Abstract
Methane gas from livestock production activities is a significant source of greenhouse gas (GHG) emissions which have been shown to influence climate change. New technologies offer a potential to manipulate the rumen biome through genetic selection reducing CH4 production. Methane production may also be mitigated to varying degrees by various dietary intervention strategies. Strategies to reduce GHG emissions need to be developed which increase ruminant production efficiency whereas reducing production of CH4 from cattle, sheep, and goats. Methane emissions may be efficiently mitigated by manipulation of natural ruminal microbiota with various dietary interventions and animal production efficiency improved. Although some CH4 abatement strategies have shown efficacy in vivo, more research is required to make any of these approaches pertinent to modern animal production systems. The objective of this review is to explain how anti-methanogenic compounds (e.g., plant tannins) affect ruminal microbiota, reduce CH4 emission, and the effects on host responses. Thus, this review provides information relevant to understanding the impact of tannins on methanogenesis, which may provide a cost-effective means to reduce enteric CH4 production and the influence of ruminant animals on global GHG emissions.
Keywords: Feed efficiency, Greenhouse gas (GHG) emission, Methanogenesis, Tannin, Ruminant
1. Introduction
Minimizing enteric methane emission from ruminant production whereas enhancing feed conversion efficiency (FCE) and dietary nutrient utilization is a goal for sustainable livestock production. Numerous studies of greenhouse gas (GHG) mitigation strategies by genetic, dietary feed additives, plant extracts and chemical supplementation have been conducted to assess their potential to reduce methanogenesis (Nagaraja et al., 1997; Beauchemin et al., 2008; Hristov et al., 2013a, b; Gerber et al., 2013; Waghorn and Hegarty, 2011). However, most proposed mitigation strategies have shown inconsistent results among studies and may even lead to increased GHG emissions and adverse effects on aspects of animal growth and performance.
Many researchers have reported the effects of plant secondary compounds, such as tannins, saponin and essential oils, as alternative feed additives to modify ruminal fermentation, antimicrobial activity, astringency to deter consumption, to improve animal productivity and mitigate CH4 production. This review is aimed at providing information on the influence of plant tannins on ruminal microbiota, CH4 production and animals’ performance. Tannins are natural polyphenolic biomolecules that can be found in the bark, wood, fruit, leaves, flowers, and roots of most plant species. Plant tannins may play a role in mitigating methanogenesis. Several studies have evaluated the relationship between tannin-rich diets and CH4 production in ruminants both in vivo and in vitro (Beauchemin et al., 2007; Jayanegara et al., 2010, 2012; Goel and Makkar, 2012; Min and Solaiman, 2018). In vitro studies have shown that tannins have anti-methanogenic activity, either directly by inhibiting methanogens or indirectly by targeting protozoa (Bhatta et al., 2009; Jayanegara et al., 2015). A meta-analysis by Jayanegara et al. (2012) showed that tannin-containing diets or tannin extracts usually reduce enteric CH4 production. In addition, the effects on animal production and efficiency of animal production need to be evaluated. Methods and practices to reduce CH4 emissions need to be evaluated in terms of effects on dry matter intake (DMI), microbial activities, and rumen fermentation efficiency (e.g., acetate-to-propionate ratio [A:P]; Goel et al., 2009).
Feed consumed by cattle and other ruminants is fermented by microbes naturally present in the rumen. Fermentation of carbohydrates into volatile fatty acids (VFA), and microbial protein synthesis, are accompanied by the release of gases such as carbon dioxide (CO2) and CH4 (Johnson and Johnson, 1995; Gerber et al., 2013). Limited studies have considered the relationship between plant tannins and CH4 emissions per unit of DMI, animal performance, rumen fermentation, and feed efficiency dynamics (Waghorn and Hegarty, 2011; Beauchemin et al., 2007). This review summarized available literature on the impact of DMI and tannins on GHG emissions, rumen fermentation, and animal growth performance associated with rumen microbial activities in different ruminant species. Opportunities for reducing rumen methanogenesis through dietary inclusion of tannins are also discussed.
2. Global challenges of livestock industry
In 2015, there were 7.5 billion peoples in the world, and the World Hunger Map estimated that 795 million of those individuals did not have an adequate food supply (WFP, 2015). Meanwhile, published models forecast the world's human population to reach 9.7 and 11.2 billion in 2050 and 2100, respectively (UN, 2015). To meet food demands for this increasing population, it was estimated that milk and meat production must increase by 63% to 76%, respectively (Alexandratos and Bruinsma, 2012). As countries move from developing to developed, there tends to be an increase in per capita protein consumption as well that should be considered in the forecast. In addition, global demand for livestock products is expected to double by 2050 (Rojas-Downing et al., 2015). Increasing food production will likely result in an increase in GHG emissions, including enteric CH4 from animals, manure, crop production (i.e., anaerobic fermentation in rice production system), and cropland with inorganic or organic fertilization, unless mitigation practices are discovered and implemented. On a global scale, it has been estimated that livestock production contributes up to 10% of total GHG emissions; however, that value does not included indirect costs associated with agricultural activities, such as fossil fuels combustion and chemical fertilizers (IPCC et al., 2013; Gerber et al., 2013).
3. Methanogenesis
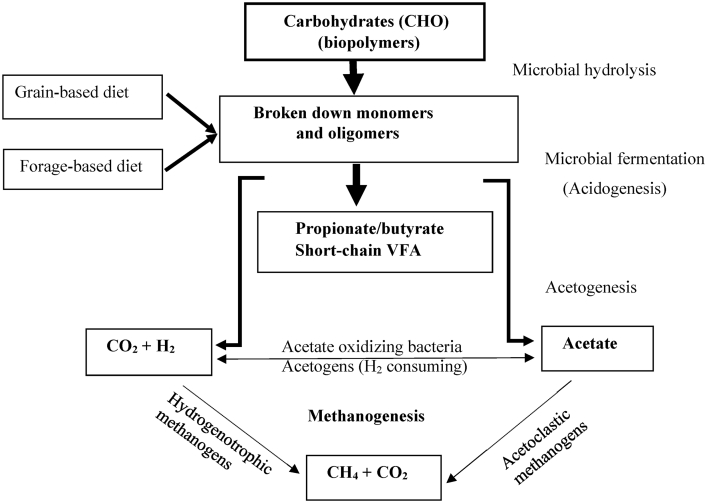
Methane gas is formed by anaerobic archaea coupled with bacteria, protozoa, and fungi in the rumen ecosystem. Up to 28 genera and 113 species of methanogens are known to exist in nature (Janssen and Kirs, 2008), and 5 of these species belong to Methanobrevibacter and Methanosarcina genera. Both Methanobrevibacter ruminantium and Methanomicrobium mobile was considered the dominant methanogen in the rumen (Yanagita et al., 2000; Whitford et al., 2001; Hristov et al., 2012; Min et al., 2014a, b). Whereas Janssen and Kirs (2008) reported that Methanobrevibacter was the dominant methanogen in the rumen (61.6%), other studies have shown that there is a diversity of methanogens. In addition, CH4 production also varies with animal species, DMI, type of forage fed, concentrate-to-forage ratio, efficiency of feed conversion, plant secondary metabolites, and rumen fermentation characteristic, e.g., VFA, hydrogen (H2) etc (Tajima et al., 2001; Wright et al., 2006, 2009; Wadhwa et al., 2016). A large population of methanogens exists in the rumen; however, there has been no straight-forward consensus on the association between the total population of methanogens and the magnitude of CH4 emissions (McSweeney and Mackie, 2012). A summary of methanogenesis and microbial fermentation of dietary components in the rumen resulting in the production of VFA, CH4, CO2, and H2 emitted through eructation is presented in Fig. 1.
Fig. 1.
Schematic microbial fermentation of polysaccharides, acetogenesis, and methanogenesis in the rumen. VFA = volatile fatty acids. Boxes with bold solid lines are potential targets for suppressing CH4 emissions. Sources: Attwood and McSweeney (2008), Beauchemin et al. (2008), Morgavi et al. (2010), Patra (2012, 2016).
It has been noted that feeding grain-based diet that are high in starch instantly lowered CH4 emission (g/d and g/kg DMI); whereas, diets with forage-based diet resulted in increased CH4 emissions (Fig. 1; Wallace et al., 2014). In ruminants, 87% to 89% of enteric CH4 production is from the rumen, 11% to 13% is produced in the hindgut of gastrointestinal tract (Murray et al., 1976, 1978; Lassey et al., 1997). The general biological reactions have been described by Van Soest (1994) as follows: Glucose (C6H12O6) + ammonia (NH3) → Rumen microbes + CH4 + CO2 + VFA. Thus, ruminants require glucose and nitrogen (N) to ensure sufficient microbial protein and VFA synthesis to meet animal requirements for growth, maintenance, and production. Multiple possible pathways of glucose fermentation result in different quantities of H2 formed; therefore, quantity of CH4 produced from glucose varies depending on microbial activity and biological reactions (Janssen, 2010). In the rumen, methanogens use H2 to reduce CO2 to CH4 (Van Soest, 1994). In this pathway, which may involve coenzyme M (Miller et al., 1986), 4 mol of H2 are used to produce CH4 (Czerkawski, 1986). Formation of butyrate + H2 or acetate + butyrate + H2 have been shown to be predominant pathways under low ruminal H2 concentrations, whereas the accumulation of acetate + propionate was predominant at higher H2 concentrations (Janssen, 2010). If ruminal VFA production favors less acetate production relative to propionate (i.e., lower A:P ratio), the net equilibrium of H2 in the rumen decreases and results in reduced CH4 formation (van Nevel and Demeyer, 1996). Most rumen methanogens attain energy for their growth through a sequence of biological reduction of CO2 with H2 (methanogenesis pathway; 4H2 + CO2 → CH4 + 2H2O), whereas some methanogens use the acetogenesis pathway (CH3COOH → CH4 + CO2) (Liu and Whitman, 2008; Attwood and McSweeney, 2008). In-depth reviews of ruminal methanogenesis were provided by Ungerfeld et al. (2015, 2018) and Nakamura et al. (2010). Alternative strategies to reduce CH4 emissions in ruminants, which are discussed in the following section, may reduce enteric GHG production and simultaneously improve FCE and profitability.
4. Methane measurement methods
Emissions of CH4 from ruminants are highly variable between animal species and detection methods (Table 1). Various methods to record CH4 emissions from individual animals have been established, each with their own advantages, disadvantages, and scope of application. The most widely used techniques are the indirect respiration chambers (IRC), the sulfur hexafluoride tracer technique (SF6), the automated head-chamber system (GreenFeed system [GF]; C-Lock Inc., Rapid City, SD, USA), and laser CH4 detectors (LMD; Laser Methane Mini, Tokyo Gas Engineering Solutions, Ltd., Tokyo, Japan). Studies that compared techniques have reported inconsistent results (Hristov et al., 2017). The standard method against which other methods are benchmarked is the IRC. However, IRC are costly and labor intensive, proving prohibitive to obtain measurements on large numbers of animals. Furthermore, individual confinement within the IRC imposes restrictions of the feeding and natural behavior of the animals under study. Recently, alternative GF methods have been developed (Zimmerman and Zimmerman, 2012). The GF system is a mass flux measurement system with clustering of animals visiting the GF at specific times, depending on the unit type and housing conditions. The instrument's mode of action is to measure emissions directly from animals by delivering a small feed treat. The animals are assigned with an instrument-recognizable identifier and emissions are measured when cattle insert their heads into the instrument to consume the delivered feed. Even though this method is less stressful for animals than IRC, the method has some shortcomings. Consideration must be given to the number of animals with access to the GF, and duration of sampling, to avoid bias associated with clustering of animals visiting the GF at specific times (Hammond et al., 2016a, Hammond et al., 2016b).
Table 1.
Selected studies of methane emissions from widely used techniques1.
