Abstract
Trypanosoma cruzi, the protozoa that causes Chagas disease in humans, is transmitted by insects from the Reduviidae family. The parasite has developed the ability to change the structure of the surface molecules, depending on the host. Among them, the mucins are the most abundant glycoproteins. Structural studies have focused on the epimastigotes and metacyclic trypomastigotes that colonize the insect, and on the mammal trypomastigotes. The carbohydrate in the mucins fulfills crucial functions, the most important of which being the accepting of sialic acid from the host, a process catalyzed by the unique parasite trans-sialidase. The sialylation of the parasite influences the immune response on infection. The O-linked sugars have characteristics that differentiate them from human mucins. One of them is the linkage to the polypeptide chain by the hexosamine, GlcNAc, instead of GalNAc. The main monosaccharide in the mucins oligosaccharides is galactose, and this may be present in three configurations. Whereas β-d-galactopyranose (β-Galp) was found in the insect and the human stages of Trypanosoma cruzi, β-d-galactofuranose (β-Galf) is present only in the mucins of some strains of epimastigotes and α-d-galactopyranose (α-Galp) characterizes the mucins of the bloodstream trypomastigotes. The two last configurations confer high antigenic properties. In this review we discuss the different structures found and we pose the questions that still need investigation on the exchange of the configurations of galactose.
Keywords: Trypanosoma cruzi, mucins, β-galactofuranose, α-galactopyranose
1. Introduction
Trypanosoma cruzi, the agent of Chagas disease [1], is an intriguing parasite, not only because of the morphological and biological changes during its life cycle but also for the drastic modifications of the sugars at the surface of the parasite. The multiple strains of T. cruzi have been grouped into seven discrete typing units (DTU) (TcI to TcVI and Tcbat) based on their phenotypic and genetic properties [2,3].
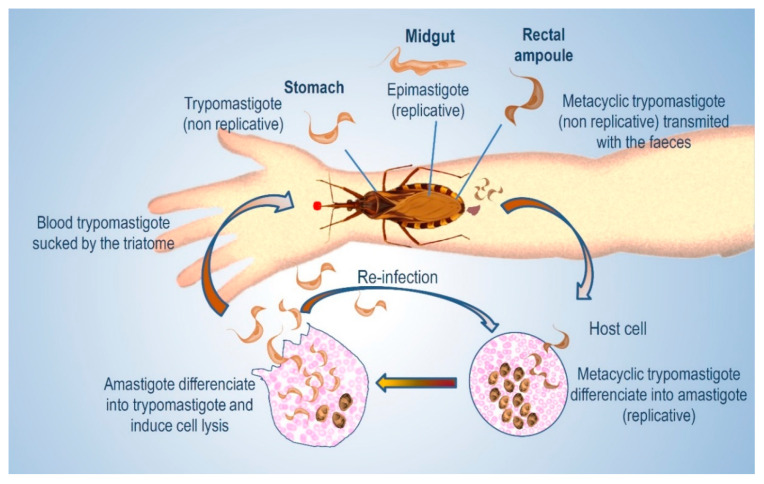
On the other hand, four main stages can be recognized in T. cruzi, depending on the host being either the triatomine insect or a mammal [4,5,6]. In each host a replicative and an infective form have been described. The replicative forms are the epimastigotes in the insect and amastigotes in the mammal, whereas trypomastigotes (metacyclic in the insect) are the infective forms. (Figure 1)
Figure 1.
Life cycle of T. cruzi.
The transmission to mammals occurs when the triatomines, while feeding, deposit feces on the skin, and parasites penetrate through the small wounds caused by scratching. Several factors influence the success of infection [7]. Oral transmission of T. cruzi followed by mucosa infection has also been characterized as highly lethal [8,9]. The mechanisms of exocytosis and endocytosis that take place in the host and favor T. cruzi invasion were studied [10]. Before cell invasion, there is an interaction of the parasite with the extracellular matrix that results in metabolic modifications. Although cell-derived trypomastigotes were used in the study, it is conceivable that changes also occur in insect-derived metacyclic trypomastigotes [11]. Once in the cell, trypomastigotes accumulate in a parasitophorus vacuole (PV) formed by fusion with the host lysosome, an essential step in order to evade the immune response of the host [12,13]. Several biological processes take place in the PV, and one of them is due to the action of the T. cruzi trans-sialidase (TcTS), referred later in this review, which mediates parasite escape from PVs to cytosol [14,15]. In the PV, the parasites start to transform into amastigotes, and differentiation is completed in the cytosol. After several cycles of binary division, they differentiate again into trypomastigotes, which upon membrane lysis are released to circulation. These blood trypomastigotes may infect other cells or be ingested by a triatomine. In the insect, the trypomastigotes must differentiate into epimastigotes to close the cycle. This process starts in the stomach; down in the rich medium of the midgut they multiply, and upon reaching the hindgut they transform into the infective metacyclic trypomastigotes, which detach from the cuticle and are excreted.
Regarding the polypeptide chains in the mucins, multiple genes encoding mucins have been described and grouped into families depending on the host, which may be an insect or a mammal. Studies from expert groups on the genes and proteins of T. cruzi mucins have been published [16,17,18,19,20,21]. A synthetic peptide from a mucin associated surface protein (MASP) was proposed as a candidate for a vaccine against Chagas disease [22].
The parasites, in the developmental stages identified in T. cruzi, express characteristic molecules that are crucial in the infection. However, the metabolic steps involved in their fate during transformations are poorly understood. The recognition of surface glycans by host cells is well documented [23,24,25]. In this review we will focus on the structure and role of the glycans at the surface of the parasite in the different developmental stages, mainly epimastigotes and trypomastigotes, because studies on glycans of amastigotes are scarce. A conclusion, with an addressing of the questions that arise from the current knowledge, is included.
2. Cell-Surface Glycans in T. cruzi
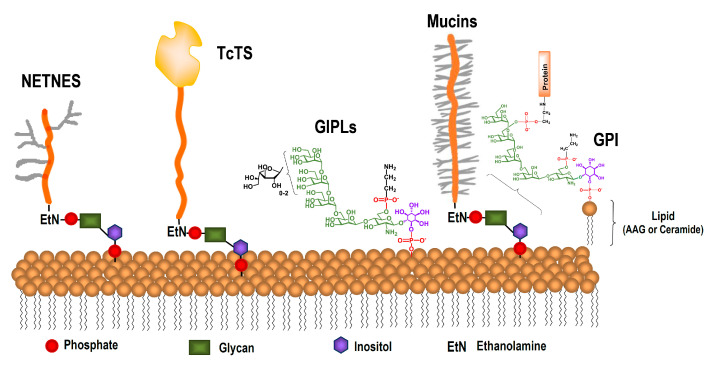
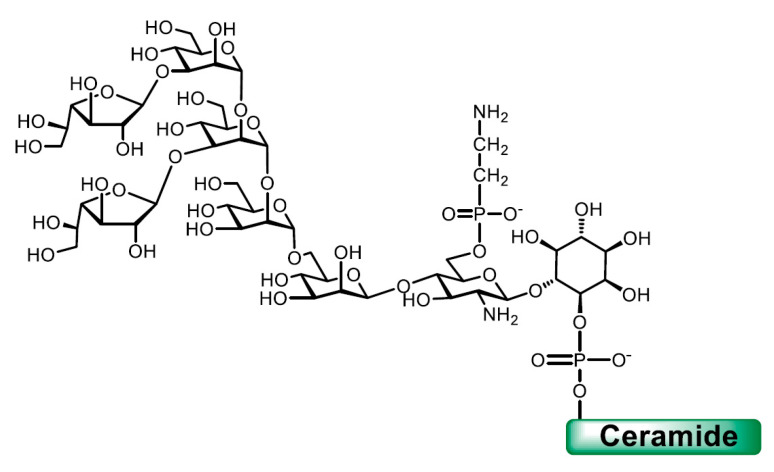
The most abundant molecules in the glycocalix that covers the parasite are glycoinositolphospholipids (GIPLs) [26,27] and glycosylphosphatidylinositol (GPI)-anchored mucins [28,29] (Figure 2). The GPI favors the dense packing of the mucins. Epimastigotes and trypomastigotes have approximately the same number of mucin molecules per cell [30], whereas a significantly higher number of GIPL molecules was detected in epimastigotes [31]. Other less abundant but unique glycoproteins have been described in T. cruzi, among them a trans-sialidase, [32,33,34] and in epimastigotes, a complex GPI-anchored glycopeptide called NETNES [35].
Figure 2.
Representative glycoconjugates in the surface of T. cruzi epimastigotes.
3. The Glycan in Mucins of T. cruzi
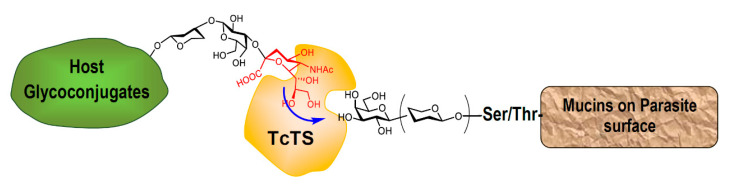
The carbohydrate in the mucins amounts to about 60% of the total mass [30]. The structure of the O-linked chains in the mucins defines their role in antigenicity and pathogenesis. They perform a crucial function as acceptors of sialic acid from host glycoconjugates in a reaction catalyzed by the parasite’s unique trans-sialidase (TcTS), an enzyme extensively studied. The negatively charged glycans protect the parasite from the action of proteases and other enzymes [32,34,36,37]. This is the only way for T. cruzi to acquire the sialic acid needed for infection [15,38]. The reaction depends on the structure of the O-linked sugars in the mucins; more precisely, on the presence of β-Galp terminal units (Figure 3). Sialylation affects the sialoglyco-profile of both the parasite and the mammal host, thus modulating immunological events. The natural change of one aminoacid in the peptidic chain leads to the inactivation of the enzyme; however, this inactive trans-sialidase (iTcTS) shows lectin properties in relation to Neu5Ac (α2-3-Gal) glycotopes influencing adhesion and invasion of the host cell [15,39].
