCP was lowest in native peas; toasting slightly increased PRCP, and highest PRCP contents were found in the ensiled and the ensiled + toasted field peas. In field beans, the highest PRCP content was found in grain silages as well, and the lowest PRCP was in the ensiled + toasted treatment. After 24 h, no differences did exist among pea or field bean treatments. The RUP contents increased after ensiling of pea grains (P < 0.05), and were highest in the ensiled + toasted treatments (P < 0.05). This was less evident in field beans. In field peas, effective PRCP was increased by ensiling (P < 0.05), but additional toasting did not have any effect; effective RUP was, however, increased by ensiling and additional toasting (P < 0.05). In field beans, only ensiling led to an increase of effective PRCP and effective RUP (P < 0.05).
Table 5.
Least squares means of post-ruminal crude protein (PRCP) and effective PRCP of native, ensiled, toasted, and ensiled + toasted field peas and field beans (g/kg DM)1.
| Item | PRCP2 |
Effective PRCP3 |
|||||
|---|---|---|---|---|---|---|---|
| 8 h | 24 h | Kp2 | Kp4 | Kp6 | Kp8 | Kp12 | |
| Native field peas | 177aBY | 116bAX | 78eAX | 116dAX | 138cBY | 155bBY | 177aBY |
| Ensiled field peas | 209aBX | 117bAX | 58eAX | 114dAX | 147cBX | 170bBX | 207aBX |
| Toasted field peas | 180aBY | 115bBX | 71eAX | 112dBX | 136cBY | 154bBY | 178aBY |
| Ensiled + toasted field peas | 206aBX | 121bAX | 64eAX | 118dAX | 150cBX | 172bBX | 203aBX |
| Native field beans | 269aAXY | 128bAX | 34eBX | 123dAX | 175cAXY | 212bAXY | 264aAXY |
| Ensiled field beans | 278aAX | 127bAX | 56eAX | 121dAX | 180cAX | 219bAX | 274aAX |
| Toasted field beans | 269aAXY | 129bAX | 39eBX | 125dAX | 176cAXY | 212bAXY | 265aAXY |
| Ensiled + toasted field beans | 260aAY | 126bAX | 37eBX | 121dAX | 170cAY | 204bAY | 253aAY |
a, b, c, d, e Within a row, different superscripts mark differences between the measuring times (for PRCP) or passage rates (Kp) (for effective PRCP) (P < 0.05).
A,B Within a column, different superscripts mark differences between legume species within same treatment (P < 0.05).
X,Y Within a column, different superscripts mark differences between treatments within same legume species (P < 0.05).
Standard errors ranged from 5.34 to 6.24 g/kg DM in PRCP, and from 3.82 to 12.5 g/kg DM in effective PRCP estimates.
Estimated from measurements after 8 and 24 h.
Estimated for Kp 0.02, 0.04, 0.06, 0.08, and 0.12/h.
Table 6.
Least squares means of rumen-undegraded protein (RUP), and effective RUP of native, ensiled, toasted, and ensiled + toasted field peas and field beans (g/kg DM).1
| Item | RUP2 |
Effective RUP3 |
|||||
|---|---|---|---|---|---|---|---|
| 8 h | 24 h | Kp2 | Kp4 | Kp6 | Kp8 | Kp12 | |
| Native field peas | 35aBZ | 30bBZ | 26cBZ | 30bcBZ | 32abBZ | 33abBZ | 35aBZ |
| Ensiled field peas | 45aBY | 39bBY | 35cBY | 39bcBY | 41abBY | 43abBY | 45aBY |
| Toasted field peas | 38aBZ | 34aBYZ | 32aBY | 35aBYZ | 36aBYZ | 37aBZ | 38aBZ |
| Ensiled + toasted field peas | 58aBX | 50bBX | 44dBX | 50cBX | 53bcBX | 55abBX | 58aBX |
| Native field beans | 56aAY | 50bAY | 47cAXY | 51bcAY | 53abAY | 54abAY | 56aAY |
| Ensiled field beans | 64aAX | 56bAX | 51dAX | 56cAX | 59bcAX | 61aAX | 64aAX |
| Toasted field beans | 53aAY | 47bAY | 44bAY | 47abAY | 49abAY | 51aAY | 52aAY |
| Ensiled + toasted field beans | 66aAX | 57bAX | 50dAX | 57cAX | 60bcAX | 63abAX | 66aAX |
a, b, c, d Within a row, different superscripts mark differences between the measuring times (for RUP) or passage rates (Kp) (for effective RUP) (P < 0.05).
A, B Within a column, different superscripts mark differences between legume species within same treatment (P < 0.05).
X, Y, Z Within a column, different superscripts mark differences between treatments within same legume species (P < 0.05).
Standard errors ranged from 1.7 to 2.2 g/kg DM in RUP, and from 2.1 to 2.6 g/kg DM in effective RUP estimates.
Estimated from measurements after 8 and 24 h.
Estimated for Kp 0.02, 0.04, 0.06, 0.08, and 0.12/h.
