Abstract
In recent years, multitarget drugs for neurological diseases such as Alzheimer’s disease have been developed and well researched. Many studies have revealed that multitarget drugs are also useful for lung cancer and respiratory diseases. Pemetrexed is a multitargeted antifolate with strong antitumor activity against mesothelioma and lung adenocarcinoma. Crizotinib is an ATP-competitive tyrosine kinase inhibitor that targets c-MET, ROS1, and ALK. Alectinib is known as an ALK inhibitor but also targets LTK, CHEK2, FLT3, PHKG2, and RET. Sorafenib is a tyrosine kinase inhibitor that targets RAF kinase, KIT, VEGFR, PDGFR1β, FLT3, and RET. Nintedanib is a multiple tyrosine kinase inhibitor that targets FGFR, PDGFR, and VEGFR. In this review, we summarize the mechanisms of action of multitarget therapies and report the results of the latest clinical trials.
Keywords: multitarget, pemetrexed, ALK inhibitors, angiogenesis inhibitors
1. Introduction
Important discoveries of new drugs have been made based on the strategy of targeting one gene with one drug in one disease [1]. This strategy is considered important to prevent the disadvantages of accidental targeting of other substances. Accordingly, drugs that interact with multiple targets have long been considered undesirable, partly because they have been associated with adverse side effects. However, owing to recent discoveries indicating the complexity of intractable diseases such as cancer and neurological diseases, single-target drugs are thought to not be sufficiently effective, and since early 2000, multitarget drugs have been rapidly developed [2]. Multitarget drugs have synergistic effects as they exhibit different modes of action, which lead to improved adherence, because the number of drugs administered to patients can be reduced. For example, because the combination of venlafaxine and fluoxetine to treat depression increases the side effects of anticholinergic activity, a multitarget drug may lead to reduced side effects [3,4,5].
Recently, multitargeted ligands have been studied in various diseases such as Alzheimer’s disease, depression, poisoning, glaucoma, and nonalcoholic steatohepatitis (NASH) [6]. In this review, we summarize the mechanisms of action of multitarget therapies and the results of the latest clinical trials and introduce novel compounds and discuss the limitations of multitarget drugs.
2. Pemetrexed
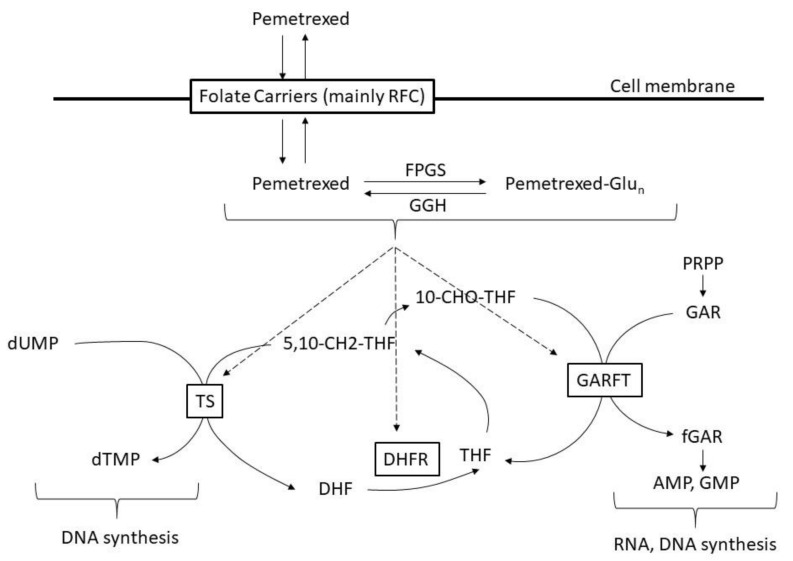
Pemetrexed is a folate antimetabolite that exhibits strong and broad antitumor activity by inhibiting multiple folate-metabolizing enzyme pathways. Pemetrexed is mainly taken up into cells by the reduced folate carrier (RFC) and undergoes polyglutamine oxidation by folyl polyglutamate synthase (FPGS). When pemetrexed is subjected to polyglutamine oxidation, its intracellular retention is increased, and its affinity for certain folate-metabolizing enzymes is also increased. Pemetrexed and its polyglutamates inhibit multiple folate-metabolizing enzymes involved in thymine and purine nucleotide biosynthetic pathways, such as thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), and thus cause imbalance in the cellular nucleotide pool, inhibit DNA and RNA synthesis, and induce growth inhibition and cell death [7] (Figure 1 [8,9]).
Figure 1.
Mechanism of pemetrexed. RFC: reduced folate carrier, FPGS: folypoly-gamma-glutamate synthetase, GGH: gamma-glutamyl hydrolase, Glun: glutamate, dUMP: deoxyyuridine monophosphate, dTMP: deoxythymidine monophosphate, 5,10-CH2-THF: 5,10-methenyl-tetrahydrofolate, DHF: dihydrofolate, THF: tetrahydrofolate, 10-CHO-THF: 10-formyl tetrahydrofolate, PRPP: phosphoribosyl pyrophosphate, GAR: glycinamide ribonucleotide, fGAR: formylglycinamide ribonucleotide, AMP: adenosine monophosphate, GMP: guanosine monophosphate.
Pemetrexed is currently used for malignant pleural mesothelioma and unresectable advanced/recurrent non-small cell lung cancer (NSCLC). Recently, low TS expression was reported to improve the therapeutic effect of chemotherapies including pemetrexed in NSCLC patients [10,11], and it is thought that further research will allow pemetrexed to be used for tailored treatment.
Malignant mesothelioma, which arises from the mesothelial cells that line the inner surface of the chest cavity, is associated with asbestos inhalation. A phase II study of pemetrexed alone showed a response rate (RR) of 14.1% and a median survival time (MST) of 10.7 months [12]. This result is better than that obtained with cisplatin [13] or gemcitabine [14]. A subsequent phase III trial comparing cisplatin alone and combination therapy with cisplatin and pemetrexed (pemetrexed/cisplatin) was conducted in 20 countries, including the United States and countries in Europe, and revealed that survival with pemetrexed/cisplatin treatment was superior to that with cisplatin alone (MST of 12.1 months vs. 9.3 months in the pemetrexed/cisplatin arm and cisplatin alone arm, respectively; p = 0.020). The median time to progression was significantly longer in the pemetrexed/cisplatin arm than in the cisplatin alone arm (5.7 months vs. 3.9 months, p = 0.001). The RR in the pemetrexed/cisplatin arm was higher than that in the cisplatin alone arm (41.3% vs. 16.7%, p < 0.0001) [15]. Additionally, in this trial, a relationship between folic acid and vitamin B12 deficiencies and the occurrence of severe toxicity graded by the Common Terminology Criteria for Adverse Events (CTCAE, grade 4 myelosuppression, grade 3/4 diarrhea, mucositis, infection, etc.) was found, and supplementation with folic acid and vitamin B12 reduced toxicity. Therefore, beginning in the middle of the test, investigators used supplemental vitamin B12 and folic acid. Pemetrexed/cisplatin was subsequently approved by the US Food and Drug Administration (FDA) in February 2004 and is now the standard treatment for malignant mesothelioma. In addition, the RR was 32%, and the MST was promising at 451 days in a phase I study of pemetrexed/carboplatin [16]. In a phase II trial of pemetrexed/carboplatin, the RR ranged from 18.6% to 25%, and the MST was 12.7–14.1 months, showing favorable results. Thus, pemetrexed/carboplatin is a treatment option against malignant mesothelioma [17,18].
