Abstract
The larvicidal potential of crude leaf extracts of Rhizophora mucronata, the red mangrove, using diverse solvent extracts of the plant against the early fourth instar larvae of Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti mosquito vectors was analyzed. The acetone extract of R. mucronata showed the greatest efficacy: for Cx. quinquefasciatus (LC50 = 0.13 mg/mL; LC90 = 2.84 mg/mL), An. stephensi (LC50 = 0.34 mg/mL; LC90 = 6.03 mg/mL), and Ae. aegypti (LC50 = 0.11 mg/mL; LC90 = 1.35 mg/mL). The acetone extract was further fractionated into four fractions and tested for its larvicidal activity. Fraction 3 showed stronger larvicidal activity against all the three mosquito larvae. Chemical characterization of the acetone extract displayed the existence of several identifiable compounds like phytol, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 1-hexyl-2-nitrocyclohexane, eicosanoic acid etc. Enzyme assay displayed that R. mucronata active F3-fractions exert divergent effects on all three mosquitos’ biochemical defensive mechanisms. The plant fractions displayed significant repellent activity against all the three mosquito vectors up to the maximum repellent time of 210 min. Thus, the bioactive molecules in the acetone extract of R. murconata leaves showed significant larvicidal and enzyme inhibitory activity and displayed novel eco-friendly tool for mosquito control.
Keywords: mangrove, larvicidal activity, enzyme inhibition, Rhizophora mucronata, repellent, mosquitoes
1. Introduction
Mosquitoes are central to the spreading of many infections including dengue fever, malaria, yellow fever and lymphatic filariasis, especially in areas with ecosystems that favor their breeding [1]. Mosquitoes are the primary arthropod vectors of different blood borne illness that cause millions of mortalities per year in humans [2]. Mosquitoes can also cause allergic reactions in human beings such as angioedema [3]. In India alone, there were 1.5 million cases and more than 1500 deaths caused due to malaria cases in the past decades due to mosquito vectors [4].
Malaria is chiefly spread by six mosquito species in India, with Anopheles stephensi the most prevalent in urban society [5]. Aedes aegypti are the major vectors for dengue and dengue hemorrhagic fever, which are prevalent in Africa, the Americas and India [6]. Culex quinquefasciatus, which often breeds in contaminated water, is the major domestic mosquito in many tropical countries and a significant lymphatic filariasis vector, with lymphatic filariasis being the highest growing vector borne illness in the tropical countries affecting more than 146 million people [7]. Larval mosquitoes are particularly striking targets for major insecticides since their breeding site is located in water, an accessible habitat [4]. Among the approaches to reduce mosquito populations and entomological inoculation rates, larvicidal represent an attractive tool to be developed for mosquito control [8,9].
Phytochemicals with higher mosquitocidal actions are now recognized as effective natural pesticides due to their exceptional larvicidal, repellent, pupicidal, and adulticidal actions [7]. Mangrove plants are generally suggested as an alternative source of bioactive ingredients, because they are thought to contain bioactive substances that are considered safe and environmentally friendly to both humans and animals [10]. The red mangrove plant, Rhizophora mucronata Lam. (Malpighiales: Rhizophoraceae) is indigenous to East Africa, India, Indonesia, wet tropical regions of Australia and other prominent countries of Asia [11]. The major advantage of choosing this plant is it can be collected easily and cost effective for preparing mosquitocides as compared to commercial pesticides, is easily degradable and, most importantly, has active blends of bio-active chemicals with diverse pharmacological activity. The chief tactic of the present research is to target the developmental stages, especially the Laval stage, of blood sucking pests which keen to block the adult emergence and blocks spreading dreadful diseases. Furthermore, an aim is to detect the repellent activity of the bio-active fractions of R. mucronata since any green based pesticides delivering higher repellent activity has higher commercial value in the market for developing better insecticides. It is crucial to investigate the toxic range of any botanical extracts or its derivatives, just as estimated for commercial pesticides, to provide safer dealings to non-targets, especially humans [3]. Thus, the present research was to investigate the chemical composition of leaf extracts of the mangrove R. mucronata and larvicidal, enzyme inhibitory activity and repellent against the major medically challenging pest larvae of An. stephensi, Ae. aegypti, and Cx. quinquefasciatus.
2. Results and Discussion
2.1. Larvicidal Activity of R. mucronata Crude Extract
Extracts isolated from the plant leaves and flowers deliver novel visions into bio-rational mosquitocidal development. Previous research displayed that botanicals are a dynamic resource with significant biological activities, such as antiviral, antifungal, phytotoxic and, most importantly, larvicidal actions [2,3,12]. An increment number of plant molecules were stated in the previous research, suggesting that there is the atmosphere possible to provide a fertile source of compounds for medicinal or therapeutic use. The synthetic chemical usage has been an essential agent for arthropod management, but they deliver harmful outcomes, including higher tenacity in the surrounding, non-target impact to other well beings, polluting the natural sources, insecticide residues being found on food, the development of insecticide resistance by the targeted pests and impact on non-targets [13]. The aquatic regions are a unique reservoir of bio-active compounds, most of them delivers inimitable structures [13]. Plant-derived chemicals are likely to be part of the future arsenal of mosquito control programs, as they can act as common toxins, reproduction and growth inhibitors or as active repellents and also oviposition deterrent [3]. Investigating the natural botanicals and their active deliverables against the mosquito larvae may ultimately prime to their practicing green based pesticides [14]. Larval management using bio-active compounds is a major vector for the effective management of blood sucking arthropods. Plants are considered to be a viable and preferred substitute to commercial larvicides for managing mosquitoes at the community level [7,14]. For these reasons, we decided to explore our local environment for possible sources of botanic larvicides that might provide effective and safe alternatives to synthetic anti-mosquito products. Here, the larvicidal action of mangrove leaf extracts was determined against three important mosquito vectors. Ethyl acetate, acetone, benzene and methanol leaf extracts of R. mucronata (Figure S1) were assayed against the fourth instars. Among them, acetone extracts against Cx. quinquefasciatus (LC50 = 0.129; LC90 = 2.8417 mg/mL), An. stephensi (LC50 = 0.378; LC90 = 6.035 mg/mL), and Ae. aegypti (LC50 = 0.113; LC90 = 1.334 mg/mL) produced the highest larval mortality (Table 1). Similarly, the lethal dosage (1.5 mg) of crude volatile oil derived from the Piper betle leaf exhibited a significant mortality rate against the dengue vector with more than 94% of larval mortality and the LC50 was observed at 0.63 mg/L [15].
Table 1.
