Abstract
Caesalpinia ferrea C. Mart., popularly known as “Jucá” or “Pau-ferro”, belongs to the Fabaceae family, and is classified as a native and endemic species in Brazil. Numerous studies that portray its ethnobotany, chemical composition, and biological activities exist in the literature. The present study aimed to systematically review publications addressing the botanical aspects, uses in popular medicine, phytochemical composition, and bioactivities of C. ferrea. The searches focused on publications from 2015 to March 2020 using the Scopus, Periódicos Capes, PubMed, Google Scholar, and ScienceDirect databases. The leaves, fruits, seeds, and bark from C. ferrea are used in popular medicine to treat disorders affecting several systems, including the circulatory, immune, cardiovascular, digestive, respiratory, genitourinary, musculoskeletal, and conjunctive systems. The most commonly found chemical classes in phytochemical studies are flavonoids, polyphenols, terpenoids, tannins, saponins, steroids, and other phenolic compounds. The biological properties of the extracts and isolated compounds of C. ferrea most cited in the literature were antibacterial, antifungal, antioxidant, antiproliferative, anti-inflammatory, and healing potential. However, further studies are still needed to clarify a link between its traditional uses, the active compounds, and the reported pharmacological activities, as well as detailed research to determine the toxicological profile of C. ferrea.
Keywords: Caesalpinia ferrea, ethnoknowledge, bioactivities, phytochemicals, HPLC
1. Introduction
Caesalpinia ferrea C. Mart, formerly known as Libidibia ferrea, is a native and endemic species of Brazil, belonging to the Fabaceae family, popularly known as “Jucá” or “Pau-ferro”, and with several phytogeographic domain distributions, including the Cerrado, Caatinga, and Atlantic Forest [1].
Several parts from this species, such as the bark, leaves, fruits, and seeds, have been widely used by human beings in popular medicine through teas, decoctions, infusions, syrup, and macerations for countless therapeutic purposes, including: cicatrizing, anti-inflammatory, homeostatic, antiseptic, respiratory disorders, rheumatism, gastritis, among other purposes [2,3,4,5].
Therefore, several studies have investigated the phytochemicals and bioactivities attributed to C. ferrea, where, in terms of its chemical composition, studies have shown a diversity of chemical classes and compounds in the extracts from different C. ferrea organs. Flavonoids, organic acids, saponins, coumarins, phenols, and tannins are among the secondary metabolite chemical classes found in its extracts, while the phenolic acids, ellagic acid, and gallic acid, are the most commonly found compounds [6,7,8].
C. ferrea leaves, fruits, pods, and bark have been reported in the literature to have antibacterial, antifungal, anti-inflammatory, antioxidant, antidiabetic, and antiulcerogenic properties [9,10,11,12,13,14].
In the literature, there is a review about the biological properties of C. ferrea [15]; however, this review aimed to list the scientific information, covering five years, about the botanical aspects, traditional uses, phytochemistry, bioactivities, and the toxicity of C. ferrea, as well as discuss perspectives for new researches.
2. Results and Discussion
Following the database searches, 4069 studies were counted. Once the established inclusion and exclusion criteria were implemented, 87 articles were selected and analyzed for data extraction and result interpretation. It is worth noting that some publications analyzed both the chemical composition of the extract, as well as its biological effects.
2.1. Botanical Characterization
Caesalpinia ferrea is characterized for presenting an arboreal habit, with a height ranging from 10 to 15 m, alternating leaves and composed with alternating oval shaped leaflets with a hydrophobic character [16,17,18]. Inflorescences have flowers with yellow petals, an obovate shape and reddish spots [17,19]. The flowering period starts at the end of November and extends to the month of January, while the fruit ripening period comprises the months from July to August [20].
The fruits are flattened pods, which when immature are a green color and when ripe are a brown color, with this behavior being repeated with the seeds [19]. Table 1 represents the summary of the C. ferrea botanical characteristics. Its seeds are a determinant for the diffusion of the species; however, the seeds present dormancy caused by the impermeability of the tissue integument [21].
Table 1.
Summary of the C. ferrea botanical characteristics.
According to a study performed to evaluate the viability of C. ferrea seeds using the tetrazolium test, viable seeds had the following characteristics: bright light pink color, tissues with a normal and firm appearance, an intense red embryonic axis that did not reach the central cylinder, less than 50% of cotyledons were discolored or had necrotic regions, which did not interfere with the embryonic axis attachment area [22].
C. ferrea is a water demanding species for its growth, since when subjected to hydric stress conditions a relevant reduction in its height and leaf number was observed, indicating that water supply restrictions impaired the development of its morphological and physiological characteristics [23].
2.2. Ethnobotany
The ethnobotanical studies were selected according to the data provided for the plant part that was used, including preparation methods or the uses and therapeutic indications of C. ferrea, or its synonym C. ferrea. Table 2 summarizes the 19 articles addressing the medicinal uses of C. ferrea.
Table 2.
Traditional uses of C. ferrea for curing diseases.
| Part Used | Method of Preparation or Use | Therapeutic Indication | Citation |
|---|---|---|---|
| Leaf | Tea | Vermifuge | [24] |
| Leaf, bark and fruit | Decoction, “lambedor”, maceration, medicinal wine | Asthma, bones pain, flu, kidney pain, cough, shaking | [25] |
| Bark | Decoction | Liver/bleeding | [26] |
| Pod, fruit, seed, and bark | Tanned in wine, tea, bath, macerated, cooked beaten with water | Anti-inflammatory and healing | [27] |
| Bark and fruit | Tea, “lambedor” and syrup | Flu, kidney inflammation and soothing | [28] |
| Fruit | “Lambedor” | Flu | [29] |
| Bark and seeds | Hurt the seed and soak it in the water | Pneumonia, anemia, diarrhea, colic and gastritis | [30] |
| Stem bark, fruit, and seed | Maceration | Anti-inflammatory, kidneys, bruises, back pain, healing, analgesic | [31] |
| Bark | Decoction | Malaria | [32] |
| Bark and root | Tea and bottles | Rheumatism and diabetes | [33] |
| Bark and fruit | Bottles | Anti-inflammatory | [34] |
| Fruit | Tea | Diarrhea, liver and healing | [35] |
| Stalk | Tea | Anti-inflammatory | [36] |
| Roots | Decoction | Hemorrhoids, inflammation of the eyes and injuries | [37] |
| Whole shell | Immersed in water | Hemorrhage, anti-inflammatory, infection and pain | [38] |
| Dry bark | Decoction | Back pain | [39] |
| Stem bark and fruit | Maceration and cooking | Back pain, vision problems, anti-inflammatory and healing | [40] |
| Fruit | Tea (decoction), tea (maceration), maceration in a bath | Sore throat, hoarseness, leg pain, toothache, uterine inflammation, wounds, anemia, gastritis | [41] |
| Fruit, bark, roots and seed | Tea and tincture | Asthma, bronchitis, flu, fever, sore throat, sinusitis, diarrhea, rheumatism, blood clearance, kidneys and soothing | [42] |
| Fruits | Tea | Urinary infection | [43] |
| Fruits | Bottles | Infection | [44] |
| Stem bark, bast, fruits and seeds | Tea, “lambedor”, in natura and in powder | Infectious diseases, parasitic, circulatory, immune, cardiovascular, digestive, respiratory, genitourinary, musculoskeletal, conjunctive, injuries and poisoning | [45] |
| Seeds | Tea and immersed in water | Skin cuts, cough, flu and depression | [3] |
| Bark, fruit, and seeds | Decoction, infusion, maceration and syrup | Syphilis, cancer, depurative, diabetes, asthma, gastritis, bronchitis, sinusitis, stomach ache, rheumatism, sexual impotence, healing, bone fracture, headache, fever and throat infection | [46] |
| Whole plant and fruits | Infusion and maceration | Spine, blow, inflammation and kidneys | [47] |
| Leafs | Tea | All kinds of infection and inflammation | [5] |
Ethnobotanical researches are important to understand the use of this plant by traditional communities and the general population for medicinal purposes and to maintain the folk culture. The results of ethnobotanical studies contribute with the association between traditional and modern knowledge, being an important tool to the investigation of the biological properties of medicinal plants.
Traditionally, Caesalpinia ferrea is used by traditional communities in northern and northeastern regions of Brazil to medicinal purposes [27,32,37,43,45,46,47]. The barks of C. ferrea are used by the native communities Cunuri, Tapira Ponta, Ilha das Flores, Curicuriari, and São Jorge, and riverside communities and rubber collectors located in the Amazon region, to combat malaria symptoms [25,32].
Given the analysis of the articles, the leaves were indicated as a vermifuge and for the treatment of infections and general inflammation, both of which used tea as a preparation method [5,24]. The bark from C. ferrea is one of the most commonly used organs in traditional medicine through the process of decoction, tea preparations, syrups and bottles for the treatment of various conditions which includes: flus, coughs, kidney and liver inflammation, anxiolytic, rheumatisms, diabetes, hemorrhages, inflammations, infections, and general pains [26,28,32,33,38,39].
The traditional use of the bark alongside other plant parts such as the bark, roots, and leaves, especially its fruits and seeds, has also been reported in the literature for various therapeutic indications, namely: syphilis, cancer, as a depurative, diabetes, gastritis, stomach pain, rheumatism, sexual impotence, cicatrizing, bone fractures, headaches, respiratory system disorders, fever, diarrhea, kidney problems, anxiolytic, vision problems, anti-inflammatory, analgesic, hematomas, anemia, colic, and shaking [27,31,34,40,42,45,46].
After the bark, the fruits are the most commonly used plant parts in the form of teas, bottled and macerated, for the treatment of diarrhea, liver, and kidney problems, pain (throat, legs, spine, and tooth), uterine inflammation, anemia, gastritis and urinary infection, as well as cicatrizing [29,35,41,43,44,47]. The seed tea is used to treat flus and coughs, and when immersed in water it is used as a cicatrizing agent [3].
2.3. Phytochemical Aspects
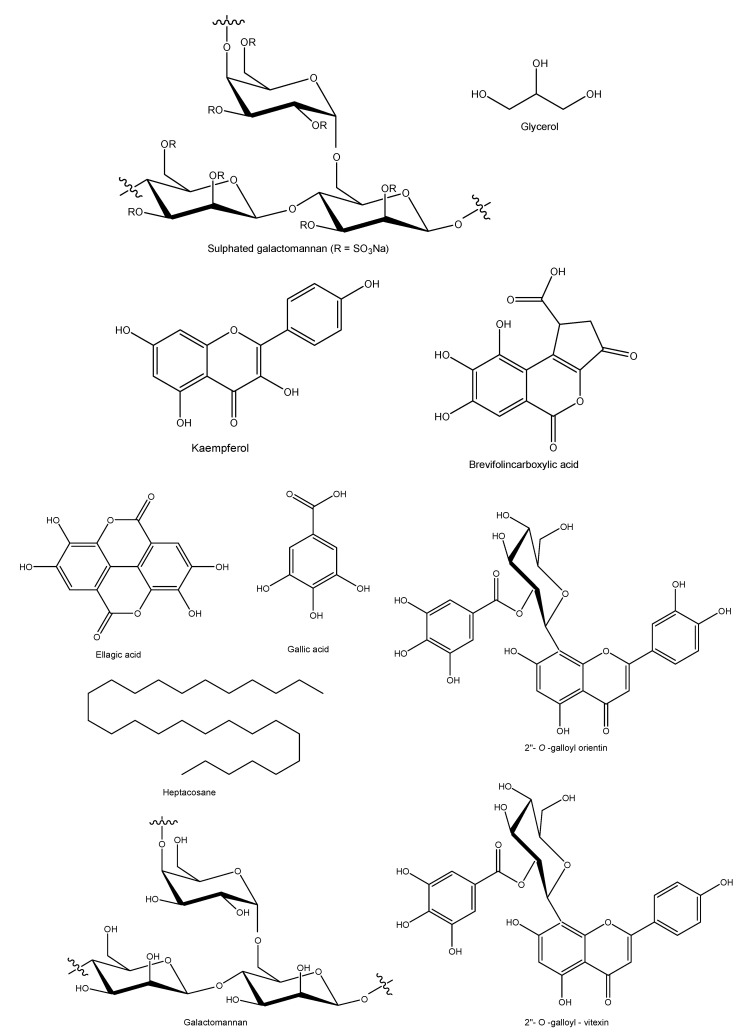
Extracts from C. ferrea leaves, seeds, pods, and basts have been widely studied for having several secondary metabolites such as flavonoids, polyphenols, terpenoids, tannins, saponins, steroids, and other phenolic compounds that present a variety of bioactivities. In this review, 23 articles that investigated the phytochemicals in C. ferrea extracts were found (Table 3). Figure 1 represents the main phytocompounds from C. ferrea.
Table 3.
