Abstract
NAFLD is a global epidemic. The prevalence of NAFLD is 20–30% in North America, northern Europe, Australia, Japan, India and China. It is crucial that patients with NAFLD receive an assessment for their risk of advanced fibrosis, which increases the risk of hepatocellular carcinoma and other complications of cirrhosis. Risk stratification that is efficient, cost-effective, patient-centred and evidence-based is one of the most important issues facing clinicians who care for those with liver disease. Given patients’ preference to avoid liver biopsy, noninvasive alternatives to assess liver fibrosis are in high demand. The most accurate noninvasive methods are based on liver elastography. Research on these techniques — which include vibration- controlled transient elastography (VCTE), magnetic resonance elastography (MRE), shear-wave elastography and acoustic radiation force impulse — has proliferated. Unfortunately, the literature has not kept pace with clinical practice. There is limited guidance for how clinicians should anticipate and manage the pitfalls of these tests. Furthermore, guidance is unavailable for clinicians regarding the optimal incorporation of VCTE, MRE or the emerging elastographic techniques into their clinical strategy, particularly for patients with NAFLD. In this Review, we summarize the available evidence, highlight gaps to address in further research and explore optimization of these techniques in clinical practice.
NAFLD, prevalent in >30% of the US population1,2, is becoming the major cause of liver disease-related morbidity and mortality3,4. In a spectrum of disease ranging from steatosis to steatohepatitis and advanced fibrosis, it is those patients with advanced fibrosis who are most at long-term risk of adverse outcomes such as hepato cellular carcinoma and hepatic decompensation5,6. Owing to the sheer volume of at-risk patients, there is a substantial unmet need for efficient and cost-effective means to risk stratify patients with NAFLD. Liver biopsy, long the gold standard for this purpose, is impractical to satisfy these needs. Expensive, risky and frequently refused by patients7–9, liver biopsy is further limited by sampling error10 and poor inter-rater reliability11. Spurred by these limitations, validated and noninvasive alternatives to the liver biopsy are an area of intense research interest in the field12.
Among the noninvasive biomarkers of disease severity in NAFLD, imaging-based biomarkers are emerging as the lead candidates in clinical development. There are several imaging-based biomarkers, but the most promising and also most widely applied are techniques that perform elastography — that is, liver stiffness measurement (LSM).
However, data are limited regarding how the different approaches compare, their trade-offs and how they complement each other in the context of real-world clinical practice. In this Review, we discuss each modality with reference to its inherent properties, diagnostic performance and caveats associated with clinical application. We also compare their performance and pitfalls and lay out our view of the most urgent items on the agenda of this vital area of research.
The landscape of biopsy alternatives
Four devices that perform liver elastography have been evaluated in cohorts of patients with NAFLD: vibration- controlled transient elastography (VCTE), shear-wave elastography (SWE), acoustic radiation force impulse (ARFI) imaging and magnetic resonance elastography (MRE) (FIG. 1). Each modality varies in terms of its evidence base in the setting of NAFLD (although published experience in hepatitis C, and often hepatitis B, is more extensive, particularly for VCTE), cost and technical considerations (TABLE 1). Only one technique is used at the point of care (VCTE), whereas the others require technical expertise for interpretation and therefore consultation from a radiologist. We refer the readers to a 2017 technical review of liver elastography for in-depth description of the physics and performance of each technique13. Herein, we review the studies that included large, identifiable cohorts of patients with NAFLD who were evaluated using these four elastographic techniques.
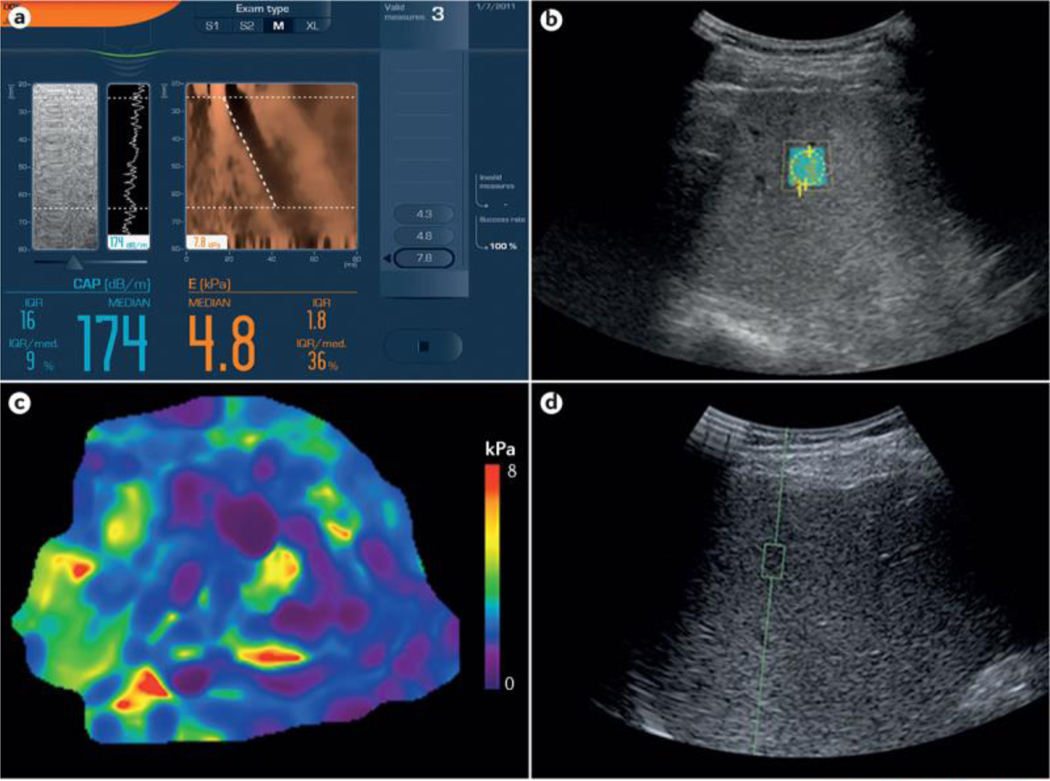
Figure 1 |. Example images from elastographic techniques.
