Abstract
Age-related macular degeneration (AMD) is the leading cause of blindness in the industrialized world. AMD is associated with dysfunction and atrophy of the retinal pigment epithelium (RPE), which provides critical support for photoreceptor survival and function. RPE transplantation is a promising avenue towards a potentially curative treatment for early stage AMD patients, with encouraging reports from animal trials supporting recent progression toward clinical treatments. Mature RPE cells have been reported to be superior, but a detailed investigation of the specific changes in the expression pattern of key RPE genes during maturation is lacking. To understand the effect of maturity on RPE, we investigated transcript levels of 19 key RPE genes using ARPE-19 cell line and human embryonic stem cell-derived RPE cultures. Mature RPE cultures upregulated PEDF, IGF-1, CNTF and BDNF—genes that code for trophic factors known to enhance the survival and function of photoreceptors. Moreover, the mRNA levels of these genes are maximized after 42 days of maturation in culture and lost upon dissociation to single cells. Our findings will help to inform future animal and human RPE transplantation efforts.
Keywords: retinal pigment epithelium (RPE), maturation, differentiation, embryonic stem cells, pigment epithelium derived factor (PEDF), cell culture
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the industrialized world, affecting more than 20% of adults over the age of 65 [1]. Visual impairment leads to a significantly reduced quality of life and accounts for billions of dollars in direct and indirect annual healthcare costs in the USA alone [2,3,4]. The earliest signs of AMD are often abnormalities of the retinal pigmented epithelium (RPE) monolayer and accumulation of drusen (complex, poorly understood protein/lipid deposits) immediately adjacent to it. In dry (or geographic) AMD, which accounts for ~90% of AMD patients, RPE dysfunction and atrophy is followed by a local, progressive and irreversible loss of photoreceptors [5,6]. In wet (or neovascular) AMD, acute penetration of the choroidal vasculature through the RPE into the eye results in rapid vision loss
The RPE secretes cytokines and growth factors essential for maturation and survival of both the retina and the choroid, and is also responsible for maintaining a barrier between these compartments, ensuring that choroidal signals (such as vascular endothelial growth factor, VEGF) do not enter the retina, and vice versa [7,8,9,10]. RPE cells also maintain photoreceptor survival and function directly, phagocytosing damaged outer segments, transporting nutrients and waste products and regenerating visual pigment [11]. Thus, it is reasonable to hypothesize that loss of normal RPE function is a central driver of AMD.
RPE transplantation has been shown to rescue photoreceptors from death in the RCS rat model of retinal degeneration that is caused by RPE atrophy [12,13,14,15,16]. This is an attractive approach to treat AMD as we are capable of efficiently differentiating stem cells into RPE for transplantation [17,18,19], and clinical trials are now underway [20,21,22,23,24,25]. With RPE cell therapy rapidly making its way to the clinic, important concerns regarding the exact phenotype of the transplanted RPE remain [26].
One such consideration is RPE maturation, which has been reported to enhance the function of RPE from various sources including: a primary cell-derived human cell line (ARPE-19) [27,28]; embryonic stem cells [20,29,30,31]; induced pluripotent stem cells [23] and adult stem cells [26]. ARPE-19 monolayers have been shown to exhibit substantially increased epithelial resistance and more physiological morphology after maturation for more than twenty days [27,28], while adult-stem cell-derived RPE cells demonstrated superior post-transplant function when transplanted into rats at the fourth week of differentiation [26]. RPE maturity has been reported to affect cellular morphology [27,28], pigmentation [23,32] and the production of several functional proteins such as pigment epithelium-derived factor (PEDF), bestrophin-1 (BEST1) and cellular retinaldehyde-binding protein (CRALBP) [20]. However, despite these demonstrations of the significance of RPE maturation, its molecular signature has not yet been established, nor is there a consensus on the culture duration required to achieve maturity in vitro.
In this study, we employed ARPE-19 and embryonic stem cell-derived (E-)RPE in vitro cultures to investigate the transcript profile of 19 genes (identified from the literature as important to RPE function) during maturation. We found increased transcript levels for 13 and 16 of these genes in matured ARPE-19 and E-RPE monolayers respectively, over nonmatured control cultures. ARPE-19 and E-RPE monolayers matured for 8 weeks demonstrated robust expression of these key functional genes which was largely and rapidly lost after single cell passaging. Our findings emphasize the potential benefits of transplanting RPE as a tissue rather than dissociated cells; allowing that tissue to mature prior to use and including confirmation of the molecular fingerprint of mature RPE tissue as part of clinical release criteria.
2. Results
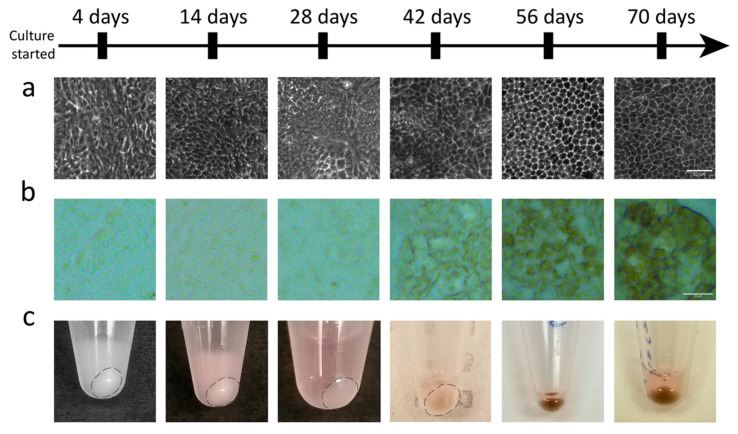
2.1. E-RPE Demonstrates Development in Morphology and Pigmentation as they Mature
Cellular morphology and pigmentation have been used in the field as qualitative criteria to evaluate RPE maturity and suitability for transplantation [20,23,26]. To assess the relationship of these phenotypes with maturation, ARPE-19 and E-RPE monolayers were cultured for up to 70 days and sampled at 14-day intervals. As E-RPE cultures mature, a distinct hexagonal or cobble-stone cell morphology progressively emerges over days 28–56, along with a parallel gain in pigmentation (Figure 1). ARPE-19 cultures were sampled on the same experimental timeline but, as previously reported, this spontaneously immortalized human cell line does not exhibit these markers of primary RPE [33].
Figure 1.
Embryonic stem cell-derived retinal pigment epithelium (E-RPE) cultures demonstrate morphological changes and become pigmented as they mature. (a) Cellular morphology of E-RPE cultures develops over time to make the consistent hexagonal shaped cells seen at 56 and 70 days. (b) Representative micrographs of the progressive pigmentation of the E-RPE culture as it matures. (c) Cell pellets in microcentrifuge tubes of E-RPE cultures sampled at confluency (4 days), 14 days, 28 days, 42 days, 56 days and 70 days show increasing pigmentation over time. Scale bars represent lengths of 50 µm in (a) and (b).
