Abstract
The kidney function of patients with chronic kidney disease (CKD) is impaired irreversibly. Organ transplantation is the only treatment to restore kidney function in CKD patients. The assessment of new potential therapeutic procedures relies heavily on experimental animal models, but it is limited by its human predictive capacity. In addition, the frequently used two-dimensional in vitro human renal cell models cannot replicate all the features of the in vivo situation. In this study, we developed a three-dimensional (3D) in vitro human renal organoid model from whole kidney cells as a promising drug screening tool. At present, the renal tissue generated from human pluripotent stem cells (hPSCs) exhibits intrinsic tumorigenicity properties. Here we first developed a 3D renal organoid culture system that originated from adult differentiated cells without gene modification. Renal organoids composed of multiple cell types were created under optimal experimental conditions and evaluated for morphology, viability and erythropoietin production. As a novel screening tool for renal toxicity, 3D organoids were exposed to three widely used drugs: aspirin, penicillin G and cisplatin. The study results showed this 3D renal organoid model can be used as a drug screening tool, a new in vitro 3D human kidney model, and provide hope for potential regenerative therapies for CKD.
Keywords: drug screening, human kidney, organoid, toxicity
Introduction
Chronic kidney disease (CKD) is a healthcare problem worldwide and affects 9–14% of the adult population in the USA1. CKD often leads to low glomerular filtration rate, high urinary albumin excretion, interstitial fibrosis, anemia, hyperphosphatemia and additional complications like cardiovascular disease and hypertension2–4. The typical pathological progression of CKD is gradual, irreversible loss of nephrons, the distinct functional compartments of kidneys5. The process of nephrogenesis, the formation of new nephrons, ceases shortly before birth6. Thus, the loss of nephrons can only be attenuated but not reversed, and eventually results in end-stage renal disease (ESRD)7.
Generally, the only therapeutic options for patients with ESRD are dialysis or kidney transplantation. Life-long dialysis reduces the quality of life and has side effects such as hypotension, high infection rate, and loss of erythropoietin and activated vitamin D4. In addition, there are problems related to transplantation including donor organ shortage, transplant rejection, high cost, increased infections and potential tumor formation caused by immunosuppression8.
The growing number of CKD/ESRD cases and the shortage of transplantable kidneys have caused increasing interest in renal regenerative treatment, renal disease models and bioartificial kidneys9. Drug injury assessment relies heavily on experimental animal models, and it is difficult to precisely reflect the same predictive capacity in humans. Current human two-dimensional (2D) in vitro models are limited in accurately replicating the in vivo organ system10,11. Organoids are considered as complex three-dimensional (3D) structures that develop from stem cells or organ-specific progenitors through a self-organization process12. As a functional unit, an organoid is composed of multiple cell types and contains multicellular organ structures, displaying architectures and functionalities similar to in vivo organs, and is thought to closely reproduce the in vivo environment13. Recent studies have demonstrated that organoids can be used to model organ development and disease, and have a wide range of applications in basic research, drug screening and regenerative medicine12.
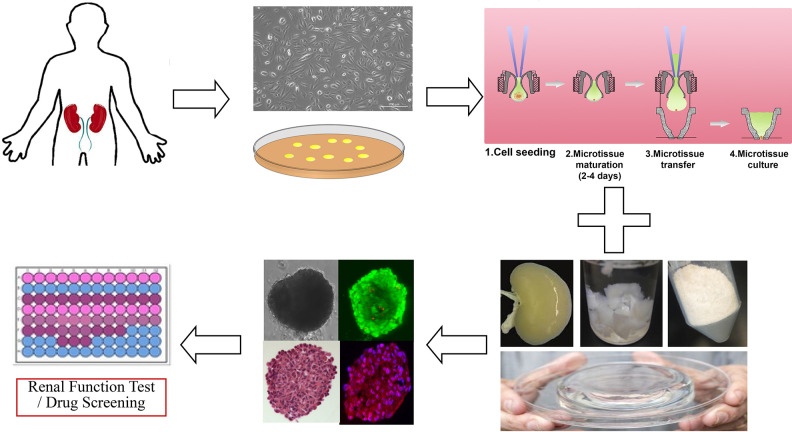
The kidney is a complex organ and consists of over 20 different types of cells which are constructed into individual anatomical and functional units including the glomerulus, and proximal and distal tubules14. It is hard to establish functional human renal cells and tissues composed of different cell types, compared with liver, heart or cartilage. In recent years, various protocols have been developed and published for the differentiation of human pluripotent stem cells (hPSCs), such as human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs), into different renal-like cell types and self-organizing kidney organoids5,15–17. However, undifferentiated hiPSCs are known to possess intrinsic tumorigenicity properties, and the applications of hESCs are also limited due to medical ethical issues18. In this study, we demonstrated a method to generate renal organoids directly from whole renal differentiated cells from adult human kidney tissues, which could be obtained by renal biopsy or kidney resection surgery (Fig. 1). We hypothesized that the renal organoids made from whole kidney cells had similar characteristics with renal organs in vivo and could be considered as a high throughput, personalized tool for nephrotoxicology testing. Human renal cell therapy seems to show great potential, though it is still in the experimental stage7. The in vitro culture of whole renal differentiated cells derived organoids could be used as an autologous cell source for renal cell therapy which targets the inherent ability of renal cells for repair and regeneration, especially for patients with CKD or ESRD.