Figure 3.
Trans-sialidase from T. cruzi transfers sialic acid from host sialoglycoconjugates to β-Galp-containing glycoproteins expressed on the parasite’s surface.
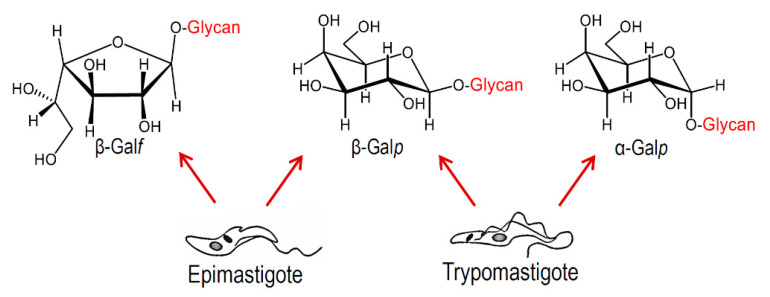
As will be discussed below, the O-linked carbohydrate chains display great variability, and galactose being the main monosaccharide constituent, it may be present as β-galactofuranoside or as galactopyranoside in α- and/or β- anomeric configurations, depending on the strain and the stage of the parasite studied (Figure 4). The microheterogeneity and highly diverse structures of the O-linked glycans have been described. The higher amount of β-Galp terminal units, available for sialylation, may determine the virulence of the parasite. On the other hand, β-Galf and α-Galp are highly antigenic, and thus could provoke an immune response by the host.
Figure 4.
Configurations of Galactose (Gal) found in T. cruzi mucins.
The glycan structures of human and T. cruzi mucins show striking differences [40]. First, the sugar chains are linked to the protein by α-GlcNAc instead of GalNAc, like in vertebrate mucins [41,42]. Moreover, apparently GalNAc was not found in T. cruzi glycoconjugates. Accordingly, the UDP-Glc4′-epimerase is unable to convert UDP-GlcNAc to UDPGalNAc, in contrast to the human epimerase [43]. The gene encoding the transferase which incorporates the α-GlcNAc from the nucleotide was identified and called TcOGNT-2 [44]. This gene is not equally expressed in all stages of the life cycle of T. cruzi. On the differentiation of trypomastigotes to amastigotes inside the mammal cell, the expression levels of TcOGNT-2 decrease and increase again when, after replication, the amastigotes differentiate again into trypomastigotes [45]. Several studies have been published on the sugar structures of mucins, particularly those of epimastigote strains [19,46,47,48,49,50,51]. Unusual for surface glycoproteins, in some strains, a significant amount of non-substituted O-linked GlcNAc, which amounted to about 20% of the glycosylation sites, was found [47,52]. In all of them, the next sugar added is galactose, and the disaccharide may be further elongated with galactoses to afford lineal or branched structures [28]. The nucleotide for the incorporation of galactose must be formed from UDPGlcp by the action of UDPGlcp-4 epimerase, since a galactose transporter could not be identified [53]. The suppression of the epimerase activity caused important changes in the morphology and membrane cell structure of the parasite [54].
The configuration of galactose in epimastigotes varies among strains and from the cell-derived trypomastigotes. Galactofuranose (in the β-configuration) has only been found in the epimastigote mucins of strains belonging to DTU I or hybrid strains, whereas α-Galp is only present in trypomastigote mucins. The other anomer, β-Galp, was identified in all strains of epimastigotes and trypomastigotes, and is recognized by the antibody 2B10 [55]. Apparently, there are no reports on the structure of glycans from amastigote mucins in agreement with the fact that the enzyme that incorporates GlcNAc, the first sugar of the O-linked chain, decays upon the differentiation of trypomastigotes into amastigotes [45]. A stage-specific GPI-anchored glycoprotein called Ssp4 has been characterized in extracellular amastigotes. These may be found in the extracellular milieu due to the early lysis of infected cells [56,57], or to cytolysis at sites of infection during the chronic stage of Chagas disease [58]. The extracellular amastigotes share morphological, immunological and infective properties with the intracellular counterparts. β-Galactopyranosides were identified as determinants in Ssp4 of host–cell interactions [59], but the linkage to the protein and the structure of the oligosaccharides were not defined.
4. Galactofuranose in Glycoconjugates of T. cruzi Epimastigotes
d-galactose in the pyranose configuration is a common constituent of the oligosaccharides, glycoproteins and glycolipids of mammals, whereas Galf is found in bacteria, protozoa and fungi, some of which are pathogenic for humans that lack Galf [60]. In T. cruzi, Galf was first detected in GIPLs [61], and there are several reports on its presence in the Leishmania species [62,63]. The selective presence of Galf in mucins of some strains of T. cruzi insect forms [28] is particularly interesting; it was not described in T. brucei. β-Galactofuranosyl-containing conjugates are also constituents of other important human pathogens, like Mycobacterium tuberculosis [64] and Aspergillus fumigatus [65,66]. Being absent in mammals, the enzymes involved in the biosynthesis of galactofuranosides are good targets for chemotherapy [67]. As with other monosaccharides, Galf is incorporated from the nucleotide UDPGalf, which in turn is produced from UDPGalp by the action of a mutase (UGM) that was first described in E. coli [68,69] and has been extensively studied [70,71]. Candidate genes (GLF) were identified in eukaryotes, among them T. cruzi, by combinatorial bioinformatics screening. When GLF were expressed in E. coli, the proteins showed UGM activity [72]. The crystal structure of T. cruzi UGM showed differences with the bacterial UGMs [73]. The galactofuranosyl transferases (GALFT) working for the introduction of Galf from the nucleotide were less studied. Although genes encoding GALFT have been detected in the genoma of T. cruzi [74], the cloning of proteins with enzymatic activity was not reported. Comparative analyses of the amino acid sequence of a GALFT from T. rangeli (TrGALFT), a non-pathogenic trypanosome, revealed identities between 73% and 55% with T. cruzi orthologs, however antibodies raised against TrGALFT did not recognized proteins in a T. cruzi extract [75].
In T. cruzi, the GIPLs, originally named lipopeptidophophoglycan (LPPG) [76], are the most abundant glycoconjugates in epimastigotes and metacyclic trypomastigotes (107 molecules/cell) [35]. The full structures were determined for GIPLs of different strains [26,27,77]. A microheterogeneity in the glycan structure was described. The major structure (65%), found in GIPLs of the Y strain, with two β-Galf units is shown in Figure 5 [27]. Different from the mucins, as discussed below, Galf was identified in the GIPLs of epimastigote strains belonging to DTU I and DTU II. An exo β-galactofuranosidase able to remove Gal from the GIPLs was reported [78].
Figure 5.
Major structure found in glycoinositolphospholipids (GIPLs) of the Y strain.
In T. cruzi, GIPLs, commonly called GPI anchors, are also found attaching proteins like the mucins to the cell membrane [79,80]. However, no Galf was detected in the GPI anchor of mucins from the Y strain [81] or the G strain [47]. In the last case, the result is not conclusive since the glycan analyzed was obtained after hydrofluoric acid treatment, which could cleave the labile Galf.
Antibodies directed towards Galf epitopes have been obtained from GIPLs in rabbits [31]. A monoclonal antibody named 10D8 recognizes β-Galf in the mucins of insect-stage strains from DTU I and some hybrid strains [82,83]; mucins from DTU II are not reactive. Moreover, a single-chain variable fragment (scFv) derived from mAb-10D8 was engineered to target the mucins of the metacyclic infective forms. It was proven that scFv-10D8 specifically inhibited parasite invasion of mammalian cells [84].
5. Structure of O-Glycans in Mucins of Epimastigotes and Metacyclic Trypomastigotes
In the mucins, the O-linked oligosaccharides may be derived from two cores, β-d-Galp (1→4) GlcNAc (core 1) or β-d-Galf (1→4) GlcNAc (core 2). Higher oligosaccharides, formed by elongation and/or branching with more galactoses, contribute to the microheterogeneity of the mucins. Only Galp was found in oligosaccharides derived from core 1. Interestingly, oligosaccharides with core 2 may contain β-Galf and β-Galp.