3.2. Total gas production, gas production kinetics, and production of methane
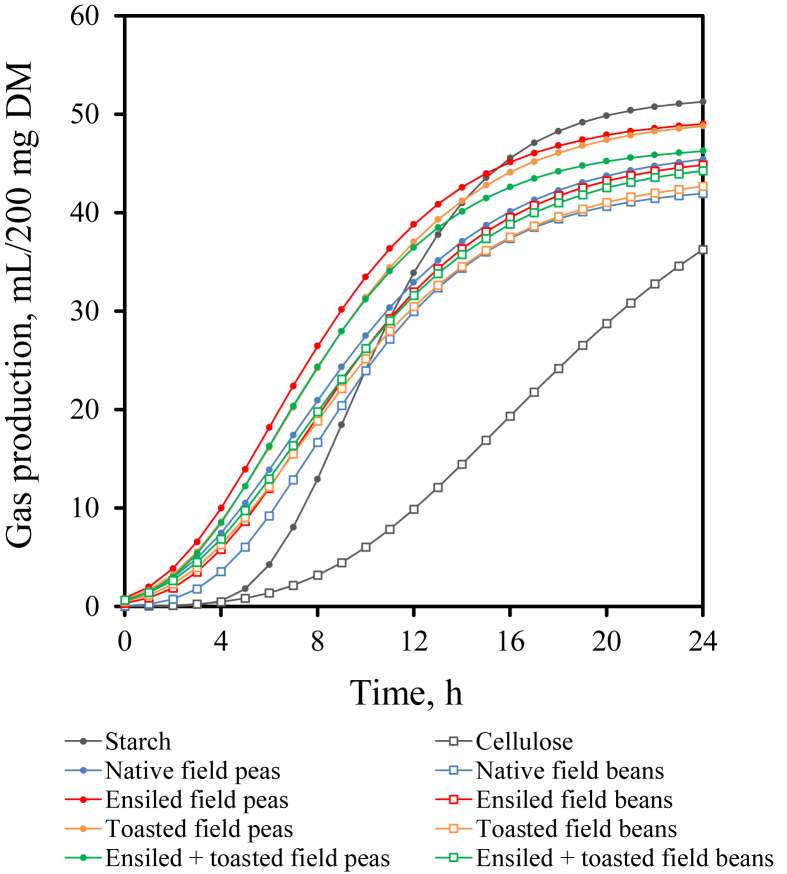
Gas production after 24 h of incubation was highest with starch (54 mL/200 mg DM) and lowest with cellulose (36 mL/200 mg DM). It was higher with field peas than with field beans (P < 0.001). Total gas production did not differ among field pea or field bean treatments. Gas production significantly increased in the course of incubation time stages (P < 0.05). Starting with the 5th hour of incubation, peas generally had a higher gas production than the field beans (P < 0.05); grain silages had a higher gas production than the toasted or ensiled + toasted treatments, and native field peas and field beans had the lowest gas production, respectively (Table 7, Fig. 3). However, treatment effects were not evident (Table 7). Starch fermentation started after a lag time of 4 h, from which on the produced gas increased rapidly. Gas production from cellulose increased slowly and did not reach their asymptote after 24 h (Fig. 3). The curve fitting parameters and convergence success for gas production kinetics of starch, cellulose, as well as pea and field bean treatments are summarized in Table 8.
Table 7.
Least squares means of gas production (mL/200 mg DM) from native, ensiled, toasted, and ensiled + toasted field peas and field beans and reference substrates (starch and cellulose) within different stages of the 24-h incubation.1
| Item | 0 to 2 h | 3 to 4 h | 5 to 6 h | 7 to 8 h | 9 to 12 h | 13 to 24 h |
|---|---|---|---|---|---|---|
| Maize starch | 0.9dA | 2.1dBC | 4.1cdB | 8.5cC | 24.0bB | 46.5aA |
| Cellulose | 1.1cA | 1.3cC | 1.7cC | 2.7cD | 5.9bC | 23.9aD |
| Native field peas | 2.2cAX | 5.7cABX | 10.6cAX | 16.9cAY | 27.2bBY | 41.4aABY |
| Ensiled field peas | 3.8fAX | 7.7eAX | 13.0dAX | 21.6cAX | 33.6bAX | 46.0aABX |
| Toasted field peas | 2.8fAX | 6.4eABX | 11.9dAX | 19.9cAXY | 31.2bAX | 45.2aABX |
| Ensiled + toasted field peas | 3.0fAX | 6.8eABX | 11.7dAX | 19.3cAXY | 31.1bAX | 43.4aABXY |
| Native field beans | 2.8eAX | 3.4eABCX | 6.9dABCX | 12.2cACX | 23.7bBX | 38.4aBX |
| Ensiled field beans | 2.4fAX | 5.2eABX | 8.8dBX | 14.3cBX | 25.9bBX | 40.8aCX |
| Toasted field beans | 2.4fAX | 5.2eABX | 9.0dABX | 14.6cBX | 24.9bBX | 38.8aCX |
| Ensiled + toasted field beans | 3.5fAX | 6.4eABCX | 9.7dAX | 14.8cBX | 25.8bBX | 40.2aBX |
a, b, c, d, e, f Within a row, different superscripts mark differences between time stages (P < 0.05).