Chemical classes or constituents found in C. ferrea extracts.
| Part Used | Solvent | Analytical Technique | Constituents | Citations |
|---|---|---|---|---|
| Leafs | Cyclohexane | CG/MS | Octacosane, docosane, and heptacosan | [8] |
| Leafs | Water | HPLC | Ellagic acid and gallic | [7] |
| Leafs | Water at 25 and 100 °C | HPLC-DAD | Gallic acid, caffeic and ellagic epicatechin, quercetin, and luteolin and catechin | [48] |
| Leafs | Ethanol at 70% | HPLC | Ellagic acid and gallic, orientin and isovitexin | [49] |
| Leafs | Ethanol at 70% | NMR 1D e 2D | Gallic acid, brevifolin carboxylic acid, and brevifolin | [50] |
| Barks and seeds | Ethanol at 70% | HPLC | Ellagic acid | [51] |
| Barks | Water at 25 and 100 °C | HPLC-DAD | Gallic, caffeic and ellagic acids, catechin, epicatechin and quercetin | [48] |
| Barks | Water | RP-HPLC | Ellagic acid and gallic | [52] |
| Barks | Ethanol and water | LC-MS/MS | Kaempferol, quinolinic acid and gallic | [53] |
| Fruit | Ethanol at 96% | LC-HRMS/MS | Corilagin and ellagic acid and gallic | [1] |
| Fruit | Water, ethanol at 20–80% | HPLC-DAD | Ellagic acid and gallic | [7] |
| Fruit | Ethanol | HPLC | Ellagic acid and gallic | [54] |
| Fruit | Water | HPTLC e HPLC | Ellagic acid and gallic | [55] |
| Pods | N-hexane | GC-MS | N-dodecanol, myristic acid, methyl palmitate, palmitic acid | [56] |
| Pods | Chloroform | GC-MS | n-valeric acid, caproic acid, heptanoic acid, and octanoic acid | [56] |
| Pods | Ethyl acetate | GC-MS | Oxalic acid, butanedioic acid, pyrotartaric acid, and pentanoic acid | [56] |
| Pods | Alcohol at 70% | GC-MS | Glycerol, D-fructose, myo-inositol, and glucopyranose | [56] |
| Pods | Alcohol at 40% | HPLC-MS | Valonium dilactone acid, gallic acid derivatives, and ellagic acid | [12] |
| Pods | Ethanol and Water | LC-MS/MS | Ellagic acid, chlorogenic acid, and rutin | [54] |
| Pods | Water at 25 and 100 °C | HPLC-DAD | Ellagic acid and gallic, catechin, epicatechin, quercetin, and luteolin | [48] |
GC-MS = thin-layer chromatography and Gas Chromatography-Mass Spectrometry; HPLC-DAD = High-Performance Liquid Chromatography with Diode Array Detection; RP-HPLC = Reverse Phase High-Performance Liquid Chromatography; HPLC = High Performance Liquid Chromatography; LC-HRMS/MS = Liquid Chromatography-High Resolution Tandem Mass Spectrometry; NaOH = Sodium hydroxide; LC-MS/MS = Liquid Chromatography Coupled to Tandem Mass Spectrometry; HPLC-MS = High-Performance Liquid Chromatography coupled to Mass Spectrometry; HPTLC = High-Performance Thin Layer Chromatography; NMR = Nuclear Magnetic Resonance.
Figure 1.
Chemical structures of the main compounds from C. ferrea. Ellagic acid: C14H6O8 and MW: 302.19 g/mol; Gallic acid: C7H6O5 and MW: 170.12 g/mol; Heptacosan: C27H56O and MW: 396.7 g/mol; Galactomannan: C18H32O16 and MW: 504.4g/mol; Kaempferol: C15H10O6 and MW: 286.24 g/mol; 2”-O-Galloylorientin: C28H24O15 and MW: 600.5 g/mol; 2”-O-Galloylvitexin: C28H24O14 and MW: 584.5 g/mol; Glycerol: C3H8O3 and MW: 92.09 g/mol.
2.3.1. Leaves
The qualitative phytochemical investigation of C. ferrea leaf extracts showed the presence of several chemical classes such as flavonoids, tannins, alkaloids, cinnamic derivatives, terpenes, saponins, organic acids, reducing sugars, steroids, triterpenoids, phenols, glycosides, phenolic compounds, and carbohydrates [8,9,57].
Gallic acid, brevifolin carboxylic acid, ellagic acid, brevifolin, tellimagrandin-I, 2”-O-galloylvitexin, vitexin, 2”-O-galloylorientin, orientin, isovitexin 2”-O-β- [xylopyranosyl-(1”” --- 2”’)-O-β-xylopyranosyl], isovitexin, orientin 2”-O-β- [xylopyranosyl-(1”” --- 2”’)-O-β-xylopyranosyl] were identified in the phytochemical composition of the leaf hydroethanolic extract using HPLC and NMR 1 and 2D techniques [49,50].
The leaves were subjected to two treatments, one with water at 100 °C and the other with water at 25 °C. After a HPLC phytochemical analysis of these extracts, gallic acid, caffeic acid, epicatechin, quercetin and luteolin were identified in both extracts, with ellagic acid and catechin also being identified in the hot extract (100 °C) [48]. Meanwhile, GC-MS analyzes of the cyclohexanic extract revealed the presence of n-dodecanal, octacosane, docosane, pentadecane, and heptacosane [8].
2.3.2. Bark
The High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) analysis of the C. ferrea aqueous extract showed the presence of gallic, caffeic, and ellagic acids, catechin, epicatechin, and quercetin [47]. Whereas qualitative phytochemical analyzes of extracts with different solvent bases (ethanol, methanol, ethyl acetate and NaOH) presented different chemical classes such as flavonoids, tannins, saponins, steroids, terpenoids, coumarins, carbohydrates and proteins [6,51,53,58].
The phytochemical profile of the stem bark hydromethanolic extract was traced using the LC-MS/MS technique, detecting the presence of 15 compounds: quinolinic acid, gallic acid, 2-(2-ethyl-3-hydroxy-6-propionylcyclo-hexyl) acetic acid, ellagic acid, 12-oxo-phytodienoic acid, catechin, epicatechin, chlorogenic acid, rutin, taxifolin, myricetin, quercetin, kaempferol, apigenin, and isorhamnetin [57]. The chemical compounds ellagic and gallic acids were also found by HPLC in the aqueous and hydromethanolic extract [59,60].
2.3.3. Fruits or Pods
The phytochemical analyzes of the aqueous and hydroalcoholic fruit extracts identified the presence of ellagic acid and gallic acid with the HPLC technique [13,55,61]. Meanwhile, qualitative chemical analyzes of the hydroalcoholic fruit extract showed the presence of seven chemical classes: saponins, organic acids, reducing sugars, phenols, tannins, sesquiterpene lactones, and anthraquinones [56].
LC-HRMS/MS analysis of the hydromethanolic fruit extract revealed the presence of phytochemicals such as gallic acid, galloyl-glucose ester, gallic acid methyl glycoside, hexose, di-O-galloyl-d-hexose, corilagin, ellagic acid, eriodictyol-O-hexoside and naringenin-O-hexoside [1].
Characterization of the pod hydroalcoholic extract chemical constituents revealed the presence of polyphenols (7.3%) and HPLC-MS chromatographic analyzes revealed the presence of nine compounds: galiliquinoic acid, galloyl-HHDP-hex, brevifolin carboxylic acid, valonium dilactone acid, gallic acid derivatives, ellagic acid derivatives (hex-ellagic acid), ellagic acid, and dihydroisovaltrate [12,14].
The LC-MS/MS phenolic composition investigation of the hydroethanolic pod extract showed the presence of gluconic acid, gallic acid, caffeic acid, 2-(2-ethyl-3-hydroxy-6-propionylcyclohexyl) acetic acid, ellagic acid, 12-oxo-phytodienoic acid, catechin, epicatechin, chlorogenic acid, rutin, taxifolin, myricetin, quercetin, kaempferol, apigenin, and isorhamnetin [55].
Phytochemical studies of the hydroalcoholic, chloroformic, n-hexane, and ethyl acetate extracts from C. ferrea pods showed the presence of numerous compounds such as glycerol, d-fructose, myo-inositol, chemical acid, glucopyranose, glucose, 1,2-benzenedicarboxylic acid, oxalic acid, butanedioic acid, pyrotartaric acid, pentanoic acid, malic acid, pentanedioic acid, arabinonic acid, octanedioic acid, azelaic acid, d-galactopyranosyl, benzoic acid, alpha-d-glucopyranose, palmitic acid, stearic acid, 2-bromosbacic acid, tetracosanoic acid, n-valeric acid, alpha hydroxyisobutyric acid, caproic acid, heptanoic acid, octanoic acid, maleic acid, pyrotartaric acid, pelargonic acid, pimelic acid, tetradecanoic acid, suberic acid, myristic acid, D-mannose, n-pentadecanoic acid, palmitic acid, cholesterol, 2-bromosbacic acid, monopalmitin, docosanoic acid, N-dodecanol, myristic acid, methyl palmitate, palmitic acid, methyl oil, methyl stearate, vapor acid, methyl arachidate, arachidonic acid, methyl benzoate, methyl lignocerate, tetracosanoic acid, nonacosane, octacosanol, and campesterol [56].
2.3.4. Seeds and Roots
The seed hydroalcoholic extract presented ellagic acid as its main phytochemical constituent and Thin-Layer Chromatography (TLC) qualitative chemical analyzes showed the presence of fatty acids, coumarins, as well as total and hydrolyzable tannins [59,62]. The root aqueous extract made with water at different temperatures (100 and 25 °C) presented the following constituents in common: gallic and ellagic acid, epicatechin, quercetin and luteolin, while the hot extract (100 °C) also presented catechin in its composition [48].
2.3.5. Compostos De Valor Quimiotaxônomico
The species of the subfamily Caesalpiniaceae present hydrophilic polysaccharides such as galactomannans with a the mannose/galactose ratio ranging from 2.5:1 to 4.3:1 [63]. In C. ferrea, the presence of these polysaccharides was detected by different authors, and these biomolecules are chemotaxonomic markers used to the analysis and identification of these species [64,65]. Other chemical compounds useful as chemotaxonomic markers of Caesalpiniaceae are flavonoids, terpenoids, and isoflavonoids [12,14,48,66].
2.4. Bioactivities
A total of 57 articles investigating the bioactivity of the extracts and compounds isolated from C. ferrea were found. Table 4 represents the summary of the extracts’ bioactivities and Table 5 corresponds to those of the isolated compounds.
Table 4.
Bioactivities evaluated with different extracts of C. ferrea.
| Parts Used/Solvents | Target or Model | Bioactivities Evaluated | Formulations/Dosage | Control (s) | Results | Citations |
|---|---|---|---|---|---|---|
| Full pod/Methanol | Parvimonas micra and Porphyromonas gingivalis | Antibacterial and anti-halitosis | In vitro 50–400 μg/mL for 72 h |
Positive: chlorhexidine Negative: liquid medium |
MIC: 50 and 120 µg/mL, respectively MBC: >50 and 130 µg/mL, respectively | [14] |
| Leafs/ Cyclohexane |
Bacillus subtilis, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, and Staphylococcus aureus | Antibacterial | In vitro 0.04–25 mg/mL for 24 h |
Positive: ampicillin Negative: DMSO 10% |
MIC: 0.039, 0.039, 0.039, 0.39, 0.078 mg/mL, respectively | [8] |
| Leafs/Chloroform | B. subtilis, E. coli, P. vulgaris, P. aeruginosa, and S. aureus | Antibacterial | In vitro 0.04–25 mg/mL for 24 h |
Positive: ampicillin Negative: DMSO 10% |
MIC: 1.56, 6.25, 12.5, 3.12, 0.78 mg/mL, respectively | [8] |
| Leafs/Ethyl acetate | B. subtilis, E. coli, P. vulgaris, P. aeruginosa, and S. aureus | Antibacterial | In vitro 0.04–25 mg/mL for 24 h |
Positive: ampicillin Negative: DMSO 10% |
MIC: 0.78, 6.25, 12.5, 3.12, 1.56 mg/mL, respectively | [8] |
| Leafs/Methanol | B. subtilis, E. coli, P. vulgaris, P. aeruginosa, and S. aureus | Antibacterial | In vitro 0.04–25 mg/mL for 24 h |
Positive: ampicillin Negative: DMSO |
MIC: 6.25, 12.5, 25, 3.12, 3.12 mg/mL, respectively | [8] |
| Barks/Alcohol |
S. aureus ATCC 10390, P. aeruginosa ATCC 9721, and E. coli ATCC 25,922 Wistar rats |
Antibacterial and healing activity | In vitro 10 mg/mL for 24 h In vivo10 mg/mL for 28 days |
Negative: bacterial nanocellulose membranes with extract | MIC: 0.39, 0.79 and 0.19 mg·mL−1, respectively | [67] |
| Fruits/Alcohol | S. aureus, E. coli, Klebsiella pneumoniae, and P. aeruginosa | Antibacterial | In vitro 20 μL of the crude extracts and in dilutions 1:2, 1:4, 1:8; 1:16 for 24 h |
Negative: sterile water | Inhibition halos: 18, 12, 10 and 11 mm, respectively | [68] |
| Leafs and Fruits/Water | Ralstonia solanacearum | Antibacterial | In vitro 0.4–4.0 mg/mL for 24 h |
Negative: water | 70% inhibition at a concentration of 0.4 mg/mL | [69] |
| Pods and bark/Ethanol | S. aureus, E. coli, and P. aeruginosa | Antibacterial | In vitro 512–8 μg/mL for 24 h |
Positive: amikacin, gentamicin, and clindamycin | MIC: 1024 µg/mL for all strains | [70] |
| Barks/Alcohol | Staphylococcus spp. | Antibacterial | In vitro Crude extract, 70% and 50% for 24 h |
Positive: ampicillin, cephalexin, gentamicin, oxacillin, and penicillin Negative: saline |
Inhibition halos: 61.1; 27.78 and 5.56% for the crude extract and concentrations of 70 and 50%, respectively | [71] |