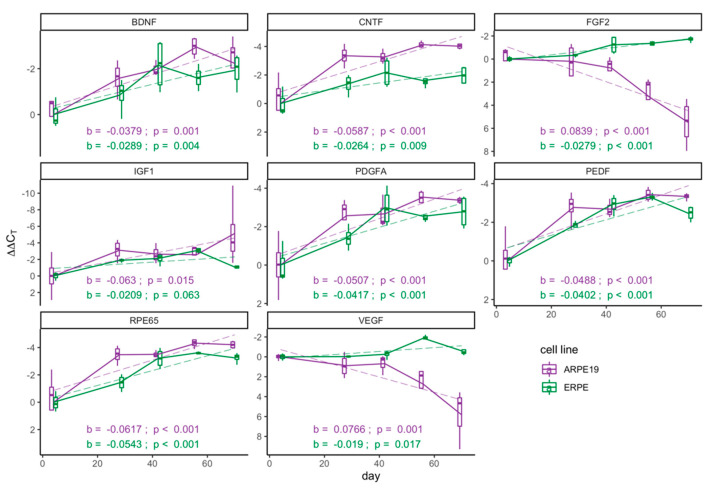
2.2. E-RPE and ARPE-19 Cultures Demonstrate Progressive Increase in mRNA Levels of Key Functional RPE Genes as They Mature
As many transplantation studies use morphology and pigmentation as an indicator for RPE maturity, and ultimately utilize RPE after a range of culture durations [20,21,23], we investigated the mRNA levels of PEDF, VEGF, PDGFA, CNTF, BDNF, FGF2, IGF1 and RPE65 over time in culture. We focused our initial exploration on this subset of genes, as they have implications for various retinal degenerative diseases [34,35]. By day 42, most investigated E-RPE transcripts had attained 75% of their maximum level (5/8) with the greatest change taking place between four and 28 days of maturation (Figure 2). ARPE-19 cultures followed a similar trend, with six of eight investigated genes increasing with time. The exception is that unlike during E-RPE maturation, VEGF and FGF2 mRNA levels in ARPE-19 progressively dropped with days in culture.
Figure 2.
Key functional genes progressively increase with maturation. Real-time polymerase chain reaction (RT-qPCR) analysis of mRNA levels across 70 days of culture in E-RPE culture (green) and ARPE-19 culture (purple). Results were normalized to an endogenous reference gene (PPIA) and are presented as ΔΔCt means (n = 4) ± standard deviation at each time point. A linear regression model (dashed line) was used to describe the relationship between ΔΔCt values and days of maturation of each gene for both RPE cell sources; regression coefficients, denoted as b, and associated p-values, to reject the null hypothesis that b = 0, are shown on each graph.
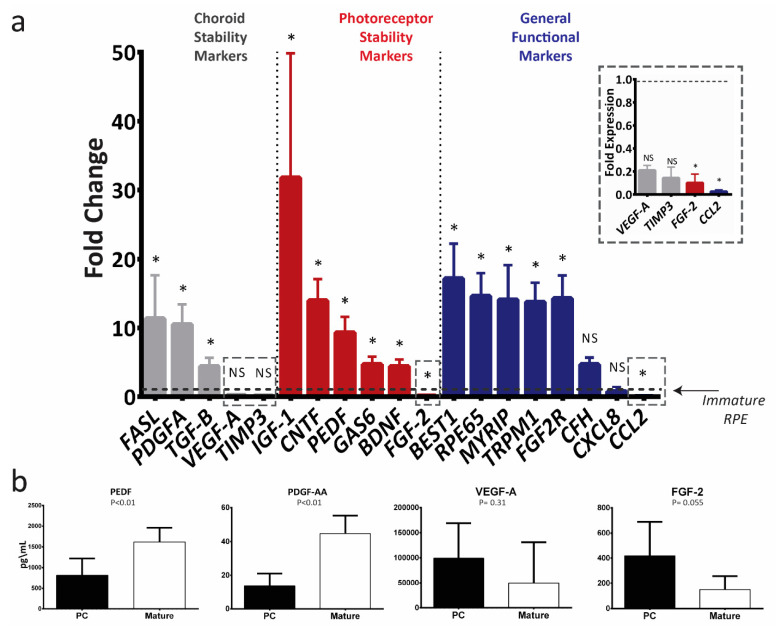
2.3. Mature ARPE-19 Upregulates Transcript Levels of Key RPE Genes
After identifying 42 days as a critical point in RPE maturation, we expanded our analysis to 19 genes that code for proteins that carry essential and specific RPE functions such as photoreceptor support, choroid support, visual recycling and immune modulation for analysis (all investigated genes are described Table A1). Mature ARPE-19 significantly upregulates the mRNA levels of 13 of 19 investigated RPE functional genes (Figure 3a), including PEDF, BDNF, CNTF, GAS6 and IGF1—genes that code for RPE-secreted factors known to enhance photoreceptor survival and function in retinal degenerative animal models [36,37,38,39]. Mature ARPE-19 also showed increased mRNA levels of genes that code basally secreted RPE factors known to contribute to the stability of the choroid, namely PDGFA, FASL, and TGFB [35,40,41,42]. Levels of some factors, including VEGFA and FGF2, were lower in mature ARPE-19. To confirm that the differential expression seen in mRNA levels was reflected by secreted proteins, we investigated the conditioned media taken from immature and mature cultures for levels of PEDF, PDGF-AA (a homodimer of two ‘A’ subunits coded for by PDGFA), VEGF-A and FGF-2. We observed that mature ARPE-19 secreted significantly more PEDF and PDGF-AA protein. Levels of secreted VEGF-A and FGF-2 appeared to decrease with maturation, but the change was not statistically significant (Figure 3b).
Figure 3.
In vitro maturation of ARPE-19 upregulates the expression of therapeutically relevant photoreceptor and choroid trophic factors. (a) RT-qPCR analysis of mRNA levels of key RPE genes in mature ARPE-19 cells (>42 days in culture; n = 4). The results were normalized to an endogenous reference gene (PPIA) and are presented as mean fold change (2−ΔΔCT) relative to immature ARPE-19 cultures (dotted line) ± standard deviation. Data were compared with a Mann–Whitney U test of ΔCt values; * p < 0.05; NS: not significant. (b) Protein secreted by the post-confluent (PC) immature and mature ARPE-19 cultures into the conditioned media presented as mean concentration ± standard deviation (n = 5), Mann–Whitney U test.
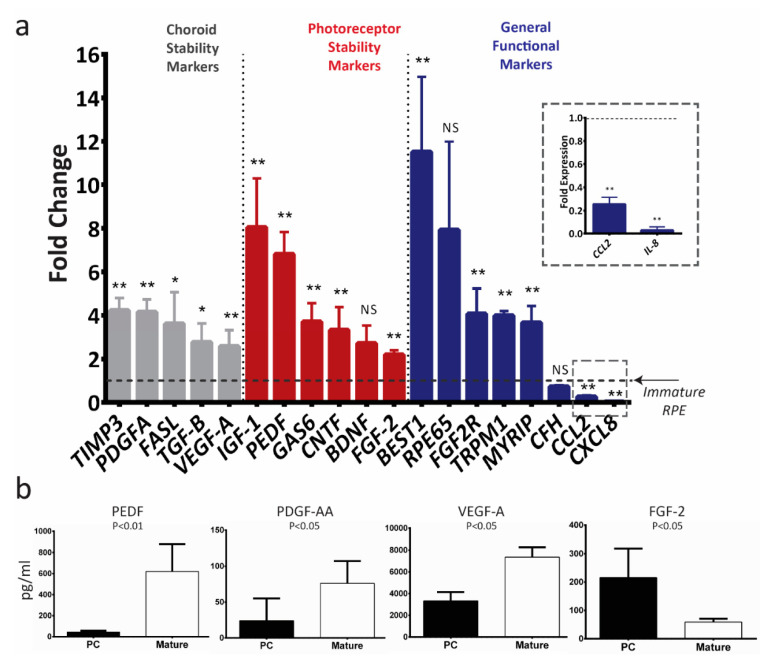
2.4. Mature E-RPE Culture Upregulates Transcript Levels of Key RPE Genes
Compared to nonmatured control cultures, mature E-RPE cultures upregulate mRNA levels of 16 out of 19 investigated RPE genes, and significantly downregulate mRNA levels of CXCL8 and CCL2 (Figure 4a). As with ARPE-19, we investigated the conditioned media of both cultures and found mature RPE secreted significantly more PEDF, PDGF-AA and VEGF-A, and less FGF-2 when compared to immature culture (Figure 4b). Notably, the lower secretion of FGF-2 in mature ES-RPE cultures is not in line with the mRNA levels; it is in line with the trend in both mRNA and protein seen in mature ARPE-19 cultures (Figure 3). Finally, we compared the rank correlation of fold-change in transcript levels for mature cultures between ARPE-19 and E-RPE and found a strong relationship, suggesting that genes are similarly upregulated in both cell types (Spearman’s rank correlation coefficient, rs = 0.7; p < 0.001).