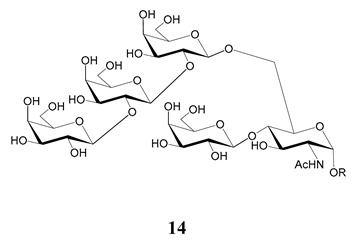
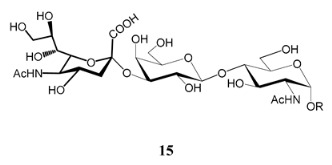
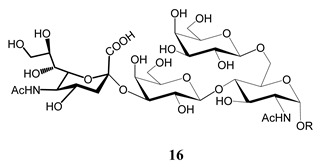
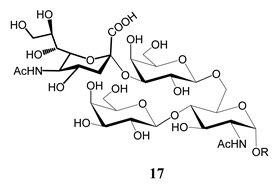
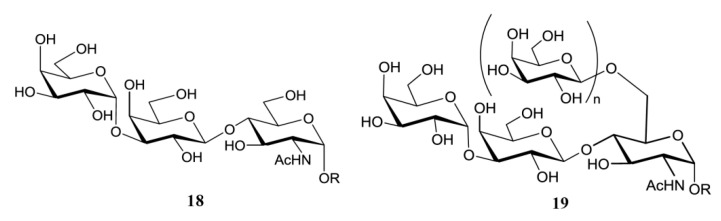
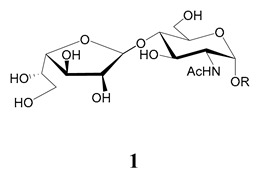
On studying the fine structure of the glycans in several strains, it was concluded that core 2 was found in the mucins of strains belonging to DTU I isolated from sylvatic hosts, namely G strain [47,48], Colombiana [50], Dm28c [49] and Silvio X10/1 [85], and also in the hybrid Tulahuen strain [51], classified as DTU VI. A sialylated oligosaccharide was also identified in the mucins from Dm28c. The structures of the oligosaccharides in the mucins are shown in Table 1.
Table 1.
O-linked carbohydrates derived from core 2 in the mucins of T. cruzi.
| Structure | Strain (Ref) | Chemical Synthesis (Ref) |
|---|---|---|
|
G [47,48] Dm28c [49] Colombiana [50] Tulahuen [51] |
[86] |
|
Tulahuen [51] | [87] |
|
Tulahuen [51] | [87] |
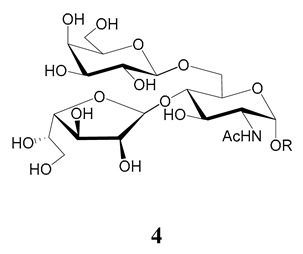
|
G [47,48] Dn28c [49] Colombiana [50] Tulahuen [51] |
[88] |
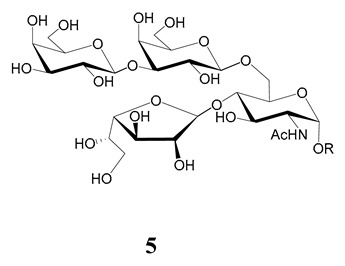
|
G [47,48] Dn28c [49] Colombiana [50] Tulahuen [51] |
[89] |
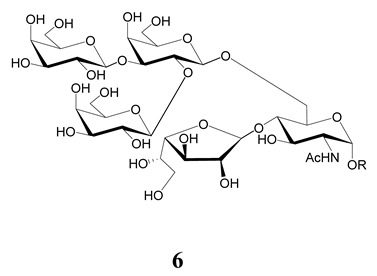
|
G [47,48] Dn28c [49] Colombiana [50] Tulahuen [51] |
[90] |
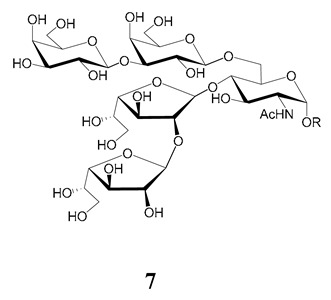
|
Tulahuen [51] | - |
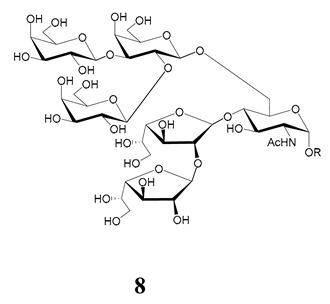
|
Dm28c [49] Tulahuen [51] |
[91] |
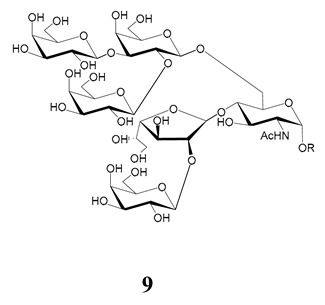
|
G [47,48] Dm28c [49] Colombiana [50] Tulahuen [51] |
[92,93] |
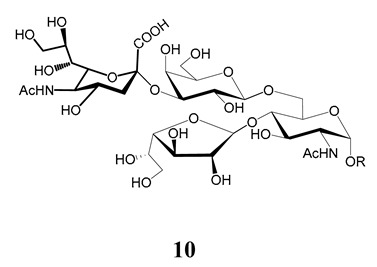
|
Dm28c [49] | - |
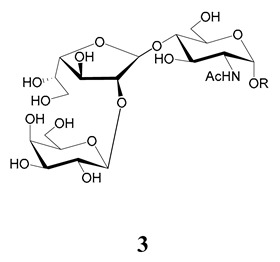
In the mucins of the Y strain belonging to DTU II (with a domestic transmission), only β-Galp was found in the oligosaccharides, which was derived from core 1 (Table 2) [81]. On analyzing the oligosaccharides obtained from mucins of the Tulahuen strain and CL clones, included in DTU VI [3,94], controversial results were obtained. In the Tulahuen strain, both cores were identified, giving rise to a high diversity of oligosaccharides (Table 1). Besides the core 1 disaccharide, the trisaccharide resulting from the incorporation of sialic acid was identified, whereas the more complex chains in the Tulahuen mucins were derived from core 2 [51]. In the CL clones, only core 1 was identified, and the derived oligosaccharides lack Galf and are similar to those found in the Y strain [19,46,52] (Table 2).
Table 2.
O-linked carbohydrates derived from core 1 in the mucins of T. cruzi.
The role of the glycan structure of the GIPLs and mucins of epimastigotes in the interaction with the insect host was studied. Purified GIPLs from the Y strain inhibit the adhesion of epimastigotes to the insect midgut [95]. It was proven that, at least in part, the interaction with the midgut was due to the presence of Galf.
The mucins oligosaccharides 1–9 of Table 1 were chemically synthesized as benzyl glycosides [22,86,87,88,89,90,91,92,93] and used for studies on the adhesion of the parasites to insect tissues [96]. Diverse results were obtained depending on the strain. It was shown that the branched (Galf)-containing trisaccharide β-d-Galf(1→4)[β-d-Galp(1→6)]-α-d-GlcNAc is a determinant for the adhesion of Dm28c (DTU I) parasites to the rectal ampoule of the triatomine vector. Higher oligosaccharides bearing the same motif were even better competitive inhibitors. In contrast, the synthetic oligosaccharides did not show any inhibitory effect on the binding of CL Brener (DTU VI) epimastigotes to the triatomine hindgut, which is in agreement with the lack of Galf in mucins of the CL strain [46]. Moreover, studies showed that the epimastigote mucins do not bind to the insect midgut [96], which however could attach GIPLs [95]. Although Galf contributes to both interactions, these results point to different receptors for GIPLs and mucins. Adhesion to the rectal ampoule is the step before the differentiation into infective metacyclic trypomastigotes, which then detach from the cuticle. The process would be caused by changes in the composition of the surface molecules on metacyclogenesis [5,97]. However, in a comparative analysis of the GPI anchors and the O-linked chains from the mucins of epimastigotes and metacyclic trypomastigotes of the G strain, it was found that the only difference was in the lipid structure, which changes from an alkylacyl glycerol to a ceramide in the infective forms [47]. These results suggest that different moieties mediate the adhesion of epimastigotes and the release of the metacyclic forms.
6. Structure of Glycans in Mucins from Mammalian Cell-Derived Trypomastigotes. Immunogenicity of the α-Gal Epitope
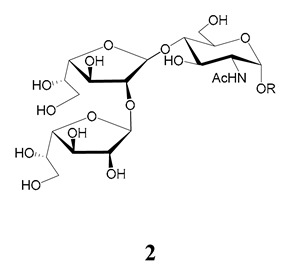
The unique feature of the O-linked sugars in the mammal-stage parasites is the presence of the α-d-Galp (1→3) Gal epitope. The hexosamine that links the carbohydrate to the polypeptide chain is the same in parasites of all stages and strains analyzed. Thus, the smallest oligosaccharide found in the mucins of mammal trypomastigotes was the trisaccharide α-d-Galp(1→3)-β-d-Galp(1→4)-GlcNAc (18) (Figure 6). Higher oligosaccharides were released from the mucins via a reductive β-elimination reaction [98]. Although the structure of the branched oligosaccharides was not fully described, the methylation studies pointed to branching at C-6 of the GlcNAc with Galp, which should be in the β-configuration so as to function as an acceptor in the transfer reaction of sialic acid from host glycoconjugates [33,34,38]. This process is crucial for the pathogenesis of T. cruzi, since the lysis of trypomastigotes is prevented by sialylation [30,99]. The β-galactose in the branch may be further substituted with more β-Galp (19) units, since 2,6-di-O-substituted Galp was also identified in the methylation analysis. The highly relative amount of tetramethyl galactose detected in the mixture agrees with the presence of terminal Galp necessary for the trans-sialidase reaction [98].
Figure 6.
O-linked sugars in mucins of T. cruzi cell-derived trypomastigotes. 19 were assigned based on methylation analysis
Variations in the expression of α-Gal in strains belonging to different DTUs were reported, with higher expressions of this sugar in Y T. cruzi populations, followed by Colombiana and CL strains [100].
The oligosaccharides are highly immunogenic due to the presence of the terminal α-Galp units, which elicit the lytic anti α-Gal antibodies found in sera from patients with chronic Chagas disease. The α-d-Galp(1→3)Gal epitope is common in the glycoconjugates of non-primate mammals, prosimians and New World monkeys, but is not found in Old World monkeys, apes or humans due to an evolutionary mutation, which resulted in the inactivation of the α-1,3-galactosyltransferase gene [101]. For that reason, healthy humans also produce α-Gal antibodies as a response to carbohydrate antigens from bacteria in normal gastrointestinal flora [102], however the response to the α-Gal epitope is much stronger in patients infected with T. cruzi [98,103].