A, B, C, D Within a column, different superscripts mark differences between the legume species within same treatment (P < 0.05); starch and cellulose were compared to all other substrates/treatments, respectively.
X, Y Within a column, different superscripts mark differences between the treatments within same legume species (P < 0.05).
Standard errors ranged from 0.799 to 2.14 mL/200 mg DM.
Fig. 3.
Cumulative gas production within 24 h of in vitro incubation of maize starch, cellulose, as well as native, ensiled, toasted, and ensiled + toasted field peas and field beans, modelled using the Gompertz non-linear regression function.
Table 8.
Curve fitting parameters for gas production kinetics from native, ensiled, toasted, and ensiled + toasted field peas and field beans and reference substrates (maize starch and cellulose) modelled with the Gompertz function.
| Item | Gas production kinetics parameters1 |
R2 | ||
|---|---|---|---|---|
| a | b | b + c | ||
| Maize starch | 51.9 | 9.1 | 12.5 | 0.89 |
| Cellulose | 50.5 | 15.7 | 23.2 | 0.90 |
| Native field peas | 46.8 | 7.0 | 11.8 | 0.97 |
| Ensiled field peas | 49.8 | 6.0 | 10.3 | 0.98 |
| Toasted field peas | 49.8 | 6.5 | 11.0 | 0.98 |
| Ensiled + toasted field peas | 46.9 | 6.3 | 10.4 | 0.97 |
| Native field beans | 42.8 | 7.8 | 11.9 | 0.88 |
| Ensiled field beans | 46.1 | 7.4 | 12.0 | 0.96 |
| Toasted field beans | 44.0 | 7.2 | 12.0 | 0.94 |
| Ensiled + toasted field beans | 45.7 | 7.1 | 12.0 | 0.96 |
a, asymptotic maximal gas production (mL/200 mg DM); b, time (h) until which one third of a is produced; b + c, time (h) until which 70% of a is produced.
Methane production distinctly increased from 8 to 24 h (P < 0.05; Table 9). After 8 h, microbes produced more methane on field peas (1.26 to 1.32 mmol/L) than on field beans (0.78 to 1.03 mmol/L) (P < 0.05). After 24 h, this difference was widely abolished. Treatments of field beans differed in methane production after 8 h (P < 0.05; Table 9). Treatments of peas and field beans did not differ among each other, respectively, after 24 h of incubation. The 24-h methane production of the legume grains was similar to that amount produced by cellulose (2.45 mmol/L), and higher than that of pure maize starch (1.39 mmol/L; P < 0.05).
Table 9.
Least squares means of methane production (mmol/L) from native, ensiled, toasted, and ensiled + toasted field peas and field beans and reference substrates (starch and cellulose) measured after 8 and 24 h of incubation.1
| Item | 8 h | 24 h |
|---|---|---|
| Maize starch | 0.09bD | 1.39aC |
| Cellulose | 0.40bC | 2.45aB |
| Native field peas | 1.28bAX | 2.51aABX |
| Ensiled field peas | 1.26bAX | 2.47aABX |
| Toasted field peas | 1.32bAX | 2.55aABX |
| Ensiled + toasted field peas | 1.31bAX | 2.39aABX |
| Native field beans | 0.79bBYZ | 2.62aAX |
| Ensiled field beans | 0.96bBXY | 2.49aABX |
| Toasted field beans | 1.03bBX | 2.58aABX |
| Ensiled + toasted field beans | 0.78bBZ | 2.52aABX |
a, b Within a row, different superscripts mark differences between measuring time (P < 0.05).
A, B, C, D Within a column, different superscripts mark differences between the legume species within same treatment (P < 0.05); starch and cellulose were compared to all other substrates/treatments, respectively.
X, Y, Z Within a column, different superscripts mark differences between the treatments within same legume species (P < 0.05).
Standard errors ranged from 0.0667 to 0.0797 mmol/L.
