Abstract
Cell therapy with a variety of stem populations is increasingly being investigated as a promising regenerative strategy for cardiovascular (CV) diseases. Their combination with adequate scaffolds represents an improved therapeutic approach. Recently, several biomaterials were investigated as scaffolds for CV tissue repair, with decellularized extracellular matrices (dECMs) arousing increasing interest for cardiac tissue engineering applications. The aim of this study was to analyze whether dECMs support the cardiac differentiation of CardiopoieticAF stem cells. These perinatal stem cells, which can be easily isolated without ethical or safety limitations, display a high cardiac differentiative potential. Differentiation was previously achieved by culturing them on Matrigel, but this 3D scaffold is not transplantable. The identification of a new transplantable scaffold able to support CardiopoieticAF stem cell cardiac differentiation is pivotal prior to encouraging translation of in vitro studies in animal model preclinical investigations. Our data demonstrated that decellularized extracellular matrices already used in cardiac surgery (the porcine CorTMPATCH and the equine MatrixPatchTM) can efficiently support the proliferation and cardiac differentiation of CardiopoieticAF stem cells and represent a useful cellular scaffold to be transplanted with stem cells in animal hosts.
Keywords: cardiac differentiation, cardiac tissue engineering, decellularized extracellular matrix, amniotic fluid stem cells, CardiopoieticAF cells, scaffolds, induced pluripotent stem cells, biomaterials, sarcomeric proteins, L-type calcium channels
1. Introduction
Cardiovascular (CV) diseases represent some of the main causes of mortality in worldwide [1], comprising 31% of global death [2,3,4]. The generation of fully differentiated cardiomyocytes (CMs) and cell-based tissue engineering approaches are among the goals of regenerative medicine. Cell therapy, with a variety of stem populations, is being increasingly investigated as a promising therapeutic approach to CV diseases [5,6].
The transplantation of beating CMs is expected to be more effective for serious heart diseases. The identification of the appropriate engineering processes and suitable culture substrates to induce stem cell differentiation into beating CMs is therefore pivotal [3]. Data obtained with human induced pluripotent stem cells (hiPSCs) showed that these cells can efficiently differentiate into patient-specific beating CMs in vitro [6,7,8], but their clinical use must be tempered due to their tumorigenic potential [5]. Recently, we proposed an alternative and safer stem cell source, demonstrating that human amniotic fluid cells that express the multipotency markers SSEA4, OCT4, and CD90 (CardiopoieticAF cells), if properly cultured on Matrigel® in presence of differentiative factors, can give rise to a morphologically homogeneous population of CMs that, similar to hiPSC-derived CMs, express cardiac-specific proteins and may contract spontaneously [2,9,10].
Nevertheless, even if CardiopoieticAF cells represent a promising source of CMs, the translation of their use in animal model preclinical studies is hampered by the need to identify an appropriate cellular scaffold. Indeed, despite its large use in “in vitro”, Matrigel® cannot be used in “in vivo” human studies due to its murine origins and potential tumorigenicity [11]. The realization of an appropriate scaffold for the cardiac tissue engineering presents unique challenges, since native CMs are continuously exposed to cyclic mechanical (stress and strain) and electrical stimuli that must be properly transmitted to neighboring cells. In addition to these peculiar characteristics, tissue scaffolds for heart regeneration must also comply with the general requirements for all tissue scaffolds, such as degradation time, cell–scaffold interactions, vascularization/nutrient delivery, and implantation method [12,13]. Among the biological scaffolds, xenogenic, decellularized extracellular matrix (dECM) recently aroused a great deal of interest [3,14,15,16,17,18,19] with some matrices already being used in clinical practice; in fact, the elimination of cells from the tissues significantly reduces the risk of inflammatory response and immunological rejection, while preserving the natural three-dimensional structure of the ECM.
CorTM PATCH is a decellularized, porcine, small intestinal, submucosal extracellular matrix approved by the Federal Drug Administration to repair cardiovascular injury by providing a support for host cells to repair the damaged heart [20]. Evidence shows that the CorTM PATCH is resorbed in animal models, allowing the formation of nonfibrotic connective tissue without an accompanying inflammatory response [21,22,23].
The equine Matrix PatchTM is a cell-free, equine-derived, pericardial patch routinely used in pediatric cardiac surgery, providing excellent mechanical properties, however, no calcification shrinkage has yet been observed [24].
The aim of this study was to evaluate dECM as a scaffold suitable for the adhesion, proliferation, and differentiation toward the cardiac lineage of CardiopoieticAF. For this reason, we analyzed the biological response of the CardiopoieticAF to two different dECM, the CorTM PATCH and the Matrix PatchTM., which were obtained from different animal origins (porcine and equine, respectively). The abilities of CardiopoieticAF or hiPSCs (as a positive control) to adhere, proliferate, and differentiate in CMs when seeded onto the dECM were tested in comparison with the same cells cultured in Matrigel®, a substrate matrix known to support the process but which is not transplantable.
2. Results
We first investigated the capacity of seeding and the viability and differentiation potential toward the cardiac lineage of CardiopoieticAF on the porcine CorTM PATCH and the equine Matrix PatchTM. We performed the experiments in parallel using hiPSCs as a control of differentiation ability.
CardiopoieticAF and hiPSC cells were expanded until 60% confluency and dissociated and replated on the dECMs prior to cardiac differentiation induction.
Since we could not predict how the scaffolds would affect the viability or the differentiation potential of the cells, the evaluation was carried out via two parallel experimental procedures, which are summarized in Figure 1 and described as follows:
-
(1)
The CardiopoieticAF and the hiPSCs were differentiated in a monolayer for 15 days, as previously described [2,25] either on one of the two dECMs or on the Matrigel® control;
-
(2)
The CardiopoieticAF and the hiPSCs were differentiated in a monolayer onto Matrigel® for 12 days and then dissociated and moved to the dECMs for 72 h before analysis.
