Abstract
The normal cellular isoform of prion protein, designated PrPC, is constitutively converted to the abnormally folded, amyloidogenic isoform, PrPSc, in prion diseases, which include Creutzfeldt-Jakob disease in humans and scrapie and bovine spongiform encephalopathy in animals. PrPC is a membrane glycoprotein consisting of the non-structural N-terminal domain and the globular C-terminal domain. During conversion of PrPC to PrPSc, its 2/3 C-terminal region undergoes marked structural changes, forming a protease-resistant structure. In contrast, the N-terminal region remains protease-sensitive in PrPSc. Reverse genetic studies using reconstituted PrPC-knockout mice with various mutant PrP molecules have revealed that the N-terminal domain has an important role in the normal function of PrPC and the conversion of PrPC to PrPSc. The N-terminal domain includes various characteristic regions, such as the positively charged residue-rich polybasic region, the octapeptide repeat (OR) region consisting of five repeats of an octapeptide sequence, and the post-OR region with another positively charged residue-rich polybasic region followed by a stretch of hydrophobic residues. We discuss the normal functions of PrPC, the conversion of PrPC to PrPSc, and the neurotoxicity of PrPSc by focusing on the roles of the N-terminal regions in these topics.
Keywords: prion protein, prion, prion disease, neurodegeneration, protein conformation
1. Introduction
Conformational conversion of the normal cellular isoform of prion protein, designated PrPC, to the abnormally folded, amyloidogenic isoform, PrPSc, is a key pathogenic event in prion diseases, a group of fatal neurodegenerative disorders that include Creutzfeldt–Jakob disease (CJD) in humans, scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle, and chronic wasting disease in deer [1,2,3,4]. These diseases are pathologically characterized by neuronal cell loss, spongiform degeneration, gliosis, and PrPSc accumulation in the brain [5]. Prions, or proteinaceous infectious particles, are the causative agents of these diseases [6,7]. It is believed that prions consist of, if not entirely, PrPSc molecules, and catalyze conformational conversion of PrPC to PrPSc through a seeded protein polymerization mechanism, eventually propagating PrPSc or prions themselves [6,7]. Indeed, it has been shown that mice devoid of PrPC (Prnp0/0) are resistant to prion infection, neither propagating prions nor PrPSc in their brains nor developing disease even after intracerebral inoculation with prions [8,9,10,11].
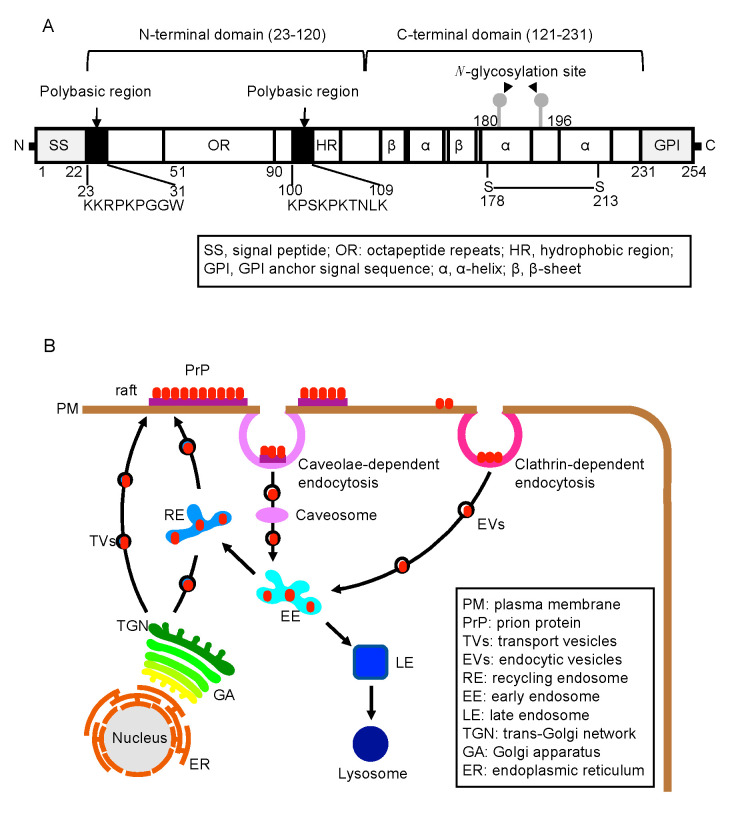
PrPC is a highly conserved, glycosylphosphatidylinositol (GPI)-anchored membrane glycoprotein among mammalian species [12]. It is expressed most abundantly in the central nervous system, particularly by neurons, and to a lesser extent in other non-neuronal tissues, such as the lymphoreticular system, lung, and kidney [13]. PrPC consists of two domains; the highly flexible, nonstructural N-terminal (residues 23–120) and the globular C-terminal (residues 121–231) [14,15,16] (Figure 1A). The globular C-terminal domain is composed of three α-helices and two short anti-parallel ß-sheets. Upon conversion to PrPSc, PrPC undergoes marked structural changes in its 2/3 C-terminal region to form a proteinase K (PK)-resistant structure, while most regions of the N-terminal domain remain PK-sensitive [13]. Reverse genetic studies using reconstituted Prnp0/0 mice and various mutant PrP molecules have revealed that the N-terminal domain has an important role not only in the normal function of PrPC but also in the conversion of PrPC to PrPSc. The N-terminal domain includes several characteristic regions, such as the so-called polybasic region (residues 23–31), which is rich in positively charged residues, the octapeptide repeat (OR) region (residues 51–90) consisting of five repeats of an octapeptide sequence, and the post-OR region (residues 91–120) including the second polybasic region followed by a stretch of hydrophobic amino acid residues [1,2,3,4] (Figure 1A). Here we discuss the role of each N-terminal region in the normal function of PrPC, the conversion of PrPC to PrPSc, and the neurotoxicity of PrPSc.
Figure 1.
Structure and biosynthesis of PrPC. (A) Structural configuration of PrPC. Arabic numbers indicate positions of amino acids. (B) Biosynthetic pathways of PrPC, including the vesicle transport pathway from the ER to the plasma membrane, particularly raft domains, and the clathrin- or caveolae-dependent endocytic pathway, which connects to recycling pathway or degradation pathway to lysosomes.
2. The N-Terminal Domain in the Function of PrPC
2.1. Biosynthesis of PrPC
The gene for PrPC, termed Prnp, in human and mouse consists of 2 and 3 exons and resides on chromosome 20 and 2, respectively. The protein coding sequence lies within the last single exon [17,18]. PrPC is synthesized as a precursor protein in the endoplasmic reticulum (ER). The N-terminal and C-terminal sequences, which are rich in hydrophobic residues, are removed as a signal peptide sequence and a GPI-anchor signal sequence, respectively, in the ER (Figure 1A) [17,18]. PrPC also undergoes several post-translational modifications en route to the cell surface, including a GPI anchor attachment at the C-terminus, N-glycosylation at two sites, and formation of a disulfide bond in the C-terminal domain (Figure 1A) [19,20,21,22,23,24]. On the cell surface, PrPC is predominantly localized at the so-called “raft” domains and constitutively internalized via clathrin- and caveolae-dependent endocytosis (Figure 1B) [25,26,27]. Some of the internalized PrPC molecules are recycled to the cell surface and others are trafficked to lysosomes for degradation (Figure 1B) [28,29].
