Abstract
Cardiovascular disease (CVD) is closely related to chronic kidney disease (CKD), and patients with CKD have a high risk of CVD-related mortality. Traditional CVD risk factors cannot account for the higher cardiovascular risk of patients with CKD, and standard CVD interventions cannot reduce the mortality rates among patients with CKD. Nontraditional factors related to mineral and vitamin-D metabolic disorders provide some explanation for the increased CVD risk. Non-dialyzable toxins, indoxyl sulfate (IS) and p-cresol sulfate (PCS)—produced in the liver by colonic microorganisms—cause kidney and vascular dysfunction. Plasma trimethylamine-N-oxide (TMAO)—a gut microbe-dependent metabolite of dietary L-carnitine and choline—is elevated in CKD and related to vascular disease, resulting in poorer long-term survival. Therefore, the modulation of colonic flora can improve prospects for patients with CKD. Managing metabolic syndrome, anemia, and abnormal mineral metabolism is recommended for the prevention of CVD in patients with CKD. Considering nontraditional risk factors, the use of resveratrol (RSV), a nutraceutical, can be helpful for patients with CVD and CKD. This paper discusses the beneficial effects of RSV on biologic, pathophysiological and clinical responses, including improvements in intestinal epithelial integrity, modulation of the intestinal microbiota and reduction in hepatic synthesis of IS, PCS and TMAO in patients with CVD and CKD.
Keywords: cardiovascular disease, chronic kidney disease, indoxyl sulfate, microbiota, p-cresol sulfate, resveratrol, trimethylamine-N-oxide
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death in patients with chronic kidney disease (CKD). The strong causality between CKD and CVD risk means that preventing the progression of CKD can also prevent CVD. In patients with CKD, increased CVD risk is multifactorial, and a targeted intervention for a single traditional risk factor is inadequate. Therefore, research on innovative therapeutic strategies and CVD risk factors is essential [1]. We evaluated traditional and nontraditional risk factors of CVD in CKD and analyzed their relationship. Nutraceuticals are derived from foods and have been demonstrated to have physiological benefits, establishing them as likely the most suitable option for preventing CVD in patients with CKD.
Resveratrol (RSV), a nutraceutical, is a naturally occurring polyphenol that can be found in red wine and grapes [2]. Reports have indicated that it is beneficial for treating or preventing CVD by improving metabolic syndrome components [3,4]. Moreover, RSV reduces the amount of plasma protein-bound uremic toxins and trimethylamine-N-oxide (TMAO) levels by maintaining balance in the gut microbiota [5]. RSV has been demonstrated to be safe and tolerable in humans. This overview focuses on how RSV can influence traditional and nontraditional cardiovascular risk factors and provide beneficial effects by attenuating the effect of uremic toxins in patients with CKD.
2. Relationship between CVD and CKD
The traditional risk factors common for CVD and CKD are advanced age, hypertension, diabetes, dyslipidemia, smoking habit, family history of CVD or CKD and male sex. Toxic metabolites produced during uremia, such as indoxyl sulfate (IS), account for most cases of CVD in patients with CKD. Changes in how these chemicals are metabolized constitute nontraditional risk factors [6,7]. Other nontraditional factors, including TMAO, accumulate in patients with CKD and aggravate CVD. In addition, dysregulation of calcium, phosphorus, PTH or vitamin D metabolism was evaluated.
Screening and testing in the early stages of CKD can aid the development of interventions that can delay disease progression. In fact, the focus of treatments and interventions should be shifted to the early stage of CKD because early identification through screening may substantially attenuate the impact of CKD and delay or even prevent its development [8].
2.1. Oxidative Stress in Chronic Kidney Disease
Oxidative stress (OS) is caused by the overproduction of reactive oxygen/nitrogen species (RONS) and weakened antioxidant defense and may cause blood vessel and tissue damage and DNA damage in patients with CKD [9]. Patients with CKD who have increased RONS production, impaired nonenzymatic or enzymatic antioxidant defense, increased uremic toxins (IS), severe isoform cystine deficiency, insufficient biocompatibility of dialysis-related membranes and the inhibition of normal cell function by endotoxins [9]. The uncoupling of uremic toxin-induced endothelial nitric oxide synthase (eNOS) [10], increased activity of nicotinamide adenine dinucleotide phosphate-oxidases [11,12], and loss of antioxidants due to insufficient dietary intake and reduced intestinal absorption [13] contribute to OS in CKD.
Chronic inflammation and OS are intrinsically linked because they mutually enhance each other. Nuclear factor κB (NFκB), a redox-sensitive transcription factor, regulates proinflammatory cytokine and chemokine production. OS can promote the activation of leukocytes and resident cells, thereby initiating inflammatory response [14]. Renal mitochondria are rich in oxidative reactions and are therefore susceptible to OS. Evidence suggests that OS can accelerate the progression of renal function. In advanced CKD, elevated OS is associated with numerous metabolic complications [15]. This results in the formation of a vicious cycle, which ultimately leads to a high death rate in patients with CKD [16].
2.2. Inflammation in Chronic Kidney Disease
Although CKD is related to systemic inflammation, many other factors may cause CKD. Recently, persistent low-grade inflammation has been identified as a critical component of CKD that plays a unique role in its pathophysiology and, to a certain extent, leads to the development of cardiovascular disease, all-cause death and wastage of protein energy [17]. In mice with progressive CKD associated with Alport syndrome, the production of intestinal bacteriocins is unregulated, live bacteria are transported through the gut barrier into the liver, and levels of microbial endotoxins increase in the serum [18]. Therefore, uremia is related to enteral malnutrition, gut barrier dysfunction and microbial translocation, all of which cause persistent systemic inflammation in CKD [19].
Cohen et al. described patients with heart disease who had a proinflammatory patterns of high levels of IL-1, IL-6 and TNF-α combined with low anti-inflammatory parameters—including IL-2, IL-4, IL-5 and T cell number. The patients with high proinflammatory cytokines had a lower survival rate than patients without a characteristic cytokine pattern [20]. In addition, external factors such as impurities in dialysis water, quality of the dialysate and biologically incompatible factors in the dialysis circuit, act as dialysis-related factors [21]. On a histological level, regardless of initial injury, inflammation resulted in renal fibrosis. Mounting evidence suggests that renal inflammation plays a central role in the development and progression of CKD. Therefore, restoring balance between profibrotic and antifibrotic signaling pathways can be used as a strategy to develop antifibrotic strategies that target several pathways simultaneously [22].
3. Effect of Nontraditional Risk Factors (Uremic Toxins) on the Development of Cardiovascular Disease
In patients with CKD, CVD is highly prevalent and traditional risk factors cannot adequately predict cardiovascular events. In CKD, the influx of urea and other residual toxins alters the gut microbiota. The number of beneficial microbes that produce short-chain fatty acids (epithelial energy source) decreases, whereas the number of microbes that produce uremic toxins increases [23].
Indoxyl sulfate and p-cresol sulfate (PCS) accumulate in the organs of patients with CKD. The local and systemic disturbances caused by the toxins in biologic metabolism and cellular signal transduction result in uremic syndrome. Chronic kidney disease perturbs inter-organ communication through small molecules, including uremic solutes and signaling molecules [24]. Moreover, IS plays a role in regulating drug-metabolizing enzymes (DMEs) and transporters during inter-organ communication [25]. In healthy individuals, the concentration of IS is 0.1–2.39 μM in healthy individuals but exceeds 500 μM in patients with advanced CKD [26]. In addition, IS is related to the progression of CVD in patients with CKD, which may be due to increased OS in the myocardium and vasculature [27]. Barreto et al. demonstrated that IS may be related to a higher CVD mortality in patients with CKD [28].