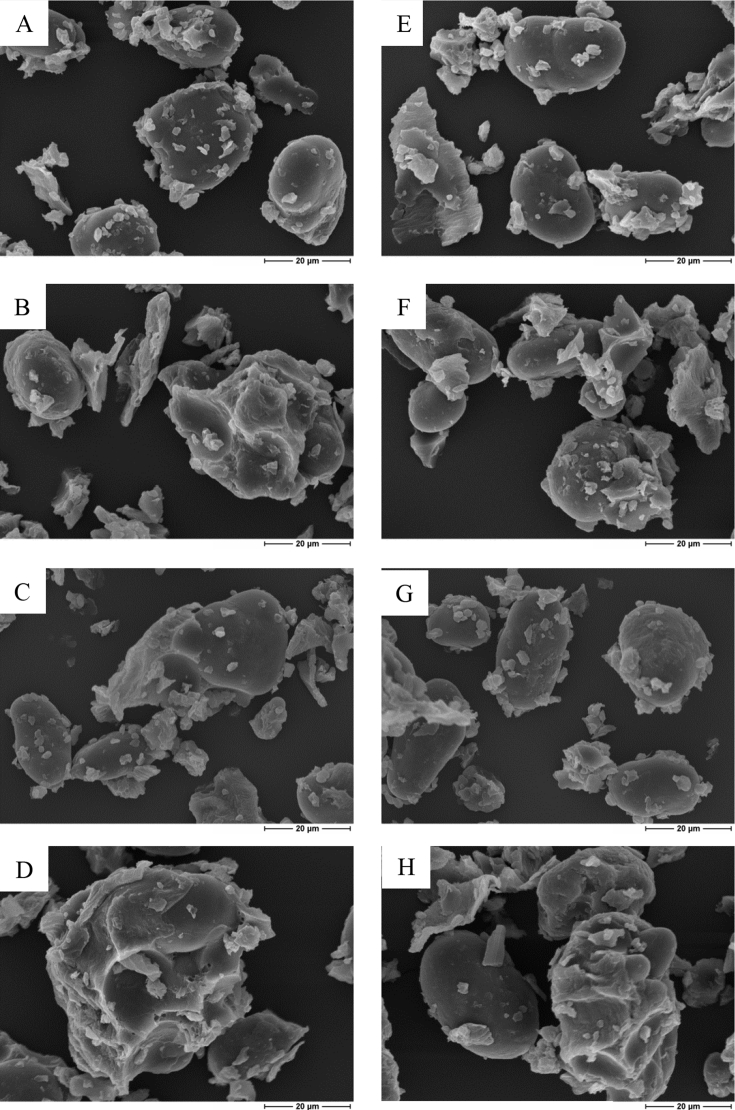
3.3. Scanning electron microscopic imaging
Scanning electron micrographs of field pea and field bean treatments are shown in Fig. 4. In the native legumes, the starch granules had a predominantly oval, reniform, or irregular shape, with smooth surfaces, and clear demarcations to the surrounding matrix structures. In the micrographs, the matrices were visible as sharp-edged fragments onto the granules’ surfaces, which have arisen from milling of the substrates before imaging. The pea starches were found at a length range of 17.9 to 25.3 μm, and a width range of 8.8 to 23.6 μm. The starch granules of the field bean cultivar were found at a length range of 10.4 to 29.1 μm, and a width range of 8.7 to 10.6 μm. Overall, no structural changes were observed that could show any treatment effects.
Fig. 4.
Scanning electron micrographs of starch granules and their embedding, covering, or surrounding matrices of native field peas (A), ensiled field peas (B), toasted field peas (C), ensiled + toasted field peas (D), native field beans (E), ensiled field beans (F), toasted field beans (G), and ensiled + toasted field beans (H), made by 1,500× magnification and 15 keV.
4. Discussion
4.1. PRCP and RUP in native field peas and field beans
The field beans had generally higher concentrations of essential and non-essential amino acids than the peas; however, the concentrations of sulfurous amino acids cysteine and methionine did not differ between peas and field beans. For PRCP or RUP, comparable results deriving from the same methods are less available for field peas and field beans from the literature. Masoero et al. (2005) reported a bit lower RUP contents in native field pea and field bean meals using the S. griseus protease test (13% and 18% of CP, respectively). In the current study, in vitro PRCP and RUP contents of native field peas at Kp8 were 153 g/kg DM, and 17% of CP, respectively. This was similar to in situ PRCP (187 g/kg DM) and RUP data (15% of CP) given in the German feed value tables (DLG, 1997). The current in vitro PRCP and RUP contents of field beans were 212 g/kg DM and 26% of CP, respectively, which was higher than the tabulated in situ PRCP (195 g/kg DM) and in situ RUP contents (15% of CP) (DLG, 1997). On a DM basis, field peas and field beans had lower PRCP and RUP contents than SBM. For SBM, PRCP ranged between 298 and 436 g/kg DM, and RUP of CP ranged between 24% and 79% (DLG-Futterwerttabellen, 1997, NRC, 2001). They were quite similar compared to in situ data tabulated for rapeseed meal that PRCP ranged from 212 to 219 g/kg DM and RUP of CP ranged from 16% to 21% by DLG (1997) and NRC (2001). Proportional to CP, field peas had approximately 77% PRCP at Kp8, which was higher than in SBM (59% of CP), and in rapeseed meal (55% of CP) (DLG, 1997). Field beans had, with about 72% of CP at Kp8, a likewise higher amount of PRCP than given for SBM and rapeseed meal. Especially intra-species differences in PRCP and RUP are likely due to comparison of different cultivars.
4.2. In vitro gas and methane production in native field peas and field beans
The current results have confirmed that field pea grains have a higher gas production capacity than field beans (Abreu and Bruno-Soares, 1998, Blümmel et al., 1999, Masoero et al., 2005). Both were found to range in front of rapeseed, soybeans, and lupines (Abreu and Bruno-Soares, 1998, Blümmel et al., 1999, Masoero et al., 2005, Calabrò et al., 2009), primarily caused by higher amounts of readily fermentable starch, simple sugars, and oligomeric carbohydrates (Adamidou et al., 2011). Differences between peas and field beans in proportions of soluble and insoluble non-starch polysaccharides (Adamidou et al., 2011) might have had an additional effect on gas production. Methane production from the in vitro batch cultures was faster with peas than with field beans, but the final volume did not differ. The initially lower methane formation in field beans was probably because of an elevated propionate formation in contrast to acetate formation in the peas, which had higher concentrations of sugars. Unlike acetate, propionate acts as hydrogen sink, which has made more hydrogen available for methane production during fermentation of the field peas.