Figure 1.
Schematic diagram of the study.
The objective of this study was to develop a 3D human renal organoid culture system originating from whole adult kidney cells as a new predictive first-line drug-screening tool in vitro that could have immense potential in renal cell regenerative therapy for CKD and ESRD patients.
Materials and Methods
Preparation for Human Whole Kidney Cell
Human whole kidney cells used in this study were taken from cryogenically stored cells that we isolated previously4. Donor human kidneys not used for transplantation were obtained from Carolina Donor Services at Winston-Salem, USA, with written consent from the donors and ethical approval by the Institutional Review Board of Wake Forest University Health Sciences. The renal medulla was discarded and the cortex was removed and placed in Krebs-Ringer buffer (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 1% antibiotic (penicillin-streptomycin, Gibco Invitrogen, Carlsbad, CA, USA). The renal capsule and connective tissue were removed and the remaining tissue was minced using scissors. The tissue was digested using Liberase Blendzyme (Roche, Indianapolis, IN, USA) for 1 h in a 37°C shaking water bath. After filtration through a 100 µm cell strainer (BD Falcon, San Jose, CA, USA) and centrifugation at 1500 rpm for 5 min, the cell pellet was re-suspended in kidney culture medium (KCM). The KCM consisted of equal parts of keratinocyte serum-free medium (KSFM, premixed with 2.5% fetal bovine serum (FBS), 1% penicillin-streptomycin, 0.4% insulin transferrin selenium, 0.2% EGF and bovine pituitary extract) and Dulbecco’s Modified Eagle’ s Medium(DMEM, supplemented with 1% penicillin-streptomycin and 10% FBS). The cells were plated in a 15 cm2 cell culture plate and incubated at 37°C in an atmosphere containing 5% CO2 in air. The medium was refreshed every 2 days. The cells were sub-cultured for expansion at a ratio of 1:3 when confluent.
Preparation of Kidney Extracellular Matrix (ECM) Extraction
To include all important factors that may support renal cell function in vivo, human kidney ECM was extracted and added to the culture medium. Fresh kidneys were first rinsed in pre-cooled Dulbecco phosphate-buffered saline (PBS), cut into 2 cm × 2 cm blocks, and then flash frozen at –80°C. The frozen tissue was sliced into 3 mm pieces and then transferred to 500 ml double distilled water (ddH2O). The tissue was shaken on a rotary shaker at 4°C for 3 days at 200 rpm, during which water was refreshed every 8 h. After 3 days, the sliced tissue was moved to 500 ml 2% Triton-X-100 and shaken for approximately 4 days. This solution was then changed to 2% Triton-X-100 containing 0.1% NH4OH and shaken at 200 rpm for an additional day. The decellularized tissues were washed for two more days to remove any remaining Triton-X-100. After rinsing, the ECM was lyophilized for 48 h at –80°C and ground into powder using a freezer mill. The ECM (1 mg) was mixed with 100 mg Pepsin (porcine gastric mucosa, 3400 units, Fisher Scientific, Fair Lawn, NJ, USA) and 0.1 N hydrochloric acid was added. The mixture was incubated for 48 h at room temperature followed by gamma irradiation sterilization and centrifugation three times at 3000 rpm for 15 min. The supernatant was neutralized to pH 7.2 and stored at –80°C for further use after filtration using a 0.2 µm syringe filter (Fisher Scientific, Fair Lawn, NJ, USA).
Generation and Culture of Renal Organoids in Different Cell Numbers
Human renal cells at passage 1 were resuspended in KCM, 30% FBS, and 1 μg/ml solubilized human renal tissue ECM at a ratio of 8:1:1 and seeded into 96-well format GravityPLUS™ Hanging Drop Plate (InSphero AG, Zurich, Switzerland) at a density of 250, 500,1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000, 9000, 10,000 cells/40 μl drop volume, respectively. After 3 days, compact organoids were transferred to 96-well format GravityTRAP™ Plate (InSphero AG, Zurich, Switzerland) following the manufacturer’s protocols for long-term cultivation. Renal organoids were cultured in KCM supplemented with 1 μg/ml solubilized human renal ECM extract at 37°C in an atmosphere of 5% CO2 in air. Half of the culture medium was removed and replaced with fresh medium every day.
Proliferation and Viability of Renal Organoids
Live/Dead cell viability kit (Molecular Probes) and ATP measurement (CellTiter-Glo Luminescent Cell Viability Assays, Promega, Madison, WI, USA) were applied to assess proliferation and viability of renal organoids. For the Live/Dead assay, live cells and necrotic cells were stained green and red fluorescent, respectively, in serum free medium and assessed using an Olympus FV10i Confocal microscope. The number of viable cells was also evaluated based on the quantitation of the presence of ATP. Medium was discarded and CellTiter-Glo 3D reagent was added to each well. After incubating 30 min at room temperature on a rotary shaker, bioluminescence activity was assessed by plate luminometer. Viability was monitored at days 1, 3, 7, 14, 21 and 28.