TMAO is derived from the metabolites of the gut microbiota, dietary choline, lecithin and L-carnitine. In patients with CKD, serum TMAO levels are high, which contributes to lower survival. In a mouse model, TMAO accelerated atherosclerosis by inhibiting cholesterol transfer from tissues to the liver [29]. Studies have shown that TMAO is independently related to cardiovascular events and that a relationship between TMAO levels and ischemic cardiovascular events exists, especially in CKD Stages 3b and 4 [30]. For these reasons, selective inhibition of TMAO prevents renal damage and cardiovascular events in patients with CKD [31].
3.1. RSV Improves Intestinal Epithelial Integrity
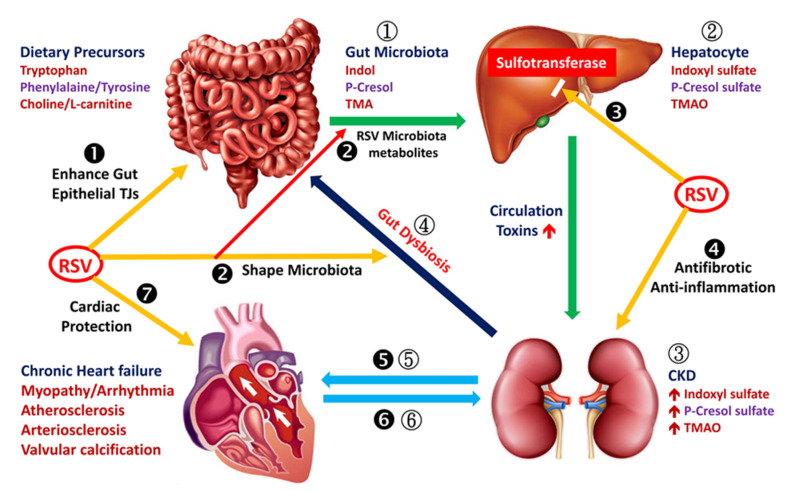
RSV Enhances Intestinal Epithelial Integrity, Shapes Microbiota and Reduces the Synthesis of Indoxyl Sulfate in the Liver (Figure 1)
Figure 1.
Resveratrol (RSV) suppresses uremic toxins and improves their effect on the cardiovascular system. Foods containing tryptophan, phenylalanine/tyrosine and choline/L-carnitine are metabolized into indole, p-cresol and trimethylamine (TMA), respectively, by gut microbiota (1). Indole, p-cresol and TMA are absorbed in the gut and metabolized into indoxyl sulfate (IS), p-cresol or p-cresyl sulfate (PCS) and trimethylamine-N-oxide (TMAO), respectively, in the liver (2). Thereafter, IS, PCS and TMAO enter the circulation and are excreted through the renal proximal tubules. These toxins accumulate in the body when kidney function declines (3). Chronic kidney disease (CKD) and related uremic toxins may induce changes in normal gut microbiota (4). Circulating IS and PCS and possibly TMAO cause electrical and structural remodeling of the myocardial tissue, which may lead to heart failure, atherosclerosis or arrhythmia (5). Cardiovascular abnormality accelerates the progression of renal function decline (6). Resveratrol (RSV) treatment restores intestinal epithelial tight junction proteins, thereby enhancing epithelial integrity (❶). Both RSV-altered gut microbiota and microbial metabolites of RSV contribute to decreased indole, p-cresol and TMA levels in the intestinal lumen (❷). RSV inhibits hepatic sulfotransferase to reduce IS and PCS production (❸); protects the kidney through its anti-inflammatory and antifibrotic effects (❹); protects the heart from chronic injury caused by IS, PCS and TMAO (❺); and prevents exacerbation of renal deterioration caused by failed cardiovascular function (❻). Resveratrol provides direct cardiac protective effects (❼).
The main functions of intestinal mucosa are nutrient absorption, waste secretion and the prevention of waste absorption [32]. The apical junctional complex, which includes the tight junction (TJ) and the adherens junction, prevents intercellular permeation of intestinal contents [33]. The TJ consists of different transmembrane and cytosolic components [33,34]. Transmembrane proteins include occludin, claudin and junctional adhesion molecule-A; cytosolic proteins include members of the zonula occludens (ZO) protein family, of which ZO-1 plays a crucial role in TJ assembly and function [33]. CKD causes the disintegration and reduction of colonic TJ proteins [35].
Urolithin A (UroA), derived from polyphenolics, demonstrates anti-inflammatory and antioxidative activities [36]. Urolithin A and its effective synthetic analog (UAS03) improve intestinal barrier function and inflammation significantly. UroA and UAS03 activate aryl hydrocarbon receptor/Nrf2-dependent pathways to increase the production of epithelial TJ proteins [37]. RSV is one of the most researched natural polyphenols and may enhance intestinal epithelial integrity through the increased production of epithelial TJ proteins [38]. It has also been shown that RSV increases the expression of TJ, desmosomes in colorectal cancer cells. Thus, the epithelization is an important property of RSV, even in tumor cells [39].
3.1.1. RSV Shapes Intestinal Microbiota
RSV modulates the gut microbiota to reduce body weight and fat and improve glucose metabolism and obesity-related indices [40]. Moreover, it increases the abundance of beneficial probiotics, but reduces the growth of Enterococcus faecalis [41,42], which generates oxygen radicals that damage colonic epithelial cell DNA. RSV supplementation can increase butyrate-producing microbes that alleviate OS [43]. Furthermore, polyphenols inhibit the growth of TMAO-producing bacteria, thereby reducing TMAO levels [44].
In the colon, the bacterial metabolites of polyphenols can affect microbial composition and function [45]. Studies suggest that either shaping the intestinal microbiota through specific bacterial species or reducing the Firmicutes/Bacteroidetes ratio can provide crucial benefits for the host [46].
Moreover, RSV attenuates TMAO-induced atherosclerosis by reducing TMAO levels through shaping of the gut microbiota [42]. Moreover, RSV increases bile salt hydrolase (BSH) activity, which promotes the generation of unconjugated bile acid (BA) and enhances fecal BA loss. Fecal BA loss leads to increased hepatic CYP7A1 expression, thereby inducing hepatic BA synthesis, which reduces hepatocyte and plasma cholesterol levels and subsequently attenuates atherosclerosis [47].
The deletion of sirtuin-1 (SIRT-1) in the gut epithelium reduces the levels of anti-inflammatory Lactobacillus. RSV activates intestinal epithelial SIRT1 by regulating the gut microbiota, thereby preventing intestinal inflammation [48].
3.1.2. RSV Reduces Hepatic Synthesis of Indoxyl Sulfate, p-Cresol Sulfate and Trimethylamine-N-oxide
Tryptophan, derived from dietary protein, is first metabolized into indole by the tryptophanase of enteric bacteria [49]. Thereafter, indole is absorbed in the intestine, converted into indoxyl by cytochrome P450 (CYP2E1, CYP2C19, CYP2A6) and coupled to IS by sulfotransferase (SULT) in the liver [50]. Indoxyl sulfate, which is mainly produced in the liver, then enters the circulatory system. Before being excreted through urine, IS is absorbed through organic anion transporters, OAT1 and OAT3, localized in the renal proximal tubular cells [51,52]. Moreover, IS production is blocked through the suppression of SULT through RSV in the rat liver S9 fraction [52].