Figure 4.
In vitro maturation of E-RPE cells upregulates the expression of therapeutically relevant photoreceptor and choroid trophic factors. (a) RT-qPCR analysis of mRNA levels of key RPE genes in mature E-RPE cells (>42 days in culture; n = 5). The results were normalized to an endogenous reference gene (PPIA) and are presented as mean fold change (2−ΔΔCT) relative to immature E-RPE culture (dotted line) ± standard deviation. Data were compared with a Mann–Whitney U test of ΔCt values; * p < 0.05; ** p < 0.01; NS: not significant. (b) Protein secreted by the post-confluent (PC) immature and mature E-RPE cultures into the conditioned media presented as mean concentration ± standard deviation (n = 5), Mann–Whitney U test.
2.5. Dissociation of the RPE Monolayer to Single Cell Suspension Resets Maturation
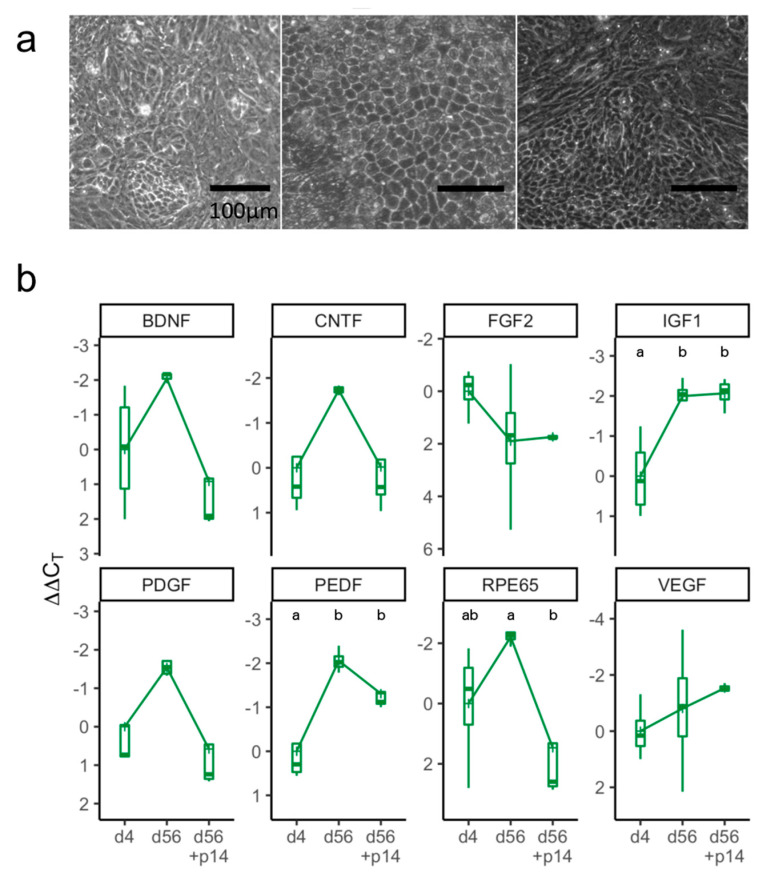
We next investigated the effect of dissociation on RPE cells, as this is a common method of transplantation [24,25]. To do this, we matured E-RPE for 56 days before dissociation and passaging, followed by another 14 days of maturation. Interestingly, after passaging, a portion of E-RPE cells quickly regained the hexagonal morphology observed on day 56 but nearly all cells quickly lost pigmentation (Figure 5a). Even at two weeks postpassage, many (5/8, 62.5%) transcripts showed levels comparable with those from RPE four days after initial seeding (Figure 5b). We observed that mRNA levels of VEGF, IGF1 and FGF2, which did not significantly increase during maturation, changed little after passaging.
Figure 5.
Markers of maturation are lost after passaging E-RPE cells. (a) Morphology of E-RPE cells at confluence, four days after seeding (left), at 56 days of maturation (center) and at a further 14 days after passaging (right). Scale bar represents 100 μm. (b) RT-qPCR analysis at three time points (4 days, 56 days and 56 + 14 days after passaging). Results were normalized to PPIA and represent mean ΔΔCt values (n = 3 or 4) ± standard deviation plotted on a negative y-axis (higher expression at the top). Kruskal-Wallis one-way ANOVA test was used to compare ΔΔCt values of the various maturation points within each gene followed by a Tukey’s honest significance test; different letters, p < 0.05.
3. Discussion
While RPE maturation has been reported to enhance RPE function both in vitro and in vivo, it is not applied consistently and preparation processes for the RPE transplanted in animal and human trials vary widely [20,26,27,28,32]. To inform the selection and development of these processes, we have, therefore, characterized the impact of in vitro maturation on transcript levels and key secreted proteins in ARPE-19 and E-RPE.
We prospectively selected 19 genes playing important roles in photoreceptor stability, choroid stability, visual recycling, immune modulation and RPE-specific functions. This latter group included retinoid isomerhydrolase (RPE65), a crucial enzyme in the sequence of reactions that recycle 11-cis retinal in RPE cells [43]; and BEST1, which codes for a membrane protein uniquely expressed in RPE that functions as an anion channel and a regulator of intracellular calcium signaling [44]. All genes were selected prior to starting experiments, and none were subsequently excluded from, or added to, the analyses. While we were not able to perform a transcriptome-wide assay, such as RNA-seq to identify additional genes that may be changing in RPE during culture maturation, this has been successfully done by others to investigate RPE subjected to oxidative stress [45] and gene expression changes associated with pigmentation [32,46]. Such an approach would reinforce the work presented here and may reveal additional noncanonical genes that further contribute to the improved performance of mature RPE and should be considered for future experiments.
Human pluripotent stem cells are a reliable, sustainable and therapeutically relevant source for producing RPE that demonstrate enhanced gene expression, morphology and function [47,48] For this reason, they are currently being utilized in clinical trials to treat retinal degenerative diseases [20,21,49].
Upon maturation, both ARPE-19 and E-RPE upregulate mRNA levels of RPE genes known to be critical for their in vivo function (Figure 3 and Figure 4). Genetic variation in 14 of the 19 genes we investigated is associated with some form of retinal degenerative disease. In particular, the upregulation of PEDF, CNTF, IGF1 and BDNF is of therapeutic value, as they are well-recognized neurotrophic factors secreted by RPE to support photoreceptors [7,37,50]. These factors have been shown to rescue photoreceptor survival and function in various retinal degeneration animal models [37,38,51], and have been identified as therapeutic targets for AMD and other retinal degenerative diseases [37,52,53,54]. We speculate that the upregulation we show here may at least partially explain the mechanism underlying the reported superiority of mature RPE in supporting photoreceptor survival [26,27,28].