Neoglycoconjugates containing the α-Gal epitope have been chemically synthesized and evaluated as diagnostic tools for Chagas disease, and also as candidates for therapeutic intervention [103,104,105,106,107,108,109]. It was shown that the trisaccharide α-d-Galp(1→3)-β-d-Galp(1→4)-β-d-Glcp, with glucose in the reducing end instead of GlcNAc, was as effective for the recognition of chagasic antibodies as the natural trisaccharide 18, and easier to synthesize [103]. α-Gal immunity has been studied in other parasites, such as Leishmania major and Plasmodium falciparum. Neoglycoproteins containing α-Gal have been described as diagnostic tools for cutaneous leishmaniasis [110] and for protection against the parasite [111,112]. In Plasmodium falciparum, the agent of malaria, terminal α-linked galactosyl units, recognized by immune sera, are present in carbohydrate chains of glycoproteins [113,114].
7. Potential Role of Galectins in the Infection of T. cruzi
In view of the relation of the terminal β-galactopyranosyl units in the parasite to sialylation by TcTS, several groups have studied the role of galectins (Gal) in the infection, mainly focusing on Galectin 3. The interactions of galectins with glycans were considered a fundamental event in pathogen recognition [115]. A mucin of 45kDa was quickly identified as a receptor for Gal 3 [116,117], and it was shown that a high Gal 3 expression enhances cell recognition of parasites [118,119]. Recent work showed that Gal 3 is important in the survival of the parasite during infection [120]. The role of Gal 1 in modulating the outcome of the infection was also studied [121,122]. In relation to the different glycan structures identified in the mucins depending on the strains and stages of T. cruzi, the work of Pineda et al. [25] investigated the binding of galectins 1, 3, 4, 7 and 8 with 14 strains of DTUs I–VI of T. cruzi in different stages of its life cycle. The binding profile for the six DTUs agreed with the genetic classification. They found that the galectins bind preferentially to amastigotes. Although amastigotes lack mucins, β-galactopyranosides have been identified as determinants for host–cell interactions in the specific glycoprotein Ssp4 [59]. The fine glycan structure of Ssp4 was not reported. The higher recognition of amastigotes could be due to the lack of sialylation of the glycans, since no TcTS was detected in these forms. The authors did not find differences between cell-derived trypomastigotes and insect metacyclic trypomastigotes.
8. Conclusions and Perspectives
Carbohydrates in the surface of microbial pathogens have received, in the last few decades, special attention as targets for diagnosis and vaccine development. In T. cruzi, the problems encountered in generating mutants defective in glycosylation leads to chemical or chemoenzymatic methods for synthesizing the oligosaccharides and neoglycoconjugates for immunological studies. Several surface molecules in the parasite and in the host have been implicated in the adhesion and infection.
The two partners of a crucial interplay in T. cruzi, the mucins and the trans-sialidase, have been the most studied. They were called mucins because of the high content of sugar in their O-linked chains, although their structures differ significantly from the human mucins, as discussed in the text. One of the differences is that GlcNAc instead of GalNAc links the O-chain to the protein [41,42,44]. The lack of UDP-GlcNAc:polypeptide α-N-acetylglucosaminyl transferase in the intracellular amastigote stage points to the absence of mucins in these forms. Accordingly, no trans-sialidase (TS) genes were reported in these intracellular parasites [123]. One intriguing feature is the presence of galactose in three possible configurations, depending on strains and life stages. Galf is only present in the stages that live in the insect vector, whereas α-Galp is characteristic of blood trypomastigotes; both may generate specific antibodies for the structures that carry them. On the other hand, Galf is not present in the mucins of all strains, and it was only found in DTU I and in the hybrid Tulahen strain (DTU VI) and is conserved when epimastigotes differentiate into metacyclic trypomastigotes. Some of the oligosaccharides of all strains and stages are branched with terminal β-Galp, which provides the site for sialylation by the TcTS. The expression of TcTS is lower in metacyclic trypomastigotes than in mammal trypomastigotes; however, both the insect and the cell-derived trypomastigotes are able to invade mammalian cells. Sialylation differently affects parasites in each infective stage [83]. Sialyl external units in metacyclic forms impair the interaction with mammal cells. In fact, it was shown that the treatment of metacyclic T. cruzi of the G strain with neuraminidase increases the infectivity [124], whereas several groups have reported on the positive effects of the sialylation of blood trypomastigotes on the adhesion and invasion of mammal cells [29]. These reports point to different receptors in the mammal cells for both kinds of trypomastigotes. On the metacyclogenesis of epimastigotes, the mucin glycans conserve their structure, which have important differences from the mammal trypomastigotes. This topic deserves further investigations. β-Galactofuranosides are the sugar epitopes of some strains, and as far as we know no human lectins for Galf have been described. The level of further substitution with β-Galp units of the O-chains, determining a glycophenotype, may modulate the infection.
The Galf in the branched oligosaccharides of epimastigote mucins is involved in the adhesion to the hindgut of the insect, where differentiation to the infective metacyclic forms takes place [96]. We also considered how to explain the adhesion of strains that lack Galf in the mucins, like the Brenner strain. One possibility is that attachment is mediated by the Galf in the GIPLs, since it is present in all the strains [77]. It was proven that upon starvation of UDP Galp, Galf incorporates into the GIPLs in preference over the mucins [54].
Several molecules on the parasite and the host are involved in mammal infection by metacyclic trypomastigotes. In fact, once in the mammal host, strains belonging to DTU I cause lower parasitemia titers than metacyclic forms from strains Y and CL, which do not contain Galf in their mucins. The first question was if the presence of Galf influences the sialylation of the β-Galp present in the oligosaccharides. In vitro studies showed that Galf does not interfere with sialylation [125]. If these results are extended to the in vivo process, it is possible that once in the cell, the Galf may trigger the production of antibodies that impair infection. Moreover, the mucins of mammal trypomastigotes of representative stages lack Galf, and it was reported that on the cell, metacyclic mucins are capped and extensively released in the parasitophorus vacuole [126]. The shedding of the mucins and other membrane components from different stages was reported [127], and this process may precede the construction of new mucins in blood trypomastigotes for adaptation to the new environments. In this respect, mucins were not detected in the intermediate amastigote stage.
The only monosaccharide transferase for mucins that has been identified is the UDP-GlcNAc:polypeptide α-N-acetylglucosaminyl transferase. An exo-β-galactofuranosidase has been purified from epimastigote lysates by affinity chromatography [78], but it was not fully characterized. This hydrolase could be important for the regulation of the interaction of the parasite with the insect tissues. The galactose transferases that introduce the galactoses in different configurations and linkages in the mucins, as well as the membrane receptors of the hosts, are interesting topics for further studies. The unique structures of the glycans point to them as selective targets for chemotherapy.