Copper is known to bind to the OR region and induce the clathrin-dependent internalization of PrPC [30]. It has been suggested that copper binding could cause conformational changes in the OR region and thereby dissociate PrPC from conjectural molecules located at raft domains, and that dissociated PrPC then moves to non-raft domains, where it interacts with other conjectural non-raft molecules through the N-terminal polybasic region to be endocytosed via clathrin-coated vesicles [30]. We have shown that sortilin, a type 1 glycoprotein in the vacuolar protein sorting 10 protein family, interacts with the N-terminal domain of PrPC and functions as a sorting receptor for lysosomal degradation of PrPC [31]. Sortilin also interacts with PrPSc and facilitates its lysosomal degradation [31]. We also have shown that sortilin-knockout mice develop prion disease with shorter incubation times and rapid brain accumulation of PrPSc after inoculation with prions, compared to control wild-type (WT) mice [31], suggesting that the sortilin-mediated trafficking of PrPC and PrPSc to lysosomes could be a host defense mechanism in prion diseases. Low-density lipoprotein receptor-related protein 1 has also been reported as a cargo receptor for PrPC for transport from the Golgi apparatus to the cell surface and from the cell surface to endosomes [32].
2.2. Various Abnormal Phenotypes Are Spontaneously Observed in Prnp0/0 Mice
Prnp0/0 mice are born with no obvious defects, indicating that PrPC could be dispensable for embryonic development [11,33,34]. However, various neurophysiological and neuropathological abnormalities have been reported in Prnp0/0 mice, including poor performance in certain behavioral tests [35], impaired long-term potentiation (LTP) in the hippocampal CA1 neurons [36], altered sleep and circadian rhythms [37], demyelination in spinal cords and peripheral nerves [38], and abnormal olfactory function [39,40]. These results suggest that PrPC is involved in various neuronal functions. However, normal LTP in Prnp0/0 mice has been reported by other investigators [41].
2.3. The OR Region in the Cell-Protective Role of PrPC
We and others have shown that Prnp0/0 mice are vulnerable to ischemic brain, heart, or kidney damage, displaying higher apoptotic cell death and higher oxidative stress in the damaged tissues [42,43,44,45,46]. We also recently reported that Prnp0/0 mice are highly sensitive to infection with influenza A viruses (IAVs), showing higher morbidity and mortality with higher inflammation, higher apoptotic cell death, and higher oxidative stress in their lungs [47]. Treatment with a scavenger for reactive oxygen species (ROS) or an inhibitor for ROS-generating xanthine oxidase rescued Prnp0/0 mice from lethal IAV infection [47]. In contrast, PrP molecules lacking the OR region failed to protect Prnp0/0 mice from lethal IAV infection and ischemic brain damage [47,48]. These results suggest that PrPC could play a cell-protective role against oxidative stress through the OR region. The OR region is known to bind copper [49]. Indeed, the copper content and enzymatic activity of copper/zinc-dependent superoxide dismutase (SOD) were lower in Prnp0/0 lungs and brains than in control WT tissues [47,49]. It is thus possible that PrPC could function as a transporter of the OR region-bound copper to copper/zinc-SOD, thereby regulating enzyme activity and eventually protecting from oxidative stress. It was reported that PrPC itself might have SOD-like activity [50]. However, other investigators have failed to detect SOD activity in PrPC in vitro and in vivo [51,52].
The OR region is also suggested to be involved in other cell-protective mechanisms of PrPC. Overexpression of PrPC, but not an OR-lacking PrP molecule, was shown to protect against Bax-mediated apoptosis in human primary neurons [53], suggesting that PrPC could function as an anti-apoptotic molecule through the OR region. Oh et al. also reported that autophagy was activated in Prnp0/0 hippocampal neuronal cultured cells under serum deprivation, and that expression of PrPC prevented the activation of autophagy in the cells, but an OR-deleted PrP mutant did not [54], suggesting that PrPC could regulate autophagy activity in neuronal cells through the OR region. It remains to be determined if these functions of PrPC are attributable to the activation of copper/zinc-SOD.
2.4. The Polybasic Region in the Function of PrPC
The polybasic region is also suggested to be involved in the anti-oxidative activity of PrPC. Oxidative stress was shown to enhance cleavage of PrPC, releasing the N-terminal fragment, termed N2, which encompasses residues 23–89 including the polybasic region [55], and the N2 fragment protected neuronal cells against oxidative stress through stimulation of MEK1 signaling [56]. Two proline residues in the polybasic region were shown to be important for the N2-mediated anti-oxidative activity [55]. Other roles have also been reported for the polybasic region including that it is involved in mediating the interaction of PrPC with tubulin or glycosaminoglycan [57,58,59,60], the ß-secretase-mediated cleavage of the Alzheimer’s amyloid precursor protein [61], and DNA repair [62].
3. The N-Terminal Domain of PrPC in Prion Disease
3.1. The Polybasic Region in Prion Disease
Reconstituted Prnp0/0 mice by transgenic introduction of a mutant PrP with a deletion of the polybasic region residues 23–31, designated Tg(PrP∆23–31)/Prnp0/0 mice, were shown to develop prion disease with markedly elongated incubation times and delayed accumulation of PrPSc∆23–31 in their brains after inoculation with RML scrapie prions (Table 1) [63]. PrPSc∆23–31 accumulated in the brains of Tg(PrP∆23–31)/Prnp0/0 mice showed similar resistance to PK to WT PrPSc [63], suggesting that the polybasic region does not affect the PK-resistance of PrPSc. These results suggest that the polybasic region could play a crucial role in the pathogenesis of prion diseases. We have shown that Tg(PrP∆25–50)/Prnp0/0 mice developed disease without elongated incubation times after infection with RML and 22L prions (Table 1) [64], suggesting that the remaining residues 23 and 24 in PrP∆25–50 could be enough for the polybasic region to support prion pathogenesis. However, it was reported that incubation times were only slightly longer or not elongated at all in Tg(PrP∆23–26)/Prnp0/0 mice after infection with 127S and LA19K scrapie prions and BSE prions (Table 1) [65]. PrP∆23–26 includes intact residues 27–31, but lacks residues 23 and 24 in the polybasic region. It is thus possible that the polybasic region might require that both residues 23–24 and 27–31 are intact to fully support prion pathogenesis [64]. Consistent with this idea, mutations of lysine residues at positions 24 and 27 together with a mutation of an arginine residue at position 25 rendered ovine PrP highly resistant to 127S and LA19K scrapie prions and BSE prions (Table 1) [65]. We also showed that Prnp0/0 mice transgenic for mouse PrP with substitutions of lysine residues at positions 23, 24, and 27 to alanine residues, or PrP3K3A, markedly reduced their susceptibility to RML and 22L scrapie prions (Table 1) [66], suggesting that positively charged residues in residues 23–24 and 27–31 could be important for the polybasic region to support prion pathogenesis. No PK-resistant PrP3K3A was spontaneously produced in the brain of uninfected Tg(PrP3K3A)/Prnp0/0 mice [66], suggesting that mutations in the polybasic region might not cause structural changes in mutant PrPs.
Table 1.