A phase III study comparing pemetrexed and docetaxel in lung cancer conducted in patients who were previously treated with platinum-based chemotherapy for NSCLC revealed that pemetrexed has an efficacy equivalent to that of docetaxel but is less toxic than docetaxel [19]. Pemetrexed was then approved as a second-line treatment for NSCLC in the US in August 2004 and in Europe, in September 2004. Another phase III trial comparing pemetrexed/cisplatin with gemcitabine/cisplatin in chemo-naïve NSCLC patients (JMDB trial) showed a prolonged MST in the pemetrexed/cisplatin group with a significant difference in MST between patients with non-squamous cell carcinoma and squamous cell carcinoma [20]. Based on this result, pemetrexed/cisplatin was approved in Europe in April 2008 and in the US, in September 2008 as a first-line treatment for NSCLC. Furthermore, a double-blind, randomized phase III trial (PARAMOUNT trial) was conducted to evaluate the efficacy of maintenance therapy with pemetrexed after induction therapy with pemetrexed/cisplatin. This trial was conducted in patients who were untreated for stage IIIB/IV NSCLC and responded to four courses of induction therapy with pemetrexed/cisplatin. The results showed a significantly prolonged progression-free survival (PFS) after maintenance therapy compared with placebo therapy (4.4 months vs. 2.8 months, respectively; hazard ratio (HR): 0.62, 95% confidence interval (CI): 0.50–0.73, p < 0.0001) and significantly better overall survival (OS) in the maintenance group than the placebo group (13.9 months vs. 11.0 months, respectively; HR: 0.78, 95%CI: 0.64–0.96, p = 0.0195). The patient’s quality of life (QOL) was not reduced despite grade 3/4 anemia (pemetrexed group: 6.4% vs. placebo group: 0.6%); neutropenia (5.8% vs. 0%); fatigue (4.7% vs. 1.1%); leukopenia (2.2% vs. 0%); nausea (0.6% vs. 0%); or vomiting (0.3% vs. 0%). Based on this study, after four courses of combination pemetrexed/cisplatin therapy, continuing maintenance therapy with pemetrexed is recommended for patients with no disease progression and acceptable toxicity [21]. Recently, a phase III trial was conducted to evaluate the efficacy of addition of pembrolizumab, an immune checkpoint inhibitor, to pemetrexed/platinum-based drugs in patients with PS 0-1 stage IV NSCLC without epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) translocation (KEYNOTE-189) [22]. In the interim analysis, PFS and OS, the primary endpoints, showed an HR of 0.52 (8.8 months vs. 4.9 months, 95%CI: 0.43–0.64, p < 0.0001) and an HR of 0.49 (median not reached vs. 11.3 months, 95%CI: 0.38–0.64, p < 0.0001), respectively. Table 1 summarizes the results of clinical trials of pemetrexed (Table 1).
Table 1.
Results of clinical trials of pemetrexed.
| Trial | Phase | Line | Type | n | RR(CR/PR) | DCR(CR/PR/SD) | Time to Progression | PFS | MST or OS |
|---|---|---|---|---|---|---|---|---|---|
| Scagliotti G.V. 2003 PEM alone |
II | 1st line | MPM | 64 | 14.1% | 65.6% | 4.7 m | 10.7 m | |
| Vogelzang N.J. 2003 PEM/CDDP vs. CDDP |
III | 1st line | MPM | 456 | 41.3% vs. 16.7% | 5.7 m vs. 3.9 m | 12.1 m vs. 9.3 m | ||
| Hughes A. 2002 PEM/CBDCA |
I | 1st line | MPM | 27 | 32% | 88% | 305 d | 451 d | |
| Ceresoli G.L. 2006 PEM/CBDCA |
II | 1st line | MPM | 102 | 18.6% | 65.7% | 6.5 m | 12.7 m | |
| Castagneto B. 2008 PEM/CBDCA |
II | 1st line | MPM | 76 | 25% | 63% | 8 m | 14.1 m | |
| Hanna N. 2004 PEM vs. DTX |
III | 2nd line | NSCLC | 571 | 9.1% vs. 8.8% | 3.4 m vs. 3.5 m | 2.9 m vs. 2.9 m | 8.3 m vs. 7.9 m | |
| Scagliotti G.V. 2008 CDDP/PEM vs. CDDP/GEM (JMDB trial) |
III | 1st line | NSCLC | 1725 | 30.6% vs. 28.2% | 4.8 m vs. 5.1 m | 10.3 m vs. 10.3 m | ||
| Non Sq | 1000 | 5.3 m vs. 4.7 m | 11.8 m vs. 10.4 m | ||||||
| Sq | 473 | 4.4 m vs. 5.5 m | 9.4 m vs. 10.8 m | ||||||
| Paz-Ares L.G. 2013 PEM maintenance vs. placebo (PARAMOUNT trial) |
III | Induction | NSCLC | 939 | 4.4 m vs. 2.8 m | 13.9 m vs. 11.0 m | |||
| Gandhi L. 2018 Platinum/PEM/ Pembrolizumab vs. Platinum/PEM/ placebo |
III | 1st line | NSCLC | 616 | 47.6% vs. 18.9% | 84.6% vs. 70.4% | 8.8 m vs. 4.9 m | Not reachedvs 11.3 m |
MPM: malignant pleural mesothelioma, NSCLC: non-small cell lung cancer, NonSq: nonsquamous cell carcinoma, RR: response rate, CR: complete response, PR: partial response, DCR: disease control rate, SD: stable disease, PFS: progression free survival, MST: medial survival time, OS: overall survival, PEM: pemetrexed, CDDP: cisplatin, CBDCA: carboplatin, DTX: docetaxel, GEM: gemcitabine.
3. Crizotinib
ALK is a cell membrane protein with a transmembrane domain, and human ALK consists of 1620 amino acids. Since ALK contains a tyrosine kinase domain in its intracellular region, it is thought to belong to the receptor tyrosine kinase family, the members of which are activated in response to extracellular stimulation. ALK is an orphan member of the insulin superfamily of receptor tyrosine kinases (RTKs), which are normally expressed in only the central nervous system, small intestine, and testis [23,24]. In 1994, it was reported that the nucleophosmin ALK (NPM1-ALK) fusion gene was present on the t (2; 5) reciprocal translocation in anaplastic large cell malignant lymphoma, and in 2007, a fusion protein consisting of echinoderm microtubule-associated protein like-4 (EML4) and ALK was found in 6.7% of NSCLC patients [25]. ALK-positive NSCLC patients were mostly non-smokers or light smokers and relatively young, had adenocarcinoma, and generally did not exhibit gene mutations such as the EGFR and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations [26].