4.3. Effects of ensiling on PRCP and RUP
Proteolysis during ensiling is usually decreasing TP while increasing non-protein nitrogen (fraction A) (Mustafa and Seguin, 2003, Gefrom et al., 2013). We observed that the A fraction increased in peas through ensiling by 49%, which is critical in terms of its unknown composition and its instability against ruminal degradation. Also, the non-soluble fractions B2 and especially B3 were increased by 32% and 431%, respectively, which led to the increased RUP contents. In field beans, neither TP, nor any of the protein fractions were considerably altered. However, protein insoluble in pepsin increased by 17%, which was also reflected by increasing RUP contents. The silages’ fermentation characteristics did not show any evidence for failed fermentation or silage heating. However, protein or amino acid fixation, respectively, through Maillard reaction evidently may also appear in legume silages (Kuhnitzsch et al., 2019b). In field beans, we found lysine to be markedly reduced after ensiling (from 18.9 g/kg DM in native beans to 15.6 g/kg DM in ensiled beans), which might be due to the formation of fructoselysine (Kuhnitzsch et al., 2019b). Even if ingested glycated proteins can partly be degraded by intestinal microbes (Hellwig et al., 2015), significant amounts of Maillard end-products were found in milk or urine (Schwarzenbolz et al., 2016). Maillard polymers, which are not absorbed and remain in the digestive tract, may decrease protein digestibility and increase immunoreactivity (Teodorowicz et al., 2018). Thus, a huge formation of Maillard polymers even after ensiling seems to be critical, and need further consideration.
4.4. Effects of ensiling on starch degradation and production of total gas and methane
Ensiling of field pea and field bean grains may force degradation of starch and other carbohydrates to fermentable substrates useable for rumen microbes and the host (Gefrom et al., 2013). Increased gas production was observed both in ensiled peas and in ensiled field beans. The in vitro production of methane was not affected by ensiling. Moreover, SEM imaging did not reveal any structural changes of starch granules and embedding matrices.
4.5. Effects of thermic treatments on PRCP and RUP
Heat and heat + pressure treatments demonstrably decrease protein degradability in the rumen (Goelema et al., 1998, Goelema et al., 1999, Theurer et al., 1999, Aufrère et al., 2001, Azarfar et al., 2008) owing to complex denaturation reactions, and the formation of Maillard polymers (Hurrell and Finot, 1985, Yu et al., 2002, Hellwig and Henle, 2014). The protective capacity of hydro-thermic treatments and the border to protein damage largely depend on temperature, time of exposure, type of substrate, substrate quantity (i.e., throughput rate in the toaster on farm scale), and the moisture content of the substrate (Yu et al., 2002). In field peas, we found slightly increased amounts of PRCP and considerably increased RUP contents after ensiling + toasting. Microbial protein is calculated as the difference between PRCP and RUP. Microbial protein was therefore arithmetically reduced due to ensiling and ensiling + toasting. Microbial protein synthesis was in parts probably limited by the availability of easily utilizable energy (Focant et al., 1990). In untreated peas, the degradation rate of starch is lower than that of protein, which means that the ammonia level in the rumen (or in the in vitro fermenter) rapidly rises, but at the same time, there is not enough energy for the microbes available to use it (Focant et al., 1990). In toasted field peas, soluble protein was decreased by 20%, B1 was decreased by 43%, A and B2 were increased by 189% and 80%, respectively. In field beans, toasting of the ensiled grains rather did not affect PRCP and RUP, but soluble protein was likewise decreased (i.e., decreasing B1, and increasing B2 by 3% and 2%, respectively). When starch was not affected by toasting at the same time, microbial protein synthesis was probably limited by both, less available nitrogen, and less available energy from starch fermentation (Focant et al., 1990). Toasting of native peas and field beans without previous ensiling had no effect on in vitro PRCP and RUP estimates, but increased the B1 fraction of protein and the soluble protein. Moreover, in field peas, the concentrations of free arginine, lysine, and glutamic acid increased after toasting the native grains. We suggest that reaching a level, on which structural changes protect the protein, requires a heavier and/or a longer heat exposure in ensiled field bean grains, while peas are much easier to affect. Again, the border to protein damage through toasting has to be known. On farm-scale, acid detergent insoluble protein (C fraction) and protein insoluble in pepsin were dramatically increased when the grain temperature exceeded 90 °C (4% to 19%, and 7% to 26% of CP, respectively, at 90 to 110 °C grain temperature, which was 190 °C supplied air temperature at 100 kg/h throughput rate) (Kuhnitzsch et al., 2019a).