Larvicidal activity of mangrove plant extracts of Rhizosphora murconata against Ae. aegypti, An. stephensi and Cx. quinquefasciatus. LC50 Lethal concentration 50% mortality, LC90 Lethal concentration 90% mortality, LCL: lower confidence limits, UCL: upper confidence limits, χ2: chi square, df: degrees of freedom.
| Species | Solvents | LC50 mg/mL (95% Confidence Limit) |
LC90 mg/mL (95% Confidence Limit) |
χ2 | df | p-Value |
|---|---|---|---|---|---|---|
| Aedes aegypti | Acetone | 0.113 | 1.334 | 1.274 | 3 | 0.763 |
| (0.1935–1.147) | (0.886–2.9626) | |||||
| Ethyl Acetate | 0.305 | 1.037 | 3.078 | 3 | 0.486 | |
| (0.125–0.556) | (1.413–8.009) | |||||
| Methanol | 0.154 | 1.1453 | 4.524 | 3 | 0.527 | |
| (0.210–0.307) | (1.258–11.809) | |||||
| Petroleum benzene | 0.502 | 1.3725 | 5.164 | 3 | 0.498 | |
| (0.271–1.026) | (1.803–8.368) | |||||
| Anopheles stephensi | Acetone | 0.378 | 6.035 | 3.500 | 3 | 0.003 |
| (0.119–0.481) | (1.1045–11.930) | |||||
| Ethyl Acetate | 0.427 | 4.418 | 2.410 | 3 | 0.395 | |
| (0.389–2.358) | (2.902–5.972) | |||||
| Methanol | 0.415 | 2.088 | 4.319 | 3 | 0.375 | |
| (0.269–1.243) | (1.202–7.169) | |||||
| Petroleum benzene | 0.504 | 5.8592 | 2.311 | 3 | 0.269 | |
| (0.304–0.895) | (1.2245–3.2839) | |||||
| Culex quinquefasciatus | Acetone | 0.129 | 2.8417 | 1.346 | 3 | 0.865 |
| (0.030–0.239) | (2.700–6.302) | |||||
| Ethyl Acetate | 0.378 | 1.7374 | 2.746 | 3 | 0.468 | |
| (0.165–0.751) | (1.861–5.499) | |||||
| Methanol | 0.295 | 1.0615 | 3.092 | 3 | 0.037 | |
| (0.116–0.539) | (1.4216–9.4711) | |||||
| Petroleum benzene | 0.584 | 1.6477 | 2.275 | 3 | 0.284 | |
| (0.324–1.302) | (2.0227–13.219) |
2.2. Mortality Bioassays of Acetone Extract Fractions
The efficacy of plant extracts varies from species to species and plant parts [16]. The variability in toxic concentrations of the various plant extracts, i.e., to achieve mosquitocidal activity, may be due to differences in concentration levels between the insecticidal components of each plant; moreover, the effect of each plant extract will vary with the time of collection and season [13]. It has been reported [15] that the seed methanol extract of Clitoria ternatea was effective against An. stephensi, Ae. aegypti, and Cx. quinquefasciatus larvae with LC50 values of 65.2, 154.5, and 54.4 ppm, respectively. In our study, the acetone extracts of R. mucronata leaves were fractioned and four different fractions were collected. These four fractions were tested for larvicidal activity against three important mosquitoes in public health terms (Table 2). When testing Fraction 3, we observed the greatest larvicidal activity against Ae. aegypti (LC50 = 0.1037; LC90 = 1.0025 mg/mL), An. stephensi (LC50 = 0.1480; LC90 = 4.6480 mg/mL) and Cx. quinquefasciatus (LC50 = 0.174348; LC90 = 16.73929 mg/mL), respectively. Earlier studies reported that methanolic extracts of plants are larvicidal against An. stephensi and Cx. quinquefasciatus [16,17]. Kamaraj and colleagues reported the highest larval mortality in leaf petroleum ether and flower methanol extracts of Cryptocoryne auriculata, flower methanol extracts of Leucas aspera, leaf and seed methanol extracts of Solanum torvum, and leaf hexane extracts of Vitex negundo against An. subpictus larvae (LC50 = 44.21, 44.69, 53.16, 41.07, 35.32, 28.90 and 44.40 ppm; LC90 = 187.31, 188.29, 233.18, 142.66, 151.60, 121.05 and 192.11 ppm, respectively) and Cx. tritaeniorhynchus larvae (LC50 = 69.83, 51.29, 81.24, 71.79, 44.42, 84.47 and 65.35 ppm; LC90 = 335.26, 245.63, 300.45, 361.83, 185.09, 351.41 and 302.42 ppm, respectively). Ansari and colleagues examined the larvicidal activity of Pinus longifolia oil against three vector mosquitoes, namely, Aedes aegypti (LC50 = 82.1 ppm), Cx. quinquefasciatus (LC50 = 85.7 ppm) and An. stephensi (LC50 = 112.6 ppm).
Table 2.
LC50, LC90, and chi square analysis of larvicidal activity of Rhizosphora murconata acetone extract column fraction against Ae. aegypti, An. stephensi and Cx. quinquefasciatus. LC50 Lethal concentration 50% mortality, LC90 Lethal concentration 90% mortality, LCL: lower confidence limits, UCL: upper confidence limits, χ2: chi square, df: degrees of freedom. Significance at p < 0.05. * denotes the predominant lethal concentration dosage of Fraction F3.
