Abstract
Although chitin is of the most available biopolymers on Earth its uses and applications are limited due to its low solubility. The deacetylation of chitin leads to chitosan. This biopolymer, composed of randomly distributed β-(1-4)-linked D-units, has better physicochemical properties due to the facts that it is possible to dissolve this biopolymer under acidic conditions, it can adopt several conformations or structures and it can be functionalized with a wide range of functional groups to modulate its superficial composition to a specific application. Chitosan is considered a highly biocompatible biopolymer due to its biodegradability, bioadhesivity and bioactivity in such a way this biopolymer displays a wide range of applications. Thus, chitosan is a promising biopolymer for numerous applications in the biomedical field (skin, bone, tissue engineering, artificial kidneys, nerves, livers, wound healing). This biopolymer is also employed to trap both organic compounds and dyes or for the selective separation of binary mixtures. In addition, chitosan can also be used as catalyst or can be used as starting molecule to obtain high added value products. Considering these premises, this review is focused on the structure and modification of chitosan as well as its uses and applications.
Keywords: chitin, chitosan, biomaterial, adsorbent, antioxidant, high added value product
1. Introduction
Chitin is considered the second most abundant polysaccharide (after cellulose) on Earth, being first described by Henri Braconnot in 1811. It appears in Nature as ordered macrofibrils in the exoskeleton of mollusks and crustaceans, as well as in fungi and insect cuticles [1] (Table 1 and Figure 1). Its natural abundance allows obtaining more than 1000 tons every year, of which about 70% comes from marine species [2].
Table 1.
Sources of chitin and chitosan.
| Sea Animals | Insects | Microorganisms |
|---|---|---|
| Crustaceans | Scorpions | Green algae |
| Coelenterata | Brachiopods | Yeast (β-type) |
| Annelida | Cockroaches | Fungi (cell walls) |
| Mollusca | Spiders | Mycelia penicillium |
| Lobster | Beetles | Brown algae |
| Shrimp | Ants | Chytridiaceae |
| Prawn | - | Ascomydes |
| Krill | - | Blastocladiacease |
| Crab | - | Spores |
Figure 1.
Structural composition and arrangement of chitin in the shell of crustaceans.
From a chemical viewpoint, chitin is a poly(β-(1-4)-N-acetyl-d-glucosamine) with β(1→4) linkages [1], and it is considered to have a cellulose-like structure, where the hydroxyl group in the C2 position has been replaced by an acetamido group (Figure 1). Depending on the orientation of polysaccharide chains, chitin displays three polymorphs, denoted as α, β and γ [3]. Among them, the α-type is the most abundant in shellfish shells. This polymorph has an antiparallel arrangement, where each chain strongly interacts with the adjacent through hydrogen bonds, which provide high thermochemical stability, as well as high insolubility [4,5]. Thus, chitin is a highly insoluble polymer, making it poorly biodegradable. Thus, only the action of chinitanase enzymes, widely distributed in nature, can degrade chitin [6]. This low solubility in water and most of organic solvents has limited its uses and applications.
Nowadays, there is a well-established protocol for the chitin extraction from shellfish wastes in industry, which includes the steps of demineralization, deproteinization and decolorization [7,8]. The deproteinization is carried out by an alkaline treatment, whereby lipids and proteins are hydrolyzed. The demineralization stage is generally performed in the presence of acids, whereas the decolorization requires an oxidative treatment (Figure 2) [9,10]. Finally, chitin, in turn, can be deacetylated by a basic treatment to give rise to chitosan, which is a soluble polymer in acid aqueous medium. Depending on the production method and species used, the degree of deacetylation ranges from 56 to 99%, but at least 85% deacetylation is required for a good solubility of chitosan [11]. Table 2 compiles most of methods employed to quantify the deacetylation degree and the molecular weight of the chitosan obtained.
Figure 2.
Purification processes of crustacean wastes.
Table 2.
Several characterization methods to evaluate the deacetylation degree and average molecular weight of chitosan.
2. Chitosan
Chitosan was discovered by Rouget in 1859 after heating chitin in an alkaline medium [26]. Several years later, Hoppe-Seyer called this material chitosan, although its chemical structure was not elucidated until 1950 [27].
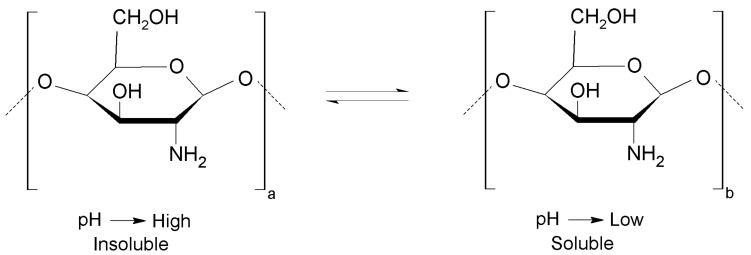
In recent years, chitosan-based materials have been developed due to their particular chemical properties, which provide it a wide range of applications as indicated in Table 3. Generally, chitosan is highly soluble in acid solution (mainly below pH = 6.0), being a weak base (pKa = 6.3) due to the presence of amine groups. At low pH values, the amine groups are positively charged due to protonation, so chitosan can be a water-soluble cationic polyelectrolyte. However, when the pH increases above 6, the amine groups of chitosan residues are deprotonated and the biopolymer loses its charge leading to an insoluble polymer (Figure 3) [29].
Table 3.
Main chemical properties of chitosan, according the information reported in [28].
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 3.
Chemical structure of chitosan as a function of pH. Insoluble (pH > 6) and soluble (pH < 6).
2.1. Modification of Chitosan by Functionalization
The presence of amine and hydroxyl groups provides interesting applications to chitosan, since these can be modified to improve certain properties of this biopolymer. Among some chemical processes to improve their properties, cross-linking, graft copolymerization, carboxymethylation, etherification, and esterification must be highlighted as the main strategies to functionalize the chitosan structure [30].
2.1.1. Cross-Linking/Hydrophobic Interactions
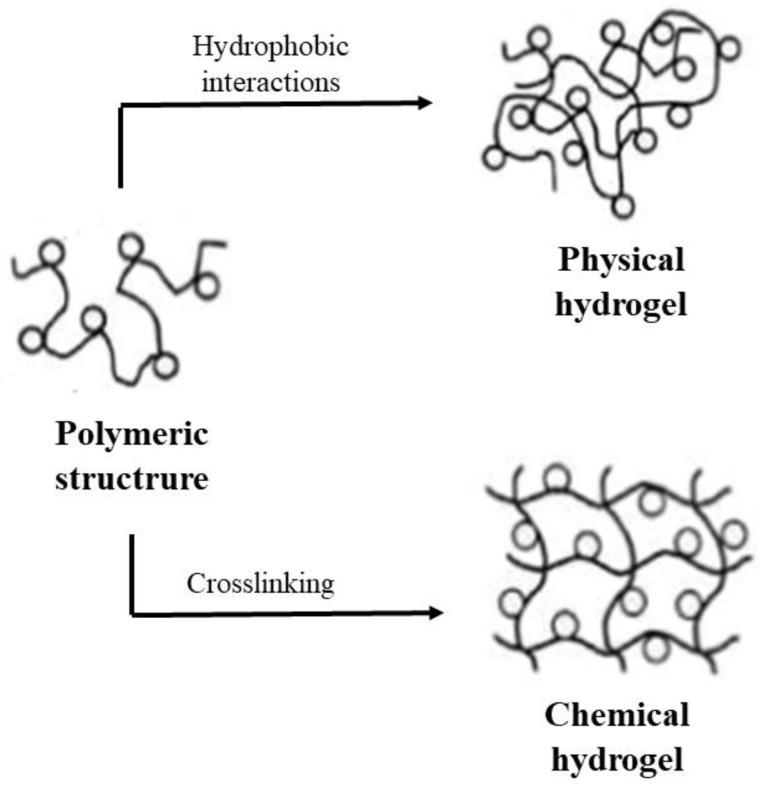
A hydrogel is defined as a polymeric structure where the chains are linked through non-covalent and/or covalent bonds to form a tridimensional network. These structures possess the ability to retain large amounts of water, causing the swelling of the structure. Chitosan is a biopolymer that can form hydrogels as a consequence of small modifications of ionic strength or pH.
As was previously indicated, the amine groups of chitosan are protonated in an acidic medium. This fact causes electrostatic repulsions, which promote the swelling of the chitosan structure [31]. The formation of hydrogels takes place mainly by electrostatic interactions of the hydroxyl groups located in C-3 and C-6 positions and the amine group located in the C-2 position of the monomers. Thus, chitosan tends to form cross-linked tridimensional structures with dialdehydes, such as glyoxal or glutaraldehyde, which are used, for example, to develop membranes with proton conductivity, which have potential use in fuel cells [32]. On the other hand, glutaric and adipic acids were also employed in the synthesis of biocompatible chitosan hydrogels [33,34]. In all cases, it has been reported that the degree of cross-linking is directly related to the properties of hydrogels, such as swelling degree, mechanical strength or pore size, among others (Figure 4). Chitosan hydrogels can also be used as controlled release systems, since they are able to maintain a constant drug concentration for a prolonged time in a particular environment [35,36]. As chitosan is a biocompatible polymer, these hydrogels have been used to prepare biodegradable sutures, hemodialysis membranes, healing of wound and burns, cells or immobilizing enzymes [2].
Figure 4.
Chitosan hydrogels obtained by hydrophobic or crosslinking interactions.
2.1.2. Graft Copolymerization
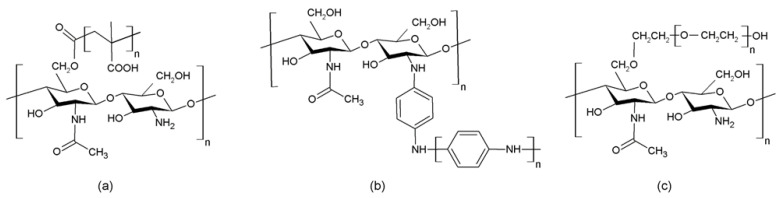
The graft copolymerization of synthetic polymers with chitosan is of great interest in several fields. It has been reported that a chitosan/methacrylate composite can be synthesized using ammonium persulfate (APS) as initiator, obtaining a copolymer whose solubility is much higher than that reported for their respective chitosan hydrogels (Figure 5A) [37]. Other authors have pointed out that the copolymerization of chitosan/aniline using, APS initiator leads to films with protonic conductivity (Figure 5B) [38]. The use of polyethylene glycol as a copolymer of chitosan has been widely studied (Figure 5C), being considered as suitable graft-forming polymers due to its good solubility both in H2O and inorganic solvents, high biodegradability and biocompatibility or low toxicity [39].
Figure 5.
Some examples of chitosan graft copolymers: (a) chitosan/PMMA, (b) chitosan/PANI and (c) chitosan/PEG.
2.1.3. Carboxymethylation
As was indicated in the previous sections, one of main disadvantages of chitosan is its low solubility. Carboxymethylation is an alternative to improve its solubility in aqueous media. This process takes place by the dispersion of chitosan in 2-propanol in basic medium.
In the next step, a 2-propanol/monochloroacetic acid mixture is added to the first suspension [40]. It must be considered that O- and N-carboxymethylation may occur simultaneously, although several parameters can be controlled in such a way the reaction takes place through one of them (Figure 6). The final product (chitosan-carboxymethyl) is an amphoteric polymer whose solubility depends on pH.
Figure 6.
Reaction of chitosan O- and N- carboxymethylation.
2.1.4. Etherification
Chitosan was also grafted with propylene epoxide under basic conditions to form a hydroxypropylchitosan composite. Other authors have reported that the etherification reaction is carried out to improve the its solubility in organic solvents and water, charge, hydrophilicity and ability to interact with other substances [41]. Chitosan ethers can be applied in pharmaceutical, biomedical, adsorption, and environmental fields [42,43]. From this etherification process, it has been noted that the hydroxypropyl chitosan copolymer improves the antimicrobial applications, showing good inhibition effect against E. coli and S. aureus [37]. On the other hand, trimethylchitosan displays high solubility in water over a wide pH range in such a way that it can form stable ionic complexes with DNA and is therefore employed in DNA delivery [44]. It has been also reported that cyanoethyl chitosan improves solubility in organic solvents and can be used in dialysis, filtration and insulating papers [45] while hydroxyethyl chitosan and hydroxypropyl chitosan membranes improves the ionic conductivity about one order of magnitude in comparison to the pristine chitosan membrane [41].
2.1.5. Esterification
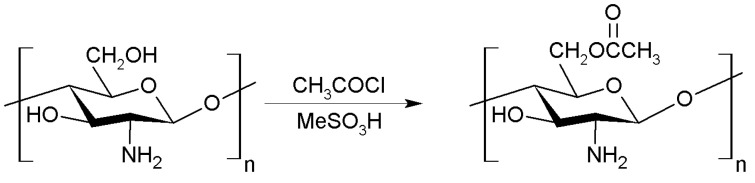
Another alternative to improve certain properties of chitosan is to carry out esterification reactions with the chitosan molecule. Previous research have stablished the synthesis of N,O-acyl chitosan with acetyl chloride by using MeSO3H as solvent (Figure 7). This reaction can lead to O- and N-acetylation although O-acetylated chitosan is the main product. In any case, the acetylation of chitosan substantially improves its antifungal activity [46,47].
Figure 7.
O-acylation of chitosan.
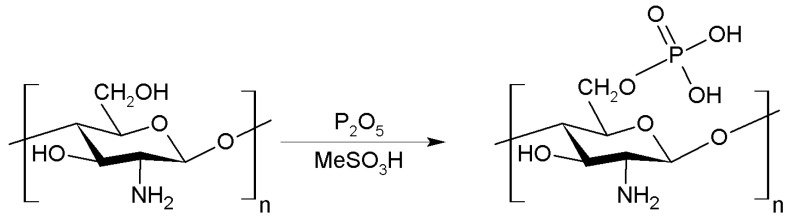
2.1.6. Phosphorilation
The phosphorylation of chitosan modifies their biological and chemical properties due to this treatment improves its bactericidal and osteoinductive properties. Generally, the phosphorylation of chitosan takes place in the C-3 and C-6 positions (Figure 8). Phosphorylated chitosan can be obtained by heating chitosan with phosphoric acid using N,N-dimethylformamide (DMF) as solvent. Another alternative to synthesize phosphorylated chitosan is through the reaction of chitosan with phosphorous pentoxide in the presence of methanesulphonic acid [48].
Figure 8.
Phosphorylation of chitosan using P2O5.
In its anionic form, phosphonic chitosan can interact with some amphoteric cations such as Ca2+, Cu2+, Cd2+ or Zn2+ [49]. This complexation process protects the surface of metals againts corrosion processes [50]. On the other hand, these phosphoriled derivatives can also be grafted with alkyl groups to improve their amphiphilic properties, being used in the cosmetic field [51].
2.1.7. Sulphatation
Both O-sulfated derivative [52] and N-sulfated chitosan [53] are of interest in biomedical applications as anticoagulants. In this sense, it has been reported that the degreed of sulfate substitution influence in the anticoagulant activity of chitosan, obtaining a similar anticoagulant activity to that reported for the heparin due its structural similarity [54]. On the other hand, sulfated chitosans are potent scavengers of free radical ions, including hydroxyl and superoxide ions [55].
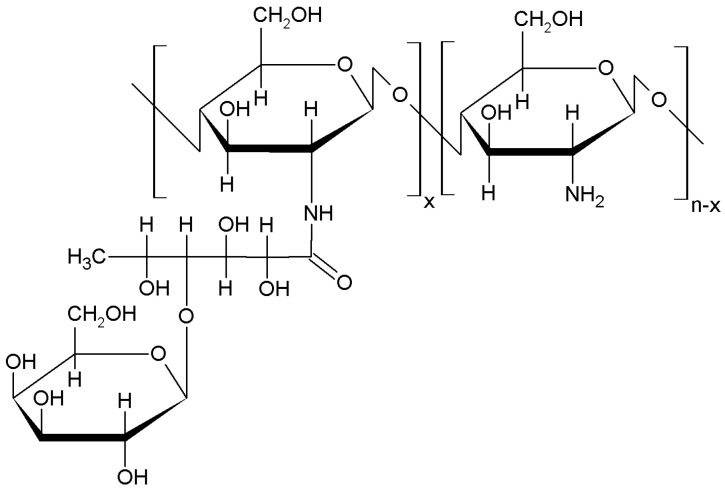
2.1.8. Guanidinylation/Biguanidinylation
Both guanidinylation and biguanidinylation of chitosan by grafting are effective and easy way to prepare composites which interact strongly with plasmic DNA in such a way it enhances the gene delivery [56]. In the same way, other authors reported that the guanidinylation of chitosan also has an excellent antibacterial activity although the grafting reaction between guanidine and chitosan requires high temperature [57,58]. More recently, other authors established that the microwave assisted preparation of antimicrobial chitosan with guanidine oligomers diminishes the temperature of the grafting reaction [59]. The obtained composite also showed a significant increases of the antimicrobial activity in comparison to the raw chitosan [59]. In the same way, the guanidinylation reaction of chitosan with dicyandiamide to form chitosan biguanidine hydrochloride has also reported excellent antibacterial and antifungal applications [60].
3. Applications of Chitosan
Taking into account that chitosan can be modified to improve some of its physical, chemical or biological properties, these chitosan-based materials have been highly tested in a wide range of applications. Some of the main applications will be highlighted in Table 4 and the next sections.
Table 4.
Some applications of chitosan in biomedical and pharmaceutical material.
| ● Treating major burns |
| ● Preparation of artificial skin |
| ● Surgical sutures |
| ● Contact lenses |
| ● Blood dialysis membranes |
| ● Artificial blood vessels |
| ● Antitumor |
| ● Blood anticoagulant |
| ● Antigastritis |
| ● Haemostatic |
| ● Hypochlesterolaemic agent |
| ● Antithrombogeic agent |
| ● Drug and gene-delivery systems |
| ● Dental therapy |
3.1. Uses in Pharmacy and Medicine
As was previously described, chitosan is a biopolymer with interesting biomedical applications due to its low toxicity, biodegradability and biocompatibility. This biopolymer is prone to be degraded by enzymatic hydrolysis with lysozyme, which is a proteolytic enzyme that is present in the tissues of all humans. In the same way, lipase present in the human gastric, pancreatic fluid or saliva is also able to degrade chitosan. In all cases, the obtained product were non-toxic [61].
The contact of the chitosan amine groups with the acid groups of the blood cells leads to the formation of clots as a consequence of thrombogenic and/or hemolytical response. The mechanism of chitosan-blood interaction starts with an adsorption of the plasma on the surface of chitosan, followed by the adhesion and activation of platelets that form thrombus [62]. It has been reported in the literature that sulfatation of the hydroxyl or amine groups of chitosan improves its behavior of the raw chitosan as anticoagulant, antioxidant, antimicrobian and hemagglutination inhibition [63].