Histological Evaluation of Renal Organoids by Whole Mounting Staining
After culturing for 14 days, organoids were pooled and fixed in 4% paraformaldehyde (PFA) for 30 min at room temperature. Fixed organoids were embedded in Histon Gel and paraffin and then sliced into 5 µm sections. Deparaffinization and Hematoxylin and Eosin (H&E) staining was performed based on standard protocols. Organoid slides were observed using a Zeiss Axiovert 200 M microscope.
Whole Mount Immunofluorescence for Specific Markers of Renal Cells
Renal organoids in GravityTRAPTM were harvested by aspirating the culture medium carefully and transferred to chamber slides. After washing twice in PBS for 5 min, the organoids were fixed in 4% PSA for 60 min, permeabilized in 0.2% Triton-100 for 30 min, washed with PBS and then incubated in protein blocking buffer (Dako, Carpinteria, CA, USA) for 1 h at room temperature. Specific primary anti-human antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for Aquaporin-1 (AQP1, L-19), Aquaporin-3 (AQP3, C-18), Podocin (H-130), Synaptopodin (P-19), Nephrin (B-12) and erythropoietin (EPO, H-162) were diluted in blocking buffer. After incubation with primary antibody overnight at 4°C, corresponding diluted secondary antibodies were applied to the slides for 4 h in the dark at room temperature. Tissue sections were then incubated with HRP-conjugated streptavidin (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature and signals were visualized using the AEC Peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA). 2D renal cells were also stained by immunofluorescence under the same conditions as the control group. All slides were imaged with FV1200 Laser Scanning Confocal Microscope.
Function Test and Drug Toxicity Test
Organoids cultured in hypoxia (3% and 1% oxygen) condition were used for erythropoietin (EPO) excretion assessment after incubation for 1 h, 3 h, 6 h, 12 h, 24 h 36 h and 48 h. Following incubation, cell culture supernatants were collected from 3% oxygen and 1% oxygen cultured organoids and concentrated 4× using an Eppendorf Vacufuge for EPO production measurement using a Human EPO ELISA kit (RayBiotech, Norcross, GA, USA) according to the manufacturer’s instructions. EPO levels were determined via optical density at 450 m wavelength absorbance. All samples were assayed in duplicate. A bar plot was generated to illustrate EPO level.
Drugs excreted by the kidneys, aspirin, penicillin G+ and cisplatin were used for nephrotoxicity testing. The organoids were treated with 200 μM aspirin, 200 μM penicillin G and 200 μM cisplatin for 2 days in vitro. Drug-treated and normal renal organoids, as well as 2D cultured renal cells were homogenized in 200 μl ice-cold GGT Assay Buffer. The γ-Glutamyltransferase (GGT) Activity Colorimetric Assay Kit (Sigma-Aldrich, St. Louis, MO, USA) was used according to the manufacturer’s protocol.
Dose–Response Curves and IC50 Estimation
Renal cells and organoids were seeded into 96-well plates. Twelve hours later, the cells and organoids were treated with DMSO and different concentrations of cisplatin at 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 and 6.4 mM. Twenty-four hours after this treatment, the relative cell survival of each well was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl) -2-(4-sulfophenyl)-2 H tetrazolium, inner salt (MTS) method (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The “relative cell survival” was defined as the number of viable cells in the drug-containing medium/the number of viable cells in the drug-free medium. The “relative cell survival” values and drug concentrations were fitted to dose–response curves to estimate the IC50 values of the drug using the SPSS software.
RNA Extraction and Quantitative Real-Time PCR
Total RNAs were extracted by MagZol (Invitrogen, Carlsbad, CA, USA) and cDNAs were synthesized by using SYBR Premix Ex TaqTM (TaKaRa, Kusatsu, Shiga, Japan). Realtime PCR was performed by SYBR Green Realtime PCR Master Mix (TOYOBO, Kita-ku, Osaka, Japan). Primers for the reaction are provided as follows: GAPDH (forward 5’-GTCATCATCTCCGCCCCTTCTGC-3’, reverse 5’-GATGCCTGCTTCACCACC TTCTTG-3’); KIM-1 (forward 5’-CTGCAGGGAGCAATAAGGAG-3’, reverse 5’-TCCAAAGGCCATCTGAAGAC-3’); AQP1 (forward 5’-CTGGGCATCGAGAT CATCGG-3’, reverse 5’-ATCCCACAGCCAGTGTAGTCA-3’); AQP3 (forward 5’-GGGGAGATGCTCCACATCC-3’, reverse 5’-AAAGGCCAGGTTGATGGTGAG-3’); Podocin (NPHS2) (forward 5’-ACCAAATCCTCCGGCTTAGG-3’, reverse 5’-CAACCTTTACGCAGAACCAGA-3’).
Statistical Analysis
Statistical analysis results are presented as mean ± standard deviation (SD). Comparisons between groups were performed using a two-tailed Student’s t-test; p<0.05 was considered significant. Statistical results were calculated using GraphPad Prism 6 software.