| Species | Column Fraction | LC50 mg/mL (95% Confidence Limit) |
LC90 mg/mL (95% Confidence Limit) |
χ2 | df | p-Value |
|---|---|---|---|---|---|---|
| Culex quinquefasciatus | F1 | 0.245333 | 2.322 | 1.77430 | 3 | 0.187 |
| (0.159992–2.886752) | (5.002817–7.2119) | |||||
| F2 | 0.341783 | 12.58869 | 3.647 | 3 | 0.476 | |
| (0.212881–0.493197) | (4.985508–86.38909) | |||||
| F3 | 0.174348 * | 16.73929 | 3.919 | 3 | 0.528 | |
| (0.061645–0.291515) | (5.101697–366.6364) | |||||
| F4 | 0.217996 | 8.073846 | 1.82923 | 3 | 0.098 | |
| (0.115948–0.323425) | (3.511051–45.48615) | |||||
| Aedes aegypti | F1 | 0.314289 | 39.94815 | 3.41649 | 3 | 0.461 |
| (0.148417–0.511897) | (9.04682–2474.55) | |||||
| F2 | 0.130 | 1.1158 | 65.1 | 3 | 0.782 | |
| (0.119–0.1561) | (0.9422–2.2120) | |||||
| F3 | 0.1037 * | 1.0025 | 4.84 | 3 | 0.521 | |
| (0.069–0.112) | (0.8871–2.1147) | |||||
| F4 | 0.20831 | 22.581621 | 3.082 | 3 | 0.391 | |
| (0.48172–1.19820) | (20.1682–24.8216) | |||||
| Anopheles stephensi | F1 | 0.266881967 | 3.1525 | 1.938 | 3 | 0.207 |
| (0.0021–0.469229) | (2.9900–3.1211) | |||||
| F2 | 0.31175 | 3.3851 | 0.992 | 3 | 0.034 | |
| (0.01451–0.22655) | (2.44364–7.5935) | |||||
| F3 | 0.1480 * | 4.6480 | 3.147 | 3 | 0.218 | |
| (0.2957–0.767397) | (3.2585–9.4680) | |||||
| F4 | 1.358 | 5.8546 | 0.678 | 3 | 0.004 | |
| (0.0198–3.2210) | (4.3215–7.5842) |
2.3. Chemical Characterization of R. mucronata Extract
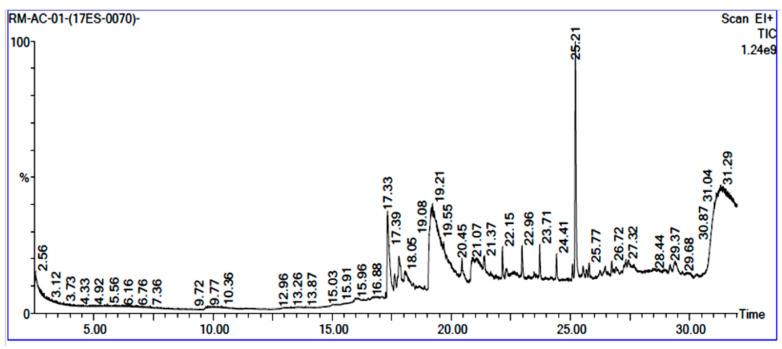
GC-MS analysis of acetone solvent extracts of R. mucronata leaves (Figure 1) showed 13 peaks representing 13 compounds. The 13 compounds were characterized and identified as shown in Table 3. phytol, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 1-hexyl-2-nitrocyclohexane, eicosanoic acid, estra-1,3,5(10)-trien-17, betaol, sulfurous acid, octadecyl 2-propyl ester, 2-heptadecenal, 1-hexyl-2-nitrocyclohexane, 17-pentatriacontene, tritetracontane, urs-12-en-28-ol and squalene. The peak area percentage was prominent in Eicosanoic Acid (38.24%) with retention time at 19.205. Since the Peak area percentage was prominent in Eicosanoic Acid and this might have played a key role in mosquitocidal activity against all the three arthropod vectors. Preliminary phytochemical screening of whole plant extracts revealed the presence of phenol, flavonoids, alkaloids, saponins, tannins, glycosides, amino acids, quinones and carbohydrates in the plant extracts (Table 4).
Figure 1.
Chemical composition of GC-MS analysis using acetone leaf extract of R. murconata.
Table 3.
Chemical characterization of acetone leaf extract of R. murconata through GC-MS analysis.
| S.No. | Name of the Compounds | RI Polar Column Exp | Lit | RI Polar Column Exp | Lit | Peak Area % | Formula | Structure |
|---|---|---|---|---|---|---|---|---|
| 1 | Phytol | 925 | 919 | 2622 | 2617 | 9.704 | C20H40O |
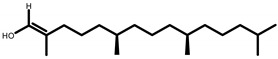
|
| 2 | 3,7,11,15-Tetramethyl-2-Hexadecen-1-ol | 957 | 900 | 2114 | 2116 | 3.738 | C20H40O |

|
| 3 | 1-Hexyl-2-Nitrocyclohexane | 817 | 814 | 1054 | 1060 | 1.338 | C12H23O2N |
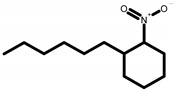
|
| 4 | Eicosanoic Acid | 913 | 912 | 2442 | 2445 | 38.246 | C20H40O2 |
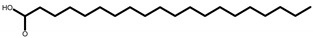
|
| 5 | Estra-1,3,5(10)-Trien-17-Beta-ol | 871 | 869 | 1145 | 1152 | 15.447 | C18H24O |
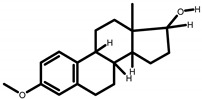
|
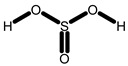
| 6 | Sulfurous acid, Octadecyl 2-Propyl Ester | 915 | 911 | 1231 | 1237 | 3.108 | C21H44O3S |
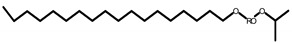
|
| 7 | 2-Heptadecenal | 918 | 909 | 1174 | 1183 | 3.406 | C17H32O |
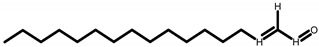
|
| 8 | 1-Hexyl-2-Nitrocyclohexane | 923 | 916 | 1214 | 1217 | 5.675 | C12H23O2N |
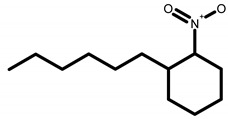
|
| 9 | 17-Pentatriacontene | 929 | 921 | 1063 | 1066 | 2.450 | C35H70 |
|
| 10 | Sulfurous acid, Octadecyl 2-Propyl Ester | 952 | 947 | 1118 | 1120 | 1.310 | C21H44O3S |
|
| 11 | Tritetracontane | 943 | 939 | 4297 | 4300 | 1.433 | C43H88 |

|
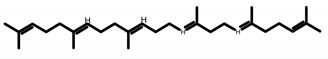
| 12 | 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-Hexamethyl-, (all-e)- | 983 | 975 | 2814 | 2819 | 12.222 | C30H50 |
|
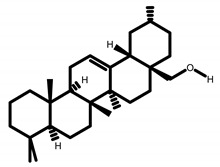
| 13 | Urs-12-En-28-ol | 749 | 748 | 987 | 992 | 1.923 | C30H50O |
|
Table 4.
Phytochemical constituent’s analysis of different solvent extracts of R. murconata. Where + indicates presence, - indicates absence.
| S. No. | Phytochemical Test | Petroleum Benzene | Ethyl Acetate Extract | Acetone Extract | Methanol Extract |
|---|---|---|---|---|---|
| 1 | Phenols | + | + | + | + |
| 2 | Flavonoids | + | + | + | + |
| 3 | Alkaloids | - | + | + | + |
| 4 | Saponins | + | + | + | + |
| 5 | Tannins | + | + | + | + |
| 6 | Glycosides | + | + | + | + |
| 7 | Proteins | - | - | - | - |
| 8 | Amino Acid | - | + | + | - |
| 9 | Quinones | + | + | + | + |
| 10 | Carbohydrates | - | - | - | + |
Plant-based compounds are known to be eco-friendly and can be used in mosquito larval control safely. Furthermore, these natural products are readily biodegradable and safe to other organisms [18]. The potent larvicidal activity of R. murconata could be attributed to the presence of tannins, phenols, flavonoids, saponins, glycosides and quinones (Figure 2). The isolation of these compounds, as defined here, could provide the basis for developing natural mosquitocidal products as a substitute for synthetic insecticides- or for developing mosquito repellents. The fractions obtained from the Adhatoda vasica were an effective larvicidal agent against the Cx. quinquefasciatus and Ae. aegypti larvae [19]. It was highly toxic to mosquito larvae and also inhibited the development of pupae. The high rate of larval mortality of mosquitoes observed at higher concentrations (250 ppm of A. vasica) within a 24 h exposure indicates the high toxicity of the product. The plants tested in the present study are reported to be eco-friendly and toxic to agriculturally important insect pest [20]. For example, essential oils from Citronella and Eucalyptus are often used in repellents sold under several brand names [21]. Recent studies have indicated that phytol, a precursor of synthetic vitamin E and vitamin K (which was identified in our analysis as well), exhibits antioxidant and antinociceptive effects and was cytotoxic against the MCF−7 breast cancer cell line [22].