Our observation of increased PEDF expression is also relevant for its effects on the choroid, where it has been demonstrated to prevent angiogenic retinopathies such as wet AMD by antagonizing VEGF signaling [55,56,57]. Increased VEGF expression by E-RPE over time (Figure 4) and particularly the high transcript levels following single-cell dissociation (Figure 5), suggest a spontaneous epithelial to mesenchymal transition, which has been observed in E-RPE cells that are routinely passaged [58]. Alternatively, it may relate to the fact that in vivo, RPE forms a well-defined boundary between the choroidal and retinal compartments—future studies focused on identifying the directionality of VEGF secretion may yield greater understanding. Mature ARPE-19 and E-RPE also both demonstrated increased transcription of choroid stability factors including TGFB, TIMP3 and PDGFA. TGF-β is known to have an immune suppressive effect, which enables RPE to establish and maintain the immune privilege of the eye [59]. TIMP3 is secreted by RPE basally to remodel Bruch’s membrane, inhibit angiogenesis and regulate inflammation [60], and mutations and deficiencies in TIMP3 have been linked to various retinal degenerative diseases including AMD and Sorsby’s fundus dystrophy [61,62]. PDGF-AA is secreted by RPE basally to support the choroid and regulate angiogenesis [35]. In addition to supporting photoreceptors with trophic factors, we speculate that mature RPE may better regulate blood vessel growth and control neovascularization.
We also observed downregulation of CCL2 and CXCL8, which code for monocyte chemoattractant protein (MCP)-1 and interleukin (IL)-8, respectively. Both are proinflammatory chemokines secreted by the RPE that act on immune cells and modulate the immune response under physiological and pathological conditions [63]. The expression of these proteins in healthy animals and individuals is quite low, and clinical studies have reported a direct correlation between their expression and incidence of AMD [64,65]. We speculate that these factors may recruit immune cells as a secondary line of defense in the event that the RPE is unable to provide a complete physical barrier between the choroid and retina, sacrificing potential inflammatory damage in favor of immediate response to injury or pathogens. This would explain their elevated expression in both immature and deteriorating RPE as compared to mature RPE. Further investigations are required to confirm specific roles played by these upregulated transcripts in the functional improvement observed in matured RPE cells.
Changes in gene expression during maturation were broadly similar between ARPE-19 and E-RPE, supporting a generalizable effect of RPE maturation on gene expression. However, FGF2 and VEGFA mRNA levels did not follow this trend as they increased in mature E-RPE but decreased or remained stable in mature ARPE-19 cells (Figure 3 and Figure 4). This is not unexpected, as previous studies have identified differences in gene expression and behavior between ARPE-19, a spontaneously immortalized cell line, and E-RPE [18,27,33]. Moreover, we have observed that, unlike E-RPE, ARPE-19 cells do not exhibit the traditional hexagonal RPE morphology and pigmentation in culture, even after 70 days of maturation (data not shown). While our observation is consistent with the majority of literature accounts, newly emerging evidence from Samuel and colleagues indicate that ARPE-19 can adopt the traditional hexagonal RPE morphology when they are aged longer than 120 days in culture [46]. Our observations that ARPE-19 and E-RPE respond largely similarly to maturation do generally support the continued use of the ARPE-19 cell line model. One limitation of this study was the absence of endogenous RPE derived from adult donors, although others have shown considerable similarities in the transcriptome between both ARPE-19 and E-RPE, particularly after maturation [32,46].
The expression kinetics of IGF1, PEDF and RPE65 in E-RPE are intriguing as they demonstrate increased expression as the culture ages up until 56 days (Figure 2). While it is possible that the expression of these genes has reached a maximum, additional time points beyond 70 days would be required to confirm this observation. Our findings are generally consistent with Da Cruz and colleagues who reported that PEDF secretion increases as RPE matures and then declines after ~80 days in culture [20]. The drop in PEDF, IGF-1 and RPE65 transcription after 56 days in E-RPE is, however, not seen in ARPE-19. While the expression of most examined genes in E-RPE peaked at 42–56 days, further in vivo investigations are required to determine if that would be the optimal maturation age for RPE transplantation.
Given the reversion of maturation we observed following dissociation, we anticipate that the transplantation of RPE as multicellular tissues [20,21,23,31] will improve the effectiveness of treatments over single cell suspensions by maintaining RPE maturity. Indeed, transplantation of E-RPE monolayers (or “sheets”) survived better, and led to significant improvements in visual acuity and photoreceptor survival, compared to transplantation of single cell suspensions [29,31]. Future work would also benefit from an investigation of RPE tissue characteristics, like the establishment of tight junctions and subsequent increased transepithelial resistance, to determine whether the improved gene expression profile of mature RPE we observed requires assembly into a coherent tissue.
In this work, we demonstrated that the in vitro maturation of ARPE-19 and E-RPE leads to increased expression of genes crucial for a range of critical RPE functions. Matured RPE cultures had higher transcript levels of therapeutically relevant neurotrophic and choroid stability factors; increased production of PDGF-AA and PEDF proteins and lower transcript levels of cytokines that modulate the recruitment of immune cells to the retina. Taken together, our results provide plausible mechanisms for the reported superiority of mature RPE in therapeutic transplantation.
While further investigations are needed to conclusively relate maturation to clinical outcomes, based on our findings we recommend that RPE cultures be matured for at least 42 days for best in vitro and in vivo performance and that expression levels of factors such as PEDF, TIMP3 and CXCL8 be considered when developing release criteria for RPE batches intended for clinical use.
4. Materials and Methods
4.1. Tissue Culture
ARPE-19 cells were seeded in a 6-well plate (VWR, Mississauga, ON, Canada. cat # 82050-842) at 600,000 cells/well (60,000 cells/cm2) and reached confluency at ~120,000 cells/cm2. Cultures were maintained for up to 70 days in 2 mL of ARPE-19 culture media that consisted of: DMEM/F-12, HEPES (Thermo Fisher Scientific, Mississauga, ON, Canada. cat # 11330057), 10% FBS (VWR. cat # 97068-085), and 1% Pen/strip (Thermo Fisher cat # 15140122). Media was changed every 48 h by replacing the entire old media volume with 2mL of fresh media. For all experiments, E-RPE cells were cultured in an identical manner to ARPE-19 cells, using ES-RPE culture media that consisted of: 70% DMEM; 30% F12; 2% B-27 supplement and 1% Pen/Strip (Thermo Fisher. cat # 11965, 11765, 17504, and 15140122).
To assess the oxygenation status of the cells under the outlined culture conditions, oxygen delivery was calculated using our previously published method [66]. A RPE oxygen consumption rate of 42 amol∙cell−1∙s−1 was used for the calculation [67]. We determined that cultured RPE cells should be receiving an adequate amount of oxygen, with a local oxygen concentration of 1.42 × 10−4 mol/L at the cells and a maximum oxygen delivery rate of 191 amol∙cell−1∙s−1.