| Leafs/ Propylene Glycol |
S. aureus ATCC 6538 | Antibacterial | In vitro Glycolic extract in concentrations of 3%, 5%, and 10% for 24 and 48 h |
Negative: liquid soap | Average inhibition halo: 0.97 cm | [51] |
| Pods/Ethanol | Streptococcus mutans, Streptococcus mitis, Streptococcus sanguis, Streptococcus sobrinus, and Lactobacillus casei | Antibacterial | In vitro 0.97–500 mg/mL for 24 h |
Positive: chlorhexidine gluconate | MIC: 15, 14, 14, 15, 15 mg/mL, respectively, and MICA: 31.2 mg/mL for all strains | [72] |
| Pods/Ethanol | Staphylococcus aureus, Enterococcus faecalis, B. subtilis, E. coli, K. pneumoniae, and P. aeruginosa | Antibacterial and antioxidant | In vitro 500–25 μg/mL for 24 h; 100–500 μg/mL for 30 min; 20 to 120 μg/mL for 10 min respectively |
Positive: ascorbic acid and Trolox. Negative: specific medium |
MIC: 125, 50, 50, 50, 125, 50 μg/mL, respectively; DPPH: EC50 4.4 μg/mL and ABTS: EC50 2.5 μg/mL | [73] |
| Pods/Alcohol |
Helicobacter pylori Wistar rats |
Antibacterial, antioxidant, antiulcerogenic and toxicity | In vitro 32–1024 μg/mL for 24 h In vivo 200 mg/kg for 14 days |
Positive: amoxicillin, trolox, ranitidine, respectively. Negative: NaCl |
MIC: 512 µg/mL; DPPH and ABTS: IC50 of 28.96 and 145.10 μg/mL, respectively; ED: 113 and 185.7 mg/kg; LD greater than 2000 mg/kg | [12] |
| Pods | Proteobacteria and Bacteroidetes | Antibiofilm | In vitro 0.5, 1, 2, 4, and 8 mg/mL for 48 h |
Negative: sterile water | Inhibited growth by 82% at a concentration of 4 mg·mL−1 | [74] |
| Seeds/ Ethanol |
Candida albicans ATCC 10231, Candida glabrata CCT 0728, Candida krusei CCT 1517, and Candida guilliermondii CCT 1890 | Antifungal | In vitro 4.8–5000 μg/mL for 48 h |
Positive: ethanol 70%; amphotericin B and nystatin. Negative: specific medium |
MIC: 9.7, 19.53, 78 and 39.06 µg/mL, respectively | [11] |
| Seeds/Ethanol | C. albicans ATCC 10231, C. glabrata CCT 0728, C. krusei CCT 1517, and C. guilliermondii CCT 1890; L929 fibroblast cells | Antifungal and Cytotoxicity | In vitro 7.81–1.000 μg/mL for 48 h |
Positive: ethanol 70% | MIC: 9.7; 19; 78 and 4.8 µg/mL, respectively; toxicity at concentrations of 1000; 500 and e 250 µg/mL | [75] |
| Leafs/Water | Colletotrichum sp. | Antifungal | In vitro 0.156 mg/200 mL for 24 h |
Positive: captan | Up to 96% inhibition at a concentration of 0.075 mg.mL−1 | [76] |
| Leafs/Alcohol | Colletotrichum sp. | Antifungal | In vitro 0.156 mg/200 mL for 24 h |
Positive: captan | 100% inhibition of symptoms in treated seeds | [77] |
| Stem bark/Water, Ethanol and acetone | Trichophyton rubrum ATCC 28,189 and Trichophyton mentagrophytes ATCC 11481 | Antifungal | In vitro 1.96–1000 mg/mL for 7 days |
Positive: terbinafine Negative: DMSO |
MIC: 62.5 and 31.3 μg/mL, respectively | [78] |
| --- | Lasiodiplodia theobromae | Antifungal | In vitro 10, 20, and 30% for 5 days |
Negative: sterile distilled water | Inhibited mycelial growth by 85.6% at a concentration of 30% | [79] |
| Leafs/Water | Wistar rats | Anti-inflammatory and antioxidant | In vitro 100, 200 and 300 mg for 24 h |
Negative: saline 0.9% Positive: diclofenac 100 mg/kg |
Effective doses: 100, 200 and 300 mg/kg; | [7] |
| Seeds/Water or Ethanol (20 - 80%) | Swiss mice and mouse embryonic fibroblast 3T3 cell line | Anti-inflammatory, antioxidant, antinociceptive, and cytotoxicity | In vitro 10, 15, 20, 25, and 30 µg/mL for 24, 48, and 72 h In vivo 50, 100, and 200 mg/kg for 20 and 30 min |
Positive: diclofenac, cisplatin and ascorbic acid, and morphine Negative: saline |
Effective doses: 50, 100 and 200 mg/kg; | [13] |
| Pods/Alcohol and ethyl acetate | ACP02 gastric adenocarcinoma cell line | Antioxidant and antimetastatic | In vitro 6.25 or 400 µg/mL for 20 min 6.25, 12.5, 25, 50, 100, 200, and 400 μg/mL for 24 and 48 h |
Positive: doxorubicin Negative: medium RPMI |
DPPH: IC50 74.36 and 116.10 μg/mL ABTS: IC50 9.76 and 29.13 μg/mL Decreased cell migration at concentrations of 50 µg/mL |
[56] |
| Leafs/Ethanol | HaCaT and Wistar rats | Antioxidant, cytotoxicity, and hypolipidemic activity | In vitro 12.45 mg/L for 90 min; extract 50% for 45 min In vivo 300 mg/kg for 4 weeks |
Positive: trolox, etoposide, lipanthyl, respectively | ED50: 12.5 µg/mL, IC50, 114.4 µg/mL | [50] |
| Leafs/Ethanol | Male Sprague–Dawley rats | Antioxidant, antihyperglycemic, and toxicity | In vitro 1 µg/mL for 30 min In vivo 250–500 mg/kg for 72 h and 1600, 2900, and 5000 mg/kg for 24 h |
Positive: ascorbic acid Negative: normal rats |
ED50: 12.45 µg/mL; reduced levels of liver function, serum glucose and a-amylase; non-toxic profile; | [9] |
| Leafs/Ethanol | --- | Antioxidant | In vitro 0.39–100 μg/mL for 30 min |
Positive: trolox | DPPH: IC50 10.57 µg/mL e ABTS: IC50 2.77 µg/mL | [10] |
| Leafs, branches and fruits/Ethanol and hexane | Leishmania (Leishmania) amazonensis and Leishmania (Viannia) guyanensis | Antileishmanial | In vitro 32–500 μg·mL−1 for 24, 48 and 72 h |
Positive: pentamidine Negative: DMSO | Methanol extract from fruits and hexane from leaves: IC50 of 15.04 and 53.09 μg·mL−1L. (L.) amazonensis | [80] |
| Fruits/Ethanol | HT-29 e HEK-293 | Antiproliferative, apoptotic and antioxidant | In vitro 12.5; 25; 50; 100 µg/mL for 24 and 48 h |
Negative: untreated cells | Effective doses: 25–100 μg/mL | [54] |
| Barks and pods/Ethanol | B16F10 e NHF | Anti-wrinkle, anti-whitening and cytotoxicity | In vitro 0–250 μg/mL for 48 h |
Negative: IBMX 25 μM | Effective doses: 25 and 250 μg/mL | [53] |
| Bark and seed/ethanol | Wistar rats | Acute toxicity maternal and fetal | In vivo 1.0; 2.5 and 5.0 g/kg for 14 days |
Positive: 0.9% saline solution | Increase in creatinine levels in maternal serum and morphological changes in the fetus | [59] |
| Fruit/Ethanol | Danio rerio (Zebrafish) | Toxicity | In vivo 25, 50, 75, 125, 250, and 500 mg/L |
Positive: water Negative: 1% propylene glycol | Concentrations of 25, 50, and 125 mg/L caused lethality in the embryos | [1] |
| Bark/Alcohol | Larvae of Artemia salina L. | Toxicity | In vitro 50, 100, 250, 500, 750, and 1000 µg/mL; 750 µg/mL for 24 h | Positive: sea water | CL50 of 822.6334 µg/mL | [81] |
| Fruit/Alcohol | Wistar rats | Toxicity and healing activity | In vivo C. ferrea 12.5 and 50% for 9 days |
Positive: chlorhexidine digluconate Negative: NaCl 0.9% |
Concentration of 12.5% exhibited epidermis constituted in all animals | [61] |
| Seed/Ethanol | Astyanax sp. | Genotoxicity | In vivo and In vitro 5, 10 and 20 mg/L for 96 h |
Negative: not exposed | Increase of 2.5× in the level of DNA strands breaks in erythrocytes exposed to doses of 5, 10, and 20 mg/L | [82] |
| Leafs/Ethanol | HepG-2, Hep2, MCF-7, and HCT-116 | Cytotoxicity | In vitro 5, 12.5, 25, and 50 μg/mL for 48 h |
Positive: not exposed | IC50 of 19.3, 20, 21.8, and 24.47 μg/mL, respectively | [49] |
| Pods/Water | Meristematic roots cells of Allium cepa | Cytotoxic, genotoxic, and cytoprotective potential | In vitro 1 g/500 mL and 1 g/1000 mL for 24 and 48 h |
Positive: water | Cytotoxic at concentrations 1 g/500 mL and 1 g/1000 mL after times 24 and 48 h of exposure | [83] |
| Pods | Oryctolagus cuniculus | Healing activity | In vivo Ointment in 16 and 24% for 21 days |
Negative: glycerin and water | Ointment in 24% inhibited the lesion area | [84] |
| Stem barks/NaOH | Wistar rats | Healing activity | In vivo 0.025–0.1% for 21 days |
Positive: collagenase 0.1 mL; negative: NaCl 0.9% | Effective concentrations: contractions 0.025, 0.05, 0.75, and 0.1% | [58] |
| Pods | Wistar rats | Healing activity | In vivo Ointment in 50% for 21 days |
Positive: ointment collagenase | Significant reduction in the lesion area | [85] |
| Barks/Ethanol | --- | Photoprotective activity and antioxidant | In vitro 0.005; 0.025; 0.050 e 0.100 mg/mL |
Positive: Ascorbic acid | SPF of 3.29 in concentration 0.100 mg/mL and IC50 27.53 µg/mL | [6] |
| Stem barks/Methanol | --- | Arginase inhibitory activity | In vitro 10 μL for 30 min |
Positive: Nor-NOHA | Inhibited 12.81% in the concentration 100 μg/mL | [51] |
| Seeds/water | Swiss mice | Inhibition of the hemorrhagic activity | In vitro Two venom to plant extract ratios 1:12 and 1:48 for 1 h |
Positive: crude venom + saline Negative: crude venom + plant extract + saline | Showed no activity | [62] |
| Pods, bark and leafs/Methanol | Wistar rats | Edematogenic effect | In vivo 0.01, 0.1, 1 mg/Kg for 8 h |
Negative: NaCl 0.9% | Effects at doses of 0.01–1 mg/kg | [86] |
| Stem barks/Water | Human third molars | Erosive potential | In vitro 50 mL tea + 0.1 mL 0.1 mol/L NaOH for 5 days |
Positive: 1% citric acid | Loss of 37.03% dental enamel | [87] |
| Fruit barks | Flies of the Calliphoridae family | Repellent action | In vivo 20 and 50 % for 24 h |
Positive: deteriorated bovine liver | Repellency of 97.5 and 100% in the concentrations 20 and 50% | [88] |
| Leafs and pods/Water and methanol | Nasutitermes corniger (Termite) | Insecticidal activity | In vitro 10, 25, 50, and 100 mg·mL−1 for 11 days |
Negative: 0.1% Tween 80 | Workers: CL50 0.255–1.279 mg·mL−1 Soldiers: CL50 0.146–8.003 mg·mL−1 |
[89] |
| Leafs/Alcohol | Aphis craccivora (Black aphid) | Insecticidal activity | In vivo 2.5 and 5 % |
Positive: insecticide Negative: water |
Efficiency of 51.71% | [90] |
| Leafs and pods/Water and methanol | Dactylopius opuntiae (Carmine cochineal) | Insecticidal activity | In vivo 200 mg/mL |
Negative: 0.1% Tween 80 | 72.46–99.33% of mortality | [91] |
| Leafs and pods/Water and methanol | Dactylopius opuntiae (Carmine cochineal) | Insecticidal activity | In vivo 100, 50, 25, and 10 mg/mL for 10 days |
Positive: chlorpyrifos, acetamiprid and thiamethoxam | Nymphs: CL50 20–150 mg/mL Adults: CL50 43–50 mg/mL |
[92] |
| Leafs/Ethanol | Alternaria alternata | Control of brown spot of Alternaria | In vitro 100, 50, 25, and 10 mg/mL for 10 days for 12 days | Positive: cibenzolar-S-methyl Negative: water |
Concentration of 500 μg/mL reduced in 52.0% the severity of disease | [93] |
| Leafs/Water and ethanol | Alternaria alternata | Control of brown spot of Alternaria | In vitro 1.0 mg/mL for 96 h |
Positive: acibenzolar-S-methyl Negative: water |
Concentration of 1 mg/mL reduced in 96.49 and 99.12% the severity of disease | [94] |
| Leafs | Sorghum bicolor L. (Sorghum) | Fertilizer | --- | --- | Increased the levels of potassium, calcium, and magnesium in the soil | [95] |
| Leafs and seeds/Ethanol | Seeds of Cucumis melo L. | Allelopathic potential | In vitro 1; 0.5; 0.25 and 0.125% for 8 days |
Positive: water | 30% abnormal seedlings at the concentration of 1% | [96] |
| Leafs, barks and roots/Water | Calotropis procera and Cenchrus echinatus | Allelopathic potential | In vitro Crude extract for 5 and 7 days |
Negative: water | Inhibition of germination of both species | [48] |
| Dry leaves | Vigna unguiculata | Allelopathic potential | In vivo Proportion of sand: leaves 1:1/2; 1:1 e 1:2 for 70 days |
Positive: water | Abnormalities in seedlings | [97] |
| Fruits | Meio aquoso contendo MB | Biosorbent | --- | --- | Fast kinetics and good adsorption in the removal of MB | [98] |
| Residues of pods | Captopril aqueous solutions | Biosorbent | Proportion of pod waste: ZnCl2 0.5: 1; 1: 1 and 1.5:1 | --- | 97.67% removal | [99] |
MIC = Minimum Inhibitory Concentration; MBC = Minimum Bactericidal Concentration; MB = methylene blue; HT-29 = human colorectal cancer cell line; HEK-293 = embryonic renal cell line; NaOH = Sodium hydroxide; B16F10 = murine melanoma cell lines; NHF = normal human fibroblasts; HaCaT = keratinocyte cell line; IC50: Half Maximal Inhibitory Concentration; LC50: Median Lethal Concentration; ED50: Half Effective Maximum Dose; EC50: Half Maximal Effective Concentration; DPPH: 2,2-Diphenyl-1-picrylhydrazyl radical; ABTS: 2,2’-azino-bis (3-ethylbenzothiazoline); IBMX: 3-isobutyl-1-methylxanthine; RPMI: Roswell Park Memorial Institute (cell culture medium).