Results
Formation of Multicellular Human Renal Organoids
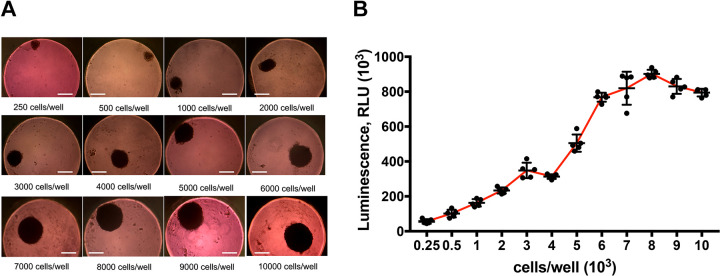
Multiple renal cells were seeded in a 96-well format GravityPLUS™ Hanging Drop Plate with renal organoid formation media at concentrations of 250, 500, 1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000, 9000 and 10,000 cells per well. After 3 days’ incubation, organoids of multiple cell numbers were formed and visible spherical solid organoids of different sizes could be observed in all wells at day 7 (Fig. 2A). The diameter of the organoids grew larger with the increase in cell number, from about 125 µm (250 cells/well) to more than 400 µm (10,000 cells/well).
Figure 2.
Optimization of the organoid production. (A) Morphology and size of renal organoids generated from different cell amounts. The 8000 cells/well concentration was chosen as the optimal initial cell amount for organoid culture (scale bar: 200 μm). (B) ATP measurement in renal organoids of different cell amount for proliferation.
Organoids were harvested after 14 days in culture for viability examination. Renal organoid proliferation of different cell amount at the beginning was assessed by ATP assays (CellTiter-Glo Promega). The results demonstrated that the viability of organoids increased with elevation of initial cell amount and peaked at 8000 cells/well, which suggested that the optimum initial cell concentration for renal organoids was 8000 cells per well (Fig. 2B). The 8000 cells/well concentration was set as the standard initial cell concentration for all following organoid culture experiments.
Viability of Multicellular Human Renal Organoids in Vitro
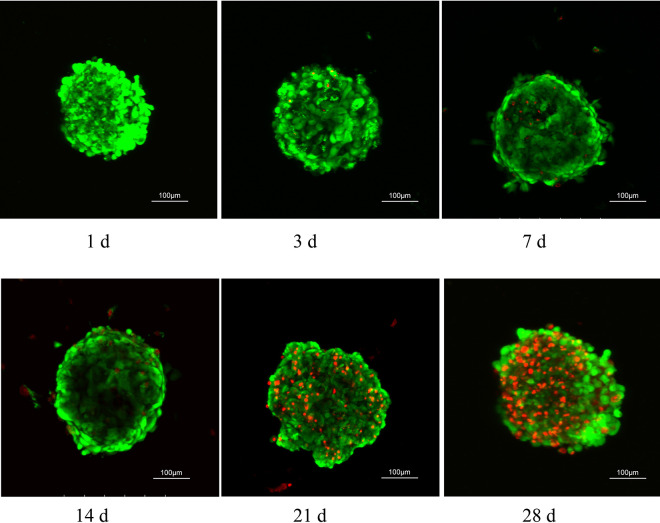
Organoid viability at days 1, 3, 7, 14, 21 and 28 was assessed using confocal microscopy after Live/Dead cell staining (Invitrogen). Renal organoids were well organized and no significant cell death was observed within a 14-day culture period (Fig. 3). After 21 days’ incubation, detectable necrosis and apoptosis of renal organoid cells could be observed.
Figure 3.
Optimization of the organoid culture period. 14 days was selected as optimum culture period for organoids. LIVE/DEAD fluorescence assay in different culture periods for viability. Renal organoids were well self-organized and kept viability in 14 days under our protocol.
Long-Term Morphological Characteristics of Renal Organoids and Expression of Renal Cell Markers during Extended Culture
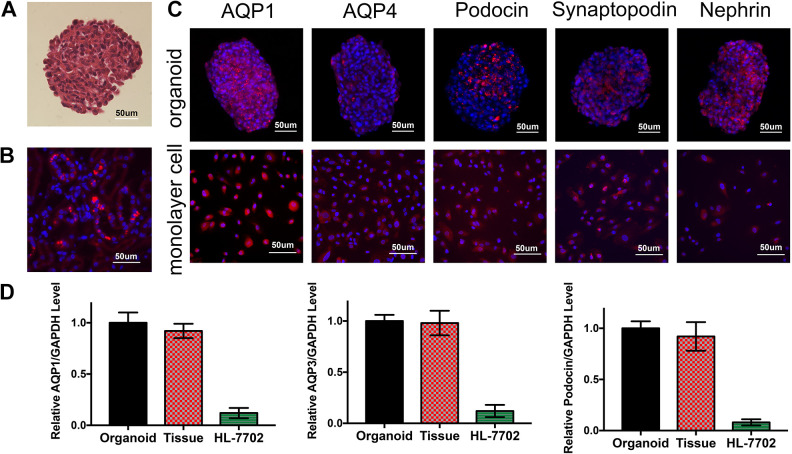
For the evaluation of internal morphology, organoids were harvested, paraffin embedded, and sectioned for H&E staining at day 14 (Fig. 4A). We can see that a compact, tight organoid was generated with no detectable necrosis in the center. The multicellular 3D organoids showed a specific structure of self-aggregation which was not found in 2D cultures, and ECM was visible within the structure as well. Meanwhile, comparing with normal kidney slides, the morphology of the renal organoids was close to that of normal glomeruli.