Four methods have been investigated for the assessment of liver fibrosis in patients with NAFLD. a | Vibration-controlled transient elastography (VCTE). The screen (captured directly from a Fibroscan machine; Echosens, France) shows a limited ultrasonic window with a reconstructed view of the wavefront (in brown) that passes through the liver from the handheld probes (M or XL). b | Shear-wave elastography (SWE). SWE is performed in the defined region of interest (ROI). The liver stiffness readout, in m/s, is provided by the software. c | Magnetic resonance elastography (MRE). MRE provides liver stiffness values, in kPa, across the hepatic parenchyma from an average of multiple ROIs. d | Acoustic radiation force impulse (ARFI). This panel is a view of a standard liver ultrasonography image with ARFI performed in the defined ROI (green square). The readout, in m/s, is provided by the software. Image for VCTE (part a) courtesy of Echosens, France. Images for SWE (part b), MRE (part c) and ARFI (part d) were all taken from the same patient at the NAFLD Research Center of the University of California at San Diego, USA.
Table 1 |.
Technical considerations for and experience with elastographic modalities
| Imaging technique | Strength of published experience in NAFLD | Cost per test | Confounders | Quality criteria | ||
|---|---|---|---|---|---|---|
| Inflammation | Obesity | Other | ||||
| VCTE | +++ | $ | ++ | ++ | Congestion and possibly steatosis | Standardized by interquartile range and/or median values and shot acquisition percentage |
| MRE | ++ | $$$ | + | - | Iron overload and congestion | Individualized operator assessment of slice quality |
| ARFI | + | $$$ | + (assumed from limited data) | + (assumed from limited data) | Expected to be similar to VCTE (limited data) | Individualized operator assessment of window quality |
| SWE | + | $$ | + (assumed from limited data) | + (assumed from limited data) | Expected to be similar to VCTE (limited data) | Individualized operator assessment of window quality |
Although data are limited on the effect of meal ingestion, it is assumed to cause transient increases in liver stiffness; therefore, patients should be fasting for 2–3 h before an elastographic examination. Similarly, although data are limited, passive congestion can be assumed to increase liver stiffness as determined by SWE and ARFI. Cost estimates are inexact and range from <USD$100 ($) to >$1,000 ($$$). ARFI, acoustic radiation force imaging; MRE, magnetic resonance elastography; SWE, shear-wave elastography; VCTE, vibration-controlled transient elastography
Although these techniques are very different, each produces an LSM. Although LSMs are continuous values, by analysing their statistical receiver operating characteristics they can be dichotomized into optimal cut-offs for the presence or absence of advanced fibrosis. LSM cut-off values carry three levels of uncertainty. First, they vary substantially among underlying liver diseases and even among studies of the same disease. Second, LSMs can be confounded by any process that influences the viscoelastic properties of the liver. These confounders are best characterized in patients undergoing VCTE and include congestion (that is, heart failure), post-prandial hepatic hyperaemia (patients should fast for 2–3 hours), severe hepatic inflammation (particularly in patients with serum alanine aminotransferase (ALT) levels greater than three times the upper limit of normal)14 and possibly the presence or degree of steatosis15. Third, as with any discrete cut-off, there is a range of indeterminate results within confidence intervals of ~10–20% above and below. For example, whereas an LSM that is 10 kPa above a published cut-off is more likely to be a true positive, an LSM 2 kPa below the cut-off should be considered within a grey zone of indeterminate meaning. Any strategy that utilizes elastography in clinical practice should have a deliberate approach to managing uncertainty. This consideration applies both before the test, through patient selection and counselling, and after the test, by considering the effect of confounding factors and having a plan for indeterminate results including repeat LSM or employing alternative tests such as serological indices of fibrosis.
Vibration-controlled transient elastography
Assessment of fibrosis and quality criteria.
VCTE (Fibroscan; Echosens) is perhaps the most widely used and the first FDA-approved elastographic modality. Using a handheld probe, a mechanical shear wave (a ‘shot’) is introduced into the liver across the chest wall from within the intercostal space. Wave propagation is then evaluated by a receiver in the probe at a fixed distance: 25–65 mm for the ‘M probe’ and 35–75 mm for the ‘XL probe’. The deeper extension possible with the XL probe can overcome chest walls that are thickened by adipose tissue, a particular issue in patients who are obese. The velocity of the shear wave is then converted into an LSM rendered in kPa by Hooke’s law of solid-elastic properties. VCTE software provides feedback to the user as to whether the probe lies over liver tissue, aborting the shot if liver tissue is not appreciated. Although inadequate shots can be determined to be failures according to the computer, the waveform can be further assessed for quality with respect to the solidity of the wave-front visualized.
In general, although definitions of quality or reliability vary slightly16,17, an adequate examination includes ten valid shots (>60% success rate) with an interquartile range (IQR)-to-median LSM ratio of ≤0.3. LSM, in turn, correlates with fibrosis burden16,18,19. There can be substantial overlap in the confidence intervals of the representative LSMs obtained from any of the elastographic techniques for patients with adjacent fibrosis stages (for example, F1 versus F2). LSM, therefore, cannot accurately stage fibrosis. Instead, LSM offers good test characteristics, particularly high negative predictive values for the presence of advanced fibrosis (discussed later). TABLE 2 reviews the performance of each modality in the discrimination of patients with advanced fibrosis (METAVIR scoring of F3–F4 or NASH Clinical Research Network stage 3 or stage 4). In general, VCTE provides an accurate per-protocol risk assessment of advanced fibrosis (stage 3 and stage 4 fibrosis) in NAFLD (area under the receiver operating characteristic (AUROC) ranging from 0.80 to 0.94)). The central issues with VCTE are the technical failure rate, which varied from 6.7% to 27.0% in the lar gest American study (with a notably high mean BMI), and limited precision18,20. For technically successful examinations, the optimal LSM cut-off to maximize sensitivity and specificity varies from 7.2 to 11.4 kPa (REFS 21, 22). Cassinoto et al.19 provide LSM cut-offs to provide 90% sensitivity and specificity, which are 8.2 kPa and 12.5 kPa, respectively. Notably, only three published studies include measurements using the XL probe18,21,23, two of which have lower cut-offs for advanced fibrosis (7.2–7.6 kPa), and one of which had a lower failure rate than reported using the M probe alone (6.7%)18.