Figure 2.
Phytochemical analysis of R. murconata using different solvent extracts. (A) Phenol; (B) flavonoids; (C) alkaloids; (D) saponins; (E) tannins; (F) glycosides; (G) protein; (H) amino acids; (I) quinones; (J) carbohydrates.
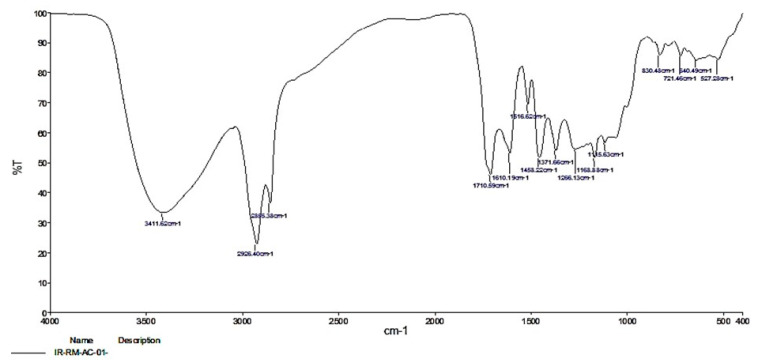
The FT-IR spectrum analysis of acetone solvent extract of R. mucronata (Figure 3) showed 13 peaks indicating the presence of functional groups. The functional groups of the detected peak values were identified (Table 5). Alkane, Amine amd Alkyl were invariably present in acetone extracts. The column separation was eluted the different fractions of R. mucronata. Besides the active fraction of F3 was compared with a standard of squalene. The retention time of fraction F3 got eluted in 4.8 min at 220 nm and it was comparable to the standard peak of squalene (Figure 4). Correspondingly, Octacosane, derived from Couroupita guianensis, showed the highest percentage, at 31.86% peak area in the active fraction F6 and was previously shown to have toxicity to early third instars of Cx. quinquefasciatus (Say.) [23]. Octacosane was also shown to be present in the unsaponifiable phase of common chicory, Cichorium intybus L. at 1.34% peak area, exhibiting larvicidal activity against Anopheles pharoensis (Theobald) with LC50 value of 13.62 mg/kg_1 [23]. Spirostan-3,15-diol,3-(4-methylbenzenesulfo-nate) at 5.20% of peak area, was isolated by Soule et al. [24] as a spirostane glycoside from the Solanum laxum (Steud.) aerial parts were reported to have toxicity against the aphid, Schizaphisgra minum (Rondani) [25]. FractionF6 also contains hexacosane at 17.54% peak area. Hexacosane, in the leaf oil of Solanum sarrachoides (Sendt.), exhibits oviposition deterrence against red spidermites, (Tetranychus evansi) [26,27]. The sensitivity of adults of Ae. aegypti has been shown as resulting from the presence of 1,8-cineole, α-pinene and p-cymene and is correlated to the amount of 1,8-cineole in the tested extract [28]. The insecticidal activity of the essential oil of Eucalyptus tereticornis observed in the current work, might be explained by the presence of one of its major components (p-cymene) but also by a minor compound (1,8-cineole) in our extracts, which both have demonstrated insecticidal activity against An. gambiae [27].
Figure 3.
FT-IR spectrum analysis of acetone extract of R. murconata.
Table 5.
FT-IR analysis of peak values of R. mucronata acetone extract.
| S.No. | Peak (Wave Number cm−1) | Intensity | Bond | Functional Group Assignment |
|---|---|---|---|---|
| 1 | 3411.62 | 39.09 | N-H Stretch | Amine |
| 2 | 2926.40 | 23.06 | C-H Stretch | Alkyl |
| 3 | 2855.38 | 36.55 | C-H Stretch | Alkyl |
| 4 | 1710.59 | 42.02 | C=O Stretch | Aldehyde |
| 5 | 1610.19 | 50.93 | C=O Stretch | Amide |
| 6 | 1516.62 | 59.60 | C=C Bending | Aromatic |
| 7 | 1458.22 | 49.94 | C-H Bending | Alkane |
| 8 | 1371.66 | 53.83 | C-H Bending | Alkane |
| 9 | 1266.13 | 47.76 | C-N Stretch | Amine |
| 10 | 1168.88 | 49.26 | C-N Stretch | Amine |
| 11 | 1115.63 | 53.74 | C-N Stretch | Amine |
| 12 | 830.48 | 80.77 | C-H Bending | Aromatic |
| 13 | 721.46 | 85.12 | C-Cl Stretch | Alkyl Halide |
Figure 4.
HPLC chromatogram analysis for acetone extract active F3-fractions of R. murconata.
2.4. Enzyme Assay
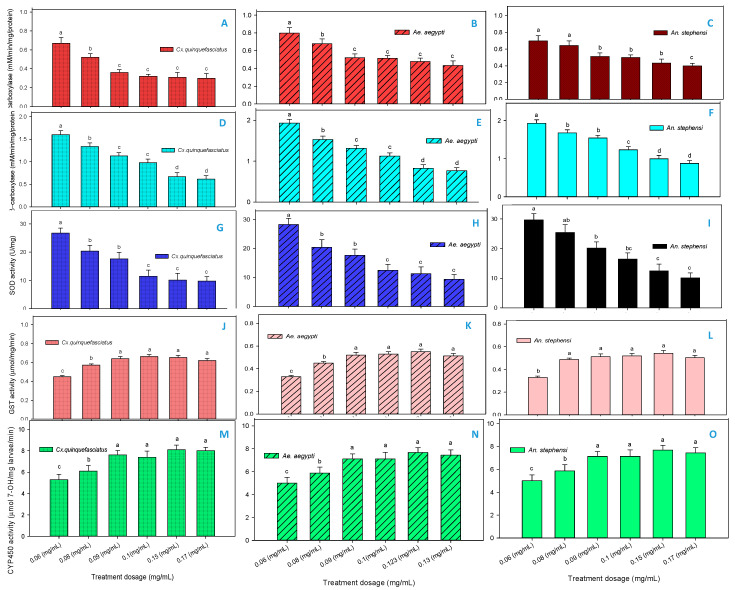
The active acetone extract F3-fractions of R. murconata inhibited α-carboxylesterase activity of fourth instar larvae tested at 24 h. The activity decreased in dose dependent manner across Cx. quinquefasciatus (Figure 5A, F4,20 = 41.62; p < 0.0001), Ae. aegypti (Figure 5B, F4,20 = 32.12; p < 0.0001) and An. stephensi (Figure 5C, F4,20 = 27.22; p < 0.0001), respectively. Similarly, β carboxylesterase activity decreased significantly to the sub-lethal dosages of F3-fractions against all the three mosquito vectors. However, the reduction rate was prominent in Cx. quinquefasciatus (Figure 5D, F4,20 = 27.22; p < 0.0001) as compared to Ae. aegypti (Figure 5E) and An. stephensi (Figure 5F). Likewise, the level of SOD activity was also declined at the maximum sub-lethal dosages of all the three mosquito vectors (Figure 5G–I). However, the reduction rate was minimal at the dosage of 0.6 mg/mL in Cx. quinquefasciatus (F4,20 = 13.11; p < 0.0001), Ae. aegypti (F4,20 = 15.11; p < 0.0001) and An. stephensi (F4,20 = 17.26; p < 0.0001), respectively. It has been well known that many bio-insecticides affects the insect metabolism by inhibiting or stimulating the activity of digestive enzymes. Generally, SOD (superdioxide) expressed in several regions especially in anal gills of mosquitoes to catalyzes the superoxide radical dismutation into hydrogen peroxide [28].