| Reference | Animal breed | BW, kg/animal | Ration type | DMI2, kg/d per animal | CH42, g/kg DMI | Technique used |
|---|---|---|---|---|---|---|
| Beef cattle | ||||||
| Alemu et al. (2017) | heifer | 380 to 404 | low-RFI | 7.4 | 27.4 | GF |
| high-RFI | 7.9 | 28.12 | GF | |||
| low-RFI | 6.0 | 26.5 | IRC | |||
| high-RFI | 6.3 | 26.5 | IRC | |||
| Beauchemin and McGinn (2005) | steer | 328.0 | corn-based | 6.83 | 9.2 | IRC |
| barley-based | 6.17 | 13.1 | IRC | |||
| Beauchemin and McGinn (2006) | heifer | 328.3 | high-forage (70%) | 6.2 | 21.35 | IRC |
| high-grain (56%) | 7.5 | 20.13 | IRC | |||
| Dini et al. (2019) | steer | 536.2 | high-RFI | 10.6 | 28.1 | SF6 |
| low-RFI | 9.33 | 20.3 | SF6 | |||
| Hales et al. (2015) | steer | 223.5 | steam-flake corn | 5.1 | 11.65 | IRC |
| dry-rolled corn | 5.3 | 14.06 | IRC | |||
| beef TMR | 5.2 | 12.88 | IRC | |||
| beef TMR + WDGS | 5.2 | 12.83 | IRC | |||
| Hammond et al. (2015) | heifer | 317 to 339 | grazing | |||
| period I | 7.62 | 26.6 | GF | |||
| 7.66 | 28.3 | IRC | ||||
| period II | 7.6 | 27.8 | GF | |||
| 7.54 | 27.7 | IRC | ||||
| period III | 9.15 | 18.8 | GF | |||
| 9.15 | 21.5 | SF6 | ||||
| ryegrass | 8.28 | 24.1 | GF | |||
| 10.0 | 17.3 | GF | ||||
| 8.13 | 28.4 | IRC | ||||
| 10.0 | 21.8 | SF6 | ||||
| RC | 6.86 | 29.5 | GF | |||
| 8.69 | 18.5 | GF | ||||
| 7.10 | 28.1 | IRC | ||||
| 8.69 | 23.0 | SF6 | ||||
| BT | 7.93 | 28.9 | GF | |||
| 7.51 | 29.2 | IRC | ||||
| wild flowers3 | 7.34 | 28.8 | GF | |||
| 8.78 | 19.7 | GF | ||||
| 7.42 | 25.7 | IRC | ||||
| 8.78 | 19.5 | SF6 | ||||
| Hammond et al. (2015) | heifer | 317 to 339 | ryegrass | 10.0 | 21.8 | SF6 |
| RC | 8.69 | 23.0 | SF6 | |||
| wild flowers3 | 8.78 | 19.5 | SF6 | |||
| steer | 519 | feedlot | 7.2 | 15.0 | GF | |
| Herd et al. (2016) | roughage | 7.6 | 19.0 | GF | ||
| Jonker et al. (2016) | Hereford/Friesian heifer | 382 | alfalfa silage-based | |||
| period I | 5.9 | 24.0 | IRC | |||
| 5.9 | 22.7 | SF6 | ||||
| 5.9 | 24.3 | GF | ||||
| period II | 7.5 | 24.5 | IRC | |||
| 7.1 | 22.2 | SF6 | ||||
| 7.2 | 24.7 | GF | ||||
| period III | 8.3 | 24.6 | IRC | |||
| 8.4 | 22.6 | SF6 | ||||
| 8.3 | 26.6 | GF | ||||
| period IV | 10.9 | 24.5 | IRC | |||
| 12.2 | 22.4 | SF6 | ||||
| 12.1 | 26.8 | GF | ||||
| McCaughey et al. (1997) | steer | 356.2 | rotational stocking | SF6 | ||
| HSR | 14.94 | 17.65 | ||||
| LSR | 13.61 | 20.85 | ||||
| continuous stocking | ||||||
| HSR | 13.51 | 17.92 | ||||
| LSR | 13.20 | 23.23 | ||||
| McGinn et al. (2009) | steer | 381.2 | barley grains (35%) | 9.5 | 23.8 | SF6 |
| corn DDGS (35%) | 9.0 | 19.9 | ||||
| Pedreira et al. (2013) | steer | 444.3 | with no concentrate, 100% SS | 5.52 | 22.76 | SF6 |
| 30% concentrate + 70% SS | 7.9 | 18,97 | ||||
| 60% concentrate + 40% SS | 8.7 | 16.13 | ||||
| Tomkins et al. (2015) | steer | 226.8 | chopped Rhodes grass | 5.4 | 14.60 | IRC |
| Dairy cattle | ||||||
| Aguerre et al. (2011) | Holstein | 620.7 | forage-to-concentrate ratio | IRC | ||
| 47:53 | 18.2 | 25.9 | ||||
| 54:46 | 18.4 | 28.2 | ||||
| 61:39 | 17.6 | 29.1 | ||||
| 68:32 | 17.5 | 31.9 | ||||
| Arbre et al. (2016) | dairy cow | 723 to 729 | 60% hay + 40% grains | 9.7 | 23.6 | SF6 |
| silage-based diet | 23.8 | 17.4 | GF | |||
| Bharanidharan et al. (2018) | Holstein | 540.3 | TMR | 12.3 | 11.3 | IRC |
| roughage, concentrate | 10.2 | 10.3 | ||||
| Dini et al. (2012) | Holstein | 536.2 | legume-dominated | 17.3 | 29.4 | SF6 |
| grass-dominated | 16.8 | 31.0 | ||||
| Hammond et al. (2014) | Holstein/Friesian | 339.8 | rye grass | 8.03 | 28.4 | SF6 |
| RC | 7.06 | 28.0 | ||||
| BT | 7.61 | 28.9 | ||||
| Hristov et al. (2015) | dairy cow | 653 | control (n = 7) | 26.7 | 20.4 | GF |
| TCNSL4 (n = 7) | 26.6 | 18.9 | GF | |||
| Grainger et al. (2007) | Holstein-Friesian | 496.6 | ryegrass | 19.9 | 21.8 | SF6 |
| Lee et al. (2004a) | Holstein/Friesian | ryegrass with no WC | 15.6 | 21.7 | SF6 | |
| ryegrass + 15% WC | 17.6 | 20.9 | ||||
| ryegrass + 30% WC | 18.6 | 18.6 | ||||
| ryegrass + 60% WC | 20.5 | 18.1 | ||||
| Odongo et al. (2006) | Holstein cow | 620.6 | dairy TMR | 19.7 | 23.3 | IRC |
| dairy TMR + 24 mg Rumensin | 19.1 | 22.4 | ||||
| Olijhoek et al. (2017) | Holstein | 647.6 | high-RFI/low-concentrate | 20.9 | 30.7 | IRC |
| high-RFI/high-concentrate | 23.7 | 21.4 | ||||
| low-RFI/low-concentrate | 18.6 | 32.4 | ||||
| low-RFI/high-concentrate | 21.6 | 24.5 | ||||
| Jersey | 469.2 | high-RFI/low-concentrate | 15.0 | 32.6 | ||
| high-RFI/high-concentrate | 17.8 | 28.2 | ||||
| low-RFI/low-concentrate | 14.9 | 32.5 | ||||
| low-RFI/high-concentrate | 17.0 | 27.9 | ||||
| Rischewski et al. (2017) | Holstein | 655 | TMR + silages | |||
| period I | 16.9 | 20.0 | GF | |||
| period II | 20.6 | 18.4 | ||||
| period III | 20.1 | 20.7 | ||||
| Wims et al. (2010) | Holstein/Friesian | 493.8 | mixed forage | SF6 | ||
| low-forage mass5 | 16.9 | 17.0 | ||||
| high-forage mass5 | 15.4 | 18.7 | ||||
| Woodward et al. (2002) | Friesian/Jersey | ryegrass | 13.1 | 19.3 | SF6 | |
| sulla | 10.7 | 24.3 | ||||
| Woodward et al. (2004) | Friesian | 538 | ryegrass | 14.9 | 24.2 | SF6 |
| ryegrass + PEG | 14.9 | 24.7 | ||||
| BT | 17.4 | 19.7 | ||||
| BT + PEG | 17.1 | 22.9 | ||||
| Waghorn et al. (2016) | Holstein/Friesian | 520 | LSR6 (n = 4 periods) | 15.4 | 21.8 | GF |
| HSR6 (n = 4 periods) | 13.7 | 22.7 | ||||
DMI = dry matter intake; RFI = residual feed intake; GF = GreenFeed system (C-Lock Inc., Rapid City, SD, USA); IRC = indirect respiratory chamber; SF6 = sulfur hexafluoride tracer technique; TMR = total mixed ration; WDGS = wet distiller's grains with solubles; DDGS = dry distillers' grains with solubles; SS = sorghum silage; RC = red clover (Trifolium pratense); BT = birdsfoot trefoil (Lotus corniculatus); WC = white clover (Trifolium repens); PEG = polyethylene glycol.
All data are presented in their original units of the literature. A graphical comparison of calculated CH4 missions per DMI is presented in Fig. 2.
Dry matter intake and CH4 yield were used to calculate daily CH4 emissions. Animals of different ages and BW were used in the dairy and beef data sets, with correspondingly different DMI and CH4 production values.
Wild flowers are mixtures of a ryegrass (Lolium perenne) and flowers (Hammond et al., 2014).
TCNSL is the basal diet with 30 g/cow per day of technical grade cash-nut-shell liquid (data collected from 7 cows).
Low-forage mass, 100 kg DM/ha; High-forage mass, 2,200 kg DM/ha.
LSR and HSR means low- and high-stocking rates.
Another extensively used technique to determine enteric CH4 emissions is the SF6 tracer method (Zimmerman, 1993). For this method, a bolus containing SF6 is deposited in the rumen and concentrations of SF6 and CH4 in the breath are analyzed by gas chromatography. The concentration of CH4 is corrected for dilution by external air from the measured SF6 concentration. Results with the SF6 technique have been inconsistent (Pinares-Patino and Clark, 2008; Pinares-Patino et al., 2011), but the modifications by Deighton et al. (2014) decreased the most serious sources of error.
Another new measurement method of CH4 is the LMD, a hand-held open path laser measuring device, namely tunable diode laser absorption spectroscopy. It was originally developed for the detection of gas leaks, and therefore, discriminates between high CH4 concentrations and the low background concentration in the atmosphere (Crowcon, 2014). However, there was not a strong relationship LMD and the dynamics of CH4 concentrations exhaled by ruminants (Chagunda, 2013) and dairy wastewater (Todd et al., 2011). When it is used to study the CH4 emission of animals, an operator points the device a fixed distance from the snout of a cow for a duration of several minutes, once or multiple times a day, and the cumulative CH4 concentration along the laser path is quantified and recorded in real-time for large groups of animals. A variant of the LMD method is to determine atmospheric CH4 concentrations down-wind and up-wind of a group of animals grazing a paddock or in large confined areas such as a beef feed yard (Todd et al., 2016). Therefore, it is of great value for researchers to know and understand the extent of variability and comparability among the available measurement methods prior to investing considerable time and instrumentation/labor expense in recording emissions from a large number of animals.
5. Interrelationships between methane production, DMI and FCE
Results from a known set of published experiments examining the effects of CH4 detection method, cattle type (beef vs. dairy) and DMI were assembled and summarized in Table 1. A simple regression analysis using Proc Reg in SAS (SAS, 2004) was conducted to evaluate the extent to which diets and DMI were related to CH4 emissions from cattle.
5.1. Dry matter intake and methane emissions
When the regression analysis was conducted using the data in Table 1, CH4 production was strongly correlated with DMI as described by Eq. (1).
| Eq. (1) |
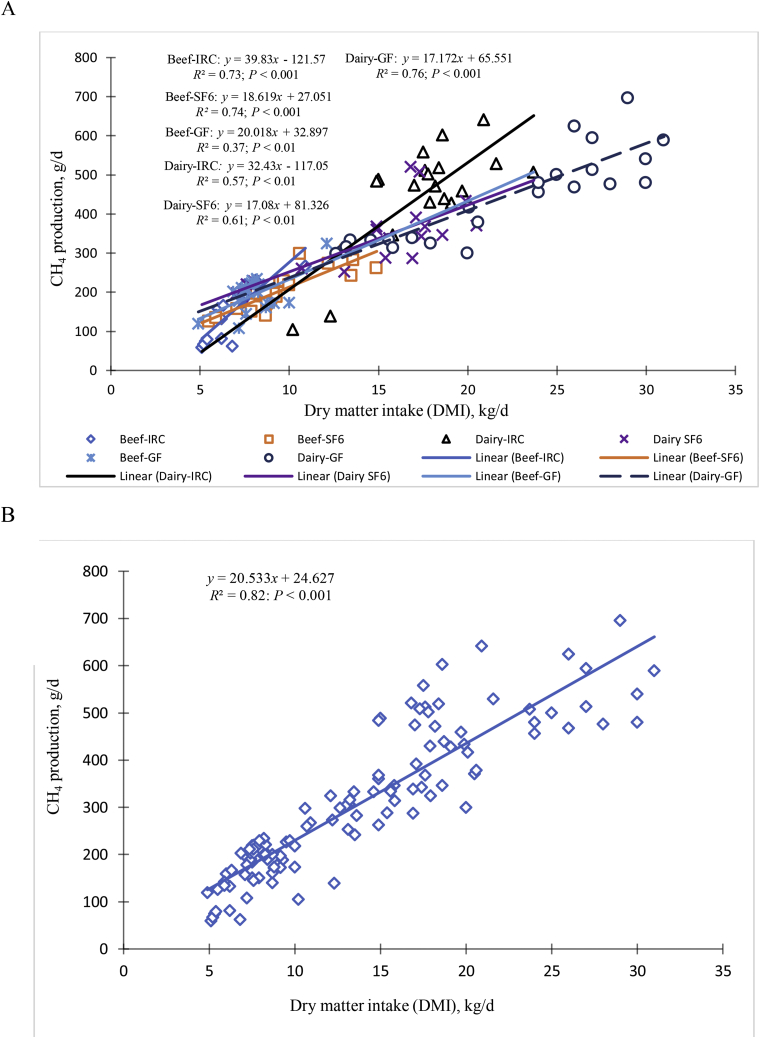
Each 1.0-kg increase in DMI increased CH4 production by an average of 20.53 (±0.87) g per kg of DMI in beef and dairy cattle. However, there was not a strong relationship (Fig. 2A) between different CH4-measurement methods (IRC, SF6, and GF). The slope of the proposed general relationship between CH4 and DMI was similar to previously published values which range from 17.0 to 25.0 g/kg DMI (Clark et al., 2005; Grainger et al., 2007; Yan et al., 2009; Dijkstra et al., 2011; Charmley et al., 2016). Our average predicted relationship (CH4 at 20.53 g/kg DMI) was similar to the 20.7 g/kg DMI found in Australian cattle (Charmley et al., 2016) and by other researchers (19.1 g/kg DMI; Hristov et al., 2013a, b; Clark et al., 2005) for CH4 estimation from beef and dairy cattle. Animals of different ages and body weights were used in the dairy and beef data sets, with correspondingly different DMI and CH4 production values. Measurements in the dairy dataset were from lactating Holstein-Friesian dairy cows with a high DMI and high CH4 production, whereas measurements in the beef dataset were from growing/finishing steers or non-lactating heifers with lower DMI and low CH4 production. Therefore, it makes direct comparisons more difficult because the DMI ranges are varied between dairy and beef cattle. It is also difficult to measure the DMI on animals grazing in natural environment. However, Eq. (1), based on the data from Table 1 and presented in Fig. 2B, provides a rather simple means to estimate daily CH4 production by cattle based solely on their DMI when no other information is available. An R2 of 82% implied that DMI strongly influenced CH4 production. Since most dairies and feedlots know their cattle's DMI, an inventory of CH4 emission is possible.
Fig. 2.
Selected studies of methane emissions from widely used techniques. (A) Effects of dry matter intake (DMI) on daily CH4 emissions in dairy and beef cattle associated with detection methods, and (B) effects of DMI on average daily CH4 emissions in dairy and beef cattle. SF6 = sulfur hexafluoride tracer technique; IRC = indirect respiration chamber; GF = GreenFeed system (C-Lock Inc., Rapid City, SD, USA). Source: adapted from Table 1.