Table 2 |.
Performance of elastography modalities in patients with NAFLD
| Study | n | Mean BMI (kg/m2) | Failure and/or unreliable rate (%)a | Patients with advanced (F3-F4) fibrosis (%) | AUROC for F3-F4 | Optimal cut-off for F3-F4 | Sensitivity/specificity | Refs |
|---|---|---|---|---|---|---|---|---|
| VCTE | ||||||||
| Chen | 111 | 40.3 | 22.7 | 20 | 0.87 | 7.6 kPa (M and XL) | 84/64 | 23 |
| Tapper | 164 | 32.3 | 27 | 18 | 0.93 | 9.9 kPa | 95/77 | 20 |
| Park | 104 | 30.4 | 6.7 | 17 | 0.8 | 7.3 kPa (M and XL) | 78/78 | 18 |
| Boursier | 588 | 31.7 | 9.3 | 39 | 0.83 | 8.7 kPa | 88/63 | 16 |
| Imajo | 142 | 28.1 | 10.5 | 32 | 0.88 | 11.4 kPa | 86/84 | 22 |
| Petta | 324 | 39.5% >30 | NA | 35 | 0.86 | 10.1 kPa | 78/78 | 54 |
| Cassinottob | 291 | 60.1% >30.0 | 23.4 | 43 | 0.86 | 8.2 and 12.5 kPa | 90/61 and 57/90 | 19 |
| Kumar | 120 | 26.1 | 14.9 | 23 | 0.94 | 9.0 kPa | 85/88 | 55 |
| Chan | 153 | 29.4 | 3.9 | 21 | NA | 8.0 kPa | 95/66 | 56 |
| Aykut | 88 | 30.3 | NA | 41 | 0.94 | 7.9 kPa | 96/90 | 57 |
| Naveau | 100 | 42.3 | 19 | 9 | 0.85 | 7.6 kPa | 100/74 | 58 |
| Gaia | 72 | 27.5 | NA | 34 | 0.76 | 8.0 kPa | 65/80 | 59 |
| Mahadeva | 131 | 32.8% >30.0 | 8 | 22 | 0.77 | 7.1 kPa | 70/67 | 60 |
| Wong | 193 | 28.9 | 25 | 30 | 0.78 (ITT) and 0.85 (PP) | 7.2 kPa (XL) | 74/75 (ITT) and 78/78 | 21 |
| Wong | 246 | 28.5% >30.0 | 10.2 | 23 | 0.93 | 8.7 kPa | (PP) 84/83 | 61 |
| MRE | ||||||||
| Chen | 111 | 40.3 | 4.5 | 20 | 0.92 | 3.6 kPa | 84/83 | 23 |
| Park | 104 | 30.4 | 0 | 17 | 0.87 | 3.0 kPa | 78/80 | 18 |
| Loomba | 100 | 32.1 | 0 | 15 | 0.92 | 3.8 kPa | 80/95 | 36 |
| Imajo | 142 | 28.1 | 0 | 32 | 0.89 | 4.8 kPa | 75/87 | 22 |
| Loomba | 117 | 32.4 | 0 | 19 | 0.92 | 3.6 kPa | 82/90 | 37 |
| Cui | 102 | 31.7 | 0 | 19 | 0.96 | 3.6 kPa | 92/90 | 39 |
| ARFI | ||||||||
| Cassinotto | 291 | 60.1% >30.0 | 19 | 43 | 0.84 | 1.15 and 1.53 m/s | 90/63 and 59/90 | 19 |
| Cui | 125 | 31.8 | 2.4 | 17 | 0.9 | 1.34 m/s | 95/74 | 31 |
| Palmeri | 172 | 68.6% >30.0 | 21.5 | 33 | 0.9 | 4.24 kPa (2.06 m/s) | 90/90 | 32 |
| SWE | ||||||||
| Cassinotto | 291 | 60.1% >30.0 | 20.3 | 43 | 0.89 | 8.3 and 10.7 kPa | 91/71 and 71/90 | 19 |
| Ochi | 181 | 26.5 | NA | 28 | 0.88 | 3.02c | 89/97 | 62 |
All relevant studies with >50 patients with NAFLD were included in this table. Some studies provided only the proportion of patients with BMI >30 kg/m2.
Failure and unreliable rates for VCTE studies are combined in this column; reliability criteria have not yet been determined for MRE, SWE or ARFI.
Used two cut-offs to maximize sensitivity and specificity.
Study used elastic ratio (the ratio of strain distribution in two selected regions of interest). ARFI, acoustic radiation force imaging; AUROC, area under the receiver operating characteristic; ITT; intention to treat; MRE, magnetic resonance elastography; NA, not available; PP, per protocol; SWE, shear-wave elastography; VCTE, vibration-controlled transient elastography.
Assessment of hepatic steatosis by controlled attenuation parameter.