Figure 4.
Expression of renal cell markers in 3D renal organoids. (A) H&E staining of renal organoid. (B) AQP1 immunofluorescence of normal kidneys. (C) Organoids whole mount staining and monolayer renal cells immunofluorescence test for renal cell markers (AQP1, AQP3, Podocin, Synaptopodin, Nephrin. Red-Alexa Fluor 594, Blue: DAPI) (D) The expression level of kidney marker genes AQP1, AQP3 and Podocin in renal organoids, human kidney primary tissue and human normal liver cells HL-7702.
The expression of aquaporin-1(AQP1), aquaporin-3 (AQP3), Podocin, Synaptopodin, and Nephrin, the marker proteins for renal tubular epithelial cells, collecting duct, and glomerular podocytes, was assessed by immunofluorescence staining in paraffin-embedded sections. Organoids and monolayer renal cells were assessed. Red staining indicated the corresponding biomarkers and blue staining for DAPI (Fig. 4C).
The expression levels of kidney marker genes AQP1, AQP3 and Podocin (NPHS2) in renal organoids, human kidney primary tissue and human normal liver cells HL-7702 were detected by qPCR (Fig. 4D). The results showed that the expression levels of kidney marker genes in renal organoids and kidney tissues were not significantly different, but the expression levels were significantly higher in renal organoids and kidney tissues than in HL-7702 cell lines.
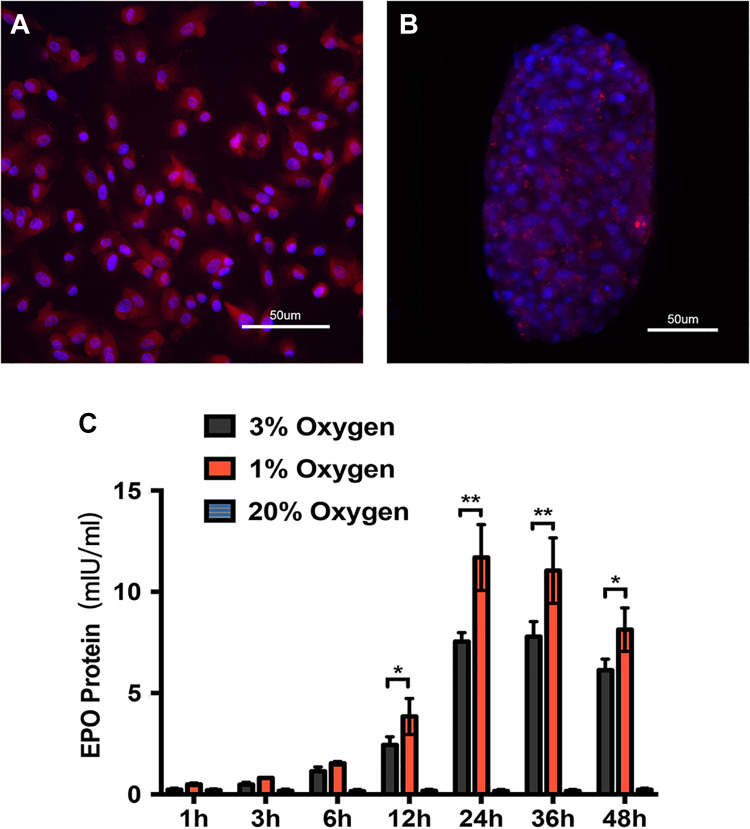
Detection of EPO expression level was carried out using an ELISA kit. We found the renal organoids assembled only from first passage kidney cells could secret EPO protein under condition of hypoxia (Fig. 5A, B). The primary renal cells from kidney tissues were incubated in 1%, 3% and 20% oxygen conditions. EPO expression increased with prolonged hypoxic culture time, reached maximum level at 24 h and decreased gradually afterwards. EPO was not detected in organoids which were cultured in the 20% oxygen condition, and the EPO production level was higher in 1% than 3% oxygen condition at each time point (Fig. 5C).
Figure 5.
EPO expression and secretion in renal organoids in a hypoxic environment. (A, B) The expression of EPO in monolayer renal cells and renal organoids in hypoxia environment was evaluated by immunofluorescence test. (C) The secretion level of renal organoids was upregulated in hypoxia circumstances (*p<0.05, **p<0.01).