Figure 5.
(A–C) α- carboxylestrase (D–F) β carboxylestrase (G–I) SOD activity (J–L) Glutathione S-transferase (M–O) CYP450 enzyme activity of Cx. quinquefasciatus, Ae. aegypti, An. stephensi fourth instar larvae after treatment with active acetone extract F3- fractions of R. murconata. Mean (± SEM) followed by the same letter in the above bars indicate no significant difference (p < 0.05) in a Tukey’s test.
In contrast to the above enzyme levels, GST and CYP450 enzyme regulations upregulated significantly in dose dependent manner. The enzyme ratio increased significantly initially in the lower doses (0.06 mg/mL) and relics constant at the higher sub-lethal dosages across all the mosquito vectors. Besides, the GST (Figure 5J–L) and CYP450 (Figure 5M–O) enzyme regulations uplifted significantly in Cx. quinquefasciatus (F4,20 = 11.21; p < 0.0001) as compared to Ae. aegypti (F4,20 = 16.71; p < 0.0001) and An. stephensi (F4,20 = 14.55; p < 0.0001). Our enzyme analysis data was interpreted to display that R. mucronata active F3-fractions exert divergent effects on all three mosquitos’ biochemical defensive mechanisms.
In addition to their potential for mosquito control, the R. mucronata extracts may exhibit enzyme inhibitory activity at currently unknown bioactivities and further studies are required to investigate the possibility of the herein identified constituents of R. mucronata extracts for the development of environmentally-friendly applications. Indeed, phytochemicals from plant sources may also act as insect growth regulators, repellents, ovipositor attractants, among others [26,27].
2.5. Repellent Activity
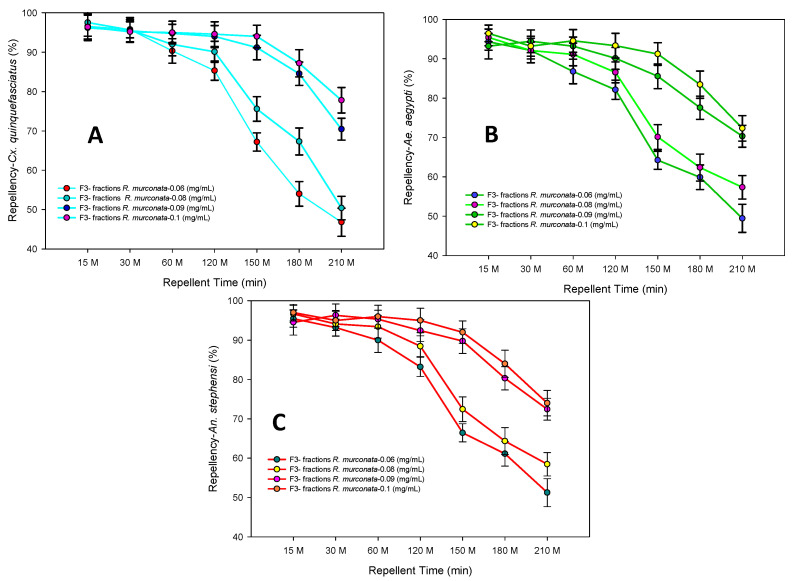
The active fractions of R. mucronata showed significant repellent activity against Cx. quinquefasciatus (96.4%-F4,20 = 24.22, p ≤ 0.0001), Ae. aegypti (94.32%-F4,20 = 18.82, p ≤ 0.0001) and An. stephensi (97.2%-F4,20 = 21.88, p ≤ 0.0001), respectively, at the maximum repellent dosage of 0.1 (mg/mL) at the maximum repellent time of 210 min (Figure 6. Similarly, the sub-lethal dosage (100 ppm) of crude seed extracts of Terminalia chebula Retz. displayed significant protection time at the maximum time of 210 min [29].
Figure 6.
Repellency of acetone extract F3- fractions of R. murconata against Cx. Quinquefasciatus (A), Ae. Aegypti (B), and An. Stephensi (C). Mean (± SEM) followed by the same letter in the above bars indicate no significant difference (p < 0.05) in a Tukey’s test.
3. Materials and Methods
3.1. Plant Harvesting
The fresh leaves of R. mucronata were harvested from the region of Pichavaram, Rameshwaram (Latitude: 13.129991; Longitude: 79°18′46.54” E), Tamil Nadu, India, with taxonomy confirmed by an expert (Prof. Kathiresan, Department of Marine Biology, Annamalai University, Tamil Nadu) (Figure S1). The specimen voucher was preserved in the herbarium (Ref. No. BC¬/2016/Rm- 07) for further assays.
3.2. Crude Extract Preparation
Collected fresh leaves were air dried under shadow at room temperature for 7–10 days. The leaves (250 g) were dried and mechanically powdered using a mixer and crushed (Mixer Grinders Stylo 750) to well particle size. Leaf powdered extracts of R. mucronata (250 g) was prepared by using soxhlet device utilizing diverse solvents including acetone, ethyl acetate, petroleum benzene, and methanol. Further the solvents were evaporated by using rotary evaporator and the remaining were preserved under 4 °C for further assays. The total yields were observed 2.15, 1.76, 1.95 and 2.02 g, respectively.
3.3. Mosquitoes
Ae. aegypti, An. stephensi, and Cx. quinquefasciatus larvae were collected from the local areas of Pallipalayam (Latitude: 11.3450° N; Longitude: 77.7309° E), Tiruchengode, Tamil Nadu, India. Furthermore, the culture were maintained in the laboratory were kept in sterile vessels filled with water, maintained at 28 ± 2 °C and 75%–80% relative humidity (RH) under a fixed photoperiod (14:10 L/D). The emerged adult mosquitoes were maintained under the same conditions as the larvae.