4.2. RNA Extraction and cDNA Synthesis
Adherent cultures of ARPE-19 and E-RPE were collected by adding 1 mL of TrypLE Express Enzyme (Thermo. cat # 12604013) into each well and incubating for 5 min at room temperature. Next, collected samples were centrifuged for 5 min at 200× g and cell pellets were stored at −80 °C for up to one month before RNA isolation using a Total RNA Purification kit (Norgen Biotek Corp., Thorold, ON, Canada. cat # 37500). For each cDNA reaction, 1 µg RNA was used as input for the iScript Reverse Transcription Supermix kit (Bio-Rad Laboratories Ltd., Mississauga, ON, Canada. cat # 1708841). The cDNA was then used for real-time polymerase chain reaction (RT-qPCR) using PowerUp™ SYBR™ Green Master Mix (Thermo Fisher cat # A25777) to analyze and compare expression levels of selected genes in immature and mature RPE cultures using primer sequences detailed in Table A1. SYBR Green RT-qPCR was carried out with technical duplicates using the 7500 Fast Real-Time PCR System (Thermo Fisher) with PPIA as a housekeeper gene, as has been used previously [68,69]. Analyses were performed on either ∆CT values or ∆∆CT values where the reference sample was set at 4 days of culture.
4.3. Conditioned Media Analysis of Secreted Protein
Conditioned media (CM) was collected during the regular media change (every 48 h) for both ARPE-19 and E-RPE cells. The conditioned media was stored at −80 °C before being assayed. Multiplexing Laser Bead Technology (Eve Technologies, Calgary, AB, Canada) was performed on CM to estimate the concentration of proteins of interest. PEDF ELISA (Abcam, Cambridge, MA, USA. cat # ab213815) was utilized to measure the amount of PEDF in conditioned media from E-RPE as the original PEDF multiplex assay had been discontinued.
4.4. E-RPE Differentiation
RPE cells were differentiated from human embryonic stem cells as previously described by Maruotti and colleagues [70] with the following two modifications: (1) the HES-2 cell line was grown to confluence under 5% CO2 and 5% O2 in mTeSR1 and (2) during induced differentiation, a concentration of 50 nM chetomin was used. Cells were grown to a low passage (3–5) before being expanded and cryogenically stored.
4.5. Statistical Analysis
All experimental units (n) are biologically distinct repeats conducted from fresh thawed vials of banked cell lines and were repeated a minimum of three times. Data were analyzed with GraphPad Prism (v.7, San Diego, CA, USA) and R statistical software (v.3.5.1, Vienna, Austria [71]) Specific statistical methods are described in the text and figure legends. We considered p-values of less than 0.05 statistically significant.
Acknowledgments
The authors would like to thank Kayla Giles and Jarin Thundathil for their help collecting preliminary data for this project, as well as Carol Schuurmans and Yacine Touahri for their guidance.
Abbreviations
| AMD | Age-related macular degeneration |
| RPE | Retinal pigmented epithelium |
| E-RPE | Embryonic-stem-cell-derived RPE |
Appendix A
Table A1.
RT-qPCR primers chosen to evaluate the functions of human RPE.
| Gene | Primers | Function | Reference |
|---|---|---|---|
| PEDF | TCATTCACCGGGCTCTCTACT | Antiangiogenesis and antiapoptotic photoreceptor trophic factor with a potent neurotrophic effect. | [72] |
| GGGCAGTGACCGTGTCAAG | |||
| IGF1 | GCTCTTCAGTTCGTGTGTGGA | Anti-apoptotic and photoreceptor prosurvival factor. | [36] |
| GCCTCCTTAGATCACAGCTCC | |||
| FGF2 | AGAAGAGCGACCCTCACATCA | Neurotrophic and angiogenic factor required for photoreceptor and choroid maintenance. | [73] |
| CGGTTAGCACACACTCCTTTG | |||
| BDNF | CTACGAGACCAAGTGCAATCC | Neurotrophic factor with a prosurvival effect on photoreceptors. | [50] |
| AATCGCCAGCCAATTCTCTTT | |||
| GAS6 | CCGGGGACTTGTTCCAACC | Involved in the phagocytosis of photoreceptor outer segments. | [74] |
| CTGCACGAGGTCCTTCTCAT | |||
| FASL | TGCCTTGGTAGGATTGGGC | Antiangiogenic factor that also allows RPE to uphold and maintain the immune privilege feature of the eye by inactivating immune cells. | [40,75] |
| GCTGGTAGACTCTCGGAGTTC | |||
| TGFB | CAATTCCTGGCGATACCTCAG | Angiogenic factor that induces VEGF secretion by RPE and choroid cells. | [41] |
| GCACAACTCCGGTGACATCAA | |||
| TIMP3 | TGGGTTGTAACTGCAAGATCAAG | Involved in the maintenance and remodeling of Bruch’s membrane. | [76] |
| GGTCCAGAGACACTCGTTCT | |||
| VEGFA | AGGGCAGAATCATCACGAAGT | Angiogenic factor secreted by RPE to maintain the choriocapillaris. | [9] |
| AGGGTCTCGATTGGATGGCA | |||
| PDGFA | GCAAGACCAGGACGGTCATTT | Angiogenic factor with an autocrine growth effect on RPE. | [77,78] |
| GGCACTTGACACTGCTCGT | |||
| KDR | GTGATCGGAAATGACACTGGAG | VEGF receptor and plays a crucial role eye development. | [79] |
| GTGATCGGAAATGACACTGGAG | |||
| BEST1 | CTGGGCTTCTACGTGACGC | Calcium-activated anion channel and a regulator of intracellular calcium signaling. | [44,80] |
| TTGCTCGTCCTTGCCTTCG | |||
| CCL2 | CAGCCAGATGCAATCAATGCC | Chemokine involved in the regulation and modulation of an immune response. | [81] |
| TGGAATCCTGAACCCACTTCT | |||
| CFH | GTGAAGTGTTTACCAGTGACAGC | Regulates and modulates the complement pathway. | [82] |
| AACCGTACTGCTTGTCCAAAA | |||
| FGF2R | AGCACCATACTGGACCAACAC | FGF-2 receptor. | [46] |
| GGCAGCGAAACTTGACAGTG | |||
| CXCL8 | ACTGAGAGTGATTGAGAGTGGAC | Encodes a chemokine (IL-8) that initiates and amplifies inflammation. It has also been implicated in the pathogenesis of AMD. | [65] |
| AACCCTCTGCACCCAGTTTTC | |||
| RPE65 | CCTGCTGGTGGTTACAAGAAA | Encodes an essential isomerohydrolase enzyme in the retinoid visual cycle. | [43] |
| CCTGCCTGTTACATGAGCTGT | |||
| LHX2 | ATGCTGTTCCACAGTCTGTCG | Highly involved in early eye development and regulates the expression of multiple visual cycle genes. | [83,84] |
| GCATGGTCGTCTCGGTGTC | |||
| LOXL1 | CCACTACGACCTACTGGATGC | RPE signature gene that has been reported to be involved in ocular disorders. | [85] |
| GTTGCCGAAGTCACAGGTG | |||
| MYRIP | CTAAGAGCGGGACGTTTCAGG | Encodes for a protein that enables the trafficking of melanosomes in RPE. | [86] |
| TCTTCCTCGCTATCGGAGCC | |||
| TRPM1 | GTTCACCAACCAGCATATCCC | Encodes a permeable cation channel and plays a role in melanin synthesis in the RPE. | [46] |
| AGCCTTGATCAGGCCTTTCC | |||
| CNTF | ACAGAGCATTCACCGCTGAC | Encodes neurotrophic cytokine with a photoreceptor prosurvival effect. It was determined to be the most stable gene between the 2D and 3D RPE cultures. | [38] |
| TCAGGTCTGAACGAATCTTCCTT |
Author Contributions