Chitosan displays excellent applications as a hypocholesterolemic and hypolipidemic agent, in such a way chitosan can reduce the risk of cardiovascular diseases. In addition, chitosan shows interesting antimicrobial and antioxidant properties. All these properties has led to this biopolymer and its derivatives have applications beyond medicine since it can also be applied biodegradable sponges, surgical sutures, membranes, microspheres, tablets, delivery drugs [64,65].
3.2. Biomaterial
Chitosan displays a wide range of applications as a biomaterial due to its good behavior in the human body since it has been reported that this biopolymer displays antimicrobial activity, bioactivity, chemotactic action, immunostimulaion, enzymatic biodegradability, mucoadhesion, or epithelial permeability, favoring its adhesion with different types of cells [66]. Due to this excellent behavior, on one hand, chitosan together with chitin have been used in treatment of wounds, burns and ulcers due to its haemostatic characteristics and hastening wound healing effect. On the other hand, chitosan has been also used in tissue regeneration and restoration due to its biodegradability and cell affinity [67].
3.3. Tissue Engineering
Several materials are developed according to the wound type and healing mode. These biocomposites may contain synthetic polymers like polyurethane rubber and natural polymers such as collagen, chitosan, gelatin and alginate. Bioactive dressings are reported to have better quality as compared to synthetic dressings (Table 5).
Table 5.
Some applications of chitosan in tissue engineering.
| ● Cell growth and proliferation in tracheal cartilage, nerve |
| ● Bone tissue repair and regeneration materials for cartilage repair |
| ● Porous 3-D scaffold of chitosan-hydroxyapatite composites for bone regeneration |
| ● Chitosan-chondroitin sulfate sponges in bone regeneration |
| ● Chitosan-calcium alginate capsules to develop artificial pancreas for diabetes mellitus treatment |
Tissue engineering involves the use of living cells, which are generally manipulated from their extracellular environment to synthesize tissue that can be implanted into the body [68,69]. Generally, the tissue engineering is employed to repair, maintain, replace or enhance the function of a specific tissue or organ [70]. Chitosan has been used as polymer scaffold in tissue engineering due to the fact these structures display some properties such as high porosity, biodegradability, structural integrity and non-toxic to cell as well promoting the interaction with the cells to favor its adhesion and it should also encourage cell function [71]. The materials obtained from tissue engineering have shown excellent results as cartilage membranes [72], nerve [72], bone [73] and tracheal tissue [74].
Bones consist of mainly hydroxyapatite (Ca10(PO4)6(OH)2), together with other components such as collagen, keratin sulfate, chondroitin sulfate and lipids. The treatment of broken bone or damage can be carried out using a biodegradable compound, which is used as temporary skeleton to substitute the lost bones or the defective sites. Then, this bone support is progressively degraded and replaced by the new bone tissue without having any adverse effect on health throughout the treatment. Bioactive ceramics are chemically similar to natural bone, which allows osteogenesis to occur and can provide a bony contact or bonds with the host bone; however, the limitations of these bioceramic compounds are related to its low biodegradability and brittleness [75]. In order to minimize these drawbacks, several biopolymers such as chitosan have been studied in bone tissue engineering due to its capacity to promote growth and mineral rich matrix deposition by osteoblasts in culture [76]. Thus, the synthesis of composite/hydroxyapatite composites have shown interesting applications in the bone reparation since the hydroxyapatite structure strengthened the chitosan matrix and adjust the release burst effect [65].
Articular cartilage is a connective tissue whose principal functions is to facilitate the lubrication of the articulations and the transmission of loads to diminish the friction coefficient. Articular cartilage consists of isolated articular chondrocytes as well as their precursor cells that may be expanded in vitro and then seeded into a biocompatible matrix or scaffold for cultivation and subsequent implantation into a joint. The selection of a suitable biomaterial is a key factor for the successful repair of cartilage [77]. In this context, it has been reported that cartilage-specific extracellular matrix components such as collagen and glycosaminoglycans can play an important role in the regulation of the chondrodrocytic phenotype in supporting chondrogenesis both in vivo and in vitro (Figure 9). As chitosan displays a similar structure to glycosaminoglycans, it is a biopolymer with potential to be used as scaffolding material in articular cartilage engineering [78]. Thus, chitosan-chondroitin sulphate membranes and collagen-glycosamino glycans-chitosan have shown good results in the repair of cartilage and human skin [78,79]. Other authors prepared chitosan-based scaffolds by combining it with alginate. In this work, chitosan was modified with lactobionic acid to produce galactosylated chitosan, which was added to cross-linked alginated gel and finally freeze-drying lyophilization. These materials display pores whose size depends on the freeze-drying treatment, the molecular weight and the proportion of galactosylated chitosan. These authors carried out a study on human endothelial cells on chitosan, which had cell-adhesive peptides photochemically grafted onto their surfaces.
Figure 9.
Chemical structure of galactosylated chitosan.
Nerve injuries are one of most complicated to repair due to the fact neurons have scant ability to undergo cell division. All attempts to repair fibrous nerves have been focused on the regenerating nerve fiber into the proper endoneurial tubes. Several materials have been proposed for generating artificial tubes to repair nervous fibers [80]. These materials must be biocompatible and biodegradable as well as provide a modulated cellular, structural and molecular framework. Considering the required properties, chitosan is considered as potential biomaterial to repair nerve injuries due to its special properties such as antibacterial activity, biodegradability, biocompatibility and antitumoral activity [81]. It has been reported that chitosan membranes can grow and repair neurons of the peripheral nervous system [82]. In the same way, other authors have pointed out that chitosan fiber can also reinforce the adhesion, migration and proliferation of Schwann cells, which provide a similar effect for regenerating axons in the nervous system [83].
Several methods to generate three-dimensional chitosan scaffolds for initial cell attachment and the formation of the subsequent tissue It has been reported (Figure 10). Among them, the following can be highlighted:
Phase separation and lyophilization. Firstly, a chitosan solution is introduced into a mold and then a freezing step makes it ready for phase separation with acetic acid as solvent and chitosan acetate salt.
Particulate leaching techniques. A porogen, usually gelatin, is mixed with a chitosan solution prior to phase separation and lyophilization steps. When is submerged in a solvent, the scaffold is formed through porogen leaching. This fact implies that the obtained scaffolds can have an additional porosity.
Gas foaming. A chitosan solution contains a cross-linked, mainly glutaraldehyde, which is saturated with CO2 under high pressure, favoring the cross-linking. When the system is depressurized, the thermodymamic instability leads to nucleation and gas bubble growth. The porosity is formed by the bubbled space of the polymer solution).
Freeze gelation. The obtained scaffolds is placed in a gelation solution of NaOH and ethanol below the chitosan freezing temperature. Then, the gel is air-dried to remove the residual liquid.
Figure 10.
Different methodologies to synthesize three-dimensional chitosan scaffolds.
3.4. Wounds and Burns
The healing of wound and burns is a biological process related with growth and tissue regeneration. The wound healing process has five important steps [84]:
Homeostasis
Inflammation
Migration
Proliferation
Maturation
Several studies have reported the use of chitosan membranes to facilitate wound healing with potential application in patients with severe burns or wounds. It has been reported that the use of polyvinyl alcohol-chitosan composite membranes accelerates the mechanical properties of the obtained composite. In the same way, glycerol-oleic acid-chitosan composites have shown to be cyto-compatibility, biocompatible, bioadsorbable and provide sustained drug release. Chitosan-based composites have great importance in the field of wound care to avoid complications (infections or poor wound healing); thus, Ag-chitosan has been used as an antibacterial agent [84,85]. Similarly, Cu-chitosan or ZnO-chitosan have also shown good wound healing properties since these composites favor collagen deposition, fibroblast proliferation and re-epithelialization [86,87].
Blazevic et al. synthesized chitosan-lecithin nanoparticles for release of melatonin to improve the wound healing, obtaining good results for wound epithelialization [88]. Archana et al. have synthesized a composite formed by chitosan/TiO2/N-vinylpyrrolidone. This composite had excellent antimicrobial efficiency against several pathogenic bacteria as a consequence of a good biocompatibility, antibacterial ability, wound appearance, elevated swelling properties and hydrophilic nature [89].
The treatment of the skin after burns can also be carried out using chitosan since this biopolymer forms resistant films which can be directly deposited on the burned skin by the application of an aqueous solution of chitosan acetate [90]. In addition, chitosan can also facilitate the oxygen permeability, which is essential for the healing of burns. Moreover, chitosan film has the ability to adsorb water and then it can be automatically degraded by body enzymes [91].
Those individuals who have suffered extensive losses skin by burns have a high risk of dying from fluid loss as well as massive infections. Several studies have reported that chitosan/ glycoaminoglycan-based composites have potential to be used in a skin replacement. Thus, some authors have developed a wound covering material from polyelecrolyte complexes of chitosan and sulfonated chitosan, which accelerates the wound healing due to the easy chitosan degradation by tissue enzymes, achieving the regeneration the skin tissue of the wound area [92]. In the same way, other authors synthesized a chitosan/gelatin composite which showed good mechanical properties, to be used as artificial skin. This composite does not elicit any adverse inflammatory reactions as a consequence of its biodegradability and biocompatibility [93].
Commercially available suture materials are composed by materials as catgut, chromic catgut, polyglycolic acid and polylactic acid. The main drawback of these materials is related to their low degradation capacity. Considering that chitosan displays a higher biodegradability, chitosan based-composites are sustainable materials for use in suturing wounds.
3.5. Drug Delivery
The development of drugs in the clinical field is a great challenge due to the fact most drugs do not achieve desired clinical effects as a result of their inability to reach the target site of action. A high proportion of the dosed drug is disseminated over organs and tissues, which are not involved in the pathological processes, leading to severe effects.
Chitosan can also be employed as potential excipient to a sustained release for oral drugs in the form of granules or beads due to its abundant availability, inherent pharmacological properties and other biological properties (biodegradability, biocompatibility, non-toxic profile or low-immunogenicity) without side effects in the human body (Table 6). Chitosan can also be a suitable matrix in different forms (beads, films, microcapsules, coated, tables). Membranes or films can be prepared with different hydrophilic behavior by the formation of mixtures or semi-interpenetrated and interpenetrated networks of chitosan with highly hydrophilic polymers, such as polyvinyl alcohol, polyvinyl pyrrolidone or gelatin, which have controlled swelling. It has been reported that glutaraldehyde-crosslinked chitosan-gelation is pH-sensitive to drug delivery since this gel can swells and de-swells in a wider range of pH [94]. Intelligent drug delivery system can release them in reaction after some change in environmental conditions (temperature, pH, electric field, light and some chemicals). This drug delivery is administrated by the diffusion coefficient and relaxation time, which is highly dependent on the pH and the drug solubility [95].
Table 6.
Chitosan-based drug delivery systems.
| Drug | Dosage Form |
|---|---|
| Aspirin | Wet granulation formulation |
| Chlorpheniramine maleate | Tablet |
| Dapsone | Gel |
| Oxyphenbutazone | Coated tablet |
| Prednisolone | Granules |
| Pullulan | Film |
Several chitosan-based systems have been used for these applications. Among them, chitosan/polyethyleneglycol/alginate microspheres have been described as suitable material for the delivery of compounds with low molecular weight such as heparin, which displays anti-thrombotic properties [96]. In the same way, chitosan nanoparticles have been used for the nasal administration of vaccines and drugs due to it favors the penetration of the active molecules through the nasal barrier [97]. As was previously indicated, it is possible to generate chitosan scaffolds to obtain three-dimensional supports, so these chitosan-based composites are appropriate candidates to be used as drug reservoirs. In this sense, it has been reported that chitosan-based composites can be non-viral gene delivery systems owing to the fact chitosan (positively charged) can be complexed with the DNA plasmid genes or antigens (negatively charged) [98].
Chitosan also displays great potential in cancer treatment due to its use in the design of suitable anticancer drug delivery systems as well as tracking the path of the drug carrier though a bio-friendly heavy metal free quantum dot [99]. As an example, the quantum dot formed by folic acid/carboxymethyl chitosan/ZnxMn1-xS is used for the targeting controlled release of drugs as well as the obtaining cancer cell images [100]. In the same way, it has been reported in the literature that chitosan can activate caspase-3 protein causing apoptotic death of bladder tumor cells [101]. Some experiments with animals have demonstrated that the use of chitosan can inhibit the advance of metastatic breast cancer as well as force macrophages to mature into cytotoxic macrophages [102], which favors the suppression the growth of tumors [103]. In the same way, chitosan can favor the necrotic death of liver cancer cells due to neutralization of the cell surface charge [104]. Moreover, chitosan inhibits Enrlich ascites tumor growth due to the decrease of the glucose uptake and ATP live, diminishing the glycolysis, in tumor cells [105].
3.6. Artificial Kidney Membrane
Commercially both cuprophan and cellulose are used as semipermeable artificial kidneys due to their mechanical strength and good permeability. In order to improve the dialysis rates for medium and large size molecules, many polymeric membranes have been designed and developed. Considering these premises, chitosan has shown to be a promising artificial kidney membrane due to it displays an appropriate permeability to urea and creatinine as well as impermeability to serum proteins [106]. Taking into account that chitosan has widely used as resistant film, several chitosan membranes have been proposed for reverse osmosis, metal ion uptake, ion exchange, diffusion of dyes and separation of several binary systems [107]. In fact, the properties of these membranes can be optimized by the addition of some water-soluble polymers or by the grafting of copolymers that can improve the dialysis properties. Thus, it has been reported that the mixture chitosan/polyvinyl acetate displays a great tensile strength. Several authors studied the diffusion of bovine serum albumin (BSA) in highly cross-linked chitosan/polyvinyl alcohol membranes while other authors have studied the efficiency of these polymeric membranes in transporting alkaline ions and low molecular weight molecules [108]. In turn, Hirano et al. prepared chitosan-based membranes, which displayed good properties in dialysis treatment [109].
3.7. Blood Vessel
Vascular diseases are one of the highest causes of mortality worldwide. One of main treatment for vascular diseases is vascular transplantation. Nowadays, expanded polytetrafluoroethylene (ePTFE) and polyethylene terephthalate (PET) have been the main biomaterials used for prosthetic vascular grafts [110]. These materials display however some limitations for small-diameter applications. In this sense, complexation of glycosaminoglycans with porous chitosan scaffolds inhibits the anti-coagulant activity and vascular smooth muscle cells [111]. Other authors also fabricated a chitosan/heparin scaffolds with potential applications in blood vessel tissue engineering as hemostatic agent for vascular grafts [112].
Catheters are tubes, generally long, thin and flexible, made of different materials (rubber, plastic or metal), which is used in medicine and surgery for therapeutic or diagnostic purposes. These tubes is inserted into a duct, blood vessel, organ, or cavity to explore, widen, unclog, evacuate or inject a fluid. The use of heparin/chitosan in catheters has been very promising due to its physical compatibility and good clinical performance [113]. It has been reported that heparin/chitosan cyanoborohydride surface is a very promising catheter due to its higher compatibility with surrounding fluids and tissues. In addition, the heparin/chitosan coated polymers display excellent thromboresistence properties as well as blood compatibility for period as long as four days [114].
3.8. Ophthalmology
Chitosan has also interesting applications in the field of ophthalmology due to its mechanical stability, optical clarity, gas permeability-partially towards oxygen, immunologically compatibility, wettability, tear strength, tensile strength, elongation capacity and biodegradability, making this biopolymer a potential material to be used for the perfect contact lens [115].
3.9. Cosmetics
Chitosan is the only natural cationic polymer that turns viscous on being neutralized with acid. This fact together with the fungicidal and fungi static properties favor its use in creams, lotions, permanent waving lotions and nail lacquers [116].
3.10. Agricultural Applications
The use of both chitin and chitosan in agriculture is focused on four directions:
Plant protection against diseases and plagues (pre- and post-harvest).
Support of beneficial microorganism-plant symbiotic relationships.
Enhancing biological control and antagonist microorganism action.
Plant growth development and regulation.
Chitosan has shown an interesting fungicidal activity against many phytopathogenic fungi as well as antiviral and antibacterial activity. In this sense, the antimicrobial properties of chitosan and its outstanding film-creating aptitude have been exploited in the post-harvest preservation of fruits and vegetables, generating antimicrobial protection and enhancing the shelf life [117].
The presence of chitosan on soil favors symbiotic interactions between plant and microorganisms, as takes place for example in micorrizas. They also improve the action of plague-controlling biological organisms such as Tricoderma sp. and Bacilus sp. and are suitable for the encapsulation biocides, improving the efficiency in pathogenic plagues. Chitosan can also improve the metabolism of fruit or plant, which enhances germination and higher crop yields [118].
3.11. Food and Nutrition Applications
N-Acetylglucosamine coming from human milk improves the growth of bifidobacteria, which inhibit the growth of other microorganisms and produce lactase that is necessary for milk digestion. Cows’ milk has a limited amount of N-acetylglucosamine so the infants fed with this milk may suffer from indigestion. Some studies have reported that the addition of a small amount of chitosan to the diet improves the digestion and the intestinal microflora [119]. Other applications of chitosan related to food and nutrition are compiled in Table 7 [120].
Table 7.
Applications of chitosan in the field of food and nutrition.
| Chitosan Application | Example |
|---|---|
| Additive | Clarification and deacilification of fruits and beverages |
| Color stabilization | |
| Emusifying agent | |
| Food mimetic | |
| Natural flavor extender | |
| Texture controlling agent | |
| Thickening and stabilizing agent | |
| Antimicrobial agent | Bactericidal |
| Fungicidal | |
| Measure of mold contamination in agricultural commodities | |
| Edible film industry | Controlled release of antimicrobial substances |
| Controlled release of antioxidants | |
| Controlled release of nutrients, flavors and drugs | |
| Controlled moisture transfer between foo and surrounding environment | |
| Nutritional quality | Antigastritis agent |
| Dietary fiber | |
| Hypocholesterolemic effect | |
| Infant feed ingredient | |
| Livestock and fish feed additive | |
| Production of single cell protein |
3.12. Antioxidant and Antimicrobial Properties
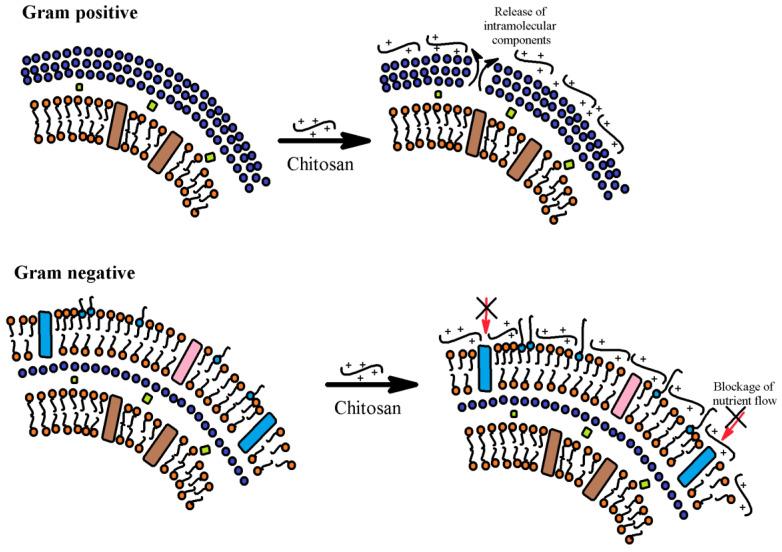
Chitosan is a polysaccharide with antimicrobial properties. Chitosan and its derivatives exhibit differential activity towards Gram positive and Gram negative bacteria, as is evident in most studies (Figure 11) [26,121].