Gut microbiota dysbiosis results in the development of proteolytic fermentation through the growth of bacteria that produce uremic toxins (IS, PCS and TMAO). Oral RSV can alter the composition of the intestinal microbiota to reduce TMA production, then the hepatic synthesis of TMAO and finally plasma TMAO levels [47]. Thus, RSV can reduce the serum levels of IS or PCS through the inhibition of hepatic SULT and reduce TMAO levels by reducing the gut microbial production of TMA.
3.2. Signaling Pathway Involved in Inflammation and OS
Pharmacokinetic analysis revealed that RSV is rapidly metabolized in the body. Although 70% of RSV is absorbed, its bioavailability after oral intake is low [53]. The beneficial effects of RSV may be due to its ability to protect the body from reactive oxygen species (ROS) injury [54], suppression of cyclooxygenase [55] or activation of anti-inflammatory signaling pathways such as the SIRT-1 pathway [56]. SIRT-1 inhibits TLR4/nuclear factor κB (NF-κB)/STAT signaling, which reduces the production of cytokines [57] and macrophage-derived proinflammatory factors [58].
3.2.1. Epithelial Nitric Oxide Synthase/Inducible Nitric Oxide Synthase Balance
NO synthesis occurs through the nitric oxide synthase (NOS)-dependent or NOS-independent pathways. The reduction of nitrite into NO mainly occurs in the NOS-independent pathway [59]. NOS catalyzes the oxidation of L-arginine to L-citrulline, which is then used to synthesize NO.
Neuronal NOS and eNOS are mainly expressed during physiological processes; however, inducible NOS (iNOS) is more likely to be expressed in a pathologic state [60]. Depletion of L-arginine or cofactor tetrahydrobiopterin and the uncoupling of eNOS increase superoxide production [60]. The increase in OS could lead to vascular endothelial dysfunction.
The vascular endothelium determines aortic permeability for macromolecules and leukocytes, regulates vascular tone and prevents coagulation. Endothelial cell–derived NO reduces smooth muscle cell (SMC) contractility, which regulates vascular tone [61]. In a Marfan syndrome mouse model, NO synthesis was impaired [61]. Endothelium dysfunction is defined as either the loss or overproduction of NO [62]. Excess production of NO, driven by iNOS, aggravates OS and cellular damage through the accumulation of peroxynitrites [63]. For this reason, RSV has been identified as the trigger for NO synthesis in endothelial cells [64].
Studies have demonstrated that RSV increases the expression of Krüppel-like factor 2 (KLF2) [65] through the activation of SIRT-1 [66] and production of eNOS [67,68,69], thereby promoting endothelial cell function. Studies on rats with STZ-induced diabetes have revealed that RSV accentuates endothelial dysfunction through the attenuation of OS, thereby enhancing the expression of NOS3, enriching NO bioavailability and diminishing transforming growth factor β expression [70]. Notably, overexpression of endothelial microRNA-21 (miR-21) induces eNOS production; thus, the induction of miR-21 by RSV likely plays a crucial role in preventing vascular endothelial dysfunction [71].
3.2.2. NADPH Oxidase 4 and Reactive Oxygen Species
The biologic function of NADPH oxidase (NOX) involves the generation of reactive oxygen species [72] through electron transfer across biologic membranes. NOX-dependent ROS production causes persistent OS, eNOS uncoupling and poor mitochondrial function, thereby increasing ROS production and causing tissue injury, which is related to the progression of CVD [11].
The antioxidant characteristic of RSV is likely attributable to its role as a gene regulator. RSV inhibits NOX-mediated production of ROS by downregulating gene expression and thus the activity of the oxidase. Moreover, RSV attenuates mitochondrial superoxide production and increases the expression of various antioxidant enzymes; some gene-regulating effects of RSV are mediated by histone/protein deacetylase SIRT-1 or by nuclear factor E2-related factor 2 [6].
3.2.3. NAD+-Dependent Protein Deacetylase Sirtuin-1 Activation
SIRT-1 deacetylases various transcription factors that are involved in lifespan prolongation. The expression of NO synthase is upregulated by SIRT-1, which also upregulates fork head box O to maintain vascular endothelial morphology [73]. RSV also augments eNOS expression through SIRT-1 activation. Moreover, the upregulation of SIRT-1 and eNOS in response to calorie restriction has been reported [74]. Over expression of SIRT-1 leads to an increase in eNOS expression [75]. In coronary arterial endothelial cells, SIRT-1 knockdown attenuates RSV-induced eNOS expression [76]. Fourny et al. showed that RSV ameliorates ischemia-reperfusion injury in the heart by increasing energy utilization and prolonging eNOS/SIRT-1 expression in female rats with type 2 diabetic [77].
3.2.4. Heme Oxygenase
Heme oxygenase (HO), an antioxidant enzyme, modulates intracellular pro-oxidant heme, carbon monoxide and biliverdin, which have been reported to be upregulated during stress. Thus, to protect cells from stress, HO-1 promotes detrimental effects such as neurological disease and malignancy [78].
We have shown that HO-1 therapy attenuates the severity of membranous nephropathy (MN) through its antioxidative and immune modulatory effects [79]. Furthermore, we proved that RSV increased HO-1 expression and attenuated MN in a murine model. Therefore, RSV can potentially be used as a treatment for MN [80].
4. Effect of RSV on Traditional Cardiovascular Risk Factors
4.1. Effect of RSV on Vascular Function
Angiotensin II (Ang II) acts on the angiotensin II type 1 receptor (AT1R) to induce the production of ROS and subsequent OS. By contrast, angiotensin 1–7 (Ang 1–7) acts on the Mas receptor (MasR) and provides protective effects. Previous studies have shown that RSV can prevent renal function deterioration, ameliorate proteinuria and improve renal histological findings in aged mice by downregulating Ang II/AT1R activity and promoting Ang 1–7/MasR activity [81]. Another study demonstrated that RSV inhibits Ang II and increases the effects of angiotensin-converting enzyme 2 (ACE2) to prevent arterial aging [82]. RSV promotes the synthesis of nitric oxide (NO) from endothelial cells and suppresses the production of endothelin-1, thereby reducing OS. Mounting evidence suggests that RSV treatment protects against the detrimental effects of induced vascular damage. Thus, RSV has protective effects on vascular function and blood pressure [83].
4.2. Effect of RSV on Myocardial Function
Myocardial inflammation causes cardiac damage. Increased activation of NF-κB, a nuclear transcription factor responsible for the production of proinflammatory cytokines, is involved in the immune and inflammatory response of the myocardium, thereby aggravating myocardial injury [84].
In a rat model of sepsis, RSV was reported to inhibit related inflammatory factors and the NF-κB signaling pathway and activate the PI3K/mTOR signaling pathway, thereby protecting the myocardium during sepsis [85]. RSV therapy increases the expression of the autophagy biomarkers beclin-1 and LC-3II but reduces the expression of IL-6. These findings show that RSV has protective effects in ischemia-reperfusion injury in a diabetic rat model [86].