3.4. Larval Mortality Assay
Larval bioassays were executed on the fourth instars of Cx. quinquefasciatus, An. stephensi and Ae. aegypti with diversified dosages (0.5, 0.10, 0.15, 0.20 and 0.25 mg/mL) of R. mucronata leaf extracts. Minimal of 20 larvae/each concentration were utilized for all the assays, and the procedure were three times replicated. The lethal dosage (LC50 and LC90) was calculated based on Probit analysis [30]. Twenty larvae of fourth instars were presented to a 200 mL glass jar supplemented with discriminating dosage of leaf extracts along with 50 mg/L of yeast extract. A group of control was also kept alone treated with methanol. Three times were replicated along with control in each replication. Mortality percentage in the treatments was corrected whenever required using Abbott formula [31].
3.5. Preparation of Whole-Body Homogenates for Enzyme Assay
The control and plant extract exposed fourth instar larvae were rinsed with sterile distilled water, and the adhering water was distant by using blotting tissue paper from the surface. The larval homogenized were distinct using a handy homogenizer in Eppendorf tubes containing ice-cold sodium phosphate buffer (500 μL−20 mM, pH 7.0). Homogenates were further spin for 20 min at 8000× g at 4 °C) and the supernatants separated were utilized for enzyme experiments. The homogenates separated were reserved at −4 °C for further experiments.
3.6. Carboxyl Esterase Assays
The α- and β- carboxylesterase activity was estimated using the larval extracts in phosphate potassium buffer (0.2 M; pH = 7.1) were set to (20 μL; 84 μg protein). Further, it was assorted with 500 μL buffer (0.3 mMα- or β-naphthyl acetate in 0.1 M phosphate potassium at pH 7.2 containing 1% acetone). Enzyme activity of one unit was definite as the enzyme amount essential to generate 1 μmol of α- or β-naphthol/minute.
3.7. Superoxide Dismutase Activity
The Superoxide Dismutase (SOD) assays were carried out by using the Superoxide dismutase determination kit (Sigma-Aldrich, Bangalore, India). Activity of SOD per 1 unit was resolute as the required enzyme to slab the increment absorbance by 50% at 440 nm.
3.8. Glutathione-S-Transferase Activity
A total of 250 μL of fourth instars were homogenized in sodium phosphate solution (50 mM; pH = 7.2) and spin at 10,000× g at 4 °C for 20 min. The Glutathione-S-Transferase (GST) assay Kit (Sigma-Aldrich, Catalog 0410, Bangalore, India) was utilized to investigate the conjugation of the thiol group of glutathione to the 1-chloro-2,4-dinotrobenzene (CDNB) substrate. Further assays were performed based on the standard protocol prescribed in the Kit. The GST activity was stated as µmol/mg protein/min substrate conjugated.
3.9. Cytochrome P450 Activity
Fourth instar visible to bioactive fractions3 were rinsed in sterile water and separated. Further the larvae were kept in 40 mM sodium phosphate solution with maintained pH: 7.2 and cooled earlier separation. The abdominal segments, heads, and digestive tissues were extracted. For determining the enzyme activity, carcasses were taken. The enzymes were measured by using ethoxycoumarin-O-deethylase existing in the body walls and it was stated as mol 7-OH/mg larvae/min.
3.10. Phytochemical Analysis
Chemical testing for the presence of carbohydrates, alkaloids, saponins, phenolics, tannins, terpenes and flavonoids was evaluated in the mangrove extracts and the most active extract using the standard procedure of Harborne [32].
3.10.1. Phenols: Ferric Chloride Test
Extracts were treated with 3–4 drops of ferric chloride solution. Formation of bluish black color indicates the presence of phenols.
3.10.2. Flavonoids Test
Ammonia solution (5 mL) was added to a portion of the crude extract followed by addition of concentrated H2SO4. Formation of a yellow coloration in the extract indicates the presence of flavonoids. The yellow coloration disappears after some time.
3.10.3. Alkaloids: Wagner Reagent
Extracts were dissolved individually in dilute Hydrochloric acid and filtered. Filtrates were treated with Wagner’s reagent (1.27 g Iodine in 2 g of Potassium Iodide). The formation of a brown/reddish precipitate indicates the presence of alkaloids.
3.10.4. Saponin
Half mg of the extract was shaken with 2 mL of distilled water. If foam produced persists for ten min, it indicates the presence of saponins.
3.10.5. Tannin Test: Gelatin Test
To the extract, 1% gelatin solution containing sodium chloride was added. Formation of white precipitate indicates the presence of tannins.
3.10.6. Glycosides Test
Minimum quantities of the extracts were hydrolyzed with hydrochloric acid for a few minutes on a water bath and the hydrolysate was subjected to the following tests.
The extracts were treated with chloroform and evaporate it to dryness. Separately, 0.4 mL of glacial acetic acid containing a trace amount of ferric chloride was added and transferred to a small test tube added with carefully 0.5 mL of concentrated sulfuric acid by the side of the test tube; blue color appeared in the acetic acid layer, indicating the presence of glycosides.
3.10.7. Ninhydrin Test
To the extract, 0.25% w/v Ninhydrin reagent was added and boiled for few minutes. Formation of blue or blue to violet indicates the presence of amino acid.
3.10.8. Benedict Test
Two mL of crude extracts were dissolved individually in 5 mL distilled water and filtered. Filtrates were treated with Benedict’s reagent and heated gently. Orange-red precipitate indicates the presence of reducing sugars.
3.10.9. Starch Test
To 5 mL of the extract, a few drops of iodine was added. The presence of starch was indicated by the formation of blue color.
3.10.10. Flavonoids Test
Ammonia solution (5 mL) was added to a portion of the crude extract followed by addition of concentrated H2SO4. Formation of a yellow coloration in the extract indicates the presence of flavonoids. The yellow coloration disappears after some time.
3.11. Repellent Assay
The active fractions F3 derived from R. mucronata extracts evaluated for its repellency against all the three mosquito vectors with sub-lethal dosages (0.06, 0.08, 0.09 and 0.1 mg/mL). Further repellent assay experiments were adapted from our previous research protocol Thanigaivel et al. [29].
3.12. GC–MS Analysis and Compound Identification
Among the different extracts (acetone, petroleum benzene, ethyl acetate and methanol) which were tested for their larvicidal toxic, and based on our preliminary tests, the highest toxicity was observed in acetone extracts of R. mucronata. Therefore, the acetone extract was used for further experiments. The isolated R. mucronata acetone extract was dissolved in (1:1) ratio with ethyl alcohol. From this, 2 μL of crude solution was dissolved in HPLC-grade methanol and subjected to GC and MS JEOL GC (Agilent Technologies 6890N, PerkinElmer, Bangalore, India) mate equipped with secondary electron multiplier. The downstream procedure of chemical characterization was carried out by our previous methodology (Agilent Technologies 6890N, PerkinElmer, Bangalore, India) [29]. The molecular weight, molecular formula and structure of the compounds of tested materials were ascertained by interpretation on mass spectrum of GC-MS using the database of the National Institute Standard and Technology (NIST).
