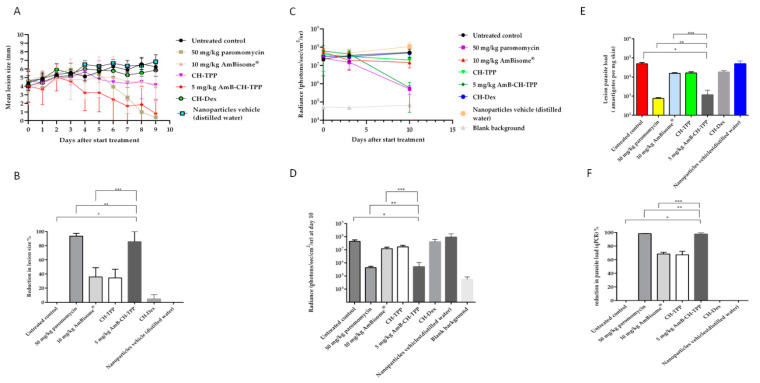
Figure 2.
AmB nanoparticles efficacy in the lesion cure model in BALB/c mice infected with luciferase-expressing L. major parasites. L. major infected mice were allocated into 8 groups: (G1) represents untreated infected group, (G2) paromomycin as a positive control (50 mg/kg/QD for 10 days; i.p.), (G3) AmBisome® as a comparison group (10 mg/kg/QAD for 10 days; i.v.), (G4) CH-TPP nanoparticles (mass of nanoparticles in the blank formulations reflected the related AmB-loaded ones) (QAD for 10 days; i.v.), (G5) AmB-CH-TPP nanoparticles (5 mg of AmB/kg/QAD for 10 days; i.v.), (G6) CH-Dex nanoparticles (mass of nanoparticles in the blank formulations reflected the related AmB-loaded ones) (QAD for 10 days; iv), (G7) AmB-CH-Dex nanoparticles (10 mg of AmB/kg/one dose; i.v.), 24 h after the first (and only) dose of the formulation, the mice looked unwell with piloerection and weight loss, therefore data of G7 are not represented in the figure or (G8) the nanoparticles vehicle (distilled water, QAD for 10 days; i.v.). QAD: every other day, QD: once a day. The average lesion size and parasite load represent the mean ± SD. One way-ANOVA for parasite load (bioluminescence signal), parasite load (qPCR) and repeated measures for lesion size followed by Tukey’s multiple-comparison tests was used to compare outcomes among the groups. A p-value < 0.05 was considered statistically significant ((*) p < 0.05, (**) p > 0.05 and (***) p < 0.05). (A) represents mean lesion size progression in function of time since the start of treatment, (B) represents the % reduction in lesion size compared with G1 (untreated infected group) at day 10, (C) represents the bioluminescence signal in function of time since the start of treatment, (D) represents the bioluminescence signal compared with G1 (untreated infected group) at day 10, (E) represents the parasite load (qPCR-DNA) at day 10 and (F) represents the % reduction in the parasite load (qPCR) compared with G1 (untreated infected group) at day 10.