4.3. Effect of RSV on Metabolic Syndrome Components
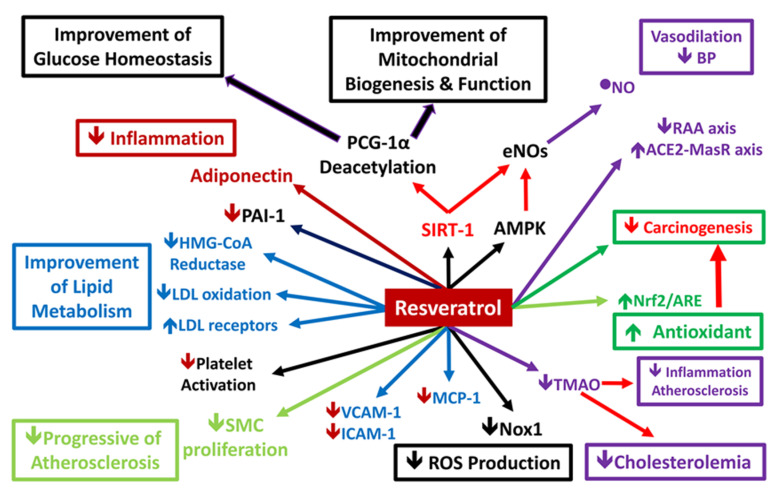
Metabolic syndrome increases the risk of CVD, stroke and type II diabetes. RSV acts through different mechanisms to improve the symptoms of metabolic syndrome and related disorders [87]. RSV activates SIRT-1, which activates eNOS, thereby resulting in cardioprotective, antioxidant and anti-inflammatory effects [88,89]. Tamaki et al. reported that in addition to SIRT-1, RSV activated the adenosine monophosphate-activated kinase (AMPK) signaling and Nrf2/ARE antioxidant pathways in a rat periodontitis model [90]. In addition, RSV treatment markedly reduces profibrotic protein expression and increases the expression of the ACE2/MasR axis components to increase vasodilatation and reduce blood pressure. Moreover, RSV inhibits the migration of vessel SMCs, which have important antiatherogenic and antiatherosclerotic effects [82,91]. Thus, RSV plays an important role in metabolic syndrome (Figure 2).
Figure 2.
Potential mechanism of resveratrol action on metabolic syndrome. Resveratrol (RSV) improves the plasma lipid profile by reducing LDL cholesterol and triglyceride levels and increasing HDL cholesterol levels. In hepatocytes, RSV can increase HMG-CoA reductase activity and potentiate LDL receptor expression to reduce plasma LDL cholesterol. RSV also inhibits the migration of vascular smooth muscle cells, which have important antiatherogenic and antiatherosclerotic effects. RSV can also activate Nrf2, endothelial nitric oxide synthase and other antioxidant response components and attenuate the production of TNFα. RSV induces structural alterations in Keap1 protein, thereby inhibiting it from sequestering Nrf2 in the cytoplasm. Increased cytoplasmic Nrf2 translocate to the nucleus, where it binds the response elements and initiates transcription of multiple antioxidant genes90. Thus, RSV has antioxidative and anti-inflammatory effects. In the vascular endothelium, RSV attenuates the expression of adhesion molecules by inhibiting the NF-κB activation pathway. In vascular macrophages, RSV reduces the formation of foam cells by inhibiting nitric oxide synthase 1 and reducing monocyte chemoattractant protein-1 production. RSV prevents vascular aging by reducing the activity of the renin/angiotensin II system and stimulating the angiotensin-converting enzyme 2/mas receptor axis. RSV also provides additional anti-carcinogenic effects parallel with cardioprotective effects.
4.3.1. RSV and High Blood Pressure
The effects of RSV on blood pressure have been explored in numerous animal models [92,93,94]. RSV can reduce levels of serum Ang II and ACE. RSV treatment also increases serum Ang-(1e7) levels, which is accompanied by the increased expression of ACE2, angiotensin II type 2 receptor and MasR [82]. The blood pressure-lowering effects of RSV are attributable to the increased production of endothelial NO, which reduces vascular OS [83].
4.3.2. Fat Accumulation and Cholesterolemia
RSV affects the lipid profile by increasing the expression of the cholesterol transporter protein. RSV enhances Apo-A1 synthesis and ameliorates foam cell formation through the PPAR-γ and adenosine 2A receptor pathways [95]. RSV enhances gut microbiota remodeling and BSH activity. In addition, RSV-induced fecal BA loss leads to increased expression of CYP7A1 in the liver, thereby inducing hepatic BA synthesis. Hepatic BA reduces the levels of hepatocyte and plasma cholesterol [47].
4.3.3. RSV and Glucose Intolerance and Insulin Resistance
The effect of RSV on cellular glucose metabolism has been well studied. RSV improves insulin resistance (IR) in adipose tissue and the liver [96], improves hepatic IR by regulating long noncoding RNAs (lncRNAs) [97] and upregulating the miRNA mmu-miR-363-3p [98] and reduces hepatic endoplasmic reticulum stress, thereby improving insulin sensitivity [99].
In skeletal muscles, RSV promotes the phosphorylation of the α-subunit of AMPK to improve glucose metabolism [100] and increases glucose uptake by increasing the expression of the cell membrane localized glucose transporter type 4 [101,102,103]. RSV improves skeletal muscle fatty acid oxidation and reduces OS, thereby reducing IR [104]. Moreover, RSV ameliorates ROS levels in the muscle and liver cells in high-fat diet-induced IR [105].
5. Biologic Role of RSV in Atrial Fibrillation
Atrial fibrillation (AF) and CKD usually occur together, which poses a medical dilemma because of the risk of thromboembolism and bleeding episodes [106]. RSV directly affects heart function and rhythm through cardiac remodeling and ion channel activity [107]. RSV also suppresses hypertrophic heart remodeling through the activation of SIRT-1/AMPK and the subsequent inhibition of NFAT activation, which is implicated in the evolution of AF [108], cardiac myopathy and congestive heart failure [109]. In a study investigating the therapeutic efficacy of RSV in ameliorating AF in an animal model, RSV was found to attenuate atrial fibrosis and modulate ion channels to reduce AF through the PI3K/eNOS signaling pathway [110]. Thus, the cardiovascular protective effects of RSV include reducing OS and alleviating inflammation through Nrf2 and SIRT-1 activation, upregulating the PI3K/eNOS pathway and downregulating the NF-κB pathway [111].
6. RSV with Strong Anti-Carcinogenic Effect via Cardiovascular Protective Effects
Reactive oxygen species (ROS) play a pivotal role in the pathogenesis of both heart disease and tumor progression. Any disturbances in ROS metabolism results an increase in OS which initiates subcellular changes and resultant cardiomyopathy and heart failure. A previous study indicated that exogenous antioxidant RSV is of value in preventing both the development of heart disease and cancer by acting as ROS scavenger [112]. RSV reverses multidrug resistance in cancer cells and sensitizes cancer cells to standard chemotherapeutic agents. The proposed mechanisms of RSV to prevent carcinogenesis include the inhibition of OS, inflammation and cancer-cell proliferation and the activation of tightly regulated cell-death mechanisms [113]. RSV possesses a wide range of preventive and therapeutic options against different types of cancer [114] through its proapoptotic, antiproliferative and anti-inflammatory actions [115,116]. RSV also suppresses the malignant biologic behaviors of cancer cells, including proliferation, antiapoptosis, invasion, migration, EMT progress, levels of ROS and stemness [117]. Recently, RSV is proved be chemopreventive from tumorigenesis by targeting Sirt1 and suppression of NF-κB activation [118]. In brief, RSV provides additional anti-carcinogenic effects parallel with cardioprotective effects.