Figure 1.
Scheme of the experimental design: (1) The CardiopoieticAF and the human induced pluripotent stem cells (hiPSCs) were differentiated in a monolayer for 15 days either on the Matrix PatchTM,the CorTM PATCH, or on the Matrigel® control; (2) the CardiopoieticAF and the hiPSCs were differentiated in monolayer onto THE Matrigel® for 12 days and then dissociated and moved to the Matrix PatchTM or CorTM PATCH for 72 h.
In the first experimental condition, both the undifferentiated hiPSCs and the CardiopoieticAF engrafted and proliferated easily on the dECMs and, when induced to differentiate, the viability was always comparable to the control cells differentiated on the Matrigel®.
Although a slight foreseeable drop in cell numbers was observed when the cells were cultured under cardiac permissive induction conditions (24 h of BMP4 and ActA followed by 72h of VEGF stimulation for mesodermal and cardiac induction, respectively), the cell vitality remained high (>82 ± 5% in hiPSCs and >76 ± 4% in CardiopoieticAF) after 15 days of differentiation in all experimental conditions.
In contrast, the second experimental condition did not give the same encouraging results; only a small percentage of hiPSC- and CardiopoieticAF-derived CMs were able to attach to the dECMs once dissociated and no viable cells were present on the matrices after 72 h. Dissociated CardiopoieticAF-derived CMs were also unable to adhere and proliferate when reseeded on the Matrigel® control (Figure 2). These observations suggest that both hiPSCs the CardiopoieticAF-derived CMs are deeply threatened by enzymatic dissociation, hampering their abilities to restore their cell–matrix and cell–cell interactions, thereby affecting their viability.
Figure 2.
Percentage of hiPSCs and CardiopoieticAF viability, obtained by 3(4,5-Dimethylthiazol-2yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) assay performed under proliferation conditions once cells reached 60% confluency and under differentiation conditions at day 0, 4, 12, and 15 in all experimental conditions, as indicated. The graphs show the mean ± SD of five independent experiments; * p < 0.05.
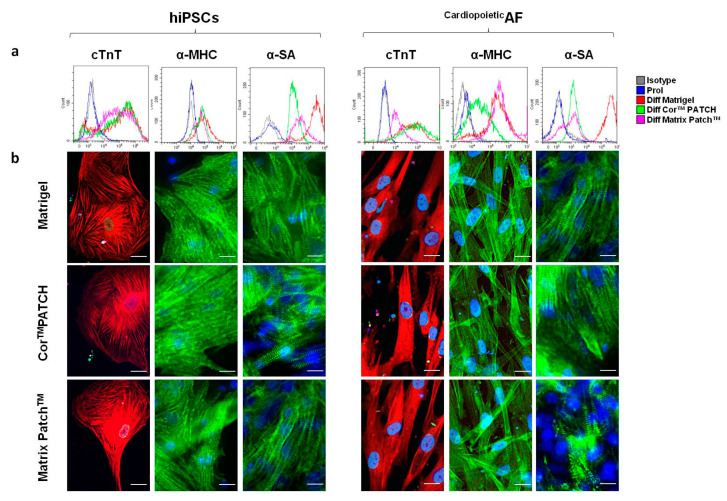
After 15 days of differentiation culture following the first experimental condition either on the dECMs or on the Matrigel® internal control, we analyzed hiPSC- and CardiopoieticAF-derived CM expression of cTnT, α-MHC, and α-SA, specific sarcomeric proteins recognized as “late” cardiac markers. Sarcomeric proteins represent the structural building blocks of heart muscle, which are essential for contraction and relaxation [26]. Interestingly, differentiated cells obtained from both hiPSCs and CardiopoieticAF expressed high levels of cTnT, α-MHC, and α-SA, comparable in all the experimental conditions (Figure 3 and Table 1). In particular, the percentage and fluorescence intensity of the analyzed proteins were stackable for cTnT and a-MHC in hiPSCs and CardiopoieticAF cells cultured in the dECMs and Matrigel®, while α-SA of CardiopoieticAF cultured in the Matrix PatchTM was less expressed, although still at a high percentage, than the same cells differentiated in Matrigel®. α-SA of the hiPSCs and CardiopoieticAF cells cultured in the CorTM PATCH remaiedn comparable to those differentiated in Matrigel®, although slightly less expressed (Table 1 and Figure 3). Nevertheless, immunofluorescence images displayed clearly that the sarcomeric striations typical of α-SA and cTnT localization were present in all culture conditions, indicating that both dECMs are permissive to cardiac differentiation (Table 1 and Figure 3).
Figure 3.
Detection of cTNT, α-MHC, and α-SA in hiPSC- and CardiopoieticAF-derived cardiomyocytes (CMs), obtained after 15 days of differentiation using CoreTM PATCH, Matrix PatchTM, and Matigel, as indicated. (a) Flow cytometry analysis of TnT, α-MHC, and α-SA in hIPSCs and CardiopoieticAF cells, as indicated. Legend of histogram colors: Grey: isotype control; blue: proliferating cells (undifferentiated); red: differentiation on Matrigel®; green: differentiation on CorTM Patch; purple: differentiation on Matrix PatchTM. (b) Immunofluorescent staining for cTnT (red), α-MHC (green), and α-SA (green) in hIPSCs and CardiopoieticAF cells on CoreTM PATCH, Matrix PatchTM, or Matrigel®, as indicated. The nuclei were counterstained with DAPI (blue). Scale bar: 10 μm. Data are representative of five independent experiments.
Table 1.