Conceptualization; conceptualization, A.A.-A. and M.U.; formal analysis, A.A.-A., S.S., D.T. and M.U.; investigation, A.A.-A., S.S. and B.H.; writing—original draft, A.A.-A. and D.T.; writing—review & editing, A.A.-A., S.S., D.T. and M.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from Brain Canada (M.U.) and the Canadian National Institute for the Blind (D.T., M.U.). A.A.-A. and D.T. were supported by fellowships from the Alberta Children’s Hospital Research Institute; A.A.-A. also received funding from the University of Calgary Biomedical Engineering Graduate Program and Canadian Institutes of Health Research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Heal. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Chou C.-F., Cotch M.F., Vitale S., Zhang X., Klein R., Friedman D.S., Klein B.E., Saaddine J.B. Age-Related eye diseases and visual impairment among U.S. adults. Am. J. Prev. Med. 2013;45:29–35. doi: 10.1016/j.amepre.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frick K.D., Gower E.W., Kempen J.H., Wolff J.L. Economic Impact of Visual Impairment and Blindness in the United States. Arch. Ophthalmol. 2007;125:544. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- 4.Rein D.B., Zhang P., Wirth K.E., Lee P.P., Hoerger T.J., McCall N., Klein R., Tielsch J.M., Vijan S., Saaddine J. The Economic Burden of Major Adult Visual Disorders in the United States. Arch. Ophthalmol. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 5.Lim L.S., Mitchell P., Seddon J.M., Holz F.G., Wong T.Y. Age-Related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 6.Zając-Pytrus H.M., Pilecka A., Turno-Kręcicka A., Adamiec-Mroczek J., Misiuk-Hojło M. The Dry Form of Age-Related Macular Degeneration (AMD): The Current Concepts of Pathogenesis and Prospects for Treatment. Adv. Clin. Exp. Med. 2015;24:1099–1104. doi: 10.17219/acem/27093. [DOI] [PubMed] [Google Scholar]
- 7.Barnstable C.J., Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: Molecular targets and therapeutic potential. Prog. Retin. Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Bouck N. PEDF: Anti-Angiogenic guardian of ocular function. Trends Mol. Med. 2002;8:330–334. doi: 10.1016/S1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 9.Saint-Geniez M., Kurihara T., Sekiyama E., Maldonado A.E., D’Amore P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauß O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sparrow J.R., Hicks D., Hamel C.P., Sparrow J.R. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algvere P.V., Berglin L., Gouras P., Sheng Y. Transplantation of RPE in age-related macular degeneration: Observations in disciform lesions and dry RPE atrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997;235:149–158. doi: 10.1007/BF00941722. [DOI] [PubMed] [Google Scholar]
- 13.Castillo B.V., Del Cerro M., White R.M., Cox C., Wyatt J., Nadiga G., Del Cerro C. Efficacy of Nonfetal Human RPE for Photoreceptor Rescue: A Study in Dystrophic RCS Rats. Exp. Neurol. 1997;146:1–9. doi: 10.1006/exnr.1997.6534. [DOI] [PubMed] [Google Scholar]
- 14.Li L., Turner J.E. Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp. Eye Res. 1988;47:911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 15.Little C.W., Castillo B., DiLoreto A.D., Cox C., Wyatt J., Del Cerro C., Del Cerro M. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Investig. Ophthalmol. Vis. Sci. 1996;37:204–211. [PubMed] [Google Scholar]
- 16.Lund R., Wang S., Klimanskaya I., Holmes T., Ramos-Kelsey R., Lu B., Girman S., Bischoff N., Sauvé Y., Lanza R. Human Embryonic Stem Cell–Derived Cells Rescue Visual Function in Dystrophic RCS Rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 17.Buchholz D.E., Hikita S.T., Rowland T.J., Friedrich A.M., Hinman C.R., Johnson L.V., Clegg D.O. Derivation of Functional Retinal Pigmented Epithelium from Induced Pluripotent Stem Cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 18.Klimanskaya I., Hipp J., Rezai K.A., West M., Atala A., Lanza R. Derivation and Comparative Assessment of Retinal Pigment Epithelium from Human Embryonic Stem Cells Using Transcriptomics. Cloning Stem Cells. 2014;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 19.Tan Y.S.E., Shi P.J., Choo C.-J., Laude A., Yeong W.Y. Tissue engineering of retina and Bruch’s membrane: A review of cells, materials and processes. Br. J. Ophthalmol. 2018;102:1182–1187. doi: 10.1136/bjophthalmol-2017-311390. [DOI] [PubMed] [Google Scholar]
- 20.Da Cruz L., Fynes K., Georgiadis O., Kerby J., Luo Y.H., Ahmado A., Vernon A., Daniels J.T., Nommiste B., Hasan S.M., et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018;36:328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 21.Kashani A.H., Lebkowski J.S., Rahhal F.M., Avery R.L., Salehi-Had H., Dang W., Lin C.-M., Mitra D., Zhu D., Thomas B.B., et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao4097. [DOI] [PubMed] [Google Scholar]
- 22.Klassen H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin. Boil. 2015;16:7–14. doi: 10.1517/14712598.2016.1093110. [DOI] [PubMed] [Google Scholar]
- 23.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y., et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz S.D., Hubschman J.-P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz S.D., Regillo C.D., Lam B.L., Eliott D., Rosenfeld P.J., Gregori N.Z., Hubschman J.-P., Davis J.L., Heilwell G., Spirn M., et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 26.Davis R.J., Alam N.M., Zhao C., Müller C., Saini J.S., Blenkinsop T.A., Mazzoni F., Campbell M., Borden S.M., Charniga C.J., et al. The Developmental Stage of Adult Human Stem Cell-Derived Retinal Pigment Epithelium Cells Influences Transplant Efficacy for Vision Rescue. Stem Cell Rep. 2017;9:42–49. doi: 10.1016/j.stemcr.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn K., Aotaki-Keen A., Putkey F., Hjelmeland L. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996;62:155–170. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 28.German O.L., Buzzi E., Rotstein N.P., Rodríguez-Boulan E., Politi L.E. Retinal pigment epithelial cells promote spatial reorganization and differentiation of retina photoreceptors. J. Neurosci. Res. 2008;86:3503–3514. doi: 10.1002/jnr.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diniz B., Thomas P., Thomas B., Ribeiro R., Hu Y., Brant R., Ahuja A., Zhu D., Liu L., Koss M., et al. Subretinal Implantation of Retinal Pigment Epithelial Cells Derived From Human Embryonic Stem Cells: Improved Survival When Implanted as a Monolayer. Investig. Opthalmol. Vis. Sci. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koss M.J., Falabella P., Stefanini F.R., Pfister M., Thomas B.B., Kashani A.H., Brant R., Zhu D., Clegg D.O., Hinton D.R., et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: A feasibility and safety study in Yucatán minipigs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016;254:1553–1565. doi: 10.1007/s00417-016-3386-y. [DOI] [PubMed] [Google Scholar]
- 31.M’Barek K.B., Habeler W., Plancheron A., Jarraya M., Regent F., Terray A., Yang Y., Chatrousse L., Domingues S., Masson Y., et al. Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai7471. [DOI] [PubMed] [Google Scholar]