VCTE can also provide an estimate of the amount of hepatic steatosis through a measure called controlled attenuation parameter (CAP). CAP is acquired simultaneously with LSM by any CAP-equipped VCTE machine. While LSM is obtained, the M probe emits a 3.5 MHz signal (the XL probe uses a 2.5 MHz signal), and the receiver grades the return wave in dB/m (100–400 dB/m) with the median (IQR) of valid shots presented as the final result. The optimal cut-offs for the presence or severity of steatosis are unknown. Karlas et al. performed a large meta-analysis, including data from 19 of the 21 available studies of CAP performance24. Of the 2,735 patients included, 537 (19.6%) had NAFLD. For these patients, the AUROC was 0.82 for the detection of steatosis on liver histology, with false negative and false positive rates of 31% and 18%, respectively. The cut-off for mild steatosis (>5% hepatocytes affected) was 248 dB/m (95% CI 237–261), moderate steatosis (>33% hepatocytes affected) was 268 dB/m (95% CI 257–284), and severe (>66% hepatocytes affected) steatosis was 280 dB/m (95% CI 268–294)). Research to determine quality criteria for CAP measurements is ongoing, with two reports on M probe measurements available (and one involving XL probe measures)25,26. Although validation might be required by further study, Caussy et al. argue that by using steatosis burden determined by MRI as a gold standard, CAP-assisted detection of hepatic steatosis is optimized when the IQR of CAP is <30 dB/m (REF. 25).
Effect of steatosis on liver stiffness assessment.
Any histo logical feature that alters the viscoelastic properties of the liver also changes the LSM. Conflicting evidence suggests that steatosis affects the LSM27. However, how best to account for this effect using CAP, and whether the CAP measurements obtained from M and XL probes have similar effects on LSM, remains an area of active investigation. The clearest current example is in a study by Petta et al. in which the risk of false positive diagnosis of advanced liver fibrosis was stratified by CAP score in a cohort of 324 patients with NAFLD27. The risk of false positive results for possible advanced fibrosis was highest in patients with a CAP score of 300–339 dB/m and an LSM of 10.1–12.5 kPa (but not >12.5 kPa) and an LSM of 10.1–13.6 kPa in patients with a CAP score >340 dB/m. Although additional data are needed to confirm and clarify these findings, it is possible that LSM cut-offs and the definition of indeterminate values could require modification by CAP values. Furthermore, additional data on CAP values using the XL probe are needed.
Shear-wave elastography
Assessment of fibrosis.
SWE is an FDA-approved technique that adapts ultrasonography imaging to produce an LSM. The operator, using the B-mode (2D mode) ultrasonography image, targets the liver to find an area of homogeneous filling free of large vascular structures using the colour map. A region of interest of variable depth and diameter is then defined within the visualized liver. As the shear-wave front propagates within the region of interest away from the emitting probe, the speed of the shear wave is recorded. By converting shear-wave speed into stiffness measurements, the software constructs a colour map of the interpreted stiffness superimposed upon the B-mode images within the region of interest28.
Quality criteria.
Generalizable quality criteria for SWE have not been fully developed13. The validity of the measures provided are dependent on the operator’s assessment that the region of interest is representative liver tissue and is absent of blood vessels, and the number of acquisitions deemed adequate for accurate measurement varies from three29 to five19 to ten per patient28. Cassinoto et al., for example, applied standards from the VCTE experience to SWE and considered the data adequate when IQR/LSM was <0.30 (REF.19). Failure to obtain a reliable result is typically associated with a BMI >30 kg/m2, waist circumference >102 cm or increased parietal wall thickness19.
Performance in NAFLD.
Only one study has evaluated advanced fibrosis assessment using SWE in large numbers (n = 291) of patients with NAFLD (where their data are separate from patients with alcoholic liver disease)19. In this study, Cassinoto et al. examined SWE performance along with ARFI and VCTE (TABLE 2). The combined technical failure and unreliable examination rates were similar to VCTE (20.3% versus 23.4%), as was the per-protocol advanced fibrosis assessment (AUROC 0.89). Cut-offs of 8.7 kPa and 10.3 kPa provided 90% sensitivity and specificity, respectively19.
ARFI imaging
Assessment of fibrosis.
ARFI elastography is integrated into a conventional ultrasound device. Similar to SWE, the ARFI operator must define a large, vessel-free region of interest themselves in ultrasonic B-mode imaging using a curved abdominal probe during apnoea. Thereafter, the targeted tissue is subject to 262 μs acoustic pulses at a frequency of 1.0–4.5 MHz. The resulting displacements form a shear-wave propagation front that is tracked by the ultrasound receiver, which follows many 3.08 MHz ultrasound beams laterally adjacent to the push beam within a central window 5 mm by 4 mm. The time to peak displacement measured by each lateral beam is used to estimate the shear-wave speed, which itself is proportional to the square root of the shear moduli (measured in kPa). The results are expressed in m/s but can also be described in terms of the shear moduli. The operator determines an adequate acquisition according to several criteria including excessive motion artefacts, poor signal-to-noise ratio and inadequate imaging ‘windows’. Some ARFI devices provide an error message for inadequate windows without providing a shear-wave speed30.
Performance in NAFLD.
ARFI performance has been evaluated in three studies of patients with NAFLD (TABLE 2). Similar to VCTE and SWE, the per-protocol AUROC for advanced fibrosis stratification is excellent at ~0.90 but the modality also suffers from a risk of technical failure (2.4%, 19.0% and 21.5% in each of the three studies)19,31,32. The rates of technical failure or unreliable results are much higher in patients with elevated BMI (28.0% in patients with a BMI >30 kg/m2 versus 5.2% in patients with lower BMI). Whether inflammation is a confounder has not been explored in NAFLD but can be inferred from studies in viral hepatitis in which elevated ALT or histological necroinflammatory activity are both linked with falsely elevated ARFI values33,34. Although cut-offs also vary, a shear-wave speed of roughly 1.34 m/s is closely associated with advanced fibrosis. Cassinoto et al. provide cut-offs to yield 90% sensitivity and specificity of 1.15 m/s and 1.53 m/s, respectively19.