Figure 11.
Action modes of chitosan on Gram positive and Gram negative bacteria. Structural composition of the outer envelope of Gram positive and Gram negative bacteria and effect of chitosan binding to the outer envelope of Gram positive and Gram negative bacteria.
In Gram positive bacteria, the cell wall is made up of a thick peptidoglycan layer where negatively charged teichoic acids are covalently linked to N-acetylmuramic acid, while lipolyteichoic acids form covalent bonds with the cytoplasmic membrane. These teichoic acids perform functions such as providing strength to the cell wall and arranging uniform high density charges in the cell wall, thereby affecting the passage of ions across the outer surface layers [122].
In the case of Gram negative bacteria, a thin peptidoglycan layer above the cytoplasmic membrane is further covered by an additional outer envelope called the outer membrane (OM). Lipoprotein and lipopolysaccharide (LPS) are the principal components of the OM and therefore the hydrophilic O-specific side chains present in the LPS help in identifying bacteria. Hydrophobic compounds and macromolecules are usually not active towards Gram negative bacteria, and in order to interact with the Gram negative bacteria it is therefore essential to overcome the outer membrane barrier. The mode of antibacterial action of chitosan is presumably due to interactions with the bacterial surface (either cell wall or outer membrane), and to explain this mechanism [122].
This interaction modifies their barrier properties thereby preventing the entry of nutrients or causing the leakage of intracellular contents. As chitosan has the ability to form films, this biopolymer can be used to produce food packaging as a potential food preservative [123]. However, the low solubility of the chitosan film limits its applicability.
Chitosan can improve its solubility and its antimicrobial properties by the functionalization with several functional groups such as quaternary ammoniumyl, carboxyalkyl, guanidinyl, hydroxyalkyl, thiol-containing groups or hydrophobic groups (alkyl chains or benzyl rings) [124,125,126].
In the following Table 8, several antimicrobial applications are indicated.
Table 8.
Some microbial applications of chitosan and chitosan derivatives.
| Chitosan/Chitosan Derivative | Microbial Strain | Application | Ref. |
|---|---|---|---|
| Chitosan | Streptococcus | Dental materials | [127] |
| Listeria monocytogenes, Pseudomonas aeruginosa and S. aureus | Dairy food packaging | [128] | |
| F. acuminatum, Cylindrocladium floridanum, Aspergillus flavus, Magnaporthe grisea, Bipolaris sorokiniana, F. graminearum, Phytophthora parasitica, Sclerotinia sclerotiorum | Plant protection | [129] | |
| Chitosan-polyphosphate-silver | P. aeruginosa and S. aureus | Wound dressing | [130] |
| Chitosan acetate | P. aeruginosa, Proteus mirabilis and S. aureus | Wound dressing | [131] |
| Carboxymethyl chitosan | E. coli | Fruit preservation | [132] |
| Chitosan-sulfonamide derivatives | Staphylococcus aureus, Sarcina lutea, Bacillus cereus, Bacillus subtilis, Escherichia coli, Candida albicans, Candida glabrata and Candida sake | Wound dressing and wound healing | [133,134] |
| N,N,N-trimethylchitosan polylactide/polypropylene fibers | S. aureus | Wound dressing | [135] |
| N-(Carboxymethyl) chitosan | F. solani and C. lindemuthianum | Plant protection | [129] |
| N,N,N-dimethylalkyl chitosans | A. tumefaciens, E. carotovora, fungi B. cinerea, F. oxysporum, and P. debaryanum | Crop protection | [136] |
| N-(o,p-Diethoxybenzyl)chitosan | F. oxysporum and P. debaryanum | Crop protection | [136] |
| N-(o,o-Dichlorobenzyl) chitosan, N-(o,o-dichloro-benzyl) chitosan, N,O-(p-chlorobutyryl) chitosan, N,O-decanoyl chitosan, N,O-cinnamoyl chitosan and N,O-(p-methoxy-benzoyl) chitosan | B. cinerea | Crop protection | [136] |
| N-Phenylalanine-O-carboxymethyl chitosan | S. aureus and E. coli | Food preservative coating | [137] |
| Chitosan/quaternary chitosan-polylactide | S. aureus and E. coli | Wound healing | [138] |
| Chitosan, chitosan-hydroxyapatite, N-[1-hydroxy-3-(trimethylammonium) propyl]chitosan chloride, carboxymethyl chitosan | Streptococcus | Dental care | [139,140] |
| N-[1-Hydroxy-3-(trimethylammonium) propyl]chitosan | Bacillus subtilis | Paper packaging | [141] |
| Carboxymethyl chitosan | E. coli and S. aureus | Cotton fabric | [142] |
| Poly(n-butyl acrylate)-chitosan | S. aureus | Cotton fabric | [143] |
| Chitosan-cellulose | E. coli and S. aureus | Membranes | [144] |
| O-Hydroxyethylchitosan-cellulose | E. coli | Textile | [145] |
| Chitosan-lauric acid-starch | B. subtilis and E. coli | Antimicrobial film | [123] |
| Dodecenyl succinylated phthaloyl chitosan | E. coli, S. aureus and B. subtilis | Antimicrobial film | [146] |
3.13. Adsorption of Pigments, Dyes and Metals
As chitosan displays a polycationic structure, this biopolymer has been used as a flocculating agent but also as a chelating agent and to trap metals. Both chitin and chitosan are highly used in the treatment of wastewater. Already in 2000, chitosan was used for the removal of color in effluents coming from dye-houses [147]. In this sense, it has been reported that chitosan can agglomerate anionic wastes in solution to generate precipitates in such a way it can be used as flocculent for recycling of food processing waste [147]. Some of adsorption techniques using chitosan composites, usually with clays or polymers, have been developed to adsorb dyes as an alternative to conventional wastewater processes [148]. This interaction with the pigments and/or dyes takes place through electrostatic interaction so the pH is a key parameter in the adsorption capacity. The list of chitosan-based composites that have been tested to remove dyes in wastewater is compiled in Table 9.
Table 9.
Chitosan-based composites used in the adsorption of dyes.
| Adsorbent | Dye | Adsorption Capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| Chitosan/activated clay | Methylene blue | 330 | 7.1 | 30 | [149] |
| Reactive dye RR222 | 1912 | 6.5 | 30 | ||
| Chitosan/bentonite | Tartrazine | 294 | 2.5 | 47 | [150] |
| Malachite green | 435.0 | 6.0 | 37 | [151] | |
| Chitosan/kaolin/γ-Fe2O3 | Methyl orange | - | 6.0 | - | [152] |
| Chitosan/montmorillonite | Congo red | 53 | 7.0 | 30 | [153] |
| Chitosan/oil palm | Reactive Blue 19 | 909 | 6.0 | 50 | [154] |
| Chitosan/polyurethane | Acid violet 48 | 30 | 7.0 | 30 | [155] |
In the same way, chitosan has demonstrated to be an efficient biopolymer to remove oil droplets from water. In addition, the adsorption capacity of chitosan can be improved by grafting reactions or by the formation of nanocomposites. As example, chitosan is very effective in the removal of petroleum products from wastewater by chemical interaction with the chitosan molecules [156].
As chitosan can generate resistant films in acid pH, Yang et al. synthesized reverse osmosis membranes. The obtained membrane has a NaCl rejection of 78.8%, using a flux rate of 1.67 × 103 gm3 cm−2 s−1, a pressure of 680 psi and a salt solution of 0.2% [157].
One of the main applications of chitosan is related with the intrinsic ability of chitosan molecules to interact with transition metals through the free electron pairs that nitrogen of the amino group presents. In this sense, chitosan has been combined with clay minerals, some polymers (poly-urethane, polyvinyl alcohol) or cellulose to be employed in the metal adsorption, being highly efficient and easily regenerable [158,159,160]. In Table 10, data on the metal adsorption capacity of several chitosan-based composites are compiled.
Table 10.
Chitosan-based composites used in the adsorption of cations.
| Adsorbent | Adsorbate | Maximum Adsorption Capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| Chitosan/alginate | Cu2+ | 68 | 4.5 | - | [161] |
| Chitosan/calcium arginate | Ni2+ | 222 | 5 | - | [162] |
| Chitosan/cellulose | Cu2+ | 26 | - | 25 | [163] |
| Zn2+ | 20 | ||||
| Cr6+ | 13 | ||||
| Ni2+ | 13 | ||||
| Pb2+ | 26 | ||||
| Chitosan/ceramic alumina | As3+ | 56 | 4.0 | - | [164] |
| As5+ | 96 | 4.0 | 25 | ||
| Cu2+ | 86 | -0- | - | [165] | |
| Ni2+ | 78 | 4 | 25 | ||
| Cr6+ | 154 | 4 | 25 | [166] | |
| Chitosan/clinoptilolite | Cu2+ | 574 | 5.0 | - | [167] |
| Cu2+ | 719 | 5.0 | 25 | [168] | |
| Co2+ | 468 | ||||
| Ni2+ | 247 | ||||
| Chitosan/cotton fibers (via C-N single bond) |
Hg2+ | 96 | 5.0 | 25 | [169] |
| Au3+ | 89 | 3.0 | 25 | [170] | |
| Chitosan/cotton fibers (via Schiff base bon) |
Hg2+ | 104 | 5.0 | 35 | [169] |
| Au3+ | 77 | 3.0 | 25 | [170] | |
| Cu2+ | 25 | 6.5 | 25 | [171] | |
| Ni2+ | 8 | ||||
| Pd2+ | 102 | ||||
| Cd2+ | 16 | ||||
| Chitosan/magnetite | Cr6+ | 69 | 4.0 | - | [172] |
| Pb2+ | 63 | 6.0 | - | [173] | |
| Ni2+ | 53 | ||||
| Chitosan/perlite | Cu2+ | 196 | 5.0 | - | [174] |
| Ni2+ | 115 | ||||
| Cd2+ | 179 | 6.0 | 25 | [175] | |
| Cr6+ | 154 | 4.0 | 25 | [176] | |
| Cu2+ | 104 | 4.5 | 25 | [177] | |
| Chitosan/polyvinyl alcohol | Cd2+ | 143 | 6.0 | 50 | [178] |
| Cu2+ | 48 | 6.0 | - | [179] | |
| Chitosan/polyvinyl chloride | Cu2+ | 88 | 4.0 | - | [180] |
| Ni2+ | 120 | 5.0 | |||
| Chitosan/silica | Ni2+ | 254 | 5.0 | - | [161] |
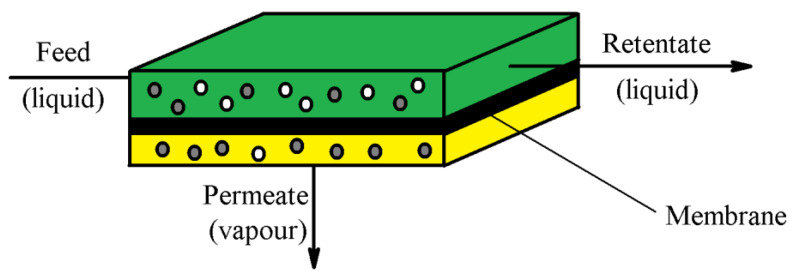
3.14. Pervaporation
Pervaporation is a separation method where a liquid is transported through a non-porous liophilic membrane (Figure 12). This membrane is responsible for removing some components in their vapor state into a vacuum or inert carrier gas [181]. An efficient pervaporation membrane should be characterized by a good mechanical durability, chemical resistance, high selectivity and high permeate rates [181].
Figure 12.
General scheme of a pervaporation.
Taking into account these considerations, chitosan is a hydrophilic material under acid conditions, which can react, through its hydroxyl and amine groups, with several functional groups, such as epoxy groups. The hydrophilic groups can play a key role in the preferential H2O sorption and diffusion through the chitosan membrane. Thus, chitosan has been proven to have good film forming properties, high water permeability and chemical resistance [182].
Many studies have reported that the chitosan membranes were highly water permselectivity or the pervaporation of aqueous alcohol solutions, as is compiled in Table 11.
Table 11.
Chitosan-based composites and its uses in separation processes.
| Application | Membrane | Ref. |
|---|---|---|
| Water/ethanol mixture separation | Chitosan salt | [183] |
| Crosslinked chitosan | [184] | |
| Chitosan/N-methyol nylon 6 blend | [185] | |
| HY zeolite-filled chitosan | [186] | |
| Crossline quaternized chitosan composite | [187] | |
| Chitosan-hydroxyethylcellulose composite | [182] | |
| Isopropanol-water separation | Chitosan | [188] |
| Chitosan-hydroxyethylcellulose blended | [189] | |
| Crosslinked chitosan | [190] | |
| Chitosan/NaY zeolite composite | [191] | |
| Blended chitosan/polyvinyl alcohol | [192] | |
| Chitosan-poly(tetrafluoroethylene) composite | [193] | |
| Crosslinked carboxymethyl chitosan-PSF-hollow-fiber composite | [194] | |
| Diisocyanate crosslinked chitosan | [195] | |
| Chitosan-polyacrylonitrile hollow fiber | [196] | |
| Poyelectrolyte complexes of chitosan and phosphotungstic acid | [197] | |
| Chitosan g-polyaniline | [198] | |
| Sodium alginate and chitosan-wrapped MWCNT | [199] | |
| Ethylene glycol/H2O separation | Surface crosslinked chitosan | [200] |
| Chitosan-poly(acrylic acid) polyelectrolyte complex | [201] | |
| Chitosan polysulfone composite | [202] | |
| Chitosan poly(vinyl alcohol) blend | [203] | |
| Separation methanol/methyl t-butyl ether | Chitosan-poly (N.vinyl-2-pyrrolidone) blend | [204] |
| Chitosan composite (modified with surfactants) | [205] | |
| Chitosan-anionic surfactant complex | [206] | |
| Separation alcohol-toluene | N-acetylated chitosan | [207] |
| Silicate zeolite embedded chitosan mixed matrix | [208] | |
| Separation dimethyl carbonate-methanol | Chitosan | [209] |
| ZSM-5 zeolite-filled chitosan | [210] | |
| Separation benzene-cyclohexane | Poly(vinyl alcohol) chitosan blend | [211] |
| Chitosan/Ag+-carbon nanotubes | [212] | |
| Dehydration of 1,4-dioxane | Poly(vinyl alcohol)/chitosan | [213] |
| Chitosan/nylon 66 | [214] | |
| Crosslinked calcium alchinate-chitosan blend | [215] | |
| Poly(3-hydroxybutyrate)-functionalized multiwalled carbon nanotubes-chitosan composite | [216] | |
| Dehydration of caprolactam | Crosslinked PVA/chitosan | [217] |
| Chitosan-konjac glucomannan blending | [218] | |
| Chitosan-poly(acrylic acid) composite | [219] |
3.15. Catalytic Applications
Chitosan can be used for both homogeneous and heterogeneous catalysis. In recent years, the scientific community is developing environmentally benign and sustainable catalysts. In this sense, the use of natural catalysts such as biopolymers is an excellent alternative to develop for the synthesis of sustainable catalysts.
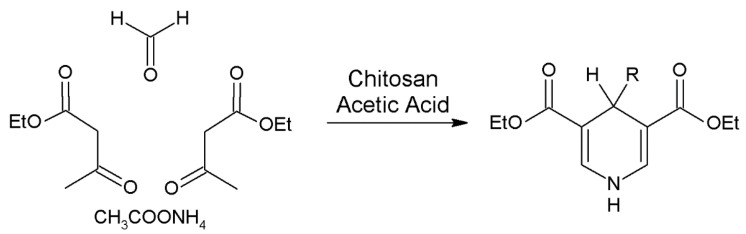
Taking into account that chitosan is soluble in acetic acid solutions (minimum 1%), chitosan can act as homogeneous catalyst in the synthesis of nitrogen heterocyclic derivatives by one-pot three-component reaction of substituted aromatic aldehydes, dicarbonyl compounds and 2-aminobenzothiazole/3-amino-1,2,4-triazole/urea/thiourea in aqueous medium at 60–65 °C (Figure 13) [220]. These authors reported that free amino groups in chitosan distributed on the surface of chitosan activate the carbonyl group of benzaldehyde through nucleophilic attack to produce the corresponding intermediate [220].
Figure 13.
Preparation of 4H-pyrimido [2,1-b] benzothiazole derivatives.
Other authors have functionalized chitosan with sulphonic groups with chlorosulfonic acid obtaining a biodegradable and biocompatible acid catalyst which has been used in the condensation reaction of aldehydes, ethyl acetoacetate and ammonium acetate through the Hantzsch reaction, obtaining 1,4-dihydropyridines (Figure 14) [221].
Figure 14.
Synthesis of 1,4-dihydropyridines from the Hantzsch reaction.
Chitosan-based preparations loaded with metal ions and complexes as well as metal nanoparticles can be successfully used to induce different reactions. This fact favors the dispersion of the active sites (transition metal), enhancing the amount of available and increasing the availability of active sites. On the other hand, as the solubility of chitosan in water is dependent on pH conditions, these catalysts can be transformed into homogeneous in acid conditions and then it can become heterogeneous after the reaction with an increase in pH (pH > 6) to recover the catalyst. The main drawback of chitosan-based catalysts may be related to their lower thermochemical stability compared to oxides such as silica, alumina, zeolites or carbons, among others, and their low surface area. Nowadays, there are several methodologies to synthesize three-dimensional chitosan scaffolds to increase the surface area and pore volume (Figure 10).
These organocatalysts are efficient in a wide range of transformations such as organic synthesis, electrochemical reactions and decontamination reactions [222]. The presence of functional groups of the backbone affords a strong interaction with metallic species and nanoparticles. The possibility to obtain a varied chitosan-hybrid preparation further widens the options for catalyst synthesis and their use.
As was previously indicated, chitosan is a biopolymer that can be transformed into membranes, flakes, disks, hollow spheres or fibers. In addition, it can be treated to form particles of appropriate size, to develop porous structures or to increase the surface area. In the same way, chitosan can be modified, via cross-linking with different organic compounds (glutaraldehyde, glyoxal, hexamethylene diisocyanate or epichlorohydrin). This fact improves both the thermal and mechanical stabilities for chitosan [223]. In the next subsection, the chitosan-based metal catalysts involved in organic transformations are shown.
3.15.1. Carbon-Carbon Coupling Reactions
Carbon-carbon coupling reactions enable the construction of organic molecules with a wide range of applications. Most of catalysts studied in the literature are based on palladium although works by using other metals such as nickel, cobalt, copper or gold have also been reported.