Most national inventories assume CH4 production is linear with DMI (Hristov et al., 2013b; Miller et al., 2013; Niu et al., 2018). This assumes that CH4 production is constant for all DMI values and there is a 0-intercept in the prediction equation (Charmley et al., 2016). However, Cottle and Eckard (2018) reported that the differences in CH4 production values from beef cattle studies using different CH4-measurement methods, cattle breeds, diets, and geographic location are so diverse that a universal CH4 production value may not be recommended at this stage. This agrees with our current study which indicated that the 3 different CH4-measurement methods (IRC, SF6, and GF) may misrepresent the relationship between daily CH4 production and DMI (g/kg DMI; Fig. 2A). Based on the present study, average estimate of CH4 production (g/d) varied among the 3 measurement techniques, mean (±SE) values were 39.8 ± 5.48 (beef-IRC), 18.6 ± 2.36 (beef-SF6), 20.0 ± 6.86 (beef-GF) for beef cattle, and 32.4 ± 3.33 (dairy-IRC), 17.1 ± 6.40 (dairy-SF6), and 17.2 ± 2.22 (dairy-GF) for dairy cattle. Overall CH4 emissions determined using GF and SF6 were significantly lower (P < 0.05) than those measured using IRC technique. Differences in daily CH4 production between GF and other techniques are likely due to the short duration of the CH4 measurements obtained for each animal (Hammond et al., 2015). The variation in DMI for beef cattle with the GF was much smaller than the variation in measured CH4 production with that system, which likely was a large contributor to the low R2 value. An argument in support of the GF measurement systems is that data are collected several times over the course of a day to arrive at an estimated daily emission rate. The GF measurements can be made over days and weeks, whereas other techniques are difficult to implement for more than a few days due to high labor costs. A limitation of GF and SF6 breath measurement systems is how could CH4 production from the hind gut be measured with GF and SF6 (Murray et al., 1976, 1978). Using an isolated tracer method and cannulated sheep, Murray et al. (1976, 1978) estimated that the hindgut of ruminants produces approximately 11% to 13% of total enteric CH4 emissions. Two explanations for lower values of GF and SF6 are possible: 1) CH4 production by the hindgut is much greater impact; 2) CH4 losses from hindgut are being measured (Murray et al., 1978) despite the precautions that are taken to prevent such occurrence. As with all short-term CH4-measurement techniques, cumulative daily CH4 production may be under- or over-estimated because of diurnal patterns in CH4 emission rate over a 24-h period — emissions will differ based on animal activity, time since feeding, and other factors (Hammond et al., 2016b). Strong diurnal patterns of ruminal concentrations of VFA, pH, and bacterial community changes have been reported in sheep (Kristensen et al., 1996) and dairy cows (Palmonari et al., 2010). However, all 3 methods support that: 1) DMI is a strong determinant of CH4 production, and 2) the average rate of CH4 production is between 15 and 25 g per kg of DMI.
5.2. Feed conversion efficiency and CH4 emissions
In one investigation, Fox et al. (2001) confirmed that a 10% improvement in FCE had a much greater impact on feedlot profitability than a similar improvement in average daily gain (ADG; Table 2). Results from Table 2 indicated that a 10% increase in ADG improved estimated profits by 18% compared to a control. In contrast, when DMI remained the same and there was a 10% improvement in FCE, ADG increased by 11%, and resulted in a 43% increase in estimated profits. Therefore, genetic selection for animals that have a superior FCE (e.g., efficient cattle) could potentially increase profits more than selection for a higher ADG.
Table 2.
Effect of improvement in average daily gain (ADG) and feed conversion efficiency (FCE) on steer profitability1.
| Item | Average | 10% higher ADG | 10% higher FCE2 |
|---|---|---|---|
| DMI, kg/d | 8.5 | 9.1 | 8.5 |
| ADG, kg/d | 1.45 | 1.60 | 1.63 |
| Feed to gain | 5.86 | 5.68 | 5.21 |
| Feed cost, $ | 176 | 172 | 157 |
| Non-feed cost, $ | 98 | 91 | 89 |
| Total cost of gain3, $ | 274 | 263 | 246 |
| Profit, $ | 65 | 77 | 93 |
DMI = dry matter intake.
Computed with Cornell Value Discovery System (Tedeschi et al., 2001; Fox et al., 2001).
FCE is the ratio of feed to gain.
Total cost of gain = Feed cost + Non-feed cost.
It has been shown that both FCE (heritability [h2] = 0.29) and net feed efficiency (NFE; h2 = 0.39) are moderately heritable in growing Angus cattle (Table 3). Therefore, it may be possible to selectively breed cattle that ingest less feed without reduced performance because of improvements in feed efficiency (Carstens, 2019), which would lead to increased cost-effectiveness. However, selecting dairy cows for feed efficiency and lower emissions may be more difficult than it is for beef cattle because of a host of factors need to be considered, such as stage of lactation, milk components, somatic cell counts, body condition score, feed efficiency and feed intake. Therefore, one alternative strategy of mitigating CH4 emission might involve selective breeding for animals that are lower CH4 emitters because of higher FCE, resulting in lower energy losses as CH4. However, such a strategy has yet to be evaluated.
Table 3.
Heritability (h2) estimates (±SE) and genetic correlations among growth and efficiency traits in Angus cattle1.
| Trait | ADG | BW | DMI | FCE | NFE or RFI |
|---|---|---|---|---|---|
| ADG | 0.28 ± 0.04 | 0.53 ± 0.07 | 0.54 ± 0.06 | −0.63 ± 0.06 | −0.04 ± 0.08 |
| BW | – | 0.04 ± 0.01 | 0.65 ± 0.03 | −0.01 ± 0.07 | −0.06 ± 0.06 |
| DMI | – | – | 0.39 ± 0.03 | 0.31 ± 0.07 | 0.69 ± 0.08 |
| FCE | – | – | – | 0.29 ± 0.04 | 0.66 ± 0.05 |
| NFE | – | – | – | – | 0.39 ± 0.03 |
ADG = average daily gain; BW = body weight; DMI = dry matter intake; FCE = feed conversion efficiency (feed DMI per unit weight gain); NFE = net feed efficiency; RFI = residual feed intake.
Adapted from Arthur et al. (2001). n = 1,180 young Angus bulls and heifers.
5.3. Residual feed intake (RFI) and methane emissions
Traditionally, beef cattle efficiency measures were dependent on FCE, the ratio of feed intake to ADG. Animals with a low FCR consume less feed per kilogram of ADG, whereas animals with higher FCR consume more feed per unit of ADG. However, the primary limitation of FCR is that it represents a gross measure of feed intake; it does not evaluate yet between maintenance and growth requirements (Carstens and Tedeschi, 2006). In contrast, RFI is a measure of feed efficiency that is calculated as the difference between actual and expected feed requirements, which is obtained from feeding beef total mixed ration diet, or regression for BW maintenance against some measure of production for meat and milk (Koch et al., 1963; Arthur et al., 2001; Basarab et al., 2003; Nkrumah et al., 2006). The RFI is identified as the measure of method when determining efficiency in beef cattle (Table 3; Nkrumah et al., 2006; Hegarty et al., 2007; Herd and Arthur, 2009). However, the relationship between RFI and CH4 emissions in beef and dairy cattle is weak (Waghorn and Hegarty, 2011). Hegarty et al. (2007) reported a significant relationship between CH4 emission and RFI for Angus steers. However, RFI accounted for only a small proportion of the variations in CH4 production. The data suggest that animal selection could only reduce CH4 loss per kilogram of DMI by 10% to 20% (Waghorn et al., 2006). Myer et al. (2017) also reported that genetic markers associated with RFI and feed efficiency have been difficult to identify, and differing genetics, feed supplementation, and environments among studies contribute to great variation and elucidation of results.
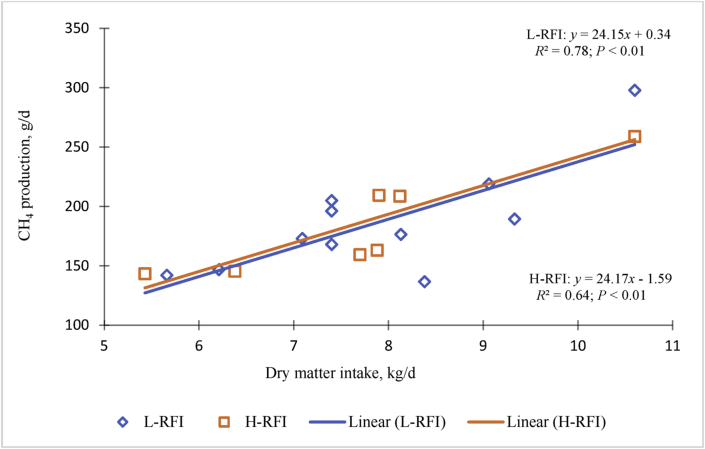
The relationships between DMI and CH4 emissions (g/d) in low-RFI (y = 24.5x + 0.34; R2 = 0.64; P = 0.01) and high-RFI (y = 24.17x – 1.59; R2 = 0.64; P = 0.01) beef cattle are presented in Fig. 3. The results indicate that there was no significant reduction in CH4 as a function low-RFI (<0; more efficient) and high-RFI (>0; less efficient) beef cattle. These findings demonstrate that differences in CH4 production may not be directly associated with RFI, but rather they are due to RFI-induced differences in DMI (Freetly et al., 2015). However, several studies have shown a positive relationship between RFI and CH4 production but the effect of RFI on CH4 is not consistent across all studies (Freetly and Brown-Brandi, 2013; Carberry et al., 2014; McDonnell et al., 2016; Flay et al., 2019). If animals are selected for reduced methane production, feed efficiency will be increased by the amount of energy conserved from CH4 production, which is small. However, there are several physiological mechanisms, which have no effect on CH4 production, that can result in increased feed efficiency as discussed by Herd and Arthur (2009). Selection for efficiency is probably mostly by these mechanisms and in some cases by reduction in CH4 production. Waghorn and Hegarty (2011) reported no differences in CH4 production among dairy cows with differing RFI, which is similar to the findings in Fig. 3. Comparable results were reported by McDonnell et al. (2016), in which CH4 production did not differ between heifers with high- and low-RFI. When adjusted for DMI, CH4 yields (g/kg DMI) were similar for high- and low-RFI heifers, using GF method (27.7 and 28.5 g/kg DMI, respectively) and respiration chambers (26.5 and 26.5 g/kg DMI, respectively; Alemu et al., 2017). Recently, Flay et al. (2019) reported that RFI did not affect either CH4 emission per day or CH4 emission per kilogram BW in dairy heifers; however CH4 emission per kilogram of DMI was higher in low-RFI heifers than high-RFI heifers because of their lower DMI. No differences in abundances of methanogen species were observed between animals ranked as both substrates have a higher or lower RFI across 2 dietary energy concentrations (a low energy + high forage vs. a high energy + low forage) (Carberry et al., 2014). However, Zhou et al. (2009) reported a greater proportion of Methanosphaera stadtmanae and Methanobrevibacter sp. AbM 4 in high-RFI cattle compared to low-RFI cattle. Miller et al. (1986) explained that M. stadtmanae utilizes methanol, whereas Methanobrevibacter sp. AbM4 utilizes acetate as its main substrate for CH4 production (Zhou et al., 2009, 2010). These results suggest beef cattle with microbiomes prefer organic methanogenesis substrates with a higher RFI (Basarab et al., 2013). It is also important to note that differences in dietary energy concentration can affect associations between RFI and overall methanogen profiles in Hereford × Aberdeen Angus steers (Zhou et al., 2010).
Fig. 3.
Feed dry matter intake and methane production from beef cattle selected for variance in residual feed intake (RFI). Low (L)-RFI are efficient, high (H)-RFI are inefficient. RFI is expected feed requirements for maintenance and growth, with the expected feed requirements obtained by regression of feeding standards formula. IRC = indirect respiratory chamber; GF = GreenFeed system (C-Lock Inc., Rapid City, SD, USA). Sources: Dini et al. (2019; beef, BW = 357 kg BW; SF6); Hegarty et al. (2007; beef, BW = 590 kg; SF6); Alemu et al. (2017; beef heifers, BW = 380 kg; IRC and GF); Mercadante et al. (2015; cattle, BW = 238 to 326 kg BW; SF6); Flay et al. (2019; dairy cattle, BW = 448 kg; GF); Lansink, (2018; beef, BW = 269 kg; GF).
5.4. Interaction of rumen microbiota with other parameters
Animals that consumed a concentrate-based diet had lower CH4 emissions than those fed a forage-based diet (Wallace et al., 2014; Roehe et al., 2016). This variation was due to higher propionic acid production [decrease A:P ratio] from digestible carbohydrates in the rumen, which leads to reduction of H2 available for typical CH4 producing pathway (Beauchemin and McGinn, 2005; Cottle and Eckard, 2018). Thus, CH4 reduction strategies that reduce available H2 may be antagonistic to cellulose digestion (Wolin et al., 1997). In addition, lower A:P ratios and higher phylum Firmicutes populations related to higher ADG (Waghorn and Barry, 1987; Myer et al., 2015). Recently, Kim et al. (2018) reported that supplementation of acetogenic bacteria isolated from Korean native goats decreased methanogenic archaea. Acetogens undertake reductive acetogenesis, which is a substitute for the typical H2-using pathway; therefore, acetogens may function as a net H2 sink that reduces CH4 emissions (van Nevel and Demeyer, 1996). However, the primary cellulolytic bacterial species and protozoa in the rumen are H2 producing microbes; thus, counteracting CH4 reduction strategies that reduce available H2 may slow cellulose digestion (Latham and Wolin, 1977; Ungerfeld, 2015).
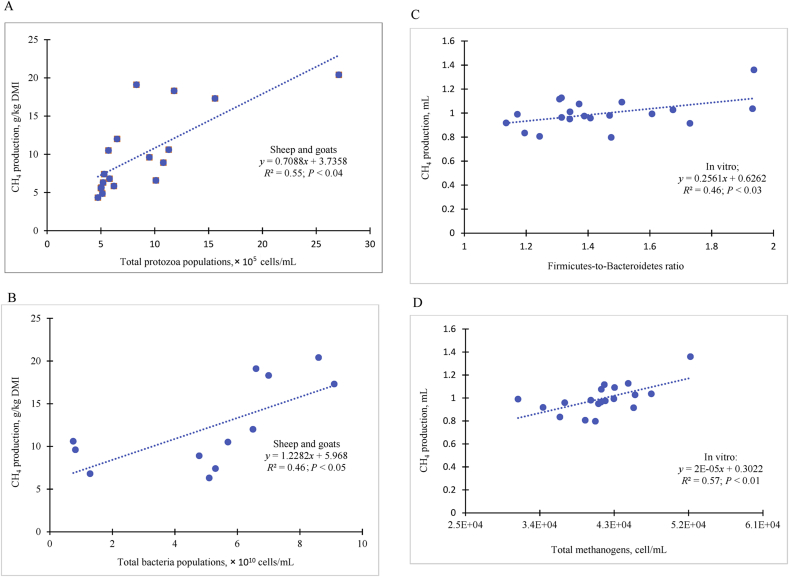
The number of methanogenic archaea may not be a strong determinant of CH4 production, but rather the metabolic activity of individual methanogenic species (Shi et al., 2014). However, Wallace et al. (2014, 2015) reported that the ratio of archaea to bacteria in the rumen could be used to estimate CH4 emissions (R2 = 0.49) in beef cattle fed high- and medium-levels of concentrate in their diets. These authors argue that methanogenesis is the only mechanism of ATP synthesis for methanogens and therefore, there should be a relationship between CH4 production and the concentration of methanogens in the rumen. A positive correlation between CH4 production and abundance of Methanobrevibacter species has been reported in dairy cows (Danielsson et al., 2012, 2017). This agrees with data from present study which indicated that a positive correlation exists between populations of total protozoa (R2 = 0.55; P < 0.04; Fig. 4A) and total bacterial population (R2 = 0.46; P < 0.05; Fig. 4B) per unit of forage-based DMI and CH4 emissions (g/d) in sheep and goats. In addition, CH4 production was strongly correlated with Firmicutes-to-Bacteroidetes ratio (F:B) (Fig. 4C) and total methanogens (Fig. 4D). A similar relationship between the relative abundance of M. gottschalkii and high CH4 production, and the relative abundance of M. ruminantium and low CH4 production, have been reported (Shi et al., 2014; Danielsson et al., 2017) in sheep and dairy cattle.