Percentage of cells positive for cTnT, α-MHC, α-SA, CACNA1C, and SERCA2 in hiPSC- and CardiopoieticAF-derived CMs after 15 days of differentiation.
| cTnT | α-MHC | α-SA | CACNA1C | SERCA2 | |
|---|---|---|---|---|---|
| hiPSCs | |||||
| Prol | 1.0 ± 0.5 | 1.2 ± 0.5 | 1.1 ± 1.1 | 6.5 ± 5.8 | 26.6 ± 9.5 |
| Diff Matrigel® | 83.5 ± 6.6 * | 83.4 ± 3.1 * | 95.3 ± 3.1 * | 79.9 ± 6.8 * | 93.4 ± 8.6 * |
| Diff CorTM PATCH | 88.1 ± 11.1 * | 69.2 ± 9.9 * | 88.5 ± 7.3 * | 76.2 ± 3.1 * | 83.6 ± 7.3 * |
| Diff Matrix PatchTM | 86.2 ± 5.6 * | 81.4 ± 7.5 * | 81.5 ± 6.4 *& | 75.9 ± 6.2 * | 86.1 ± 7.2 * |
| CardiopoieticAF | |||||
| Prol | 2.2 ± 2.1 | 1.1 ± 0.5 | 4.1 ± 2.1 | 12.5 ± 8.8 | 45.1 ± 12.5 |
| Diff Matrigel® | 85.5 ± 5.6 * | 88.4 ± 8.1 * | 89.3 ± 7.9 * | 88.9 ± 5.9 * | 83.5 ± 6.8 * |
| Diff CorTM PATCH | 78.6 ± 9.6 * | 79.5 ± 6.9 * | 78.5 ± 7.4 * | 96.2 ± 4.1 * | 84.5 ± 9.3 * |
| Diff Matrix PatchTM | 75.2 ± 6.6 * | 74.4 ± 8.8 * | 66.5 ± 9.4 *& | 92.4 ± 7.2 * | 83.5 ± 6.1 * |
* p < 0.01 relative to cells under proliferation conditions (Prol); & p < 0.05 relative to cells differentiated in Matrigel (Diff Matrigel).
Flow cytometry and immunofluorescence revealed the dramatic induction of the L-type calcium channel CACNA1C and SERCA2 proteins, two calcium pumps essential for excitation–contraction coupling that reside in the sarcolemma and sarcoplasmic reticulum of CMs, respectively (Table 1 and Figure 4a). In the hiPSCs and CardiopoieticAF cells differentiated in the dECMs and in Matrigel®, CACNA1C was localized mainly in the perinuclear area and marked the cytoplasmic membrane in some cells, probably indicating a homing of the protein in the sarcolemma of more mature CMs. On the other hand, SERCA2 immunolabeling showed the typical reticulated organization of the sarcoplasmic reticulum (Figure 4b).
Figure 4.
Detection of calcium pumps CACNA1C and SERCA2 in hiPSC- and CardiopoieticAF-derived CMs obtained after 15 days of differentiation. (a) Flow cytometry analysis of CACNA1C and SERCA2 on hIPSCs and CardiopoieticAF cells, as indicated. Legend of histogram colors: Grey: isotype control; blue: proliferating cells; red: differentiation on Matrigel®; green: differentiation on CorTM Patch; purple: differentiation on Matrix PatchTM. (b) Immunofluorescent staining for CACNA1C and SERCA2 (green) in hiPSCs and CardiopoieticAF cells, as indicated. Nuclei were counterstained with DAPI (blue). Scale bar: 10 μm. Data are representative of five independent experiments.
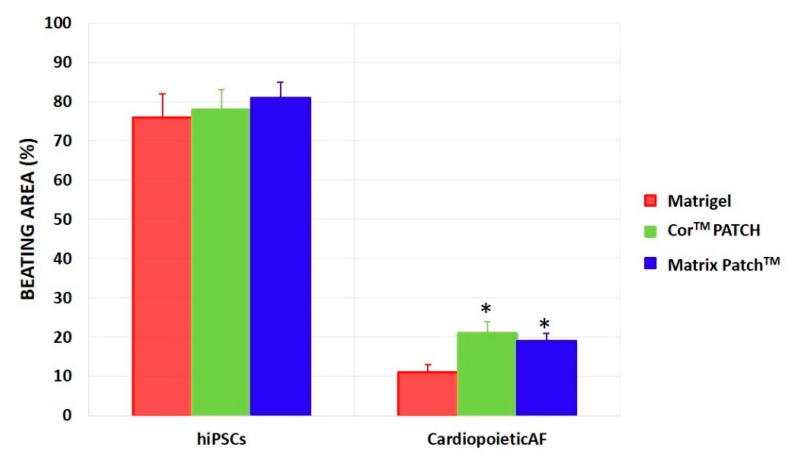
Finally, the beating colonies appeared from 8 days of culture and increased until 15 days on almost all of the substrates. hiPSC- and CardiopoieticAF-derived CMs differentiated in both dECMs showed percentages of spontaneous beating foci comparable to or even higher than the same cells differentiated in Matrigel (Figure 5), demonstrating once again that both the CorTM PATCH and Matrix PatchTM could be considered suitable scaffolds for the adhesion and the proliferation of stem cells from different sources and permissive for differentiation toward cardiac lineage.
Figure 5.
Percentage of dish area of hiPSC- and CardiopoieticAF-derived CMs that presented spontaneous beating foci after 15 days of differentiation in Matrigel® (red), CorTM PATCH (green), or Matrix PatchTM (blue). hiPSC-derived CMs were lower compared to CardiopoieticAF-derived CMs differentiated on the CorTM PATCH and the Matrix PatchTM, compared to Matrigel®. The graphs show the mean ± SD of five independent experiments; * indicates statistically differences (p < 0.05) of the CorTM PATCH or the Matrix PatchTM culture vs. the same cells cultured in Matrigel®.