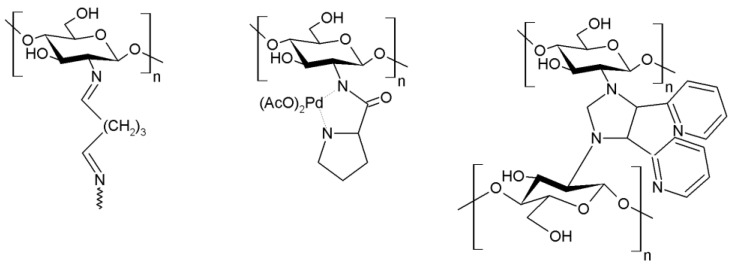
O-Carboxymethyl chitosan has been used as support to graft PdCl2 or Ni(OAc)2, obtaining high yields in the Heck coupling [224]. In another research, chitosan was cross-linked with glutaraldehyde to the immobilization of Pd2+-species in its structure, reaching yields very close to 100% (Figure 15) [225]. Other authors also synthesized proline-loaded chitosan beads and gelatin chitosan beads by cross-linking with similar results in couplings reactions of iodo- and bromoarenes, attaining conversion values between 68–100% [226,227].
Figure 15.
Some examples of chitosan-based catalysts loaded with Pd2+.
In the same way, 6-caboxymethylchitosan was also modified with various Schiff bases loaded with Pd2+, obtaining catalysts with high activity in the Suzuki coupling reaction, although these reactions required long reaction times [228] (Figure 16).
Figure 16.
Suzuki couplings using catalysts modified with 6-carboxymethylchitosan.
In the same way, Zeng et al. prepared porous chitosan microspheres by cross-linking and subsequent CuI deposition, obtaining catalysts with high activity in the Heck reaction of iodobenzenes, although they required severe catalytic conditions (140 °C and 10 h) in such a way that they detected a partial leaching after the third cycle [229].
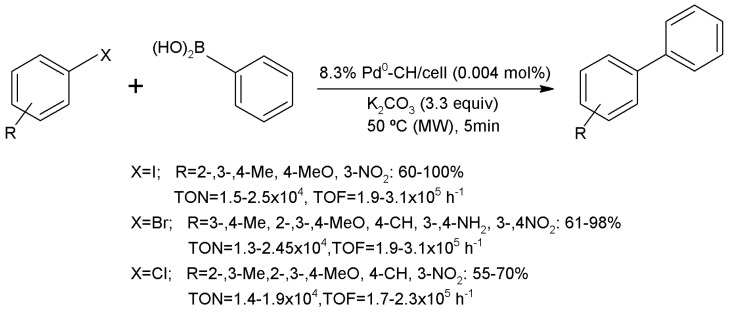
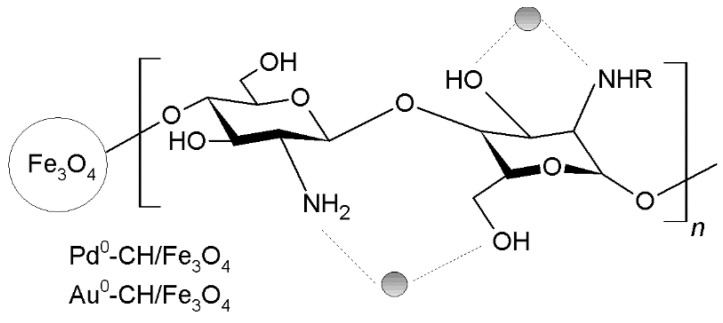
In order to recover these chitosan-based metal catalysts, Raffiee and Hosseini [230] carried out a reflux chitosan with cyanuric chloride in toluene and then, the product was treated with N-methylimidazones i-Pr2EtN and with magnetic Fe3O4 particles in acetic acid to recover the catalyst magnetically (Figure 17). These catalysts provided high yields (90–98%) in the Suzuki coupling of iodo- and bromobenzenes. In the same way, other authors also synthesized other magnetical chitosan-based metal catalysts (Pd2+, Cu2+ or Pd2+-Co2+) with high activity in the Heck coupling reaction [231]. Other authors have employed silica nanospheres to disperse the active phase involved in the coupling reaction. Jadhav et al. dispersed silica in an acid solution of chitosan and then induced the chitosan deposition. In the next step, the solid was treated with Pd(OAc)2 to obtain a mixture of Pd2+/0-species [232]. The obtained material exhibited a good behavior in the Heck coupling reaction with a yield of 81–92%.
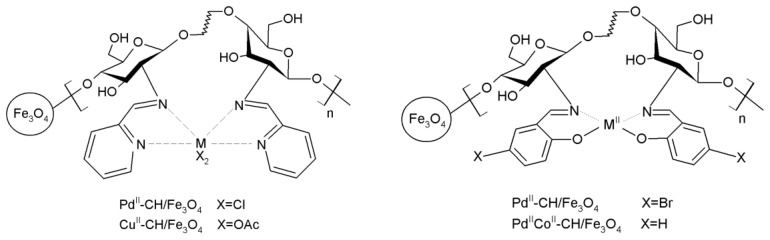
Figure 17.
Magnetic chitosan-based metal catalysts with high activity in the Heck coupling reaction.
Clay minerals were also used to disperse the chitosan-based catalyst. Zeng et al. synthesized a composite catalyst by the mixing of chitosan, montmorillonite and Na2PdCl4, obtaining good results in the Heck coupling of iodo- and bromo arenes, although they observed significant losses of Pd-species after each cycle [233]. Most metal-based catalysts show improved catalytic behavior in the metallic state so the complexed metal ions should be treated with a reductant such as alcohols, hydrazine or NaBH4 (Figure 18) [234,235].
Figure 18.
Suzuki reaction with Pd0-chitosan based catalysts.
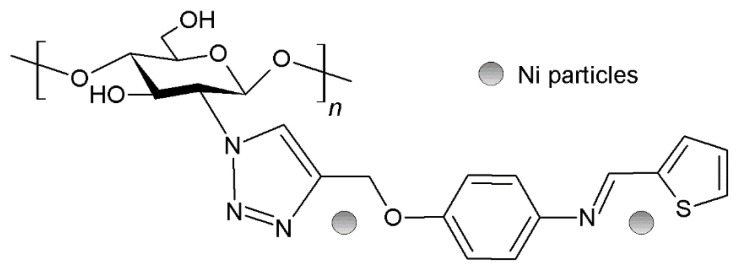
Similarly to Pd0, other transition metals have been employed to synthesize chitosan-based catalysts. Thus, both Au and Au-Pd bimetallic catalyst were synthesized by co-reducing of HAuCl4 and H2PdCl4 with NaBH4 in an acidic chitosan solution and then were used in the oxidative homocoupling of phenylboronic acid, reaching yields of 96–99% (Figure 19) [236]. Ni-nanoparticles have been rarely used in coupling chemistry. However, Hajipour and Abolfathi synthesized alkynylated imino thiophene ligand, transforming chitosan with azidation and then combining these two components via a click reaction (Figure 20) [237].
Figure 19.
Magnetic chitosan based catalysts loaded with Pd0 or Au0 used in the oxidative homocoupling of phenylboronic acid.
Figure 20.
Chitosan-based catalysts loaded with Ni0 used in C-C. coupling reactions.
3.15.2. Carbon-Nitrogen Coupling Reactions
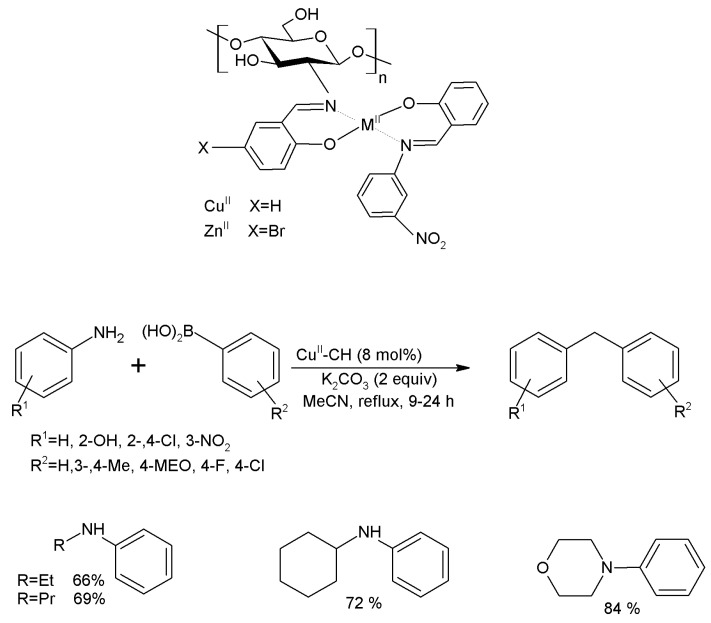
The most important and frequently used method to form C-N bonds via is the coupling of haloarenes. From this reaction it is possible to synthesize a wide range of amines through Buchwald-Hartwig amination. The first studies were carried out by mixing of chitosan with an ionic liquid (IL[bmin]BF4) and grafting with Pd(OAc)2 and a ligand, reaching a conversion of 98% [238]. Later, other authors reported that the incorporation of Co2+-species improved the yield and the stability of the catalysts in these C-N coupling reactions [239]. In another study, chitosan was functionalized with salicylaldehyde, obtaining a complex, which was stirred with 3-nitroaniline in ethanolic solutions of Cu(AcO)2, obtaining a catalyst with high yields in the reaction of primary amines with phenylboronic acids [240]. In the same way, Bodhak et al. treated chitosan with CuSO4 in aqueous solutions to form a catalyst, which was employed in the coupling of iodo- and bromo-benzene with aliphatic 1,2- and 1,3-diamines, achieving a yield of 86–94% (Figure 21) [241].
Figure 21.
C-N coupling of amines with phenylboronic acids using chitosan-based catalysts loaded with Cu2+.
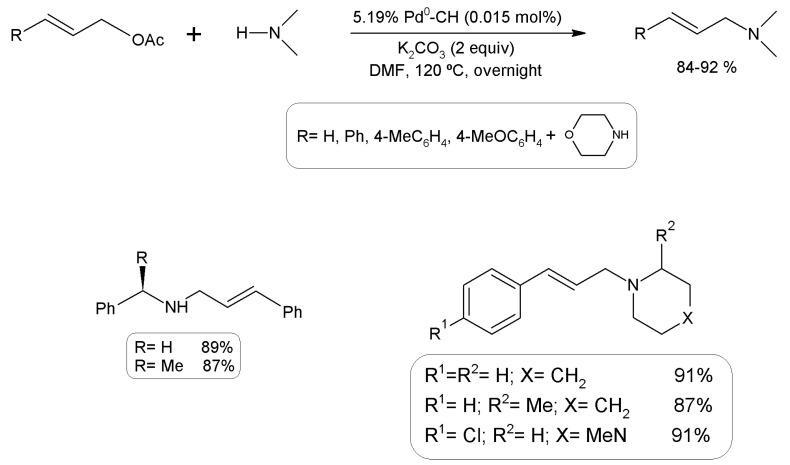
Palladium particles generated by the reduction of PdCl2 with NaBH4 in the presence of chitosan followed by drying afforded catalysts, obtaining high yields in the coupling of allylic acetates with phenylethylamines and N-heterocycles, reaching yields of 78–86% after five cycles (Figure 22) [242].
Figure 22.
Coupling of allyl acetates with amines using chitosan-based catalysts loaded with Pd0.
3.15.3. Carbon-Sulfur Coupling Reactions
Recently, Cu-based catalysts have been employed in the carbon-sulfur coupling reactions. Zhang et al. synthesized a catalyst from Cu(OAc)2 and chitosan, obtaining Cu-particles of 3–8 nm [243]. These catalysts showed a good catalytic behavior in the reaction of iodo- and bromoarenes with sodium sulfinates to produce sulfones.
In the same way, García et al. prepared Cu nanoparticles of about 0.5 nm y solvothermal reduction of an acidic chitosan/Cu(NO3)2 solution. Then, the gel was treated with an alkaline solution to form microspheres. The obtained materials showed a high surface area and a good activity in the coupling of iodobenzene and thiophenol, reaching a yield of 96% (Figure 23) [244].
Figure 23.
Coupling of haloarenes and sodium sulfonates using chitosan-based catalysts loaded with Cu0.
3.15.4. Oxidation Reactions
Chitosan-based metal catalysts have also been employed in oxidation reactions. One of the biggest challenges that must be addressed is related to the stability of chitosan under oxidative conditions. This has been prolifically studied with the aim of transforming chitosan, insoluble in water, to low-molecular weight products through hydrolysis. In this sense, high degradation was achieved with H2O2 under acidic conditions (pH = 5). The first studies of oxidation with the aid of chitosan-based catalysts was reported by Pispisa applying immobilized Fe3+ and Cu2+ complexes in alkaline solutions for the oxidation of catecholamines via intramolecular electron transfer [245].
Several studies have used porphyrin-chitosan in preparation of the oxidation of cyclohexane. In all cases, several cations (Fe3+, Mn3+ and Co2+) were complexed in the tetraphenylprophyrin ring, obtaining the highest activity for the Fe-based catalyst. In later studies, this group immobilized Fe3+-tetrakis(4-carboxyphenyl)porphyrin, obtaining a material with higher porosity and higher amount of available active sites [246]. This catalyst showed better activity values in the oxidation reaction of cyclobenzene or ethylbenzene [247]. In the same way, Shaabani et al. synthesized chitosan-based cobalt catalyst by reducing the aqueous solution of chitosan mixed with CoCl2 with NaBH4, being active in the aerobic oxidation of benzylic carbon atoms to form their corresponding ketones and aldehydes with a yield of 90–95% [241].
Crucianelli et al. synthesized silica-supported preparations with chitosan bearing O-acyl, N-acyl and N-alkyl moieties by complexation with methyltrioxorhenium. Oxidation of alkenes were carried out with urea-H2O2 adduct providing high epoxide yield in ethanol at room temperature [248]. In the same way, Ru3+-based catalyst also showed interesting results in epoxidation reactions with NaIO4 [249].
Thiol-functionalized chitosan was prepared by modification with methyl acrylate and then the adduct was functionalized with ethane-1,2-thiol. The obtained product was loaded with AuCl3 and reduced with NaBH4. The obtained catalyst was very active and stable for several cycles in oxidation of cyclohexene [250].
The oxidation of alcohols by using chitosan-based metal catalysts was firstly described by the Shaabani group in the aerobic oxidation of benzylic alcohol [251]. It has been reported in the literature that Fe3+ are active species in the oxidation of benzyl alcohol to their respective carbonyl compounds (Figure 24). In the same way, both Au and bimetallic AuPd catalysts in the oxidation of 4-hydroxybenzyl alcohol to obtain 4-hydroxybenzaldehyde in the presence of K2CO3 [252]. On the other hand, porous chitosan/polyacrylamide interpenetrating polymer network with entrapped silver nanoparticles was active in the oxidation of 1-phenylethanol for at least 7 runs [253].
Figure 24.
Selective oxidation of benzyl alcohols to carbonyl compounds using chitosan-based catalysts loaded with Fe3+.
3.15.5. Hydrogenation Reactions
In this section, chitosan-based metal catalysts involved in hydrogenation of C-C and C-X are summarized. RhCl3(H2O)3 was mixed with chitosan and then reduced with NaBH4 under supercritical CO2 conditions to afford a catalyst with high surface area [254]. This catalyst showed good catalytic activity in the hydrogenation of buta-1,3-diene and but-1-yne both in gas and liquid phases. On the other hand, chitosan solution in 3 wt.% formic acid treated with H2PdCl4 or H2PtCl6 were employed in the hydrogenation of unsaturation of palm oil [255].
Adlim and Bakar reported mono- and bimetallic Pd, Au and Pd-Au catalysts stabilized by chitosan and subsequently reduced with methanol or NaBH4 [256]. The obtained catalysts, mainly the bimetallic ones, exhibited relevant values of dispersion and activity in the hydrogenation of octa-1-ene. In the same way, other authors synthesized a magnetic chitosan-coated Fe3O4, with H2PdCl4, and then, Pd2+-species were reduced with NaBH4. This catalyst displayed a high dispersion of the Pd0 (Figure 25), which provided a high activity in the hydrogenation of nitrobenzenes to form the corresponding amines [257].
Figure 25.
Hydrogenation of nitroarenes using chitosan-based catalysts loaded with Pd0.
It has been reported in the literature that chitosan-based Ru catalysts show interesting results in transfer hydrogenation reactions. A Ru2+-complex was prepared by esterification of chitosan with pivaloyl chloride (Figure 26). Then, this adduct was treated with [Ru(p-cymene)Cl2]2 in methanol. The obtained product was tested in the asymmetric transfer hydrogenation of acetophenone [258,259]. One of the main drawbacks of this catalyst were its degradation and precipitation under basic conditions [258].
Figure 26.
Chitosan-based catalysts loaded with Ru used in transfer hydrogenation reactions.
3.15.6. Hydrogenolysis Reactions
Likewise, a slurry containing chitosan with PdCl2 adsorbed in ethanolic solution was reduced with NaBH4 at room temperature, being active in the transformation of 2-phenyloxirane to 2-phenyl-ethanol, which is used in the fragrance industry [260]. On the other hand, Santes et al. synthesized a multicomponent (Mo, Ni and P) chitosan-based catalyst by pore-filling impregnation, being tested in the hydrodesulfurization of dibenzothiophene to obtain high proportion of biphenyl [261].
3.16. Catalytic Processes to Valorize Chitosan into Valuable Products
In recent years, the scientific community is developing processes to valorize lignocellulosic biomass to obtain high-added value products. These processes are encompassed in the biorefinery concept. Considering that chitosan has a structure analogous to the sugars obtained from cellulose and hemicellulose, chitosan has great potential to obtain products with high commercial interest.
The hydrolysis and dehydration processes of chitosan to produce 5-hydroxymethylfurfural (HMF, Figure 27) were firstly performed using mineral liquid acids, such as H2SO4 [262]. The optimization of reaction parameters led to an HMF yield of 14% after 37 min of reaction by using 2.2 wt.% of H2SO4 [263]. Ionic liquids have shown a higher activity than mineral acids, giving rise to a HMF yield of 29.5% from chitosan and 19.3% from chitin, after 5 h of reaction at 180 °C using N-methylimidazolium hydrogen sulfate ([Mim]HSO4) as catalyst [264]. Other authors have added ZnCl2 as a homogeneous catalyst, where Zn2+ ions are coordinated with -OH in C1 and -NH2 in C2, obtaining a HMF yield between 2.8–10.1% after 1.5 h of reaction at 120 ° C from the chitin monomers (GlcNAc) and chitosan (GlcNH2) [264]. Similarly, other authors evaluated several metal chlorides and boric acid as co-catalysts, obtaining the highest conversion values for AlCl3 and boric acid, with a maximum yield towards HMF of 26.5% from GlcNH2 [264]. Another catalyst with high activity is SnCl4-5H2O, which is hydrolyzed under reaction conditions to form SnO2, with a HMF yield of 13.2%, using chitosan with high molecular weight [265].