Evaluation of Human Renal Organoids as a Potential Nephrotoxicity Model
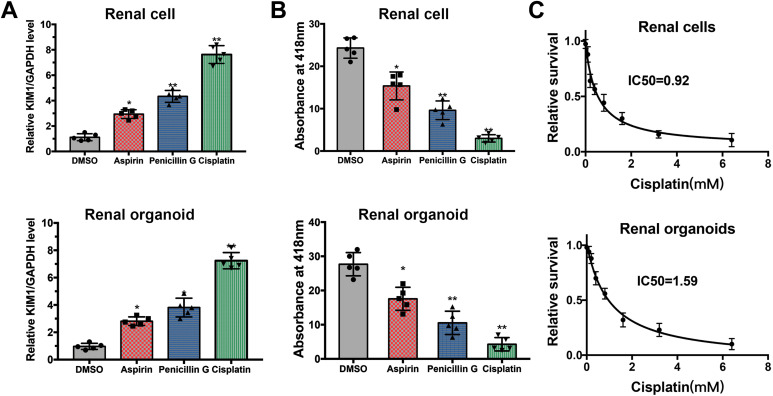
To evaluate the use of human renal organoids as a novel screening tool for nephrotoxicity, 2D renal cells and 3D organoids were exposed to 200 μM aspirin, 200 μM penicillin G and 200 μM cisplatin, which are excreted at the glomerulus and renal tubules. After a 48-h treatment with drugs, drug-treated and control monolayer renal cells were collected for real-time PCR to evaluate the expression of Kidney injury molecule1 (KIM1) (Fig. 6A), and organoids were harvested for evaluating nephrotoxicity by GGT activity colorimetric assay (Fig. 6B). KIM1 is a transmembrane glycoprotein that is normally undetectable in non-injured kidney, and up-regulated in proximal tubule injury19. GGT plays a key role in the γ-glutamyl cycle, a critical pathway for glutathione homeostasis as well as the detoxification of xenobiotics. The absorbance at 418 nm in aspirin (+) and penicillin G (+) and cisplatin (+) organoids was significantly lower than that in control organoids (Fig. 6B), the viability of organoids was adversely affected by aspirin, penicillin and cisplatin, and the trend of change was consistent with the real-time PCR results of KIM1 expression. The “relative cell survival” values and drug concentrations were fitted to dose–response curves to estimate the IC50 values of cisplatin in 2D monolayer renal cells and 3D renal organoids. The IC50 of cisplatin in renal cells was 0.92 mM, and it was 1.59 mM in organoids (Fig. 6C).
Figure 6.
Application of renal organoids in kidney function and drug screening. (A) Real-time PCR demonstrate that treatment of aspirin, penicillin G and cisplatin upregulated mRNA levels of KIM1 in renal cells and in renal organoids. (B) GGT activity measurement for kidney function and drug screening in 2D renal cells and renal organoids. (C) Dose–response curves of cisplatin in renal cells and organoids, and the IC50 of cisplatin was 0.92 mM and 1.59 mM, respectively.
Discussion
This study demonstrated the development of a simple protocol for multicellular human 3D renal organoids from human tissue fragments. The 3D organoids represent a better model than the routine 2D cell cultures or animal models. The 3D organoids may ultimately be developed into an efficient research and clinical application for drug screening on renal cells and regenerative therapy for CKD and ESRD.
In previous studies, many types of kidney organoids have been generated from hESCs or hiPSCs5,15,20–22; to our knowledge, kidney organoids formed by adult differentiated renal cells obtained from primary adult kidney tissues are reported for the first time in this study. Adult differentiated cell-derived organoids may have many advantages and wider clinical applications. These organoids are easier to produce, because there is no need for long differentiation time and extra differentiation induction factors for stem cells to differentiate. Kidney tissues obtained from renal biopsy, kidney resection or even urinary sediment cells can be used to generate patient-specific organoids which can be used for high-throughput drug nephrotoxicity testing, drug screening or other subsequent applications. Undifferentiated hPSCs are considered to be potentially tumorigenic and may cause tumor formation. Renal cells isolated from human kidney tissues could be a safer source for human cell-based therapeutic products.
In the present study, human cells were isolated from donor kidneys and successfully integrated into 3D multicellular organoids using hanging drop plates. The concentration and amount of the primary cells can be precisely controlled with the hanging drop approach, so that it is easy to manipulate the subsequent experimental procedures and assays. To facilitate the formation of renal organoids and maintain long-term viability of organoids, isolated human kidney ECM containing growth factors and other essential proteins was added into the optimized organoid culture medium in vitro. The optimal initial cell amount and culture period were determined by ATP production assay and Live/Dead cell viability assay. It was found that 8000 cells per well and 14-day incubation time resulted in the highest cell proliferation, and this was set as routine for organoid culture. Multiple types of renal cells were verified to be present in the compact organoid by detecting the expression of specific renal glomerular markers (Podocin, Synaptopodin and Nephrin), proximal tubule marker (AQP1) and collecting duct marker (AQP3)15,23.
EPO is an indispensable glycoprotein hormone primarily produced from renal fibroblasts including tubular epithelial cells, glomerular mesangial cells and interstitial fibroblasts24, and its production is induced by the presence of hypoxia to maintain tissue oxygen homeostasis25. EPO expression is a complex process regulated by multiple transcription factors, including hypoxia-induced factor (HIF), prolyl hydroxylase domain-containing protein (PHD) and GATA binding protein (GATA)26. The hypoxic induction of EPO production resulted in an increase in the number of EPO-producing cells rather than an elevation of EPO expression level per cell27. Little et al.16 proved functional maturity of organoids by detecting megalin-mediated and cubilin-mediated endocytosis, and the endocrine function of renal organoids was validated by EPO test in this study. Unfortunately, EPO was only detected in the hypoxia-cultured renal organoids generated from the first passage of renal cells. There was no hint of EPO excretion in organoids derived from second or further passage cells. Further studies are needed to reveal the mechanism of this phenomenon.