4.6. Effects of thermic treatments on starch degradation and production of total gas and methane
Legume starches seem to have high resistance against swelling, rupture of matrices, and gelatinization induced by heat treatments (Yu et al., 2002). In the current study, we have not found any effect of toasting on gas or methane production. The SEM images also have not shown any structural changes in the starch granules or the matrices. The DSB was not measurably altered in any treatment. Considerable gelatinization and/or retrogradation of starch did probably not occur.
5. Conclusion
Especially in field pea grain silages, RUP was increased after ensiling and ensiling + toasting at 160 °C for 30 min, thus, pea protein seemed to be better protected against ruminal degradation. Microbial protein synthesis was arithmetically reduced, possibly by limited nitrogen and energy availability. Post-ruminal crude protein, gas, and methane production were not significantly altered. For farm-scale application and the feeding of high-yielding ruminants, optimal processing conditions (i.e., temperatures, durations, and throughput rates) still need to be tested and defined to avoid significant protein damage. The observed reduction of soluble protein during ensiling is not fully clarified.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
The project was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the Federal Programme Protein Crop Strategy (grant no. 2815EPS058).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abreu J.M.F., Bruno-Soares A.M. Chemical composition, organic matter digestibility and gas production of nine legume grains. Anim Feed Sci Technol. 1998;70:49–57. [Google Scholar]
- Adamidou S., Nengas I., Grigorakis K., Nikolopoulou D., Jauncey K. Chemical composition and antinutritional factors of field peas (Pisum sativum), chickpeas (Cicer arietinum), and faba beans (Vicia faba) as affected by extrusion preconditioning and drying temperatures. Cereal Chem. 2011;88:80–86. [Google Scholar]
- Aufrère J., Graviou D., Melcion J.P., Demarquilly C. Degradation in the rumen of lupin (Lupinus albus L.) and pea (Pisum sativum L.) seed proteins. Effect of heat treatment. Anim Feed Sci Technol. 2001;92:215–236. [Google Scholar]
- Azarfar A., Tamminga S., Pellikaan W.F., van der Poel A.F.B. In vitro gas production profiles and fermentation end-products in processed peas, lupins and faba beans. J Sci Food Agric. 2008;88:1997–2010. [Google Scholar]
- Bachmann M., Kuhnitzsch C., Martens S.D., Steinhöfel O., Zeyner A. Lutherstadt Wittenberg; 2019. Einfluss des Silierens und Toastens auf antinutritive Inhaltsstoffe von Erbsen und Ackerbohnen. 15. Tagung Schweine- und Geflügelernährung; pp. 146–148. [Abstract] [Google Scholar]
- Blümmel M., Aiple K.-P., Steingass H., Becker K. A note on the stoichiometrical relationship of short chain fatty acid production and gas formation in vitro in feedstuffs of widely differing quality. J Anim Physiol Anim Nutr. 1999;81:157–167. [Google Scholar]
- Calabrò S., Tudisco R., Balestrieri A., Piccolo G., Infascelli F., Cutrignelli M. Fermentation characteristics of different grain legumes cultivars with the in vitro gas production technique. Ital J Anim Sci. 2009;8:280–282. [Google Scholar]
- De Cicco A. Eurostat; 2017. Dry pulses in EU agriculture - statistics on cultivation, production and economic value.http://ec.europa.eu/eurostat/statistics-explained/index.php/Dry_pulses_in_EU_agriculture_-_statistics_on_cultivation production_and_economic_value. [Google Scholar]
- Corbett R.R., Okine E.K., Goonewardene L.A. Effects of feeding peas to high-producing dairy cows. Can J Anim Sci. 1995;75:625–629. [Google Scholar]
- Cutrignelli M.I., Messina M., Tulli F., Randazzo B., Olivotto I., Gasco L., Loponte R., Bovera F. Evaluation of an insect meal of the Black Soldier Fly (Hermetia illucens) as soybean substitute: intestinal morphometry, enzymatic and microbial activity in laying hens. Res Vet Sci. 2018;117:209–215. doi: 10.1016/j.rvsc.2017.12.020. [DOI] [PubMed] [Google Scholar]
- DLG. DLG-Futterwerttabellen . 7th ed. 1997. Wiederkäuer. Frankfurt (Main) [Google Scholar]
- Edmunds B., Südekum K.-H., Spiekers H., Schuster M., Schwarz F.J. Estimating utilisable crude protein at the duodenum, a precursor to metabolisable crude protein for ruminants, from forages using a modified gas test. Anim Feed Sci Technol. 2012;175:106–113. [Google Scholar]
- Edmunds B., Südekum K.-H., Spiekers H., Schwarz F.J. Estimating ruminal crude protein degradation of forages using in situ and in vitro techniques. Anim Feed Sci Technol. 2012;175:95–105. [Google Scholar]