Figure 27.
Scheme for the production of HMF and LA from chitosan.
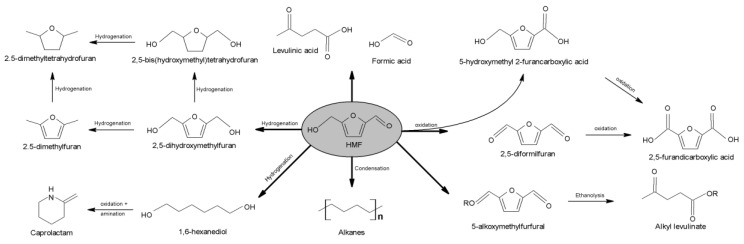
HMF is considered one of the most versatile biological-based compounds [266], since it can be transformed into a wide range of chemicals (Figure 28) [267]. In this context, together with cellulose and hemicellulose, both chitin and chitosan are also appropriate biopolymers for the synthesis of valuable furan derivatives.
Figure 28.
Scheme showing possible reactions of 5-hydroxymethylfurfural to obtain high value-added products.
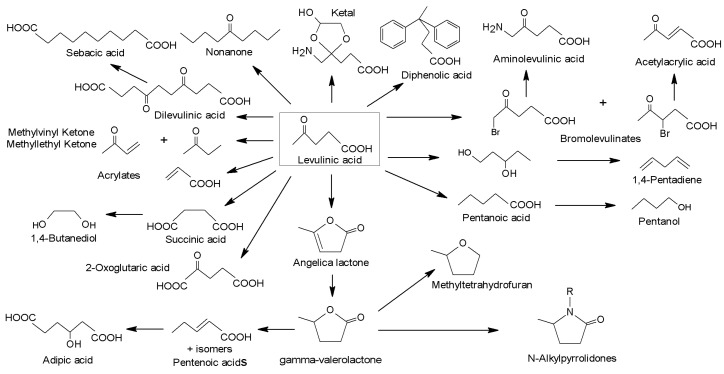
The subsequent rehydration of HMF could also give rise to levulinic acid (LA), which is considered another useful building block molecule, since it can be converted in many different value-added products (Figure 29). In this sense, it has been reported that LA can be obtained from chitin and chitosan in the presence of H2SO4 (2 M) at 190 °C for 30 min [268]. LA was also the major product when SnCl4 was used as catalyst [269], achieving yields of 34.7% and 59.4% from chitosan and chitosan monomer respectively, under microwave irradiation at 220 °C. In another study, a LA yield of 39% was achieved through the hydrothermal transformation of GlcNH2, and 29% when chitin was hydrothermally treated in 4 wt.% H2SO4 solution at 190 °C [270].
Figure 29.
Scheme of the possible reaction of levulinic acid to obtain high value-added products.
Chitin and chitosan can also undergo oxidative reactions in the presence of noble metal nanoparticles, generally Au, dispersed on metal oxides, leading to 2-amino-2-deoxy-d-gluconic acid (GlcNA) (Figure 30), which is used in the asymmetric synthesis of amino acids, besides its potential in biomedical applications [271].
Figure 30.
Oxidation of GlcNH2 to GlcNA.
Traditionally, Au nanoparticles have shown to be active in the oxidation of alcohols. Thus, the oxidation of glucose to gluconic acid has been extensively reported. However, this oxidation reaction has hardly studied using an analogous monomer, such as GlcNH2, where one –OH group is replaced by –NH2. As this field is very little explored, this task has a great potential to design novel catalysts to synthesize these valuables starting products for fine chemistry.
4. Conclusions
Chitosan is a natural polymer with tremendous biological properties such as biocompatibility, biodegradability and anti-infective activity, among others, due its high charge density, the existence of reactive hydroxyl and amine groups or its capacity of interact with other polymeric fractions through hydrogen bonds. These interesting physicochemical properties and the capacity to functionalize the amine and hydroxyl groups for specific uses has attracted the interest of the scientific community in the last decades to study and develop interesting applications for chitosan.
In this review, it has been highlighted that chitosan and chitosan-based composites have enormous potential in medical applications such as burn treatment, artificial kidneys, blood anticoagulation and bone, tendon or blood vessel engineering. However, the tunable capacity of chitosan takes this biopolymer to applications beyond medicine. Thus, other challenges for the future are focused on the developing of nanocomposites for applications in biosensors, packaging, separation processes, the food or agricultural industry and catalytic processes. Another challenge is related to the valorization of chitosan into valuable organic compounds, which usually are obtained as alternatives to traditional fossil fuels.
One of the main challenges for the use of chitosan in the future must be the optimization of its degree of deacetylation using environmentally benign reagents. Another key parameter is the design of modulated tridimensional chitosan structures by crosslinking processes, which improve its use in specific applications. From a catalytic point of view, a main challenge is how to design porous chitosan-based catalysts to increase the amount of available active sites and thus improve the efficiency of catalytic processes.
Acknowledgments
J.A.C. and C.P.J.-G. thank the University of Malaga for PhD incorporation contracts.
Author Contributions
Conceptualization: J.A.C.; methodology: C.P.J.-G. and J.A.C.; validation: C.P.J.-G. and J.A.C.; investigation: J.A.C.; data curation: C.P.J.-G.; writing—original draft preparation: J.A.C.; writing—review and editing: C.P.J.-G.; visualization: J.A.C.; supervision: J.A.C.; project administration: J.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to financial support from the Spanish Ministry of Innovation, Science and Universities (Project RTI2018-094918-B-C44 and RTI2018-099668-B-C22) and FEDER (European Union) funds.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kaur S., Dhillon G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014;40:155–175. doi: 10.3109/1040841X.2013.770385. [DOI] [PubMed] [Google Scholar]
- 2.Islam S., Bhuiyan M.A.R., Islam M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017;25:854–866. doi: 10.1007/s10924-016-0865-5. [DOI] [Google Scholar]
- 3.Khoushab F., Yamabhai M. Chitin research revisited. Mar. Drugs. 2010;8:1988–2012. doi: 10.3390/md8071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peter M.G. Chitin and Chitosan in Fungi. Wiley; Honoken, NJ, USA: 2005. [Google Scholar]
- 5.Shahidi F., Abuzaytoun R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005;49:93–135. doi: 10.1016/S1043-4526(05)49003-8. [DOI] [PubMed] [Google Scholar]
- 6.Elieh-Ali-Komi D., Hamblin M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016;4:411–427. [PMC free article] [PubMed] [Google Scholar]
- 7.Shahidi F., Synowiecki J. Isolation and Characterization of Nutrients and Value-Added Products from Snow Crab (Chinoecetes Opilio) and Shrimp (Pandalus Borealis) Processing Discards. J. Agric. Food Chem. 1991;39:1527–1532. doi: 10.1021/jf00008a032. [DOI] [Google Scholar]
- 8.Beaney P., Lizardi-Mendoza J., Healy M. Comparison of chitins produced by chemical and bioprocessing methods. J. Chem. Technol. Biotechnol. 2005;80:145–150. doi: 10.1002/jctb.1164. [DOI] [Google Scholar]
- 9.Navard P. The European Polysaccharide Network of Excellence (EPNOE) Springer; Wien, Austria: 2012. [DOI] [PubMed] [Google Scholar]
- 10.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong K., Meyers S.P. Preparation and characterization of chitin and chitosan—A review. J. Aquat. Food Prod. Technol. 1995;4:27–52. [Google Scholar]
- 12.dos Santos Z.M., Caroni A.L.P.F., Pereira M.R., da Silva D.R., Fonseca J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009;344:2591–2595. doi: 10.1016/j.carres.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X., Chen L., Zhong W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003;54:457–463. doi: 10.1016/j.carbpol.2003.05.004. [DOI] [Google Scholar]
- 14.Muñoz G., Valencia C., Valderruten N., Ruiz-Durántez E., Zuluaga F. Extraction of chitosan from Aspergillus niger mycelium and synthesis of hydrogels for controlled release of betahistine. React. Funct. Polym. 2015;91–92:1–10. doi: 10.1016/j.reactfunctpolym.2015.03.008. [DOI] [Google Scholar]
- 15.Sajomsang W., Rungsardthong Ruktanonchai U., Gonil P., Nuchuchua O. Mucoadhesive property and biocompatibility of methylated N-aryl chitosan derivatives. Carbohydr. Polym. 2009;78:945–952. doi: 10.1016/j.carbpol.2009.07.020. [DOI] [Google Scholar]
- 16.Urreaga J.M., de la Orden M.U. Chemical interactions and yellowing in chitosan-treated cellulose. Eur. Polym. J. 2006;42:2606–2616. doi: 10.1016/j.eurpolymj.2006.05.002. [DOI] [Google Scholar]
- 17.Nwe N., Stevens W.F., Tokura S., Tamura H. Characterization of chitosan and chitosan-glucan complex extracted from the cell wall of fungus Gongronella butleri USDB 0201 by enzymatic method. Enzyme Microb. Technol. 2008;42:242–251. doi: 10.1016/j.enzmictec.2007.10.001. [DOI] [Google Scholar]
- 18.Kasaai M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008;71:497–508. doi: 10.1016/j.carbpol.2007.07.009. [DOI] [Google Scholar]
- 19.Heux L., Brugnerotto J., Desbrières J., Versali M.F., Rinaudo M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules. 2000;1:746–751. doi: 10.1021/bm000070y. [DOI] [PubMed] [Google Scholar]
- 20.Hirai A., Odani H., Nakajima A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991;26:87–94. doi: 10.1007/BF00299352. [DOI] [Google Scholar]
- 21.Lavertu M., Xia Z., Serreqi A.N., Berrada M., Rodrigues A., Wang D., Buschmann M.D., Gupta A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003;32:1149–1158. doi: 10.1016/S0731-7085(03)00155-9. [DOI] [PubMed] [Google Scholar]
- 22.Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 23.Kasaai M.R. Calculation of Mark-Houwink-Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007;68:477–488. doi: 10.1016/j.carbpol.2006.11.006. [DOI] [Google Scholar]
- 24.Zielinska K., Shostenko A.G., Truszkowski S. Analysis of chitosan by gel permeation chromatography. High Energy Chem. 2014;48:72–75. doi: 10.1134/S0018143914020143. [DOI] [Google Scholar]
- 25.De Benedictis V.M., Soloperto G., Demitri C. Correction of MHS viscosimetric constants upon numerical simulation of temperature induced degradation kinetic of chitosan solutions. Polymers (Basel) 2016;8:210. doi: 10.3390/polym8060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raafat D., Sahl H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009;2:186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khor E. Chitin: Fulfilling a Biomaterials Promise. Elsevier Ltd.; Amsterdam, The Netherlands: 2001. [Google Scholar]
- 28.Thakur V.K., Thakur M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014;2:2637–2652. doi: 10.1021/sc500634p. [DOI] [Google Scholar]
- 29.Yi H., Wu L.Q., Bentley W.E., Ghodssi R., Rubloff G.W., Culver J.N., Payne G.F. Biofabrication with chitosan. Biomacromolecules. 2005;6:2881–2894. doi: 10.1021/bm050410l. [DOI] [PubMed] [Google Scholar]
- 30.Rafique A., Mahmood Zia K., Zuber M., Tabasum S., Rehman S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016;87:141–154. doi: 10.1016/j.ijbiomac.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Gil E.S., Hudson S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004;29:1173–1222. doi: 10.1016/j.progpolymsci.2004.08.003. [DOI] [Google Scholar]
- 32.Dashtimoghadam E., Hasani-Sadrabadi M.M., Moaddel H. Structural modification of chitosan biopolymer as a novel polyelectrolyte membrane for green power generation. Polym. Adv. Technol. 2010;21:726–734. doi: 10.1002/pat.1496. [DOI] [Google Scholar]
- 33.Bodnar M., Hartmann J.F., Borbely J. Preparation and characterization of chitosan-based nanoparticles. Biomacromolecules. 2005;6:2521–2527. doi: 10.1021/bm0502258. [DOI] [PubMed] [Google Scholar]
- 34.Valderruten N.E., Valverde J.D., Zuluaga F., Ruiz-Durántez E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014;84:21–28. doi: 10.1016/j.reactfunctpolym.2014.08.006. [DOI] [Google Scholar]
- 35.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: Progress and challenges. Polymer (Guildf) 2008;49:1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- 36.Peppas N.A., Bures P., Leobandung W., Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50:27–46. doi: 10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 37.Xie W., Xu P., Wang W., Liu Q. Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohydr. Polym. 2002;50:35–40. doi: 10.1016/S0144-8617(01)00370-8. [DOI] [Google Scholar]
- 38.Yang S., Tirmizi S.A., Burns A., Barney A.A., Risen W.M. Chitaline materials: Soluble Chitosan-polyaniline copolymers and their conductive doped forms. Synth. Met. 1989;32:191–200. doi: 10.1016/0379-6779(89)90841-2. [DOI] [Google Scholar]
- 39.Gorochovceva N., Makuška R. Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft copolymers. Eur. Polym. J. 2004;40:685–691. doi: 10.1016/j.eurpolymj.2003.12.005. [DOI] [Google Scholar]
- 40.De Abreu F.R., Campana-Filho S.P. Preparation and characterization of carboxymethylchitosan. Polímeros. 2005;15:79–83. doi: 10.1590/S0104-14282005000200004. [DOI] [Google Scholar]
- 41.Cao J., You J., Zhang L., Zhou J. Homogeneous synthesis and characterization of chitosan ethers prepared in aqueous alkali/urea solutions. Carbohydr. Polym. 2018;185:138–144. doi: 10.1016/j.carbpol.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Meng G., He J., Wu Y., Wu F., Gu Z. Antibiotic-loaded chitosan hydrogel with superior dual functions: Antibacterial efficacy and osteoblastic cell responses. ACS Appl. Mater. Interfaces. 2014;6:10005–10013. doi: 10.1021/am502537k. [DOI] [PubMed] [Google Scholar]
- 43.Tseng T.C., Tao L., Hsieh F.Y., Wei Y., Chiu I.M., Hsu S.H. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015;27:3518–3524. doi: 10.1002/adma.201500762. [DOI] [PubMed] [Google Scholar]
- 44.Liu H., Ojha B., Morris C., Jiang M., Wojcikiewicz E.P., Rao P.P.N., Du D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules. 2015;16:2363–2373. doi: 10.1021/acs.biomac.5b00603. [DOI] [PubMed] [Google Scholar]
- 45.Abou-Zeid N.Y., Waly A.I., Kandile N.G., Rushdy A.A., El-Sheikh M.A., Ibrahim H.M. Preparation, characterization and antibacterial properties of cyanoethylchitosan/cellulose acetate polymer blended films. Carbohydr. Polym. 2011;84:223–230. doi: 10.1016/j.carbpol.2010.11.026. [DOI] [Google Scholar]
- 46.Badawy M.E.I., Rabea E.I., Rogge T.M., Stevens C.V., Smagghe G., Steurbaut W., Höfte M. Synthesis and fungicidal activity of new N,O-acyl chitosan derivatives. Biomacromolecules. 2004;5:589–595. doi: 10.1021/bm0344295. [DOI] [PubMed] [Google Scholar]
- 47.Badawy M.E.I., Rabea E.I., Rogge T.M., Stevens C.V., Steurbaut W., Höfte M., Smagghe G. Fungicidal and insecticidal activity of O-acyl chitosan derivatives. Polym. Bull. 2005;54:279–289. doi: 10.1007/s00289-005-0396-z. [DOI] [Google Scholar]
- 48.Jayakumar R., Selvamurugan N., Nair S.V., Tokura S., Tamura H. Preparative methods of phosphorylated chitin and chitosan-An overview. Int. J. Biol. Macromol. 2008;43:221–225. doi: 10.1016/j.ijbiomac.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Heras A., Rodríguez N.M., Ramos V.M., Agulló E. N-methylene phosphonic chitosan: A novel soluble derivative. Carbohydr. Polym. 2001;44:1–8. doi: 10.1016/S0144-8617(00)00195-8. [DOI] [Google Scholar]
- 50.Ramos V.M., Rodríguez N.M., Rodríguez M.S., Heras A., Agulló E. Modified chitosan carrying phosphonic and alkyl groups. Carbohydr. Polym. 2003;51:425–429. doi: 10.1016/S0144-8617(02)00211-4. [DOI] [Google Scholar]
- 51.Ramos V.M., Rodríguez N.M., Henning I., Díaz M.F., Monachesi M.P., Rodríguez M.S., Abarrategi A., Correas-Magaña V., López-Lacomba J.L., Agulló E. Poly(ethylene glycol)-crosslinked N-methylene phosphonic chitosan. Preparation and characterization. Carbohydr. Polym. 2006;64:328–336. doi: 10.1016/j.carbpol.2005.12.025. [DOI] [Google Scholar]
- 52.Terbojevich M., Carraro C., Cosani A., Focher B., Naggi A.M., Torri G. Solution studies of chitosan 6-O-sulfate. Die Makromol. Chemie. 1989;190:2847–2855. doi: 10.1002/macp.1989.021901116. [DOI] [Google Scholar]
- 53.Holme K.R., Perlin A.S. Chitosan N-sulfate. A water-soluble polyelectrolyte. Carbohydr. Res. 1997;302:7–12. doi: 10.1016/S0008-6215(97)00117-1. [DOI] [PubMed] [Google Scholar]
- 54.Fan L., Wu P., Zhang J., Gao S., Wang L., Li M., Sha M., Xie W., Nie M. Synthesis and anticoagulant activity of the quaternary ammonium chitosan sulfates. Int. J. Biol. Macromol. 2012;50:31–37. doi: 10.1016/j.ijbiomac.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Subhapradha N., Ramasamy P., Srinivasan A., Madeswaran P., Shanmugam V., Shanmugam A. Sulfation of β-chitosan and evaluation of biological activity from gladius of Sepioteuthis lessoniana. Int. J. Biol. Macromol. 2013;62:336–340. doi: 10.1016/j.ijbiomac.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Zhai X., Sun P., Luo Y., Ma C., Xu J., Liu W. Guanidinylation: A Simple Way to Fabricate Cell Penetrating Peptide Analogue-Modified Chitosan Vector for Enhanced Gene Delivery. J. Appl. Polym. Sci. 2011;121:3569–3578. doi: 10.1002/app.34156. [DOI] [Google Scholar]
- 57.Hu Y., Du Y., Yang J., Kennedy J.F., Wang X., Wang L. Synthesis, characterization and antibacterial activity of guanidinylated chitosan. Carbohydr. Polym. 2007;67:66–72. doi: 10.1016/j.carbpol.2006.04.015. [DOI] [Google Scholar]