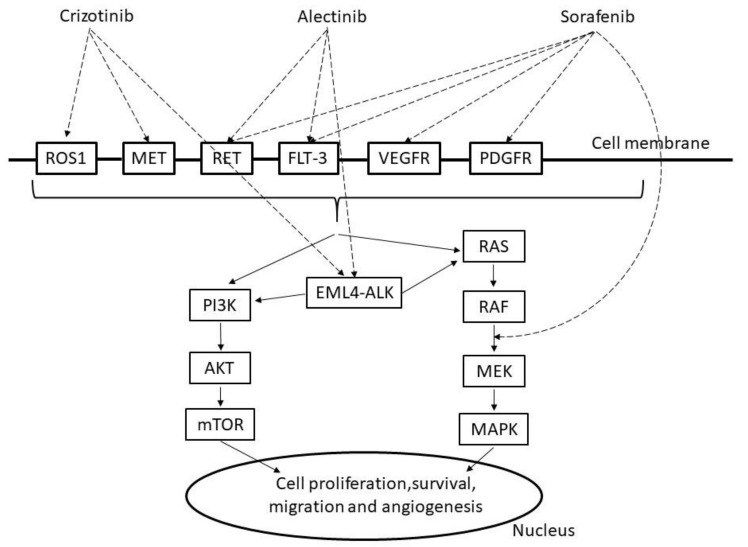
Crizotinib is an ATP-competitive tyrosine kinase inhibitor (TKI) that selectively inhibits the activities of anaplastic lymphoma kinase (ALK); c-MET/hepatocyte growth factor receptor (HGFR); and c-ROS oncogene 1 (ROS1) and their oncogenic variants (ALK fusion protein, c-MET/HGFR variant, and ROS1 fusion protein), leading to inhibition of phosphoinositide 3 kinase (PI3K)/v-act murine thymoma viral oncogene homolog (AKT)/mammalian target of rapamycin (mTOR) or rat sarcoma protein (RAS)/v-raf murine viral oncogene homolog (RAF)/mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) kinase MAPK (MEK)/MAPK signaling (Figure 2). The ALK fusion protein and some ROS1 fusion proteins are constitutively activated by dimerization and activate many downstream signaling factors to promote the cell cycle, proliferation, and survival [27]. Crizotinib is believed to exhibit antitumor effects by inhibiting the kinases ALK, ROS1, and c-MET and suppressing the activation of these factors, tumor cell proliferation, and tumor angiogenesis.
Figure 2.
Mechanism of crizotinib, alectinib and sorafenib. ROS1: c-ROS oncogene1, MET: mesenchymal epithelial transition factor, RET: proto-oncogene ret, FLT-3: Fms-like tyrosine protein kinase, VEGFR: vascular endothelial growth factor receptor, PDGFR: platelet-derived growth factor receptors, PI3K: phosphoinositide 3 kinase, AKT: v-act murine thymoma viral oncogene homolog, mTOR: mammalian target of rapamycin, EML4-ALK: echinoderm microtubule associated protein like 4, RAS: rat sarcoma protein, RAF: v-raf murine viral oncogene homolog, MAPK: mitogen activated protein kinase, MEK:,MAPK/extracellular signal regulated kinase(ERK) kinase.
An overseas phase I study (PROFILE 1001) [28] and an international joint phase II study (PROFILE 1005) were conducted in patients with ALK-positive NSCLC and showed favorable results by the fluorescence in situ hybridization (FISH) method. Then, an international phase III study (PROFILE 1007) conducted in previously treated ALK-positive NSCLC patients to compare crizotinib with conventional standard treatments using docetaxel or pemetrexed showed a significantly prolonged PFS [29]. Further, even for patients with untreated ALK-positive NSCLC, a strong antitumor effect with an RR of 74% and a median PFS of 10.9 months was observed (PROFILE 1014) [30]. Based on these results, crizotinib became a first-line ALK inhibitor for use in ALK-positive NSCLC. Although most of the adverse events such as digestive symptoms and visual impairment were grade 1, other serious adverse events such as interstitial pneumonia, liver injury, and QT prolongation were also reported. Notably crizotinib acts as a TKI of ALK as well as ROS1 [31]. In the expanded cohort of the PROFILE 1001 trial, three out of 50 patients with ROS1-positive NSCLC had a complete response, as determined by the Response Evaluation Criteria in Solid Tumors, and 33 patients had a partial response with an RR of 72% (95%CI: 58–84%), median response time of 17.6 months (95%CI: 14.5 months to not reached), and a median PFS of 19.2 months (95%CI: 14.4 months to not reached) [32]. Based on these results, the expanded use of crizotinib for ROS1-positive NSCLC was approved by the US FDA in March 2016 and the European EMA, in August of the same year. A clinical phase II trial of crizotinib for ROS1-positive NSCLC was conducted in four East Asian countries—Japan, China, South Korea, and Taiwan. A total of 127 patients were enrolled, and among these patients, 17 showed complete response and 74 showed partial response, with an RR of 71.7% (95%CI: 63–79.3%). The median duration of response was 19.7 months (95%CI: 14.1 months to not reached), and the median PFS was 15.9 months (95%CI: 12.9–24 months), demonstrating the high efficacy of crizotinib [33]. Based on these results, an application to extend the use of crizotinib to ROS1 fusion gene-positive unresectable advanced recurrent NSCLC was filed in Japan.
4. Alectinib
Alectinib, an ALK inhibitor developed in Japan, is a low-molecular-weight compound that is also effective for cell lines with the L1196M (gatekeeper) and C1156Y mutations, which have been implicated in resistance to crizotinib [34]. From the results of crystal structure analysis, it was confirmed that alectinib binds the AFG-binding site DFG-in of ALK. Alectinib was originally regarded as an ALK-TKI with a high selectivity for ALK, but Kodama et al. investigated 451 biochemical kinases and found that in addition to ALK and leukocyte receptor tyrosine kinase (LTK), checkpoint kinase (CHEK2), Fms-like tyrosine protein kinase (FLT3) (D835Y), phosphorylase kinase gamma submit 2 (PHKG2), proto-oncogene ret (RET), and RET (M918T) were also inhibited. In particular, RET kinase activity was strongly inhibited, leading to blocking PI3K or RAS signaling [35]. Notably, since alectinib does not block vascular endothelial growth factor receptors (VEGFR2), unlike multikinase inhibitors, alectinib has fewer side effects associated with its antiangiogenetic properties [36].