Fig. 4.
Effects of predominant bacterial phylum, total protozoa, and methanogen on methane emissions (A) Relationships between CH4 productions and populations of total protozoa, (B) total bacteria, (C) Firmicutes-to-Bacteroidetes ratio, and (D) total methanogens in the rumen and in vitro rumen incubations. Source: Animut et al. (2008a, b; respiratory chamber), Liu et al. (2011; respiratory chamber), Puchala et al. (2005, 2012a, b; respiratory chamber), Min et al. (2019a; in vitro).
A possible explanation for this could be competition for the same substrate, as Methanobrevibacter species are hydrogenotrophs (Leahy et al., 2013) and use H2 and/or formates as substrates for CH4 production. Therefore, different methanogenic species could have an advantage at different H2 concentrations and/or respond differently (because of different methanogenic enzymes; Reeve et al., 1997) to produce CH4 (Kittelman et al., 2014). These results implied that the dominant types in the rumen microbial community (F:B ratio), total protozoa, and total methanogen populations might have a role in adapting host biological parameters to reduce CH4 production and can potentially be utilized to estimate CH4 emissions (Chen et al., 2017). Chen et al. (2017) reported that the abundances of Firmicutes and the F:B ratio were strongly correlated with reduced CH4 production. These same authors stated that Firmicutes populations were linked to lower VFA levels when CH4 production was high, indicating that the F:B ratio could be used as an indicator to study gut microbiome and GHG emissions. Addition of tannins in the diets increased Firmicutes and F:B ratio in the rumen (Min et al., 2014a; Carrasco et al., 2017), which improved ADG due to lower A:P ratio and CH4 production (Min et al., 2019a, b). However, Wright et al. (2009) attributed modifications in methanogenic diversity to dietary alteration, whereas other studies reported that variations in methanogenic diversity were due to DMI (Ungerfeld, 2018), diet composition (Wright et al., 2009), host traits (Zhou et al., 2010; Roehe et al., 2016), and geographical range (Henderson et al., 2015). Recently, Roehe et al. (2016) reported that methanogenesis genes (e.g., methyl coenzyme M reductase [mcrA] and molybdenum formylmethanofuran dehydrogenase B [fmdB]) were coupled with CH4 emissions, but host microbiome cross talk genes (e.g., GDP-l-fucose synthetase [TSTA3] and l-fucose isomerase [Fucl]) were related to FCE. Published data also showed that higher rumen particulate passage was linked with lower rumen H2 concentrations, reduced CH4 generation, and increased propionate production (Janssen, 2010). Dairy cows which consumed a white clover legume silage had a higher level of milk production, higher rates of rumen passage and fermentation and higher levels of voluntary feed intake than cows consuming grass silage (Thomson et al., 1985; Auldist et al., 1999; Dewhurst et al., 2003). Different feeds produce different ruminal passage rate (Owens and Hanson, 1992). To understand digestion of different feeds, it is important to know rates of passage. However, little work has been done on differences in passage rates among types of forage diets and CH4 production per unit of DMI per day.
5.5. Other strategies for methane emissions
Several potential enteric CH4 mitigation strategies (Fig. 5) have been proposed, including use of CH4 inhibitors (e.g., halogenated compounds, nitrate), probiotics (e.g., yeast, acetogen probiotics), oilseeds, essential oils, dietary fat, micro-algae, plant constituents (e.g., tannins, saponins), propionate enhancers, immunization against CH4 oxidation, improvements in forage quality, and genetic selection of low CH4 producing ruminants (Beauchemin et al., 2008; Hristov et al., 2013a, b). Ionophores have not been proposed but have been used for more than 40 years commercially. The most effective strategy for reducing CH4 emissions will likely incorporate several of these mitigation strategies (Beauchemin et al., 2008). These approaches have emerged as means to decrease CH4 production; however, additional studies are needed before these practices can be recommended to livestock producers.
Fig. 5.
Methane emission abatement strategies for ruminants. FCE = feed conversion efficiency, RFI = residual feed intake. Low (L)-RFI are efficient, high (H)-RFI are inefficient. RFI is expected feed requirements for maintenance and growth, with the expected feed requirements obtained by regression of feeding standards formula. Sources: Arthur et al. (2001), Beauchemin et al. (2007, 2008), Broucek (2018), Charmley et al. (2016), Carstens, (2019), Hegarty et al. (2007), Hristov et al. (2013a, b), Min and Solaiman (2018), Nkrumah et al. (2006), Patra et al. (2017), Roeche et al. (2016), Ross et al. (2013), Tavendale et al. (2005), and Woodward et al. (2001).
6. Plant tannins and methanogenesis
6.1. Tannins
Plant tannins occur primarily as condensed (CT) and hydrolysable tannins (HT) (Hagerman et al., 1992). Condensed tannins (or proanthocyanidins) are polyphenolic compounds of flavan-3-ol units (e.g., catechin subunits). The numerous phenolic groups in tannins can bind to various substrates (e.g., proteins, metal ions and polysaccharides) to form indigestible complexes (Haslam, 1989; Hagerman et al., 1992). Both CT and HT are varied among forages. Tannins are thought to have both beneficial and detrimental effects on feed nutritive value and animal performance. The influence of CT in the diet on the ruminal microbiota, CH4 emissions, and ruminal fermentation have been reported (Min et al., 2003b; Carulla et al., 2005; Grainger et al., 2009). Plant tannins, as feed supplements or as tanniferous forage diets, have shown a potential for reducing enteric CH4 emissions by up to 20% (Woodward et al., 2001; Waghorn et al., 2002). However, tannin-rich diets (>5% DM) can negatively affect animal production when dietary crude protein (CP) is a limiting factor, because tannins reduce absorption of amino acids in the small intestine (Waghorn, 2008).
Unlike CT, HT (e.g., gallic acid or ellagic acid) are hydrolyzed after ingestion, gallic acid and its degradation products are absorbed from the small intestine of animals and are possibly poisonous to ruminants (Hagerman et al., 1992). Strategies for formulating optimal tannin-rich diets for mitigation of enteric CH4 emissions from ruminants, without biological impacts on ruminant animal productivity, have not been established. Therefore, attention must be given so that the advantages of decreased CH4 emissions are not offset by negative properties of tannins on feed intake, digestion, metabolism, and animal productivity.
6.2. Tannin-rich diets as a potential methane mitigation strategy
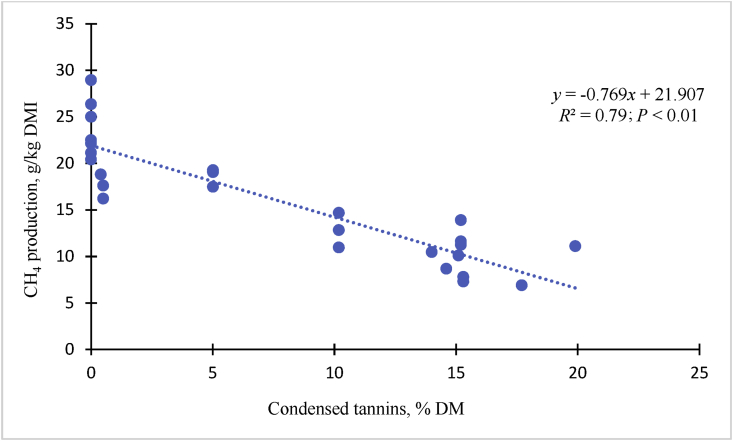
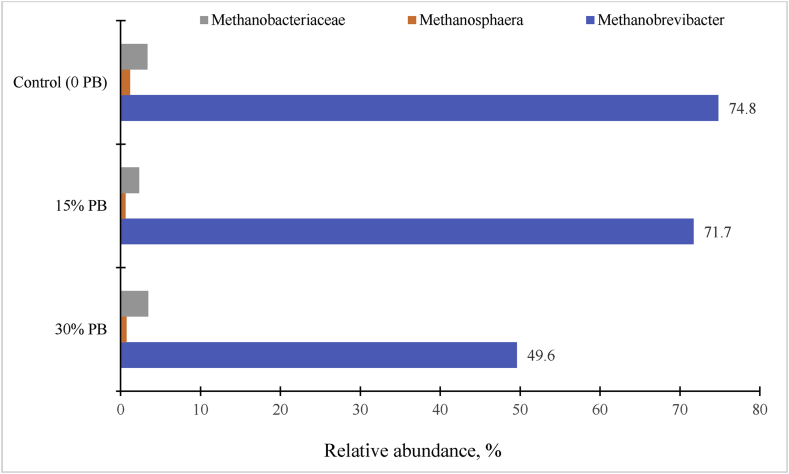
Research on CH4 inhibition strategies associated with tannin-rich diets or CT extracts in vivo have been conducted with sheep (Waghorn et al., 2002; Woodward et al., 2001, 2002; Sliwinski et al., 2004; Tiemann et al., 2008), dairy cows (Woodward et al., 2001, 2002; Beauchemin et al., 2007), goats (Puchala et al., 2005; Animut et al., 2008a, b), and beef cattle (Krueger et al., 2010). Despite this research, mechanisms of associative effects of CT and methanogenesis are not well understood. A reference scaling factor per unit of DMI is needed to compare CH4 emissions of varying tannin-rich diets (e.g., Sericea lespedeza) in both in vitro and in vivo settings. The relationship between CT (% DM) and reduces CH4 production per unit of DMI (Fig. 6) indicated that increasing CT in the diets linearly reduced CH4 emissions in meat goats (y = −0.769x + 21.91: R2 = 0.79; P < 0.01). This has been confirmed by the findings that predominant species of Methanobrevibacter spp (75%) were linearly decreased with increasing CT-containing pine bark (1.6% to 3.2% CT DM) concentration (Fig. 7), similar to that reported by Liu et al. (2011) and Min et al. (2015a) in sheep, goats, and beef cattle.
Fig. 6.
Effect of condensed tannin-rich diets on rumen methane production per kilogram of dry matter intake for meat goats. Sources: Animut et al. (2008a, b); Puchala et al. (2005, 2012a, b).
Fig. 7.
Relative abundance (%) of major gastrointestinal methanogenic archaea diversity (>0.9%) present in meat goats (n = 6) fed condensed tannins (CT)-containing ground pine bark (PB) supplementation with grain mixed diets as analyzed using pyrosequencing. Control, 0 PB, 19% CT DM; 15% PB, 1.63% CT DM; and 30% PB, 3.20% CT DM, as-fed basis (Min et al., 2014b).
Dairy cows fed diets composed of CT-rich birdsfoot trefoil (Lotus corniculatus; 2.62% CT DM) had reduced CH4 production (g/kg DMI) by 13% to 16% (Woodward et al., 2004). When wattle tannins (Acacia mearnsii; 2.5% CT DMI) were offered to sheep fed a ryegrass-based diet (Carulla et al., 2005), CH4 emissions were decreased by 13%. Grainger et al. (2009) reported that CH4 production (g/d) dropped by 14% at a low level of CT supplementation (163 g/d) and by 29% when fed at a higher level (CT at 244 g/d) in grazing dairy cows. Types of CT may not only affect CH4 production, but also have effect on the microbial community and ruminal fermentation. In addition, diets composed of CT-rich birdsfoot trefoil (Turner et al., 2005) and other CT-rich pasture species (Strom, 2012) can increase the concentration of omega-3 fatty acids (e.g., linoleic and linolenic acids) in beef adipose tissue via changing ruminal biohydrogenation of fatty acids, suggesting that CT in diets may produce potentially value-added milk in the future (Khiaosa-Ard et al., 2015).
Dairy cows fed diets composed of CT-rich birdsfoot trefoil silage or perennial ryegrass (Lolium perenne) silage had similar total CH4 emissions, but total CH4 emissions were 13% lower (g DM) from cows and 15% lower per unit of milk solids (378 vs. 434 g/kg milk solids; Woodward et al., 2001). Woodward et al. (2002) reported CH4 production of 24.6 g/kg DMI when dairy cows were grazing perennial ryegrass pasture, compared to CH4 (19.5 g/kg DMI) with CT-rich sulla (Sulla coronaria; 3.5% to 6.7% CT DM). In addition to the impact on methanogenesis, CT-rich diets fed to ruminants can have other beneficial effects on animal production (Min et al., 2003b, 2005a, b; Hoskin et al., 2003), smaller populations of gastrointestinal nematodes (Min and Hart, 2003) and improved milk quality through increased concentrations of unsaturated fatty acids (Turner et al., 2005).
Generally, the most successful enteric CH4 mitigation strategies utilizing tannins, without any detrimental effects on animal productivity, have been documented with diets that contained high levels of CP in the forages, this was 15% to 25% CP from birdsfoot trefoil, big trefoil (Lotus pedunculatus), sulla, sericea lespedeza (Lespedeza cuneate), and high-quality perennial ryegrass (L. perenne). Min et al. (2005a) studied steers grazing winter wheat forage (15% to 18% CP) with quebracho CT extract at 10 to 20 g/kg DMI. In vitro CH4 production was reduced by 25% to 51% (Min et al., 2005a, 2006b). It appears that a CT-rich diet can effectively decrease CH4 emissions per unit of DMI over a range of CP from 15% to 25%. Previous in vitro research showed that addition of plant secondary metabolites, such as CT extract (quebracho) and saponin, reduced CH4 production by 6% to 40% per unit of DM (Min et al., 2015a; Goel and Makkar, 2012). Min et al. (2015a) reported linear reduction of CH4 in the presence of quebracho (Schinopsis lorentzii), mimosa (Albizia julibrissin), chestnut (Castanea dentata) and saponin (Yucca schidigera) extracts, with increasing concentrations of plant-derived secondary metabolites. Similarly, Becker et al. (2014) reported an inhibition of CH4 production in an in vitro experiment that was linearly related with the concentration of extracted CT (e.g., catechin). They also reported that 6 hydrogen atoms per catechin molecule were retained by purified CT, and CH4 production was decreased at a rate of 1.2 mol of CH4 per mole of catechin (Becker et al., 2014).