3.13. FT-IR Analysis
Dried acetone extract was used for FT-IR analysis according to the combined methods reported in [33,34]. A total of 2 mg sample were mixed with 100 mg KBr (FT-IR grade) and then compressed to prepare a translucent sample disc (3 mm diameter), which was immediately kept in the sample holder. The sample was scanned and the FT-IR spectra recorded in the absorption range of 400 and 4000 cm−1. FT-IR analysis was performed using a Perkin-Elmer spectrophotometer (Perkin-Elmer FT-IR, Spectrum 2 Singapore, L160000A) to detect characteristic peaks, types of chemical bonds, and probable functional groups present in the sample. FT-IR peak values were recorded and the analysis was repeated twice for spectrum confirmation.
3.14. High Performance Liquid Chromatography (HPLC) Analysis
The HPLC (Flexar HPL, Perkin Elmer, Chennai, India) analysis was carried out with a C18 column (220 nm) (250 × 4.6 mm). Acetonitrile and water were used as mobile phase at flow rate of 1 mL/min. Peaks obtained were detected at 220 nm. Fraction F3 was analyzed and compared with a purchased standard of squalene (Sigma-Aldrich, Analytical Standard). The yield of the collected fraction dry weight is 0.57 mg.
3.15. Statistical Analysis
Average larval mortality data were analyzed by probit analysis to calculate the LC50, LC90, and other statistics with 95% confidence intervals of upper confidence limit (UCL) and lower confidence limit (LCL) standards and the chi-squared test. SPSS 14.0 (IBM Inc., Chicago, IL, USA) was used to analyze the data.
4. Conclusions
As an endnote, the phyto-chemical characterization of acetone extracts of R. mucronata through GC-MS analysis displayed 13 major phyto-chemicals and the individual bio-active fractions were purified and characterized through HPLC and FT-IR assays which signified that active fraction-F3 was prominent as compared to other fractions and the larvicidal activity of active fraction F3 delivers that they are lethal against larvae of all three mosquito vectors. Moreover, the enzyme assays signify that the acetone extract F3-fractions significantly drifted the enzyme pattern in all five major digestive and detoxifying enzymes. Furthermore, the sub-lethal dosage of active fraction F3 also showed significant repellent activity in a dose dependent manner across all the three arthropod vectors. From this research, it is suggested that the mangrove leaf extract of R. murconata contains several bioactive molecules that might be useful as eco-friendly larvicides and repellent for managing blood sucking pests of medical importance. Further functional and mechanistic studies on how these compounds exert larvicidal activity may pave the way for environmentally safe botanical insecticides to control mosquito populations.
Acknowledgments
We would like to thank the Department of Biochemistry, K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, Namakkal, Tamil Nadu, India for providing the infrastructural facility for carrying out this research work and also, this work was partially supported Post-Doctoral Program, Chiang Mai University, Thailand. We also thank the anonymous reviewers for their critical and constructive comments which helped a lot in improving the draft.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/17/3844/s1, Figure S1: Rhizophora mucronata (A) whole plant and (B) stem and leaf.
Author Contributions
S.K., M.V., K.U., V.M. and P.K. designed the research plan and drafted the manuscript. M.V., K.U., V.M., P.V.-S., K.C., performed the experimental works and data compilation. M.V., K.U., P.K., and S.K. coordinated the work and discussed the results. S.K. and P.K. revise the manuscript. All authors read and approved the final manuscript.
Funding
This research was partially funded by Post-Doctoral Program, Chiang Mai University, Thailand.
Conflicts of Interest
The authors declare that no competing interests exist.
Footnotes
Sample Availability: Samples of the compounds are not available.
References
- 1.Narendhiran S., Mohanasundaram S., Arun J., Saravanan L., Catherine L., Subathra M., Rannjith R. Comparative study in larvicidal efficacy of medicinal plant extracts against Culex quinquefasciatus. Int. J. Res. Plant Sci. 2014;4:22–25. [Google Scholar]
- 2.Chellappandian M., Thanigaivel A., Vasantha-Srinivasan P., Edwin E., Ponsankar A., Selin-Rani S., Kalaivani K., Senthil-Nathan S., Benelli G. Toxicological effects of Sphaeranthus indicus Linn. (Asteraceae) leaf essential oil against human disease vectors, Culex quinquefasciatus Say and Aedes aegypti Linn. and impacts on a beneficial mosquito predator. Environ. Sci. Pollut. Res. Int. 2017;1110:294–10306. doi: 10.1007/s11356-017-8952-2. [DOI] [PubMed] [Google Scholar]
- 3.Chellappandian M., Vasantha-Srinivasan P., Senthil-Nathan S., Karthi S., Thanigaivel A., Ponsankar A., Kalaivani K., Hunter W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018;113:214–230. doi: 10.1016/j.envint.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac. J. Trop. Med. 2018;3:74–877. doi: 10.1016/S1995-7645(10)60210-6. [DOI] [Google Scholar]
- 5.Panneerselvam C., Murugan K., Kovendan K., Kumar P.M., Ponarulselvam S., Amerasan D. Larvicidal efficacy of Catharanthus roseus Linn. (Family: Apocynaceae) leaf extract and bacterial insecticide Bacillus thuringiensis against Anopheles stephensi Liston. Asian Pac. J. Trop. Biomed. 2013;6:847–853. doi: 10.1016/S1995-7645(13)60151-0. [DOI] [PubMed] [Google Scholar]
- 6.Edwin E., Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Ponsankar A., Pradeepa V., Selin-Rani S., Kalaivani K., Hunter W.B., Abdel-Megeed A., et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae) Acta Trop. 2016;163:167–178. doi: 10.1016/j.actatropica.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Pavela R., Maggi F., Iannarelli R., Benelli G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019;193:236–271. doi: 10.1016/j.actatropica.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Elumalai D., Hemalatha P., Kaleena P. Larvicidal activity and GC–MS analysis of Leucas aspera against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. J. Saudi Soc. Agric. Sci. 2017;16:306–313. doi: 10.1016/j.jssas.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Kalaivani K., Senthil-Nathan S., Murugesan A.G. Biological activity of selected Lamiaceae and Zingiberaceae plant essential oils against the dengue vector Aedes aegypti L. (Diptera: Culicidae) Parasitol. Res. 2012;110:1261–1268. doi: 10.1007/s00436-011-2623-x. [DOI] [PubMed] [Google Scholar]