Phase I/II clinical trials of alectinib include the AF-001JP trial, targeting crizotinib in untreated cases, and the AF-002JG trial, targeting crizotinib in treated cases. Both of these were single-group trials, and these trials showed high RRs of 93.5% in untreated cases and 55% in previously treated cases [37,38]. In the J-ALEX trial, a phase III trial conducted in Japan, crizotinib was compared with alectinib as a first-line treatment, which showed an RR of 92% in the alectinib group vs. 79% in the crizotinib group [39]. The final report showed a median PFS of 10.2 months with crizotinib vs. 34.1 months with alectinib (HR: 0.37, 95%CI: 0.26–0.52) in 2019. Furthermore, PFS was significantly improved in the alectinib group compared to the crizotinib group [40]. Subsequently, in an international phase III clinical trial (ALEX trial), crizotinib and alectinib were compared as first-line treatments for ALK-positive NSCLC. The median PFS was 11.1 months in the crizotinib group, and the median PFS was not reached in the alectinib group (HR: 0.47, 95%CI: 0.34–0.65) [41]. The results of the J-ALEX study showed that the main adverse events that occurred with alectinib were constipation, nasopharyngitis, and dysgeusia, and fewer adverse events of grade 3 or higher were reported in the alectinib group (36.9%) than in the crizotinib group (60.6%). Table 2 summarizes the results of clinical trials of crizotinib and alectinib (Table 2).
Table 2.
Results of clinical trials of crizotinib and alectinib.
| Trial | Phase | Line | Type | n | RR(CR/PR) | DCR(CR/PR/SD) | PFS | MST or OS | |
|---|---|---|---|---|---|---|---|---|---|
| Crizotinib | Kwak E.L. 2010 (PROFILE 1001) |
I | 1st line | ALK-positive NSCLC | 82 | 57% | 90% | 6.4 m | |
| Shaw A.T. 2013 Crizotinib vs. PEM or DTX (PROFILE 1007) |
III | 2nd line | ALK-positive LC | 347 | 65% vs. 20% | 84% vs. 56% | 7.7 m vs. 3.0 m | 20.3 m vs. 22.8 m | |
| Solomon B.J. 2014 Crizotinib vs. PEM/platinum (PROFILE 1014) |
III | 1st line | ALK-positive NSCLC(NonSq) | 343 | 74% vs. 45% | 91% vs. 82% | 10.9 m vs. 7.0 m | Not reached | |
| Shaw A.T. 2014 Crizotinib alone |
I | 1st line | ROS-1-positive NSCLC | 50 | 72% | 90% | 19.2 m | Not reached | |
| Wu Y-L. 2018 Crizotinib alone |
II | 4th line or later | ROS-1-positive NSCLC | 127 | 71.7% | 80.3%(16 w) | 15.9 m | 32.5 m | |
| Alectinib | Seto T. 2013 Alectinib alone (AF-001JP study) |
I | 3rd line or later | ALK-positive NSCLC(ALK inhibitor-naïve) | 24 | ||||
| II | 2nd line or later | 46 | 93.5% | 95.7% | |||||
| Gadgeel S.M. 2014 (AF-002JG trial) Alectinib alone |
I/II | any | ALK-positive NSCLC(crizotinib-treated) | 47 | 55% | 91% | |||
| With CNS metastases | 21 | 52% | 90% | ||||||
| Hida T. 2017 Alectinib vs. crizotinib (J-ALEX trial) |
III | 1–2nd line | ALK-positive NSCLC(ALK inhibitor-naïve Japanese) | 207 | 92% vs. 79% | 96% vs. 92% | Not reached vs. 10.2 m | Not reached | |
| Nakagawa K. 2020 Alectinib vs. crizotinib (J-ALEX trial) |
III | 1–2nd line | ALK-positive NSCLC(ALK inhibitor-naïve Japanese) | 207 | 34.1 m vs. 10.2 m | Not reached vs. 43.7 m | |||
| Peters S. 2017 Alectinib vs. crizotinib (ALEX trial) |
III | 1st line | ALK-positive NSCLC | 303 | 82.9% vs. 75.5% | 89% vs. 91% | Not reached vs. 11.1 m | Not reached |
NSCLC: non-small cell lung cancer, NonSq: nonsquamous cell carcinoma, CNS: central nervous system, RR: response rate, CR: complete response, PR: partial response, DCR: disease control rate, SD: stable disease, PFS: progression free survival, MST: medial survival time, OS: overall survival, PEM: pemetrexed, DTX: docetaxel.
5. Sorafenib
Angiogenesis consists of multiple processes including sprouting, invasion, migration, proliferation, lumen formation, and the maturation of endothelial cells from existing vascular endothelial cells and vascular endothelial progenitor cells, each of which is regulated by single or multiple angiogenic factors. In addition, the extracellular matrix, adhesion molecules, and various proteases have important functions in the process of angiogenesis [42]. Tumors measuring up to a few millimeters in size can acquire the oxygen and nutrients they need by spreading from their environment. However, when the size of the tumor becomes large, blood vessels (tumor blood vessels) that supply oxygen and nutrients to the tumor are required [43]. Many tumors secrete angiogenic factors to build tumor blood vessels; among the many angiogenic factors, the VEGF family is the most studied. The VEGF signaling pathway consists of three subtypes of receptor tyrosine kinases (VEGFR-1, VEGFR-2, and VEGFR-3); five subtypes (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E); and two subtypes of placental growth factor (PlGF-1 and P1GF-2) as ligands [44].
The angiogenic effect of VEGF is thought to be exerted mainly through VEGFR2 on the vascular endothelium, and VEGF is a target molecule for many drugs. Specifically, it is thought to promote growth, survival, acquisition of migration ability, and acquisition of invasion ability of vascular endothelial cells, and a new blood vessel for the tumor is created [45]. Furthermore, VEGF enhances the permeability of existing blood vessels to create a microenvironment in which vascular endothelial cells can easily migrate and promote the chemotaxis of vascular endothelial cells and progenitor cells of pericytes to promote tumor angiogenesis. As in other carcinomas like colon cancer and gastric cancers, overexpression of VEGF is a poor prognostic factor for lung cancer [46]. Tumor angiogenesis inhibitors can be broadly classified into two groups: drugs that inhibit the binding of VEGF-A and VEGFR-2 and multikinase inhibitors, which are small-molecule compounds that inhibit the kinase activity of VEGFR. The VEGF-A or VEGFR-2 inhibitors that have been approved for the treatment of NSCLC in Japan include bevacizumab, which binds VEGF-A and inhibits its activity, and ramucirumab, which binds VEGFR-2 and inhibits its activity. Pazopanib, regorafenib, sorafenib, sunitinib, and nintedanib among others are known to be multikinase inhibitors [47,48,49]. Pazopanib is used in soft tissue tumors and renal cell carcinoma; regorafenib is used in rectal cancer and gastrointestinal stromal tumors (GISTs); and sunitinib is used in GIST, renal cell carcinoma, and pancreatic neuroendocrine tumors.