Dominant ruminal cellulolytic bacterial species, including Fibrobacter succinogens, Ruminococcus albus, and Ruminococcus flavefaciens (Koike and Kobayashi, 2009), may influence CH4 production (Chaucheyras-Durand et al., 2010). Min et al. (2006a) reported that cultures from R. albus and R. flavefaciens produced the most H2 among dominant ruminal cellulolytic bacterial strains. In addition, these cellulolytic bacteria resulted in greater CH4 production when cultured with M. smithii compared with other co-cultured combinations. More recent research indicated that tannin-rich diets resulted in ruminal CH4 suppression through reduced methanogen population size (Min et al., 2014a, b; Christensen et al., 2017) and decreased H2 production in the rumen (Tavendale et al., 2005). Tavendale et al. (2005) reported that CT extracted from big trefoil inhibited methanogen growth rates in broth cultures, especially M. ruminantium strains.
6.3. Effects of tannin-rich diets on rumen fermentation and microbiota
The CT bind with plant proteins in the rumen because of its neutral pH, but CT-protein complexes dissociate in the acidic pH of the abomasum (Hagerman et al., 1992; Min et al., 2003). The extent to which CT interferes with protein digestion is a function of astringency, concentration, and potential sites for binding (Haslam, 1989; Hagerman et al., 1992; Waghorn, 2008). In vitro and in vivo studies have consistently shown a reduction in the growth rate of select strains, as well as increased proteolysis, as a consequence of dietary CT (McNabb et al., 1996; Molan et al., 2001; Min et al., 2005a, b). However, some strains (Clostridium proteoclasticum B316T and R. albus 8) showed transient increases in their growth rate at low concentrations (50 to 100 μg/mL) but not at high (>200 μg/mL) concentrations of CT (Min et al., 2005b).
In general, concentrations of VFA are known to affect CH4 production; higher concentrations of propionate and lower concentrations of acetate have been found to reduce CH4 emissions (Monteny et al., 2006). Rumen A:P ratio may also be associated with a lower CH4 production per unit of DMI in ruminants. As ruminal VFA production changed towards less production of acetate relative to propionate (i.e., lower A:P ratio), the net concentrations of H2 in the rumen decreased via physical intracellular hydrogenosomes, resulting in less CH4 being formed (van Nevel and Demeyer, 1996).
Tannin-rich diets can modify the rumen fermentation profiles and ruminal bacterial community diversity. Complexes of CT-protein are most commonly based on hydrophobic and hydrogen bonding in a pH dependent manner (Haslam, 1989). When CT-containing forage is consumed, CT-substrate complexes form during the processes of chewing and ruminating (Jones and Mangan, 1977). Once it across the rumen, CT can also bind to substrates (e.g., protein) and bacterial cell surfaces (Jones et al., 1994). Dietary CT (e.g., sainfoin) has been shown to induce changes in the activity of endoglucanase, the enzyme responsible for breaking internal glycoside bonds in a glucose polymer, and morphology (physical) of several species of rumen bacteria (Chiquette et al., 1988; Bae et al., 1993). Inhibition of rumen bacteria by CT is probably due to interactions between CT present in the tannin structure and the specific substrate to which it binds (e.g., protein, bacterial cell walls, etc.) (Bae et al., 1993). The addition of CT extracts to the diet reduced populations of CH4-producing archaea and some cellulolytic bacteria (R. flavefaciens) (Bhatta et al., 2009). Ruminal fungi and protozoa have been linked to CH4 formation (Khiaosa-Ard et al., 2015). The process of protozoal CH4 formation is via hydrogenosomes. This protozoa-derived H2 was associated with methanogens in the rumen (Mosoni et al., 2011). To enhance access to H2, these methanogens may be involved in a mutually beneficial relationship with rumen protozoa (Finlay and Fenchel, 1989). It has been shown that nearly 37% of CH4 from ruminants is produced by protozoa-associated methanogens (Finlay et al., 1994). Tymensen et al. (2012) confirmed that numbers of Methanobrevibactor spp. were high among the community of protozoa-associated methanogens. Furthermore, the population of methanogens was reduced when ruminants were fed diets containing CT-enriched from birdsfoot trefoil (2.7% to 4.9% CT DM; Christensen et al., 2017) and pine (Pinus) bark CT (1.6% to 3.3% CT DM) (Min et al., 2014a, b). However, a reduction in CH4 production is not always concomitant with decreased protozoa (Bhatta et al., 2009), as some tannins, e.g. peanut (Arachis hypogaea) skin CT, may decrease methanogens that are not associated with protozoa (Min et al., 2019a). Inclusion of wattle tannin extracts (a mixture of CT and HT) inhibited CH4 production in sheep by 10% and in cattle by up to 30% (Carulla et al., 2005; Grainger et al., 2009), but Min et al. (2005a) found that quebracho CT extract included at concentrations of 1 to 2 mg/mL decreased CH4 production by 12.3% to 32.6% in vitro.
Goel and Makkar (2012) have noted that the anti-methanogenic effect of tannin-rich diets depends on both the tannin concentration and the number of hydroxyl groups present in the tannin structure. Pellikaan et al. (2011a, b) reported that in vitro ruminal gas and CH4 production were highly related to the specific chemical structure of tannins, such as type of tannins (i.e., CT vs. HT), solubility, and cis–trans configuration. Several earlier studies have noted that procyanidin (PC) and prodelphinidin (PD)-types of CT may disrupt methanogenesis (Min et al., 2015a; Naumann et al., 2018).
Hydrolysable tannins (e.g., gallic acid subunits) directly constrain methanogens, but the action of CT on rumen CH4 production is variable (Goel and Markkar, 2012; Aboagye and Beauchemin, 2019). A meta-analysis from 30 experiments comprising 171 treatments showed a linear decrease in both in vitro (R2 = 0.69; n = 91) and in vivo (R2 = 0.47; n = 39) in CH4 production with increasing tannin concentrations (Jayanegara et al., 2012). However, some of the CH4 decrease was due to the concomitant decline of in vivo digestibility (R2 = 0.29) of organic matter (Jayanegara et al., 2012). Min et al. (2015a) reported that reduction rates of ruminal in vitro gas and CH4 emissions were greater in chestnut (mainly HT; gallic acids) and mimosa (black wattle; mainly catechol) tannins than in quebracho (mainly CT). This difference was probably due to greater sensitivities of some microbial species to these compounds and/or different affinities with other dietary components (e.g., binding capacity with protein) (Haslam, 1989; Hagerman et al., 1992). Wolin (1979) reported that more H2 and CH4 were produced during fermentation of fiber than starch, which related to greater propionate synthesis in starch-based diets than fiber fermentation. Vasta et al. (2019) and Min and Solaiman (2018) hypothesized that plant tannins could directly inhibit CH4 production through decreased methanogenesis pathways and reduced activities of selected rumen microbes (such as cellulolytic bacteria and protozoa) that modify conversion of substrate to H2 and acetates. Dietary fiber performs to interact with tannins through hydrogen bonds formed with free phenolic groups (Silanikove et al., 2001). Any reduction in dietary fiber digestibility is likely to reduce CH4 production because fibrolysis provides H2 as a substrate for methanogenesis in forming acetate from pyruvate (Moss et al., 2000; Tavendale et al., 2005). Therefore, plant tannins could be a useful tool for mitigation of enteric GHG emissions as a potential anti-methanogenic agent. Further research is needed to assure a sustainable supply of abundant and safe food and other livestock products, whereas reducing emissions of GHG.
7. Areas for future research
Comprehensive in vivo research on ruminants is required to assess the applicability of various dietary interventions in reducing enteric CH4 gas emissions whereas improving ruminant production without negative effects on the animal. In addition, research is needed that will deliver insight on the potential benefits of plant secondary compounds that produce animals with both reduced CH4 production and increased feed efficiency, and host-gut microbiome interactions associated with enteric CH4 emissions. Additional large-scale investigations should be carried out to find optimal tannin levels, types, and conditions to reduce GHG emissions in commercial settings.
8. Summary of findings
The potential to beneficially manipulate the rumen microbiome community structure and meet sustainable with reduce GHG emissions of ruminant production systems through an animal selection program for both reduced CH4 production and increased feed efficiency, and introduction of dietary interventions has recently progressed toward application of new technologies. Animal DMI is the single important predictor of CH4 production; however, total methanogens, total protozoa populations, and F:B ratio can significantly affect this relationship. The idea that the host animal controls its own microbiota to significant extent shows potential for implementation of effective breeding strategies. The use of relative abundance of microbial genes in the gastrointestinal tract can affect potential CH4 emissions. Strategies to mitigate GHG emissions from ruminant livestock production can improve animal performance and feed efficiency while help reducing livestock-induced atmospheric GHG emissions that contribute to global warming. One possible strategy to reduce GHG emissions is dietary modifications that include feeding tannin-rich diets to cattle and other ruminants. Properly designed CT-rich diets can reduce GHG emissions as enteric CH4 production without detrimental impacts on animal production. Therefore, GHG reduction strategies should be established to increase ruminant production efficiency, whereas minimizing losses of CH4 and volatile organic compounds from animal agriculture.
Conflict of interest
We state that we have no financial or personal relationships with other people or organizations that can improperly influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be contributed as influencing the content of this paper.
Footnotes
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA/ARS.
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aboagye I.A., Beauchemin K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: a review. Animals. 2019;9(856):1–18. doi: 10.3390/ani9110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguerre M.J., Wattliaux M.A., Powell J.M., Broderic G.A., Arndt C. Effect of forage-to-concentrate ratio in dairy cow diets on emission of methane, carbon dioxide, ammonia, lactation performance, and manure excretion. J Dairy Sci. 2011;94:3081–3093. doi: 10.3168/jds.2010-4011. [DOI] [PubMed] [Google Scholar]
- Alemu A.W., Vyas D., Manafiazar G., Basarab J.A., Beauchemin K.A. Enteric methane emissions from low– and high–residual feed intake beef heifers measured using GreenFeed and respiration chamber techniques. J Anim Sci. 2017;95:3727–3737. doi: 10.2527/jas.2017.1501. [DOI] [PubMed] [Google Scholar]
- Alexandratos N., Bruinsma J. FAO, Food and Agriculture Organization of the United Nations; 2012. World agriculture towards 2030/2050: the 2012 revision. [Google Scholar]
- Animut G., Puchala R., Goetsch A.L., Patra A.K., Sahlu T., Varel V.H., Wells J. Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Anim Feed Sci Technol. 2008;144:212–227. [Google Scholar]
- Animut G., Puchala R., Goetsch A.L., Patra A.K., Sahlu T., Varel V.H., Wells J. Methane emission by goats consuming different source of condensed tannins. Anim Feed Sci Technol. 2008;144:228–241. [Google Scholar]
- Arbre M., Rochette Y., Guyader J., Lascoux C., Gomez L.M., Eugene M., Morgavi D.P., Renand G., Doreau M., Martin C. Repeatability of enteric methane determinations from cattle using either the SF6 tracer technique or the GreenFeed system. Anim Prod Sci. 2016;56:238–243. [Google Scholar]
- Arthur P.F., Archer J.A., Johnston D.J., Herd R.M., Richardson E.C., Parnell P.F. Genetic and phenotypic variance and covariance components for feed intake, feed efficiency and other postweaning traits in Angus cattle. J Anim Sci. 2001;79:2805–2811. doi: 10.2527/2001.79112805x. [DOI] [PubMed] [Google Scholar]
- Attwood G., McSweeney C. Methanogen genomics to discover targets for methane mitigation technologies and options for alternative H2 utilization in the rumen. Aust J Exp Agric. 2008;48:28–37. [Google Scholar]
- Auldist D.E., Atkinson K.L., Silvapulle M.J., Dellow D.W., McDowell G.H. Utilization of white clover silage fed alone or with maize silage by lactating dairy cows. Aust J Exp Agric. 1999;39:237–246. [Google Scholar]
- Bae H.D., McAllister T.A., Yanke J., Cheng K.J., Muir A.D. Effects of condensed tannins on endoglucanase activity and filter paper digestion by Fibrobacter succinogenes S85. Appl Environ Microbiol. 1993;59:2131–2138. doi: 10.1128/aem.59.7.2132-2138.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarab J.A., Price M.A., Aalhus J.L., Okine E.K., Snelling W.M., Lyle K.L. Residual feed intake and body composition in young growing cattle. Can J Anim Sci. 2003;83:189–204. [Google Scholar]
- Basarab J.A., Beauchemin K.A., Baron V.S., Ominski K.H., Guan L.L., Miller S.P., Crowley J.J. Reducing GHG emissions through genetic improvement for feed efficiency: effects on economically important traits and enteric methane production. Animal. 2013;7:303–3015. doi: 10.1017/S1751731113000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin K.A., McGinn S.M. Methane emissions from feedlot cattle fed barley or corn diets. J Anim Sci. 2005;83:653–661. doi: 10.2527/2005.833653x. [DOI] [PubMed] [Google Scholar]
- Beauchemin K.A., McGinn S.M. Enteric methane emissions from growing beef cattle as affected by diet and level of intake. Can J Anim Sci. 2006;86:401–408. [Google Scholar]