Effects of various mutations in the polybasic region of PrPC on acquired prion diseases.
| Disease Type | PrPs | Amino Acid Sequence of the Polybasic Region (Residues 23–31) 1 | Susceptibility to Prions | References |
|---|---|---|---|---|
| Acquired prion disease | WT PrP | KKRPKPGGW | • Normal. | |
| PrP∆23–31 | − − − − − − − − − | • Markedly reduced to RML scrapie prions. | [63] | |
| PrP∆25–50 | KK− − − − − − − | • Not reduced to RML and 22L scrapie prions | [64] | |
| PrP∆23–26 | − − − −KPGGW | • Only slightly or not reduced to 127S and LA19K scrapie prions and BSE prions. | [65] | |
| PrP-M | KQHPHPGGW | • Markedly reduced to 127S and LA19K prions and BSE prions | [65] | |
| PrP3K3A | AARPAPGGW | • Markedly reduced to RML and 22L scrapie prions. | [66] |
1 Amino acids are indicated by single letters. Underline letters indicate amino acids mutated.
3.2. The OR Region in Prion Disease
Insertion of various numbers of an OR sequence, ranging from one to nine, and deletion of one OR sequence in the OR region have been identified in patients with hereditary CJD [67]. Brain homogenates from patients with five, seven, or eight extra OR sequences in PrP can transmit the disease to animals after intracerebral inoculation [68]. This suggests that disruption of the integrity of the OR region by the insertion or deletion of the OR sequence could cause structural instability of mutated PrPs, ultimately leading to their spontaneous conversion to pathogenic, infectious PrPs. We failed to detect PK-resistant PrP in the brains of Tg(PrPΔOR)/Prnp0/0 mice, which express PrP with a deletion of the OR region alone (Table 2) [69,70], suggesting that spontaneous conversion of mutated PrPs with extra OR sequences to PK-resistant PrPs could be due to gain-of-function, but not due to loss-of-function, of the mutated OR region. Consistent with this, Tg(PG14)/Prnp0/0 mice, which express a PrP mutant with nine extra OR sequences in the OR region, developed spontaneous cerebellar neurodegeneration including granule cell death, with very slight but substantial accumulation of PK-resistant PrPScPG14 in their brains (Table 2) [71,72]. However, PrPScPG14 had no prion infectivity in animal bioassays (Table 2) [73]. Also, transgenic expression of bovine PrP with four extra OR sequences, or bo10OR-PrP, caused a slowly progressive neurological disorder with ataxia, vacuolization, gliosis, and cerebellar granule cell loss in Prnp0/0 mice (Table 2) [74]. Insoluble and slightly PK-resistant 10OR-PrPSc molecules accumulated in their brains, but no prion infectivity was found associated with the insoluble 10OR-PrPSc (Table 2) [74]. These results indicate that PrPPG14 and bo10OR-PrP spontaneously convert to PrPScPG14 and 10OR-PrPSc, respectively, with structural features shared with PrPSc that are responsible for the neurotoxicity but not prion infectivity. These results also suggest that the structural features of PrPSc that contribute to its neurotoxicity and prion infectivity are not identical.
Table 2.
Effects of various mutations in the OR region of PrPC on hereditary and acquired prion diseases.
| Disease Type | PrPs | Number of the OR Sequence | Clinicopathological Features | References |
| Hereditary prion disease | PG14 | 14 1 | • Spontaneously develop cerebellar neurodegeneration. • Accumulate very slightly but substantially PK-resistant PrPScPG14 in the brain. • No prion infectivity associated with PrPScPG14. |
[71,72,73] |
| Bo10OR-PrP | 10 2 | • Spontaneously develop cerebellar neurodegeneration. • Accumulate insoluble and slightly PK-resistant 10OR-PrPSc in their brains. • No prion infectivity associated with 10OR-PrPSc. |
[74] | |
| Disease Type | PrPs | Number of the OR Sequence | Susceptibility to Prions | References |
| Acquired prion disease | PrP∆OR | 0 1 | • Reduced to BSE prions, but not to RML and 22L scrapie prions. | [70] |
| Bo7OR-PrP | 7 2 | • Increased to BSE prions. | [75] | |
| Bo10OR-PrP | 10 2 | • Increased to BSE prions. | [74] | |
| PrP(TetraH>G) | 51 (with 4 histidine residues mutated to glycine residues) | • Reduced to RML prions. | [76] |
1 Normal mouse PrPC contains 5 repeats of the OR sequence. 2 Normal bovine PrPC contains 6 repeats of the OR sequence.
The OR region is also involved in prion infection. We have shown that Tg(PrPΔOR)/Prnp0/0 mice are highly resistant to BSE prions (Table 2) [70]. They developed the disease with markedly elongated incubation times with delayed accumulation of PrPScΔOR in their brains after inoculation with BSE prions (Table 2) [70]. Consistent with our results, an increasing number of OR insertions contrarily enhances BSE pathogenesis in mice. Prnp0/0 mice expressing bovine PrP with one extra OR sequence had shortened incubation times when compared with Prnp0/0 mice expressing WT bovine PrP, or bo6OR-PrP, after infection with BSE prions (Table 2) [75]. BSE-inoculated Tg(bo10OR-PrP)/Prnp0/0 mice were also shown to have further shortened incubation times when compared to BSE-inoculated Tg(bo6OR-PrP)/Prnp0/0 mice (Table 2) [74]. These results suggest that the OR region could play a crucial role in BSE prions during the conversion of PrPC to PrPSc. In contrast, Tg(PrPΔOR)/Prnp0/0 mice remained susceptible to RML and 22L scrapie prions, developing the disease without elongated incubation times with slightly less PrPScΔOR in their brains after infection with RML and 22L prions (Table 2) [70], suggesting that the OR region might be involved in prion pathogenesis in a strain-dependent manner. However, Prnp0/0 mice expressing PrP with histidine residues in the OR region replaced by glycine residues, termed PrP(TetraH>G), showed significantly prolonged incubation times after infection with RML prions (Table 2) [76]. Further studies are needed to clarify whether or not the OR region might mediate strain-dependent prion pathogenesis.
3.3. The Post-OR Region in Prion Diseases
Three mutations in the post-OR region, including P102L (substitution of a proline residue to a leucine residue at position 102), P105L (substitution of a proline residue to a leucine residue at position 105), and A117V (substitution of an alanine residue to a valine residue at position 117), are associated with inherited human prion diseases [67], suggesting that the post-OR region also plays a role in prion diseases. Tg(PrP-P101L) mice, which express high levels of mouse PrP-P101L, the analogous mutation to human PrP-P102L, have been shown to spontaneously develop prion disease-like diseases, with amyloid plaques, spongiform degeneration, and gliosis in their brains (Table 3) [77]. Brain homogenates from ill Tg(PrP-P101L) mice transmitted the disease to 40% of Tg(PrP-P101L) mice, which never spontaneously developed disease due to lower expression of the mutant protein, and 10% of hamsters, but not to WT CD-1 mice, after intracerebral inoculation (Table 3) [78], indicating that PrPSc-P101L could be infectious. Tg mice expressing mouse PrP-A116V (the human homologue of PrP-A117V) at six times the endogenous levels of PrPC also spontaneously developed progressive ataxia with vacuolation and PrP amyloid plaques in their brains (Table 3) [79]. The PrP molecules from Tg(PrP-A116V) brains were partly insoluble and weakly protease-resistant (Table 3) [79]. No data are available regarding whether PK-resistant PrP-A116V is infectious.
Table 3.