Magnetic resonance elastography
Assessment of fibrosis.
MRE requires a special adaptation and proprietary software instalment for conventional MRI scanners. During an MRI scan, shear waves at 60 Hz are generated by a circular device (19 cm in diameter), attached to the patient anterior to the liver, which is coupled to an active acoustic driver outside of the MRI room. The resulting shear waves are then visualized using a 2D gradient-recalled-echo pulse sequence. Noncontiguous axial slices (each roughly 10 mm thick) are acquired during 16-second apnoeic episodes over 2 min of scan-time. Wave images are then interpreted using an inversion algorithm processed by commercial software to generate ‘elastograms’ — quantitative, multicolour cross-sectional maps of liver stiffness. Each elastogram, in turn, can be divided into regions of interest where clear shear-wave propagation can be observed along with liver tissue free of vessels and imaging artefacts35. The mean LSM is a function of the average per-pixel stiffness measurements from regions of interest in at least four axial slice locations35–37. Although LSMs from VCTE and SWE are numerically similar, they are not equivalent. The technical failure rate of MRE is lower than those of the other modalities discussed in this Review. In the largest series of MRE examinations (n = 691), the failure rate in patients with NASH was 7.7%38. In general, MRE outperforms all ultrasound-based modalities and has a lower risk of failure in patients with severe obesity31,39. Notably, however, hepatic iron burden — common in haemochromatosis or patients with chronic iron infusions such as those on haemodialysis — can be a cause of failed examinations35. Acute hepatic inflammation and chronic passive congestion typically affect all elastography-based methods and can be reliably excluded only with careful history and clinical assessment23. There is a small subset of patients who will not be able to tolerate an MRE scan owing to claustrophobia. MRE might also be precluded in patients with metal implants or in those who are be too obese for the MRI scanner (typically a body weight >160 kg).
Assessment of hepatic steatosis.
As with CAP for VCTE, additional procedures can be performed at the same time as MRE to quantify steatosis. Proton density fat fraction (PDFF) has emerged as the leading noninvasive quantitative biomarker for liver fat quantification. The approach uses the different resonance frequencies of water and fat protons to determine the proportion of total hepatic protons bound to fat. This parameter is accurate, reproducible and precise and has excellent inter-rater and intrarater reproducibility. MRI-PDFF, reviewed in detail elsewhere40, is a set of sequences that are performed by the MRI scanner in addition to the MRE examination. MRI-PDFF has a robust correlation with MRI spectroscopy-based quantification (the gold standard to quantify liver triglyceride content) for liver fat, with a correlation coefficient ranging from 0.98 to 0.99 (REFS 40–44).
MRI-PDFF is superior to CAP for the quantification of liver steatosis, as shown by two independent studies in patients with biopsy-proven NAFLD18,22. Whereas the AUROCs for the identification of steatosis grades 1, 2 and 3 were 0.99, 0.90 and 0.92 for PDFF, respectively, they were 0.85, 0.70 and 0.73 for CAP18. An estimate of <5% liver fat by PDFF has excellent (100% in one study) negative predictive value for the presentation of histological steatosis burden >10%45. The exact cut-off point of CAP that corresponds to 5% steatosis by MRIPDFF remains to be established. Similar indices of liver fat are lacking for the ultrasound-based modalities. Emerging studies suggest that the backscatter coefficient, measured using ultrasound waves, can be utilized to improve liver fat quantification, and this is an area of intense investigation46.
Performance in NAFLD.
MRE has been evaluated in six studies with large cohorts of patients with NAFLD (TABLE 2). The technical failure rate for MRE was 7.7% (of 49 patients with NAFLD) in the only study to explicitly evaluate failure rates38. The other studies reported failure rates from 0 to 4.5%. In all studies, the AUROC for advanced fibrosis discrimination typically ranged between 0.92 and 0.94, with optimal LSM cut-offs that varied from 2.99 to 4.80 kPa. In one of the earliest studies on MRE in patients with NAFLD (published in 2014), Loomba et al. enrolled 100 patients with an average BMI of 32.4 kg/m2 and a 22% prevalence of F3–F4 fibrosis as determined by liver biopsy and found that 3.63 kPa was the optimal cut-off for advanced fibrosis37. Later, in 2016, Imajo and colleagues found a higher optimal cutoff (4.8 kPa) in a cohort of 142 patients in Japan with an average BMI of 28.1 kg/m2 and 45% prevalence of F3–F4 fibrosis22. In another study published in 2016, Park et al. compared MRE and VCTE in a cohort of 104 patients in the USA with an average BMI of 30.4 kg/m2 and a lower F3–F4 prevalence (18%) and found an optimal cut-off of 2.99 kPa (REF. 18).
Head to head comparisons
Few studies compare multiple elastographic techniques in large (n >100) cohorts of patients with NAFLD. VCTE has been compared with ARFI and SWE in one study of 291 patients by Cassinoto et al.19 In this study, no one modality was more likely to produce more reliable results overall, although for the subgroup of patients with BMI <30 kg/m 2, ARFI might be associated with more reliable results. The AUROCs for advanced fibrosis were comparable (SWE 0.89, VCTE 0.86 and ARFI 0.84), with no statistically significant differences.
VCTE has been compared with MRE in three studies. In a study conducted in Japan with a cohort of patients with NAFLD, Imajo et al. showed that MRE was able to classify the fibrosis stage of more patients owing to the higher technical failure rate of VCTE (TABLE 2). However, there was no statistically significant difference between modalities in the per-protocol risk discrimination of advanced fibrosis (although there a significant difference when assessing for cirrhosis presence (AUROC 0.97 versus 0.92; P = 0.049))22. The studies conducted by Chen et al.23 and Park et al.18 confirmed these data by showing that, in the per-protocol comparison patients who underwent both MRE and VCTE examinations, MRE was superior to VCTE, especially in discriminating between early stages of fibrosis. In determining the risk of F3–F4 fibrosis, Chen et al. found that MRE outperformed VCTE (AUROC 0.97 versus 0.87; P = 0.046), although neither study found a difference in performance for the evaluation for cirrhosis. Finally, ARFI has been compared with MRE in one study by Cui and colleagues31. This study also found that MRE is a superior technique in the assessment of fibrosis burden overall, although ARFI is statistically equivalent to MRE for the discrimination of advanced (F3–F4) fibrosis from F0–F2 fibrosis.