Table 5.
Biological activities of compounds isolated from C. ferrea extracts.
| Compound | Target or Model | Bioactivities Evaluated | Formulations/Dosage | Control(s) | Results | Citations |
|---|---|---|---|---|---|---|
| Galactomannan | Wistar rats | Antihyperglycemic and toxicity | In vivo10 mg/kg for 5 weeks | Positive: non-diabetic animals | Efficient dose of 10 mg/kg; No toxicity | [65] |
| Sulfated galactomannan | DENV-2 virus in Vero cells | Antiviral, antioxidant and cytotoxicity | In vitro 25, 50 and 100 μg/mL for 7 days |
Positive: Vero cells infected DENV-2 Negative: Normal Vero Cells |
96% inhibition against DENV-2 in the concentration of 25 g/mL; IC50 of 0.94 μg/mL | [100] |
| Brevifolin carboxylic acid | HaCaT | Antioxidant and cytotoxicity | In vitro 1–500 μg/mL for 72 h |
Positive: Not exposed | ED50 5 µg/mL and IC50 124.9 μg/mL | [50] |
| 2″-O-Galloylorientin | HaCaT | Antioxidant and cytotoxicity | In vitro 1–500 μg/mL for 72 h |
Positive: Not exposed | ED50 1.9 µg/mL and IC50 67.5 μg/mL | [50] |
| 2″-O-Galloylvitexin | HaCaT | Antioxidant and cytotoxicity | In vitro 1–500 μg/mL for 72 h |
Positive: Not exposed | ED50 3.8 µg/mL and IC50 59.7 μg/mL | [50] |
| 2″-O-Galloylvitexin | HepG-2, HCT-116, Hep2 and MCF-7 | Cytotoxicity | In vitro 5, 12.5, 25, and 50 μg/mL for 48 h |
Positive: Not exposed | IC50: 18.5; 22.6; 24.2 and 28.4 μg/mL, respectively | [49] |
HaCaT = keratinocyte cell line; liver HepG-2, larynx Hep2, colon HCT-116, breast MCF-7 and prostate PC3, human cell line; ED50: Half Effective Maximum Dose; IC50: Half Maximal Inhibitory Concentration.
2.4.1. Antimicrobial Activity
The antibacterial activity of the C. ferrea pod hydromethanolic extract was evaluated against oral bacteria that are commonly associated with bad breath, where a Minimum Inhibitory Concentration (MIC) of 50 µg/mL and a Minimum Bactericidal Concentration (MBC) >50 µg/mL were obtained for Parvimonas micra, whereas the Porphyromonas gingivalis microorganism obtained a MIC of 120 µg/mL and a MBC of 130 µg/mL [14]. Moreover, the pod hydroalcoholic extract presented an antibacterial activity against Helicobacter pylori with a MIC and MBC of 512 µg/mL [12].
Ethanol extracts from C. ferrea bark and pods show MIC values of 1024 µg/mL to Staphylococcus aureus (ATCC 25293), Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 9027) strains, as well as multi-drug resistant S. aureus (SA10), E. coli (EC06), and P. aeruginosa (PA 32) strains [70].
The bark hydroalcoholic extract presented an antimicrobial potential with species from the Staphylococcus spp., obtaining inhibition halo sizes of 61.1%, 27.78%, and 5.56% for the crude extract at the 70% and 50% concentrations, respectively [70]. The aforementioned extract also presented an antimicrobial activity against S. aureus ATCC 10390, P. aeruginosa ATCC 9721, and E. coli ATCC 25,922 strains with MIC values of 0.39, 0.79, and 0.19 mg·mL−1, respectively [67].
The antibacterial activity of C. ferrea leaf extracts was evaluated against several bacterial strains: Bacillus subtilis, E. coli, Proteus vulgaris, P. aeruginosa, S. aureus. The C. ferrea methanolic extract showed a MIC of 6.25, 12.5, 25, 3.12, 3.12, 12.5, 0.78, 3.12, and 12.5 mg/mL, respectively, while the cyclohexane extract obtained MIC values of 0.039, 0.039, 0.039, 0.39, 0.078, 0.312, 0.039, 0.078, and 0.039 mg/mL, respectively. The chloroform extract presented MIC values of 1.56, 6.25, 12.5, 3.12, 0.78, 6.25, 0.78, 12.5, and 12.5 mg/mL, respectively. The ethyl acetate extract showed MIC values of 0.78, 6.25, 12.5, 3.12, 1.56, 6.25, 0.39, 3.12, and 3.12 mg/mL, respectively [8].
The C. ferrea leaf and fruit aqueous extracts presented a 70% growth inhibition potential for Ralstonia solanacearum [69]. Meanwhile the glycolic leaf extract presented an antimicrobial potential against the following strains: Streptococcus mutans ATCC 25175, Streptococcus mitis ATCC 9811, Streptococcus sanguis ATCC10556, Streptococcus sobrinus ATCC 27,609, and Lactobacillus casei ATCC 7469 [72].
The C. ferrea fruit hydroalcoholic extract presented antimicrobial potential against the following strains: S. aureus, E. coli, Klebsiella pneumoniae, and P. aeruginosa with inhibition halo averages of 18, 12, 10 and 11 mm, respectively [68]. The C. ferrea pod ethanolic extract also presented antimicrobial activity against Gram-positive and Gram-negative strains with MIC values ranging from 50 to 125 μg/mL [73]. The glycolic leaf extract on the other hand exhibited an average inhibition halo of 0.97 cm over S. aureus ATCC 6538 [57].
The C. ferrea fruit aqueous extract presented antibiofilm activity for biofilms formed by bacteria from the Proteobacteria and Bacteroidetes phylum, inhibiting their growth by 82% at a concentration of 4 mg·mL−1 [74].
2.4.2. Anti-Halitosis Activity
Volatile sulfuric compounds are produced by bacteria present in the oral cavity, with these compounds being responsible for unpleasant breath odors. Tests using a salivary sediment model have shown the C. ferrea pod hydromethanolic extract inhibited the formation of these odors, reducing the concentration of volatile sulfuric compounds associated with halitosis [14].
2.4.3. Antifungal Activity
The C. ferrea seed hydroethanolic extract presented antifungal activity against Candida albicans ATCC 10231, C. glabrata CCT 0728, C. krusei CCT 1517 and C. guilliermondii CCT 1890 strains with MIC values that varied between 4.8–78 µg/mL [11,75].
Extracts from the C. ferrea stem bark using three different solvents (water, ethanol, and acetone) exhibited antifungal activity over dermatophyte fungi species, presenting the same MIC value of 62.5 μg/mL for Trichophyton rubrum ATCC 28,189 and 31.3 μg/mL for Trichophyton mentagrophytes ATCC 11481, with the author classifying MIC values ≤ 75.0 μg/mL as having an effective antifungal activity. The stem bark aqueous extract also showed antifungal activity over clinical isolates (T. rubrum and T. mentagrophytes) with a MIC50 value of 31.3 μg/mL and MIC90 value of 62.5 μg/mL for both species, where MIC50 and MIC90 refer to the concentration (μg/mL) of the extract that inhibited growth of all isolates by 50% and 90%, respectively [78].
Tests using the C. ferrea leaf ethanolic extract as an alternative control for Alternaria brown spots, caused by the fungus Alternaria alternata in ‘Dancy’ mandarin fruits, showed the 500 μg/mL concentration presented a 52.0% disease severity reduction [93]. The aqueous and ethanolic extracts also showed 96.49% and 99.12% disease severity reduction in ‘Ponkan’ tangerine seedling leaves, respectively, at the 1 mg/mL concentration [94].
The C. ferrea leaf alcoholic extract demonstrated an antifungal potential over Colletotrichum sp. in Sideroxylon obtusifolium (“quixaba”) seeds, since all seeds treated with the extract did not present symptomatic seedlings percentages or pathogen transmission rates [77]. The aqueous extract also demonstrated an antifungal activity over Colletotrichum sp. in S. obtusifolium seeds, since pathogen incidence decreased by up to 96% at the 0.075 mg·mL−1 concentration [76]. C. ferrea extracts also showed antifungal activity over Lasiodiplodia theobromae, inhibiting mycelial growth by 85.6 and 78.9% at 30% and 20% concentrations, respectively [79].
2.4.4. Anti-Inflammatory Activity
The C. ferrea leaf aqueous extract decreased leukocyte accumulation (76 ± 2%) and myeloperoxidase levels (85 ± 7%) in the articular fluid of rats at 100, 200, and 300 mg/kg doses when compared to the zymosan control group, a substance used to stimulate intra-articular inflammation. In addition, the extract significantly reduced inflammatory cytokine levels such as beta interleukins (IL-1β) and tumor necrosis factor alpha (TNF-α) in the articular tissue of rats treated with 200 and 300 mg/kg doses [7]. The fruit aqueous and hydroalcoholic extracts (20–80%) also showed an anti-inflammatory activity at all tested doses (50, 100, and 200 mg/kg), decreasing the migration of inflammatory cells and myeloperoxidase activity levels [13].
2.4.5. Antioxidant Activity
Generally, two parameters are used to assess the integrity of the body’s antioxidant defense: the first is the quantity of glutathione, which is responsible for promoting detoxification and free radical elimination, the second is malondialdehyde (MDA) content, which is characterized as a lipid peroxidation marker. The fruit and leafs aqueous and hydroalcoholic extracts of Pau-ferro showed antioxidant potential at all evaluated doses (50, 100, and 200 mg/kg), increasing total glutathione levels and decreasing MDA levels [7,13,54].
The C. ferrea leaf hydroethanolic extract and the compounds isolated from this extract (brevifolin carboxylic acid, 2”-O-Galloylvitexin and 2”-O-Galloylorientin) presented an antioxidant activity through its ability to absorb oxygen radicals in HaCaT keratinocytes, presenting effective dose values (ED50) of 12.5, 5, 3.8, and 1.9 µg/mL, respectively [50].
The C. ferrea leaf ethanolic extract demonstrated antioxidant potential, where an ED50 of 12.45 ± 2.86 µg/mL was obtained, exhibiting a marked activity in radical elimination in the 2,2-diphenyl-1-picrylhydrazil (DPPH) assay using male Sprague–Dawley rats as an experimental model [9]. Other positive results were also found using the same extract with an EC50 of 4.4 μg/mL (DPPH) and 2.5 μg/mL 2,2’-azino-bis (3-ethylbenzothiazoline) (ABTS) demonstrating high free radical scavenging activity [10,73].
The leaf hydroalcoholic extract also showed high antioxidant activity in the DPPH and ABTS free radical elimination assays with Half Maximal Inhibitory Concentration (IC50) values of 28.96 and 145.10 μg/mL, respectively [12]. Four pod extracts (chloroformic, n-hexane, hydroalcoholic, and ethyl acetate) were evaluated for their antioxidant activity; however, only two extracts presented free radical elimination activity, these being the hydroalcoholic extract, with IC50 values of 74.36 μg/mL for DPPH and ABTS IC50 of 9.76 μg/mL, and the ethyl acetate extract, with IC50 values of 116.10 and 29.13 μg/mL for DPPH and ABTS, respectively [56].
2.4.6. Antileishmanial Activity
The fruit methanolic extract and leaf hexanic extract showed antileishmanial activity, with IC50 values of 15.04 and 53.09 μg·mL−1 against Leishmania (L.) amazonensis promastigotes. Meanwhile, the leaf and fruit methanol extracts showed an IC50 activity of 129.42 and 173.11 μg·mL−1 against Leishmania (V.) guyanensis promastigotes. The fruit methanol extract showed low in vitro toxicity on infected macrophages, and was thus selected for antileishmanial activity tests with intracellular infected macrophages, the results of which showed the 500 μg.mL−1 concentration inhibited 62% and 54% of L. (V.) guyanensis and L. (L.) amazonensis amastigotes survival, respectively [80].
2.4.7. Antiproliferative and Apoptotic Effects
C. ferrea fruit ethanol extracts were evaluated for their antiproliferative effects on human colorectal cancer cells (HT-29), with a potential for inhibiting cancer cell proliferation being observed for extracts with 40, 60, and 80% ethanol, where the following results can be highlighted: 15–25% proliferation inhibition between 25 and 100 μg/mL doses of the 40% ethanolic extract, while the 60% ethanolic extract showed a 50% rate of proliferation inhibition at the 25 μg/mL dose, and the 80% ethanolic extract showed 43.7% inhibition at the 12.5 μg/mL dose, where all of results were observed in the first 24 h of the experiment. During this same period, the extracts did not present embryonic renal cell line (HEK-293) toxicity, these being tumor-free [54].
As for apoptotic effects, the fruit extract with 40% ethanol at a 25 μg/mL dose presented a high percentage of cells undergoing apoptosis (38.7%) in the HT-29 tumor line, while the number of cells undergoing apoptosis in HEK-293 non-tumor cell line did not differ statistically from the control [54].
2.4.8. Anti-Wrinkle and Anti-Melanogenic Activity
The C. ferrea pod and bark ethanolic extract showed high elastase inhibitory activity, with 35.99% inhibition at 250 µg/mL for the bark extract and 19.6% for the pod extract. In terms of collagenase activity, the extracts did not show significant inhibitory potentials, whereas for hyaluronidase, the two extracts obtained better inhibitory potentials than the control [53].