- Focant M., Van Hoecke A., Vanbelle M. The effect of two heat treatments (steam flaking and extrusion) on the digestion of Pisum sativum in the stomachs of heifers. Anim Feed Sci Technol. 1990;28:303–313. [Google Scholar]
- Fontaine J., Bech-Andersen S., Butikofer U., de Froidmont-Görtz I. Determination of tryptophan in feed by HPLC – development of an optimal hydrolysis and extraction procedure by the EU Commission DG XII in three international collaborative studies. Agribiol Res. 1998;51:97–108. [Google Scholar]
- France J., Dijkstra J., Dhanoa M.S., Lopez S., Bannink A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr. 2000;83:143–150. doi: 10.1017/s0007114500000180. [DOI] [PubMed] [Google Scholar]
- Gatrell S., Lum K., Kim J., Lei X.G. Non ruminant nutrition symposium: potential of defatted microalgae from the biofuel industry as an ingredient to replace corn and soybean meal in swine and poultry diets. J Anim Sci. 2014;92:1306–1314. doi: 10.2527/jas.2013-7250. [DOI] [PubMed] [Google Scholar]
- Gdala J., Buraczewska L. Chemical composition and carbohydrate content of several varieties of faba bean and pea seeds. J Anim Feed Sci. 1997;6:123–135. [Google Scholar]
- Gefrom A., Ott E.M., Hoedtke S., Zeyner A. Effect of ensiling moist field bean (Vicia faba), pea (Pisum sativum) and lupine (Lupinus spp.) grains on the contents of alkaloids, oligosaccharides and tannins. J Anim Physiol Anim Nutr. 2013;97:1152–1160. doi: 10.1111/jpn.12024. [DOI] [PubMed] [Google Scholar]
- Goelema J.O., Spreeuwenberg M.A.M., Hof G., van der Poel A.F.B., Tamminga S. Effect of pressure toasting on the rumen degradability and intestinal digestibility of whole and broken peas, lupins and faba beans and a mixture of these feedstuffs. Anim Feed Sci Technol. 1998;76:35–50. [Google Scholar]
- Goelema J.O., Smits A., Vaessen L.M., Wemmers A. Effects of pressure toasting, expander treatment and pelleting on in vitro and in situ parameters of protein and starch in a mixture of broken peas, lupins and faba beans. Anim Feed Sci Technol. 1999;78:109–126. [Google Scholar]
- Hellwig M., Henle T. Baking, ageing, diabetes: a short history of the Maillard reaction. Angew Chem Int Ed. 2014;53:10316–10329. doi: 10.1002/anie.201308808. [DOI] [PubMed] [Google Scholar]
- Hellwig M., Bunzel D., Huch M., Franz C.M.A.P., Kulling S.E., Henle T. Stability of individual Maillard reaction products in the presence of the human colonic microbiota. J Agric Food Chem. 2015;63:6723–6730. doi: 10.1021/acs.jafc.5b01391. [DOI] [PubMed] [Google Scholar]
- Hoedtke S., Zeyner A. Comparative evaluation of laboratory-scale silages using standard glass jar silages or vacuum-packed model silages. J Sci Food Agric. 2011;91:841–849. doi: 10.1002/jsfa.4255. [DOI] [PubMed] [Google Scholar]
- Honig H. EUROBAC conference. 1990. Evaluation of aerobic stability; pp. 72–78. Uppsala. [Google Scholar]
- Hurrell R.E., Finot R.A. Effect of food processing on protein digestibility and amino acid availability. In: Finley J.W., Hopkins D.T., editors. Digestibility and amino acid availability in cereals and oilseeds. American Association of Cereal Chemists; St. Paul, MN: 1985. pp. 516–527. [Google Scholar]
- ISAAA . ISAAA; 2016. Global status of commercialized biotech/GM crops: 2016. ISAAA brief No. 52. Ithaca, NY. [Google Scholar]
- Jurášek L., Johnson P., Olafson R.W., Smillie L.B. An improved fractionation system for pronase on CM-Sephadex. Can J Biochem. 1971;49:1195–1201. doi: 10.1139/o71-171. [DOI] [PubMed] [Google Scholar]
- Krupa U. Main nutritional and antinutritional compounds of bean seeds – a review. Pol J Food Nutr Sci. 2008;58:149–155. [Google Scholar]
- Kuhnitzsch C., Martens S.D., Bachmann M., Zeyner A., Hofmann T., Steinhöfel O. Forum angewandte Forschung in der Rinder- und Schweinefütterung. Fulda; 2019. Vergleichende Untersuchungen zum Einsatz siliert und getoasteter Erbsen bzw. Erbsenschröpfschnitt-GPS in der Fütterung hochleistender Milchkühe; pp. 61–64. [Abstract] [Google Scholar]
- Kuhnitzsch C., Hofmann T., Bachmann M., Martens S.D., Henle T., Zeyner A., Steinhöfel O. Effect of ensiling and toasting of field pea grains on formation of Maillard polymers from lysine and arginine. Adv Anim Biosci. 2019;10:540. (Abstract) [Google Scholar]
- Licitra G., Hernandez T.M., Van Soest P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol. 1996;57:347–358. [Google Scholar]
- Licitra G., Lauria F., Carpino S., Schadt I., Sniffen C.J., Van Soest P.J. Improvement of the Streptomyces griseus method for degradable protein in ruminant feeds. Anim Feed Sci Technol. 1998;72:1–10. [Google Scholar]