Effects of various mutations in the post-OR region of PrPC on hereditary and acquired prion diseases.
| Disease Type | PrPs | The Post-OR Sequence | Clinicopathological Features | References |
| Hereditary prion disease | PrP-P101L | Proline residue at position 101 mutated to leucine residue in mouse PrP | • Spontaneously develop prion disease-like diseases. • Accumulate weakly protease-resistant PrP-P101L in the brain. • Accumulate prion infectivity associated with weakly protease-resistant PrP-P101L. |
[77,78] |
| PrP-A116V | Alanine residue at position 116 mutated to valine residue in mouse PrP | • Spontaneously developed prion disease-like diseases. • Accumulate partly insoluble and weakly protease-resistant PrP-A116V in the brain. • No data available as to infectivity associated with protease-resistant PrP-A116V. |
[79] | |
| Disease Type | PrPs | The Post-OR Sequence | Susceptibility to Prions | References |
| Acquired prion disease | PrP∆32–80 | Intact | • Fully susceptible to RML scrapie prions. | [80] |
| PrP∆32–93 | The post-OR residues 91–93 deleted | • Partially reduced to RML scrapie prions. | [81] | |
| PrP∆32–106 | The post-OR residues 91–106 deleted | • Resistant to RML scrapie prions. | [82] |
The post-OR region could be also involved in prion infection. Tg(PrP∆32–80)/Prnp0/0 mice developed disease without elongation in incubation times and accumulated PrPSc∆32–80 in their brains after infection with RML prions (Table 3) [80], suggesting that residues 32–80 are dispensable for PrPC to convert to PrPSc after prion infection. However, Tg(PrP∆32–93)/Prnp0/0 mice, which express PrP with a deletion extending to the post-OR region at position 93 from the OR region at position 88, developed disease with longer incubation times and with lower levels of infectivity and PrPSc∆32–93 in their brains after infection with RML prions (Table 3) [81]. Moreover, PrP with a deletion further extending to the post-OR region at position 106, or PrP∆32–106, neither converted to PrPSc nor supported prion pathogenesis in Prnp0/0 mice after intracerebral inoculation with RML prions (Table 3) [82]. These results suggest that the post-OR residues 91–106, which are completely deleted in PrP∆32–106 and partially in PrP∆32–93, but intact in PrP∆32–80, could have a crucial role in prion infection. However, it remains to be determined if the resistance of Tg(PrP∆32–106)/Prnp0/0 mice to RML prions could be due to deletion of the post-OR residues 91–96 alone or together with deletion of other residues.
4. The N-Terminal Domain in Conversion of PrPC to PrPSc
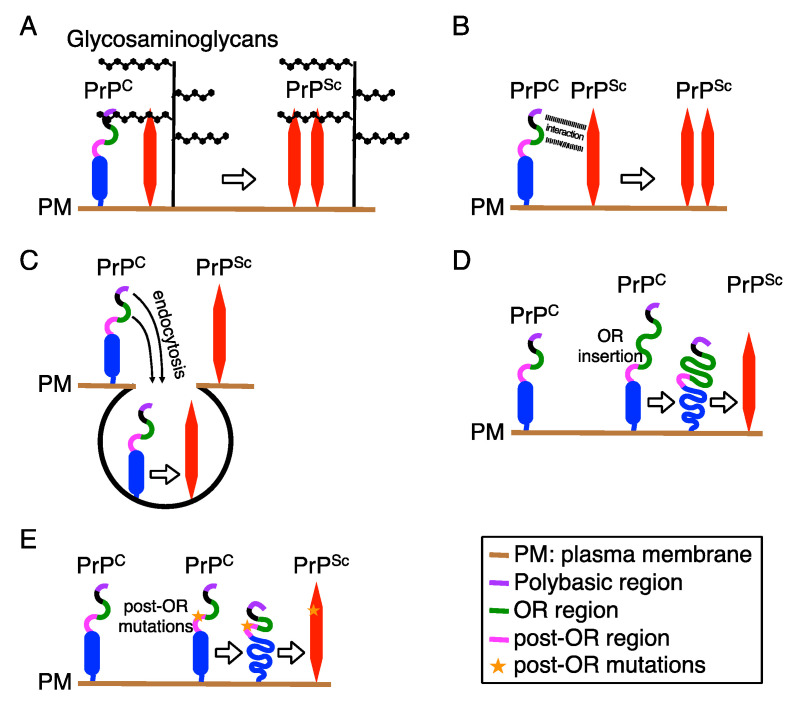
The first step for conversion of PrPC to PrPSc is an intermolecular interaction between both molecules. The polybasic region has been suggested to be involved in the binding of PrPC and/or PrPSc to the extracellular matrix proteins glycosaminoglycans through the positively charged residues [58,59,60]. It is thus possible that the polybasic region might promote interaction between PrPC and PrPSc by recruiting both molecules to glycosaminoglycans, thereby supporting conversion of PrPC to PrPSc (Figure 2A). The polybasic region has also been suggested to mediate a direct interaction between PrPC and PrPSc, thereby promoting the conversion of PrPC to PrPSc [63] (Figure 2B).
Figure 2.
Possible roles of the N-terminal regions in the conversion of PrPC into PrPSc. Upon conversion of PrPC into PrPSc, PrPC might interact with PrPSc through glycosaminoglycans (A) or through the polybasic and OR regions (B). (C) The polybasic and OR regions are also involved in endocytosis of PrPC to endosomal compartments, where PrPC is considered to convert into PrPSc. Extra OR sequences in the OR region (D) and point mutations in the post-OR region (E) might render mutated PrPs structurally unstable, ultimately leading to their spontaneous conversion to pathogenic PrPs.
The next step for conversion is a structural unfolding of the interacting PrPC. PrPC is rich in α-helix structures and soluble in non-ionic detergents [83]. In contrast, PrPSc is abundant in ß-sheet structures and insoluble in non-ionic detergents, forming fibrils [83], suggesting that structural transition of α-helices to ß-sheets in PrPC is an underlying mechanism of the conversion to PrPSc. Several structural models have been proposed for PrPSc fibrils. The 4-rung ß-solenoid model postulates that a PrPSc fibril consists of two intertwined protofilaments of PrPSc molecules [84,85]. In this model, single PrPSc molecules adopt a solenoid structure of four rungs, each rung including three ß-strands, running perpendicular to fibril axis, stacking each other. The upper and lower ß-solenoid rungs of PrPSc protofibrils could template an incoming unfolded PrPC molecule to create additional ß-solenoid rungs. Once a new ß-solenoid rung has formed, it continues to template until the unfolded PrPC molecule is completely converted to PrPSc conformer. In the parallel in-register intermolecular ß-sheet model, single PrPSc molecules comprise the entire cross-section of a fibril, with many hairpins defined by natural and artificial disulfide bonds [86,87]. They are stacked parallel in-register and perpendicular to the fibril axis by forming intermolecular ß-sheet interactions between them. Endocytic/lysosomal compartments are considered to be a site for conversion of PrPC to PrPSc [88,89], suggesting that acidic conditions in the endosomal/lysosomal compartments might promote the structural unfolding of PrPC. The polybasic and OR regions are involved in endocytosis of PrPC [30,90]. It is thus possible that these regions might play a role in conversion of PrPC to PrPSc by mediating endocytosis of PrPC to acidic endocytic/lysosomal compartments (Figure 2C). Insertion of extra OR sequences in the OR region or mutations in the post OR region are associated with spontaneous conversion of mutated PrPs to pathogenic PrPs, causing hereditary prion diseases in humans [67], suggesting that structural instability of the OR region or in the post-OR region might also be involved in the unfolding of the mutant PrPs (Figure 2D,E). Indeed, recombinant human PrPs with three or five extra OR sequences have been reported to spontaneously form aggregates [91]. Copper binding to recombinant mouse PrP was reported to cause novel intramolecular interactions, including those between the N-terminal residues 90–120 and the C-terminal residues 144–147 and its nearby residues 139–143, and between the N-terminal region comprising the OR region and the C-terminal residues 174–185 [92], suggesting that copper binding might also be involved in the unfolding of PrPC. Copper is able to bind to histidine residues located in the OR and post-OR regions [76]. We have shown that, while Tg(PrP∆OR)/Prnp0/0 mice were highly resistant to BSE prions, they still remained susceptible to RML and 22L prions [70], suggesting that copper binding to histidine residues in the OR region might be irrelevant to the unfolding of PrPC. Indeed, it has been shown that histidine residues in the post-OR could be important for conversion of PrPC to PrPSc in acidic conditions [93].