The anti-melanogenic effect of the two extracts were analyzed in B16F10 cells (murine melanoma), where the cells were pre-treated with 3-isobutyl-1-methylxanthine (IBMX) and showed an increase in tyrosinase activity before receiving treatment with the extracts at the 25 μg/mL concentration for 48 h. After treatment with the extracts, significant reductions of 99.0 and 96.4% in tyrosinase activity were observed when treated with the bark and pod extracts, respectively [53]. Tests analyzing the photoprotective activity of the bark ethanolic extract demonstrated a sun protection factor (SPF) of 3.29 at the 0.100 mg/mL concentration [6].
2.4.9. Anti-Hyperglycemic Activity
A galactomannan extracted from C. ferrea seeds demonstrated an anti-hyperglycemic activity when orally administered to streptozotocin-induced diabetic rats at a 10 mg/kg dose. During the first days of treatment, a reduction in blood glucose and blood triacylglycerol levels was observed, in addition to a boost in adipose tissue insulin sensitivity, contributing to the functional recovery of the tissue [65].
Oral administration of the C. ferrea leaf ethanolic extract also showed anti-hyperglycemic activity in streptozotocin-induced diabetic rats, reducing liver function levels, elevated serum glucose and a-amylase, while, in contrast, increasing serum insulin levels, total proteins and body weight [9].
2.4.10. Antiviral Activity
Sulfated galactomannan extracted from C. ferrea exhibited 96% inhibition at a concentration of 25 g/mL against the dengue virus (DENV-2), as well as showing strong antioxidant activity in Vero cells infected with the dengue virus (DENV-2), with an IC50 of 0.94 μg/mL [100].
2.4.11. Antinociceptive Activity
The C. ferrea fruit aqueous extract exhibited an analgesic activity in rats during the hot plate test at 100 and 200 mg/kg doses at 90 and 60–90 min, respectively [13].
2.4.12. Antiulcerogenic Activity
The C. ferrea dry extract exhibited an antiulcerogenic activity in Wistar rats with lesions induced by absolute ethanol obtaining inhibition values of 46.36, 87.56, and 95.99% at 100, 200, and 400 mg/kg doses, respectively. In ulcers induced by acidified ethanol administration, the extract showed a protection of 59.12 and 96.61% in the group treated with 200 and 400 mg/kg doses. At the end of the tests used to evaluate the gastroprotective potential of the extract, Half Effective Maximum Dose (ED50) values of 113 and 185.7 mg/kg were obtained for the groups with ulcers induced by absolute ethanol and acidified ethanol, respectively. In addition, the 200 mg/kg dose decreased the area of chronic ulcers induced by acetic acid by 77.44% [12].
2.4.13. Hypolipidemic Effects
The C. ferrea leaf hydroethanolic extract was evaluated for its in vitro hypolipidemic effect on the activity of HMG CoA-reductase, an enzyme responsible for cholesterol biosynthesis. The extract showed an enzymatic inhibitory activity of 86%, a result similar to that of the medication Lipantil used as the positive control. In in vivo tests with Wistar rats, the group treated with the extract showed significant reductions in total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), triglycerides (TG) and total lipids of 53.08, 25.03, 48.84 and 23.28%, respectively, while HDL-C showed a significant increase of 158.71% compared to the untreated group, that is, the hypercholesterolemic group. Histopathological analyzes showed that rats treated with the extract had normal-looking livers and a very mild presence of congestion and degenerative changes [50].
2.4.14. Toxicity
Pregnant rats treated with C. ferrea bark and seed extracts showed changes in some biochemical parameters, among which increases in creatinine levels in the maternal serum stood out when compared to the control. Additionally, the group of rats exposed to the seed extract presented amniotic fluid rich in glucose and aspartate aminotransferase and low levels of calcium, which as a result, the fetuses had shorter head and body section lengths when compared to the control, as well as exhibiting visceral and skeletal anomalies [59].
No behavioral changes were observed through toxicological tests using adult zebrafish (Danio rerio) as a model organism when these were exposed to the C. ferrea fruit ethanolic extract, however, histopathological analyzes of different tissues showed changes in their gills, intestines, and livers. In contrast, assays using 25, 50, and 125 mg/L concentrations of the extract showed embryonic lethality rates of 30, 33.3, and 10%, respectively, while higher concentrations (250 and 500 mg/L) triggered edema in the heart, yolk sac and scoliosis [1]. Meanwhile, the bark hydroalcoholic extract showed an LC50 of 822.6334 µg/mL in the toxicity evaluation assay over Artemia salina L., this being considered a low toxicity [81]. The evaluation of the C. ferrea leaf ethanolic extract toxicity demonstrated a non-toxic profile, since the rats subjected to the extract did not exhibit significant behavioral changes, neurological responses or mortality rates in any of the doses until the end of the assays [9]. Similar results were found in the acute toxicity evaluation of the fruit ethanol extract and the pod hydroalcoholic extract in Wistar rats, with a lethal dose (LD) greater than 2000 mg/kg for the pod extract [12,61].
2.4.15. Genotoxicity
The genotoxic activity of the C. ferrea seed ethanol extract on Astyanax sp. erythrocytes was measured through the comet assay, where the tail length increased the level of DNA breaks in red cells by 2.5× when exposed to 5, 10, and 20 mg/L doses compared to the negative control; thus, demonstrating a genotoxic potential over Astyanax sp. erythrocytes, in addition, a clastogenic response following exposure to the 20 mg/L dose, as evidenced by a decrease in tail length [82].
2.4.16. Cytotoxicity
The cytotoxic potential of the C. ferrea leaf hydroethanolic extract over human cancer cell lines (liver HepG2, breast MCF-7, colon HCT-116, larynx Hep2 and prostate PC3) was analyzed using the sulforhodamine B (SRB) assay. The results showed the extract presented cytotoxic activity over the five tumor cell lines analyzed with an IC50of 19.3 μg/mL for the HepG-2 liver cell line, this being considered the most efficient cytotoxic activity. As for the other lines, larynx Hep2, breast MCF-7, and colon HCT-116, IC50 values of 20, 21.8, and 24.47 μg/mL, respectively, were obtained, while a negative cytotoxic activity was observed with the PC3 prostate cell line [49].
The 2”-O-galloylvitexin compound was isolated from the C. ferrea leaf hydroethanolic extract and was analyzed for its cytotoxicity potential over the aforementioned strains using the same methodology, presenting similar results with more effective cytotoxic activity on the HepG-2 liver cell line with an IC50 of 18.5 μg/mL, followed by the HCT-116 colon, Hep2 larynx, and MCF-7 breast cell lines with IC50 values of 22.6, 24.2, and 28.4 μg/mL, respectively [49].
The cytotoxicity of the C. ferrea bark and pod ethanolic extracts were evaluated against B16F10 cells (murine melanoma) and normal human fibroblasts (NHF). The pod extract obtained an IC50 of 50.1 µg/mL for the B16F10 cells after the 48-h treatment period, while the bark extract showed a 47% viability percentage of B16F10 cells. Neither extract presented significant cytotoxicity in over NHF [53].
The fruit aqueous and hydroalcoholic extracts (20–80%) had no cytotoxic effect over mouse embryonic fibroblast (3T3) cell lines at any of the periods analyzed (24, 48, and 72 h) [13]. Tests using the C. ferrea seed hydromethanolic extract demonstrated toxicity in fibroblast cells (L929) when used at concentrations of 1000, 500, and 250 μg/mL, while concentrations below 250 μg/mL did not show cytotoxicity [75].
The cytotoxicity potential of galactomannan extracted from C. ferrea seeds was evaluated, in vitro, in human neutrophils through the lactate dehydrogenase (LDH) assay, where it is possible to detect cell death, such as necrosis. The results showed that galactomannan did not increase LDH activity at any of the analyzed concentrations (10–200 μg/mL) when compared to the negative control data [65]. Assays with sulfated galactomannans at concentrations of 25, 50, and 100 g/mL also did not show cytotoxicity in Vero cells [100].
The cytotoxic potential of the C. ferrea leaf hydroethanolic extract and its isolated compounds such as brevifolin carboxylic acid, 2”-O-Galloylvitexin and 2”-O-Galloylorientin were investigated in HaCaT keratinocytes by the neutral red uptake (NRU) test. The results from the cytotoxic activity showed IC50 values of 114.4, 124.9, 59.7, and 67.5 μg/mL for the extract, brevifolin carboxylic acid, 2”-O-Galloylvitexin and 2”-O-Galloylorientin, respectively [50].
The C. ferrea pod aqueous extract exhibited cytotoxicity over meristematic cells from Allium cepa roots, inhibiting cell division at concentrations of 1 g/500 mL and 1 g/1000 mL following 24 and 48 h of exposure. Moreover, the extract showed a cytoprotective effect at both concentrations in tests used to evaluate their cytoprotective potential in cells treated with paracetamol at a concentration of 0.008 mg/mL. In addition, the extract did not contribute to the antiproliferative activity caused by a mutagenic compound in meristematic cells from A. cepa roots [83].
2.4.17. Cicatrizing Activity
Powder and ointment formulations developed from C. ferrea pods were evaluated for their cicatrizing potential in clean rabbit (Oryctolagus cuniculus) wounds, following three times a day administration. The results showed that animals treated with the 24% ointment showed a better lesion area percentage inhibition, while inefficiency at reducing the final cicatrizing period was observed with the other treatments [84].
C. ferrea bark extracts that are rich in polysaccharides at concentrations of 0.025, 0.05, 0.75, and 0.1% decreased the wound area and increased wound contraction in Wistar rats, in addition to reducing the infiltration of inflammatory mediators, such as TNF-α and IL-1β, contributing to the acceleration of the wound healing process, as evidenced by the presence of attenuated clinical signs (edema, hyperemia, exudate). Likewise, ulcers treated with the extract showed the formation of organized connective tissue and collagen deposition, as well as a layer of epithelial tissue protecting the granulation tissue [58].
Positive results were found for the wound cicatrizing process in Wistar rats using the C. ferrea pod powder, with a significant reduction in the lesion area occurring and the wounds drying with no exudate from the third day of treatment, exhibiting a regular, thick crust with the presence of mononuclear red blood cells and fibrin, in the form of a blood clot at the edges of the wound [85].
Cutaneous wound treatment in Wistar rats with the C. ferrea fruit ethanolic extract stimulated the formation of dark brown to black crusts over the wounds of treated animals at concentrations of 50 and 12.5%, where crust detachment was also observed during topical treatment days with the extract. In the group treated with the extract at a concentration of 12.5%, all animals exhibited a constituted epidermis, this being more efficient at skin wound treatment in rats than the 50% concentration [61].
Diabetic and non-diabetic Wistar rats with lesions induced by thermal contact were treated with the C. ferrea bark hydroalcoholic extract incorporated with bacterial nanocellulose membranes, where the non-diabetic rat group exhibited epithelialization after 14 days of treatment, while the remaining animals presented epithelialization after 21 days of treatment [67].
2.4.18. Repellent Action
The repellent action of the C. ferrea fruit bark powder against fly species from the Calliphoridae family was analyzed using traps containing deteriorating bovine liver as an attractant for the flies. The treatments that had the powder at the 20 and 50% tested concentrations presented a higher repellency percentage of 97.5 and 100%, respectively [88].
2.4.19. Insecticidal Activity
The insecticidal activity of the C. ferrea leaf hydroalcoholic extract was evaluated using Aphis craccivora (black aphid) nymphs, which demonstrated the 5% concentration showed insecticidal activity with 51.71% efficiency [90]. The insecticidal activity of the leaf and pod aqueous and methanolic extracts against Dactylopius opuntiae (“Cochonilha-do-carmin”) were also verified, with a 72.46–99.33% of adult female mortality being observed [91]. Results using the same extract and D. opuntiae demonstrated LC50 values ranging from 20–150 mg/mL for the nymphs and 43–50 mg/mL for the adults, while other tests using termites as a study model (Nasutitermes corniger) presented LC50 values ranging from 0.255–1.279 mg·mL−1 for the workers and 0.146–8.003 mg·mL−1 for the soldiers [89,92].
2.4.20. Fertilizer
The C. ferrea leaf litter was used to evaluate its fertilizing potential against Sorghum bicolor L. (Sorgo) cultures, where following a period of 75 days the C. ferrea litter increased potassium, calcium, and magnesium soil content; however, it did not increase the dry matter production of the S. bicolor L. aerial part [95].
2.4.21. Allelopathic Potential
The C. ferrea seed hydroalcoholic extract presented a 30% rate of abnormal melon seedlings (Cucumis melo L.) at the highest concentration (1%), where the seedlings had imperfect roots, including the absence of absorbent hair, a necrotic, dark, and hard apex, in addition to negative gravitropism. The leaf hydroalcoholic extract on the other hand contributes to the growth of the melon seedling aerial parts at the highest tested concentration (1%), while it negatively interfered with root growth compared to the control [96].
Decomposing C. ferrea leaves exhibited an allelopathic potential over Vigna unguiculata L. seedlings, affecting the length of the aerial part and root system, as well as the total seedling dry mass [97]. The hot leaf, bark, and root extracts exhibited allelopathic potential over Calotropis procera, preventing its germination, while Cenchrus echinatus germination was inhibited by the hot leaf extract [48].
2.4.22. Biosorbent
Tests with a biosorbent produced from C. ferrea fruits used to remove methylene blue from aqueous media showed rapid kinetics coupled to good adsorption, demonstrating that the fruits can become an excellent lower cost alternative used for the removal of pollutants in wastewater [98]. Activated coals prepared from pod waste showed a removal percentage of up to 97.67% of the pharmaceutical captopril from aqueous media [99].
2.4.23. Other Bioactivities
The C. ferrea stem bark tea presented an erosive potential on human third molars, with a pH value of 0.28 ± 0.05, this being considered close to the pH value that promotes tooth enamel demineralization, with 37.03% of enamel demineralization [87].