3. Discussion
The ideal goal of stem cell therapy is to substitute necrotic or dysfunctional cardiac tissue with new competent CMs derived from stem cells [27,28]. Currently, the only cells with proven promising results regarding cardiogenic potential are hiPSCs. The possibility to obtain functionally mature CMs from patient-specific hiPSCs is a big challenge for clinical use in tailored cell therapy [5,29,30,31,32,33], with their epigenetic instability still considered an unsolved issue [10]. The recent discovery that CardiopoieticAF could circumvent any ethical and safety concerns and provide effective cell replacement therapy for cardiac diseases, particularly in congenital heart defect repair applications, [2,34,35] opened the door to a new scenario.
For the first time to our knowledge, CardiopoieticAF (a new and safe source of stem cells) were demonstrated to be able to proliferate on dECMs and generate CMs with high efficiency in this work. In line with the literature [7,36] cardiac differentiation from hiPSCs and CardiopoieticAF cells was observed to be optimal in terms of both efficacy and efficiency when cultured in a monolayer on Matrigel®, a 3D matrix known to support cells in “in vitro” studies, but is unfortunately not transplantable for safety reasons [37]. In this study, we replaced the Matrigel® with dECMs and investigated the biological response of CardiopoieticAF cells to two engineered patches, the pericardial CorTM PATCH and the Matrix PatchTM. Both technologies are decellularized membranes, one derived from porcine intestinal submucosa, the other from equine pericardium, which are commonly used in clinical practice, mainly as patches to restore valves and vascular tissue in need of repair; when resorbed, they allow the formation of nonfibrotic connective tissue. Neither were previously analyzed as possible transplantable scaffolds for CMs generated “in vitro”.
dECMs were widely explored as natural scaffolds for cardiac tissue engineering applications because they offer many unique advantages, such as preservation of organ-specific ECM microstructure and composition, retention of tissue-mimetic mechanical properties, and biochemical cues in favor of subsequent recellularization [12,38,39]. In this study, we exploited two dECMs from small intestinal submucosa and bovine-derived pericardium that were previously successfully tested in clinical studies [40] dECM scaffolds can be implanted during cardiac surgery intervention onto injured myocardial regions since they offer a structural support to the damaged myocardium and stimulate endogenous mechanisms of tissue repair, such as vasculogenesis [40]. Our data suggest their potential use as biological scaffolds to support CardiopoieticAF cell proliferation and cardiac differentiation, with the ideal goal of implantation at the site of the infarction to restore tissue function. Interestingly, dECMs derived from both cardiac and intestinal tissues showed similar results, with no substantial differences in cellular adhesion or differentiation efficiency.
Another important observation was that both hiPSCs and CardiopoieticAF needed to be cultured from the beginning on the dECMs (first experimental condition of this manuscript) in order to guarantee proper engrafting and to maintain their differentiation features. This was unsurprising, as previous studies showed that most of the murine embryonic stem cell (mESC)-derived CMs failed to attach to a variety of polymer substrates at the end of the differentiation process. It is therefore probable that CMs need a more complex environment than 2D or 3D scaffolds enriched in cardioprotective factors to attach and survive after dissociation [41]. In addition, Van Deel et al. showed that the commonly used enzymatic detaching methods, such as Trypsin, Detachin, and Accutase, are lethal to adult CMs, altering the integrity of the cells [42]. Thus, the failure of CMs obtained from hiPSCs and CardiopoieticAF cells to attach onto dECMs was probably due to the fact that, after enzymatic digestion, the CMs were not able to recreate cell–cell and cell–scaffold connections. However, further studies are necessary to address this point.
CardiopoieticAF stem cells attached to and proliferated easily on the two engineered scaffolds, and, if induced to differentiate, gave rise to CMs that showed typical sarcomere striation, expressed mature CMs hallmark proteins (α-AS, α-MHC, and cTnT) [5,43] and showed strong CACNA1 and SERCA2 expression, two L-type calcium channels essential for the so-called “Ca2+-induced Ca2+ release”, the mechanism crucial for excitation–contraction coupling in mammalian cardiac muscle. CardiopoieticAF-derived CMs cultured on dECMs showed a proper homogeneous cardiac phenotype [43] and expressed cardiac-specific functional markers comparable to those obtained using the Matrigel® culture.
Finally, hiPSC-derived CMs differentiated using both dECMs showed percentages of spontaneous beating foci comparable to the same cells differentiated using Matrigel®, while the percentage of the contracting area in CardiopoieticAF-derived CMs was surprisingly significantly higher in both the CorTM PATCH and Matrix PatchTM than in the control cells. Even if this percentage never exceeded 30% of the total area, it was still a promising result, since very few groups were able to obtain beating CMs from amniotic stem cells without prior reprogramming [2,44,45]. This was possibly because, as expected [5,46,47] CMs with different levels of maturity were obtained, and a scaffold with a rigid elasticity niche may strongly promote beating induction, in line with what was reported by Hirata and colleagues [48].
In conclusion, these data confirm the efficacy of both the analyzed dECMs as cell carriers for ensuring phenotypic and functional CardiopoieticAF-derived CMs, driving new challenges for producing biomimetic cardiac patches in tailored regenerative medicine.
The main limitation of this study was the lack of specific analysis of the calcium-handling capabilities of CardiopoieticAF-derived CMs differentiated using the CorTM PATCH and the Matrix PatchTM. More efforts are needed to characterize the electrophysiological properties and to promote of the homogeneity of CardiopoieticAF-derived CMs prior to encouraging their transplantation in animal models.
4. Materials and Methods
4.1. Isolation and Culture of CardiopoieticAF and hiPSC Cells
AF samples were obtained from 8 women undergoing amniocentesis for prenatal diagnosis at 16–17 weeks of pregnancy after written informed consent, in accordance with the Declaration of Helsinki. All samples presented normal diploid male karyotypes, as evidenced by cytogenetic investigation. The mean (±SD) maternal age at amniocentesis was 37.9 ± 2.6 years. The study was approved by the local ethics committee and all experiments were performed in accordance with relevant guidelines and regulations.