Organoids are cell-derived 3D structures which recreate important aspects of the 3D anatomy of the original organs and recapitulate basic tissue-level function12. Notably, the organoid model better mimics in vivo organ structure because it possesses many features that 2D cell models are missing, including multiple renal cell types, cell–cell communication, the presence of ECM and growth factors and cell-specific gene expression9,28. As a means to assess the potential of this model system for nephrotoxicity screening, organoids were exposed to aspirin and penicillin G, which are widely used in clinical practice. Aspirin is a non-steroid anti-inflammatory drug and is rapidly absorbed through the gastrointestinal tract, hydrolyzed to salicylic acid29 and widely distributed throughout the body, with the highest concentration in the renal cortex, plasma and liver30. Aspirin and salicylates are filtrated through the glomerulus and primarily excreted by the kidneys31. Therefore, high-dose or long-term consumption of aspirin demonstrates an adverse effect on renal function32. β-lactam antibiotics (penicillins, cephalosporins and carbapenems) are among the main groups of drugs excreted by kidneys, and are not only filtered through the glomerulus but are also actively secreted by the proximal tubules33. β-lactam antibiotics are likely to be taken up by the proximal tubule cells and cause acute proximal tubular necrosis34. Cisplatin is a well-known and characterized nephrotoxicant which can lead to acute kidney injury in clinical use35. The activity of GGT, which is expressed in the proximal tubule, was used to reflect the functional characters of the renal cells15. GGT activity in aspirin, penicillin G and cisplatin-treated organoids was significantly lower than that in the control group, which is consistent with expectations.
This study verified that renal organoids made from whole kidney cells is a promising tool for drug nephrotoxicity testing, and construction of the organoids fills the gap between currently accepted in vitro cell culture models and in vivo functional analysis36,37. In addition, utilization of organoids should reduce the reliance on experimental animals13. Renal disease modeling would be another important area for applications of these renal organoids, which could be helpful to improve our understanding of the underlying pathophysiology of many different types of chronic kidney diseases9,38.
Currently, there are no effective therapies to cure and reverse severe terminal kidney dysfunction, and treatment is limited to alleviating symptoms and delaying the progression of the disease39. For restoring full physiological organ function, kidney transplantation is the only definitive cure for CKD. However, it is impossible for every patient to have a matched kidney because of the shortage of donor organs40. Renal organoids are attractive with respect to regenerative therapies, and they contain various renal-like cell types arranged into structures resembling the micro-structure of a kidney. However, these structures have not been shown to be functional, and organoids also do not recapitulate the overall tissue organization of an entire kidney9,41. Much research is needed before transplantable kidneys can be grown from renal organoids; however, this endeavor is worth pursuing.
In conclusion, our study developed a multicellular 3D renal organoid model which exhibited long-term viability and originated from differentiated cells of adult kidney tissues. We demonstrated a method to generate renal organoids from differentiated cells and optimized the culture parameters, conditions and procedures. This model may be useful as a drug screening tool, drug nephrotoxicity assay, potential kidney disease model, and potential supplement or replacement of kidney transplantation in regenerative medicine.
Footnotes
Ethical Approval: The study was approved by the Institutional Review Board of Wake Forest University Health Sciences, North Carolina, USA.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institutional Review Board of Wake Forest University Health Sciences approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the donors for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Liang Chen  https://orcid.org/0000-0002-5779-6148
https://orcid.org/0000-0002-5779-6148
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Cross-sectional 8. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. [DOI] [PubMed] [Google Scholar]
- 2. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. [DOI] [PubMed] [Google Scholar]
- 3. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AYM, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. [DOI] [PubMed] [Google Scholar]
- 4. George SK, Abolbashari M, Jackson JD, Aboushwareb T, Atala A, Yoo JJ. Potential use of autologous renal cells from diseased kidneys for the treatment of renal failure. PLoS One. 2016;11(10):e0164997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre J V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Little MH, Kairath P. Regenerative medicine in kidney disease. Kidney Int. 2016;90(2):289–299. [DOI] [PubMed] [Google Scholar]