7. New Therapeutic Interventions
The poor bioavailability of RSV limits its use as a therapeutic drug. RSV has rapid Phase II metabolism in the liver and intestine [119,120] and sufficient blood levels cannot be reached through intravenous nor oral administration of RSV [121]. To overcome this challenge, the use of natural or synthetic analogs with better bioavailability or higher potency or a combination of drugs that exhibit a synergistic effect are favorable strategies [122].
8. Potential Adverse Effects of RSV
Mounting evidence suggests that RSV has health benefits and plays a role in cardiovascular protection [123]. However, human clinical studies have reported conflicting results regarding the conservative effects of RSV in various diseases and their sequelae [124]. The reasons for these conflicting findings are unknown. However, dissimilarity in the characteristics of the study subjects, the prescribed RSV dosage and the duration of RSV treatment are possible causes [125]. Numerous studies have shown that RSV has paradoxical dose-dependent effects. At low concentrations, RSV acts as an antioxidant and protects against lipid, protein and DNA damage. However, at high concentrations, RSV acts as a pro-oxidant and promotes cellular damage [4]. The optimal RSV dosage for maximizing the benefit of RSV to cardiovascular health without increasing toxicity requires further investigation [126].
9. Conclusions
The findings of this study suggest that RSV is a possible therapeutic option for patients with CKD with or without CVD. In this regard, RSV can influence the traditional and nontraditional CVD risk factors and alleviate the effects of uremic toxins in patients with CKD.
One of the nontraditional risk factors for CVD is uremic toxin-related cardiovascular side effects. IS causes electrical and structural remodeling in myocardial tissue, leading to atherosclerotic vascular disease. RSV treatment restores intestinal epithelial TJ proteins to increase epithelial integrity. RSV alters the gut microbiota to reduce indole levels in the intestinal lumen. Moreover, RSV inhibits hepatic SULT to reduce the production of uremic toxins such as IS.
In addition, RSV treatment markedly reduces the expression of pro-fibrotic proteins and increases the expression of the components of the ACE2/MasR axis to cause vasodilatation and reduce blood pressure. RSV inhibits the migration of vascular SMCs, which have important antiatherogenic and antiatherosclerotic effects [82,91]. Moreover, RSV exhibits anti-sclerotic activity in CKD by delaying disease progression. The development of RSV derivatives to reduce the occurrence of CV events in patients with CKD is warranted.
Abbreviations
| chronic kidney disease | CKD |
| cardiovascular disease | CVD |
| resveratrol | RSV |
| oxidative stress | OS |
| reactive oxygen species | ROS |
| indoxyl sulfate | IS |
| zonula occludens | ZO |
| urolithin A | UroA |
| erythroid 2-related factor 2 | Nrf2 |
| tight junction | TJ |
| p-cresol or p-cresyl sulfate | PCS |
| drug-metabolizing enzymes | DMEs |
| sulfotransferase | SULT |
| sirtuin-1 | SIRT-1 |
| nitric oxide synthase | NOS |
| endothelial NOS | eNOS |
| inducible NOS | iNOS |
| smooth muscle cell | SMC |
| Krüppel-like factor 2 | KLF2 |
| reactive oxygen/nitrogen species | RONS |
| trimethylamine-N-oxide | TMAO |
| nuclear factor κB | NF-κB |
| nicotinamide adenine dinucleotide phosphate | NADPH |
| NADPH oxidase | NOX |
| NADPH oxidase 4 | NOX4 |
| heme oxygenase | HO |
| membranous nephropathy | MN |
| angiotensin II | Ang II |
| angiotensin II type 1 receptor | AT1R |
| angiotensin 1–7 | Ang 1–7 |
| mas receptor | MasR |
| angiotensin-converting enzyme | ACE |
| nuclear factor (erythroid-derived 2)-like 2 | Nrf2 |
| adenosine monophosphate-activated kinase | AMPK |
| bile salt hydrolase | BSH |
| bile acid | BA |
Funding
This research received on external funding.
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., Jafar T.H., Heerspink H.J.L., Mann J.F., Matsushita K., Wen C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 2.Saldanha J.F., Leal Vde O., Stenvinkel P., Carraro-Eduardo J.C., Mafra D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell. Longev. 2013;2013:963217. doi: 10.1155/2013/963217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saldanha J.F., Leal V.O., Rizzetto F., Grimmer G.H., Ribeiro-Alves M., Daleprane J.B., Carraro-Eduardo J.C., Mafra D. Effects of Resveratrol Supplementation in Nrf2 and NF-kappaB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016;26:401–406. doi: 10.1053/j.jrn.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Shaito A., Posadino A.M., Younes N., Hasan H., Halabi S., Alhababi D., Al-Mohannadi A., Abdel-Rahman W.M., Eid A., Nasrallah G.K., et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020;21:2084. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang W.W., Wang Z., Kennedy D.J., Wu Y., Buffa J.A., Agatisa-Boyle B., Li X.S., Levison B., Hazen S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2014;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia N., Daiber A., Förstermann U., Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2016;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez-Garza S.L., Laveriano-Santos E.P.L., Marhuenda-Muñoz M., Storniolo C.E., Tresserra-Rimbau A., Vallverdú-Queralt A., Lamuela-Raventós R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutr. 2018;10:1892. doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallianou N., Mitesh S., Gkogkou A., Geladari E. Chronic Kidney Disease and Cardiovascular Disease: Is there Any Relationship? Curr. Cardiol. Rev. 2018;15:55–63. doi: 10.2174/1573403X14666180711124825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung C.-C., Hsu Y.-C., Chen C.-C., Lin Y.-F., Wu C. Oxidative Stress and Nucleic Acid Oxidation in Patients with Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2013;2013:1–15. doi: 10.1155/2013/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibal L., Agarwal S.C., Home P.D., Boger R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010;6:82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Murugesan P., Huang K., Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2019;17:170–194. doi: 10.1038/s41569-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu M., Kim Y.J., Kang D.H. Indoxyl Sulfate–Induced Endothelial Dysfunction in Patients with Chronic Kidney Disease via an Induction of Oxidative Stress. Clin. J. Am. Soc. Nephrol. 2010;6:30–39. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankowska M., Rutkowski B., Slizien A.D. Vitamins and Microelement Bioavailability in Different Stages of Chronic Kidney Disease. Nutrients. 2017;9:282. doi: 10.3390/nu9030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cachofeiro V., Goicochea M., De Vinuesa S.G., Oubiña P., Lahera V., Luño J., Oubi P. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008;74:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 15.Daenen K., Andries A., Mekahli D., Van Schepdael A., Jouret F., Bammens B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2018;34:975–991. doi: 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
- 16.Ravarotto V., Simioni F., Pagnin E., Davis P.A., Calò L.A. Oxidative stress—Chronic kidney disease—Cardiovascular disease: A vicious circle. Life Sci. 2018;210:125–131. doi: 10.1016/j.lfs.2018.08.067. [DOI] [PubMed] [Google Scholar]