5. The N-Terminal Domain and Neurotoxic PrP Molecules
The neurotoxic mechanism of PrPSc remains largely unknown. However, there have been several reports of neurotoxic PrP molecules causing prion disease-like neurodegeneration, giving rise to an interesting possibility that these neurotoxic PrP molecules might share their neurotoxic mechanism with PrPSc. In addition to a GPI-anchored extracellular form of PrPC, another form of PrP, termed CtmPrP, has been reported [94]. CtmPrP is a transmembrane form of PrP, with the N-terminus facing the cytoplasm and the C-terminus exposed extracellularly. Increased hydrophobicity in the post-OR region by mutations that cause residues to become hydrophobic, including the mutation in hereditary prion disease (A117V), increase the ratio of CtmPrP to total forms of PrP molecules in neuronal cells [94]. Interestingly, transgenic mice expressing these mutant PrPs spontaneously develop prion disease-like neurodegeneration with focal vacuolar degeneration in the neuropil and astrocytic gliosis [94]. Moreover, the ratio of CtmPrP was also reported to increase in the brains of mice infected with prions [95]. These results suggest that CtmPrP might be responsible for neurodegeneration in prion diseases. However, CtmPrP from transgenic mice is not infectious [94].
Other neurotoxic PrP molecules have also been reported. It was shown that Prnp0/0 mice transgenic for PrP with a deletion of the N-terminal residues 32–121 or 32–134, which includes the OR region and a section of the post-OR region, spontaneously developed cerebellar neurodegeneration, with marked granule cell death [96]. Other investigators also showed that Prnp0/0 mice expressing a PrP molecule, designated ΔCR, that harbors a deletion of residues 105–125, developed cerebellar neurodegeneration [97], suggesting that deletion of the post-OR residues 105–125 alone could be responsible for the neurodegeneration in Prnp0/0 mice expressing PrP∆21–121 and PrP∆32–134. Interestingly, the neurotoxicity of these mutant PrPs in Prnp0/0 mice is abrogated by co-expression of WT PrPC [96,97], suggesting that, while the toxic PrP molecules generate a neurotoxic signal, WT PrPC transduces a neuroprotective signal to antagonize the neurotoxic signal of the mutant PrPs. It was shown that, in contrast to PrP∆32–134, PrP∆23–134 was not neurotoxic in Prnp0/0 mice, suggesting that the polybasic region residues 23–31, which remain intact in toxic PrP∆32–134 but not in non-toxic PrP∆23–134, are critical for the neurotoxicity of mutant PrPs [98,99]. Patch-clamp electrophysiological experiments revealed that ΔCR induced abnormal spontaneous ionic currents in various cultured cells and neurons through the polybasic region, and that these currents were suppressed by co-expression of WT PrPC [100,101], suggesting that the abnormal ionic currents might be the neurotoxic signal of the mutant PrPs. It would be thus worthy to investigate whether PrPSc could generate similar abnormal currents in neurons.
6. Conclusions
It has been shown that the non-structural, flexible N-terminal domain, which includes various specific regions such as the polybasic region, OR regions, and post-OR region, has a role in not only the normal function of PrPC but also in the pathogenesis of prion diseases through regulation of the conversion of PrPC to PrPSc and the neurotoxicity of PrPSc. Further elucidation of the exact mechanism of how each of the N-terminal regions could regulate the normal function of PrPC and prion pathogenesis would be of great help for understanding the function of PrPC and prion pathogenesis, and eventually for developing therapeutics for prion diseases.
Abbreviations
| PrP | Prion protein |
| OR | Octapeptide repeat |
| WT | Wild-type |
| CJD | Creutzfeldt-Jakob disease |
Author Contributions
Conceptualization, H.H. and S.S.; writing, H.H. and S.S.; funding acquisition, H.H. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Number 18K07499, Brain Science Foundation, Takeda Science Foundation, and The Ichiro Kanehara Foundation to H.H. and JSPS KAKENHI Grant Number 19H03548 to S.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Aguzzi A., Baumann F., Bremer J. The prion’s elusive reason for being. Annu. Rev. Neurosci. 2008;31:439–477. doi: 10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner S.B. The prion diseases. Brain Pathol. 1998;8:499–513. doi: 10.1111/j.1750-3639.1998.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheckel C., Aguzzi A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018;19:405–418. doi: 10.1038/s41576-018-0011-4. [DOI] [PubMed] [Google Scholar]
- 4.Giles K., Olson S.H., Prusiner S.B. Developing Therapeutics for PrP Prion Diseases. Cold Spring Harb. Perspect. Med. 2017;7:a023747. doi: 10.1101/cshperspect.a023747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prusiner S.B. Early evidence that a protease-resistant protein is an active component of the infectious prion. Cell. 2004;116:S109. doi: 10.1016/S0092-8674(03)01032-8. [DOI] [PubMed] [Google Scholar]
- 6.Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 7.Igel-Egalon A., Bohl J., Moudjou M., Herzog L., Reine F., Rezaei H., Beringue V. Heterogeneity and Architecture of Pathological Prion Protein Assemblies: Time to Revisit the Molecular Basis of the Prion Replication Process? Viruses. 2019;11:429. doi: 10.3390/v11050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueler H., Aguzzi A., Sailer A., Greiner R.A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 9.Prusiner S.B., Groth D., Serban A., Koehler R., Foster D., Torchia M., Burton D., Yang S.L., DeArmond S.J. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc. Natl. Acad. Sci. USA. 1993;90:10608–10612. doi: 10.1073/pnas.90.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manson J.C., Clarke A.R., McBride P.A., McConnell I., Hope J. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration. 1994;3:331–340. [PubMed] [Google Scholar]
- 11.Sakaguchi S., Katamine S., Shigematsu K., Nakatani A., Moriuchi R., Nishida N., Kurokawa K., Nakaoke R., Sato H., Jishage K., et al. Accumulation of proteinase K-resistant prion protein (PrP) is restricted by the expression level of normal PrP in mice inoculated with a mouse-adapted strain of the Creutzfeldt-Jakob disease agent. J. Virol. 1995;69:7586–7592. doi: 10.1128/JVI.69.12.7586-7592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schatzl H.M., Da Costa M., Taylor L., Cohen F.E., Prusiner S.B. Prion protein gene variation among primates. J. Mol. Biol. 1995;245:362–374. doi: 10.1006/jmbi.1994.0030. [DOI] [PubMed] [Google Scholar]