The pod hydroalcoholic extract showed an antimetastatic potential, since it decreased the migration of ACP02 cells (gastric adenocarcinoma) following 24 h of treatment, presenting a dose-response effect that increased from 50 µg/mL concentrations [56]. The C. ferrea seed aqueous extract did not have the potential to inhibit hemorrhages induced by Bothrops jararaca venom in Swiss mice [62].
Polysaccharide extracts from C. ferrea leaves, pods and barks showed an edematogenic effect in Wistar rats at 0.01–1 mg/kg doses, with these effects being inferior to those developed by the drugs used as control (carrageenan and dextran) [86].
3. Materials and Methods
The plant’s name was checked on the www.theplantlist.org/ (The List Plant) website to check for synonyms. For data collection, a comprehensive article search was performed using the Scopus, Periódicos Capes, PubMed, Google Scholar, and ScienceDirect databases, using the following descriptors: Caesalpinia ferrea and its synonym Libidibia ferrea. Studies published between 2015 and March 2020 were reviewed. Full-text articles were selected if the title, abstract, or keywords included the aforementioned descriptors.
Articles duplicated between the search engines, as well as review articles, were removed in the first step. The remaining articles were then selected based on their title, abstract, and keywords. Lastly, full articles were analyzed according to the following criteria (1): botanical aspects; (2): phytochemistry; (3): ethnobotanical uses; (4): bioactivities; (5): bioactivities of compounds isolated from C. ferrea. The chemical structures of the isolated secondary metabolites of this plant were drawn using the software ChemDraw Ultra 7.0.
4. Critical Analysis
Phytochemical investigations are fundamental for understand the chemical basis of the compounds from medicinal plants; however, many of these studies still use colorimetric techniques that have limitations. By this fact, is necessary the usage of metabolomic techniques to trace the phytochemical profiles. The phytocompounds of C. ferrea have a wide range of therapeutic properties, however only few compounds were isolated and evaluated by in vivo and in vitro assays, as evidenced in this review. The studies that evaluated pharmacological activities did not show results about the mechanisms of action of the crude extracts and the isolated compounds. By this fact, investigations to evaluate the toxicological in vivo effects are necessary to understand the pharmacokinetic and pharmacodynamic mechanisms of these products. Pharmacological studies have focused on the antibacterial, antioxidant, anti-inflammatory, antihyperglycemic and healing effects, demonstrating the ethnomedicinal uses of this plant. However, many traditional uses are not supported to experimental results, as the effects against malaria, anemia, and the calming properties of this plant.
5. Conclusions
In this work, we reported the ethnobotanical, phytochemical, and pharmacological aspects of C. ferrea. This medicinal plant is used in traditional practices to treat certain diseases and has interesting biological properties. Different pharmacological assays had demonstrated antibacterial, antioxidant, anti-inflammatory, anti-diabetic, and healing activities. However, these pharmacological investigations have focused mainly on the organic fractions of the crude extracts, with few attentions to the aqueous extracts, since that are the mainly used formulations. Another point is the necessity of more comprehensive clinical trials. Regarding the phytochemical analysis of this species, different compounds as flavonoids, polyphenols, terpenoids, tannins, saponins, steroids, and other phenolic compounds were reported. However, the reports about the pharmacological properties cited in this review have not demonstrated the mechanisms molecular of C. ferrea extracts. Therefore, it is necessary to develop new research to establish a link between traditional uses and pharmacological activities, mainly studies to determine the toxicological profile of C. ferrea.
Acknowledgments
Graduate Program in Biological Sciences at the Federal University of Pernambuco.
Author Contributions
Conceptualization, M.V.d.S.; F.A.B.d.C., J.G.M.d.C.; methodology, N.S.M.; A.H.B.; Writing—original draft preparation, N.S.M.; M.V.d.S.; Writing—review and editing, B.R.; Z.d.S.S.; F.A.B.d.C.; Supervision, M.V.d.S.; F.A.B.d.C.; Coordination of the project, R.C. and H.D.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico-FUNCAP [BP3-0139-00077.01.00/18].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ferreira D.Q., Ferraz T.O., Araújo R.S., Cruz R.A.S., Fernandes C.P., Souza G.C., Ortiz B.L.S., Sarquis R.S.F.R., Miranda J.C.M.M., Garrett R., et al. Libidibia ferrea (Jucá), a traditional anti-inflammatory: A study of acute toxicity in adult and embryos Zebrafish (Danio rerio) Pharmaceuticals. 2019;12:175. doi: 10.3390/ph12040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bueno N.R., Campos É.P., Silva M.S., Rezende K.S., Lima B.B.M. Levantamento Etnofarmacológico e Farmacológico de Plantas Medicinais Comercializadas em Rondonópolis (MT) Biodiversidade. 2019;2:2–20. [Google Scholar]
- 3.Leal J.B., Silva M.M., Costa J.M., Albuquerque L.C.S., Pereira M.D.G.S., Sousa R.L. Etnobotânica de plantas medicinais com potencial anti-inflamatório utilizadas pelos moradores de duas comunidades no município de Abaetetuba, Pará. Biodiversidade. 2019;3:110–125. [Google Scholar]
- 4.Sacramento A.A., Martins Filho I.E., Dos Reis L.A. Estudo etnobotânico das plantas medicinais comercializadas numa feira livre num município do interior da Bahia. Rev. Enferm. Atual Derme. 2019;89:27. doi: 10.31011/reaid-2019-v.89-n.27-art.455. [DOI] [Google Scholar]
- 5.Santos E.Q., Costa J.F.D.S., Pereira M.D.G.D.S., Costa J.M., Sousa R.L. Etnobotânica da flora medicinal de quintais na comunidade Mamangal, Rio Meruú, Igarapé-Miri, Pará. Sci. Plena. 2019;15:1–11. doi: 10.14808/sci.plena.2019.051202. [DOI] [Google Scholar]
- 6.Andrade B.A., Corrêa A.J.C., Gomes A.K.S., Neri P.M.S., Sobrinho T.J.S.P., Araújo T.A.S., Castro V.T.N.A., Amorim E.L.C. Photoprotective Activity of Medicinal Plants from the Caatinga Used as Anti-inflammatories. Pharmacogn. Mag. 2019;15:356–361. doi: 10.4103/pm.pm_482_18. [DOI] [Google Scholar]
- 7.Falcão T.R., Rodrigues C.A.O., Araújo A.A., Medeiros C.A.C.X., Soares L.A.L., Ferreira M.R.A., Vasconcelos R.C., Araújo-Júnior R.F., Sousa Lopes M.L.D., Guerra G.C.B. Crude extract from Libidibia ferrea (Mart. ex. Tul.) L.P. Queiroz leaves decreased intra articular inflammation induced by zymosan in rats. BMC Complement. Altern. Med. 2019;19:1–10. doi: 10.1186/s12906-019-2454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna M.S.M., Paula R.A., Brandão Costa R.M.P., Anjos J.V., Silva M.V., Correia M.T.S. Bioprospection of Libidibia ferrea var. ferrea: Phytochemical properties and antibacterial activity. South. African J. Bot. 2020;130:103–108. doi: 10.1016/j.sajb.2019.12.013. [DOI] [Google Scholar]
- 9.Hassan S.K., El-Sammad N.M., Mousa A.M., Mohammed M.H., Farrag A.E.R.H., Hashim A.N.E., Werner V., Lindequist U., Nawwar M.A.E.-M. Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015;5:462–471. doi: 10.1016/j.apjtb.2015.03.004. [DOI] [Google Scholar]
- 10.Nascimento J., Reatgui W., Araújo L., Ribeiro M.E., Maia D., Giacomin L., Kitagawa R., Baratto L. Avaliação do potencial antioxidante e anti-Helicobacter pylori in vitro de extratos de plantas medicinais utilizadas popularmente na região amazônica. Rev. Fitos. 2017;11:140–152. doi: 10.5935/2446-4775.20170023. [DOI] [Google Scholar]
- 11.Soares M.R.P.S., Caneschi C.A., Chaves M.D.G.A.M., Mota M., Stroppa P.H.F., Barbosa W., Raposo N.R.B. In Vitro Antifungal Activity and Cytotoxicity Screening of Dry Crude Extracts from Brazilian Amazonia Plants. Afr. J. Tradit. Complement. Altern. Med. 2018;15:13. doi: 10.21010/ajtcam.v15i4.2. [DOI] [Google Scholar]
- 12.Prazeres L.D.K.T., Aragão T.P., Brito S.A., Almeida C.L.F., Silva A.D., Paula M.M.F., Farias J.S., Vieira L.D., Damasceno B.P.G.L., Rolim L.A., et al. Antioxidant and Antiulcerogenic Activity of the Dry Extract of Pods of Libidibia ferrea Mart. ex Tul. (Fabaceae) Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1983137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcão T.R., Araújo A.A., Soares L.A.L., Farias I.B., Silva W.A.V., Ferreira M.R.A., Araújo R.F., Medeiros J.S., Lopes M.L.D.D.S., Guerra G.C.B. Libidibia ferrea Fruit Crude Extract and Fractions Show Anti-Inflammatory, Antioxidant, and Antinociceptive Effect in Vivo and Increase Cell Viability in Vitro. Evid.-Based Complement. Altern. Med. 2019 doi: 10.1155/2019/6064805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veloso D.J., Abrão F., Martins C.H.G., Bronzato J.D., Gomes B.P.F.A., Higino J.S., Sampaio F.C. Potential antibacterial and anti-halitosis activity of medicinal plants against oral bacteria. Arch. Oral Biol. 2020;110 doi: 10.1016/j.archoralbio.2019.104585. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira M.R.A., Soares L.A.L. Libidibia ferrea (Mart. Ex Tul.) LP Queiroz: A review of activities biological and phytochemical composition. J. Med. Plants Res. 2015:140–145. doi: 10.5897/JMPR2014.5706. [DOI] [Google Scholar]
- 16.Andrade G.C., Silva L.C. Responses of tropical legumes from the Brazilian Atlantic Rainforest to simulated acid rain. Protoplasma. 2017;254:1639–1649. doi: 10.1007/s00709-016-1054-z. [DOI] [PubMed] [Google Scholar]
- 17.Matos S.S., Melo A.L., Santos-Silva J. Caesalpinioideae e Cercidoideae (Leguminosae) no Parque Estadual Mata da Pimenteira, Semiárido de Pernambuco, Brasil. Rodriguesia. 2019;70 doi: 10.1590/2175-7860201970017. [DOI] [Google Scholar]
- 18.Medeiros J.G.F., Araújo-Neto A.C., Silva E.C., Huang M.-F.N., Nascimento L.C. Qualidade Sanitária de Semente de Caesalpinia ferrea: Incidência de Fungos, Controle e Efeitos na Qualidade Fisiológica com o Uso de Extratos Vegetais. Rev. Floresta. 2015;45:163–174. doi: 10.5380/rf.v45i1.34074. [DOI] [Google Scholar]
- 19.Bragante R.B., Hell A.F., Paulo J.P., Silva N.D.C., Centeno R.D.C.L., Figueiredo-Ribeiro C.J.B. Physiological and metabolic responses of immature and mature seeds of Libidibia ferrea ((Mart. ex Tul.) L.P. Queiroz) under contrasting storage temperatures. Braz. J. Bot. 2018;41:43–55. doi: 10.1007/s40415-018-0442-3. [DOI] [Google Scholar]
- 20.Santos S.F., Santos A.S., Corpes R.S., Leão N.V.M. Aspectos do cultivo in vitro de Libidibia ferrea (Mart. Ex Tul.) L. P. QUEIROZ (Leguminosae-Caesalpinioideae) como fonte alternativa para produção de metabólitos secundários. Rev. Espac. 2018;39:17–24. [Google Scholar]
- 21.Matos A.C.B., Ataíde G.M., Borges E.E.L. Physiological, physical, and morpho-anatomical changes in Libidibia ferrea ((Mart. ex Tul.) L.P. Queiroz) seeds after overcoming dormancy. J. Seed Sci. 2015;37:26–32. doi: 10.1590/2317-1545v37n1140433. [DOI] [Google Scholar]
- 22.Carvalho S.M.C., Torres S.B., Benedito C.P., Nogueira N.W., Souza A.A.T., Souza Neta M.L. Viability of Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz var. ferrea) seeds by tetrazolium test. J. Seed Sci. 2017;39:7–12. doi: 10.1590/2317-1545v39n1163784. [DOI] [Google Scholar]