- Beauchemin K.A., McGinn S.M., Martinez T.F., McAllister T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J Anim Sci. 2007;85:1990–1996. doi: 10.2527/jas.2006-686. [DOI] [PubMed] [Google Scholar]
- Beauchemin K.A., Kreuzer M., O'Mara F., McAllister T.A. Nutritional management for enteric methane abatement: a review. Aust J Exp Agric. 2008;48:21–27. [Google Scholar]
- Becker P.M., Wikselaar P.G., Franssen M.C.R., Vos R.C.H., Hall R.D., Beekwilder J. Evidence for a hydrogen-sink mechanism of (+) catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics. 2014;10:179–189. [Google Scholar]
- Bharanidharan R., Arokiyaraj S., Kim E.B., Lee C.H., Yang W.W., Kim N.Y., Kim K.H. Ruminal methane emissions, metabolic, and microbial profile of Holstein steers fed forage and concentrate, separately or as a total mixed ration. PloS One. 2018;13:1–19. doi: 10.1371/journal.pone.0202446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta R., Uyeno Y., Tajima K., Takenaka A., Yabumoto Y., Nonaka I., Enishi O., Kurihara M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J Dairy Sci. 2009;92:5512–5522. doi: 10.3168/jds.2008-1441. [DOI] [PubMed] [Google Scholar]
- Broucek J. Options to methane production abatement in ruminants: a review. J Anim Plant Sci. 2018;28:348–364. [Google Scholar]
- Carberry C.A., Kenny D.A., Kelly A.K., Waters S.M. Quantitative analysis of ruminal methanogenic microbial populations in beef cattle divergent in phenotypic feed intake (RFI) offered contrasting diets. J Anim Sci Biotechnol. 2014;5:1–9. doi: 10.1186/2049-1891-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco J.M., Cabral D.C., Redondo L.M., Visco N.D.P., Miyakawa M.E.F. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. BioMed Res Int. 2017:1–11. doi: 10.1155/2017/9610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens G.E. 2019. Net feed intake: potential selection tool to improve feed efficiency in beef cattle.www.agribsa.co.za/Documents/BreeplanTraits/Texas AandM.pdf [Google Scholar]
- Carstens G.E., Tedeschi L.O. Beef improvement federation conference, Choctaw, MS, USA. 2006. Defining feed efficiency in beef cattle; pp. 12–21. April 18-21. [Google Scholar]
- Carulla J.E., Kreuzer M., Machmuller A., Hess H.D. Supplementation of Acasia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust J Agric Res. 2005;56:961–970. [Google Scholar]
- Chagunda M.G.G. Opportunities and challenges in the use of the Laser Methane Detector to monitor enteric methane emissions from ruminants. Animal. 2013;7:394–400. doi: 10.1017/S1751731113000724. [DOI] [PubMed] [Google Scholar]
- Charmley E., Williams S.R.O., Moate P.J., Hegarty R.S., Herd R.M., Oddy V.H., Reyenga P., Staunton K.M., Anderson A., Hannah M.C. A universal equation to predict methane production of forage-fed cattle in Australia. Anim Prod Sci. 2016;56:169–180. [Google Scholar]
- Chaucheyras-Durand F., Masséglia S., Fonty G., Forano E. Influence of the composition of the cellulolytic flora on the development of hydrogenotrophic microorganisms, hydrogen utilization, and methane production in the rumens of gnotobiotically reared lambs. Appl Environ Microbiol. 2010;76:7931–7937. doi: 10.1128/AEM.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Cheng H., Wyckoff K.N., He Q. Linkages of Firmicutes and Bacteroidetes populations to methanogenic process performance. J Int Micro Biotech. 2017;43:771–781. doi: 10.1007/s10295-016-1760-8. [DOI] [PubMed] [Google Scholar]
- Chiquette J., Cheng K.J., Costerton J.W., Milligan L.P. Effect of tannins on the digestibility of two isosynthetic strains of birdsfoot trefoil (Lotus corniculatus L.) using in vitro and in sacco techniques. Can J Anim Sci. 1988;68:751–760. [Google Scholar]
- Christensen R.G., Eun J.S., Yang Y.S., Min B.R., MacAdams J.W. In vitro effects of birdsfoot trefoil (Lotus corniculatus L.) pasture on ruminal fermentation, microbial population and methane production. Prof Anim Sci. 2017;33:451–460. [Google Scholar]
- Clark H., Pinares-Patiño C.S., de Klein C.A.M. Methane and nitrous oxide emissions from grazed grasslands. In: McGilloway D.A., editor. Grassland: a global resource. Wageningen Academic; Wageningen, the Netherlands): 2005. pp. 279–293. [Google Scholar]
- Cottle D.J., Eckard R.J. Global beef cattle methane emissions: yield prediction by cluster and meta-analyses. Anim Prod Sci. 2018;58:2167–2177. [Google Scholar]
- Crowcon . 2014. Monitoring and analysis of landfill gasses.www.crowcon.com/blog/monitoringandanalysisoflandfillgasses [Google Scholar]
- Czerkawski J.W. Pergamon Press; Oxford, New York, Toronto, Sydney, Frankfurt: 1986. An introduction to rumen studies. [Google Scholar]
- Danielsson R., Schnurer A., Arthurson V., Bertilsson J. Methanogenic population and CH4 production in Swedish dairy cows fed different levels of forage. Appl Environ Microbiol. 2012;78:6172–6179. doi: 10.1128/AEM.00675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson R., Dicksved J., Sun L., Gonda H., Muller B., Schnurer A., Bertilsson J. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton M.H., Williams S.R.O., Hannah M.C., Eckard R.J., Boland T.M., Wales W.J., Moate P.J. A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants. Anim Feed Sci Technol. 2014;197:47–63. [Google Scholar]
- Dewhurst R.J., Evans R.T., Scollan N.D., Moorby J.M., Merry R.J., Wilkins R.J. Comparison of grass and legume silages for milk production. 1. Production responses with different levels of concentrate. J Dairy Sci. 2003;86:2598–2611. doi: 10.3168/jds.S0022-0302(03)73855-7. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Zijderveld S.M., van Apajalahti J.A., Bannink A., Gerrits W.J.J., Newbold J.R., Perdok H.B., Berends H. Relationships between methane production and milk fatty acid profiles in dairy cattle. Anim Feed Sci Technol. 2011;166–167:590–595. [Google Scholar]
- Dini Y., Gere J., Briano C., Manetti M., Juliarena P., Picasso V., Gratton R., Astigarraga L. Methane emission and milk production of dairy cows grazing pastures rich legumes or rich in grasses in Uruguay. Animals. 2012;2:288–300. doi: 10.3390/ani2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini Y., Cajarville C., Gere J.I., Fernandez S., Fraga M., Pravia M.I., Navajas E.A., Ciganda V.S. Association between residual feed intake and enteric methane emissions in Hereford steers. Trans Anim Sci. 2019;3:239–246. doi: 10.1093/tas/txy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B.J., Fenchel T. Hydrogenosomes in some anaerobic protozoa resembling mitochondria. FEMS Microbiol Lett. 1989;65:311–314. [Google Scholar]
- Finlay B.J., Esteban G., Clarke K.J., Williams A.G., Embley T.M., Hirt R.P. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol Lett. 1994;117:157–161. doi: 10.1111/j.1574-6968.1994.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Flay H.E., Sherlock B.K., Macdonald K.A., Camara M., Villalobos N.L., Donaghy D.J., Roche J.R. Selecting cattle for low residual feed intake did not affect daily methane production but increased methane yield. J Dairy Sci. 2019;102:2708–2713. doi: 10.3168/jds.2018-15234. [DOI] [PubMed] [Google Scholar]
- Fox D.G., Guuiroy P.J., Tedeschi L.O. Proc. 33rd Beef Improvement Federation meeting; San Antonio, TX: 2001. Determining feed intake and feed efficiency of individual cattle fed in groups; pp. 1–18. [Google Scholar]
- Freetly H.C., Brown-Brandi T.M. Enteric methane production from beef cattle that vary in feed efficiency. J Anim Sci. 2013;91:4826–4831. doi: 10.2527/jas.2011-4781. [DOI] [PubMed] [Google Scholar]
- Freetly H.C., Lindholm-Perry A.K., Hales K.E., Brown-Brandl T.M., Kim M.S., Myer P.R., Wells J. Methane production and methanogen levels in steers that differ in residual gain. J Anim Sci. 2015;93:2375–2381. doi: 10.2527/jas.2014-8721. [DOI] [PubMed] [Google Scholar]
- Gerber P.J., Henderson B., Makkar H.P.S. Mitigation of greenhouse gas emissions in livestock production. A review of technical options for non-CO2 emissions. Anim. Prod. Health. 2013 doi: 10.1017/S1751731113000876. [FAO, United Nations, Rome] [DOI] [PubMed] [Google Scholar]
- Goel C., Makkar H.P.S., Becker K. Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentation. Br J Nutr. 2009;101:1484–1492. doi: 10.1017/S0007114508076198. [DOI] [PubMed] [Google Scholar]
- Goel G., Makkar H.P.S. Methane mitigation from ruminants using tannins and saponins. Trop Anim Health Prod. 2012;44:729–739. doi: 10.1007/s11250-011-9966-2. [DOI] [PubMed] [Google Scholar]
- Grainger T., Clarke M.J., Auldist K.A., Beauchemin K.A., McGinn S.M., Waghorn G.C. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can J Anim Sci. 2009;89:241–251. [Google Scholar]
- Grainger T., Clarke M.J., McGinn S.M., Auldist M.J., Beauchemin K.A., Hannah M.C. Methane emissions from dairy cows measured using the sulfur hexafluoride (SF6) tracer and chamber techniques. J Dairy Sci. 2007;90:2755–2766. doi: 10.3168/jds.2006-697. [DOI] [PubMed] [Google Scholar]
- Hagerman A.E., Robins C.T., Weerasuriya Y., Wilson T.C., Mcarthur V. Tannin chemistry in relation to digestion. J Range Manag. 1992;45:57–62. [Google Scholar]
- Hales K.E., Cole N.A., MacDonald J.C. Effects of corn processing method and dietary inclusion of wet distiller's grains with solubles on energy metabolism, carbon-nitrogen balance, and methane emissions of cattle. J Anim Sci. 2015;90:3174–3185. doi: 10.2527/jas.2011-4441. [DOI] [PubMed] [Google Scholar]
- Hammond K.J., Humphries D.J., Westbury D.B., Thompson A., Crompton L.A., Kirton P., Green C., Reynolds C.K. The inclusion of forage mixtures in the diet of growing dairy heifers: impacts on digestion, energy utilization, and methane emissions. Agric Ecosyst Environ. 2014;197:88–95. [Google Scholar]
- Hammond K., Humphries D., Crompton L., Green C., Reynolds C. Methane emissions from cattle: estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulfur hexafluoride tracer. Anim Feed Sci Technol. 2015;203:41–52. [Google Scholar]
- Hammond K.J., Jones A.K., Humphries D.J., Crompton L.A., Reynolds C.K. Effects of diet forage source and neutral detergent fiber content on milk production of dairy cattle and methane emission determined using GreenFeed and respiration chamber techniques. J Dairy Sci. 2016;99:7904–7917. doi: 10.3168/jds.2015-10759. [DOI] [PubMed] [Google Scholar]
- Hammond K.J., Waghorn G.C., Hegarty R.S. The GreenFeed systems measurement of enteric methane emission from cattle. Anim Prod Sci. 2016;56:181–189. [Google Scholar]
- Haslam E. Cambridge University Press; Cambridge, UK: 1989. Plant polyphenols. Vegetable tannins revisited. [Google Scholar]
- Hegarty R.S., Goopy J.P., Herd R.M., McCorkell B. Cattle selected for lower residual feed intake have reduced daily methane production. J Anim Sci. 2007;85:1479–1486. doi: 10.2527/jas.2006-236. [DOI] [PubMed] [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5:1–13. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd R.M., Arthur P.F. Physiological basis for residual feed intake. J Anim Sci. 2009;87:E64–E71. doi: 10.2527/jas.2008-1345. [DOI] [PubMed] [Google Scholar]
- Herd R.M., Velazco J.I., Arthur P.F., Hegarty R.S. Proxies to adjust methane production rate of beef cattle when the quantity of feed consumed is unknown. Anim Prod Sci. 2016;56:213–217. [Google Scholar]
- Hoskin S.O., Barry T.N., Wilson P.R. The role of plants containing secondary compounds in sustainable deer farming – a review. The Nutrition and Management of Deer on Grazing Systems. 2003:101–112. [Google Scholar]
- Hristov A., Callaway T., Lee C., Dowd S.E. Rumen bacterial, archaeal, and fungal diversity of dairy cows in response to ingestion of lauric or myristic acid. J Anim Sci. 2012;90:4449–4457. doi: 10.2527/jas.2011-4624. [DOI] [PubMed] [Google Scholar]
- Hristov A.N., Oh J., Firkins J.L., Dijkstra J., Kebreab E., Waghorn G.C., Makkar A.P.S., Adesogan A.T., Yang W., Lee C., Gerber P.J., Henderson B., Tricarico J.M. Mitigation of methane and nitroxide emissions from animal operations: I. A review of enteric methane mitigation options. J Anim Sci. 2013;91:5045–5069. doi: 10.2527/jas.2013-6583. [DOI] [PubMed] [Google Scholar]
- Hristov A.N., Oh J., Lee C., Montes M.R., Ott T., Firkins J., Dell C., Adesogan A. In: Mitigation of greenhouse gas emissions in livestock production: a review of technical options for non-CO2 emissions. FAO animal production and health. Gerber P., Henderson B., Makkar H., editors. FAO; Rome, Italy: 2013. p. 177. [Google Scholar]
- Hristov A.N., Oh J., Giallongo F., Frederic T.W., Harper M.T. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc Natl Acad Sci USA. 2015;34:10663–10668. doi: 10.1073/pnas.1504124112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov A.N., Harper M., Meinen R., Day R., Lopes J., Ott T., Venkayesch A., Randles C.A. Discrepancies and uncertainties in bottom-up gridded inventories of livestock methane emissions for the continuous United States. Environ Sci Technol. 2017;51:13668–13677. doi: 10.1021/acs.est.7b03332. [DOI] [PubMed] [Google Scholar]
- IPCC . In: Intergovernmental panel on climate change (IPCC). The physical science basis. Stocker T.F., Qin D., Plattner G.K., Tignor M., Allen S.K., Bosehung J., editors. Cambridge University Press; Cambridge, UK and NY, USA: 2013. p. 1535. [Google Scholar]
- Janssen P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol. 2010;160:1–22. [Google Scholar]
- Janssen P.H., Kirs M. Structure of the aechaeal community of the rumen. Appl Environ Microbiol. 2008;74:3619–3625. doi: 10.1128/AEM.02812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanegara A., Goel G., Makkar H.P.S., Becker K. Reduction in methane emissions from ruminants by plant secondary metabolites: effects of polyphenols and saponins. In: Odongo N.E., Garcia M., Viljoen G.J., editors. 151–157 in sustainable improvement of animal production and health. Food and Agricultural Organization of the United Nations; Rome, Italy: 2010. [Google Scholar]