Sorafenib is a multitargeted TKI that targets v-raf murine viral oncogene homolog (RAF) kinase, c-KIT, VEGFR, platelet-derived growth factor receptors (PDGFR)-1β, FLT-3, and RET leading to inhibition of PI3K or RAS signaling [50,51]. It is currently approved by the FDA for renal cell cancer (2005), hepatocellular carcinoma (2007), and thyroid cancer (2013). Adverse events of sorafenib include hypertension, skin disorders (particularly hand-foot syndrome), liver disorders, elevated lipase and amylase levels, and interstitial pneumonia. In NSCLC, a single-agent phase II clinical trial of sorafenib was conducted in 54 previously treated patients with a schedule of 400 mg of sorafenib twice daily. Although neither complete nor partial response was observed in 51 evaluable patients, stable disease was observed in 30 patients (58.5%), of which 15 (28.8%) also showed tumor shrinkage. The median PFS was 2.7 months, and the median OS was 6.7 months. The main adverse events of grade 3 or higher were hand-foot syndrome, hypertension, fatigue, and diarrhea. Death from pulmonary hemorrhage was observed in one patient with squamous cell carcinoma [52]. Subsequently, Paz-Ares et al. published the MISSION study confirming the efficacy of sorafenib as a third/fourth-line treatment in patients with advanced and recurrent NSCLC. Although PFS was clearly prolonged with sorafenib compared to placebo, the OS did not change. PFS was prolonged in both patients with wild-type KRAS and patients with KRAS mutations, but OS was unchanged and has not been clinically used [53]. To examine sorafenib as a combination therapy with an existing standard treatment regimen, a phase III clinical trial (ESCAPE trial) for untreated NSCLC was conducted to evaluate the effect of the addition of sorafenib to paclitaxel/carboplatin therapy. However, because the interim analysis did not show extension of the OS, which was the primary endpoint, and an increase in mortality was observed in squamous cell carcinoma, the trial was terminated early [54].
6. Nintedanib
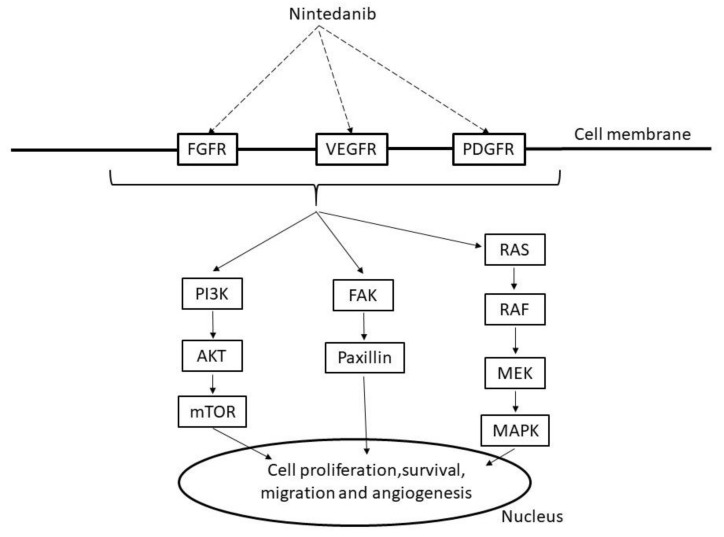
Nintedanib is an antifibrotic drug that inhibits multiple tyrosine kinases, including fibroblast growth factor receptors (FGFRs), PDGFRs, and VEGFRs, including PDGFRα, PDGFRβ, FGFR1, FGFR12, VEGFR1, VEGFR2, and VEGFR3, leading to PI3K, RAS, or focal adhesion kinase (FAK)/paxillin signaling (Figure 3) [55,56]. Nintedanib also inhibits nonreceptor kinases such as FLT-3, RET, lymphocyte-specific tyrosine kinase (LCK), tyrosine-protein kinase lyn (LYN), and proto-oncogene tyrosine protein kinase src (SRC) [57].
Figure 3.
Mechanism of nintedanib. FGFR: fibroblast growth factor receptors, VEGFR: vascular endothelial growth factor receptor, PDGFR: platelet-derived growth factor receptors, PI3K: phosphoinositide 3 kinase, AKT: v-act murine thymoma viral oncogene homolog, mTOR: mammalian target of rapamycin, FAK: focal adhesion kinase, RAS: rat sarcoma protein, RAF: v-raf murine viral oncogene homolog, MAPK: mitogen activated protein kinase, MEK:,MAPK/extracellular signal regulated kinase(ERK) kinase.
Through inhibition of fibrotic growth factor receptor as described above, the progress of fibrosis is expected to be delayed by nintedanib, and nintedanib is used as a therapeutic drug for idiopathic pulmonary fibrosis (IPF). In a phase II trial in IPF patients, a decrease in forced vital capacity (FVC), which was the primary endpoint, was suppressed in the nintedanib group compared with the placebo group (FVC: −0.06 L per year vs. −0.19 L per year, p = 0.06). The frequency of acute exacerbation, which was the secondary endpoint, was significantly lower in the nintedanib group than in the placebo group (2.4% per year vs. 15.7% per year) [58]. Following this trial, two phase III trials (INPULSIS-1 and INPULSIS-2 trial) were conducted [59,60]. The primary endpoint was the annual FVC decline rate (mL/year), and important secondary endpoints were time taken to the first acute exacerbation of IPF at 52 weeks (reported by the investigator) and changes in the total St. George’s Respiratory Questionnaire (SGRQ) score at 52 weeks from baseline. As a result, the adjusted annual change in FVC was significantly lower in the nintedanib group than in the placebo group (p < 0.001) for both trials. In the INPULSIS-1 trial, no significant difference in the time to first acute exacerbation (reported by the attending physician) between the nintedanib group and placebo group was observed (HR: 1.15; 95%CI: 0.54–2.42; p = 0.67), but in the INPULSIS-2 trial, the nintedanib group showed significantly prolonged survival compared with the placebo group (HR: 0.38; 95%CI: 0.19–0.77; p = 0.005). In addition, combined analysis of the INPULSIS-1 and INPULSIS-2 trials showed no significant difference in the time to the first acute exacerbation (reported by the attending physician) (HR: 0.64, 95%CI: 0.39–1.05, p = 0.08). However, sensitivity analysis of acute exacerbation/suspicion of acute exacerbation by an independent committee using integrated data showed that the time until first acute exacerbation (HR: 0.32, 95%CI: 0.16–0.65, p = 0.001) and frequency of acute exacerbation were significantly different between the groups. In the INPULSIS-1 trial, the most common adverse event in the nintedanib group was diarrhea, and the incidence of diarrhea was 61.5% in the nintedanib group and 18.6% in the placebo group. In the INPULSIS-2 trial, the incidence of diarrhea in the nintedanib and placebo groups was 63.2% and 18.3%, respectively, but the severity was low.
Recently, a phase III international joint trial (SENSCIS trial), announced in 2019, targeting patients with interstitial lung disease associated with systemic scleroderma (SSc-ILD) showed nintedanib to be effective and safe for SSc-ILD patients [61]. This trial, the largest international, placebo-controlled, randomized, double-blind study on nintedanib was conducted in 576 patients with SSc-ILD in more than 32 countries, including the US, Canada, China, Japan, Germany, France, and the United Kingdom. The 52-week adjusted rate of annual reduction (mL/year) in FVC (mL) (the main endpoint) was −52.4 mL/year in the nintedanib group and −93.3 mL/year in the placebo group. The difference between groups was 41.0 mL/year (95%CI: 2.9–79.0; p = 0.04), which was similar to the results of the INPULSIS trials. The most common adverse event was diarrhea with an incidence of 75.7% in the nintedanib group and 31.6% in the placebo group; 49.5% of the incidences of diarrhea in the nintedanib group were mild, while 45% were moderate. As a result, nintedanib was approved by the FDA in the US in September 2019 and in Japan, in December 2019.