MRE is the best noninvasive biomarker for the quantification of liver fibrosis in NAFLD owing to the low technical failure rate in patients who are obese and high prevalence of obesity in this patient population. In the setting of severe obesity, VCTE and all ultrasound-based modalities can have unreliable readings. However, owing to the cost of and limited access to MRE, as well as the need to confirm its performance in the community outside of expert centers, the other modalities discussed here are typically attempted first. Whether there is a specific BMI cut-off below which VCTE or the ultrasound techniques should be used first, and above which MRE should be used first, is a matter for future study.
Clinical strategy
Each modality reviewed here is most reliable in excluding the presence of advanced (F3–F4) fibrosis. Beyond that determination, positive predictive values diminish in proportion to known confounders such as inflammation and obesity. For this reason, positive results must be evaluated carefully with consideration of patient factors including age, comorbidities, laboratory tests and serological indices, such as FIB-4 and the NAFLD fibrosis scores (FIG. 2). For example, patients with very low FIB-4 scores (suggestive of a low risk of advanced liver fibrosis) might not benefit from elastographic evaluation; conversely, young patients with a long life expectancy but indeterminate FIB-4 scores need highly accurate elastographic testing to provide greater diagnostic certainty and optimize future resource utilization. Overall, the available evidence for elastographic techniques suggest that MRE is the most reliable and accurate modality for the noninvasive determination of advanced fibrosis on an intentionto-diagnose basis. However, its cost (thousands of US dollars) and restricted availability can require that the strategy for risk assessment in NAFLD incorporates, if not relies on, other modalities (which can cost <USD$100)47. MRE is best suited for special cases (for example, those with indeterminate FIB-4 scores and unreliable VCTE readings) or in patients with substantially higher BMIs (perhaps >35 kg/m2). Further research is needed to develop an optimal algorithm for the step-wise utilization of multiple elastographic methods and clinical prediction rules to develop a cost-effective strategy to screen for advanced fibrosis in NAFLD. Similarly, although SWE and ARFI seem to be as promising as VCTE, data are currently limited for these modalities regarding the determination of advanced fibrosis in NAFLD. Furthermore, as VCTE provides real-time results and is performed by clinicians and medical assistants at the point of care, this approach is clinically expedient and enables in-clinic counselling. Where VCTE is not available in clinic, the use of SWE or ARFI versus VCTE in another facility should be based on a few factors, including a discussion with radiologists to decide on the preferred techniques, requirements for operator experience and prespecified criteria for reporting standards to ensure correctly interpreted, valid results. MRE should be considered as the next step in a selected group of individuals, including patients at increased risk of advanced fibrosis (for example, those with indetermin ate FIB-4 scores or LSMs, and those that have a BMI >35 kg/m2). Additionally, there might be cases in which the diagnostic precision offered by MRE is necessary to optimize health-care utilization and outcomes (such as in young patients with LSMs just below the cut-off for advanced fibrosis).
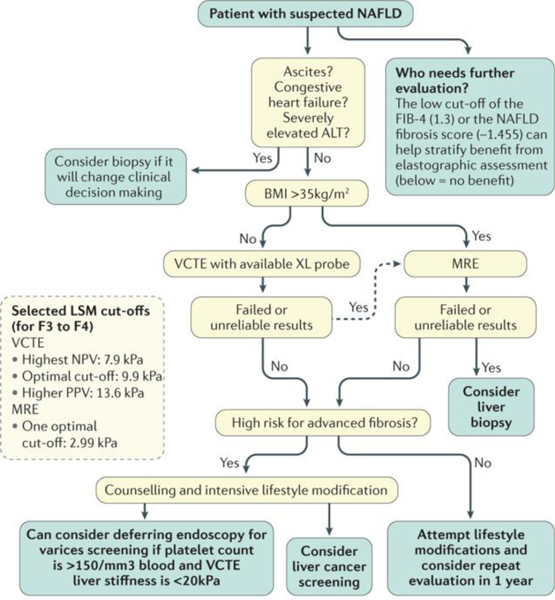
Figure 2 |. A suggested strategy for risk stratification of patients with NAFLD using multiple elastographic methods.
Many patients with low-risk FIB-4 (<1.3) and NAFLD Fibrosis scores (<–1.455) can be excluded from further evaluation by elastography53. For higher-risk patients, for example, patients with indeterminate or high FIB-4 or NAFLD fibrosis scores, we suggest using vibration-controlled transient elastography (VCTE) — and potentially shear-wave elastography (SWE) or acoustic radiation force imaging (ARFI) — for patients with a BMI ≤35 kg/m2 followed by magnetic resonance elastography (MRE) for a BMI >35 kg/m2 or patients with failed and/or unreliable VCTE exams. Selected cut-offs for liver stiffness by modality are chosen from TABLE 2. As patients with advanced fibrosis and cirrhosis are at increased risk of liver cancer, we suggest consideration of screening. Guidelines from the American Association for the Study of Liver Disease suggest that for patients with platelet counts >150/mm3 blood and liver stiffness <20 kPa, one can consider forgoing screening for varices by endoscopy49. ALT, alanine aminotransferase; LSM, liver stiffness measurement; NPV, negative predictive value; PPV, positive predictive value.