CardiopoieticAF cells were isolated as previously described on the basis of the phenotypic pattern [2] cultured in Iscove’s modified Dulbecco’s medium (IMDM, Lonza Group Ltd., Basel, Switzerland), supplemented with 20% fetal bovine serum (FBS, GE Healthcare HyClone Defined Fetal Bovine Serum, Buckinghamshire, UK), 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mM L-glutamine, (all Sigma-Aldrich, Saint Louis, MO, USA), and 5 ng/mL basic fibroblast growth factor (bFGF, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), and incubated at 37 °C with 5% humidified CO2.
hiPSCs [49] were cultured on a monolayer of irradiated murine fibroblasts (Thermo Fischer Scientific, Waltham, MA, USA) in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM/F12) supplemented with 20% KnockOut Serum Replacement (KO) (Thermo Fischer Scientific, Waltham, MA, USA), 1% penicillin/streptomycin (Thermo Fischer Scientific, Waltham, MA, USA), 2 mM L-glutamine (Corning, New York, NY, USA), 5 ng/mL basic fibroblast growth factor (bFGF) (Thermo Fischer Scientific, Waltham, MA, USA), and 1% MEM nonessential amino acid (Corning, New York, NY, USA).
4.2. Culture in Monolayer and Cardiac Differentiation
CardiopoieticAF and hiPSCs were seeded either onto Matrigel® (Corning, Flintshire, UK)-coated plates or onto two dECMs ((CorTM PATCH (CorMatrix, Roswell, GA, USA) or Matrix PatchTM (Auto Tissue Berlin GmbH)) using two methods: a) The cells were seeded onto the scaffold before starting the differentiation protocol and were kept on the dECMs for the duration of the process, followed by analysis; b) the cells were differentiated onto Matrigel® and after 12 days of differentiation were detached using 0.025% Tryspin-EDTA (Invitrogen Carlsband, CA, USA) and distributed on each dECM or back to the Matrigel® control. Sample were cultured in differentiation conditions for 72 h and subsequently analyzed. The cells were cultured onto the dECM as described above, then, when 80–90% confluence was reached, the medium was changed to RPMI 1640 (Thermo Fischer Scientific, Waltham, MA, USA) supplemented with B-27 minus insulin (GIBCO, Thermo Fischer Scientific, Waltham, MA, USA), 50 μg/mL ascorbic acid (Sigma-Aldrich, Saint Louis, MO, USA) and 10 μM 5-aza-2′-deoxycytidine (5-Aza), and differentiation was induced by exposure for 24 h to activin A (50 ng/mL, R&D Systems, Minneapolis, MN, USA) and BMP4 (25 ng/mL, R&D Systems, Minneapolis, MN, USA), followed by treatment for 72 h with VEGF (10 ng/mL, R&D Systems, Minneapolis, MN, USA). Cells were analyzed after 15 days of differentiation. Beating foci were observed and quantified microscopically as the percentage of the beating area inside the whole plate [50,51].
4.3. MTT(3(4,5-Dimethylthiazol-2yl)-2,5-Diphenyl Tetrazolium Bromide) Assay
Cell viability and metabolic activity were measured using an MTT (3(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide) growth assay (Sigma-Aldrich, Saint Louis, MO, USA), as previously described [52]. The cultured medium was removed and washed once with 1X PBS. MTT solution (0.5 mg/mL; Sigma-Aldrich, Saint Louis, MO, USA) was added into each well and incubated for 4 h at 37 °C for formazan crystal development. Formazan crystals were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Saint Louis, MO, USA) and read at 540 nm in a microplate reader.
The values obtained in the absence of cells were considered to be background and subtracted from the optical density values of the samples. Five independent experiments were performed under the same experimental conditions.
4.4. Flow Cytometry and Imaging Flow Cytometry
For flow cytometry and imaging flow cytometry, cells were treated with the FIX and PERM® Kit (Thermo Fischer Scientific, Waltham, MA, USA) and incubated for 1 h at RT with anti-α-myosin heavy chain (α-MHC; 1:100; Abcam, Cambridge, UK), anti-cardiac troponin T (cTnT, 1:100; Abcam, Cambridge, UK), anti-α-sarcomeric actin (α-SA; 1:100; Abcam, Cambridge, UK), anti-sarcolemmal Ca2+ channel (CACNA1C, 1:100; Abcam, Cambridge, UK), and anti-sarcoendoplasmic reticulum Ca2+ ATPase protein (Serca2, 1:100; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), followed by incubation with the appropriate secondary antibody conjugated with Alexa Fluor 488, PE-Alexa Fluor 647, or PE-Cy7 (1:200; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), as previously reported [53]. Cells incubated with isotypes (all from BD) were used as negative controls [54].
Cytometric analysis was performed with a Cytoflex cytometer (Beckman Coulter, Brea, CA, USA) and data were analyzed using FlowJo (TreeStar, Ashland, OR, USA) or CytExpert Acquisition and Analysis Software (Beckman Coulter, Brea, CA, USA).
4.5. Immunofluorescence Staining and Microscopy
Cells fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 were incubated with anti-cTnT (1:100, Abcam, Cambridge, UK), anti-α-MHC (1:100, Abcam, Cambridge, UK)), anti-α-SA (1:100, Abcam, Cambridge, UK), anti-CACNA1C (1:100, Abcam, Cambridge, UK), and anti-SERCA2 (1:100; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), followed by the appropriate secondary antibody conjugated with Alexa Fluor 488 or PE (1:200; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), as previously reported [51]. Nuclei were counterstained with DAPI (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Samples treated only with the secondary antibody were used as controls. Images were acquired using an Axio Vert A1 microscope. Images were analyzed by ZEN 2009 (Carl Zeiss, Oberkochen, Germany).