In an in vitro study, nintedanib inhibited angiogenesis and suppressed tumor cell proliferation; and as nintedanib is expected to be an antitumor drug, it is still under development [55]. A phase III trial (LUME-Lung 1 trial) to evaluate the efficacy and safety of the combination of docetaxel and nintedanib [62] showed that the median PFS (the primary endpoint) was significantly longer in the nintedanib plus docetaxel group than in the placebo plus docetaxel group (3.4 months vs. 2.7 months, HR: 0.79; 95%CI: 0.68–0.92, p = 0.0019). An analysis of 658 already diagnosed adenocarcinomas showed that the median OS in the nintedanib group was longer than that in the placebo group (12.6 months vs. 10.3 months, HR: 0.83, 95%CI: 0.70–0.99, p = 0.0359). The proportion of cases with squamous cell carcinomas was high in this study (42% of the total). Adverse events included diarrhea, liver dysfunction, nausea, loss of appetite, and vomiting. Grade 3 or higher adverse events were slightly higher in the nintedanib group than in the placebo group (71.3% vs. 64.3%), and grade 5 adverse events were higher in the nintedanib group than in the placebo group (16.4% vs. 11.8%). In the LUME-Lung 2 trial, which evaluated the effects of nintedanib and pemetrexed in previously treated NSCLC patients, PFS in the nintedanib group was significantly longer than that in the placebo group (4.4 months vs. 3.6 months, HR: 0.83, 95%CI: 0.7–0.99, p = 0.04), and the disease control rate in the nintedanib group was significantly better than that in the placebo group (61% vs. 53%, odds ratio 1.37, p = 0.039). However, as a result of the interim analysis, the registration was discontinued based on futility analysis of PFS evaluated by researchers [63]. Table 3 summarizes the results of clinical trials of sorafenib and nintedanib (Table 3).
Table 3.
Results of clinical trials of sorafenib and nintedanib.
| Trial | Phase | Line | Type | n | RR(CR/PR) | DCR(CR/PR/SD) | Time to progression | PFS | MST or OS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sorafenib | Blumenschein G.R. 2009 Sorafenib alone |
II | 2nd–3rd line | NSCLC | 54 | 0% | 59% | 2.7 m | 6.7 m | |
| Paz-Ares L. 2015 Sorafenib alone vs. placebo (MISSION trial) |
III | 3rd–4th line | NSCLC | 703 | 4.9% vs. 0.9% | 47.1% vs. 24.7% | 2.9 m vs. 1.4 m | 2.8 m vs. 1.4 m | 8.2 m vs. 8.3 m | |
| EGFR mutation+ | 89 | 6.8% vs. 0% | 40.9% vs. 2.2% | 2.7 m vs. 1.4 m | 13.9 m vs. 6.5 m | |||||
| wild-type EGFR | 258 | 7.4% vs. 1.5% | 46.7% vs. 25.8% | 2.7 m vs. 1.5 m | 8.3 m vs. 8.4 m | |||||
| KRAS mutation+ | 68 | 2.9% vs. 0% | 44.1% vs. 7.6% | 2.6 m vs. 1.7 m | 6.4 m vs. 5.1 m | |||||
| wild-type KRAS | 279 | 8.3% vs. 1.4% | 45.4% vs. 20.4% | 2.7 m vs. 1.4 m | 11.0 m vs. 9.1 m | |||||
| Scagliotti G. 2010 CBDCA/PTX/sorafenib vs. CBDCA/PTX/placebo (ESCAPE trial) |
III | 1st line | NSCLC | 926 | 27% vs. 24% | 50% vs. 56% | 4.6 m vs. 5.4 m | 10.7 m vs. 10.6 m | ||
| Sq | 223 | 25% vs. 35% | 42% vs. 60% | 4.3 m vs. 5.8 m | 8.9 m vs. 13.6 m | |||||
| Other | 703 | 28% vs. 20% | 52% vs. 55% | 4.8 m vs. 5.3 m | 11.5 m vs. 10.2 m | |||||
| Nintedanib | Reck M. 2014 DTX/nintedanib vs. DTX/placebo (LUME-Lung 1 trial) |
III | 2nd line | NSCLC | 1314 | 4.4% vs. 3.3% | 54% vs. 41.3% | 3.4 m vs. 2.7 m | 10.1 m vs. 9.1 m | |
| Adeno | 658 | 4.7% 3.6% | 60.2% vs. 44% | 12.6 m vs. 10.3 m | ||||||
| Hanna N.H. 2016 Nintedanib/PEM vs. PEM (LUME-Lung 2 trial) |
III | 2nd line | NonSq NSCLC | 713 | 9.1% vs. 8.3% | 60.9% vs. 53.3% | 4.4 m vs. 3.6 m | 12.0 m vs. 12.7 m | ||
| Adeno | 670 | 9.6% vs. 9.0% | 61.8% vs. 54.6% | 4.5 m vs. 3.9 m | 12.3 m vs. 13.1 m |
NSCLC: non-small cell lung cancer, NonSq: nonsquamous cell carcinoma, RR: response rate, CR: complete response, PR: partial response, DCR: disease control rate, SD: stable disease, PFS: progression free survival, MST: medial survival time, OS: overall survival, EGFR: epidermal growth factor receptor, KRAS: kirsten rat sarcoma viral oncogene homolog, CBDCA: carboplatin, PTX: paclitaxel, DTX: docetaxel, PEM: pemetrexed.
7. Novel Compounds
Various studies on multitarget drugs for respiratory diseases are now in progress. Anlotinib, a relatively novel multitarget TKI for tumor angiogenesis and tumor cell proliferation, is effective as a third-line or beyond treatment for advanced NSCLC [64]. Entrectinib, another multitarget TKI of TRKA/B/C, ROS1, and ALK, has been studied in patients with advanced or metastatic solid tumors harboring NTRK1/2/3, ROS1, or ALK gene fusions [65].
However, some attention should be paid to multitarget therapies. Multitarget TKIs are used for NSCLC harboring RET rearrangement. Notably, these patients suffered from high-grade toxicity mainly induced by anti-VEGFR kinase activity. Therefore, selective RET inhibitors such as BLU-667, LOXO-292, and RXDX-105 have been recently investigated in early phase clinical trials and showed promising efficacy with a manageable toxicity profile [66].
8. Conclusions
We have summarized the available data regarding multitarget drugs used against respiratory diseases including lung cancer and IPF. In addition, various studies on multitarget drugs for respiratory diseases have just begun. Further advances in multitarget drugs will bring additional benefits to patients.
Acknowledgments
We thank the members of the Division of Respiratory Medicine within Kobe University Graduate School of Medicine for helpful discussions.