- 13.Oesch B., Westaway D., Walchli M., McKinley M.P., Kent S.B., Aebersold R., Barry R.A., Tempst P., Teplow D.B., Hood L.E., et al. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 14.Riek R., Hornemann S., Wider G., Glockshuber R., Wuthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231) FEBS Lett. 1997;413:282–288. doi: 10.1016/S0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 15.Donne D.G., Viles J.H., Groth D., Mehlhorn I., James T.L., Cohen F.E., Prusiner S.B., Wright P.E., Dyson H.J. Structure of the recombinant full-length hamster prion protein PrP(29–231): The N terminus is highly flexible. Proc. Natl. Acad. Sci. USA. 1997;94:13452–13457. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calzolai L., Lysek D.A., Perez D.R., Guntert P., Wuthrich K. Prion protein NMR structures of chickens, turtles, and frogs. Proc. Natl. Acad. Sci. USA. 2005;102:651–655. doi: 10.1073/pnas.0408939102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prusiner S.B. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 18.Hackl S., Becker C.F.W. Prion protein-Semisynthetic prion protein (PrP) variants with posttranslational modifications. J. Pept. Sci. 2019;25:e3216. doi: 10.1002/psc.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl N., Borchelt D.R., Hsiao K., Prusiner S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 20.Stahl N., Baldwin M.A., Hecker R., Pan K.M., Burlingame A.L., Prusiner S.B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemical. 1992;31:5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- 21.Hebert D.N., Molinari M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport T.A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- 23.Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., Luis A., McCarthy N., Montibeller L., More S., et al. Endoplasmic reticulum stress signalling - From basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Needham P.G., Guerriero C.J., Brodsky J.L. Chaperoning Endoplasmic Reticulum-Associated Degradation (ERAD) and Protein Conformational Diseases. Cold Spring Harb. Perspect. Biol. 2019;11:a033928. doi: 10.1101/cshperspect.a033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puig B., Altmeppen H.C., Linsenmeier L., Chakroun K., Wegwitz F., Piontek U.K., Tatzelt J., Bate C., Magnus T., Glatzel M. GPI-anchor signal sequence influences PrPC sorting, shedding and signalling, and impacts on different pathomechanistic aspects of prion disease in mice. PLoS Pathog. 2019;15:e1007520. doi: 10.1371/journal.ppat.1007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wulf M.A., Senatore A., Aguzzi A. The biological function of the cellular prion protein: An update. BMC Biol. 2017;15:34. doi: 10.1186/s12915-017-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters P.J., Mironov A., Jr., Peretz D., van Donselaar E., Leclerc E., Erpel S., DeArmond S.J., Burton D.R., Williamson R.A., Vey M., et al. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J. Cell Biol. 2003;162:703–717. doi: 10.1083/jcb.200304140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campana V., Sarnataro D., Zurzolo C. The highways and byways of prion protein trafficking. Trends Cell Biol. 2005;15:102–111. doi: 10.1016/j.tcb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Vilette D., Courte J., Peyrin J.M., Coudert L., Schaeffer L., Andreoletti O., Leblanc P. Cellular mechanisms responsible for cell-to-cell spreading of prions. Cell Mol. Life Sci. 2018;75:2557–2574. doi: 10.1007/s00018-018-2823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor D.R., Watt N.T., Perera W.S., Hooper N.M. Assigning functions to distinct regions of the N-terminus of the prion protein that are involved in its copper-stimulated, clathrin-dependent endocytosis. J. Cell Sci. 2005;118:5141–5153. doi: 10.1242/jcs.02627. [DOI] [PubMed] [Google Scholar]
- 31.Uchiyama K., Tomita M., Yano M., Chida J., Hara H., Das N.R., Nykjaer A., Sakaguchi S. Prions amplify through degradation of the VPS10P sorting receptor sortilin. PLoS Pathog. 2017;13:e1006470. doi: 10.1371/journal.ppat.1006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D.R., Hooper N.M. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem. J. 2007;402:17–23. doi: 10.1042/BJ20061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bueler H., Fischer M., Lang Y., Bluethmann H., Lipp H.P., DeArmond S.J., Prusiner S.B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 34.Manson J.C., Clarke A.R., Hooper M.L., Aitchison L., McConnell I., Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 1994;8:121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 35.Nishida N., Katamine S., Shigematsu K., Nakatani A., Sakamoto N., Hasegawa S., Nakaoke R., Atarashi R., Kataoka Y., Miyamoto T. Prion protein is necessary for latent learning and long-term memory retention. Cell Mol. Neurobiol. 1997;17:537–545. doi: 10.1023/A:1026315006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collinge J., Whittington M.A., Sidle K.C., Smith C.J., Palmer M.S., Clarke A.R., Jefferys J.G. Prion protein is necessary for normal synaptic function. Nature. 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- 37.Tobler I., Gaus S.E., Deboer T., Achermann P., Fischer M., Rulicke T., Moser M., Oesch B., McBride P.A., Manson J.C. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- 38.Nishida N., Tremblay P., Sugimoto T., Shigematsu K., Shirabe S., Petromilli C., Erpel S.P., Nakaoke R., Atarashi R., Houtani T., et al. A mouse prion protein transgene rescues mice deficient for the prion protein gene from purkinje cell degeneration and demyelination. Lab. Investig. 1999;79:689–697. [PubMed] [Google Scholar]
- 39.Kim C.K., Sakudo A., Taniuchi Y., Shigematsu K., Kang C.B., Saeki K., Matsumoto Y., Sakaguchi S., Itohara S., Onodera T. Late-onset olfactory deficits and mitral cell loss in mice lacking prion protein with ectopic expression of Doppel. Int. J. Mol. Med. 2007;20:169–176. doi: 10.3892/ijmm.20.2.169. [DOI] [PubMed] [Google Scholar]
- 40.Le Pichon C.E., Valley M.T., Polymenidou M., Chesler A.T., Sagdullaev B.T., Aguzzi A., Firestein S. Olfactory behavior and physiology are disrupted in prion protein knockout mice. Nat. Neurosci. 2009;12:60–69. doi: 10.1038/nn.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lledo P.M., Tremblay P., DeArmond S.J., Prusiner S.B., Nicoll R.A. Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc. Natl. Acad. Sci. USA. 1996;93:2403–2407. doi: 10.1073/pnas.93.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weise J., Crome O., Sandau R., Schulz-Schaeffer W., Bahr M., Zerr I. Upregulation of cellular prion protein (PrPC) after focal cerebral ischemia and influence of lesion severity. Neurosci. Lett. 2004;372:146–150. doi: 10.1016/j.neulet.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 43.McLennan N.F., Brennan P.M., McNeill A., Davies I., Fotheringham A., Rennison K.A., Ritchie D., Brannan F., Head M.W., Ironside J.W., et al. Prion protein accumulation and neuroprotection in hypoxic brain damage. Am. J. Pathol. 2004;165:227–235. doi: 10.1016/S0002-9440(10)63291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai-Yamashita Y., Sakaguchi S., Yoshikawa D., Okimura N., Masuda Y., Katamine S., Niwa M. Female-specific neuroprotection against transient brain ischemia observed in mice devoid of prion protein is abolished by ectopic expression of prion protein-like protein. Neuroscience. 2005;136:281–287. doi: 10.1016/j.neuroscience.2005.06.095. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B., Cowden D., Zhang F., Yuan J., Siedlak S., Abouelsaad M., Zeng L., Zhou X., O’Toole J., Das A.S., et al. Prion Protein Protects against Renal Ischemia/Reperfusion Injury. PLoS ONE. 2015;10:e0136923. doi: 10.1371/journal.pone.0136923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanetti F., Carpi A., Menabo R., Giorgio M., Schulz R., Valen G., Baysa A., Massimino M.L., Sorgato M.C., Bertoli A., et al. The cellular prion protein counteracts cardiac oxidative stress. Cardiovasc. Res. 2014;104:93–102. doi: 10.1093/cvr/cvu194. [DOI] [PubMed] [Google Scholar]