4.6. Statistical Analysis
All quantitative data were presented as the mean ± SD. Statistical comparisons were performed using one-way analysis of variance (ANOVA) and Student’s t-test [55]. The level of significance was set at p < 0.05.
Author Contributions
Conceptualization, B.G. and A.D.B.; methodology, G.G. and A.D.C. and G.I.; software, A.D.C. and P.I.; formal analysis, M.D.M. and S.D.; investigation, G.G. and G.I.; resources, B.G., M.D.M., G.A., and A.D.B; data curation, G.A., S.S., and P.I; writing—original draft preparation, B.G. and A.D.B.; writing—review and editing, B.G. and A.D.B; supervision, B.G. and A.D.B.; project administration, B.G.; funding acquisition, B.G. and A.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Education, University and Research (Ministero dell’Università e della Ricerca-MUR), grant numbers SIR2014-RBSI140GLQ and PRIN 2017ATZ2YK.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bleumink G., Knetsch A., Sturkenboom M., Straus S., Hofman A., Deckers J., Witteman J., Stricker B. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure: The Rotterdam Study. Eur. Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Di Baldassarre A., D’Amico M.A., Izzicupo P., Gaggi G., Guarnieri S., Mariggiò M.A., Antonucci I., Corneo B., Sirabella D., Stuppia L., et al. Cardiomyocytes Derived from Human CardiopoieticAmniotic Fluids. Sci. Rep. 2018;8:12028. doi: 10.1038/s41598-018-30537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iop L., Palmosi T., Dal Sasso E., Gerosa G. Bioengineered tissue solutions for repair, correction and reconstruction in cardiovascular surgery. J. Thorac. Dis. 2018;10:S2390–S2411. doi: 10.21037/jtd.2018.04.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.-P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the spec. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 5.Di Baldassarre A., Cimetta E., Bollini S., Gaggi G., Ghinassi B. Human-Induced Pluripotent Stem Cell Technology and Cardiomyocyte Generation: Progress and Clinical Applications. Cells. 2018;7:48. doi: 10.3390/cells7060048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filardi T., Ghinassi B., Di Baldassarre A., Tanzilli G., Morano S., Lenzi A., Basili S., Crescioli C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019;20:3299. doi: 10.3390/ijms20133299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burridge P.W., Keller G., Gold J.D., Wu J.C. Production of De Novo Cardiomyocytes: Human Pluripotent Stem Cell Differentiation and Direct Reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonucci I., Di Pietro R., Alfonsi M., Centurione M.A., Centurione L., Sancilio S., Pelagatti F., D’amico M.A., Di Baldassarre A., Piattelli A., et al. Human Second Trimester Amniotic Fluid Cells are Able to Create Embryoid Body-Like Structures in Vitro and to Show Typical Expression Profiles of Embryonic and Primordial Germ Cells. Cell Transplant. 2014;23:1501–1515. doi: 10.3727/096368914X678553. [DOI] [PubMed] [Google Scholar]
- 10.Gaggi G., Di Credico A., Izzicupo P., Antonucci I., Crescioli C., Di Giacomo V., Di Ruscio A., Amabile G., Alviano F., Di Baldassarre A., et al. Epigenetic Features of Human Perinatal Stem Cells Redefine Their Stemness Potential. Cells. 2020;9:1304. doi: 10.3390/cells9051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conboy I., Freimer J., Weisenstein L., Liu Y., Mehdipour M., Gathwala R. Comprehensive Biomaterials II. Elsevier; Amsterdam, The Netherlands: 2017. 6.13 Tissue Engineering of Muscle Tissue ☆; pp. 216–235. [Google Scholar]
- 12.Kc P., Hong Y., Zhang G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019;6:185–199. doi: 10.1093/rb/rbz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajabi S., Aghdami N., Varzideh F., Parchehbaf-Kashani M., Nobakht Lahrood F. Decellularized muscle-derived hydrogels support in vitro cardiac microtissue fabrication. J. Biomed. Mater. Res. B Appl. Biomater. 2020 doi: 10.1002/jbm.b.34666. [DOI] [PubMed] [Google Scholar]
- 14.Masumoto H., Ikuno T., Takeda M., Fukushima H., Marui A., Katayama S., Shimizu T., Ikeda T., Okano T., Sakata R., et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 2015;4:6716. doi: 10.1038/srep06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perea-Gil I., Gálvez-Montón C., Prat-Vidal C., Jorba I., Segú-Vergés C., Roura S., Soler-Botija C., Iborra-Egea O., Revuelta-López E., Fernández M.A., et al. Head-to-head comparison of two engineered cardiac grafts for myocardial repair: From scaffold characterization to pre-clinical testing. Sci. Rep. 2018;8:6708. doi: 10.1038/s41598-018-25115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdi H., Planat-Benard V., Bel A., Neamatalla H., Saccenti L., Calderon D., Bellamy V., Bon M., Perrier M.-C., Mandet C., et al. Long-Term Functional Benefits of Epicardial Patches as Cell Carriers. Cell Transplant. 2014;23:87–96. doi: 10.3727/096368912X658836. [DOI] [PubMed] [Google Scholar]