- Jayanegara A., Leiber F., Kreuzer M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. Anim Physiol Anim Nut. 2012;96:365–375. doi: 10.1111/j.1439-0396.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- Jayanegara A., Goel G., Makkar H.P.S., Becker K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim Feed Sci Technol. 2015;209:60–68. [Google Scholar]
- Johnson K.A., Johnson D.E. Methane emissions from cattle. J Anim Sci. 1995;73:2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- Jones W.T., Mangan J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J Sci Food Agri. 1977;28:126–136. [Google Scholar]
- Jones G.A., McAllister T.A., Muir A.D., Cheng J. Effects of sainfoin (Onobrychis viciifolia) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl Environ Microbiol. 1994;60:1374–1378. doi: 10.1128/aem.60.4.1374-1378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker A., Molano G., Antwi C., Waghorn G.C. Enteric methane and carbon dioxide emissions measured using respiration chambers, the sulfur hexafluoride tracer technique, and a GreenFeed head-chamber system from beef heifers fed alfalfa silage at three allowances and four feeding frequencies. J Anim Sci. 2016;94:4326–4337. doi: 10.2527/jas.2016-0646. [DOI] [PubMed] [Google Scholar]
- Khiaosa-Ard R., Metzler-Zebeli B.U., Ahmed S., Muro-Reyes A., Deckardt K., Chizzola R., Böhm J., Zebeli Q. Fortification of dried distiller's grains plus solubles with grape seed meal in the diet modulates methane mitigation and rumen microbiota in Rusitec. J Dairy Sci. 2015;98:2611–2626. doi: 10.3168/jds.2014-8751. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Mamuad L.L., Choi Y.J., Sung H.G., Cho K.K., Lee S.S. Effects of reductive acetogenic bacteria and lauric acid on in vivo ruminal fermentation, microbial populations, and methane mitigation in Hanwoo steers in South Korea. J Anim Sci. 2018;96:4360–4367. doi: 10.1093/jas/sky266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelman S., Pinares-Patino C.S., Seedorf H., Kirk M.R., Ganesh S., McEwan J.C. Two different bacterial community types are linked with the low-methane emission trait in sheep. PloS One. 2014;9:1–9. doi: 10.1371/journal.pone.0103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R.M., Swiger L.A., Chambers D., Gregory K.E. Efficiency of feed use in beef cattle. J Anim Sci. 1963;22:486–494. [Google Scholar]
- Koike S., Kobayashi Y. Fibrolytic rumen bacteria: their ecology and functions. Asian-Australian J Anim Sci. 2009;22:131–138. [Google Scholar]
- Kristensen N.B., Dangfar A., Agergaard N. Diurnal patterns of ruminal concentrations and portal appearance rates of short-chain fatty acids in sheep fed a hay or a concentrate/straw diet in two meals daily. Acta Agric Scand Sect A Anim Sci. 1996;46:227–238. [Google Scholar]
- Krueger W.K., Gutierrez-Bañuelos H., Carstens G.E., Min B.R., Pinchak W.E., Gomez R.R., Anderson R.C., Krueger N.A., Forbes T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim Feed Sci Technol. 2010;159:1–9. [Google Scholar]
- Lansink N. M.Sc. Thesis. Rangeland Research Institute, University of Alberta; 2018. Performance and methane emissions of RFI selected cattle in drylot and under open range conditions. [Google Scholar]
- Lassey K.R., Ulyatt M.J., Martin R.J., Walker C.F., Shelton I.D. Methane emissions measured directly from grazing livestock in New Zealand. Atmos Environ. 1997;31:2905–2914. [Google Scholar]
- Latham M.J., Wolin M.J. Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Appl Environ Microbiol. 1977;34:297–301. doi: 10.1128/aem.34.3.297-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy S.W., Kelly R., Ronimus N., Wedlock E., Altermann A., Attwood G.T. Genome sequencing of rumen bacteria and archaea and its application to methane mitigation strategies. Animal. 2013;7:235–243. doi: 10.1017/S1751731113000700. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Woodward S.L., Waghorn G.C., Clark D.A. Methane remissions by dairy cows fed increasing proportions of white clover (Trifolium repens) in pasture. Proc NZ Grass Assoc. 2004;66:151–155. [Google Scholar]
- Liu Y., Whitman W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Anna NY Aca Sci. 2008;1125:171–189. doi: 10.1196/annals.1419.019. [DOI] [PubMed] [Google Scholar]
- Liu H., Vaddella V., Zhou D. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J Dairy Sci. 2011;94:6069–6077. doi: 10.3168/jds.2011-4508. [DOI] [PubMed] [Google Scholar]
- McCaughey K., Wittenberg K., Corrigan D. Methane production by steers on pasture. Can J Anim Sci. 1997;77:519–524. [Google Scholar]
- McDonnell R.P., Hart J.K., Boland T.M., Kelly A.K., McGee M., Kenny D.A. Effect of divergence in phenotypic residual feed intake on methane emissions, ruminal fermentation, and apparent whole-tract digestibility of beef heifers across three contrasting diets. J Anim Sci. 2016;94:303–315. doi: 10.2527/jas.2015-0080. [DOI] [PubMed] [Google Scholar]
- McGinn S.M., Chung Y.H., Beauchmin K.A., Iwaasa A.D., Grainger C. Use of corn distillers' dried grains to reduce enteric methane loss from beef cattle. Can J Anim Sci. 2009;89:409–413. [Google Scholar]
- McNabb W.C., Waghorn G.C., Peters J.S., Barry T.N. The effect of condensed tannins in Lotus pedunculatus on the solubilization and degradation of ribulose-1,5-bis phosphate carboxylase (EC 4.1.1.39; Rubisco) protein in the rumen and the sites of Rubisco digestion. Br J Nutr. 1996;76:535–554. doi: 10.1079/bjn19960061. [DOI] [PubMed] [Google Scholar]
- McSweeney C.S., Mackie R. Micro-organisms and ruminant digestion: state of knowledge, trends and future prospects. Commission on Genetic Resources for Food and Agriculture. Background Study. 2012;61:1–62. [Google Scholar]
- Mercadante M.E.Z., Caliman A.P.D.M., Canesin R.C., Bonilha S.F.M., Berndt A., Frighetto R.T.S., Branco R.H. Relationship between residual feed intake and enteric methane emission in Nellore cattle. Rev Bras Zool. 2015;44:255–262. [Google Scholar]
- Miller T.L., Wolin M.J., Zhao H., Bryant M.P. Characteristics of methanogens isolated from the bovine rumen. Appl Environ Microbiol. 1986;51:201–202. doi: 10.1128/aem.51.1.201-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.M., Steven C., Michalak A.M., Kort E.A., Andrews A.E., Biraud S.C. Anthropogenic emissions of methane in the United States. Proc Natl Acad Sci U S A. 2013;110:20018–20022. doi: 10.1073/pnas.1314392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.R., Hart S.P. Tannins for suppression of internal parasites. J Anim Sci. 2003;81(E. Suppl. 2):E102–E109. [Google Scholar]
- Min B.R., Solaiman S. Comparative aspects of plant tannins on digestive physiology, nutrition and microbial changes in sheep and goats: a review. J Anim Physiol Anim Nutr. 2018;2018:1–13. doi: 10.1111/jpn.12938. [DOI] [PubMed] [Google Scholar]
- Min B.R., Barry T.N., Attwood G.T., McNabb W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim Feed Sci Technol. 2003;106:3–19. [Google Scholar]
- Min B.R., Pinchak W.E., Fulford J.D., Puchala R. Effects of feed additives on in vitro and in vivo rumen characteristics and frothy bloat dynamics in steers grazing wheat pasture. Anim Feed Sci Technol. 2005;123–124:615–629. [Google Scholar]
- Min B.R., Attwood G.T., McNabb W.C., Molan A.L., Barry T.N. The effect of condensed tannins from Lotus corniculatus on the proteolytic activities and growth of rumen bacteria. Anim Feed Sci Technol. 2005;121:45–58. [Google Scholar]
- Min B.R., Pinchak W.E., Anderson R.C., Hume M.E. In vitro bacterial growth and ruminal microbiota populations associated with bloat in steers grazing wheat forage. J Anim Sci. 2006;84:2873–2882. doi: 10.2527/jas.2005-399. [DOI] [PubMed] [Google Scholar]
- Min B.R., Pinchak W.E., Anderson R.C., Fulford J.D., Puchala R. Effect of condensed tannins supplementation level on weight gain and in vitro and in vivo bloat precursors in steers grazing winter wheat. J Anim Sci. 2006;84:2546–2554. doi: 10.2527/jas.2005-590. [DOI] [PubMed] [Google Scholar]
- Min B.R., Wright C., Ho P., Eun J.S., Gurung N., Shange R. The effect of phytochemical tannins- containing diet on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agri Food Anal Bacteriol. 2014;4:195–211. [Google Scholar]
- Min B.R., Solaiman S., Shange R., Eun J.S. Gastrointestinal bacterial and methanogenic Archaea diversity dynamics associated with condensed tannins-containing pine bark diet in goats using 16S rDNA amplicon pyrosequencing. Internet J Microbiol. 2014:1–11. doi: 10.1155/2014/141909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.R., Pinchak W.E., Anderson R.C., Puchala R. Bloat mitigation potential of plant tannins and yucca extracts based on in vitro ruminal fermentation and methane gas production from wheat forages. Int J Res Stu Agri Sci. 2015;1:1–13. [Google Scholar]
- Min B.R., Castleberry L., Allen H., Parker D., Waldrop H., Brauer D., Willis W. Associative effect of wet distillers' grains plus solubles and tannin-rich peanut skin supplementation on in vitro rumen fermentation, greenhouse gas emissions, and microbiome changes. J Anim Sci. 2019;97:4668–4681. doi: 10.1093/jas/skz317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.R., Gurung N., Shange R., Solaiman S. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing. J Anim Sci. 2019;97:3523–3534. doi: 10.1093/jas/skz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molan A.L., Attwood G.T., Min B.R., McNabb W.C. The effect of condensed tannins from Lotus pedunculatus and Lotus corniculatus on the growth of proteolytic rumen bacteria in vitro and their possible mode of action. Can J Microbiol. 2001;47:626–633. doi: 10.1139/w01-060. [DOI] [PubMed] [Google Scholar]
- Monteny G.J., Bannink A., Chadwick D. Greenhouse gas abatement strategies for animal husbandry. Agric Ecosyst Environ. 2006;112:163–170. [Google Scholar]
- Morgavi D.P., Forano E., Martin C., Newbold C.J. Microbial ecosystem and methanogenesis in ruminants. Animal. 2010;4:1024–1036. doi: 10.1017/S1751731110000546. [DOI] [PubMed] [Google Scholar]
- Mosoni P., Martin C., Forano E., Morgavi D.P. Long-term defaunation increases the abundance of cellulolytic ruminococci and methanogens but does not affect the bacterial and methanogen diversity in the rumen of sheep. J Anim Sci. 2011;89:783–787. doi: 10.2527/jas.2010-2947. [DOI] [PubMed] [Google Scholar]
- Moss A.R., Jouan J.P., Newbold J. Methane production by ruminants: its contribution to global warming. Anim Res. 2000;49:231–253. [Google Scholar]
- Murray R.M., Bryant A.M., Leng R.A. Rates of production of methane in the rumen and large intestine in sheep. Br J Nutr. 1976;36:1–14. doi: 10.1079/bjn19760053. [DOI] [PubMed] [Google Scholar]
- Murray R.M., Brayant A.M., Leng R.A. Methane production in the rumen and lower gut of sheep given lucerne chaff: effect of level of intake. Br J Nutr. 1978;39:337–345. doi: 10.1079/bjn19780043. [DOI] [PubMed] [Google Scholar]
- Myer P.R., Smith T.P.L., Wells J.E., Kuehn L.A., Freetly H.C. Rumen microbiome from steers differing in feed efficiency. PloS One. 2015;10:1–17. doi: 10.1371/journal.pone.0129174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer P.R., Freetly H.C., Wells J.E., Smith T.P.L., Kuehn L.A. Analysis of the gut bacterial communities in beef cattle and their association with feed intake, growth, and efficiency. J Anim Sci. 2017;95:3215–3224. doi: 10.2527/jas.2016.1059. [DOI] [PubMed] [Google Scholar]
- Nagaraja T.G., Newbold C.J., van Nevel C.J., Demeyer D.I. Manipulation of ruminal fermentation. In: Hobson P.N., Stewart C.S., editors. The rumen microbial ecosystems. 2nd Eds. Blackie Academic and Professional; London. UK: 1997. pp. 523–632. [Google Scholar]
- Nakamura N., Lin H.C., McSweeney C.S., Mackie R.I., Gaskins H.R. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu Rev Food Sci Technol. 2010;1:363–395. doi: 10.1146/annurev.food.102308.124101. [DOI] [PubMed] [Google Scholar]
- Naumann H., Sepela R., Rezaire A., Masih S.E., Zeller W.E., Reinhardt L.A., Robe J.T., Sullivan M.L., Hagerman A.E. Relationships between structures of condensed tannins from Texas legumes and methane production during in vitro rumen digestion. Molecules. 2018;23:1–16. doi: 10.3390/molecules23092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Kebreab E., Hristov A., Oh J.P. Prediction of enteric methane production, yield and intensity in dairy cattle using an intercontinental database. Global Change Biol. 2018;24:3368–3389. doi: 10.1111/gcb.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkrumah D.J., Okine E.K., Mathison G.W., Schnid K., Li C., Basarab J.A., Price M.A., Wang Z., Moore S.S. Relationships of feedlot feed efficiency, performance, and feeding behavior with metabolic rate, methane production, and energy partitioning in beef cattle. J Anim Sci. 2006;84:145–153. doi: 10.2527/2006.841145x. [DOI] [PubMed] [Google Scholar]
- Odongo N.E., Bagg R., Vessie G., Dick P., Or-Rashid M.M., Hook S.E., Gray J.T., Kebreab E., France J., Mcbride B.W. Long-term effects of feeding monensin on methane production in lactating dairy cows. J Dairy Sci. 2006;90:1781–1788. doi: 10.3168/jds.2006-708. [DOI] [PubMed] [Google Scholar]
- Olijhoek D.W., Lovendahl P., Lassen J., Hellwing A.L.F., Hoglund J.K., Weisbjerg M.R., Noel S.J., McLean F., Lund P. Methane production, rumen fermentation, and diet digestibility of Holstein and Jersey dairy cows being divergent in residual feed intake and fed at 2 forage-to-concentrate ratios. J Dairy Sci. 2017;101:9926–9940. doi: 10.3168/jds.2017-14278. [DOI] [PubMed] [Google Scholar]
- Owens F.N., Hanson F.C. Symposium: external and internal markers, external and internal markers for appraising site and extent of digestion in ruminants. J Dairy Sci. 1992;75:2605–2617. doi: 10.3168/jds.S0022-0302(92)78023-0. [DOI] [PubMed] [Google Scholar]