Funding
This work was supported by grants from Universidad de Buenos Aires (UBA), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina. M.E.G. and R.M.L. are Research Members of CONICET.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Guarner J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019;36:164–169. doi: 10.1053/j.semdp.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Zingales B., Miles M.A., Campbell D.A., Tibayrenc M., Macedo A.M., Teixeira M.M., Schijman A.G., Llewellyn M.S., Lages-Silva E., Machado C.R., et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Zingales B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Kollien A., Schaub G. The development of Trypanosoma cruzi in triatominae. Parasitol. Today. 2000;16:381–387. doi: 10.1016/S0169-4758(00)01724-5. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves C.S., Ávila A.R., de Souza W., Motta M.C.M., Cavalcanti D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors. 2018;11:83. doi: 10.1186/s13071-018-2664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alba Soto C.D., González Cappa S.M. Trypanosoma cruzi journey from the insect vector to the host cell. In: Altcheh J.M., Freilij H., editors. Chagas Disease: A Clinical Approach. Springer International Publishing; Cham, Switerland: 2019. pp. 25–59. [DOI] [Google Scholar]
- 7.Monteon V. Trypanosoma cruzi: The early contact between insect-derived metacyclic trypomastigotes and the mammalian cells. Ann. Parasitol. 2019;65:193–204. doi: 10.17420/ap6503.201. [DOI] [PubMed] [Google Scholar]
- 8.Benchimol P.R.B. The oral transmission of Chagas’ disease: An acute form of infection responsible for regional outbreaks. Int. J. Cardiol. 2006;112:132–133. doi: 10.1016/j.ijcard.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 9.Steindel M., Kramer Pacheco L., Scholl D., Soares M., de Moraes M.H., Eger I., Kosmann C., Sincero T.C.M., Stoco P.H., Murta S.M.F., et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn. Microbiol. Infect. Dis. 2008;60:25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Butler C.E., Tyler K.M. Membrane traffic and synaptic cross-talk during host cell entry by Trypanosoma cruzi. Cell. Microbiol. 2012;14:1345–1353. doi: 10.1111/j.1462-5822.2012.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattos E.C., Canuto G., Manchola N.C., Magalhães R.D.M., Crozier T.W.M., Lamont D.J., Tavares M.F.M., Colli W., Ferguson M.A.J., Alves M.J.M. Reprogramming of Trypanosoma cruzi metabolism triggered by parasite interaction with the host cell extracellular matrix. PLoS Negl. Trop. Dis. 2019;13:e0007103. doi: 10.1371/journal.pntd.0007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrias E.S., de Carvalho T.M., De Souza W. Trypanosoma cruzi: Entry into Mammalian Host Cells and Parasitophorous Vacuole Formation. Front. Immunol. 2013;4:186. doi: 10.3389/fimmu.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batista M.F., Nájera C.A., Meneghelli I., Bahia D. The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival. Front. Cell Dev Biol. 2020;8:396. doi: 10.3389/fcell.2020.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freire-de-Lima L., Gentile L.B., da Fonseca L.M., da Costa K.M., Santos Lemos J., Jacques L.R., Morrot A., Freire-de-Lima C.G., Nunes M.P., Takiya C.M., et al. Role of Inactive and Active Trypanosoma cruzi Trans-sialidases on T Cell Homing and Secretion of Inflammatory Cytokines. Front. Microbiol. 2017;8:1307. doi: 10.3389/fmicb.2017.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Fonseca L.M., da Costa K.M., Chaves V.d.S., Freire-de-Lima C.G., Morrot A., Mendonça-Previato L., Previato J.O., Freire-de-Lima L. Theft and Reception of Host Cell’s Sialic Acid: Dynamics of Trypanosoma cruzi Trans-sialidases and Mucin-Like Molecules on Chagas’ Disease Immunomodulation. Front. Immunol. 2019;10:164. doi: 10.3389/fimmu.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urban I., Boiani Santurio L., Chidichimo A., Yu H., Chen X., Mucci J., Agüero F., Buscaglia C.A. Molecular diversity of the Trypanosoma cruzi TcSMUG family of mucin genes and proteins. Biochem. J. 2011;438:303–313. doi: 10.1042/BJ20110683. [DOI] [PubMed] [Google Scholar]
- 17.Di Noia J.M., Buscaglia C.A., De Marchi C.R., Almeida I.C., Frasch A.C.C. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas’ disease is due to a single parasite lineage. J. Exp. Med. 2002;195:401–413. doi: 10.1084/jem.20011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buscaglia C.A., Campo V.A., Di Noia J.M., Torrecilhas A.C., De Marchi C.R., Ferguson M.A., Frasch A.C., Almeida I.C. The surface coat of the mammal-dwelling infective trypomastigote stage of Trypanosoma cruzi is formed by highly diverse immunogenic mucins. J. Biol. Chem. 2004;279:15860–15869. doi: 10.1074/jbc.M314051200. [DOI] [PubMed] [Google Scholar]
- 19.Pollevick G.D., Di Noia J.M., Salto M.L., Lima C., Leguizamon M.S., de Lederkremer R.M., Frasch A.C. Trypanosoma cruzi surface mucins with exposed variant epitopes. J. Biol. Chem. 2000;275:27671–27680. doi: 10.1074/jbc.M000253200. [DOI] [PubMed] [Google Scholar]
- 20.Durante I.M., La Spina P.E., Carmona S.J., Agüero F., Buscaglia C.A. High-resolution profiling of linear B-cell epitopes from mucin-associated surface proteins (MASPs) of Trypanosoma cruzi during human infections. PLoS Negl. Trop. Dis. 2017;11:e0005986. doi: 10.1371/journal.pntd.0005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholomeu D.C., Cerqueira G.C., Leao A.C., daRocha W.D., Pais F.S., Macedo C., Djikeng A., Teixeira S.M., El-Sayed N.M. Genomic organization and expression profile of the mucin-associated surface protein (masp) family of the human pathogen Trypanosoma cruzi. Nucleic Acids Res. 2009;37:3407–3417. doi: 10.1093/nar/gkp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serna C., Lara J.A., Rodrigues S.P., Marques A.F., Almeida I.C., Maldonado R.A. A synthetic peptide from Trypanosoma cruzi mucin-like associated surface protein as candidate for a vaccine against Chagas disease. Vaccine. 2014;32:3525–3532. doi: 10.1016/j.vaccine.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valente M., Castillo-Acosta V.M., Vidal A.E., González-Pacanowska D. Overview of the role of kinetoplastid surface carbohydrates in infection and host cell invasion: Prospects for therapeutic intervention. Parasitology. 2019;146:1743–1754. doi: 10.1017/S0031182019001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes M.P., Fortes B., Silva-Filho J.L., Terra-Granado E., Santos L., Conde L., de Araujo Oliveira I., Freire-de-Lima L., Martins M.V., Pinheiro A.A., et al. Inhibitory effects of Trypanosoma cruzi sialoglycoproteins on CD4+ T cells are associated with increased susceptibility to infection. PLoS ONE. 2013;8:e77568. doi: 10.1371/journal.pone.0077568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pineda M.A., Corvo L., Soto M., Fresno M., Bonay P. Interactions of human galectins with Trypanosoma cruzi: Binding profile correlate with genetic clustering of lineages. Glycobiology. 2015;25:197–210. doi: 10.1093/glycob/cwu103. [DOI] [PubMed] [Google Scholar]
- 26.Previato J.O., Gorin P.A., Mazurek M., Xavier M.T., Fournet B., Wieruszesk J.M., Mendonca-Previato L. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J. Biol. Chem. 1990;265:2518–2526. [PubMed] [Google Scholar]
- 27.De Lederkremer R.M., Lima C., Ramirez M.I., Ferguson M.A., Homans S.W., Thomas-Oates J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi Epimastigotes. J. Biol. Chem. 1991;266:23670–23675. [PubMed] [Google Scholar]
- 28.De Lederkremer R.M., Agusti R. Advances in Carbohydrate Chemistry and Biochemistry. Volume 62. Academic Press; Cambridge, MA, USA: 2009. Chapter 7 glycobiology of Trypanosoma cruzi; pp. 311–366. [DOI] [PubMed] [Google Scholar]
- 29.Acosta-Serrano A., Almeida I.C., Freitas-Junior L.H., Yoshida N., Schenkman S. The mucin-like glycoprotein super-family of Trypanosoma cruzi: Structure and biological roles. Mol. Biochem. Parasitol. 2001;114:143–150. doi: 10.1016/S0166-6851(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 30.Pereira-Chioccola V.L., Acosta-Serrano A., Correia de Almeida I., Ferguson M.A., Souto-Padron T., Rodrigues M.M., Travassos L.R., Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 2000;113:1299. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 31.Golgher D.B., Colli W., Souto-Padrón T., Zingales B. Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1993;60:249–264. doi: 10.1016/0166-6851(93)90136-L. [DOI] [PubMed] [Google Scholar]
- 32.Buscaglia C.A., Campo V.A., Frasch A.C., Di Noia J.M. Trypanosoma cruzi surface mucins: Host-dependent coat diversity. Nat. Rev. Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- 33.Schenkman S., Eichinger D., Pereira M.E., Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 34.Giorgi M.E., de Lederkremer R.M. Trans-sialidase and mucins of Trypanosoma cruzi: An important interplay for the parasite. Carbohydr. Res. 2011;346:1389–1393. doi: 10.1016/j.carres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Macrae J.I., Acosta-Serrano A., Morrice N.A., Mehlert A., Ferguson M.A. Structural characterization of NETNES, a novel glycoconjugate in Trypanosoma cruzi epimastigotes. J. Biol. Chem. 2005;280:12201–12211. doi: 10.1074/jbc.M412939200. [DOI] [PubMed] [Google Scholar]