- 23.Ferreira W.N., Lacerda C.F., Costa R.C., Filho S.M. Effect of water stress on seedling growth in two species with different abundances: The importance of Stress Resistance Syndrome in seasonally dry tropical forest. Acta Bot. Bras. 2015;29:375–382. doi: 10.1590/0102-33062014abb0045. [DOI] [Google Scholar]
- 24.De David M., Pasa M.C. As plantas medicinais e a etnobotânica em Várzea Grande, MT, Brasil. Interações. 2015;16:97–108. doi: 10.1590/1518-70122015108. [DOI] [Google Scholar]
- 25.Almeida-Neto J.R., Barros R.F.M., Silva P.R.R. Uso de plantas medicinais em comunidades rurais da Serra do Passa-Tempo, estado do Piauí, Nordeste do Brasil. Braz. J. Biosci. 2015;13:165–175. [Google Scholar]
- 26.Ferreira A.B., Ming L.C., Haverroth M., Daly D.C., Caballero J., Ballesté A.M. Plants Used to Treat Malaria in the Regions of Rio Branco-Acre State and Southern Amazonas State-Brazil. Int. J. Phytocosmetics Nat. Ingredients. 2015;2:1–5. doi: 10.15171/ijpni.2015.09. [DOI] [Google Scholar]
- 27.Gonçalves K.G., Pasa M.C. O saber local e as plantas medicinais na comunidade sucuri, cuiabá, mt, brasil. Biodiversidade. 2015;14:50–73. [Google Scholar]
- 28.Silva C.G., Marinho M.G.V., Lucena M.F.A., Costa J.G.M. Levantamento etnobotânico de plantas medicinais em área de Caatinga na comunidade do Sítio Nazaré, município de Milagres, Ceará, Brasil. Rev. Bras. Plantas Med. 2015;17:133–142. doi: 10.1590/1983-084X/12_055. [DOI] [Google Scholar]
- 29.Silva M.P., Barros R.F.M., Moita Neto J.M. Farmacopeia natural de comunidades rurais no estado do Piauí, Nordeste do Brasil. Desenvolv. e Meio Ambient. 2015;33:193–207. doi: 10.5380/dma.v33i0.37241. [DOI] [Google Scholar]
- 30.Cajaiba R.L., Silva W.B., Sousa R.D.N., Sousa A.S. Levantamento etnobotânico de plantas medicinais comercializadas no município de Uruará, Pará, Brasil. Biotemas. 2016;29:115. doi: 10.5007/2175-7925.2016v29n1p115. [DOI] [Google Scholar]
- 31.Castro K.N.C., Wolschick D., Leite R.R.S., Andrade I.M., Magalhães J.A., Mayo S.J. Ethnobotanical and ethnoveterinary study of medicinal plants used in the municipality of Bom Princpio do Piau, Piau, Brazil. J. Med. Plants Res. 2016;10:318–330. doi: 10.5897/jmpr2015.6038. [DOI] [Google Scholar]
- 32.Kffuri C.W., Lopes M.A., Ming L.C., Odonne G., Kinupp V.F. Antimalarial plants used by indigenous people of the Upper Rio Negro in Amazonas, Brazil. J. Ethnopharmacol. 2016;178:188–198. doi: 10.1016/j.jep.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Lima I.E.O., Nascimento L.A.M., Silva M.S. Comercialização de plantas medicinais no município de Arapiraca-AL. Rev. Bras. Plantas Med. 2016;18:462–472. doi: 10.1590/1983-084X/15_201. [DOI] [Google Scholar]
- 34.Moraes Rego C.A.R., Rocha A.E., De Oliveira C.A., Pacheco F.P.F. Levantamento etnobotânico em comunidade tradicional do assentamento pedra suada, do município de cachoeira grande, Maranhão, Brasil. Acta Agron. 2016;65:284–291. doi: 10.15446/acag.v65n3.50240. [DOI] [Google Scholar]
- 35.Oliveira M.S., Silva E.O., Ferreira A.W.C., Guarçoni E.A.E. Conhecimento e uso tradicional das espécies madeireiras e medicinais no município de Aldeias Altas, Maranhão, Brasil. Enciclopédia Biosf. 2016;13:1160. doi: 10.18677/EnciBio_2016B_109. [DOI] [Google Scholar]
- 36.Silva F.J., Silveira A.P., Gomes V.S. Plantas medicinais e suas indicações ginecológicas: Estudo de caso com moradoras de Quixadá, CE, Brasil. Rev. Bras. Biociências. 2016;14:193–201. [Google Scholar]
- 37.Souza L.F., Dias R.F., Guilherme F.A.G., Coelho C.P. Plantas medicinais referenciadas por raizeiros no município de Jataí, estado de Goiás. Rev. Bras. Plantas Med. 2016;18:451–461. doi: 10.1590/1983-084X/15_173. [DOI] [Google Scholar]
- 38.Saraiva M.E., Ulisses A.V.R.D.A., Ribeiro D.A., Oliveira L.G.S., Macêdo D.G., Sousa F.D.F.S., Menezes I.R.A., Sampaio E.V.D.S.B., Souza M.M.D.A. Plant species as a therapeutic resource in areas of the savanna in the state of Pernambuco, Northeast Brazil. J. Ethnopharmacol. 2015;171:141–153. doi: 10.1016/j.jep.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro S.C., Melo N.D.P., Barros A.B. Etnoconhecimento de Pequenos Agricultores Tradicionais sobre Plantas Medicinais no Tratamento de Dores Provocadas pelo Trabalho. Cad. Ter. Ocup. UFSCar. 2016;24:563–574. doi: 10.4322/0104-4931.ctoAO1249. [DOI] [Google Scholar]
- 40.Cordeiro M.C., Botrel R.T., Holanda A.C. Levantamento etnobotânico de espécies arbóreas no assentamento Tabuleiro Grande, Apodi, Rio Grande do Norte. Rev. Verde Agroecol. Desenvolv. Sustentável. 2017;12:122. doi: 10.18378/rvads.v12i1.4925. [DOI] [Google Scholar]
- 41.Palheta I.C., Tavares-Martins A.C.C., Lucas F.C.A., Jardim M.A.G. Ethnobotanical study of medicinal plants in urban home gardens in the city of abaetetuba, Paá state, Brazil. Bol. Latinoam. Caribe Plantas. Med. Aromat. 2017;16:206–262. [Google Scholar]
- 42.Leandro Y.A.S., Jardim I.N., Gavilanes M.L. The use of medicinal plants in health care practices by the residents of a communitty settlement in Anapu, Pará, Brazil. Biodiversidade. 2017;16:30–44. [Google Scholar]
- 43.Pereira M.G.S., Coelho-Ferreira M. Uso e diversidade de plantas medicinais em uma comunidade quilombola na Amazônia Oriental, Abaetetuba, Pará. Biota Amaz. 2017;7:57–68. [Google Scholar]
- 44.Silva R.C., Roriz B.C., Scareli-Santos C. Etnoconhecimento sobre as espécies medicinais utilizadas pela população de Araguaína, TO. Rev. São Luís Orione. 2018;1:1–21. [Google Scholar]
- 45.Albergaria E.T., Silva M.V., Silva A.G. Levantamento etnobotânico de plantas medicinais em comunidades rurais do município de Lagoa Grande, Pernambuco, Brasil. Rev. Fitos. 2019;13:137–154. doi: 10.17648/2446-4775.2019.713. [DOI] [Google Scholar]
- 46.Ribeiro R.V., Bieski I.G.C., Balogun S.O., Martins D.T.O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017;205:69–102. doi: 10.1016/j.jep.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Lima B.B., Fernandes F.P. Uso e diversidade de plantas medicinais no município de Aracati—CE, Brasil. [(accessed on 23 August 2020)];J. Appl. Pharm. Sci. 2020 :24–42. Available online: https://www.researchgate.net/publication/340082226. [Google Scholar]
- 48.Leandro C.S., Bezerra J.W.A., Rodrigues M.D.P., Silva A.K.F., Silva D.L., Santos M.A.F., Linhares K.V., Boligon A.A., Silva V.B., Rodrigues A.S., et al. Phenolic Composition and Allelopathy of Libidibia ferrea Mart. ex Tul. in Weeds. J. Agric. Sci. 2019;11:109. doi: 10.5539/jas.v11n2p109. [DOI] [Google Scholar]
- 49.Hussein S.A.M., El-Mesallamy A.M.D., Souleman A.M.A., Mousa M.A. Cytotoxic activity of bioactive compound from Caesalpinia ferrea Martius, Fabaceae. Int. J.Pharm. Phytochem. Res. 2016;8:2080–2084. [Google Scholar]
- 50.Nawwar M.A., Hussein S.A., El-Mousallami A.M., Hashim A.N., Mousa M.A., Hetta M.H., Hamed M.A., Werner V., Becker A., Haertel B., et al. Phenolics from Caesalpinia ferrea Mart.: Antioxidant, cytotoxic and hypolipidemic activity. Pharmazie. 2015;70:553–558. doi: 10.1691/ph.2015.5526. [DOI] [PubMed] [Google Scholar]
- 51.Indriani D., Elya B., Noviani A. Arginase inhibitory activity and total flavonoid content on Caesalpinia ferrea C. Mart stem bark extracts. Pharm. J. 2018;10:1180–1183. doi: 10.5530/pj.2018.6.202. [DOI] [Google Scholar]
- 52.Alves F.S., Viturino W.A.S., Ferreira M.R.A., Soares L.A.L., Barbosa F.S.S. Bark of the Stem of Libidibia ferrea Associated with Mycorrhizal Fungi: An Alternative to Produce High Levels of Phenolic Acids. Open Microbiol. J. 2018;12:412–418. doi: 10.2174/1874285801812010412. [DOI] [Google Scholar]
- 53.Pedrosa T.N., Barros A.O., Nogueira J.R., Fruet A.C., Rodrigues I.C., Calcagno D.Q., Smith M.A.C., Souza T.P., Barros S.B.M., Vasconcellos M.C., et al. Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 2016;308:643–654. doi: 10.1007/s00403-016-1685-0. [DOI] [PubMed] [Google Scholar]
- 54.Guerra A.C.V.A., Soares L.A.L., Ferreira M.R.A., Araújo A.A., Rocha H.A.O., Medeiros J.S., Cavalcante R.S., Júnior R.F.A. Libidibia ferrea presents antiproliferative, apoptotic and antioxidant effects in a colorectal cancer cell line. Biomed. Pharmacother. 2017;92:696–706. doi: 10.1016/j.biopha.2017.05.123. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira M.R.A., Fernandes M.T.M., Silva W.A.V., Bezerra I.C.F., Souza T.P., Pimentel M.F., Soares L.A.L. Chromatographic and Spectrophotometric Analysis of Phenolic Compounds from Fruits of Libidibia ferrea Martius. Pharmacogn Mag. 2016;12:S285–S291. doi: 10.4103/0973-1296.182165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azevedo L.F.C., Ferreira T.A.A., Melo K.M., Dias C.L.P., Bastos C.E.M.C., Santos S.F., Santos A.D.S., Nagamachi C.Y., Pieczarka J.C. Aqueous ethanol extract of Libidibia ferrea (Mart. Ex Tul) L.P. Queiroz (juca) exhibits antioxidant and migration-inhibiting activity in human gastric adenocarcinoma (ACP02) cells. PLoS ONE. 2020;15:e0226979. doi: 10.1371/journal.pone.0226979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magalhães L.S., Pussente C.G., Azevedo L.R., Crespo J.M.R.S. Avaliação da atividade antibacteriana do extrato de Caesalpinia ferrea Martius e desenvolvimento de uma formulação fitocosmética. Rev. Científica Faminas. 2015;11:27–43. [Google Scholar]
- 58.Pereira L.D.P., Mota M.R.L., Brizeno L.A.C., Nogueira F.C., Ferreira E.G.M., Pereira M.G., Assreuy A.M.S. Modulator effect of a polysaccharide-rich extract from Caesalpinia ferrea stem barks in rat cutaneous wound healing: Role of TNF-α, IL-1β, NO, TGF-β. J. Ethnopharmacol. 2016;187:213–223. doi: 10.1016/j.jep.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 59.Pickler T.B., Lopes K.P., Magalhães S.A., Krueger C.M.A., Martins M.M., Filho V.C., Jozala A.F., Grotto D., Gerenutti M. Effect of Libidibia ferrea bark and seed in maternal reproductive and biochemical outcomes and fetal anomaly in rats. Birth Defects Res. 2019;111:863–871. doi: 10.1002/bdr2.1520. [DOI] [PubMed] [Google Scholar]
- 60.Lins T.R.S., Braz R.L., Silva T.C., Araujo E.C.G., Medeiros J.X., Reis C.A. Tannin Content of the Bark and Branch of Caatinga Species. J. Exp. Agric. Int. 2019;31:1–8. doi: 10.9734/jeai/2019/v31i130061. [DOI] [Google Scholar]
- 61.Kobayashi Y.T.S., Almeida V.T., Bandeira T., Alcántara B.N., Silva A.S.B., Barbosa W.L.R., Silva P.B., Monteiro M.V.B., Almeida M.B. Avaliação fotoquímica e potencial cicatrizante do extrato etanólico dos frutos de Jucá (Libidibia ferrea) em ratos Wistar. Braz. J. Vet. Res. Anim. Sci. 2015;52:34–40. doi: 10.11606/issn.1678-4456.v52i1p34-40. [DOI] [Google Scholar]
- 62.Moura V.M., Freitasa L.A.S., Santos M.C., Raposo J.D.A., Lima A.E., Oliveira R.B., Silva M.N., Mourão R.H.V. Plants used to treat snakebites in Santarém, western Pará, Brazil: An assessment of their effectiveness in inhibiting hemorrhagic activity induced by Bothrops jararaca venom. J. Ethnopharmacol. 2015;161:224–232. doi: 10.1016/j.jep.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 63.Buckeridge M.S., Dietrich S.M.C., Lima D.U. Galactomannan as the reserve carbohydrate in legume seeds. [(accessed on 23 August 2020)];Dev. Crop. Sci. 2000 26:283–316. Available online: http://www.sciencedirect.com/science/article/pii/S0378519×0080015X. [Google Scholar]