- 17.Akchurin O.M., Kaskel F. Update on Inflammation in Chronic Kidney Disease. Blood Purif. 2015;39:84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 18.Andersen K., Kesper M.S., Marschner J.A., Konrad L., Ryu M., Vr S.K., Kulkarni O.P., Mulay S.R., Romoli S., Demleitner J., et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD–Related Systemic Inflammation. J. Am. Soc. Nephrol. 2016;28:76–83. doi: 10.1681/ASN.2015111285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carney E.F. Chronic kidney disease: Microbiota trigger inflammation. Nat. Rev. Nephrol. 2016;12:376. doi: 10.1038/nrneph.2016.73. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S.D., Phillips T.M., Khetpal P., Kimmel P.L. Cytokine patterns and survival in haemodialysis patients. Nephrol. Dial. Transplant. 2009;25:1239–1243. doi: 10.1093/ndt/gfp625. [DOI] [PubMed] [Google Scholar]
- 21.Santoro A., Mancini E. Is hemodiafiltration the technical solution to chronic inflammation affecting hemodialysis patients? Kidney Int. 2014;86:235–237. doi: 10.1038/ki.2014.81. [DOI] [PubMed] [Google Scholar]
- 22.Lv W., Booz G.W., Wang Y., Fan F., Roman R.J. Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 2017;820:65–76. doi: 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau W.L., Savoj J., Nakata M.B., Vaziri N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018;132:509–522. doi: 10.1042/CS20171107. [DOI] [PubMed] [Google Scholar]
- 24.Caggiano G., Cosola C., Di Leo V., Gesualdo M., Gesualdo L. Microbiome modulation to correct uremic toxins and to preserve kidney functions. Curr. Opin. Nephrol. Hypertens. 2020;29:49–56. doi: 10.1097/MNH.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 25.Nigam S.K., Bush K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019;15:301–316. doi: 10.1038/s41581-019-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lekawanvijit S., Adrahtas A., Kelly D.J., Kompa A.R., Wang B.H., Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Hear. J. 2010;31:1771–1779. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 27.Lekawanvijit S., Kompa A.R., Wang B.H., Kelly D.J., Krum H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012;111:1470–1483. doi: 10.1161/CIRCRESAHA.112.278457. [DOI] [PubMed] [Google Scholar]
- 28.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A., European Uremic Toxin Work Group (EUTox) Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koeth R.A., Wang Z., Levison B., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim R.B., Morse B.L., Djurdjev O., Tang M., Muirhead N., Barrett B., Holmes D.T., Madore F., Clase C.M., Rigatto C., et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–1152. doi: 10.1016/j.kint.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Gupta N., Buffa J.A., Roberts A.B., Sangwan N., Skye S.M., Li L., Ho K.J., Varga J., DiDonato J.A., Tang W.W., et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arter. Thromb. Vasc. Boil. 2020;40:1239–1255. doi: 10.1161/ATVBAHA.120.314139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong S., Zhang Y.H., Zhang W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. BioMed Res. Int. 2018;2018:1–10. doi: 10.1155/2018/2819154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 34.Nusrat A., Turner J.R., Madara J.L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am. J. Physiol. Liver Physiol. 2000;279:851–857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 35.Vaziri N.D., Yuan J., Rahimi A., Ni Z., Said H., Subramanian V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2011;27:2686–2693. doi: 10.1093/ndt/gfr624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muku G.E., Murray I.A., Espín J.C., Perdew G.H. Urolithin A Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites. 2018;8:86. doi: 10.3390/metabo8040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.V., Hegde B., Kotla N.G., Hiwale A.A., Saiyed T., Patel P., Vijay-Kumar M., et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019;10:89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020;91:e13357. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buhrmann C., Shayan P., Kraehe P., Popper B., Goel A., Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- 40.Chaplin A., Carpéné C., Mercader J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients. 2018;10:1651. doi: 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Most J., Penders J., Lucchesi M., Goossens G.H., Blaak E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017;71:1040–1045. doi: 10.1038/ejcn.2017.89. [DOI] [PubMed] [Google Scholar]
- 42.Chen M.-L., Yi L., Zhang Y., Zhou X., Ran L., Yang J., Zhu J.-D., Zhang Q.-Y., Mi M.-T. Resveratrol Attenuates Trimethylamine- N -Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio. 2016;7:e02210-15. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C., Deng Q., Xu J., Wang X., Hu C., Tang H., Huang F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019;116:1202–1211. doi: 10.1016/j.foodres.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Annunziata G., Maisto M., Schisano C., Ciampaglia R., Narciso V., Tenore G.C., Novellino E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo(R)) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients. 2019;11:139. doi: 10.3390/nu11010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etxeberria U., Fernandez-Quintela A., Milagro F.I., Aguirre L., Martínez J.A., Portillo M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013;61:9517–9533. doi: 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- 46.Espín J.C., González-Sarrías A., Tomás-Barberán F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 47.Ding L., Chang M., Guo Y., Zhang L., Xue C., Yanagita T., Zhang T., Wang Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Heal. Dis. 2018;17:286. doi: 10.1186/s12944-018-0939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wellman A.S., Metukuri M.R., Kazgan N., Xu X., Xu Q., Ren N.S.X., Czopik A., Shanahan M.T., Kang A., Chen W., et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology. 2017;153:772–786. doi: 10.1053/j.gastro.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niwa T. Role of Indoxyl Sulfate in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Experimental and Clinical Effects of Oral Sorbent AST-120. Ther. Apher. Dial. 2011;15:120–124. doi: 10.1111/j.1744-9987.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- 50.Banoglu E., King R. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2002;27:135–140. doi: 10.1007/BF03190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enomoto A., Takeda M., Tojo A., Sekine T., Cha S.H., Khamdang S., Takayama F., Aoyama I., Nakamura S., Endou H., et al. Role of Organic Anion Transporters in the Tubular Transport of Indoxyl Sulfate and the Induction of its Nephrotoxicity. J. Am. Soc. Nephrol. 2002;13:1711–1720. doi: 10.1097/01.ASN.0000022017.96399.B2. [DOI] [PubMed] [Google Scholar]
- 52.Saito H., Yoshimura M., Saigo C., Komori M., Nomura Y., Yamamoto Y., Sagata M., Wakida A., Chuman E., Nishi K., et al. Hepatic Sulfotransferase as a Nephropreventing Target by Suppression of the Uremic Toxin Indoxyl Sulfate Accumulation in Ischemic Acute Kidney Injury. Toxicol. Sci. 2014;141:206–217. doi: 10.1093/toxsci/kfu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 54.Fibach E., Prus E., Bianchi N., Zuccato C., Breveglieri G., Salvatori F., Finotti A., Lipucci di Paola M., Brognara E., Lampronti I., et al. Resveratrol: Antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and beta-thalassemia patients. Int. J. Mol. Med. 2012;29:974–982. doi: 10.3892/ijmm.2012.928. [DOI] [PubMed] [Google Scholar]