- 32.Bennis A., Jacobs J.G., Catsburg L.A.E., Brink J.B.T., Koster C., Schlingemann R.O., Van Meurs J., Gorgels T.G.M.F., Moerland P.D., Heine V.M., et al. Stem Cell Derived Retinal Pigment Epithelium: The Role of Pigmentation as Maturation Marker and Gene Expression Profile Comparison with Human Endogenous Retinal Pigment Epithelium. Stem Cell Rev. Rep. 2017;13:659–669. doi: 10.1007/s12015-017-9754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ablonczy Z., Dahrouj M., Tang P.H., Liu Y., Sambamurti K., Marmorstein A.D., Crosson C.E. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Investig. Opthalmol. Vis. Sci. 2011;52:8614–8620. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim A.L., D’Amore P.A. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am. J. Pathol. 2012;181:376–379. doi: 10.1016/j.ajpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadiq M.A., Hanout M., Sarwar S., Hassan M., Do D.V., Nguyen Q.D., Sepah Y.J. Platelet derived growth factor inhibitors: A potential therapeutic approach for ocular neovascularization. Saudi J. Ophthalmol. 2015;29:287–291. doi: 10.1016/j.sjopt.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arroba A.I., Wallace D., Mackey A., De La Rosa E.J., Cotter T.G. IGF-I maintains calpastatin expression and attenuates apoptosis in several models of photoreceptor cell death. Eur. J. Neurosci. 2009;30:975–986. doi: 10.1111/j.1460-9568.2009.06902.x. [DOI] [PubMed] [Google Scholar]
- 37.Arroba A.I., Alvarez-Lindo N.L., Van Rooijen N., De La Rosa E.J. Microglia-Mediated IGF-I Neuroprotection in therd10Mouse Model of Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2011;52:9124–9130. doi: 10.1167/iovs.11-7736. [DOI] [PubMed] [Google Scholar]
- 38.Azadi S., Johnson L.E., Paquet-Durand F., Perez M.-T.R., Zhang Y., Ekström P.A., Van Veen T. CNTF + BDNF treatment and neuroprotective pathways in the rd1 mouse retina. Brain Res. 2007;1129:116–129. doi: 10.1016/j.brainres.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M., Mo X., Fang Y., Guo W., Wu J., Zhang S., Huang Q. Rescue of photoreceptors by BDNF gene transfer using in vivo electroporation in the RCS rat of retinitis pigmentosa. Curr. Eye Res. 2009;34:791–799. doi: 10.1080/02713680903086018. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan H.J., Leibole M.A., Tezel T., Ferguson T.A. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat. Med. 1999;5:292–297. doi: 10.1038/6509. [DOI] [PubMed] [Google Scholar]
- 41.Nagineni C.N., Samuel W., Nagineni S., Pardhasaradhi K., Wiggert B., Detrick B., Hooks J.J. Transforming growth factor-? Induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 42.Tosi G.M., Orlandini M., Galvagni F. The Controversial Role of TGF-β in Neovascular Age-Related Macular Degeneration Pathogenesis. Int. J. Mol. Sci. 2018;19:3363. doi: 10.3390/ijms19113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moiseyev G., Chen Y., Takahashi Y., Wu B.X., Ma J., Nathans J. RPE65 visual is the cycle in the isomerohydrolase retinoid. Proc. Natl. Acad. Sci. USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson A.A., Guziewicz K.E., Lee C.J., Kalathur R.C., Pulido J.S., Marmorstein L.Y., Marmorstein A.D. Bestrophin 1 and retinal disease. Prog. Retin. Eye Res. 2017;58:45–69. doi: 10.1016/j.preteyeres.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donato L., Scimone C., Alibrandi S., Nicocia G., Rinaldi C., Sidoti A., D’Angelo R. Discovery of GLO1 New Related Genes and Pathways by RNA-Seq on A2E-Stressed Retinal Epithelial Cells Could Improve Knowledge on Retinitis Pigmentosa. Antioxidants. 2020;9:416. doi: 10.3390/antiox9050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuel W., Jaworski C., Postnikova O.A., Kutty R.K., Duncan T., Tan L.X., Poliakov E., Lakkaraju A., Redmond T.M. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 2017;23:60–89. [PMC free article] [PubMed] [Google Scholar]
- 47.Lu B., Malcuit C., Wang S., Girman S., Francis P.R., Lemieux L., Lanza R., Lund R.D. Long-Term Safety and Function of RPE from Human Embryonic Stem Cells in Preclinical Models of Macular Degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 48.Sachdeva M.M., Eliott D. Stem Cell-Based Therapy for Diseases of the Retinal Pigment Epithelium: From Bench to Bedside. Semin. Ophthalmol. 2016;31:25–29. doi: 10.3109/08820538.2015.1115253. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz S.D., Tan G., Hosseini H., Nagiel A. Subretinal Transplantation of Embryonic Stem Cell–Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Opthalmol. Vis. Sci. 2016;57:1. doi: 10.1167/iovs.15-18681. [DOI] [PubMed] [Google Scholar]
- 50.Caffé A.R., Söderpalm A.K., Holmqvist I., Van Veen T. A combination of CNTF and BDNF rescues rd photoreceptors but changes rod differentiation in the presence of RPE in retinal explants. Investig. Ophthalmol. Vis. Sci. 2001;42:275–282. [PubMed] [Google Scholar]
- 51.Comitato A., Subramanian P., Turchiano G., Montanari M., Becerra S.P., Marigo V. Pigment epithelium-derived factor hinders photoreceptor cell death by reducing intracellular calcium in the degenerating retina. Cell Death Dis. 2018;9:560. doi: 10.1038/s41419-018-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bird A. Therapeutic targets in age-related macular disease. J. Clin. Investig. 2010;120:3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Domenici L. Translational Research on BDNF may Lead to New Research Therapy in Glaucoma. JOJ Ophthalmol. 2017;3 doi: 10.19080/JOJO.2017.03.555620. [DOI] [Google Scholar]
- 54.Singer M. Advances in the management of macular degeneration. F1000Prime Rep. 2014;6:29. doi: 10.12703/P6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhutto I.A., McLeod D.S., Hasegawa T., Kim S.Y., Merges C., Tong P., Lutty G.A. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp. Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haurigot V.A., Villacampa P., Ribera A., Bosch A., Ramos D., Ruberte J., Bosch F. Long-Term Retinal PEDF Overexpression Prevents Neovascularization in a Murine Adult Model of Retinopathy. PLoS ONE. 2012;7:e41511. doi: 10.1371/journal.pone.0041511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohno-Matsui K., Morita I., Tombran-Tink J., Mrazek D., Onodera M., Uetama T., Hayano M., Murota S.-I., Mochizuki M. Novel mechanism for age-related macular degeneration: An equilibrium shift between the angiogenesis factors VEGF and PEDF. J. Cell. Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- 58.Regent F., Morizur L., Lesueur L., Habeler W., Plancheron A., Ben M’Barek K., Monville C. Automation of human pluripotent stem cell differentiation toward retinal pigment epithelial cells for large-scale productions. Sci. Rep. 2019;9:10646. doi: 10.1038/s41598-019-47123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamiri P., Masli S., Kitaichi N., Taylor A.W., Streilein J.W. Thrombospondin Plays a Vital Role in the Immune Privilege of the Eye. Investig. Opthalmol. Vis. Sci. 2005;46:908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 60.Warwick A., Gibson J., Sood R., Lotery A. A rare penetrant TIMP3 mutation confers relatively late onset choroidal neovascularisation which can mimic age-related macular degeneration. Eye. 2015;30:488–491. doi: 10.1038/eye.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamei M., Hollyfield J.G. TIMP-3 in Bruch’s membrane: Changes during aging and in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1999;40:2367–2375. [PubMed] [Google Scholar]