Following an initial stratification based on a serological index and the patient’s clinical context, an elastographic technique should be selected. Selected cut-offs for liver stiffness by modality are chosen from TABLE 2. In addition to the statistically optimal LSM cut-offs in TABLE 2, we highlight cut-offs with higher sensitivity, or negative predictive value (7.9 kPa was associated with 100% negative predictive value in one study)20, and higher specificity, or positive predictive value (13.6 kPa accounted for contributions from confounders in another study)27. We suggest that patients identified as having advanced fibrosis or cirrhosis be screened for liver cancer, given the increased risk of this disease in these individuals48. Elastography can also quantify the benefit of screening for varices. Guidelines from the American Association for the Study of Liver Disease suggest that for patients with platelet counts >150/mm3 of blood and liver stiffness <20 kPa, one can consider forgoing screening for varices by endoscopy49.
Research frontiers
The available data on test performance for each of the reviewed modalities might be comprehensive enough to legitimize each technique for clinical use. However, multiple questions remain to clarify the specific role of each test in both the clinic and investigative endeavours. Moving forward, additional research is necessary to fill in the present gaps. Several aspects of study design should be considered.
Intention to diagnose
Although further refinement of LSM cut-offs is valuable, the most important questions are the real-world applicability of a given modality as well as the clinical implications and management of patients who are not candidates for each elastographic technique. Comparisons of each modality ought to account for its clinical usability in, for example, population subsets with high BMI, burden of liver fat or liver enzyme levels in serum. Furthermore, an effort should be taken to develop tools for clinicians to predict which modality is most likely to provide a valid measurement in a particular patient. If a patient can be identified as being at high risk of LSM failure by VCTE, ARFI or SWE, then expedient utilization of MRE is actually more cost-effective.
Quality criteria
For SWE and ARFI to become reliable options in clinical practice and away from the centres in which they were developed, effort must be taken to provide consistent, reproducible quality criteria. The expert consensus provided by the European Federation of Societies for Ultrasound in Medicine and Biology represents the most deliberate effort towards this aim and should be the basis for further study13. The test–retest reliability should be defined for modalities not yet subject to such investigation. Specifically, data are needed regarding the stability of LSMs as measured by MRE, ARFI and SWE among observers and software programs as well as over time (that is, on separate days). MRE, ARFI and SWE each require the operator to define the region of interest, a procedure that introduces a risk of human error. Studies are needed to confirm this central aspect of test interpretation, with the goal of developing consensus-defined features that can be used to adjudicate the quality of the region selected. As for VCTE, the raw images of the wave-fronts measured could also be included and adjudicated by blinded observers. If the regions of interest are not reproducible among operators or with serial imaging tests, the clinical meaning of any given test result would be rendered unclear. Reproducibility is key to safe and effective widespread use. Reported results from a patient examination could include images of the region of interest to support test validity with respect to the features that could define quality examination.
Optimal LSM cut-offs
Given mounting data on the effect of necroinflammatory activity (vis-à-vis serum ALT levels) and potentially hepatosteatosis (vis-à-vis CAP or PDFF) on LSM, further research should define and operationalize the way in which LSM cut-offs are interpreted in patients with variable inflammatory activity and steatosis. Although data along these lines is emerging for VCTE in combination with CAP27, further research is needed both for SWE, ARFI and MRE and for determining how best to incorporate these insights into clinical practice. Options could include software-based algorithms that calculate the effect of confounding factors in real-time or generate flowchart-type decision aids for clinicians.
Meaningfully reduced liver stiffness
The advent of elastographic techniques has moved patients from biopsy-based risk stratification to non-invasive testing. However, to link liver stiffness to clinical outcomes, we must establish the threshold for a minimal decline in liver stiffness in NAFLD that is clinically meaningful. At present, the FDA requires liver histology for entry into therapeutic trials for NAFLD50; convincing data are needed to change this policy. Once validated with respect to the risk of progression to cirrhosis, hepatic decompensation and liver-related mortality, we might be able to replace liver biopsy sample assessment as an end point for approval of therapies for the treatment of NASH-related liver fibrosis. A 2016 study of 39 patients in a clinical trial suggests that a >15% reduction in MRE-derived liver stiffness is seen in patients with NAFLD who experience a 5% reduction in their BMI over a 24-week period51. Further studies are needed to validate these findings and to determine a clinically meaningful reduction in MRE-derived liver stiffness for assessment of treatment response in NASH.
Cost-effectiveness
Data are needed regarding the costs associated with each modality, the effect of each modality on downstream resource utilization and clinical outcomes and patient preferences. An MRE-first strategy is a priori not cost-effective (TABLE 1), and any cost differences between VCTE, SWE or ARFI as the initial modality are marginal. Instead, the most valuable endeavour for this research methodology is to determine the most cost-effective risk-stratification strategy for various subsets of the broader NAFLD cohort (on the basis of age, BMI, ALT levels and other factors). In two cost-effectiveness analyses, patient risk stratification using VCTE was found to be more cost-effective than stratification using liver biopsy samples but not NAFLD fibrosis score47,52. In view of increasing data on this topic, updated analyses including multiple modalities are of interest. These data would aid medical decision making and also inform reimbursement schedules.
Conclusions
Elastography for patients with NAFLD is emerging as a core feature in contemporary clinical practice as well as clinical trials. It is a powerful tool, capable of providing a critical service for a growing population. However, the rapid advancement of this technology challenges clinicians and researchers alike. A deliberate clinical approach informed by knowledge of the pitfalls and preparation for multiple contingencies in case of indeterminate results is crucial. Similarly, the future research agenda should take care to answer the questions most important to the clinicians and patients whom this technology is intended to serve.