Author Contributions
Conceptualization, T.N. and M.Y.; resources, T.N. and M.Y.; writing—preparation of the original draft, T.N. and M.Y.; writing—review and editing, T.N. and M.Y.; supervision, Y.N.; project administration, Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hopkins A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 2.Ramsay R.R., Popovic-Nikolic M.R., Nikolic K., Uliassi E., Bolognesi M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018;7 doi: 10.1186/s40169-017-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proschak E., Stark H., Merk D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019;62:420–444. doi: 10.1021/acs.jmedchem.8b00760. [DOI] [PubMed] [Google Scholar]
- 4.Costa E., Giardini A., Savin M., Menditto E., Lehane E., Laosa O., Pecorelli S., Monaco A., Marengoni A. Interventional tools to improve medication adherence: Review of literature. Patient Prefer. Adherence. 2015;9:1303–1314. doi: 10.2147/PPA.S87551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J., Jiang X., He S., Jiang H., Feng F., Liu W., Qu W., Sun H. Rational Design of Multitarget-Directed Ligands: Strategies and Emerging Paradigms. J. Med. Chem. 2019;62:8881–8914. doi: 10.1021/acs.jmedchem.9b00017. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Soacha D.A., Scheiner M., Decker M. Multi-target-directed-ligands acting as enzyme inhibitors and receptor ligands. Eur. J. Med. Chem. 2019;180:690–706. doi: 10.1016/j.ejmech.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay S., Moran R.G., Goldman I.D. Pemetrexed: Biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol. Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 8.Julian R., Molina A.A.A. The Role of Pemetrexed (Alimta, LY231514) in LungCancer Therapy. Clin. Lung Cancer. 2003;5:21–27. doi: 10.3816/CLC.2003.n.017. [DOI] [PubMed] [Google Scholar]
- 9.Christoph D.C., Asuncion B.R., Mascaux C., Tran C., Lu X., Wynes M.W., Hepp R. Folypoly-glutamate synthetase expression is associated with tumor response and outcome from pemetrexed-based chemotherapy in Malignanat pleural mesothelioma. J. Thorac. Oncol. 2012;7:1440–1448. doi: 10.1097/JTO.0b013e318260deaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceppi P., Volante M., Saviozzi S., Rapa I., Novello S., Cambieri A., Lo Iacono M., Cappia S., Papotti M., Scagliotti G.V. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Yin T.J., Zhou R., Zhou S., Fan L., Zhang R.G. Expression of thymidylate synthase predicts clinical outcomes of pemetrexed-containing chemotherapy for non-small-cell lung cancer: A systemic review and meta-analysis. Cancer Chemother. Pharmacol. 2013;72:1125–1132. doi: 10.1007/s00280-013-2299-2. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti G.V., Shin D.M., Kindler H.L., Vasconcelles M.J., Keppler U., Manegold C., Burris H., Gatzemeier U., Blatter J., Symanowski J.T., et al. Phase II study of pemetrexed with and without folic acid and vitamin B 12 as front-line therapy in malignant pleural mesothelioma. J. Clin. Oncol. 2003;21:1556–1561. doi: 10.1200/JCO.2003.06.122. [DOI] [PubMed] [Google Scholar]
- 13.Berghmans T., Paesmans M., Lalami Y., Louviaux I., Luce S., Mascaux C., Meert A.P., Sculier J.P. Activity of chemotherapy and immunotherapy on malignant mesothelioma: A systematic review of the literature with meta-analysis. Lung Cancer. 2002;38:111–121. doi: 10.1016/S0169-5002(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 14.Kindler H.L., Millard F., Herndon Ii J.E., Vogelzang N.J., Suzuki Y., Green M.R. Gemcitabine for malignant mesothelioma: A phase II trial by the Cancer and Leukemia Group B. Lung Cancer. 2001;31:311–317. doi: 10.1016/S0169-5002(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 15.Vogelzang N.J., Rusthoven J.J., Symanowski J., Denham C., Kaukel E., Ruffie P., Gatzemeier U., Boyer M., Emri S., Manegold C., et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 16.Hughes A., Calvert P., Azzabi A., Plummer R., Johnson R., Rusthoven J., Griffin M., Fishwick K., Boddy A.V., Verrill M., et al. Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2002;20:3533–3544. doi: 10.1200/JCO.2002.10.073. [DOI] [PubMed] [Google Scholar]
- 17.Ceresoli G.L., Zucali P.A., Favaretto A.G., Grossi F., Bidoli P., Del Conte G., Ceribelli A., Bearz A., Morenghi E., Cavina R., et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J. Clin. Oncol. 2006;24:1443–1448. doi: 10.1200/JCO.2005.04.3190. [DOI] [PubMed] [Google Scholar]
- 18.Castagneto B., Botta M., Aitini E., Spigno F., Degiovanni D., Alabiso O., Serra M., Muzio A., Carbone R., Buosi R., et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM) Ann. Oncol. 2008;19:370–373. doi: 10.1093/annonc/mdm501. [DOI] [PubMed] [Google Scholar]
- 19.Hanna N., Shepherd F.A., Fossella F.V., Pereira J.R., Demarinis F., Von Pawel J., Gatzemeier U., Tsao T.C.Y., Pless M., Muller T., et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti G.V., Parikh P., Von Pawel J., Biesma B., Vansteenkiste J., Manegold C., Serwatowski P., Gatzemeier U., Digumarti R., Zukin M., et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Ares L.G., De Marinis F., Dediu M., Thomas M., Pujol J.L., Bidoli P., Molinier O., Sahoo T.P., Laack E., Reck M., et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 23.Iwahara T., Fujimoto J., Wen D., Cupples R., Bucay N., Arakawa T., Mori S., Ratzkin B., Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 24.Stephan W.M., Clayton N., Prasad M., Payton L.J., Mark N.K., Xue C., Witte D.P. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyto tyrosine kinase (LTK) Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 25.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S.I., Watanabe H., Kurashina K., Hatanaka H., et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 26.Shaw A.T., Engelman J.A. ALK in lung cancer: Past, present, and future. J. Clin. Oncol. 2013;31:1105–1111. doi: 10.1200/JCO.2012.44.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin L.P., Soo R.A., Soong R., Ou SH I. Targeting ROS1 with anaplastic lymphoma kinase inhibitors. J. Thorac. Oncol. 2012;7:1625–1630. doi: 10.1097/JTO.0b013e31826baf83. [DOI] [PubMed] [Google Scholar]