- 58.Sahariah P., Óskarsson B.M., Hjálmarsdóttir M., Másson M. Synthesis of guanidinylated chitosan with the aid of multiple protecting groups and investigation of antibacterial activity. Carbohydr. Polym. 2015;127:407–417. doi: 10.1016/j.carbpol.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Ye Y., Xiao H., He B., Qian L. Microwave assisted preparation of antimicrobial chitosan with guanidine oligomers and its application in hygiene paper products. Polymers (Basel) 2017;9:633. doi: 10.3390/polym9120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao X., Qiao Z.Z., He J.X. Preparation of chitosan biguanidine hydrochloride and application in antimicrobial finish of wool fabric. J. Eng. Fibers Fabr. 2010;5:16–24. doi: 10.1177/155892501000500303. [DOI] [Google Scholar]
- 61.Pantaleone D., Yalpani M., Scollar M. Unusual susceptibility of chitosan to enzymic hydrolysis. Carbohydr. Res. 1992;237:325–332. doi: 10.1016/S0008-6215(92)84256-R. [DOI] [Google Scholar]
- 62.Yeh H.Y., Lin J.C. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection-deprotection strategy. J. Biomater. Sci. Polym. Ed. 2008;19:291–310. doi: 10.1163/156856208783720985. [DOI] [PubMed] [Google Scholar]
- 63.Yang H., Luan S., Zhao J., Shi H., Li X., Song L., Jin J., Shi Q., Yin J., Shi D., et al. Improving hemocompatibility of styrene-b-(ethylene-co-butylene)-b-styrene elastomer via N-vinyl pyrrolidone-assisted grafting of poly(ethylene glycol) methacrylate. Polymer (Guildf) 2012;53:1675–1683. doi: 10.1016/j.polymer.2012.02.033. [DOI] [Google Scholar]
- 64.Peniche C., Argüelles-Monal W., Peniche H., Acosta N. Chitosan: An Attractive Biocompatible Polymer for Microencapsulation. Macromol. Biosci. 2003;3:511–520. doi: 10.1002/mabi.200300019. [DOI] [Google Scholar]
- 65.Reis R.L., Román J.S., editors. Biodegradable Systems in Tissue Engineering and Regenerative Medicine. 1st ed. CRC Press; Boca Raton, FL, USA: 2005. [Google Scholar]
- 66.Peña J., Izquierdo-Barba I., Martínez A., Vallet-Regí M. New method to obtain chitosan/apatite materials at room temperature. Solid State Sci. 2006;8:513–519. doi: 10.1016/j.solidstatesciences.2005.11.003. [DOI] [Google Scholar]
- 67.Singla A.K., Chawla M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001;53:1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 68.Francis Suh J.K., Matthew H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/S0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 69.Venkatesan J., Vinodhini P.A., Sudha P.N., Kim S.K. Chitin and chitosan composites for bone tissue regeneration. Adv. Food Nutr. Res. 2014;73:59–81. doi: 10.1016/B978-0-12-800268-1.00005-6. [DOI] [PubMed] [Google Scholar]
- 70.Jayakumar R., Jayakumar S.V., Jayakumar T., Murat H. Perspectives of Chitin and Chitosan Nanofibrous Scaffolds in Tissue Engineering. Intech; Rijeka, Croatia: 2010. [Google Scholar]
- 71.Jayakumar R., Menon D., Manzoor K., Nair S.V., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010;82:227–232. doi: 10.1016/j.carbpol.2010.04.074. [DOI] [Google Scholar]
- 72.Cheng M., Deng J., Yang F., Gong Y., Zhao N., Zhang X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871–2880. doi: 10.1016/S0142-9612(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 73.Zhao F., Yin Y., Lu W.W., Leong J.C., Zhang W., Zhang J., Zhang M., Yao K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23:3227–3234. doi: 10.1016/S0142-9612(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 74.Risbud M., Endres M., Ringe J., Bhonde R., Sittinger M. Biocompatible hydrogel supports the growth of respiratory epithelial cells: Possibilities in tracheal tissue engineering. J. Biomed. Mater. Res. 2001;56:120–127. doi: 10.1002/1097-4636(200107)56:1<120::AID-JBM1076>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 75.Fiench L.L., Wilson J. Surface-Active Biomaterials. Science. 1984;226:630–636. doi: 10.1126/science.6093253. [DOI] [PubMed] [Google Scholar]
- 76.Kim I.Y., Seo S.J., Moon H.S., Yoo M.K., Park I.Y., Kim B.C., Cho C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Grande D.A., Halberstadt C., Naughton G., Schwartz R., Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. 1997;34:211–220. doi: 10.1002/(SICI)1097-4636(199702)34:2<211::AID-JBM10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 78.Sechriest V.F., Miao Y.J., Niyibizi C., Westerhausen-Larson A., Matthew H.W., Evans C.H., Fu F.H., Suh J.K. GAG-augmented polysaccharide hydrogel: A novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 2000;49:534–541. doi: 10.1002/(SICI)1097-4636(20000315)49:4<534::AID-JBM12>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 79.Shahabeddin L., Berthod F., Damour O., Collombel C. Characterization of Skin Reconstructed on a Chitosan-Cross-Linked Collagen-Glycosaminoglycan Matrix. Ski. Pharmacol. 1990;3:107–114. doi: 10.1159/000210857. [DOI] [PubMed] [Google Scholar]
- 80.Heath C.A., Rutkowski G.E. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998;16:163–168. doi: 10.1016/S0167-7799(97)01165-7. [DOI] [PubMed] [Google Scholar]
- 81.Nair L.S., Laurencin C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32:762–798. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- 82.Haipeng G., Yinghui Z., Jianchun L., Yandao G., Nanming Z., Xiufang Z. Studies on nerve cell affinity of chitosan-derived materials. J. Biomed. Mater. Res. 2000;52:285–295. doi: 10.1002/1097-4636(200011)52:2<285::AID-JBM7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 83.Yuan Y., Zhang P., Yang Y., Wang X., Gu X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25:4273–4278. doi: 10.1016/j.biomaterials.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 84.Azuma K., Izumi R., Osaki T., Ifuku S., Morimoto M., Saimoto H., Minami S., Okamoto Y. Chitin, Chitosan, and Its Derivatives for Wound Healing: Old and New Materials. J. Funct. Biomater. 2015;6:104–142. doi: 10.3390/jfb6010104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Silva D.J.B., Zuluaga F., Carlos H. Evaluation of Biocompatibility of Chitosan Films from the Mycelium of Aspergillus niger in Connective Tissue of Rattus norvegicus. J. Mol. Genet. Med. 2015;09:1–8. [Google Scholar]
- 86.Gopal A., Kant V., Gopalakrishnan A., Tandan S.K., Kumar D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014;731:8–19. doi: 10.1016/j.ejphar.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 87.Sudheesh Kumar P.T., Lakshmanan V.K., Anilkumar T.V., Ramya C., Reshmi P., Unnikrishnan A.G., Nair S.V., Jayakumar R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces. 2012;4:2618–2629. doi: 10.1021/am300292v. [DOI] [PubMed] [Google Scholar]
- 88.Blažević F., Milekić T., Romić M.D., Juretić M., Pepić I., Filipović-Grčić J., Lovrić J., Hafner A. Nanoparticle-mediated interplay of chitosan and melatonin for improved wound epithelialisation. Carbohydr. Polym. 2016;146:445–454. doi: 10.1016/j.carbpol.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 89.Archana D., Singh B.K., Dutta J., Dutta P.K. In vivo evaluation of chitosan-PVP-titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013;95:530–539. doi: 10.1016/j.carbpol.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 90.Shigemasa Y., Minami S. Applications of chitin and chitosan for biomaterials. Biotechnol. Genet. Eng. Rev. 1996;13:383–420. doi: 10.1080/02648725.1996.10647935. [DOI] [PubMed] [Google Scholar]
- 91.Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010;56:290–299. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Kim K.Y., Min D.S. Wound covering materials from polyelectrolyte complexes of chitosan with sulphonated chitosan. Trans. Soc. Biomater. 1988;11:558. [Google Scholar]
- 93.Mao J., Zhao L., De Yao K., Shang Q., Yang G., Cao Y. Study of novel chitosan-gelatin artificial skin in vitro. J. Biomed. Mater. Res. Part A. 2003;64:301–308. doi: 10.1002/jbm.a.10223. [DOI] [PubMed] [Google Scholar]
- 94.Berger J., Reist M., Mayer J.M., Felt O., Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004;57:35–52. doi: 10.1016/S0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 95.Yao K.D., Yin Y.J., Xu M.X., Wang Y.F. Investigation of pH-sensitive drug delivery system of chitosan/gelatin hybrid polymer network. Polym. Int. 1995;38:77–82. doi: 10.1002/pi.1995.210380110. [DOI] [Google Scholar]
- 96.Chandy T., Rao G.H.R., Wilson R.F., Das G.S. Delivery of LMW heparin via surface coated chitosan/peg-alginate microspheres prevents thrombosis. Drug Deliv. J. Deliv. Target. Ther. Agents. 2002;9:87–96. doi: 10.1080/10426500290095584. [DOI] [PubMed] [Google Scholar]
- 97.Chellat F., Tabrizian M., Dumitriu S., Chornet E., Rivard C.H., Yahia L. Study of biodegradation behavior of Chitosan-Xanthan microspheres in simulated physiological media. J. Biomed. Mater. Res. 2000;53:592–599. doi: 10.1002/1097-4636(200009)53:5<592::AID-JBM20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 98.Stolnik S., Illum L., Davis S.S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 2012;64:290–301. doi: 10.1016/j.addr.2012.09.029. [DOI] [Google Scholar]
- 99.Kim S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activie and Applications. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 100.Mathew M.E., Mohan J.C., Manzoor K., Nair S.V., Tamura H., Jayakumar R. Folate conjugated carboxymethyl chitosan-manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr. Polym. 2010;80:442–448. doi: 10.1016/j.carbpol.2009.11.047. [DOI] [Google Scholar]
- 101.Hasegawa M., Yagi K., Iwakawa S., Hirai M. Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn. J. Cancer Res. 2001;92:459–466. doi: 10.1111/j.1349-7006.2001.tb01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen W.R., Adams R.L., Carubelli R., Nordquist R.E. Laser-photosensitizer assisted immunotherapy: A novel modality for cancer treatment. Cancer Lett. 1997;115:25–30. doi: 10.1016/S0304-3835(97)04707-1. [DOI] [PubMed] [Google Scholar]
- 103.Nishimura I.C., Nishimura S., Nishi N., Saiki I., Tokura S., Azuma I. Immunological Activity of Chitin. Vaccine. 1984;2:93–99. doi: 10.1016/S0264-410X(98)90039-1. [DOI] [PubMed] [Google Scholar]
- 104.Qi L., Xu Z., Chen M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer. 2007;43:184–193. doi: 10.1016/j.ejca.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 105.Gumińska M., Ignacak J., Wojcik E. In vitro inhibitory effect of chitosan and its degradation products on energy metabolism in Ehrlich ascites tumour cells (EAT) Polish J. Pharmacol. 1996;48:495–501. [PubMed] [Google Scholar]
- 106.Singh D.K., Ray A.R. Biomedical applications of chitin, chitosan, and their derivatives. J. Macromol. Sci. Polym. Rev. 2000;40:69–83. doi: 10.1081/MC-100100579. [DOI] [Google Scholar]
- 107.Blair H.S., Guthrie J., Law T.-K., Turkington P. Chitosan and modified chitosan membranes I. Preparation and characterisation. J. Appl. Polym. Sci. 1987;33:641–656. doi: 10.1002/app.1987.070330226. [DOI] [Google Scholar]
- 108.Kubota N., Kikuchi Y., Mizuhara Y., Ishihara T., Takita Y. Solid-phase modification of chitosan hydrogel membranes and permeability properties of modified chitosan membranes. J. Appl. Polym. Sci. 1993;50:1665–1670. doi: 10.1002/app.1993.070500921. [DOI] [Google Scholar]
- 109.Hirano S., Tobetto K., Hasegawa M., Matsuda N. Permeability properties of gels and membranes derived from chitosan. J. Biomed. Mater. Res. 1980;14:477–485. doi: 10.1002/jbm.820140414. [DOI] [PubMed] [Google Scholar]
- 110.Xue L., Greisler H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003;37:472–480. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]
- 111.Chupa J.M., Foster A.M., Sumner S.R., Madihally S.V., Matthew H.W. Vascular cell responses to polysaccharide materials. Biomaterials. 2000;21:2315–2322. doi: 10.1016/S0142-9612(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 112.Madihally S.V., Matthew H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/S0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 113.Irvine S.A., Yun X., Venkatraman S. Anti-platelet and tissue engineering approaches to biomaterial blood compatibilization: How well have these been translated into the clinic? Drug Deliv. Transl. Res. 2012;2:384–397. doi: 10.1007/s13346-012-0077-z. [DOI] [PubMed] [Google Scholar]
- 114.Chiellini E., Giusti P. Heparine-Like Substance and Blood Compatible Polymers Obtained from Chitin and Chitosan. Springer; Boston, MA, USA: 1983. [Google Scholar]
- 115.Hudson S.M., Jenkins D.W. Chitin and Chitosan. John Wiley & Sons; New York, NY, USA: 2002. [Google Scholar]
- 116.Mark H.F., Kroschwitz J.I. Encyclopedia of Polymer Science and Engineering. Willey; New York, NY, USA: 1989. [Google Scholar]
- 117.Gooday G.W. Advances in Microbial Ecology. Springer; Berlin/Heidelberg, Germany: 1990. [Google Scholar]
- 118.Schisler D.A., Slininger P.J., Behle R.W., Jackson M.A. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology. 2004;94:1267–1271. doi: 10.1094/PHYTO.2004.94.11.1267. [DOI] [PubMed] [Google Scholar]
- 119.Kumar M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. doi: 10.1016/S1381-5148(00)00038-9. [DOI] [Google Scholar]
- 120.Shahidi F., Arachchi J.K.V., Jeon Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999;10:37–51. doi: 10.1016/S0924-2244(99)00017-5. [DOI] [Google Scholar]
- 121.Goy R.C., De Britto D., Assis O.B.G. A review of the antimicrobial activity of chitosan. Polimeros. 2009;19:241–247. doi: 10.1590/S0104-14282009000300013. [DOI] [Google Scholar]
- 122.Sahariah P., Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules. 2017;18:3846–3868. doi: 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- 123.Dutta P.K., Tripathi S., Mehrotra G.K., Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- 124.Geisberger G., Gyenge E.B., Hinger D., Käch A., Maake C., Patzke G.R. Chitosan-thioglycolic acid as a versatile antimicrobial agent. Biomacromolecules. 2013;14:1010–1017. doi: 10.1021/bm3018593. [DOI] [PubMed] [Google Scholar]
- 125.Kaya M., Cakmak Y.S., Baran T., Asan-Ozusaglam M., Mentes A., Tozak K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioprocess Eng. 2014;19:58–69. doi: 10.1007/s12257-013-0488-9. [DOI] [Google Scholar]
- 126.Hu L., Meng X., Xing R., Liu S., Chen X., Qin Y., Yu H., Li P. Design, synthesis and antimicrobial activity of 6-N-substituted chitosan derivatives. Bioorgan. Med. Chem. Lett. 2016;26:4548–4551. doi: 10.1016/j.bmcl.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 127.Sano H., Shibasaki K.I., Matsukubo T., Takaesu Y. Effect of chitosan rinsing on reduction of dental plaque formation. Bull. Tokyo Dent. Coll. 2003;44:9–16. doi: 10.2209/tdcpublication.44.9. [DOI] [PubMed] [Google Scholar]
- 128.Coma V., Deschamps A., Martial-Gros A. Bioactive Packaging Materials from Edible Chitosan Polymer—Antimicrobial Activity Assessment on Dairy-Related Contaminants. J. Food Sci. 2003;68:2788–2792. doi: 10.1111/j.1365-2621.2003.tb05806.x. [DOI] [Google Scholar]
- 129.Badawy M.E.I., Rabea E.I. A Biopolymer Chitosan and Its Derivatives as Promising Antimicrobial Agents against Plant Pathogens and Their Applications in Crop Protection. Int. J. Carbohydr. Chem. 2011;2011:1–29. doi: 10.1155/2011/460381. [DOI] [Google Scholar]
- 130.Ong S.Y., Wu J., Moochhala S.M., Tan M.H., Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323–4332. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 131.Dai T., Tanaka M., Huang Y.Y., Hamblin M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011;9:857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao Z., Luo Y., Luo Y., Wang Q. Combined effects of sodium chlorite dip treatment and chitosan coatings on the quality of fresh-cut d’Anjou pears. Postharvest Biol. Technol. 2011;62:319–326. doi: 10.1016/j.postharvbio.2011.07.007. [DOI] [Google Scholar]
- 133.Dragostin O.M., Samal S.K., Dash M., Lupascu F., Pânzariu A., Tuchilus C., Ghetu N., Danciu M., Dubruel P., Pieptu D., et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016;141:28–40. doi: 10.1016/j.carbpol.2015.12.078. [DOI] [PubMed] [Google Scholar]
- 134.Dragostin O.M., Sama S.K., Lupascu F., Pânzariu A., Dubruel P., Lupascu D., Tuchilus C., Vasile C., Profire L. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. Int. J. Mol. Sci. 2015;16:29843–29855. doi: 10.3390/ijms161226204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stawski D., Sahariah P., Hjálmarsdóttir M., Wojciechowska D., Puchalski M., Másson M. N,N,N-trimethyl chitosan as an efficient antibacterial agent for polypropylene and polylactide nonwovens. J. Text. Inst. 2017;108:1041–1049. doi: 10.1080/00405000.2016.1218594. [DOI] [Google Scholar]