- 36.Dc-Rubin S.S., Schenkman S. Trypanosoma cruzi trans-sialidase as a multifunctional enzyme in Chagas’ disease. Cell. Microbiol. 2012;14:1522–1530. doi: 10.1111/j.1462-5822.2012.01831.x. [DOI] [PubMed] [Google Scholar]
- 37.Freire-de-Lima L., Fonseca L.M., Oeltmann T., Mendonça-Previato L., Previato J.O. The trans-sialidase, the major Trypanosoma cruzi virulence factor: Three decades of studies. Glycobiology. 2015;25:1142–1149. doi: 10.1093/glycob/cwv057. [DOI] [PubMed] [Google Scholar]
- 38.Campetella O., Buscaglia C.A., Mucci J., Leguizamón M.S. Parasite-host glycan interactions during Trypanosoma cruzi infection: Trans-Sialidase rides the show. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165692. doi: 10.1016/j.bbadis.2020.165692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cremona M.L., Sánchez D.O., Frasch A.C., Campetella O. A single tyrosine differentiates active and inactive Trypanosoma cruzi trans-sialidases. Gene. 1995;160:123–128. doi: 10.1016/0378-1119(95)00175-6. [DOI] [PubMed] [Google Scholar]
- 40.Pinzón Martín S., Seeberger P.H., Varón Silva D. Corrigendum: Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators during Infection and Targets for Diagnostics and Vaccines. Front. Chem. 2019;7:846. doi: 10.3389/fchem.2019.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Previato J.O., Sola-Penna M., Agrellos O.A., Jones C., Oeltmann T., Travassos L.R., Mendonca-Previato L. Biosynthesis of O-N-acetylglucosamine-linked glycans in Trypanosoma cruzi. Characterization of the novel uridine diphospho-N-acetylglucosamine:polypeptide N-acetylglucosaminyltransferase-catalyzing formation of N-acetylglucosamine α1→O-threonine. J. Biol. Chem. 1998;273:14982–14988. doi: 10.1074/jbc.273.24.14982. [DOI] [PubMed] [Google Scholar]
- 42.Mendonca-Previato L., Penha L., Garcez T.C., Jones C., Previato J.O. Addition of alpha-O-GlcNAc to threonine residues define the post-translational modification of mucin-like molecules in Trypanosoma cruzi. Glycoconj. J. 2013;30:659–766. doi: 10.1007/s10719-013-9469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roper J.R., Ferguson M.A. Cloning and characterisation of the UDP-glucose 4′-epimerase of Trypanosoma cruzi. Mol. Biochem. Parasitol. 2003;132:47–53. doi: 10.1016/j.molbiopara.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Heise N., Singh D., van der Wel H., Sassi S.O., Johnson J.M., Feasley C.L., Koeller C.M., Previato J.O., Mendonça-Previato L., West C.M. Molecular analysis of a UDP-GlcNAc:polypeptide α-N-acetylglucosaminyltransferase implicated in the initiation of mucin-type O-glycosylation in Trypanosoma cruzi. Glycobiology. 2009;19:918–933. doi: 10.1093/glycob/cwp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiribao M.L., Libisch M.G., Osinaga E., Parodi-Talice A., Robello C. Cloning, localization and differential expression of the Trypanosoma cruzi TcOGNT-2 glycosyl transferase. Gene. 2012;498:147–154. doi: 10.1016/j.gene.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Todeschini A.R., da Silveira E.X., Jones C., Wait R., Previato J.O., Mendonça-Previato L. Structure of O-glycosidically linked oligosaccharides from glycoproteins of Trypanosoma cruzi CL-Brener strain: Evidence for the presence of O-linked sialyl-oligosaccharides. Glycobiology. 2001;11:47–55. doi: 10.1093/glycob/11.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Acosta-Serrano A., Schenkman S., Yoshida N., Mehlert A., Richardson J.M., Ferguson M.A.J. The Lipid Structure of the Glycosylphosphatidylinositol-anchored Mucin-like Sialic Acid Acceptors of Trypanosoma cruzi Changes during Parasite Differentiation from Epimastigotes to Infective Metacyclic Trypomastigote Forms. J. Biol. Chem. 1995;270:27244–27253. doi: 10.1074/jbc.270.45.27244. [DOI] [PubMed] [Google Scholar]
- 48.Previato J.O., Jones C., Gonçalves L.P., Wait R., Travassos L.R., Mendonça-Previato L. O-glycosidically linked N-acetylglucosamine-bound oligosaccharides from glycoproteins of Trypanosoma cruzi. Biochem. J. 1994;301:151–159. doi: 10.1042/bj3010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrellos O.A., Jones C., Todeschini A.R., Previato J.O., Mendonça-Previato L. A novel sialylated and galactofuranose-containing O-linked glycan, Neu5Ac(α2→3)Galp(β1→6)Galf(β1→4)GlcNAc, is expressed on the sialoglycoprotein of Trypanosoma cruzi Dm28c. Mol. Biochem. Parasitol. 2003;126:93–96. doi: 10.1016/S0166-6851(02)00245-1. [DOI] [PubMed] [Google Scholar]
- 50.Todeschini A.R., de Almeida E.G., Agrellos O.A., Jones C., Previato J.O., Mendonça-Previato L. ±-N-acetylglucosamine-linked O-glycans of sialoglycoproteins (Tc-mucins) from Trypanosoma cruzi Colombiana strain. Mem. Inst. Oswaldo Cruz. 2009;104:270–274. doi: 10.1590/S0074-02762009000900035. [DOI] [PubMed] [Google Scholar]
- 51.Jones C., Todeschini A.R., Agrellos O.A., Previato J.O., Mendonça-Previato L. Heterogeneity in the biosynthesis of mucin O-glycans from Trypanosoma cruzi tulahuen strain with the expression of novel galactofuranosyl-containing oligosaccharides. Biochemistry. 2004;43:11889–11897. doi: 10.1021/bi048942u. [DOI] [PubMed] [Google Scholar]
- 52.Salto M.L., Gallo-Rodriguez C., Lima C., de Lederkremer R.M. Separation of Galfβ1→XGlcNAc and Galpβ1→XGlcNAc (X = 3, 4, and 6) as the Alditols by High-pH Anion-Exchange Chromatography and Thin-Layer Chromatography: Characterization of Mucins from Trypanosoma cruzi. Anal. Biochem. 2000;279:79–84. doi: 10.1006/abio.1999.4466. [DOI] [PubMed] [Google Scholar]
- 53.Tetaud E., Barrett M.P., Bringaud F., Baltz T. Kinetoplastid glucose transporters. Biochem. J. 1997;325:569–580. doi: 10.1042/bj3250569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacRae J.I., Obado S.O., Turnock D.C., Roper J.R., Kierans M., Kelly J.M., Ferguson M.A.J. The suppression of galactose metabolism in Trypanosoma cruzi epimastigotes causes changes in cell surface molecular architecture and cell morphology. Mol. Biochem. Parasitol. 2006;147:126–136. doi: 10.1016/j.molbiopara.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Mortara R.A., da Silva S., Araguth M.F., Blanco S.A., Yoshida N. Polymorphism of the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi metacyclic trypomastigotes. Infect. Immun. 1992;60:4673–4678. doi: 10.1128/IAI.60.11.4673-4678.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrews N.W., Hong K.-s., Robbins E.S., Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp. Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 57.Ley V., Andrews N.W., Robbins E.S., Nussenzweig V. Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells. J. Exp. Med. 1988;168:649–659. doi: 10.1084/jem.168.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharfstein J., Morrot A. A role for extracellular amastigotes in the immunopathology of Chagas disease. Mem. Inst. Oswaldo Cruz. 1999;94(Suppl. S1):51–63. doi: 10.1590/S0074-02761999000700005. [DOI] [PubMed] [Google Scholar]
- 59.Florentino P.T.V., Real F., Orikaza C.M., da Cunha J.P.C., Vitorino F.N.L., Cordero E.M., Sobreira T.J.P., Mortara R.A. A Carbohydrate Moiety of Secreted Stage-Specific Glycoprotein 4 Participates in Host Cell Invasion by Trypanosoma cruzi Extracellular Amastigotes. Front. Microbiol. 2018;9:693. doi: 10.3389/fmicb.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marino C., Lederkremer R. Galactose configurations in nature with emphasis on the biosynthesis of galactofuranose in glycans. In: Pomin V.H., editor. Galactose: Structure and Function in Biology and Medicine. Nova Science Publishers, Inc.; New York, NY, USA: 2014. pp. 107–133. [Google Scholar]
- 61.De Lederkremer R.M., Colli W. Galactofuranose-containing glycoconjugates in trypanosomatids. Glycobiology. 1995;5:547–552. doi: 10.1093/glycob/5.6.547. [DOI] [PubMed] [Google Scholar]
- 62.McConville M.J., Thomas-Oates J.E., Ferguson M.A., Homans S.W. Structure of the lipophosphoglycan from Leishmania major. J. Biol. Chem. 1990;265:19611–19623. [PubMed] [Google Scholar]
- 63.Späth G.F., Epstein L., Leader B., Singer S.M., Avila H.A., Turco S.J., Beverley S.M. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jankute M., Cox J.A., Harrison J., Besra G.S. Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol. 2015;69:405–423. doi: 10.1146/annurev-micro-091014-104121. [DOI] [PubMed] [Google Scholar]
- 65.Latge J.P. Galactofuranose containing molecules in Aspergillus fumigatus. Med. Mycol. 2009;47(Suppl. S1):S104–S109. doi: 10.1080/13693780802258832. [DOI] [PubMed] [Google Scholar]