- 55.Szewczuk L.M., Forti L., Stivala L.A., Penning T.M. Resveratrol is a Peroxidase-mediated Inactivator of COX-1 but Not COX-2: A mechanistic approach to the design of COX-1 selective agents. J. Boil. Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- 56.Saiko P., Szakmary A., Jaeger W., Szekeres T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Capiralla H., Vingtdeux V., Zhao H., Sankowski R., Al-Abed Y., Davies P., Marambaud P. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J. Neurochem. 2012;120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alarcón C., Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 59.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 60.Kone B.C. Nitric oxide synthesis in the kidney: Isoforms, biosynthesis, and functions in health. Semin. Nephrol. 2004;24:299–315. doi: 10.1016/j.semnephrol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Chung A.W., Yeung K.A., Cortes S.F., Sandor G.G.S., Judge D.P., Dietz H.C., Van Breemen C. Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome. Br. J. Pharmacol. 2009;150:1075–1083. doi: 10.1038/sj.bjp.0707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 63.Lomelí O., Pérez-Torres I., Márquez R., Críales S., Mejía A.M., Chiney C., Hernández-Lemus E., Soto M.E. The Evaluation of Flow-Mediated Vasodilation in the Brachial Artery Correlates With Endothelial Dysfunction Evaluated by Nitric Oxide Synthase Metabolites in Marfan Syndrome Patients. Front. Physiol. 2018;9:965. doi: 10.3389/fphys.2018.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buluç M., Demirel-Yilmaz E. Resveratrol decreases calcium sensitivity of vascular smooth muscle and enhances cytosolic calcium increase in endothelium. Vasc. Pharmacol. 2006;44:231–237. doi: 10.1016/j.vph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Hibender S., Franken R., Van Roomen C., Ter Braake A., Van Der Made I., Schermer E.E., Gunst Q., Hoff M.J.V.D., Lutgens E., Pinto Y.M., et al. Resveratrol Inhibits Aortic Root Dilatation in the Fbn1C1039G/+ Marfan Mouse Model. Arter. Thromb. Vasc. Boil. 2016;36:1618–1626. doi: 10.1161/ATVBAHA.116.307841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gracia-Sancho J., Villarreal G., Zhang Y., García-Cardeña G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 2009;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallerath T., Deckert G., Ternes T., Anderson H., Li H., Witte K., Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.CIR.0000029925.18593.5C. [DOI] [PubMed] [Google Scholar]
- 68.Leikert J.F., Rathel T.R., Wohlfart P., Cheynier V., Vollmar A.M., Dirsch V.M. Red Wine Polyphenols Enhance Endothelial Nitric Oxide Synthase Expression and Subsequent Nitric Oxide Release From Endothelial Cells. Circulation. 2002;106:1614–1617. doi: 10.1161/01.CIR.0000034445.31543.43. [DOI] [PubMed] [Google Scholar]
- 69.Dolinsky V.W., Chakrabarti S., Pereira T.J., Oka T., Levasseur J., Beker D., Zordoky B.N., Morton J.S., Nagendran J., Lopaschuk G.D., et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta. 2013;1832:1723–1733. doi: 10.1016/j.bbadis.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 70.Hammad A.S., Ahmed A.-S.F., Heeba G.H., Taye A.A. Heme oxygenase-1 contributes to the protective effect of resveratrol against endothelial dysfunction in STZ-induced diabetes in rats. Life Sci. 2019;239:117065. doi: 10.1016/j.lfs.2019.117065. [DOI] [PubMed] [Google Scholar]
- 71.Akaberi M., Hosseinzadeh H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytotherapy Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 72.Bedard K., Krause K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 73.Lu C.-L., Liao M.-T., Hou Y.-C., Fang Y.-W., Zheng C.-M., Liu W.-C., Yuan T.-H., Lu K.-C., Ng Y.-Y. Sirtuin-1 and Its Relevance in Vascular Calcification. Int. J. Mol. Sci. 2020;21:1593. doi: 10.3390/ijms21051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nisoli E., Tonello C., Cardile A., Cozzi V., Tedesco L., Falcone S., Carruba M., Bracale R., Valerio A., Cantoni O., et al. Calorie Restriction Promotes Mitochondrial Biogenesis by Inducing the Expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 75.Brandes R.P. Activating SIRT1: A new strategy to prevent atherosclerosis? Cardiovasc. Res. 2008;80:163–164. doi: 10.1093/cvr/cvn245. [DOI] [PubMed] [Google Scholar]
- 76.Csiszar A., Labinskyy N., Pinto J.T., Ballabh P., Zhang H., Losonczy G., Pearson K., De Cabo R., Pacher P., Zhang C., et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fourny N., Lan C., Eric S., Bernard M., Desrois M. Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart. Nutrients. 2019;11:105. doi: 10.3390/nu11010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waza A.A., Hamid Z., Ali S., Bhat S.A., Bhat M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018;67:579–588. doi: 10.1007/s00011-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 79.Wu C.-C., Lu K.-C., Chen J.-S., Hsieh H.-Y., Lin S.-H., Chu P., Wang J.-Y., Sytwu H.-K., Lin Y.-F. HO-1 induction ameliorates experimental murine membranous nephropathy: Anti-oxidative, anti-apoptotic and immunomodulatory effects. Nephrol. Dial. Transplant. 2008;23:3082–3090. doi: 10.1093/ndt/gfn247. [DOI] [PubMed] [Google Scholar]
- 80.Wu C.-C., Huang Y.-S., Chen J.-S., Huang C.-F., Su S.-L., Lu K.-C., Lin Y.-F., Chu P., Lin S.-H., Sytwu H.-K. Resveratrol Ameliorates Renal Damage, Increases Expression of Heme Oxygenase-1, and Has Anti-Complement, Anti-Oxidative, and Anti-Apoptotic Effects in a Murine Model of Membranous Nephropathy. PLoS ONE. 2015;10:e0125726. doi: 10.1371/journal.pone.0125726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jang I.-A., Kim E.N., Lim J.H., Kim M.Y., Ban T.H., Yoon H.E., Park C.W., Chang Y.S., Choi B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrient. 2018;10:1741. doi: 10.3390/nu10111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim E.N., Kim M.Y., Lim J.H., Kim Y., Shin S.J., Park C.W., Kim Y.-S., Chang Y.S., Yoon H.E., Choi B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 83.Li H., Xia N., Hasselwander S., Daiber A., Li X. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019;20:2155. doi: 10.3390/ijms20092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su Q., Li L., Sun Y., Yang H., Ye Z., Zhao J. Effects of the TLR4/Myd88/NF-kappaB Signaling Pathway on NLRP3 Inflammasome in Coronary Microembolization-Induced Myocardial Injury. Cell Physiol. Biochem. 2018;47:1497–1508. doi: 10.1159/000490866. [DOI] [PubMed] [Google Scholar]
- 85.Shang X., Lin K., Yu R., Zhu P., Zhang Y., Wang L., Xu J., Chen K. Resveratrol Protects the Myocardium in Sepsis by Activating the Phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway and Inhibiting the Nuclear Factor-kappaB (NF-kappaB) Signaling Pathway. Med. Sci. Monit. 2019;25:9290–9298. doi: 10.12659/MSM.918369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qu X., Chen X., Shi Q., Wang X., Wang D., Yang L. Resveratrol alleviates ischemia/reperfusion injury of diabetic myocardium via inducing autophagy. Exp. Ther. Med. 2019;18:2719–2725. doi: 10.3892/etm.2019.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bremer A.A. Resveratrol use in metabolic syndrome. Metab. Syndr. Relat. Disord. 2014;12:493–495. doi: 10.1089/met.2014.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bishayee A., Rabi T. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009;8:9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diaz-Gerevini G.T., Repossi G., Dain A., Tarres M.C., Das U.N., Eynard A.R. Beneficial action of resveratrol: How and why? Nutrition. 2016;32:174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 90.Tamaki N., Cristina Orihuela-Campos R., Inagaki Y., Fukui M., Nagata T., Ito H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014;75:222–229. doi: 10.1016/j.freeradbiomed.