- 136.Yang Z., Peng H., Wang W., Liu T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010;116:2658–2667. doi: 10.1002/app.31787. [DOI] [Google Scholar]
- 137.Sang W., Tang Z., He M.Y., Hua Y.P., Xu Q. Preparation, characterization and application of the novel chitosan derivative modified with phenylalanine. Fibers Polym. 2015;16:991–996. doi: 10.1007/s12221-015-0991-z. [DOI] [Google Scholar]
- 138.Ignatova M., Manolova N., Markova N., Rashkov I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol. Biosci. 2009;9:102–111. doi: 10.1002/mabi.200800189. [DOI] [PubMed] [Google Scholar]
- 139.Ji Q.X., Zhong D.Y., Lü R., Zhang W.Q., Deng J., Chen X.G. In vitro evaluation of the biomedical properties of chitosan and quaternized chitosan for dental applications. Carbohydr. Res. 2009;344:1297–1302. doi: 10.1016/j.carres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 140.Sano H., Shibasaki K., Matsukubo T., Takaesu Y. Comparison of the activity of four chitosan derivatives in reducing initial adherence of oral bacteria onto tooth surfaces. Bull. Tokyo Dent. Coll. 2001;42:243–249. doi: 10.2209/tdcpublication.42.243. [DOI] [PubMed] [Google Scholar]
- 141.Nechita P., Bobu E., Parfene G., Dinicə R.M., Bəlan T. Antimicrobial coatings based on chitosan derivatives and quaternary ammonium salts for packaging paper applications. Cellul. Chem. Technol. 2015;49:625–632. [Google Scholar]
- 142.Gupta D., Haile A. Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr. Polym. 2007;69:164–171. doi: 10.1016/j.carbpol.2006.09.023. [DOI] [Google Scholar]
- 143.Ye W., Leung M.F., Xin J., Kwong T.L., Lee D.K.L., Li P. Novel core-shell particles with poly(n-butyl acrylate) cores and chitosan shells as an antibacterial coating for textiles. Polymer (Guildf) 2005;46:10538–10543. doi: 10.1016/j.polymer.2005.08.019. [DOI] [Google Scholar]
- 144.Nigmatullin R., Konovalova V., Pobigay G. Development of Antimicrobial Membranes via the Surface Tethering of Chitosan. J. Appl. Polym. Sci. 2009;111:1697–1705. doi: 10.1002/app.29135. [DOI] [Google Scholar]
- 145.Xu X., Zhuang X., Cheng B., Xu J., Long G., Zhang H. Manufacture and properties of cellulose/O-hydroxyethyl chitosan blend fibers. Carbohydr. Polym. 2010;81:541–544. doi: 10.1016/j.carbpol.2010.03.011. [DOI] [Google Scholar]
- 146.Inta O., Yoksan R., Limtrakul J. Hydrophobically modified chitosan: A bio-based material for antimicrobial active film. Mater. Sci. Eng. C. 2014;42:569–577. doi: 10.1016/j.msec.2014.05.076. [DOI] [PubMed] [Google Scholar]
- 147.Sridhari T.R., Dutta P.K. Synthesis and characterization of maleilated chitosan for dye house effluent. Indian J. Chem. Technol. 2000;7:198–201. [Google Scholar]
- 148.El Mouzdahir Y., Elmchaouri A., Mahboub R., Gil A., Korili S.A. Equilibrium modeling for the adsorption of methylene blue from aqueous solutions on activated clay minerals. Desalination. 2010;250:335–338. doi: 10.1016/j.desal.2009.09.052. [DOI] [Google Scholar]
- 149.Chang M.Y., Juang R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004;278:18–25. doi: 10.1016/j.jcis.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 150.Wan Ngah W.S., Ariff N.F.M., Hanafiah M.A.K.M. Preparation, characterization, and environmental application of crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions. Water. Air. Soil Pollut. 2010;206:225–236. doi: 10.1007/s11270-009-0098-5. [DOI] [Google Scholar]
- 151.Ngah W.S.W., Ariff N.F.M., Hashim A., Hanafiah M.A.K.M. Malachite green adsorption onto chitosan coated bentonite beads: Isotherms, kinetics and mechanism. Clean Soil Air Water. 2010;38:394–400. doi: 10.1002/clen.200900251. [DOI] [Google Scholar]
- 152.Zhu H.Y., Jiang R., Xiao L. Adsorption of an anionic azo dye by chitosan/kaolin/γ-Fe2O3 composites. Appl. Clay Sci. 2010;48:522–526. doi: 10.1016/j.clay.2010.02.003. [DOI] [Google Scholar]
- 153.Wang L., Wang A. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007;147:979–985. doi: 10.1016/j.jhazmat.2007.01.145. [DOI] [PubMed] [Google Scholar]
- 154.Hasan M., Ahmad A.L., Hameed B.H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008;136:164–172. doi: 10.1016/j.cej.2007.03.038. [DOI] [Google Scholar]
- 155.Lee H.C., Jeong Y.G., Min B.G., Lyoo W.S., Lee S.C. Preparation and acid dye adsorption behavior of polyurethane/chitosan composite foams. Fibers Polym. 2009;10:636–642. doi: 10.1007/s12221-010-0636-1. [DOI] [Google Scholar]
- 156.Ahmad A.L., Sumathi S., Hameed B.H. Chitosan: A natural biopolymer for the adsorption of residue oil from oily wastewater. Adsorpt. Sci. Technol. 2004;22:75–88. doi: 10.1260/026361704323151015. [DOI] [Google Scholar]
- 157.Yang T., Zall R.R. Chitosan Membranes for Reverse Osmosis Application. J. Food Sci. 1984;49:91–93. doi: 10.1111/j.1365-2621.1984.tb13677.x. [DOI] [Google Scholar]
- 158.Wan Ngah W.S., Teong L.C., Hanafiah M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011;83:1446–1456. doi: 10.1016/j.carbpol.2010.11.004. [DOI] [Google Scholar]
- 159.Monier M. Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modified chitosan-thioglyceraldehyde Schiff’s base. Int. J. Biol. Macromol. 2012;50:773–781. doi: 10.1016/j.ijbiomac.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 160.Lü H., An H., Xie Z. Ion-imprinted carboxymethyl chitosan-silica hybrid sorbent for extraction of cadmium from water samples. Int. J. Biol. Macromol. 2013;56:89–93. doi: 10.1016/j.ijbiomac.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 161.Ngah W.S.W., Fatinathan S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008;143:62–72. doi: 10.1016/j.cej.2007.12.006. [DOI] [Google Scholar]
- 162.Vijaya Y., Popuri S.R., Boddu V.M., Krishnaiah A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008;72:261–271. doi: 10.1016/j.carbpol.2007.08.010. [DOI] [Google Scholar]
- 163.Qiao Z., Wang Z., Zhang C., Yuan S., Zhu Y., Wang J. PVAm–PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE J. 2012;59:215–228. doi: 10.1002/aic.13781. [DOI] [Google Scholar]
- 164.Boddu V.M., Abburi K., Talbott J.L., Smith E.D., Haasch R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008;42:633–642. doi: 10.1016/j.watres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 165.Boddu V.M., Abburi K., Randolph A.J., Smith E.D. Removal of copper (II) and nickel (II) ions from aqueous solutions by a composite chitosan biosorbent. Sep. Sci. Technol. 2008;43:1365–1381. doi: 10.1080/01496390801940762. [DOI] [Google Scholar]
- 166.Boddu V.M., Abburi K., Talbott J.L., Smith E.D. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003;37:4449–4456. doi: 10.1021/es021013a. [DOI] [PubMed] [Google Scholar]
- 167.Dragan E.S., Dinu M.V., Timpu D. Preparation and characterization of novel composites based on chitosan and clinoptilolite with enhanced adsorption properties for Cu2+ Bioresour. Technol. 2010;101:812–817. doi: 10.1016/j.biortech.2009.08.077. [DOI] [PubMed] [Google Scholar]
- 168.Dinu M.V., Dragan E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010;160:157–163. doi: 10.1016/j.cej.2010.03.029. [DOI] [Google Scholar]
- 169.Qu R., Sun C., Ma F., Zhang Y., Ji C., Xu Q., Wang C., Chen H. Removal and recovery of Hg(II) from aqueous solution using chitosan-coated cotton fibers. J. Hazard. Mater. 2009;167:717–727. doi: 10.1016/j.jhazmat.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 170.Qu R., Sun C., Wang M., Ji C., Xu Q., Zhang Y., Wang C., Chen H., Yin P. Adsorption of Au(III) from aqueous solution using cotton fiber/chitosan composite adsorbents. Hydrometallurgy. 2009;100:65–71. doi: 10.1016/j.hydromet.2009.10.008. [DOI] [Google Scholar]
- 171.Zhang G., Qu R., Sun C., Ji C., Chen H., Wang C., Niu Y. Adsorption for Metal Ions of Chitosan Coated Cotton Fiber. J. Appl. Polym. Sci. 2008;110:2321–2327. doi: 10.1002/app.27515. [DOI] [Google Scholar]
- 172.Huang G., Zhang H., Shi J.X., Langrish T.A.G. Adsorption of chromium(VI) from aqueous solutions using cross-linked magnetic chitosan beads. Ind. Eng. Chem. Res. 2009;48:2646–2651. doi: 10.1021/ie800814h. [DOI] [Google Scholar]
- 173.Tran H.V., Tran L.D., Nguyen T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater. Sci. Eng. C. 2010;30:304–310. doi: 10.1016/j.msec.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 174.Kalyani S., Priya J.A., Rao P.S., Krishnaiah A. Removal of copper and nickel from aqueous solutions using chitosan coated on perlite as biosorbent. Sep. Sci. Technol. 2005;40:1483–1495. doi: 10.1081/SS-200055940. [DOI] [Google Scholar]
- 175.Hasan S., Krishnaiah A., Ghosh T.K., Viswanath D.S., Boddu V.M., Smith E.D. Adsorption of divalent cadmium (Cd(II)) from aqueous solutions onto chitosan-coated perlite beads. Ind. Eng. Chem. Res. 2006;45:5066–5077. doi: 10.1021/ie0402620. [DOI] [Google Scholar]
- 176.Hasan S., Krishnaiah A., Ghosh T.K., Viswanath D.S., Boddu V.M., Smith E.D. Adsorption of chromium(VI) on chitosan-coated perlite. Sep. Sci. Technol. 2003;38:3775–3793. doi: 10.1081/SS-120024229. [DOI] [Google Scholar]
- 177.Hasan S., Ghosh T.K., Viswanath D.S., Boddu V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2008;152:826–837. doi: 10.1016/j.jhazmat.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 178.Kumar M., Tripathi B.P., Shahi V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009;172:1041–1048. doi: 10.1016/j.jhazmat.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 179.Wan Ngah W.S., Kamari A., Koay Y.J. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int. J. Biol. Macromol. 2004;34:155–161. doi: 10.1016/j.ijbiomac.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 180.Popuri S.R., Vijaya Y., Boddu V.M., Abburi K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009;100:194–199. doi: 10.1016/j.biortech.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 181.Jiang Z., Yu Y., Wu H. Preparation of CS/GPTMS hybrid molecularly imprinted membrane for efficient chiral resolution of phenylalanine isomers. J. Membr. Sci. 2006;280:876–882. doi: 10.1016/j.memsci.2006.03.006. [DOI] [Google Scholar]
- 182.Jiraratananon R., Chanachai A., Huang R.Y.M., Uttapap D. Pervaporation dehydration of ethanol-water mixtures with chitosan/hydroxyethylcellulose (CS/HEC) composite membranes I. Effect of operating conditions. J. Membr. Sci. 2002;195:143–151. doi: 10.1016/S0376-7388(01)00563-4. [DOI] [Google Scholar]
- 183.Mochizuki A., Amiya S., Sato Y., Ogawara H., Yamashita S. Pervaporation separation of water/ethanol mixtures through polysaccharide membranes. III. The Permselectivity of the Neutralized Chitosan Membrane and the Relationships between Its Permselectivity and Solid State Structure. J. Appl. Polym. Sci. 1989;37:3385–3398. doi: 10.1002/app.1989.070371210. [DOI] [Google Scholar]
- 184.Goto M., Shiosaki A., Hirose T. Separation of Water/Ethanol Vapor Mixtures through Chitosan and Crosslinked Chitosan Membranes. Sep. Sci. Technol. 1994;29:1915–1923. doi: 10.1080/01496399408002180. [DOI] [Google Scholar]
- 185.Shieh J.J., Huang R.Y.M. Preparation of N-methylol nylon-6 membranes for pervaporation of ethanol-water mixtures. J. Appl. Polym. Sci. 1997;64:855–863. doi: 10.1002/(SICI)1097-4628(19970502)64:5<855::AID-APP5>3.0.CO;2-O. [DOI] [Google Scholar]
- 186.Chen X., Yang H., Gu Z., Shao Z. Preparation and characterization of HY zeolite-filled chitosan membranes for pervaporation separation. J. Appl. Polym. Sci. 2001;79:1144–1149. doi: 10.1002/1097-4628(20010207)79:6<1144::AID-APP190>3.0.CO;2-D. [DOI] [Google Scholar]
- 187.Uragami T., Tanaka Y., Nishida S. Permeation and separation under high temperature and high pressure for ethanol/water vapors through cross-linked quaternized chitosan composite membranes. Desalination. 2002;147:449–454. doi: 10.1016/S0011-9164(02)00642-2. [DOI] [Google Scholar]
- 188.Nawawi M.G.M., Huang R.Y.M. Pervaporation dehydration of isopropanol with chitosan membranes. J. Membr. Sci. 1997;124:53–62. [Google Scholar]
- 189.Chanachai A., Jiraratananon R., Uttapap D., Moon G.Y., Anderson W.A., Huang R.Y.M. Pervaporation with chitosan/hydroxyethylcellulose (CS/HEC) blended membranes. J. Membr. Sci. 2000;166:271–280. doi: 10.1016/S0376-7388(99)00269-0. [DOI] [Google Scholar]
- 190.Devi D.A., Smitha B., Sridhar S., Aminabhavi T.M. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. Sci. 2005;262:91–99. doi: 10.1016/j.memsci.2005.03.051. [DOI] [Google Scholar]
- 191.Kittur A.A., Kulkarni S.S., Aralaguppi M.I., Kariduraganavar M.Y. Preparation and characterization of novel pervaporation membranes for the separation of water-isopropanol mixtures using chitosan and NaY zeolite. J. Membr. Sci. 2005;247:75–86. doi: 10.1016/j.memsci.2004.09.010. [DOI] [Google Scholar]
- 192.Svang-Ariyaskul A., Huang R.Y.M., Douglas P.L., Pal R., Feng X., Chen P., Liu L. Blended chitosan and polyvinyl alcohol membranes for the pervaporation dehydration of isopropanol. J. Membr. Sci. 2006;280:815–823. doi: 10.1016/j.memsci.2006.03.001. [DOI] [Google Scholar]
- 193.Liu Y.L., Yu C.H., Lee K.R., Lai J.Y. Chitosan/poly(tetrafluoroethylene) composite membranes using in pervaporation dehydration processes. J. Membr. Sci. 2007;287:230–236. doi: 10.1016/j.memsci.2006.10.040. [DOI] [Google Scholar]
- 194.Chen J.H., Liu Q.L., Fang J., Zhu A.M., Zhang Q.G. Composite hybrid membrane of chitosan-silica in pervaporation separation of MeOH/DMC mixtures. J. Colloid Interface Sci. 2007;316:580–588. doi: 10.1016/j.jcis.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 195.Choudhari S.K., Kittur A.A., Kulkarni S.S., Kariduraganavar M.Y. Development of novel blocked diisocyanate crosslinked chitosan membranes for pervaporation separation of water-isopropanol mixtures. J. Membr. Sci. 2007;302:197–206. doi: 10.1016/j.memsci.2007.06.045. [DOI] [Google Scholar]
- 196.Tsai H.A., Chen W.H., Kuo C.Y., Lee K.R., Lai J.Y. Study on the pervaporation performance and long-term stability of aqueous iso-propanol solution through chitosan/polyacrylonitrile hollow fiber membrane. J. Membr. Sci. 2008;309:146–155. doi: 10.1016/j.memsci.2007.10.018. [DOI] [Google Scholar]
- 197.Rachipudi P.S., Kittur A.A., Choudhari S.K., Varghese J.G., Kariduraganavar M.Y. Development of polyelectrolyte complexes of chitosan and phosphotungstic acid as pervaporation membranes for dehydration of isopropanol. Eur. Polym. J. 2009;45:3116–3126. doi: 10.1016/j.eurpolymj.2009.08.011. [DOI] [Google Scholar]
- 198.Varghese J.G., Kittur A.A., Rachipudi P.S., Kariduraganavar M.Y. Synthesis, characterization and pervaporation performance of chitosan-g-polyaniline membranes for the dehydration of isopropanol. J. Membr. Sci. 2010;364:111–121. doi: 10.1016/j.memsci.2010.08.007. [DOI] [Google Scholar]
- 199.Sajjan A.M., Jeevan Kumar B.K., Kittur A.A., Kariduraganavar M.Y. Novel approach for the development of pervaporation membranes using sodium alginate and chitosan-wrapped multiwalled carbon nanotubes for the dehydration of isopropanol. J. Membr. Sci. 2013;425–426:77–88. doi: 10.1016/j.memsci.2012.08.042. [DOI] [Google Scholar]
- 200.Yong Nam S., Moo Lee Y. Pervaporation of ethylene glycol-water mixtures. I. Pervaporation performance of surface crosslinked chitosan membranes. J. Membr. Sci. 1999;153:155–162. doi: 10.1016/S0376-7388(98)00263-4. [DOI] [Google Scholar]
- 201.Hu C., Guo R., Li B., Ma X., Wu H., Jiang Z. Development of novel mordenite-filled chitosan-poly(acrylic acid) polyelectrolyte complex membranes for pervaporation dehydration of ethylene glycol aqueous solution. J. Membr. Sci. 2007;293:142–150. doi: 10.1016/j.memsci.2007.02.009. [DOI] [Google Scholar]
- 202.Du J.R., Chakma A., Feng X. Dehydration of ethylene glycol by pervaporation using poly(N,N-dimethylaminoethyl methacrylate)/polysulfone composite membranes. Sep. Purif. Technol. 2008;64:63–70. doi: 10.1016/j.seppur.2008.08.004. [DOI] [Google Scholar]
- 203.Hyder M.N., Chen P. Pervaporation dehydration of ethylene glycol with chitosan-poly(vinyl alcohol) blend membranes: Effect of CS-PVA blending ratios. J. Membr. Sci. 2009;340:171–180. doi: 10.1016/j.memsci.2009.05.021. [DOI] [Google Scholar]
- 204.Cao S., Shi Y., Chen G. Properties and pervaporation characteristics of chitosan-poly(N-vinyl-2-pyrrolidone) blend membranes for MeOH-MTBE. J. Appl. Polym. Sci. 1999;74:1452–1458. doi: 10.1002/(SICI)1097-4628(19991107)74:6<1452::AID-APP18>3.0.CO;2-N. [DOI] [Google Scholar]