- 17.Di Mauro M., Gallina S., D’Amico M.A., Izzicupo P., Lanuti P., Bascelli A., Di Fonso A., Bartoloni G., Calafiore A.M., Di Baldassarre A. Functional mitral regurgitation. Int. J. Cardiol. 2013;163:242–248. doi: 10.1016/j.ijcard.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Parveen S., Singh S.P., Panicker M.M., Gupta P.K. Amniotic membrane as novel scaffold for human iPSC-derived cardiomyogenesis. Vitro Cell. Dev. Biol. Anim. 2019;55:272–284. doi: 10.1007/s11626-019-00321-y. [DOI] [PubMed] [Google Scholar]
- 19.Liguori G.R., Liguori T.T.A., de Moraes S.R., Sinkunas V., Terlizzi V., van Dongen J.A., Sharma P.K., Moreira L.F.P., Harmsen M.C. Molecular and Biomechanical Clues From Cardiac Tissue Decellularized Extracellular Matrix Drive Stromal Cell Plasticity. Front. Bioeng. Biotechnol. 2020;8:520. doi: 10.3389/fbioe.2020.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo J.S., Fishbein M.C., Reemtsen B. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc. Pathol. 2016;25:12–17. doi: 10.1016/j.carpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Zaidi A.H., Nathan M., Emani S., Baird C., del Nido P.J., Gauvreau K., Harris M., Sanders S.P., Padera R.F. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: Histologic evaluation of explanted valves. J. Thorac. Cardiovasc. Surg. 2014;148:2216–2225.e1. doi: 10.1016/j.jtcvs.2014.02.081. [DOI] [PubMed] [Google Scholar]
- 22.Stelly M., Stelly T.C. Histology of CorMatrix Bioscaffold 5 Years after Pericardial Closure. Ann. Thorac. Surg. 2013;96:e127–e129. doi: 10.1016/j.athoracsur.2013.06.114. [DOI] [PubMed] [Google Scholar]
- 23.Di Mauro M., Ghinassi B., Di Baldassarre A. Commentary: I fix what’s broken - inclusive the heart. J. Thorac. Cardiovasc. Surg. 2020:S0022522320317669. doi: 10.1016/j.jtcvs.2020.05.116. [DOI] [PubMed] [Google Scholar]
- 24.Dohmen P.M., da Costa F., Lopes S.V., Vilani R., Bloch O., Konertz W. Successful implantation of a decellularized equine pericardial patch into the systemic circulation. Med. Sci. Monit. Basic Res. 2014;20:1–8. doi: 10.12659/MSMBR.889915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson D.M., Ma Z., Fujimoto K.L., Hashizume R., Wagner W.R. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Velden J., Stienen G.J.M. Cardiac Disorders and Pathophysiology of Sarcomeric Proteins. Physiol. Rev. 2019;99:381–426. doi: 10.1152/physrev.00040.2017. [DOI] [PubMed] [Google Scholar]
- 27.Levenberg S., Huang N.F., Lavik E., Rogers A.B., Itskovitz-Eldor J., Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calafiore A.M., Lorusso R., Kheirallah H., Alsaied M.M., Alfonso J.J., Di Baldassarre A., Gallina S., Gaudino M., Di Mauro M. Late tricuspid regurgitation and right ventricular remodeling after tricuspid annuloplasty. J. Card. Surg. 2020;35:1891–1900. doi: 10.1111/jocs.14840. [DOI] [PubMed] [Google Scholar]
- 29.Lian X., Hsiao C., Wilson G., Zhu K., Hazeltine L.B., Azarin S.M., Raval K.K., Zhang J., Kamp T.J., Palecek S.P. Cozzarelli Prize Winner: Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burridge P.W., Matsa E., Shukla P., Lin Z.C., Churko J.M., Ebert A.D., Lan F., Diecke S., Huber B., Mordwinkin N.M., et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida Y., Yamanaka S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ. Res. 2017;120:1958–1968. doi: 10.1161/CIRCRESAHA.117.311080. [DOI] [PubMed] [Google Scholar]
- 32.Kanda Y., Yamazaki D., Osada T., Yoshinaga T., Sawada K. Development of torsadogenic risk assessment using human induced pluripotent stem cell-derived cardiomyocytes: Japan iPS Cardiac Safety Assessment (JiCSA) update. J. Pharmacol. Sci. 2018;138:233–239. doi: 10.1016/j.jphs.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Izzicupo P., Ghinassi B., D’Amico M.A., Di Blasio A., Gesi M., Napolitano G., Gallina S., Di Baldassarre A. Effects of ACE I/D Polymorphism and Aerobic Training on the Immune–Endocrine Network and Cardiovascular Parameters of Postmenopausal Women. J. Clin. Endocrinol. Metab. 2013;98:4187–4194. doi: 10.1210/jc.2013-2305. [DOI] [PubMed] [Google Scholar]
- 34.Guan X., Delo D.M., Atala A., Soker S. In vitro cardiomyogenic potential of human amniotic fluid stem cells. J. Tissue Eng. Regen. Med. 2011;5:220–228. doi: 10.1002/term.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cananzi M., De Coppi P. CD117 + amniotic fluid stem cells: State of the art and future perspectives. Organogenesis. 2012;8:77–88. doi: 10.4161/org.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S., et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 37.Albini A. Extracellular Matrix Invasion in Metastases and Angiogenesis: Commentary on the Matrigel “Chemoinvasion Assay”. Cancer Res. 2016;76:4595–4597. doi: 10.1158/0008-5472.CAN-16-1971. [DOI] [PubMed] [Google Scholar]