- 47.Chida J., Hara H., Yano M., Uchiyama K., Das N.R., Takahashi E., Miyata H., Tomioka Y., Ito T., Kido H., et al. Prion protein protects mice from lethal infection with influenza A viruses. PLoS Pathog. 2018;14:e1007049. doi: 10.1371/journal.ppat.1007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitteregger G., Vosko M., Krebs B., Xiang W., Kohlmannsperger V., Nolting S., Hamann G.F., Kretzschmar H.A. The role of the octarepeat region in neuroprotective function of the cellular prion protein. Brain Pathol. 2007;17:174–183. doi: 10.1111/j.1750-3639.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown D.R., Qin K., Herms J.W., Madlung A., Manson J., Strome R., Fraser P.E., Kruck T., von Bohlen A., Schulz-Schaeffer W., et al. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 50.Brown D.R., Wong B.S., Hafiz F., Clive C., Haswell S.J., Jones I.M. Normal prion protein has an activity like that of superoxide dismutase. Biochem. J. 1999;344:1–5. doi: 10.1042/bj3440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones S., Batchelor M., Bhelt D., Clarke A.R., Collinge J., Jackson G.S. Recombinant prion protein does not possess SOD-1 activity. Biochem. J. 2005;392:309–312. doi: 10.1042/BJ20051236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutter G., Heppner F.L., Aguzzi A. No superoxide dismutase activity of cellular prion protein In vivo. J. Biol. Chem. 2003;384:1279–1285. doi: 10.1515/BC.2003.142. [DOI] [PubMed] [Google Scholar]
- 53.Bounhar Y., Zhang Y., Goodyer C.G., LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J. Biol. Chem. 2001;276:39145–39149. doi: 10.1074/jbc.C100443200. [DOI] [PubMed] [Google Scholar]
- 54.Oh J.M., Shin H.Y., Park S.J., Kim B.H., Choi J.K., Choi E.K., Carp R.I., Kim Y.S. The involvement of cellular prion protein in the autophagy pathway in neuronal cells. Mol. Cell Neurosci. 2008;39:238–247. doi: 10.1016/j.mcn.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Haigh C.L., Drew S.C., Boland M.P., Masters C.L., Barnham K.J., Lawson V.A., Collins S.J. Dominant roles of the polybasic proline motif and copper in the PrP23–89-mediated stress protection response. J. Cell Sci. 2009;122:1518–1528. doi: 10.1242/jcs.043604. [DOI] [PubMed] [Google Scholar]
- 56.Haigh C.L., Tumpach C., Drew S.C., Collins S.J. The Prion Protein N1 and N2 Cleavage Fragments Bind to Phosphatidylserine and Phosphatidic Acid; Relevance to Stress-Protection Responses. PLoS ONE. 2015;10:e0134680. doi: 10.1371/journal.pone.0134680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osiecka K.M., Nieznanska H., Skowronek K.J., Karolczak J., Schneider G., Nieznanski K. Prion protein region 23–32 interacts with tubulin and inhibits microtubule assembly. Proteins. 2009;77:279–296. doi: 10.1002/prot.22435. [DOI] [PubMed] [Google Scholar]
- 58.Pan T., Wong B.S., Liu T., Li R., Petersen R.B., Sy M.S. Cell-surface prion protein interacts with glycosaminoglycans. Biochem. J. 2002;368:81–90. doi: 10.1042/bj20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warner R.G., Hundt C., Weiss S., Turnbull J.E. Identification of the heparan sulfate binding sites in the cellular prion protein. J. Biol. Chem. 2002;277:18421–18430. doi: 10.1074/jbc.M110406200. [DOI] [PubMed] [Google Scholar]
- 60.Taubner L.M., Bienkiewicz E.A., Copie V., Caughey B. Structure of the flexible amino-terminal domain of prion protein bound to a sulfated glycan. J. Mol. Biol. 2010;395:475–490. doi: 10.1016/j.jmb.2009.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parkin E.T., Watt N.T., Hussain I., Eckman E.A., Eckman C.B., Manson J.C., Baybutt H.N., Turner A.J., Hooper N.M. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bravard A., Auvre F., Fantini D., Bernardino-Sgherri J., Sissoeff L., Daynac M., Xu Z., Etienne O., Dehen C., Comoy E., et al. The prion protein is critical for DNA repair and cell survival after genotoxic stress. Nucleic Acids Res. 2015;43:904–916. doi: 10.1093/nar/gku1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turnbaugh J.A., Unterberger U., Saa P., Massignan T., Fluharty B.R., Bowman F.P., Miller M.B., Supattapone S., Biasini E., Harris D.A. The N-terminal, polybasic region of PrPC dictates the efficiency of prion propagation by binding to PrPSc. J. Neurosci. 2012;32:8817–8830. doi: 10.1523/JNEUROSCI.1103-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das N.R., Miyata H., Hara H., Uchiyama K., Chida J., Yano M., Watanabe H., Kondoh G., Sakaguchi S. Effects of prion protein devoid of the N-terminal residues 25–50 on prion pathogenesis in mice. Arch. Virol. 2017;162:1867–1876. doi: 10.1007/s00705-017-3295-3. [DOI] [PubMed] [Google Scholar]
- 65.Khalife M., Reine F., Paquet-Fifield S., Castille J., Herzog L., Vilotte M., Moudjou M., Moazami-Goudarzi K., Makhzami S., Passet B., et al. Mutated but Not Deleted Ovine PrPC N-Terminal Polybasic Region Strongly Interferes with Prion Propagation in Transgenic Mice. J. Virol. 2016;90:1638–1646. doi: 10.1128/JVI.02805-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das N.R., Miyata H., Hara H., Chida J., Uchiyama K., Masujin K., Watanabe H., Kondoh G., Sakaguchi S. The N-Terminal Polybasic Region of Prion Protein Is Crucial in Prion Pathogenesis Independently of the Octapeptide Repeat Region. Mol. Neurobiol. 2020;57:1203–1216. doi: 10.1007/s12035-019-01804-5. [DOI] [PubMed] [Google Scholar]
- 67.Prusiner S.B. Genetic and infectious prion diseases. Arch. Neurol. 1993;50:1129–1153. doi: 10.1001/archneur.1993.00540110011002. [DOI] [PubMed] [Google Scholar]
- 68.Brown P., Gibbs C.J., Jr., Rodgers-Johnson P., Asher D.M., Sulima M.P., Bacote A., Goldfarb L.G., Gajdusek D.C. Human spongiform encephalopathy: The National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 1994;35:513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi Y., Miyata H., Uchiyama K., Ootsuyama A., Inubushi S., Mori T., Muramatsu N., Katamine S., Sakaguchi S. Biological and biochemical characterization of mice expressing prion protein devoid of the octapeptide repeat region after infection with prions. PLoS ONE. 2012;7:e43540. doi: 10.1371/journal.pone.0043540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara H., Miyata H., Das N.R., Chida J., Yoshimochi T., Uchiyama K., Watanabe H., Kondoh G., Yokoyama T., Sakaguchi S. Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice. J. Virol. 2017;92:e01368-17. doi: 10.1128/JVI.01368-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiesa R., Piccardo P., Ghetti B., Harris D.A. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron. 1998;21:1339–1351. doi: 10.1016/S0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- 72.Chiesa R., Drisaldi B., Quaglio E., Migheli A., Piccardo P., Ghetti B., Harris D.A. Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation. Proc. Natl. Acad. Sci. USA. 2000;97:5574–5579. doi: 10.1073/pnas.97.10.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biasini E., Seegulam M.E., Patti B.N., Solforosi L., Medrano A.Z., Christensen H.M., Senatore A., Chiesa R., Williamson R.A., Harris D.A. Non-infectious aggregates of the prion protein react with several PrPSc-Directed antibodies. J. Neurochem. 2008;105:2190–2204. doi: 10.1111/j.1471-4159.2008.05306.x. [DOI] [PubMed] [Google Scholar]
- 74.Castilla J., Gutierrez-Adan A., Brun A., Pintado B., Salguero F.J., Parra B., Segundo F.D., Ramirez M.A., Rabano A., Cano M.J., et al. Transgenic mice expressing bovine PrP with a four extra repeat octapeptide insert mutation show a spontaneous, non-transmissible, neurodegenerative disease and an expedited course of BSE infection. FEBS Lett. 2005;579:6237–6246. doi: 10.1016/j.febslet.2005.09.099. [DOI] [PubMed] [Google Scholar]