- 66.Marino C., Rinflerch A., de Lederkremer R.M. Galactofuranose antigens, a target for diagnosis of fungal infections in humans. Future Sci. OA. 2017;3:FSO199. doi: 10.4155/fsoa-2017-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seničar M., Lafite P., Eliseeva S.V., Petoud S., Landemarre L., Daniellou R. Galactofuranose-Related Enzymes: Challenges and Hopes. Int. J. Mol. Sci. 2020;21:3465. doi: 10.3390/ijms21103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevenson G., Neal B., Liu D., Hobbs M., Packer N.H., Batley M., Redmond J.W., Lindquist L., Reeves P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 1994;176:4144–4156. doi: 10.1128/JB.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nassau P.M., Martin S.L., Brown R.E., Weston A., Monsey D., McNeil M.R., Duncan K. Galactofuranose biosynthesis in Escherichia coli K-12: Identification and cloning of UDP-galactopyranose mutase. J. Bacteriol. 1996;178:1047–1052. doi: 10.1128/JB.178.4.1047-1052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanner J.J., Boechi L., Andrew McCammon J., Sobrado P. Structure, mechanism, and dynamics of UDP-galactopyranose mutase. Arch. Biochem. Biophys. 2014;544:128–141. doi: 10.1016/j.abb.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kizjakina K., Tanner J.J., Sobrado P. Targeting UDP-galactopyranose mutases from eukaryotic human pathogens. Curr. Pharm. Des. 2013;19:2561–2573. doi: 10.2174/1381612811319140007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beverley S.M., Owens K.L., Showalter M., Griffith C.L., Doering T.L., Jones V.C., McNeil M.R. Eukaryotic UDP-galactopyranose mutase (GLF gene) in microbial and metazoal pathogens. Eukaryot. Cell. 2005;4:1147–1154. doi: 10.1128/EC.4.6.1147-1154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dhatwalia R., Singh H., Oppenheimer M., Sobrado P., Tanner J.J. Crystal Structures of Trypanosoma cruzi UDP-Galactopyranose Mutase Implicate Flexibility of the Histidine Loop in Enzyme Activation. Biochemistry. 2012;51:4968–4979. doi: 10.1021/bi300498c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Sayed N.M., Myler P.J., Bartholomeu D.C., Nilsson D., Aggarwal G., Tran A.N., Ghedin E., Worthey E.A., Delcher A.L., Blandin G., et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 75.Stoco P.H., Aresi C., Lückemeyer D.D., Sperandio M.M., Sincero T.C.M., Steindel M., Miletti L.C., Grisard E.C. Trypanosoma rangeli expresses a β-galactofuranosyl transferase. Exp. Parasitol. 2012;130:246–252. doi: 10.1016/j.exppara.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 76.De Lederkremer R.M., Alves M.J.M., Fonseca G.C., Colli W. A lipopeptidophosphoglycan from Trypanosoma cruzi (epimastigota): Isolation, purification and carbohydate composition. Biochim. Biophys. Acta Gen. Subj. 1976;444:85–96. doi: 10.1016/0304-4165(76)90226-9. [DOI] [PubMed] [Google Scholar]
- 77.Carreira J.C., Jones C., Wait R., Previato J.O., Mendonça-Previato L. Structural variation in the glycoinositolphospholipids of different strains of Trypanos. Cruzi. Glycoconj. J. 1996;13:955–966. doi: 10.1007/BF01053191. [DOI] [PubMed] [Google Scholar]
- 78.Miletti L.C., Mariño K., Marino C., Colli W., Alves M.J., de Lederkremer R.M. Evidence for exo beta-d-galactofuranosidase in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2003;127:85–88. doi: 10.1016/S0166-6851(02)00307-9. [DOI] [PubMed] [Google Scholar]
- 79.De Lederkremer R.M., Bertello L.E. Glycoinositolphospholipids, free and as anchors of proteins, in Trypanosoma cruzi. Curr. Pharm. Des. 2001;7:1165–1179. doi: 10.2174/1381612013397519. [DOI] [PubMed] [Google Scholar]
- 80.Previato J.O., Wait R., Jones C., DosReis G.A., Todeschini A.R., Heise N., Previato L.M. Glycoinositolphospholipid from Trypanosoma cruzi: Structure, biosynthesis and immunobiology. Adv. Parasitol. 2004;56:1–41. doi: 10.1016/s0065-308x(03)56001-8. [DOI] [PubMed] [Google Scholar]
- 81.Previato J.O., Jones C., Xavier M.T., Wait R., Travassos L.R., Parodi A.J., Mendonça-Previato L. Structural characterization of the major glycosylphosphatidylinositol membrane-anchored glycoprotein from epimastigote forms of Trypanosoma cruzi Y-strain. J. Biol. Chem. 1995;270:7241–7250. doi: 10.1074/jbc.270.13.7241. [DOI] [PubMed] [Google Scholar]
- 82.Yoshida N., Mortara R.A., Araguth M.F., Gonzalez J.C., Russo M. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect. Immun. 1989;57:1663–1667. doi: 10.1128/IAI.57.6.1663-1667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshida N. Molecular basis of mammalian cell invasion by Trypanosoma cruzi. Anais da Academia Brasileira de Ciencias. 2006;78:87–111. doi: 10.1590/S0001-37652006000100010. [DOI] [PubMed] [Google Scholar]
- 84.Demeu L.M.K., Soares R.J., Miranda J.S., Pacheco-Lugo L.A., Oliveira K.G., Cortez Plaza C.A., Billiald P., Ferreira de Moura J., Yoshida N., Alvarenga L.M., et al. Engineering a single-chain antibody against Trypanosoma cruzi metacyclic trypomastigotes to block cell invasion. PLoS ONE. 2019;14:e0223773. doi: 10.1371/journal.pone.0223773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fonseca L.M., Garcez T.C., Penha L., Freire D.E.L.L., Maes E., Costa K.M., Mendonça-Previato L., Previato J.O. Expanding the knowledge of the chemical structure of glycoconjugates from Trypanosoma cruzi TcI genotype. Contribution to taxonomic studies. An. Acad. Bras. Ciênc. 2016;88:1519–1529. doi: 10.1590/0001-3765201620160386. [DOI] [PubMed] [Google Scholar]
- 86.Gallo-Rodriguez C., Varela O., Lederkremer R.M. First synthesis of beta-d-Galf(1-4)GlcNAc, a structural unit attached O-Glycosidically in glycoproteins of Trypanosoma cruzi. J. Org. Chem. 1996;61:1886–1889. doi: 10.1021/jo951934m. [DOI] [PubMed] [Google Scholar]
- 87.Mendoza V.M., Kashiwagi G.A., de Lederkremer R.M., Gallo-Rodriguez C. Synthesis of trisaccharides containing internal galactofuranose O-linked in Trypanosoma cruzi mucins. Carbohydr. Res. 2010;345:385–396. doi: 10.1016/j.carres.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 88.Gallo-Rodriguez C., Varela O., de Lederkremer R.M. One-pot synthesis of β-d-Galf(1→4)[β-d-Galp(1→6)]-d-GlcNAc, a ‘core’ trisaccharide linked O-glycosidically in glycoproteins of Trypanosoma cruzi. Carbohydr. Res. 1997;305:163–170. doi: 10.1016/S0008-6215(97)00256-5. [DOI] [PubMed] [Google Scholar]
- 89.Gallo-Rodriguez C., Gil-Libarona M.A., Mendoza V.M., de Lederkremer R.M. Synthesis of β-d-Galp-(1→3)-β-d-Galp-(1→6)-[β-d-Galf-(1→4)]-d-GlcNAc, a tetrasaccharide component of mucins of Trypanosoma cruzi. Tetrahedron. 2002;58:9373–9380. doi: 10.1016/S0040-4020(02)01226-7. [DOI] [Google Scholar]
- 90.Mendoza V.M., Agusti R., Gallo-Rodriguez C., de Lederkremer R.M. Synthesis of the O-linked pentasaccharide in glycoproteins of Trypanosoma cruzi and selective sialylation by recombinant trans-sialidase. Carbohydr. Res. 2006;341:1488–1497. doi: 10.1016/j.carres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 91.Kashiwagi G.A., Mendoza V.M., de Lederkremer R.M., Gallo-Rodriguez C. Synthesis of the O-linked hexasaccharide containing β-d-Galf-(1→2)-β-d-Galf in Trypanosoma cruzi mucins. Org. Biomol. Chem. 2012;10:6322–6332. doi: 10.1039/c2ob25741f. [DOI] [PubMed] [Google Scholar]
- 92.Agustí R., Giorgi M.E., Mendoza V.M., Kashiwagi G.A., de Lederkremer R.M., Gallo-Rodriguez C. Synthesis of the O-linked hexasaccharide containing β-d-Galp-(1→2)-d-Galf in Trypanosoma cruzi mucins. Differences on sialylation by trans-sialidase of the two constituent hexasaccharides. Bioorganic Med. Chem. 2015;23:1213–1222. doi: 10.1016/j.bmc.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 93.Kashiwagi G.A., Cori C.R., de Lederkremer R.M., Gallo-Rodriguez C. Synthesis of the hexasaccharide from Trypanosoma cruzi mucins with the Galp(1→2)Galf unit constructed with a superarmed thiogalactopyranosyl donor. Carbohydr. Res. 2019;482:107734. doi: 10.1016/j.carres.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 94.Zingales B., Andrade S.G., Briones M.R., Campbell D.A., Chiari E., Fernandes O., Guhl F., Lages-Silva E., Macedo A.M., Machado C.R., et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 95.Nogueira N.F.S., Gonzalez M.S., Gomes J.E., de Souza W., Garcia E.S., Azambuja P., Nohara L.L., Almeida I.C., Zingales B., Colli W. Trypanosoma cruzi: Involvement of glycoinositolphospholipids in the attachment to the luminal midgut surface of Rhodnius prolixus. Exp. Parasitol. 2007;116:120–128. doi: 10.1016/j.exppara.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 96.De los Milagros Cámara M., Balouz V., Centeno Cameán C., Cori C.R., Kashiwagi G.A., Gil S.A., Macchiaverna N.P., Cardinal M.V., Guaimas F., Lobo M.M., et al. Trypanosoma cruzi surface mucins are involved in the attachment to the Triatoma infestans rectal ampoule. PLoS Negl. Trop. Dis. 2019;13:e0007418. doi: 10.1371/journal.pntd.0007418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Godoy L.M., Marchini F.K., Pavoni D.P., Rampazzo Rde C., Probst C.M., Goldenberg S., Krieger M.A. Quantitative proteomics of Trypanosoma cruzi during metacyclogenesis. Proteomics. 2012;12:2694–2703. doi: 10.1002/pmic.201200078. [DOI] [PubMed] [Google Scholar]