- 38.Arumugam R., Srinadhu E.S., Subramanian B., Nallani S. β-PVDF based electrospun nanofibers—A promising material for developing cardiac patches. Med. Hypotheses. 2019;122:31–34. doi: 10.1016/j.mehy.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Rajabi S., Pahlavan S., Ashtiani M.K., Ansari H., Abbasalizadeh S., Sayahpour F.A., Varzideh F., Kostin S., Aghdami N., Braun T., et al. Human embryonic stem cell-derived cardiovascular progenitor cells efficiently colonize in bFGF-tethered natural matrix to construct contracting humanized rat hearts. Biomaterials. 2018;154:99–112. doi: 10.1016/j.biomaterials.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 40.Pattar S.S., Fatehi Hassanabad A., Fedak P.W.M. Acellular Extracellular Matrix Bioscaffolds for Cardiac Repair and Regeneration. Front. Cell Dev. Biol. 2019;7:63. doi: 10.3389/fcell.2019.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfannkuche K., Neuss S., Pillekamp F., Frenzel L.P., Attia W., Hannes T., Salber J., Hoss M., Zenke M., Fleischmann B.K., et al. Fibroblasts Facilitate the Engraftment of Embryonic Stem Cell-Derived Cardiomyocytes on Three-Dimensional Collagen Matrices and Aggregation in Hanging Drops. Stem Cells Dev. 2010;19:1589–1599. doi: 10.1089/scd.2009.0255. [DOI] [PubMed] [Google Scholar]
- 42.van Deel E.D., Najafi A., Fontoura D., Valent E., Goebel M., Kardux K., Falcão-Pires I., van der Velden J. In vitro model to study the effects of matrix stiffening on Ca 2+ handling and myofilament function in isolated adult rat cardiomyocytes: Matrix stiffening alters cardiomyocyte function. J. Physiol. 2017;595:4597–4610. doi: 10.1113/JP274460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y.-K., Ng K.-M., Lai W.-H., Chan Y.-C., Lau Y.-M., Lian Q., Tse H.-F., Siu C.-W. Calcium Homeostasis in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cell Rev. Rep. 2011;7:976–986. doi: 10.1007/s12015-011-9273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang G., Herron T.J., Di Bernardo J., Walker K.A., O’Shea K.S., Kunisaki S.M. Human Cardiomyocytes Prior to Birth by Integration-Free Reprogramming of Amniotic Fluid Cells: Amniotic Cardiomyocytes. STEM CELLS Transl. Med. 2016;5:1595–1606. doi: 10.5966/sctm.2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moschidou D., Mukherjee S., Blundell M.P., Jones G.N., Atala A.J., Thrasher A.J., Fisk N.M., De Coppi P., Guillot P.V. Human Mid-Trimester Amniotic Fluid Stem Cells Cultured Under Embryonic Stem Cell Conditions with Valproic Acid Acquire Pluripotent Characteristics. Stem Cells Dev. 2013;22:444–458. doi: 10.1089/scd.2012.0267. [DOI] [PubMed] [Google Scholar]
- 46.Yang X., Pabon L., Murry C.E. Engineering Adolescence: Maturation of Human Pluripotent Stem Cell–Derived Cardiomyocytes. Circ. Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izzicupo P., Di Valerio V., D’Amico M.A., Di Mauro M., Pennelli A., Falone S., Alberti G., Amicarelli F., Miscia S., Gallina S., et al. Nad(P)H Oxidase and Pro-Inflammatory Response during Maximal Exercise: Role of C242T Polymorphism of the P22PHOX Subunit. Int. J. Immunopathol. Pharmacol. 2010;23:203–211. doi: 10.1177/039463201002300118. [DOI] [PubMed] [Google Scholar]
- 48.Hirata M., Yamaoka T. Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages. Acta Biomater. 2018;65:44–52. doi: 10.1016/j.actbio.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Amabile G., Welner R.S., Nombela-Arrieta C., D’Alise A.M., Di Ruscio A., Ebralidze A.K., Kraytsberg Y., Ye M., Kocher O., Neuberg D.S., et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prinz R.D., Willis C.M., van Kuppevelt T.H., Klüppel M. Biphasic Role of Chondroitin Sulfate in Cardiac Differentiation of Embryonic Stem Cells through Inhibition of Wnt/β-Catenin Signaling. PLoS ONE. 2014;9:e92381. doi: 10.1371/journal.pone.0092381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’amico M.A., Ghinassi B., Izzicupo P., Di Ruscio A., Di Baldassarre A. IL-6 Activates PI3K and PKCζ Signaling and Determines Cardiac Differentiation in Rat Embryonic H9c2 Cells: IL-6 AND CARDIAC DIFFERENTIATION OF H9c2 CELLS. J. Cell. Physiol. 2016;231:576–586. doi: 10.1002/jcp.25101. [DOI] [PubMed] [Google Scholar]
- 52.Rapino M., Di Valerio V., Zara S., Gallorini M., Marconi G.D., Sancilio S., Marsich E., Ghinassi B., di Giacomo V., Cataldi A. Chitlac-coated Thermosets Enhance Osteogenesis and Angiogenesis in a Co-culture of Dental Pulp Stem Cells and Endothelial Cells. Nanomaterials. 2019;9:928. doi: 10.3390/nano9070928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martelli F., Ghinassi B., Lorenzini R., Vannucchi A.M., Rana R.A., Nishikawa M., Partamian S., Migliaccio G., Migliaccio A.R. Thrombopoietin Inhibits Murine Mast Cell Differentiation. Stem Cells. 2008;26:912–919. doi: 10.1634/stemcells.2007-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghinassi B., Ferro L., Masiello F., Tirelli V., Sanchez M., Migliaccio G., Whitsett C., Kachala S., Riviere I., Sadelain M., et al. Recovery and Biodistribution of Ex Vivo Expanded Human Erythroblasts Injected into NOD/SCID/IL2R γ null mice. Stem Cells Int. 2011;2011:1–13. doi: 10.4061/2011/673752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Migliaccio A.R., Martelli F., Verrucci M., Sanchez M., Valeri M., Migliaccio G., Vannucchi A.M., Zingariello M., Di Baldassarre A., Ghinassi B., et al. Gata1 expression driven by the alternative HS2 enhancer in the spleen rescues the hematopoietic failure induced by the hypomorphic Gata1low mutation. Blood. 2009;114:2107–2120. doi: 10.1182/blood-2009-03-211680. [DOI] [PMC free article] [PubMed] [Google Scholar]