Key points:
NAFLD is the most common form of chronic liver disease, and patient-centred risk-assessment strategies are therefore needed for cost-effective care
Liver elastography — or liver stiffness measurement — is an alternative to liver biopsy to evaluate patients with NAFLD for the presence of advanced fibrosis or cirrhosis
Of the available elastographic modalities, vibration-controlled transient elastography is the most studied and magnetic resonance elastography is the most accurate; ultrasound-based elastography is promising but lacks defined examination quality criteria
Future research is needed to establish the optimal sequence of modalities for use in the clinic and the definition of clinically meaningful changes in liver stiffness
Acknowledgements
R.L. is supported in part by the grant R01-DK106419-03. Research reported in this publication was supported in part by the US National Institute of Environmental Health Sciences of the US National Institutes of Health under award number P42ES010337. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US NIH.
Footnotes
Competing interests
R.L. has received research funding support from General Electric and Siemens.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vernon G, Baranova A & Younossi Z Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Williams CD et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Mozumdar A & Liguori G Persistent increase of prevalence of metabolic syndrome among US adults: NHANES III to NHANES 1999–2006. Diabetes Care 34, 216–219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peery AF et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187. e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekstedt M et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Angulo P et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397. e10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasha T, Gabriel S, Therneau T, Dickson ER & Lindor KD Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 27, 1220–1226 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC & Smith AD Liver biopsy. Hepatology 49, 1017–1044 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Foster G et al. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ 315, 453–458 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedossa P, Dargère D & Paradis V Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38, 1449–1457 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Bedossa P Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 20, 15–20 (1994). [PubMed] [Google Scholar]
- 12.Tapper EB & Lok ASF Use of liver imaging and biopsy in clinical practice. N. Engl. J. Med. 377, 756–768 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Dietrich CF et al. EFSUMB Guidelines and Recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long version). Ultraschall Med. 34, 169–184 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Tapper EB et al. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 10, 932–937. e1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapper EB, Castera L & Afdhal NH FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin. Gastroenterol. Hepatol. 13, 27–36 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Boursier J et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 65, 570–578 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Castéra L et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 51, 828–835 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Park CC et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152, 598–607.e2 (2017).This study performs a head to head comparison of VCTE and MRE in a US cohort.
- 19.Cassinotto C et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 63, 1817–1827 (2016).This is the only study to evaluate VCTE, SWE and ARFI in patients with NAFLD.
- 20.Tapper EB, Challies T, Nasser I, Afdhal NH & Lai M The performance of vibration controlled transient elastography in a us cohort of patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 111, 677–684 (2016).The first report of VCTE use for NAFLD in the US (using the M probe).
- 21.Wong VW et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 107, 1862–1871 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Imajo K et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150, 626–637.e7 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Chen J et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 283, 418–428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlas T et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 66, 1022–1030 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Caussy C et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 10.1002/hep.29639 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VW et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J. Hepatol. 67, 577–584 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Petta S et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 65, 1145–1155 (2017).This study uses CAP during VCTE exams to account for the contribution of steatosis to liver stiffness.
- 28.Deffieux T et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 62, 317–324 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Cassinotto C et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 61, 550–557 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Friedrich-Rust M et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 252, 595–604 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Cui J et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 63, 453–461 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmeri ML et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J. Hepatol. 55, 666–672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebinuma H et al. Evaluation of liver fibrosis by transient elastography using acoustic radiation force impulse: comparison with Fibroscan®. J. Gastroenterol. 46, 1238 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Yoon KT et al. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig. Dis. Sci. 57, 1682–1691 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Venkatesh SK, Yin M & Ehman RL Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J. Magnet. Resonance Imag. 37, 544–555 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loomba R et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am. J. Gastroenterol. 111, 986–994 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomba R et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 60, 1920–1928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner M et al. Technical failure of MR elastography examinations of the liver: experience from a large single-center study. Radiology 284, 401–412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui J et al. Comparative diagnostic accuracy of magnetic resonance elastography versus eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment. Pharmacol. Ther. 41, 1271–1280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dulai PS, Sirlin CB & Loomba R MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J. Hepatol. 65, 1006–1016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui J et al. Sitagliptin versus placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J. Hepatol. 65, 369–376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loomba R et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 61, 1239–1250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noureddin M et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58, 1930–1940 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le TA et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56, 922–932 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satkunasingham J et al. Can negligible hepatic steatosis determined by MRI-proton density fat fraction obviate the need for liver biopsy in potential liver donors? Liver Transpl. 10.1002/lt.24965 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Lin SC et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin. Gastroenterol. Hepatol. 13, 1337–1345.e6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapper EB, Sengupta N, Hunink MM, Afdhal NH & Lai M Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am. J. Gastroenterol. 110, 1298–1304 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Bruix J & Sherman M Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Tsao G, Abraldes JG, Berzigotti A & Bosch J Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 65, 310–335 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Sanyal AJ et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases–US Food and Drug Administration Joint Workshop. Hepatology 61, 1392–1405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel NS et al. Weight loss decreases magnetic resonance elastography estimated liver stiffness in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 15, 463–464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tapper EB, Hunink MM, Afdhal NH, Lai M & Sengupta N Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the Primary Care Physician using the NAFLD fibrosis score. PLOS ONE 11, e0147237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chalasani N et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55, 2005–2023 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Petta S et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology 62, 1101–1110 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Kumar R et al. Liver stiffness measurements in patients with different stages of non-alcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig. Dis. Sci. 58, 265–274 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Chan W-K, Mustapha NRN & Mahadeva S A novel 2-step approach combining the NAFLD fibrosis score and liver stiffness measurement for predicting advanced fibrosis. Hepatol. Int. 9, 594–602 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Aykut UE et al. A comparison of FibroMeter™ NAFLD Score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Scand. J. Gastroenterol. 49, 1343–1348 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Naveau S et al. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes. Surg. 24, 1693–1701 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Gaia S et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J. Hepatol. 54, 64–71 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Mahadeva S et al. Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J. Digestive Diseases 14, 604–610 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Wong VWS et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51, 454–462 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Ochi H et al. Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology 56, 1271–1278 (2012). [DOI] [PubMed] [Google Scholar]