- 205.Yong Nam S., Moo Lee Y. Pervaporation separation of methanol/methyl t-butyl ether through chitosan composite membrane modified with surfactants. J. Membr. Sci. 1999;157:63–71. doi: 10.1016/S0376-7388(98)00368-8. [DOI] [Google Scholar]
- 206.Huang R.Y.M., Moon G.Y., Pal R. Chitosan/anionic surfactant complex membranes for the pervaporation separation of methanol/MTBE and characterization of the polymer/surfactant system. J. Membr. Sci. 2001;184:1–15. doi: 10.1016/S0376-7388(00)00604-9. [DOI] [Google Scholar]
- 207.Huang R.Y.M., Moon G.Y., Pal R. N-acetylated chitosan membranes for the pervaporation separation of alcohol/toluene mixtures. J. Membr. Sci. 2000;176:223–231. doi: 10.1016/S0376-7388(00)00443-9. [DOI] [Google Scholar]
- 208.Patil M.B., Aminabhavi T.M. Pervaporation separation of toluene/alcohol mixtures using silicalite zeolite embedded chitosan mixed matrix membranes. Sep. Purif. Technol. 2008;62:128–136. doi: 10.1016/j.seppur.2008.01.013. [DOI] [Google Scholar]
- 209.Won W., Feng X., Lawless D. Pervaporation with chitosan membranes: Separation of dimethyl carbonate/methanol/water mixtures. J. Membr. Sci. 2002;209:493–508. doi: 10.1016/S0376-7388(02)00367-8. [DOI] [Google Scholar]
- 210.Liu B., Cao Y., Wang T., Yuan Q. Preparation of Novel ZSM-5 Zeolite-Filled Chitosan Membranes for Pervaporation Separation of Dimethyl Carbonate/Methanol Mixtures. J. Appl. Polym. Sci. 2007;116:2117–2125. doi: 10.1002/app.26876. [DOI] [Google Scholar]
- 211.Lu L., Peng F., Jiang Z., Wang J. Poly(vinyl alcohol)/chitosan blend membranes for pervaporation of benzene/cyclohexane mixtures. J. Appl. Polym. Sci. 2006;101:167–173. doi: 10.1002/app.23158. [DOI] [Google Scholar]
- 212.Shen J., Chu Y., Ruan H., Wu L., Gao C., Van der Bruggen B. Pervaporation of benzene/cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes. J. Membr. Sci. 2014;462:160–169. doi: 10.1016/j.memsci.2014.03.040. [DOI] [Google Scholar]
- 213.Anjali Devi D., Smitha B., Sridhar S., Aminabhavi T.M. Dehydration of 1,4-dioxane through blend membranes of poly(vinyl alcohol) and chitosan by pervaporation. J. Membr. Sci. 2006;280:138–147. doi: 10.1016/j.memsci.2006.01.006. [DOI] [Google Scholar]
- 214.Smitha B., Dhanuja G., Sridhar S. Dehydration of 1,4-dioxane by pervaporation using modified blend membranes of chitosan and nylon 66. Carbohydr. Polym. 2006;66:463–472. doi: 10.1016/j.carbpol.2006.04.006. [DOI] [Google Scholar]
- 215.Reddy A.S., Kalyani S., Kumar N.S., Boddu V.M., Krishnaiah A. Dehydration of 1,4-dioxane by pervaporation using crosslinked calcium alginate-chitosan blend membranes. Polym. Bull. 2008;61:779–790. doi: 10.1007/s00289-008-1003-x. [DOI] [Google Scholar]
- 216.Ong Y.T., Ahmad A.L., Zein S.H.S., Sudesh K., Tan S.H. Poly(3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Sep. Purif. Technol. 2011;76:419–427. doi: 10.1016/j.seppur.2010.11.013. [DOI] [Google Scholar]
- 217.Li Q., Yu P., Zhu T., Zhang L., Li Q., Luo Y. Pervaporation performance of crosslinked PVA and chitosan membranes for dehydration of caprolactam solution. Desalin. Water Treat. 2010;16:304–312. doi: 10.5004/dwt.2010.1568. [DOI] [Google Scholar]
- 218.Lin W., Li Q., Zhu T. New chitosan/Konjac glucomannan blending membrane for application in pervaporation dehydration of caprolactam solution. J. Ind. Eng. Chem. 2012;18:934–940. doi: 10.1016/j.jiec.2011.09.008. [DOI] [Google Scholar]
- 219.Li Q., Lin W., Zhu T., Luo Y., Yu P. New chitosan/poly (acrylic acid) composite membrane for application in pervaporation dehydration of caprolactam solution. Desalin. Water Treat. 2012;43:43–51. doi: 10.1080/19443994.2012.672197. [DOI] [Google Scholar]
- 220.Sahu P.K., Sahu P.K., Gupta S.K., Agarwal D.D. Chitosan: An efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles. Ind. Eng. Chem. Res. 2014;53:2085–2091. doi: 10.1021/ie402037d. [DOI] [Google Scholar]
- 221.Safari J., Zarnegar Z., Sadeghi M., Azizi F. Chitosan-SO3H: An efficient and biodegradable catalyst for the green syntheses of 1,4-dihydropyridines. Curr. Org. Chem. 2016;20:2926–2932. doi: 10.2174/1385272820666160805112208. [DOI] [Google Scholar]
- 222.Guibal E., Vincent T., Navarro R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014;49:5505–5518. doi: 10.1007/s10853-014-8301-5. [DOI] [Google Scholar]
- 223.Bellich B., D’Agostino I., Semeraro S., Gamini A., Cesàro A. “The good, the bad and the ugly” of chitosans. Mar. Drugs. 2016;14:99. doi: 10.3390/md14050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lv D., Zhang M. O-carboxymethyl chitosan supported heterogeneous palladium and Ni catalysts for heck reaction. Molecules. 2017;22:150. doi: 10.3390/molecules22010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bradshaw M., Zou J., Byrne L., Swaminathan Iyer K., Stewart S.G., Raston C.L. Pd(ii) conjugated chitosan nanofibre mats for application in Heck cross-coupling reactions. Chem. Commun. 2011;47:12292–12294. doi: 10.1039/c1cc14717j. [DOI] [PubMed] [Google Scholar]
- 226.Hajipour A.R., Boostani E., Mohammadsaleh F. Proline-functionalized chitosan-palladium(ii) complex, a novel nanocatalyst for C-C bond formation in water. RSC Adv. 2015;5:24742–24748. doi: 10.1039/C5RA01187F. [DOI] [Google Scholar]
- 227.Baran T., Sargin I., Menteş A., Kaya M. Exceptionally high turnover frequencies recorded for a new chitosan-based palladium(II) catalyst. Appl. Catal. A Gen. 2016;523:12–20. doi: 10.1016/j.apcata.2016.05.012. [DOI] [Google Scholar]
- 228.Baran T., Menteş A. Microwave assisted synthesis of biarlys by C-C coupling reactions with a new chitosan supported Pd(II) catalyst. J. Mol. Struct. 2016;1122:111–116. doi: 10.1016/j.molstruc.2016.05.091. [DOI] [Google Scholar]
- 229.Zeng M., Sun X., Qi C., Zhang X.M. Preparation of a novel CuI/PCMS heterogeneous catalyst for heck-type cross-coupling reactions. Kinet. Catal. 2013;54:716–723. doi: 10.1134/S0023158413060141. [DOI] [Google Scholar]
- 230.Rafiee F., Hosseini S.A. CNC pincer palladium complex supported on magnetic chitosan as highly efficient and recyclable nanocatalyst in C─C coupling reactions. Appl. Organomet. Chem. 2018;32:1–10. doi: 10.1002/aoc.4519. [DOI] [Google Scholar]
- 231.Fakhri A., Naghipour A. Organometallic polymer-functionalized Fe3O4 nanoparticles as a highly efficient and eco-friendly nanocatalyst for C–C bond formation. Transit. Met. Chem. 2018;43:463–472. doi: 10.1007/s11243-018-0233-5. [DOI] [Google Scholar]
- 232.Jadhav S., Kumbhar A., Salunkhe R. Palladium supported on silica-chitosan hybrid material (Pd-CS@SiO2) for Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions. Appl. Organomet. Chem. 2015;29:339–345. doi: 10.1002/aoc.3290. [DOI] [Google Scholar]
- 233.Zeng M., Yuan X., Zuo S., Qi C. Novel chitosan-based/montmorillonite/palladium hybrid microspheres as heterogeneous catalyst for Sonogashira reactions. RSC Adv. 2015;5:37995–38000. doi: 10.1039/C5RA01902H. [DOI] [Google Scholar]
- 234.Affrose A., Suresh P., Azath I.A., Pitchumani K. Palladium nanoparticles embedded on thiourea-modified chitosan: A green and sustainable heterogeneous catalyst for the Suzuki reaction in water. RSC Adv. 2015;5:27533–27539. doi: 10.1039/C4RA14411B. [DOI] [Google Scholar]
- 235.Veisi H., Ghadermazi M., Naderi A. Biguanidine-functionalized chitosan to immobilize palladium nanoparticles as a novel, efficient and recyclable heterogeneous nanocatalyst for Suzuki-Miyaura coupling reactions. Appl. Organomet. Chem. 2016;30:341–345. doi: 10.1002/aoc.3437. [DOI] [Google Scholar]
- 236.Sophiphun O., Wittayakun J., Dhital R.N., Haesuwannakij S., Murugadoss A., Sakurai H. Gold/palladium bimetallic alloy nanoclusters stabilized by chitosan as highly efficient and selective catalysts for homocoupling of arylboronic acid. Aust. J. Chem. 2012;65:1238–1243. doi: 10.1071/CH12175. [DOI] [Google Scholar]
- 237.Hajipour A.R., Abolfathi P. Novel triazole-modified chitosan@nickel nanoparticles: Efficient and recoverable catalysts for Suzuki reaction. New J. Chem. 2017;41:2386–2391. doi: 10.1039/C6NJ03789E. [DOI] [Google Scholar]
- 238.Ruiz-Castillo P., Buchwald S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang L.-X., Wang Z.-W., Wang G.-S., Lin X.-D., Ren J.-G. Catalytic performance of chitosan-Schiff base supported Pd/Co bimetallic catalyst for acrylamide with phenyl halide. Polym. Adv. Technol. 2010;21:244–249. [Google Scholar]
- 240.Anuradha, Kumari S., Pathak D.D. Synthesis and development of Chitosan anchored copper(II) Schiff base complexes as heterogeneous catalysts for N-arylation of amines. Tetrahedron Lett. 2015;56:4135–4142. doi: 10.1016/j.tetlet.2015.05.049. [DOI] [Google Scholar]
- 241.Bodhak C., Kundu A., Pramanik A. An efficient and recyclable chitosan supported copper(II) heterogeneous catalyst for C-N cross coupling between aryl halides and aliphatic diamines. Tetrahedron Lett. 2015;56:419–424. doi: 10.1016/j.tetlet.2014.11.120. [DOI] [Google Scholar]
- 242.Nasir Baig R.B., Vaddula B.R., Gonzalez M.A., Varma R.S. N-Allylation of amines with allyl acetates using chitosan-immobilized palladium. RSC Adv. 2014;4:9103–9106. doi: 10.1039/C3RA47899H. [DOI] [Google Scholar]
- 243.Shen C., Xu J., Yu W., Zhang P. A highly active and easily recoverable chitosan@copper catalyst for the C-S coupling and its application in the synthesis of zolimidine. Green Chem. 2014;16:3007–3012. doi: 10.1039/C4GC00161C. [DOI] [Google Scholar]
- 244.Frindy S., El Kadib A., Lahcini M., Primo A., García H. Copper Nanoparticles Stabilized in a Porous Chitosan Aerogel as a Heterogeneous Catalyst for C-S Cross-coupling. ChemCatChem. 2015;7:3307–3315. doi: 10.1002/cctc.201500565. [DOI] [Google Scholar]
- 245.Chiessi E., Pispisa B. Polymer-supported catalysis: Oxidation of catecholamines by Fe(III) and Cu(II) complexes immobilized to chitosan. J. Mol. Catal. 1994;87:177–193. doi: 10.1016/0304-5102(93)E0235-9. [DOI] [Google Scholar]
- 246.Huang G., Guo C.C., Tang S.S. Catalysis of cyclohexane oxidation with air using various chitosan-supported metallotetraphenylporphyrin complexes. J. Mol. Catal. A Chem. 2007;261:125–130. doi: 10.1016/j.molcata.2006.08.014. [DOI] [Google Scholar]
- 247.Huang G., Luo J., Cai C.C., Guo Y.A., Luo G.W. The influence of chitosan on the performances of mono and μ-oxo dimeric iron tetraphenylporphyrins catalysts for aerobic oxidation of toluene. Catal. Commun. 2008;9:1882–1885. doi: 10.1016/j.catcom.2008.03.007. [DOI] [Google Scholar]
- 248.Di Giuseppe A., Crucianelli M., Passacantando M., Nisi S., Saladino R. Chitin- and chitosan-anchored methyltrioxorhenium: An innovative approach for selective heterogeneous catalytic epoxidations of olefins. J. Catal. 2010;276:412–422. doi: 10.1016/j.jcat.2010.10.003. [DOI] [Google Scholar]
- 249.Hatefi Ardakani M., Moghadam M., Saeednia S., Pakdin-Parizi Z. Epoxidation of alkenes with NaIO4 catalyzed by an efficient and reusable natural polymer-supported ruthenium(III) salophen catalyst. J. Iran. Chem. Soc. 2016;13:631–636. doi: 10.1007/s13738-015-0774-x. [DOI] [Google Scholar]
- 250.Ghiaci M., Dorostkar N., Martínez-Huerta M.V., Fierro J.L.G., Moshiri P. Synthesis and characterization of gold nanoparticles supported on thiol functionalized chitosan for solvent-free oxidation of cyclohexene with molecular oxygen. J. Mol. Catal. A Chem. 2013;379:340–349. doi: 10.1016/j.molcata.2013.08.030. [DOI] [Google Scholar]
- 251.Shaabani A., Boroujeni M.B., Sangachin M.H. Cobalt-chitosan: Magnetic and biodegradable heterogeneous catalyst for selective aerobic oxidation of alkyl arenes and alcohols. J. Chem. Sci. 2015;127:1927–1935. doi: 10.1007/s12039-015-0959-y. [DOI] [Google Scholar]
- 252.Murugadoss A., Sakurai H. Chitosan-stabilized gold, gold-palladium, and gold-platinum nanoclusters as efficient catalysts for aerobic oxidation of alcohols. J. Mol. Catal. A Chem. 2011;341:1–6. doi: 10.1016/j.molcata.2011.03.019. [DOI] [Google Scholar]
- 253.Shen C., Qiao J., Zhao L., Zheng K., Jin J., Zhang P. An efficient silica supported Chitosan@vanadium catalyst for asymmetric sulfoxidation and its application in the synthesis of esomeprazole. Catal. Commun. 2017;92:114–118. doi: 10.1016/j.catcom.2017.01.018. [DOI] [Google Scholar]
- 254.Barskiy D.A., Kovtunov K.V., Primo A., Corma A., Kaptein R., Koptyug I.V. Selective Hydrogenation of 1,3-Butadiene and 1-Butyne over a Rh/Chitosan Catalyst Investigated by using Parahydrogen-Induced Polarization. ChemCatChem. 2012;4:2031–2035. doi: 10.1002/cctc.201200414. [DOI] [Google Scholar]
- 255.Wu L.H., Nordin M.R., Yaakob Z., Liew K.Y., Li J.L. Effects of Fe on catalytic hydrogenation of palm oil in aqueous solution. Chem. Pap. 2017;71:119–126. doi: 10.1007/s11696-016-0063-x. [DOI] [Google Scholar]
- 256.Adlim M., Bakar M.A. The properties of Pd/Au bimetallic colloidal catalysts stabilized by chitosan and prepared by simultaneous and stepwise chemical reduction of the precursor ions. Kinet. Catal. 2013;54:586–596. doi: 10.1134/S0023158413050017. [DOI] [Google Scholar]
- 257.Zhou J., Dong Z., Yang H., Shi Z., Zhou X., Li R. Pd immobilized on magnetic chitosan as a heterogeneous catalyst for acetalization and hydrogenation reactions. Appl. Surf. Sci. 2013;279:360–366. doi: 10.1016/j.apsusc.2013.04.113. [DOI] [Google Scholar]
- 258.Liu B., Zhou H., Li Y., Wang J. Asymmetric transfer hydrogenation of acetophenone promoted by chitosan ester ruthenium complex. Chin. J. Org. Chem. 2014;34:2554–2558. doi: 10.6023/cjoc201406025. [DOI] [Google Scholar]
- 259.Szőllősi G., Kolcsár V.J. Highly Enantioselective Transfer Hydrogenation of Prochiral Ketones Using Ru(II)-Chitosan Catalyst in Aqueous Media. ChemCatChem. 2019;11:820–830. doi: 10.1002/cctc.201801602. [DOI] [Google Scholar]
- 260.Viswanadhan M., Potdar A., Divakaran A., Badiger M., Rode C. Product distribution in hydrogenation of styrene oxide over Pd/chitosan catalyst. Res. Chem. Intermed. 2016;42:7581–7595. doi: 10.1007/s11164-016-2554-3. [DOI] [Google Scholar]
- 261.Ríos-Caloch G., Santes V., Escobar J., Pérez-Romo P., Díaz L., Lartundo-Rojas L. Effect of Chitosan on the Performance of NiMoP-Supported Catalysts for the Hydrodesulfurization of Dibenzothiophene. J. Nanomater. 2016;2016:13. doi: 10.1155/2016/4047874. [DOI] [Google Scholar]
- 262.Lee S.B., Jeong G.T. Catalytic Conversion of Chitosan to 5-Hydroxymethylfurfural Under Low Temperature Hydrothermal Process. Appl. Biochem. Biotechnol. 2015;176:1151–1161. doi: 10.1007/s12010-015-1636-9. [DOI] [PubMed] [Google Scholar]
- 263.Li M., Zang H., Feng J., Yan Q., Yu N., Shi X., Cheng B. Efficient conversion of chitosan into 5-hydroxymethylfurfural via hydrothermal synthesis in ionic liquids aqueous solution. Polym. Degrad. Stab. 2015;121:331–339. doi: 10.1016/j.polymdegradstab.2015.09.009. [DOI] [Google Scholar]
- 264.Wang Y., Pedersen C.M., Deng T., Qiao Y., Hou X. Direct conversion of chitin biomass to 5-hydroxymethylfurfural in concentrated ZnCl2 aqueous solution. Bioresour. Technol. 2013;143:384–390. doi: 10.1016/j.biortech.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 265.Chen X., Yang H., Yan N. Shell Biorefinery: Dream or Reality? Chem. Eur. J. 2016;22:13402–13421. doi: 10.1002/chem.201602389. [DOI] [PubMed] [Google Scholar]
- 266.Bozell J.J., Holladay J.E., Johnson D., White J.F. [(accessed on 1 September 2020)];Top Value Added Chemicals from Biomass Volume II: Results of Screening for Potential Candidates from Biorefinery Lignin Produced. 2007 Available online: https://www.osti.gov/biblio/1216434.
- 267.van Putten R.-J., de Vries J.G., van der Waal J.C., de Jong E., Rasrendra C.B., Heeres H.J. Hydroxymethylfurfural, A Versatile Platform Chemical Made from. Chem. Rev. 2013;113:1499–1597. doi: 10.1021/cr300182k. [DOI] [PubMed] [Google Scholar]
- 268.Szabolcs Á., Molnár M., Dibó G., Mika L.T. Microwave-assisted conversion of carbohydrates to levulinic acid: An essential step in biomass conversion. Green Chem. 2013;15:439–445. doi: 10.1039/C2GC36682G. [DOI] [Google Scholar]
- 269.Omari K.W., Besaw J.E., Kerton F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012;14:1480–1487. doi: 10.1039/c2gc35048c. [DOI] [Google Scholar]
- 270.Jeong G.T. Production of levulinic acid from glucosamine by dilute-acid catalyzed hydrothermal process. Ind. Crop. Prod. 2014;62:77–83. doi: 10.1016/j.indcrop.2014.08.006. [DOI] [Google Scholar]
- 271.Ohmi Y., Nishimura S., Ebitani K. Synthesis of α-amino acids from glucosamine-HCl and its derivatives by aerobic oxidation in water catalyzed by au nanoparticles on basic supports. ChemSusChem. 2013;6:2259–2262. doi: 10.1002/cssc.201300303. [DOI] [PubMed] [Google Scholar]