- Licitra G., Van Soest P.J., Schadt I., Carpino S., Sniffen C.J. Influence of the concentration of the protease from Streptomyces griseus relative to ruminal protein degradability. Anim Feed Sci Technol. 1999;77:99–113. [Google Scholar]
- Makkar H.P.S., Tran G., Heuzé V., Ankers P. State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol. 2014;197:1–33. [Google Scholar]
- Masoero F., Pulimeno A.M., Rossi F. Effect of extrusion, expansion and toasting on the nutritional value of peas, faba beans and lupins. Ital J Anim Sci. 2005;4:177–189. [Google Scholar]
- Menke K.H., Steingass H. Estimation of the energetic feed value from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 1988;28:7–55. [Google Scholar]
- Menke K.H., Raab L., Salewski A., Steingass H., Fritz D., Schneider W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Sci. 1979;93:217–222. [Google Scholar]
- Mustafa A.F., Seguin P. Characteristics and in situ degradability of whole crop faba bean, pea, and soybean silages. Can J Anim Sci. 2003;83:793–799. [Google Scholar]
- Neumann C., Velten S., Liebert F. Improving the dietary protein quality by amino acid fortification with a high inclusion level of micro algae (Spirulina platensis) or insect meal (Hermetia illucens) in meat type chicken diets. Open J Anim Sci. 2017;8:12–26. [Google Scholar]
- NRC . 7th ed. 2001. Nutrient requirements of dairy cattle. Washington, DC. [Google Scholar]
- Schwarzenbolz U., Hofmann T., Sparmann N., Henle T. Free Maillard reaction products in milk reflect nutritional intake of glycated proteins and can be used to distinguish “organic” and “conventionally” produced milk. J Agric Food Chem. 2016;64:5071–5078. doi: 10.1021/acs.jafc.6b01375. [DOI] [PubMed] [Google Scholar]
- Szumacher-Strabel M., Stochmal A., Cieslak A., Kozłowska M., Kuznicki D., Kowalczyk M., Oleszek W. Structural and quantitative changes of saponins in fresh alfalfa compared to alfalfa silage. J Sci Food Agric. 2019;99:2243–2250. doi: 10.1002/jsfa.9419. [DOI] [PubMed] [Google Scholar]
- Tagliapietra F., Cattani M., Bailoni L., Schiavon S. In vitro rumen fermentation: effect of headspace pressure on the gas production kinetics of corn meal and meadow hay. Anim Feed Sci Technol. 2010;158:197–201. [Google Scholar]
- Teodorowicz M., Hendriks W.H., Wichers H.J., Savelkoul H.F.J. Immunomodulation by processed animal feed: the role of Maillard reaction products and advanced glycation end-products (AGEs) Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurer C.B., Huber J.T., Delgado-Elorduy A., Wanderley R. Invited review: summary of steam-flaking corn or sorghum grain for lactating dairy cows. J Dairy Sci. 1999;82:1950–1959. doi: 10.3168/jds.S0022-0302(99)75431-7. [DOI] [PubMed] [Google Scholar]
- Tillie P., Rodríguez-Crezo E. JRC science and policy report (EUR27203) 2015. Markets for non-genetically modified, identity-preserved soybean in the EU.http://publications.jrc.ec.europa.eu/repository/handle/JRC95457 [Google Scholar]
- Tufarelli V., Khan R.U., Laudadio V. Evaluating the suitability of field beans as a substitute for soybean meal in early-lactating dairy cow: production and metabolic responses. Anim Sci J. 2012;83:136–140. doi: 10.1111/j.1740-0929.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- Vaga M., Hetta M., Huhtanen P. Effects of heat treatment on protein feeds evaluated in vitro by the method of estimating utilisable crude protein at the duodenum. J Anim Physiol Anim Nutr. 2017;101:1259–1272. doi: 10.1111/jpn.12646. [DOI] [PubMed] [Google Scholar]
- Valencia D.G., Serrano M.P., Centeno C., Lázaro R., Mateos G.G. Pea protein as a substitute of soya bean protein in diets for young pigs: effects on productivity and digestive traits. Livest Sci. 2008;118:1–10. [Google Scholar]
- VDLUFA . 3rd ed. VDLUFA-Verlag; Darmstadt: 2012. Die chemische Untersuchung von Futtermitteln. Methodenbuch. [Google Scholar]
- Weber M. Lutherstadt Wittenberg; 2017. Verfügbarkeit gvo-freier Proteinfuttermittel in Deutschland. 14. Tagung Schweine- und Geflügelernährung; pp. 22–26. [Google Scholar]
- Weissbach F., Prym R., Peters G., Lengerken J.V. Pepsin-insoluble crude protein – criterion of green forage silage quality. Tierzucht. 1985;39:346–349. [Google Scholar]
- Yu P., Goelema J.O., Leury B.J., Tamminga S., Egan A.R. An analysis of the nutritive value of heat processed legume seeds for animal production using the DVE/OEB model: a review. Anim Feed Sci Technol. 2002;99:141–176. [Google Scholar]