- 75.Castilla J., Gutierrez-Adan A., Brun A., Pintado B., Parra B., Ramirez M.A., Salguero F.J., Diaz San Segundo F., Rabano A., Cano M.J., et al. Different behavior toward bovine spongiform encephalopathy infection of bovine prion protein transgenic mice with one extra repeat octapeptide insert mutation. J. Neurosci. 2004;24:2156–2164. doi: 10.1523/JNEUROSCI.3811-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eigenbrod S., Frick P., Bertsch U., Mitteregger-Kretzschmar G., Mielke J., Maringer M., Piening N., Hepp A., Daude N., Windl O., et al. Substitutions of PrP N-terminal histidine residues modulate scrapie disease pathogenesis and incubation time in transgenic mice. PLoS ONE. 2017;12:e0188989. doi: 10.1371/journal.pone.0188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsiao K.K., Scott M., Foster D., Groth D.F., DeArmond S.J., Prusiner S.B. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science. 1990;250:1587–1590. doi: 10.1126/science.1980379. [DOI] [PubMed] [Google Scholar]
- 78.Hsiao K.K., Groth D., Scott M., Yang S.L., Serban H., Rapp D., Foster D., Torchia M., Dearmond S.J., Prusiner S.B. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc. Natl. Acad. Sci. USA. 1994;91:9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang W., Cook J., Rassbach B., Lemus A., DeArmond S.J., Mastrianni J.A. A New Transgenic Mouse Model of Gerstmann-Sträussler-Scheinker Syndrome Caused by the A117V Mutation of PRNP. J. Neurosci. 2009;29:10072–10080. doi: 10.1523/JNEUROSCI.2542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fischer M., Rulicke T., Raeber A., Sailer A., Moser M., Oesch B., Brandner S., Aguzzi A., Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. doi: 10.1002/j.1460-2075.1996.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flechsig E., Shmerling D., Hegyi I., Raeber A.J., Fischer M., Cozzio A., von Mering C., Aguzzi A., Weissmann C. Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron. 2000;27:399–408. doi: 10.1016/S0896-6273(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 82.Weissmann C., Flechsig E. PrP knock-out and PrP transgenic mice in prion research. Br. Med. Bull. 2003;66:43–60. doi: 10.1093/bmb/66.1.43. [DOI] [PubMed] [Google Scholar]
- 83.Pan K.M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R.J., Cohen F.E., et al. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wille H., Bian W., McDonald M., Kendall A., Colby D.W., Bloch L., Ollesch J., Borovinskiy A.L., Cohen F.E., Prusiner S.B., et al. Natural and synthetic prion structure from X-ray fiber diffraction. Proc. Natl. Acad. Sci. USA. 2009;106:16990–16995. doi: 10.1073/pnas.0909006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vazquez-Fernandez E., Vos M.R., Afanasyev P., Cebey L., Sevillano A.M., Vidal E., Rosa I., Renault L., Ramos A., Peters P.J., et al. The Structural Architecture of an Infectious Mammalian Prion Using Electron Cryomicroscopy. PLoS Pathog. 2016;12:e1005835. doi: 10.1371/journal.ppat.1005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baskakov I.V., Caughey B., Requena J.R., Sevillano A.M., Surewicz W.K., Wille H. The prion 2018 round tables (I): The structure of PrPSc. Prion. 2019;13:46–52. doi: 10.1080/19336896.2019.1569450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spagnolli G., Rigoli M., Orioli S., Sevillano A.M., Faccioli P., Wille H., Biasini E., Requena J.R. Full atomistic model of prion structure and conversion. PLoS Pathog. 2019;15:e1007864. doi: 10.1371/journal.ppat.1007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caughey B., Raymond G.J., Ernst D., Race R.E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): Implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 1991;65:6597–6603. doi: 10.1128/JVI.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borchelt D.R., Taraboulos A., Prusiner S.B. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J. Biol. Chem. 1992;267:16188–16199. [PubMed] [Google Scholar]
- 90.Walmsley A.R., Zeng F., Hooper N.M. The N-terminal region of the prion protein ectodomain contains a lipid raft targeting determinant. J. Biol. Chem. 2003;278:37241–37248. doi: 10.1074/jbc.M302036200. [DOI] [PubMed] [Google Scholar]
- 91.Yu S., Yin S., Li C., Wong P., Chang B., Xiao F., Kang S.C., Yan H., Xiao G., Tien P., et al. Aggregation of prion protein with insertion mutations is proportional to the number of inserts. Biochem. J. 2007;403:343–351. doi: 10.1042/BJ20061592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thakur A.K., Srivastava A.K., Srinivas V., Chary K.V., Rao C.M. Copper alters aggregation behavior of prion protein and induces novel interactions between its N- and C-terminal regions. J. Biol. Chem. 2011;286:38533–38545. doi: 10.1074/jbc.M111.265645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giachin G., Mai P.T., Tran T.H., Salzano G., Benetti F., Migliorati V., Arcovito A., Della Longa S., Mancini G., D’Angelo P., et al. The non-octarepeat copper binding site of the prion protein is a key regulator of prion conversion. Sci. Rep. 2015;5:15253. doi: 10.1038/srep15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hegde R.S., Mastrianni J.A., Scott M.R., DeFea K.A., Tremblay P., Torchia M., DeArmond S.J., Prusiner S.B., Lingappa V.R. A transmembrane form of the prion protein in neurodegenerative disease. Science. 1998;279:827–834. doi: 10.1126/science.279.5352.827. [DOI] [PubMed] [Google Scholar]
- 95.Hegde R.S., Tremblay P., Groth D., DeArmond S.J., Prusiner S.B., Lingappa V.R. Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature. 1999;402:822–826. doi: 10.1038/45574. [DOI] [PubMed] [Google Scholar]
- 96.Shmerling D., Hegyi I., Fischer M., Blattler T., Brandner S., Gotz J., Rulicke T., Flechsig E., Cozzio A., von Mering C., et al. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell. 1998;93:203–214. doi: 10.1016/S0092-8674(00)81572-X. [DOI] [PubMed] [Google Scholar]
- 97.Li A., Christensen H.M., Stewart L.R., Roth K.A., Chiesa R., Harris D.A. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. EMBO J. 2007;26:548–558. doi: 10.1038/sj.emboj.7601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Westergard L., Turnbaugh J.A., Harris D.A. A nine amino acid domain is essential for mutant prion protein toxicity. J. Neurosci. 2011;31:14005–14017. doi: 10.1523/JNEUROSCI.1243-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turnbaugh J.A., Westergard L., Unterberger U., Biasini E., Harris D.A. The N-terminal, polybasic region is critical for prion protein neuroprotective activity. PLoS ONE. 2011;6:e25675. doi: 10.1371/journal.pone.0025675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solomon I.H., Khatri N., Biasini E., Massignan T., Huettner J.E., Harris D.A. An N-terminal polybasic domain and cell surface localization are required for mutant prion protein toxicity. J. Biol. Chem. 2011;286:14724–14736. doi: 10.1074/jbc.M110.214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu B., McDonald A.J., Markham K., Rich C.B., McHugh K.P., Tatzelt J., Colby D.W., Millhauser G.L., Harris D.A. The N-terminus of the prion protein is a toxic effector regulated by the C-terminus. Elife. 2017;6:e23473. doi: 10.7554/eLife.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]