- 62.Weber B.H.F., Vogt G., Pruett R.C., Sto¨hr H., Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat. Genet. 1994;8:352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- 63.Raoul W., Auvynet C., Camelo S., Guillonneau X., Feumi C., Combadière C., Sennlaub F. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J. Neuroinflamm. 2010;7:87. doi: 10.1186/1742-2094-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonas J.B., Tao Y., Neumaier M., Findeisen P. Monocyte Chemoattractant Protein 1, Intercellular Adhesion Molecule 1, and Vascular Cell Adhesion Molecule 1 in Exudative Age-Related Macular Degeneration. Arch. Ophthalmol. 2010;128:1281–1286. doi: 10.1001/archophthalmol.2010.227. [DOI] [PubMed] [Google Scholar]
- 65.Ricci F., Staurenghi G., Lepre T., Missiroli F., Zampatti S., Cascella R., Borgiani P., Marsella L.T., Eandi C.M., Cusumano A., et al. Haplotypes in IL-8 Gene Are Associated to Age-Related Macular Degeneration: A Case-Control Study. PLoS ONE. 2013;8:e66978. doi: 10.1371/journal.pone.0066978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Ani A., Toms D., Kondro D., Thundathil J., Yü Y., Ungrin M. Oxygenation in cell culture: Critical parameters for reproducibility are routinely not reported. PLoS ONE. 2018;13:e0204269. doi: 10.1371/journal.pone.0204269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calton M.A., Beaulieu M.O., Benchorin G., Vollrath D. Method for measuring extracellular flux from intact polarized epithelial monolayers. Mol. Vis. 2018;24:425–433. [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X., Xie J., Liu Z., Gong Q., Tian R., Su G.-F. Identification and validation of reference genes for quantitative RT-PCR analysis of retinal pigment epithelium cells under hypoxia and/or hyperglycemia. Gene. 2016;580:41–46. doi: 10.1016/j.gene.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Ren S., Zhang F., Li C., Jia C., Li S., Xi H., Zhang H., Yang L., Wang Y.-Q. Selection of housekeeping genes for use in quantitative reverse transcription PCR assays on the murine cornea. Mol. Vis. 2010;16:1076–1086. [PMC free article] [PubMed] [Google Scholar]
- 70.Maruotti J., Sripathi S.R., Bharti K., Fuller J., Wahlin K.J., Ranganathan V., Sluch V.M., Berlinicke C.A., Davis J., Kim C., et al. Small-Molecule–Directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2015;112:10950–10955. doi: 10.1073/pnas.1422818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.R Core Team . R: A Language and Environment for Statistical Computing. v.3.5.1. R Core Team; Vienna, Austria: 2018. [Google Scholar]
- 72.Geiger M., Wahlmüller F., Furtmüller M. The Serpin Family. Springer International Publishing; Cham, Switzerland: 2015. [Google Scholar]
- 73.Hochmann S., Kaslin J., Hans S., Weber A., Machate A., Geffarth M., Funk R.H.W., Brand M. Fgf Signaling is Required for Photoreceptor Maintenance in the Adult Zebrafish Retina. PLoS ONE. 2012;7:e30365. doi: 10.1371/journal.pone.0030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall M.O., Obin M.S., Prieto A.L., Burgess B.L., Abrams T.A. Gas6 Binding to Photoreceptor Outer Segments Requires γ-Carboxyglutamic Acid (Gla) and Ca2+ and is Required for OS Phagocytosis by RPE Cells in vitro. Exp. Eye Res. 2002;75:391–400. doi: 10.1006/exer.2002.2030. [DOI] [PubMed] [Google Scholar]
- 75.Hettich C., Wilker S., Mentlein R., Lucius R., Roider J., Klettner A. The retinal pigment epithelium (RPE) induces FasL and reduces iNOS and Cox2 in primary monocytes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014;252:1747–1754. doi: 10.1007/s00417-014-2742-z. [DOI] [PubMed] [Google Scholar]
- 76.Ruiz A., Brett P., Bok D. TIMP-3 Is Expressed in the Human Retinal Pigment Epithelium. Biochem. Biophys. Res. Commun. 1996;226:467–474. doi: 10.1006/bbrc.1996.1379. [DOI] [PubMed] [Google Scholar]
- 77.Campochiaro A.P., Hackett S.F., Vinores S.A., Freund J., Csaky C., LaRochelle W., Henderer J., Johnson M., Rodriguez I.R., Friedman Z. Platelet-Derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J. Cell Sci. 1994;107:2459–2469. doi: 10.1242/jcs.107.9.2459. [DOI] [PubMed] [Google Scholar]
- 78.Mori K., Gehlbach P., Ando A., Dyer G., Lipinsky E., Chaudhry A.G., Hackett S.F., Campochiaro P.A. Retina-Specific expression of PDGF-B versus PDGF-A: Vascular versus nonvascular proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2002;43:2001–2006. [PubMed] [Google Scholar]
- 79.Gogat K., Le Gat L., Berghe L.V.D., Marchant D., Kobetz A., Gadin S., Gasser B., Abitbol M., Menasche M. VEGF and KDR gene expression during human embryonic and fetal eye development. Investig. Opthalmol. Vis. Sci. 2004;45:7–14. doi: 10.1167/iovs.02-1096. [DOI] [PubMed] [Google Scholar]
- 80.LaVail M.M., Ash J.D., Anderson R.E., Hollyfield J.G., Grimm C. Retinal Degenerative Diseases. Springer International Publishing; Cham, Switzerland: 2012. [Google Scholar]
- 81.Chen H., Liu B., Lukas T.J., Neufeld A.H. The Aged Retinal Pigment Epithelium/Choroid: A Potential Substratum for the Pathogenesis of Age-Related Macular Degeneration. PLoS ONE. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim Y.H., He S., Kase S., Kitamura M., Ryan S.J., Hinton D.R. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009;247:651–659. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hägglund A.-C., Dahl L., Carlsson L. Lhx2 Is Required for Patterning and Expansion of a Distinct Progenitor Cell Population Committed to Eye Development. PLoS ONE. 2011;6:e23387. doi: 10.1371/journal.pone.0023387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masuda T., Wahlin K., Wan J., Hu J., Maruotti J., Yang X., Iacovelli J., Wolkow N., Kist R., Dunaief J.L., et al. Transcription Factor SOX9 Plays a Key Role in the Regulation of Visual Cycle Gene Expression in the Retinal Pigment Epithelium. J. Boil. Chem. 2014;289:12908–12921. doi: 10.1074/jbc.M114.556738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strunnikova N.V., Maminishkis A., Barb J.J., Wang F., Zhi C., Sergeev Y., Chen W., Edwards A.O., Stambolian D., Abecasis G.R., et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum. Mol. Genet. 2010;19:2468–2486. doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kopplin L.J., Igo J.R.P., Wang Y., Sivakumaran A.T., Hagstrom A.S., Peachey N.S., Francis P.J., Klein M.L., SanGiovanni J.P., Chew E.Y., et al. Genome-Wide association identifies SKIV2L and MYRIP as protective factors for age-related macular degeneration. Genes Immun. 2010;11:609–621. doi: 10.1038/gene.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]