- 10.Sakulku U., Nuchuchua O., Uawongyart N., Puttipipatkhachorn S., Soottitantawat A., Ruktanonchai U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int. J. Pharm. 2009;372:105–111. doi: 10.1016/j.ijpharm.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Senthilkumar A., Venkatesalu V. Larvicidal potential of Acorus calamus L. essential oil against filarial vector mosquito Culex quinquefasciatus (Diptera: Culicidae) Asian Pac. J. Trop. Dis. 2012;2:324–326. doi: 10.1016/S2222-1808(12)60070-X. [DOI] [Google Scholar]
- 12.Skenderidis P., Mitsagga C., Giavasis I., Petrotos K., Lampakis D., Leontopoulos S., Hadjichristodoulou C., Tsakalof A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Measur. Charact. 2019;13:2017–2031. doi: 10.1007/s11694-019-00123-6. [DOI] [Google Scholar]
- 13.Senthil-Nathan S. Physiological and biochemical effect of Neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013;4:1–17. doi: 10.3389/fphys.2013.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senthil-Nathan S. Environmental Sustainability-Role of Green Technologies. Springer; Berlin, Germany: 2015. A review of bio pesticides and their mode of action against insect pests; pp. 49–63. [Google Scholar]
- 15.Mathew N., Anitha M., Bala T., Sivakumar S., Narmadha R., Kalyanasundaram M. Larvicidal activity of Saraca indica, Nyctanthes arbortristis, and Clitoria ternatea extracts against three mosquito vector species. Parasitol. Res. 2009;104:1017–1025. doi: 10.1007/s00436-008-1284-x. [DOI] [PubMed] [Google Scholar]
- 16.Manjari S., Karthi S., Ramkumar G., Muthusamy R., Natarajan D., Shivakumar M.S. Chemical composition and larvicidal activity of plant extracts from Clausena dentata (Willd) (Rutaceae) against dengue, malaria, and filariasis vectors. Parasitol. Res. 2014;113:2475–2481. doi: 10.1007/s00436-014-3896-7. [DOI] [PubMed] [Google Scholar]
- 17.Senthil-Nathan S., Kalaivani K. Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura fabricius (Lepidoptera: Noctuidae) Biol. Control. 2005;34:93–98. doi: 10.1016/j.biocontrol.2005.03.001. [DOI] [Google Scholar]
- 18.Senthil-Nathan S., Kalaivani K., Murugan K. Effects of neem limonoids on the malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Acta Trop. 2005;96:47–55. doi: 10.1016/j.actatropica.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Sadek M.M. Antifeedant and toxic activity of Adhatoda vasica leaf extract against Spodoptera littoralis (Lepidoptera: Noctuidae) J. Appl. Entomol. 2003;127:396–404. doi: 10.1046/j.1439-0418.2003.00775.x. [DOI] [Google Scholar]
- 20.Vasantha-Srinivasan P., Senthil-Nathan S., Ponsankar A., Thanigaivel A., Edwin E., Selin-Rani S., Chellappandian M., Pradeepa V., Lija-Escaline J., Kalaivani K. Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide Temephos and plant derived essential oil derived from Piper betle L. Ecotoxicol. Environ. Saf. 2017;139:439–446. doi: 10.1016/j.ecoenv.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Vasantha-Srinivasan P., Thanigaivel A., Edwin E., Ponsankar A., Senthil-Nathan S., Selin-Rani S., Kalaivani K., Hunter W.B., Duraipandiyan V., Al-Dhabi N.A. Toxicological effects of chemical constituents from Piper against the environmental burden Aedes aegypti Liston and their impact on non-target toxicity evaluation against biomonitoring aquatic insects. Ecotoxicol. Environ. Saf. 2018;25:10434–10446. doi: 10.1007/s11356-017-9714-x. [DOI] [PubMed] [Google Scholar]
- 22.Rajkumar S., Jebanesan A. Mosquitocidal activities of octacosane from Moschosma polystachyum Linn. (lamiaceae) J. Ethnopharmacol. 2004;90:87–89. doi: 10.1016/j.jep.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 23.Mansour S.A., Ibrahim R.M., El-Gengaihi S.E. Insecticidal activity of chicory (Cichorium intybus L.) extracts against two dipterous insect-disease vectors: Mosquito and housefly. Ind. Crops Prod. 2014;54:192–202. doi: 10.1016/j.indcrop.2014.01.011. [DOI] [Google Scholar]
- 24.Soule S., Guntner C., Vazquez A., Argandona V., Moyna P., Ferreira F. An aphid repellent glycoside from Solanum laxum. Phytochemistry. 2000;55:217–222. doi: 10.1016/S0031-9422(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 25.Murungi L., Kirwa H., Torto B. Differences in essential Oil Content of berries and Leaves of Solanum sarrachoides (Solanaceae) and the effects on oviposition of the tomato spider mite (Tetranychus evansi) Ind. Crops Prod. 2013;46:73–79. doi: 10.1016/j.indcrop.2013.01.022. [DOI] [Google Scholar]
- 26.Lucia A., Licastro S., Zerba E., Audino P.G., Masuh H. Sensitivity of Aedes aegypti adults (Diptera: Culicidae) to the vapors of Eucalyptus essential oils. Bioresour. Technol. 2009;100:6083–6087. doi: 10.1016/j.biortech.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 27.Bossou D.A., Mangelinckx S., Yedomonhan H., Boko P.M., Akogbeto M.C., Kimpe N. Chemical composition and insecticidal activity of plant essential oils from Benin against Anopheles gambiae (Giles) Parasit. Vectors. 2013;6:337. doi: 10.1186/1756-3305-6-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nivsarkar M., Kumar G.P., Laloraya M., Laloraya M.M. Superoxide dismutase in the anal gills of the mosquito larvae of Aedes aegypti: Its inhibition by alpha-terthienyl. Arch. Insect Biochem. Physiol. 1991;16:249–255. doi: 10.1002/arch.940160404. [DOI] [PubMed] [Google Scholar]
- 29.Thanigaivel A., Vasantha-Srinivasan P., Senthil-Nathan S., Edwin E., Ponsankar A. Chellappandian, M.; Impact of Terminalia chebula Retz. against Aedes aegypti L. and non-target aquatic predatory insects. Ecotoxicol. Environ. Saf. 2017;137:210–217. doi: 10.1016/j.ecoenv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Perumalsamy H., Jang M.J., Kim J.R., Kadarkarai M., Ahn Y.J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasit. Vectors. 2015;8:237. doi: 10.1186/s13071-015-0848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott W. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 32.Harborne J. Phytochemical Methods. Springer; Berlin, Germany: 1973. Phenolic compounds; pp. 33–88. (Print) (Online) [Google Scholar]
- 33.Al-Tameme H.J., Hameed I.H., Idan S.A., Hadi M.Y. Biochemical analysis of Origanum vulgare seeds by fourier-transform infrared (FT-IR) spectroscopy and gas chromatography-mass spectrometry (GC-MS) J. Pharmacogn. Phytother. 2015;7:221–237. [Google Scholar]
- 34.Hussein A.O., Mohammed G.J., Hadi M.Y., Hameed I.H. Phytochemical screening of methanolic dried galls extract of Quercus infectoria using gas chromatography-mass spectrometry (GC-MS) and Fourier transform-infrared (FT-IR) J. Pharmacogn. Phytother. 2016;8:49–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.