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 91.Hou C., Tain Y.-L., Yu H.-R., Huang L.-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019;20:535. doi: 10.3390/ijms20030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H., Xia N., Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric. Oxide. 2012;26:102–110. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 93.Zordoky B.N., Robertson I.M., Dyck J.R.B. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta. 2015;1852:1155–1177. doi: 10.1016/j.bbadis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Park E.-J., Pezzuto J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta. 2015;1852:1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 95.Haghighatdoost F., Hariri M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018;129:141–150. doi: 10.1016/j.phrs.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 96.Jeon S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shu L., Hou G., Zhao H., Huang W., Song G., Ma H. Long non-coding RNA expression profiling following treatment with resveratrol to improve insulin resistance. Mol. Med. Rep. 2020;22:1303–1316. doi: 10.3892/mmr.2020.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shu L., Zhao H., Huang W., Hou G., Song G., Ma H. Resveratrol Upregulates mmu-miR-363-3p via the PI3K-Akt Pathway to Improve Insulin Resistance Induced by a High-Fat Diet in Mice. Diabetes Metab. Syndr. Obes. 2020;13:391–403. doi: 10.2147/DMSO.S240956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao H., Zhang Y., Shu L., Song G., Ma H. Resveratrol reduces liver endoplasmic reticulum stress and improves insulin sensitivity in vivo and in vitro. Drug Des. Dev. Ther. 2019;13:1473–1485. doi: 10.2147/DDDT.S203833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Musi N., Goodyear L.J. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol. Scand. 2003;178:337–345. doi: 10.1046/j.1365-201X.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 101.Furtado L.M., Somwar R., Sweeney G., Niu W., Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem. Cell Boil. 2002;80:569–578. doi: 10.1139/o02-156. [DOI] [PubMed] [Google Scholar]
- 102.Yamaguchi S., Katahira H., Ozawa S., Nakamichi Y., Tanaka T., Shimoyama T., Takahashi K., Yoshimoto K., Imaizumi M.O., Nagamatsu S., et al. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Metab. 2005;289:E643–E649. doi: 10.1152/ajpendo.00456.2004. [DOI] [PubMed] [Google Scholar]
- 103.Breen D.M., Sanli T., Giacca A., Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y.-J., Zhao H., Dong L., Zhen Y.-F., Xing H.-Y., Ma H.-J., Song G.-Y. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via ATM-AMPK axis in skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9117–9125. doi: 10.26355/eurrev_201910_19315. [DOI] [PubMed] [Google Scholar]
- 105.Gong L., Guo S., Zou Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2019;242:117212. doi: 10.1016/j.lfs.2019.117212. [DOI] [PubMed] [Google Scholar]
- 106.Bhatia H.S., Hsu J.C., Kim R.J. Atrial fibrillation and chronic kidney disease: A review of options for therapeutic anticoagulation to reduce thromboembolism risk. Clin. Cardiol. 2018;41:1395–1402. doi: 10.1002/clc.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baczkó I., Light P.E. Resveratrol and derivatives for the treatment of atrial fibrillation. Ann. N. Y. Acad. Sci. 2015;1348:68–74. doi: 10.1111/nyas.12843. [DOI] [PubMed] [Google Scholar]
- 108.Lin C.-C., Lin J.-L., Lin C.-S., Tsai M.-C., Su M.-J., Lai L.-P., Huang S.K.S. Activation of the Calcineurin-Nuclear Factor of Activated T-Cell Signal Transduction Pathway in Atrial Fibrillation. Chest. 2004;126:1926–1932. doi: 10.1016/S0012-3692(15)31443-4. [DOI] [PubMed] [Google Scholar]
- 109.Sung M.M., Das S.K., Levasseur J., Byrne N.J., Fung D., Kim T., Masson G., Boisvenue J., Soltys C.-L., Oudit G.Y., et al. Resveratrol Treatment of Mice with Pressure-Overload-Induced Heart Failure Improves Diastolic Function and Cardiac Energy Metabolism. Circ. Hear. Fail. 2014;8:128–137. doi: 10.1161/CIRCHEARTFAILURE.114.001677. [DOI] [PubMed] [Google Scholar]
- 110.Chong E., Chang S.-L., Hsiao Y.-W., Singhal R., Liu S.-H., Leha T., Lin W.-Y., Hsu C.-P., Chen Y.-C., Chen Y.-J., et al. Resveratrol, a red wine antioxidant, reduces atrial fibrillation susceptibility in the failing heart by PI3K/AKT/eNOS signaling pathway activation. Hear. Rhythm. 2015;12:1046–1056. doi: 10.1016/j.hrthm.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 111.Meng X., Zhou J., Zhao C.-N., Gan R.-Y., Li H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods. 2020;9:340. doi: 10.3390/foods9030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin J.K., Tsai S.H. Chemoprevention of cancer and cardiovascular disease by resveratrol. Proc. Natl. Sci. Counc. Repub. China Part B. 1999;23:99–106. [PubMed] [Google Scholar]
- 113.Ko J.-H., Sethi G., Um J.-Y., Shanmugam M.K., Arfuso F., Kumar A.P., Bishayee A., Ahn K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu H.-L. Resveratrol and Its Analogues: Promising Antitumor Agents. Anti. Cancer Agents Med. Chem. 2011;11:479–490. doi: 10.2174/187152011795677427. [DOI] [PubMed] [Google Scholar]
- 115.Shukla Y., Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 116.Rauf A., Imran M., Butt M.S., Nadeem M., Peters D.G., Mubarak M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2017;58:1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 117.Jiang Z., Chen K., Cheng L., Yan B., Qian W., Cao J., Li J., Wu E., Ma Q., Yang W. Resveratrol and cancer treatment: Updates. Ann. N. Y. Acad. Sci. 2017;1403:59–69. doi: 10.1111/nyas.13466. [DOI] [PubMed] [Google Scholar]
- 118.Buhrmann C., Shayan P., Popper B., Goel A., Shakibaei M. Sirt1 Is Required for Resveratrol-Mediated Chemopreventive Effects in Colorectal Cancer Cells. Nutrients. 2016;8:145. doi: 10.3390/nu8030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Walle U.K. High Absorption but very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 120.Vitaglione P., Sforza S., Galaverna G., Ghidini C., Caporaso N., Vescovi P.P., Fogliano V., Marchelli R. Bioavailability oftrans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 121.Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., et al. Properties of Resveratrol:In VitroandIn VivoStudies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015;2015:1–13. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leon D., Uribe E., Zambrano A., Salas M. Implications of Resveratrol on Glucose Uptake and Metabolism. Molecules. 2017;22:398. doi: 10.3390/molecules22030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petrovski G., Gurusamy N., Das D.K. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- 124.Ponzo V., Soldati L., Bo S. Resveratrol: A supplementation for men or for mice? J. Transl. Med. 2014;12:158. doi: 10.1186/1479-5876-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tomé-Carneiro J., Larrosa M., González-Sarrías A., Tomás-Barberán F.A., García-Conesa M.T., Espín J.C. Resveratrol and Clinical Trials: The Crossroad from In Vitro Studies to Human Evidence. Curr. Pharm. Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Oliveira Silva F., Ferreira J.V., Placido J., Chagas D., Praxedes J., Guimaraes C., Batista L.A., Laks J., Deslandes A.C. Corrigendum to “Gait analysis with videogrammetry can differentiate healthy elderly, mild cognitive impairment, and Alzheimer’s disease: A cross-sectional study”. Exp. Gerontol. 2020;135:110943. doi: 10.1016/j.exger.2020.110943. [DOI] [PubMed] [Google Scholar]