- 28.Kwak E.L., Bang Y.-J., Ross Camidge D., Shaw A.T., Solomon B., Maki R.G., Ou S.-H.I., Dezube B.J., Jänne P.A., Costa D.B., et al. Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer. N. Engl. Med. 2010;18:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw A.T., Kim D.W., Nakagawa K., Seto T., Crinó L., Ahn M.J., De Pas T., Besse B., Solomon B.J., Blackhall F., et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 30.Solomon B.J., Mok T., Kim D.W., Wu Y.L., Nakagawa K., Mekhail T., Felip E., Cappuzzo F., Paolini J., Usari T., et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 31.Yasuda H., De Figueiredo-Pontes L.L., Kobayashi S., Costa D.B. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J. Thorac. Oncol. 2012;7:1086–1090. doi: 10.1097/JTO.0b013e3182570919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw A.T., Ou S.H.I., Bang Y.J., Camidge D.R., Solomon B.J., Salgia R., Riely G.J., Varella-Garcia M., Shapiro G.I., Costa D.B., et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y.-L., Chih-Hsin Yang J., Kim D.-W., Lu S., Zhou J., Seto T., Yang J.-J., Yamamoto N., Ahn M.-J., Takahashi T., et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non–small-cell lung cancer. J. Clin. Oncol. 2018;36:1405–1411. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto H., Tsukaguchi T., Hiroshima S., Kodama T., Kobayashi T., Fukami T.A., Oikawa N., Tsukuda T., Ishii N., Aoki Y. CH5424802, a Selective ALK Inhibitor Capable of Blocking the Resistant Gatekeeper Mutant. Cancer Cell. 2011;19:679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Kodama T., Tsukaguchi T., Satoh Y., Yoshida M., Watanabe Y., Kondoh O., Sakamoto H. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol. Cancer Ther. 2014;13:2910–2918. doi: 10.1158/1535-7163.MCT-14-0274. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara R., Auger N., Auclin E., Besse B. Clinical and Translational Implications of RET Rearrangements in Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018;13:27–45. doi: 10.1016/j.jtho.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Seto T., Kiura K., Nishio M., Nakagawa K., Maemondo M., Inoue A., Hida T., Yamamoto N., Yoshioka H., Harada M., et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): A single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 38.Gadgeel S.M., Gandhi L., Riely G.J., Chiappori A.A., West H.L., Azada M.C., Morcos P.N., Lee R.M., Garcia L., Yu L., et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 39.Hida T., Nokihara H., Kondo M., Kim Y.H., Azuma K., Seto T., Takiguchi Y., Nishio M., Yoshioka H., Imamura F., et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa K., Hida T., Nokihara H., Morise M., Azuma K., Kim Y.H., Seto T., Takiguchi Y., Nishio M., Yoshioka H., et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer. 2020;139:195–199. doi: 10.1016/j.lungcan.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 41.Peters S., Camidge D.R., Shaw A.T., Gadgeel S., Ahn J.S., Kim D.W., Ou S.H.I., Pérol M., Dziadziuszko R., Rosell R., et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N. Engl. J. Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 42.Tonini T., Rossi F., Claudio P.P. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Hicklin D.J., Ellis L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 45.Dvorak H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 46.Ellis L.M., Hicklin D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 47.Abdalla A.M.E., Xiao L., Ullah M.W., Yu M., Ouyang C., Yang G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics. 2018;8:533–549. doi: 10.7150/thno.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garon E.B., Ciuleanu T.E., Arrieta O., Prabhash K., Syrigos K.N., Goksel T., Park K., Gorbunova V., Kowalyszyn R.D., Pikiel J., et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 49.Soria J.C., Mauguen A., Reck M., Sandler A.B., Saijo N., Johnson D.H., Burcoveanu D., Fukuoka M., Besse B., Pignon J.P. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2013;24:20–30. doi: 10.1093/annonc/mds590. [DOI] [PubMed] [Google Scholar]
- 50.Liu L., Cao Y., Chen C., Zhang X., McNabola A., Wilkie D., Wilhelm S., Lynch M., Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 51.Carlomagno F., Anaganti S., Guida T., Salvatore G., Troncone G., Wilhelm S.M., Santoro M. BAY 43-9006 inhibition of oncogenic RET mutants. J. Natl. Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 52.Blumenschein G.R., Gatzemeier U., Fossella F., Stewart D.J., Cupit L., Cihon F., O’Leary J., Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J. Clin. Oncol. 2009;27:4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 53.Paz-Ares L., Hirsh V., Zhang L., De Marinis F., Yang J.C.H., Wakelee H.A., Seto T., Wu Y.L., Novello S., Juhász E., et al. Monotherapy Administration of Sorafenib in Patients with Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Canc. J. Thorac. Oncol. 2015;10:1745–1753. doi: 10.1097/JTO.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 54.Scagliotti G., Novello S., Von Pawel J., Reck M., Pereira J.R., Thomas M., Miziara J.E.A., Balint B., De Marinis F., Keller A., et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 55.Hilberg F., Roth G.J., Krssak M., Kautschitsch S., Sommergruber W., Tontsch-Grunt U., Garin-Chesa P., Bader G., Zoephel A., Quant J., et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 56.Wollin L., Maillet I., Quesniaux V., Holweg A., Ryffel B. Antifibrotic and anti-inflammatory activity of the Tyrosine Kinase inhibitor Nintedanib in Experimental Models of Lung Fibrosiss. J. Pharmacol. Exp. Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 57.Roth G.J., Heckel A., Colbatzky F., Handschuh S., Kley J., Lehmann-Lintz T., Lotz R., Tontsch-Grunt U., Walter R., Hilberg F. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120) J. Med. Chem. 2009;52:4466–4480. doi: 10.1021/jm900431g. [DOI] [PubMed] [Google Scholar]
- 58.Richeldi L., Costabel U., Selman M., Soon Kim D., Hansell D.M., Nicholson A.G., Brown K.K., Flaherty K.R., Noble P.W., Raghu G., et al. Efficacy of a Tyrosine Kinase Inhibitor in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2011;12:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 59.Richeldi L., Cottin V., Flaherty K.R., Kolb M., Inoue Y., Raghu G., Taniguchi H., Hansell D.M., Nicholson A.G., Le Maulf F., et al. Design of the INPULSISTM trials: Two phase 3 trials of nintedanib in patients with idiopathic pulmonary fibrosis. Respir. Med. 2014;108:1023–1030. doi: 10.1016/j.rmed.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 60.Richeldi L., Du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 61.Distler O., Highland K.B., Gahlemann M., Azuma A., Fischer A., Mayes M.D., Raghu G., Sauter W., Girard M., Alves M., et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 62.Reck M., Kaiser R., Mellemgaard A., Douillard J.Y., Orlov S., Krzakowski M., von Pawel J., Gottfried M., Bondarenko I., Liao M., et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 63.Hanna N.H., Kaiser R., Sullivan R.N., Aren O.R., Ahn M.J., Tiangco B., Voccia I., von Pawel J., Kovcin V., Agulnik J., et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): A randomized, double-blind, phase III trial. Lung Cancer. 2016;102:65–73. doi: 10.1016/j.lungcan.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z., Zhao Y., Lu F., Hou X., Ma Y., Luo F., Zeng K., Zhao S., Zhang Y., Zhou T., et al. Multi-targeted tyrosine kinase inhibitors as third-line regimen in advanced non-small cell lung cancer: A network meta-analysis. Ann. Transl. Med. 2019;7:452. doi: 10.21037/atm.2019.08.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drilon A., Siena S., Ou S.H.I., Patel M., Ahn M.J., Lee J., Bauer T.M., Farago A.F., Wheler J.J., Liu S.V., et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7:400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ackermann C.J., Stock G., Tay R., Dawod M., Gomes F., Califano R. Targeted therapy for RET-rearranged non-small cell lung cancer: Clinical development and future directions. Onco. Targets. Ther. 2019;12:7857–7864. doi: 10.2147/OTT.S171665. [DOI] [PMC free article] [PubMed] [Google Scholar]