- 64.Gallão M.I., Normando L.D.O., Vieira Í.G., Mendes F.N., Ricardo N.M., Brito E.S. Morphological, chemical and rheological properties of the main seed polysaccharide from Caesalpinia ferrea Mart. [(accessed on 23 August 2020)];Ind. Crop Prod. 2013 47:58–62. doi: 10.1016/j.indcrop.2013.02.035. Available online: [DOI] [Google Scholar]
- 65.Cunha A.P., Ribeiro A.C.B., Ricardo N.M.P.S., Oliveira A.C., Dávila L.S.P., Cardoso J.H.L., Rodrigues D.C., Azeredo H.M.C., Silva L.M.A., Brito E.S., et al. Polysaccharides from Caesalpinia ferrea seeds – Chemical characterization and anti-diabetic effects in Wistar rats. Food Hydrocoll. 2017;65:68–76. doi: 10.1016/j.foodhyd.2016.10.039. [DOI] [Google Scholar]
- 66.Hegnauer R., Gpayer-Barkmeijer R.J. Relevance of seed polysaccharides and flavonoids for the classification of the Leguminosae: A chemotaxonomic approach. Phytochemistry. 1993;34:3–16. doi: 10.1016/S0031-9422(00)90776-3. [DOI] [Google Scholar]
- 67.Jozala A.F., Santos J.R., Santos G.R., Viroel F.J.M., Pickler T.B., Santos C.A., Rebelo M.A., Chaud M.V., Hataka A., Groto D., et al. Libidibia ferrea loaded in bacterial nanocellulose: Evaluation of antimicrobial activity and wound care. J. Biomed. Biotechnol. 2020;6:6212–6226. doi: 10.34117/bjdv6n2-066. [DOI] [Google Scholar]
- 68.Sousa A.C.J., Oliveira J.S., Porcy C., Souza M.J.C., Menezes R.A.O. Potencial antimicrobiano de extratos vegetais frente a cepas bacterianas de interesse médico em Macapá, Amapá, Amazônia Brasileira. Diagn. Trat. 2019;24:85–90. [Google Scholar]
- 69.Malafaia C.B., Jardelino A.C.S., Silva A.G., Souza E.B., Macedo A.J., Correia M.T.S., Silva M.V. Effects of Caatinga Plant Extracts in Planktonic Growth and Biofilm Formation in Ralstonia solanacearum. Microb. Ecol. 2018;75:555–561. doi: 10.1007/s00248-017-1073-0. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira J.V.A., Ferreira L.L., Gomes F.F., Matias E.F.F., Sobral E.S., Cosmo J.A., Tintino S.R., Leite N.F., Albuquerque R.S., Morais Braga M.F.B., et al. Evaluation of antimicrobial and modulatory activity of the ethanol extract of Libidibia ferrea (Mart. ex tul.) l.p. queiroz. Rev. Cuba. Plantas Med. 2016;21:71–82. [Google Scholar]
- 71.Paiva W.S., Souza Neto F.E., Bandeira M.G.L., Abrantes M.R., Batista A.C.L., Silva J.B.A. Atividade antibacteriana da casca do jucá (Libidibia ferrea (mart. ex tul.) l. p. queiroz), frente a Staphylococcus spp. isolados do leite de cabras com mastite. Arch. Vet. Sci. 2015;20:141–146. doi: 10.5380/avs.v20i2.40422. [DOI] [Google Scholar]
- 72.Conde N.C.O., Pereira M.S.V., Bandeira M.F.C.L., Venâncio G.N., Oliveira G.P., Sampaio F.C. In vitro antimicrobial activity of plants of the Amazon on oral biofilm micro-organisms. J. Dent. Sci. Orig. 2015;30:179–183. [Google Scholar]
- 73.Nascimento P., Silva T., Gomes J., Silva M., Souza S., Falcão R., Silva T., Moreira K. Antioxidant and antimicrobial properties of ethanolic extract of Libidibia ferrea pods. Rev. Fitos. 2015;9:207–216. doi: 10.5935/2446-4775.20150017. [DOI] [Google Scholar]
- 74.Agostini V.O., Macedo A.J., Muxagata E., Silva M.V., Pinho G.L.L. Non-toxic antifouling potential of Caatinga plant extracts: Effective inhibition of marine initial biofouling. Hydrobiologia. 2020;847:45–60. doi: 10.1007/s10750-019-04071-6. [DOI] [Google Scholar]
- 75.Soares R.P.S.M., Corrêa R.O., Stroppa P.H.F., Marques F.C., Andrade G.F.S., Corrêa C.C., Brandão M.A.F., Raposo N.R.B. Biosynthesis of silver nanoparticles using Caesalpinia ferrea (Tul.) Martius extract: Physicochemical characterization, antifungal activity and cytotoxicity. PeerJ. 2018:1–16. doi: 10.7717/peerj.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melo P.A.F.R., Medeiros R.L.S., Ferreira-Júnior D.C., Alves E.U.A., Valeri S.V., Mondego J.M., Araújo J.R.G.L.R.N.S.L. Protection of Sideroxylon obtusifolium seeds against Colletotrichum sp. with Caesalpinia ferrea extract. Afr. J. Agric. Res. 2017;12:3433–3440. doi: 10.5897/ajar2017.12779. [DOI] [Google Scholar]
- 77.Melo P.A.F.R., Alves E.U., Martins C.C., Anjos Neto A.P., Pinto K.M.S., Araújo L.R., Vieira C.P., Nascimento L.C. Extracts of Caesalpinia ferrea and Trichoderma sp. on the control of Colletotrichum sp. transmission in Sideroxylon obtusifolium seeds. Rev. Bras. Plantas Med. 2016;18:494–501. doi: 10.1590/1983-084X/15_191. [DOI] [Google Scholar]
- 78.Biasi-Garbin R.P., Demitto F.O., Amaral R.C.R., Ferreira M.R.A., Soares L.A.L., Svidzinski T.I.E., Baeza L.C., Yamada-Ogatta S.F. Antifungal potential of plant species from brazilian caatinga against dermatophytes. Rev. Inst. Med. Trop. 2016;58:18–22. doi: 10.1590/S1678-9946201658018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peixinho G.S., Santos C.M.G., Ribeiro V.G., Amorim E.P.R., Bispo J.S., Carvalho V.N. Avaliação da eficiência de extratos de plantas nativas da caatinga sobre o controle da podridão seca (Lasiodiplodia theobromae) em cachos da videira cv. Itália. Summa Phytopathol. 2017;43:155–157. doi: 10.1590/0100-5405/2166. [DOI] [Google Scholar]
- 80.Comandolli-Wyrepkowski C.D., Jensen B.B., Grafova I., Santos P.A., Barros A.M.C., Soares F.V., Barcellos J.F.M., Silva A.F., Grafov A., Franco A.M.R. Antileishmanial activity of extracts from Libidibia ferrea: Development of in vitro and in vivo tests. Acta Amaz. 2017;47:331–340. doi: 10.1590/1809-4392201700871. [DOI] [Google Scholar]
- 81.Lima M.F.F., Araujo Silva J.W.S., Silva J.K., Moura A.H.N., Lopes R.L.F., Cordeiro B.A., Cordeiro R.P., Melo A.F.M. Avaliação toxicológica através do bioensaio com Artemia salina Leach de espécimes vegetais pertencentes à caatinga. Braz. J. Heal. Rev. 2019;2:5950–5963. doi: 10.34119/bjhrv2n6-088. [DOI] [Google Scholar]
- 82.Sousa M.J.B., Campos C.B.M., Duarte S.S.M., Sousa D.F., Manso J.A.X., Cruz A.D., Minasi L.B., Silva C.C. Genotoxicity of Brosimum gaudichaudii (Moraceae) and Caesalpinia ferrea (Fabaceae) in Astyanax sp. (Characidae) based on a comet assay. Genet. Mol. Res. 2018;17:1–8. doi: 10.4238/gmr18100. [DOI] [Google Scholar]
- 83.Silva F., Sales M., Sá O., Santana G., Deus M., Sousa J., Ferreira P., Peron A. Potencial citotóxico, genotóxico e citoprotetor de extratos aquosos de Caesalpinia pyramidalis Tul., Caesalpinia ferrea Mart. eCaesalpinia pulcherrima Sw. Rev. Bras. Biociências. 2015:101–109. [Google Scholar]
- 84.Batista E.K.F., Trindade H.I., Farias I.S., Martins F.M.M., Silva-Filho O.F., Batista M.C.S. Avaliação da Atividade Cicatizante de Preparados à base de Jucá (Caesalpinia ferrea Mart.) Arch. Vet. Sci. 2017;22:30–39. doi: 10.5380/avs.v22i3.50360. [DOI] [Google Scholar]
- 85.Carvalho F.G., Sampaio J.P.S., Araújo M.M.S., Pinto L.S.S., Rocha A.J. Assessment of the healing activity of jucá pods [Libidibia ferrea (Mart. ex Tul.) L. P. Queiroz] in cutaneous lesions of rats. Acta Sci.-Technol. 2016;38:137–143. doi: 10.4025/actascitechnol.v38i2.28570. [DOI] [Google Scholar]
- 86.Pereira L.D.P., Queiroz C.V.G., Pereira G., Assreuy A.M.S. Uso de extratos de polissacarídicos da planta medicinal Caesalpinea ferrea na estimulação do edema na pata de ratos. Ciência Anim. 2018;28:56–69. [Google Scholar]
- 87.Martins I.C.F., Fagundes M.R., Mockdeci H.R., Amarante C.B., Chaves M.D.G.A.M., Raposo N.R.B. Ex vivo evaluation of the erosive effect of acid tea widely consumed in Brazil. J. Clin. Diagn. Res. 2018;12:ZC50–ZC53. doi: 10.7860/JCDR/2018/29966.11340. [DOI] [Google Scholar]
- 88.Fernandes C.P.M., Machado C., Lopes T.V., Cunha Filho N., Bretanha P.R., Schons S., Félix S.R., Nobre M.O. Repellent Action of Carapa guianensis and Caesalpinia ferrea for flies species of Calliphoridae family. Ciência Rural. 2016;46:867–870. doi: 10.1590/0103-8478cr20150727. [DOI] [Google Scholar]
- 89.Lopes R.S., Martins M.C.B., Oliveira L.G., Costa A.F., Santos V.F., Correia M.T.S., Silva N.H., Albuquerque A.C., Lima E.Á.L.-A., Lima V.L.M. Termiticidal Activity of Libidibia ferrea var. ferrea and of the Association with Isaria spp. against Nasutitermes corniger. J. Agric. Sci. 2020;12:159. doi: 10.5539/jas.v12n1p159. [DOI] [Google Scholar]
- 90.Gomes V.E.V., Dutra J.A.C., Almeida M.M.M. Controlling the cowpea black aphid (Aphis craccivora Koch) with botanical extracts. Biotemas. 2019;32:117–121. doi: 10.5007/2175-7925.2019v32n3p117. [DOI] [Google Scholar]
- 91.Lopes R.S., Oliveira L.G., Lima G., Costa A.F., Lima E.Á.L.A., Lima V.L.M. Controle Biológico e Alternativo de Dactylopius opuntiae por Fungo Entomopatogênico e Extratos Vegetais em Plantação de Opuntia fícus-indica (Pernambuco/Brasil) Pesqui. Agropecuária Pernambucana. 2018;23:23–26. doi: 10.12661/pap.2018.007. [DOI] [Google Scholar]
- 92.Lopes R.S., Oliveira L.G., Costa A.F., Correia M.T.S., Lima E.A.L.-A., Lima V.L.M. Efficacy of Libidibia ferrea var. ferrea and Agave sisalana Extracts against Dactylopius opuntiae (Hemiptera: Coccoidea) J. Agric. Sci. 2018;10:255. doi: 10.5539/jas.v10n4p255. [DOI] [Google Scholar]
- 93.Demartelaere A.C.F., Nascimento L.C., Abraão P.C., Gomes R.S.S., Marinho C.O., Nunes M.C. Alternativas no controle da mancha marrom de alternaria em tangerineira ‘Dancy’. Summa Phytopathol. 2018;44:164–169. doi: 10.1590/0100-5405/173580. [DOI] [Google Scholar]
- 94.Pinto K.M.S., Melo P.A.F.R., Mondego J.M., Nascimento L.C., Cortez M.I.M.M., Aires A.A.C., Anjos-Neto A.P., Medeiros R.L.S., Araujo J.R.G., Silva H.F. Plant extracts enhancers of defense response in ponkan mandarin Seedlings against Alternaria alternate f. spp. citri infection. Afr. J. Agric. Res. 2018;13:650–656. doi: 10.5897/ajar2018.13025. [DOI] [Google Scholar]
- 95.Primo A.A., Melo M.D., Pereira G.A.C., Silva L.A., Fernandes F.É.P., Souza H.A. Potencial fertilizante da serapilheira de espécies lenhosas da Caatinga na recuperação de um solo degradado. Rev. Ceres. 2018;65:74–84. doi: 10.1590/0034-737x201865010010. [DOI] [Google Scholar]
- 96.Oliveira A.K., Coelho M.F.B., Torres S.B., Diógenes F.É.P. Allelopathy by extracts of Caatinga species on melon seeds. Semin. Agrar. 2016;37:557–566. doi: 10.5433/1679-0359.2016v37n2p557. [DOI] [Google Scholar]
- 97.Alves R.M., Silva M.A.D., Silva J.N., Costa R.S., Santos B.K.L., Lima E.D.S. Efeito alelopático de Libidibia ferrea Mart. sobre o vigor das sementes de feijão-caupi. Rev. Verde Agroecol. Desenvolv. Sustentável. 2019;14:476–479. doi: 10.18378/rvads.v14i3.5974. [DOI] [Google Scholar]
- 98.Carvalho L.B., Chagas P.M.B., Pinto L.M.A. Caesalpinia ferrea Fruits as a Biosorbent for the Removal of Methylene Blue Dye from an Aqueous Medium. Water Air. Soil Pollut. 2018;229:37200. doi: 10.1007/s11270-018-3952-5. [DOI] [Google Scholar]
- 99.Kasperiski F.M., Lima E.C., Umpierres C.S., Reis G.S., Thue P.S., Lima D.R., Dias S.L.P., Saucier C., Costa J.B. Production of porous activated carbons from Caesalpinia ferrea seed pod wastes: Highly efficient removal of captopril from aqueous solutions. J. Clean. Prod. 2018;197:919–929. doi: 10.1016/j.jclepro.2018.06.146. [DOI] [Google Scholar]
- 100.Marques M.M.M., Morais S.M., Silva A.R.A., Barroso N.D., Pontes Filho T.R., Araujo F.M.D.C., Vieira I.G.P., Lima D.M., Guedes M.I.F. Antiviral and Antioxidant Activities of Sulfated Galactomannans from Plants of Caatinga Biome. Evid.-Based Complement. Altern. Med. 2015 doi: 10.1155/2015/591214. [DOI] [PMC free article] [PubMed] [Google Scholar]