- 7. Morizane R, Miyoshi T, Bonventre J V. Concise review: kidney generation with human pluripotent stem cells. Stem Cells. 2017;35(11):2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarno G, Nappi R, Altieri B, Tirabassi G, Muscogiuri E, Salvio G, Paschou SA, Ferrara A, Russo E, Vicedomini D, Vincenzo C, et al. Current evidence on vitamin D deficiency and kidney transplant: What’s new? Rev Endocr Metab Disord. 2017;18(3):323–334. [DOI] [PubMed] [Google Scholar]
- 9. Chuah JKC, Zink D. Stem cell-derived kidney cells and organoids: recent breakthroughs and emerging applications. Biotechnol Adv. 2017;35(2):150–167. [DOI] [PubMed] [Google Scholar]
- 10. John R, Herzenberg AM. Renal toxicity of therapeutic drugs. J Clin Pathol. 2009;62(6):505–515. [DOI] [PubMed] [Google Scholar]
- 11. Vincent J. Drugs and the kidneys: clinical pharmacology perspectives. Clin Pharmacol Ther. 2017;102(3):368–372. [DOI] [PubMed] [Google Scholar]
- 12. Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–687. [DOI] [PubMed] [Google Scholar]
- 13. Wang S, Gao D, Chen Y. The potential of organoids in urological cancer research. Nat Rev Urol. 2017;14(7):401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. [DOI] [PubMed] [Google Scholar]
- 15. Yamaguchi S, Morizane R, Homma K, Monkawa T, Suzuki S, Fujii S, Koda M, Hiratsuka K, Yamashita M, Yoshida T, Wakino S, et al. Generation of kidney tubular organoids from human pluripotent stem cells. Sci Rep. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takasato M, Er PX, Chiu HS, Little MH. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 2016;11(9):1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–568. [DOI] [PubMed] [Google Scholar]
- 18. Yasuda S, Kusakawa S, Kuroda T, Miura T, Tano K, Takada N, Matsuyama S, Matsuyama A, Nasu M, Umezawa A, Hayakawa T, Tsutsumi H, et al. Tumorigenicity-associated characteristics of human iPS cell lines. PLoS One. 2018;13(10):e0205022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol. 2009;238(3):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morizane R, Bonventre J V. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat Protoc. 2017;12(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16(1):118–126. [DOI] [PubMed] [Google Scholar]
- 22. Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67. [DOI] [PubMed] [Google Scholar]
- 23. Bariety J. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol. 2006;17(10):2770–2780. [DOI] [PubMed] [Google Scholar]
- 24. Souma T, Suzuki N, Yamamoto M. Renal erythropoietin-producing cells in health and disease. Front Physiol. 2015;6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537–547. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, Teng R, Noguchi CT. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15(6):10296–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaleyeva LM, Guimaraes-Souza NK, Krane LS, Agcaoili S, Gyabaah K, Atala A, Aboushwareb T, Yoo JJ. Cell therapy with human renal cell cultures containing erythropoietin-positive cells improves chronic kidney injury. Stem Cells Transl Med. 2012;1(5):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pendergraft SS, Sadri-Ardekani H, Atala A, Bishop CE. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro . Biol Reprod. 2017;96(3):720–732. [DOI] [PubMed] [Google Scholar]
- 29. Bouzenna H, Dhibi S, Samout N, Rjeibi I, Talarmin H, Elfeki A, Hfaiedh N. The protective effect of Citrus limon essential oil on hepatotoxicity and nephrotoxicity induced by aspirin in rats. Biomed Pharmacother. 2016;83:1327–1334. [DOI] [PubMed] [Google Scholar]
- 30. Fiorucci S, Antonelli E, Burgaud JL, Morelli A. Nitric oxide-releasing NSAIDs: a review of their current status. Drug Saf. 2001;24(11):801–811. [DOI] [PubMed] [Google Scholar]
- 31. Aprioku J. Renal effects of non-steroidal anti inflammatory drugs in albino rats. Br J Pharm Res. 2013;3(3):314–325. [Google Scholar]
- 32. Curiel R V, Katz JD. Mitigating the cardiovascular and renal effects of NSAIDs. Pain Med. 2013;14(Suppl 1):S23–S28. [DOI] [PubMed] [Google Scholar]
- 33. Jariyawat S, Sekine T, Takeda M, Apiwattanakul N, Kanai Y, Sophasan S, Endou H. The interaction and transport of beta-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther. 1999;290(2):672–677. [PubMed] [Google Scholar]
- 34. Goldstein RS, Smith PF, Tarloff JB, Contardi L, Rush GF, Hook JB. Biochemical mechanisms of cephaloridine nephrotoxicity. Life Sci. 1988;42(19):1809–1816. [DOI] [PubMed] [Google Scholar]
- 35. Gao L, Wu WF, Dong L, Ren GL, Li HD, Yang Q, Li XF, Xu T, Li Z, Wu BM, Ma TT, et al. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Front Pharmacol. 2016;7:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baker M. Tissue models: a living system on a chip. Nature. 2011;471(7340):661–665. [DOI] [PubMed] [Google Scholar]
- 37. Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug Discov Today. 2012;17(3–4):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun Y, Zhou H, Yang B. Drug discovery for polycystic kidney disease. Acta Pharmacol Sin. 2011;32(6):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. García-Domínguez X, Vicente JS, Vera-Donoso CD, Marco-Jimenez F. Current bioengineering and regenerative strategies for the generation of kidney grafts on demand. Curr Urol Rep. 2017;18(1):2. [DOI] [PubMed] [Google Scholar]
- 40. Morizane R, Bonventre JV. Kidney organoids: a translational journey. Trends Mol Med. 2017;23(3):246–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davies JA. Kidney tissue grown from induced stem cells. Nature. 2015;526(7574):512–513. [DOI] [PubMed] [Google Scholar]