- Palmonari A., Stevenson D.M., Mertens D.R., Cruywagen C.W., Weimer P.J. pH dynamics and bacterial community composition in the rumen of lactating dairy cows. J Dairy Sci. 2010;93:279–287. doi: 10.3168/jds.2009-2207. [DOI] [PubMed] [Google Scholar]
- Patra A.K. Enteric methane mitigation technologies for ruminant livestock: a synthesis of current research and future directions. Environ Monit Assess. 2012;184:1929–1952. doi: 10.1007/s10661-011-2090-y. [DOI] [PubMed] [Google Scholar]
- Patra A.K. Recent advances in measurement and dietary mitigation of enteric methane emissions in ruminants. Front Vet Sci. 2016;3:1–17. doi: 10.3389/fvets.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A., Park T., Kim M., Yu Z. Rumen methanogens and mitigation of methane emission by aniti- ethanogenic compounds and substances. J Anim Sci Biotechnol. 2017;8:1–18. doi: 10.1186/s40104-017-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira M.S., Oliveira S.G., Primavesi O., Lima M.A., Frighetto R.T.S., Berchielli T.T. Methane emissions and estimates of ruminal fermentation parameters in beef cattle fed different dietary concentrate levels. Rev Bras Zootec. 2013;42:592–598. [Google Scholar]
- Pellikaan W.F., Hendriks W.H., Uwimana G., Bongers L.J.G.M., Becker P.M., Cone J.W. A novel method to determine simultaneously methane production during the in vitro gas production using a fully automated equipment. Anim Feed Sci Technol. 2011;168:196–205. [Google Scholar]
- Pellikaan W.F., Stringano E., Leenaars J., Bongers D.J.G.M., van Laar-van Schuppen S., Plant J., Mueller-Harvey I. Evaluating effects of tannins on extent and rate in vitro gas and methane production using an automated pressure evaluation system (APES) Anim Feed Sci Technol. 2011;166–167:377–390. [Google Scholar]
- Pinares-Patino C.S., Clark H. Reliability of the sulfur hexafluoride tracer technique for methane emission measurement from individual animals: an overview. Aust J Exp Agric. 2008;48:223–229. [Google Scholar]
- Pinares-Patino C.S., Lassey K.R., Martin R.J., Molano G., Fernandez M., MacLean S., Sandoval E., Luo D., Clark H. Assessment of the sulphur hexafluoride (SF6) tracer technique using respiration chambers for estimation of methane emissions from sheep. Anim Feed Sci Technol. 2011;166–167:201–209. [Google Scholar]
- Puchala R., Min B.R., Goetsch A.L., Sahlu T. The effect of a condensed tannin containing forage on methane emission by goats. J Anim Sci. 2005;83:182–191. doi: 10.2527/2005.831182x. [DOI] [PubMed] [Google Scholar]
- Puchala R., Animut G., Patra A.K., Detweiler G.D., Wells F.E., Varel V.H., Sahlu T., Goetsch A.L. Methane emissions by goats consuming Sericea lespedeza at different feeding frequencies. Anim Feed Sci Technol. 2012;175:76–84. [Google Scholar]
- Puchala R., Animut G., Patra A.K., Detweiler G.D., Wells J.E., Varel V.H., Sahlu T., Goetsch A.L. Effects of different fresh-cut forages and their hays on feed intake, digestibility, heat production, and ruminal methane emissions by Boer x Spanish goats. J Anim Sci. 2012;90:2754–2762. doi: 10.2527/jas.2011-4879. [DOI] [PubMed] [Google Scholar]
- Reeve J.N., Nolling J., Morgan R.M., Smith D.R. Methanogenesis: genes, genomes, and who's on first ? J Bacteriol. 1997;179:5975–5986. doi: 10.1128/jb.179.19.5975-5986.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rischewski J., Bielak A., Nürnberg G., Derno M., Kuhla B. Ranking dairy cows for methane emissions measured using respiration chamber or GreenFeed techniques during early, peak, and late lactation. J Anim Sci. 2017;95:3154–3159. doi: 10.2527/jas.2017.1530. [DOI] [PubMed] [Google Scholar]
- Roehe R., Dewhurst R.J., Duthie C.A., Rooke J.A., McKain N., Ross D.W., Hyslop J.J., Waterhouse A., Freeman T.C., Watson M., Wallace R.J. Bovine host genetic variation influences rumen microbial methane production with best selection criterion for low methane emitting and efficiency feed converting hosts based on methagenomic gene abundance. PLoS Genet. 2016;12:1–20. doi: 10.1371/journal.pgen.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Downing R.C., Zhong Y., Saffron C.M., Liao W. Life cycle and economic assessment of anaerobic co-digestion of dairy manure and food waste. Ind Biotechnol. 2015;11:127–139. [Google Scholar]
- Ross E.M., Moate P.J., Marett L., Cocks B.G., Hayes B.J. Investigating the effect of two methane mitigating diets on the rumen microbiome using massively parallel sequencing. J Dairy Sci. 2013;96:6030–6046. doi: 10.3168/jds.2013-6766. [DOI] [PubMed] [Google Scholar]
- SAS . SAS Institute Inc.; 2004. SAS institute user's guide. Release 9.1.3. [Google Scholar]
- Shi W., Moon C.D., Leahy S.C., Kang D., Froula J., Kittelman J. Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome. Genome Res. 2014;24:1517–1525. doi: 10.1101/gr.168245.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silanikove N., Perevolotsky A., Provenza F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim Feed Sci Technol. 2001;91:69–81. [Google Scholar]
- Sliwinski B.J., Kreuzer M., Sutter F.A., Machmueller H.R. Performance, body nitrogen conversion and nitrogen emission from manure of dairy cows fed diets supplemented with different plant extracts. J Anim Feed Sci. 2004;13:73–91. [Google Scholar]
- Strom G. Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences; 2012. Effect of botanically diverse pastures on the milk fatty acid profiles in New Zealand dairy cows. Master thesis. [Google Scholar]
- Tajima K., Aminov R.I., Nagamine T., Matsui H., Nakamura M., Benno Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl Environ Microbiol. 2001;67:2766–2774. doi: 10.1128/AEM.67.6.2766-2774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavendale M.H., Meagher L.P., Pacheco D., Walker N., Attwood G.T., Sivakumaran S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim Feed Sci Technol. 2005;123–124:403–419. [Google Scholar]
- Tedeschi L.O., Fox D.G., Guiroy P.J., Baker M.J., Tylutki T.P. Cornell value discovery system for individual cattle management. Dept. of Animal Science. 2001;130 [Google Scholar]
- Thomson D.J., Beever D.E., Haines M.J., Cammell S.B., Evans R.T., Dhanoa M.S., Austin A.R. The yield and composition of the milk from Fresian cows grazing either perennial ryegrass or white clover in early lactation. J Dairy Res. 1985;52:17–31. [Google Scholar]
- Tiemann T.T., Lascano C.E., Kreuzer M., Hess H.D. The ruminal degradability of fibre explains part of the low nutritional value and reduced methanogenesis in highly taninnferous tropical legumes. J Sci Food Agri. 2008;88:1794–1803. [Google Scholar]
- Todd R.W., Cole N.A., Casey K.D., Hagevoort R., Auvermann B.W. Methane emissions from southern high plains dairy wastewater lagoons in the summer. Anim Feed Sci Technol. 2011;166–167:575–580. [Google Scholar]
- Todd R.W., Moffet C., Neel J., Turner K., Steiner J., Cole N.A. Pasture-scale methane emissions of grazing cattle. Proc. Grazing CAP Field Res Sym. 2016:46–53. [Google Scholar]
- Tomkins N.W., Denman S.E., Pilajun R., Wanapat M., McSweeney C.S., Elliott R. Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Anim Sci Technol. 2015;200:25–34. [Google Scholar]
- Turner S.A., Waghorn G.C., Woodwards S.L., Thomson N.A. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) affect the detailed composition of milk from dairy cows. Proc N Z Soc Anim Prod. 2005;65:283–289. [Google Scholar]
- Tymensen L.D., Beauchemin K.A., McAllister T.A. Structures of free-living and protozoa-associated methanogen communities in the bovine rumen differ according to comparative analysis of 16S rRNA and mcrA genes. Microbiology. 2012;158:1808–1817. doi: 10.1099/mic.0.057984-0. [DOI] [PubMed] [Google Scholar]
- UN . 2015. United nations (UN). World population prospects.http:www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html Available online at: [Google Scholar]
- Ungerfeld E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis. Front Microbiol. 2015;6:10–17. doi: 10.3389/fmicb.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerfeld E.M. Inhibition of rumen methanogenesis and ruminant productivity: a meta-analysis. Front in Vet Sci. 2018;5:1–13. doi: 10.3389/fvets.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nevel C.J., Demeyer D.I. Control of rumen methanogenesis. Environ Monit Assess. 1996;42:73–77. doi: 10.1007/BF00394043. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J. 2nd ed. Cornell University Press; Ithaca: 1994. Nutritional ecology of the ruminant; p. 476. [Google Scholar]
- Vasta V., Daghio M., Cappucci A., Buccioni A., Serra A., Viti C., Mele M. Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: experimental evidence and methodological approaches. J Dairy Sci. 2019;102:3781–3804. doi: 10.3168/jds.2018-14985. [DOI] [PubMed] [Google Scholar]
- Wadhwa M., Bakshi M.P.S., Makkar H.P.S. Modifying gut microbiomes in large ruminants: opportunities in non-intensive husbandry systems. Anim Front. 2016;6:27–36. [Google Scholar]
- Waghorn G.C. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—progress and challenges. Anim Feed Sci Technol. 2008;147:116–139. [Google Scholar]
- Waghorn G.C., Barry T.N. vol. 10. Occ. Publ.; 1987. Pasture as a nutrient source; pp. 21–37. (Feeding livestock on pasture). AM Nicol. NZ Soc Anim Prod. [Google Scholar]
- Waghorn G.C., Hegarty R.S. Lowering ruminant methane emissions through improved feed conversion efficiency. Anim Feed Sci Technol. 2011;166:290–301. [Google Scholar]
- Waghorn G.C., Tavendale M.H., Woodfield D.R. Methanogenesis from forages fed to sheep. Proc NZ Grass Assoc. 2002;64:167–171. [Google Scholar]
- Waghorn G.C., Woodward S.L., Tavendale M., Clark D.A. Inconsistencies in rumen methane production - effects of forage composition and animal genotype. Int Cong Ser. 2006;1293:115–118. [Google Scholar]
- Waghorn G.C., Jonker A., Macdonald K.A. Measuring methane from grazing dairy cows using GreenFeed. Anim Prod Sci. 2016;56:252–257. [Google Scholar]
- Wallace R.J., Rooke J.A., Duthie C.A., Hyslop J.J., Ross D.W., McKain N. Archaeal abundance in post- mortem ruminal digesta may help predict methane emissions from beef cattle. Sci Rep. 2014;4:5892. doi: 10.1038/srep05892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R.J., Rooke J.A., McKain N., Duthie C.A., Hyslop J.J., Ross D.W. The rumen microbial metagenome associated with high CH4 production in cattle. BMC Genom. 2015;16:1–14. doi: 10.1186/s12864-015-2032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WFP . 2015. World food programme (WFP) hunger Map. [Rome, Italy] [Google Scholar]
- Whitford M.F., Teather R.M., Forster R.J. Phylogenetic analysis of methanogens from the bovine rumen. BMC Microbiol. 2001;1:1–5. doi: 10.1186/1471-2180-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wims C.M., Deighton M.H., Lewis E., O'Loughlin B., Delaby L., Boland T.M., O'Donovan M. Effect of pregrazing herbage mass on methane production, dry matter intake, and milk production of grazing dairy cows during the med-season period. J Dairy Sci. 2010;93:4976–4985. doi: 10.3168/jds.2010-3245. [DOI] [PubMed] [Google Scholar]
- Wolin M.J. The rumen fermentation: a model for microbial interactions in anaerobic ecosystems. Adv Microb Ecol. 1979;3:49–77. [Google Scholar]
- Wolin M.J., Miller T.L., Stewart C.S. Microbe interactions. In: Hobson P.J., Stewart C.S., editors. The rumen microbial ecosystem. 2nd ed. Blackie Acad. Profess. London; 1997. pp. 467–491. [Google Scholar]
- Woodward S.L., Waghorn G.C., Ulyatt M.J., Lassey K.R. Early indications that feeding Lotus will reduce methane emissions from ruminants. Proc N Z Soc Anim Prod. 2001;61:23–26. [Google Scholar]
- Woodward S.L., Waghorn G.C., Lassey K.R., Laboyrie P.G. Does feeding sulla (Hedysarum coronarium) reduces methane emissions from dairy cows. Proc N Z Soc Anim Prod. 2002;62:227–230. [Google Scholar]
- Woodward S.L., Waghorn G.C., Laboyrie P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cow. Proc N Z Soc Anim Prod. 2004;64:160–164. [Google Scholar]
- Wright A.D.G., Toovey A.F., Pimm C.L. Molecular identification of methanogenic archaea from sheep in Queensland, Australia reveal more uncultured novel archaea. Anaerobe. 2006;12:134–139. doi: 10.1016/j.anaerobe.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wright A.G.G., Northwood K.S., Obi N.E. Rumen-like methanogens identified from the crop of the folivorous South American bird, the hoatzin (Opisthocomus hoazin) Int Soc Micro Ecol. 2009;3:1120–1126. doi: 10.1038/ismej.2009.41. [DOI] [PubMed] [Google Scholar]
- Yan T., Porter M.G., Mayne C.S. Prediction of methane emission from beef cattle using data measured in indirect open-circuit respiration chambers. Animal. 2009;3:1455–1462. doi: 10.1017/S175173110900473X. [DOI] [PubMed] [Google Scholar]
- Yanagita K., Kamagata Y., Kawaharasaki M., Suzuki T., Nakamura Y., Minato H. Phylogenetic analysis of methanogens in sheep rumen ecosystem and detection of Methanomicrobium mobile by fluorescence in situ hybridization. Biosci Biotechnol Biochem. 2000;64:1737–1742. doi: 10.1271/bbb.64.1737. [DOI] [PubMed] [Google Scholar]
- Zhou M., Hernandez-Sanabria E., Guan L.L. Assessment of microbial ecology of ruminal methane producers and cattle's high feed efficiency and low methane production activities. Appl Environ Microbiol. 2009;75:6524–6533. doi: 10.1128/AEM.02815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Hernandez-Sanabria E., Guan L.L. Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl Environ Microbiol. 2010;76:3776–3786. doi: 10.1128/AEM.00010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman P.R. University Corp for Atmospheric Research (UCAR); 1993. System for measuring metabolic gas emissions from animals. Assignee Pat No: 5.265.618. [Google Scholar]
- Zimmerman P.R., Zimmerman R.S. P. R. Zimmerman, assignee; United States: 2012. Method and system for monitoring and reducing ruminant methane production. Pat. No. US20090288606 A1. [Google Scholar]