- 98.Almeida I.C., Ferguson M.A., Schenkman S., Travassos L.R. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas’ disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 1994;304:793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lantos A.B., Carlevaro G., Araoz B., Ruiz Diaz P., Camara M.D.L.M., Buscaglia C.A., Bossi M., Yu H., Chen X., Bertozzi C.R., et al. Sialic acid glycobiology unveils Trypanosoma cruzi trypomastigote membrane physiology. PLoS Pathog. 2016;12:e1005559. doi: 10.1371/journal.ppat.1005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soares R.P., Torrecilhas A.C., Assis R.R., Rocha M.N., Moura e Castro F.A., Freitas G.F., Murta S.M., Santos S.L., Marques A.F., Almeida I.C., et al. Intraspecies variation in Trypanosoma cruzi GPI-mucins: Biological activities and differential expression of α-galactosyl residues. Am. J. Trop Med. Hyg. 2012;87:87–96. doi: 10.4269/ajtmh.2012.12-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galili U., Clark M.R., Shohet S.B., Buehler J., Macher B.A. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1–3Gal epitope in primates. Proc. Natl. Acad. Sci. USA. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galili U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140:1–11. doi: 10.1111/imm.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ashmus R.A., Schocker N.S., Cordero-Mendoza Y., Marques A.F., Monroy E.Y., Pardo A., Izquierdo L., Gállego M., Gascon J., Almeida I.C., et al. Potential use of synthetic α-galactosyl-containing glycotopes of the parasite Trypanosoma cruzi as diagnostic antigens for Chagas disease. Org. Biomol. Chem. 2013;11:5579–5583. doi: 10.1039/c3ob40887f. [DOI] [PubMed] [Google Scholar]
- 104.Ortega-Rodriguez U., Portillo S., Ashmus R.A., Duran J.A., Schocker N.S., Iniguez E., Montoya A.L., Zepeda B.G., Olivas J.J., Karimi N.H., et al. T. cruzi Infection. Volume 1955. Humana Press; New York, NY, USA: 2019. Purification of glycosylphosphatidylinositol-anchored mucins from Trypanosoma cruzi trypomastigotes and synthesis of α-Gal-containing neoglycoproteins: Application as biomarkers for reliable diagnosis and early assessment of chemotherapeutic outcomes of chagas disease; pp. 287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brito C.R., McKay C.S., Azevedo M.A., Santos L.C., Venuto A.P., Nunes D.F., D’Ávila D.A., Rodrigues da Cunha G.M., Almeida I.C., Gazzinelli R.T., et al. Virus-like particle display of the α-Gal epitope for the diagnostic assessment of chagas disease. ACS Infect. Dis. 2016;2:917–922. doi: 10.1021/acsinfecdis.6b00114. [DOI] [PubMed] [Google Scholar]
- 106.Pinazo M.-J., Thomas M.-C., Bustamante J., Almeida I.C.D., Lopez M.-C., Gascon J. Biomarkers of therapeutic responses in chronic Chagas disease: State of the art and future perspectives. Mem. Inst. Oswaldo Cruz. 2015;110:422–432. doi: 10.1590/0074-02760140435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schocker N.S., Portillo S., Brito C.R., Marques A.F., Almeida I.C., Michael K. Synthesis of Galα(1,3)Galβ(1,4)GlcNAcα-, Galβ(1,4)GlcNAcα- and GlcNAc-containing neoglycoproteins and their immunological evaluation in the context of Chagas disease. Glycobiology. 2016;26:39–50. doi: 10.1093/glycob/cwv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lopez R., Giorgi M.E., Melgarejo L.T., Ducrey I., Balouz V., González-Salas D., Cámara M.L.M., Buscaglia C.A., de Lederkremer R.M., Marino C. Synthesis and characterization of α-d-Galp-(1→3)-β-d-Galp epitope-containing neoglycoconjugates for chagas disease serodiagnosis. Carbohydr. Res. 2019;478:58–67. doi: 10.1016/j.carres.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 109.Portillo S., Zepeda B.G., Iniguez E., Olivas J.J., Karimi N.H., Moreira O.C., Marques A.F., Michael K., Maldonado R.A., Almeida I.C. A prophylactic α-Gal-based glycovaccine effectively protects against murine acute Chagas disease. Npj Vaccines. 2019;4:13. doi: 10.1038/s41541-019-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Subramaniam K.S., Austin V., Schocker N.S., Montoya A.L., Anderson M.S., Ashmus R.A., Mesri M., Al-Salem W., Almeida I.C., Michael K., et al. Anti-α-Gal antibodies detected by novel neoglycoproteins as a diagnostic tool for old world cutaneous leishmaniasis caused by Leishmania major. Parasitology. 2018;145:1758–1764. doi: 10.1017/S0031182018000860. [DOI] [PubMed] [Google Scholar]
- 111.Iniguez E., Schocker N.S., Subramaniam K., Portillo S., Montoya A.L., Al-Salem W.S., Torres C.L., Rodriguez F., Moreira O.C., Acosta-Serrano A., et al. An α-Gal-containing neoglycoprotein-based vaccine partially protects against murine cutaneous leishmaniasis caused by Leishmania major. PLoS Negl. Trop. Dis. 2017;11:e0006039. doi: 10.1371/journal.pntd.0006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moura A.P.V., Santos L.C.B., Brito C.R.N., Valencia E., Junqueira C., Filho A.A.P., Sant’Anna M.R.V., Gontijo N.F., Bartholomeu D.C., Fujiwara R.T., et al. Virus-like particle display of the α-Gal carbohydrate for vaccination against leishmania infection. ACS Cent. Sci. 2017;3:1026–1031. doi: 10.1021/acscentsci.7b00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramasamy R., Reese R.T. Terminal galactose residues and the antigenicity of Plasmodium falciparum glycoproteins. Mol. Biochem. Parasitol. 1986;19:91–101. doi: 10.1016/0166-6851(86)90113-1. [DOI] [PubMed] [Google Scholar]
- 114.Cabezas-Cruz A., de la Fuente J. Immunity to α-Gal: The opportunity for malaria and tuberculosis control. Front. Immunol. 2017;8:1733. doi: 10.3389/fimmu.2017.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rabinovich G.A., Gruppi A. Galectins as immunoregulators during infectious processes: From microbial invasion to the resolution of the disease. Parasite Immunol. 2005;27:103–114. doi: 10.1111/j.1365-3024.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 116.Moody T.N., Ochieng J., Villalta F. Novel mechanism that Trypanosoma cruzi uses to adhere to the extracellular matrix mediated by human galectin-3. FEBS Lett. 2000;470:305–308. doi: 10.1016/S0014-5793(00)01347-8. [DOI] [PubMed] [Google Scholar]
- 117.Turner C.W., Lima M.F., Villalta F. Trypanosoma cruzi uses a 45-kDa mucin for adhesion to mammalian cells. Biochem. Biophys. Res. Commun. 2002;290:29–34. doi: 10.1006/bbrc.2001.6189. [DOI] [PubMed] [Google Scholar]
- 118.Kleshchenko Y.Y., Moody T.N., Furtak V.A., Ochieng J., Lima M.F., Villalta F. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect. Immun. 2004;72:6717–6721. doi: 10.1128/IAI.72.11.6717-6721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vray B., Camby I., Vercruysse V., Mijatovic T., Bovin N.V., Ricciardi-Castagnoli P., Kaltner H., Salmon I., Gabius H.-J., Kiss R. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology. 2004;14:647–657. doi: 10.1093/glycob/cwh068. [DOI] [PubMed] [Google Scholar]
- 120.De Oliveira Chain M., de Medeiros Paiva C.A., de Oliveira Maciel I., Neto A.N., Castro V.F., Oliveira C.P., dos Santos Mendonça B., de Moraes G.N., dos Reis S.A., de Carvalho M.A., et al. Galectin-3 mediates survival and apoptosis pathways during Trypanosoma cruzi–host cell interplay. Exp. Parasitol. 2020;216:107932. doi: 10.1016/j.exppara.2020.107932. [DOI] [PubMed] [Google Scholar]
- 121.Poncini C.V., Ilarregui J.M., Batalla E.I., Engels S., Cerliani J.P., Cucher M.A., van Kooyk Y., González-Cappa S.M., Rabinovich G.A. Infection imparts a regulatory program in dendritic cells and T cells via Galectin-1–dependent mechanisms. J. Immunol. 2015;195:3311. doi: 10.4049/jimmunol.1403019. [DOI] [PubMed] [Google Scholar]
- 122.Benatar A.F., García G.A., Bua J., Cerliani J.P., Postan M., Tasso L.M., Scaglione J., Stupirski J.C., Toscano M.A., Rabinovich G.A., et al. Galectin-1 prevents infection and damage induced by Trypanosoma cruzi on cardiac cells. PLoS Negl. Trop. Dis. 2015;9:e0004148. doi: 10.1371/journal.pntd.0004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frasch A.C. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol. Today Pers. Ed. 2000;16:282–286. doi: 10.1016/S0169-4758(00)01698-7. [DOI] [PubMed] [Google Scholar]
- 124.Yoshida N., Dorta M.L., Ferreira A.T., Oshiro M.E., Mortara R.A., Acosta-Serrano A., Favoreto Júnior S. Removal of sialic acid from mucin-like surface molecules of Trypanosoma cruzi metacyclic trypomastigotes enhances parasite-host cell interaction. Mol. Biochem. Parasitol. 1997;84:57–67. doi: 10.1016/S0166-6851(96)02783-1. [DOI] [PubMed] [Google Scholar]
- 125.Agustí R., Giorgi M.E., Mendoza V.M., Gallo-Rodriguez C., de Lederkremer R.M. Comparative rates of sialylation by recombinant trans-sialidase and inhibitor properties of synthetic oligosaccharides from Trypanosoma cruzi mucins-containing galactofuranose and galactopyranose. Bioorg. Med. Chem. 2007;15:2611–2616. doi: 10.1016/j.bmc.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 126.Schenkman S., Ferguson M.A.J., Heise N., Cardoso de Almeida M.L., Mortara R.A., Yoshida N. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1993;59:293–303. doi: 10.1016/0166-6851(93)90227-O. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe Costa R., da Silveira J.F., Bahia D. Interactions between Trypanosoma cruzi secreted proteins and host cell signaling pathways. Front. Microbiol. 2016;7:388. doi: 10.3389/fmicb.2016.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]