Typhoid toxin is a virulence factor of Salmonella enterica serovar Typhi, the causative agent of typhoid fever, and is thought to be responsible for the symptoms of severe disease. This toxin has a unique A2B5 architecture with two active subunits, the ADP ribosyl transferase PltA and the DNase CdtB, linked to a pentameric B subunit, which is alternatively made of PltB or PltC. Here, we describe the generation and characterization of typhoid toxin-neutralizing human monoclonal antibodies by immunizing genetically engineered mice that have a full set of human immunoglobulin variable region genes.
KEYWORDS: typhoid fever, bacterial pathogenesis, Salmonella Typhi, Salmonella Paratyphi, infectious diseases, bacterial toxins, monoclonal antibodies, therapeutics
ABSTRACT
Typhoid toxin is a virulence factor of Salmonella enterica serovar Typhi, the causative agent of typhoid fever, and is thought to be responsible for the symptoms of severe disease. This toxin has a unique A2B5 architecture with two active subunits, the ADP ribosyl transferase PltA and the DNase CdtB, linked to a pentameric B subunit, which is alternatively made of PltB or PltC. Here, we describe the generation and characterization of typhoid toxin-neutralizing human monoclonal antibodies by immunizing genetically engineered mice that have a full set of human immunoglobulin variable region genes. We identified several monoclonal antibodies with strong in vitro and in vivo toxin-neutralizing activity and different mechanisms of toxin neutralization. These antibodies could serve as the basis for the development of novel therapeutic strategies against typhoid fever.
INTRODUCTION
Salmonella enterica serovar Typhi and Paratyphi A are the cause of typhoid and paratyphoid fever, respectively, which affect ∼20 million people annually, leading to an estimated 200,000 deaths (1–5). Disease occurs mostly in developing countries and largely results from the consumption of contaminated food or water. Children under 15 years of age and the elderly are considered most at risk (6, 7). The emergence of multiple antibiotic-resistant S. Typhi and S. Paratyphi, which are becoming endemic in some parts of Asia, is a major concern, as it raises the prospect of untreatable typhoid fever (8, 9). Currently available vaccines to protect against typhoid fever caused by S. Typhi include the parenteral unconjugated Vi polysaccharide (ViPS) and oral live-attenuated Ty21a vaccines, which confer partial protection and are not approved for use in children (10, 11). Recently, a typhoid conjugate vaccine (TCV) has been shown to confer protection to children, and the World Health Organization (WHO) has recommended its deployment in areas of endemicity (12, 13). However, there are currently no available vaccines to protect against typhoid fever caused by S. Paratyphi A. This is a major concern, as paratyphoid fever is quickly emerging and currently accounts for more than 20% of all typhoid fever cases, a proportion expected to increase substantially as current vaccines to protect against S. Typhi infection are deployed (14–16). Consequently, there is an urgent need for the development of novel therapeutic strategies that can target both S. Typhi and S. Paratyphi A.
Typhoid toxin is a virulence factor carried by both S. Typhi and S. Paratyphi A and is absent from nontyphoidal serovars (17–22). Toxins with homology to typhoid toxin are present in some clade B serovars of S. enterica, which are rarely associated with disease in humans (23). However, when they infect humans, some of these serovars can cause a disease that is clinically indistinguishable from typhoid fever (24). Typhoid toxin is an unusual AB5 toxin with a unique architecture composed of a pentameric B subunit (PltB) associated with two A subunits, an ADP ribosyl transferase (PltA) and a DNase (CdtB), which are covalently linked to one another by a disulfide bond (19). Recently, an alternative form of typhoid toxin assembled by S. Typhi and S. Paratyphi A has been described in which the PltB subunit is replaced by a different subunit (PltC) that is present away from the typhoid toxin locus (22). Studies have shown that typhoid toxin may have emerged recently, from the combination of two toxins, namely, an ArtAB-like toxin broadly distributed in many S. enterica serovars, which showed amino acid sequence similarity to PltB/PltC and PltA; and cytolethal distending toxin (CDT), whose A subunit exhibits amino acid sequence similarity to CdtB (25). Two cell surface glycoproteins, CD45 and podocalixin 1, have been shown to serve as typhoid toxin receptors in myelocytic and epithelial cells, respectively (19). More importantly, typhoid toxin specifically recognizes acetyl neuraminic acid (Neu5Ac)-terminated sialoglycans on these receptor molecules (26). This observation is relevant since, unlike most other mammals whose sialoglycans are terminated in glycolyl neuraminic acid (Neu5Gc), humans display sialoglycans terminated in Neu5Ac (27). In humans, this is due to the presence a loss-of-function mutation in CMAH, which encodes the enzyme that converts Neu5Ac into Neu5Gc (28). Consequently, typhoid toxin is specialized to exert its function in human tissues, which is consistent with the strict human host specificity exhibited by both S. Typhi and S. Paratyphi A.
Administration of typhoid toxin into mice displaying Neu5Ac-terminated glycans can reproduce many of the symptoms of severe typhoid, including stupor, encephalopathy, hemodynamic shock, and leukopenia, strongly suggesting a role for typhoid toxin in the pathogenesis of severe typhoid fever (19, 22, 29). A recent study in human volunteers detected no differences in the onset and severity of disease between patients challenged with either wild-type S. Typhi or a typhoid toxin-deficient S. Typhi mutant (30). However, for ethical reasons, in this model, the disease process is interrupted immediately upon appearance of early symptoms of disease (i.e., fever), thus precluding the development of the more severe typhoid fever symptoms commonly seen in hospital settings in areas of endemicity that appear later in the disease progression (31–34). In fact, the average length of time from disease onset (fever) to hospitalization in areas of endemicity is most often more than 10 days (31, 32, 35). This time course allows typhoid toxin to exert its effects, thus explaining the differences in the manifestation of typhoid fever in the natural setting compared with controlled infection volunteer studies. In fact, it has been determined that the frequency of occurrence of severe typhoid fever is strongly correlated with the duration of illness preceding hospitalization (31–34). Furthermore, the occurrences of multiple antibiotic-resistant S. Typhi or S. Paratyphi A often lengthens the time to proper diagnosis and treatment, thus increasing the risk of severe typhoid (35). Consequently, there is an urgent need for the development of novel therapeutic interventions that could be deployed in areas of endemicity and that could aid in the treatment of severe typhoid. Typhoid toxin, which is conserved in both S. Typhi and S. Paratyphi A, offers unique opportunities for the development of such therapeutic strategies.
Here, we describe the generation of human monoclonal antibodies directed to typhoid toxin using genetically engineered mice that have a full set of human immunoglobulin variable region genes (36). We identified several monoclonal antibodies that effectively neutralize the toxin and are able to protect mice challenged with a lethal dose of typhoid toxin. We found that different monoclonal antibodies neutralize the toxin utilizing different mechanisms, preventing the toxin from entering cells or from reaching its intracellular targets. These antibodies could therefore serve as the basis for the development of effective antitoxin strategies that could aid in the treatment of severe typhoid fever.
RESULTS
Generation of human monoclonal antibodies against typhoid toxin.
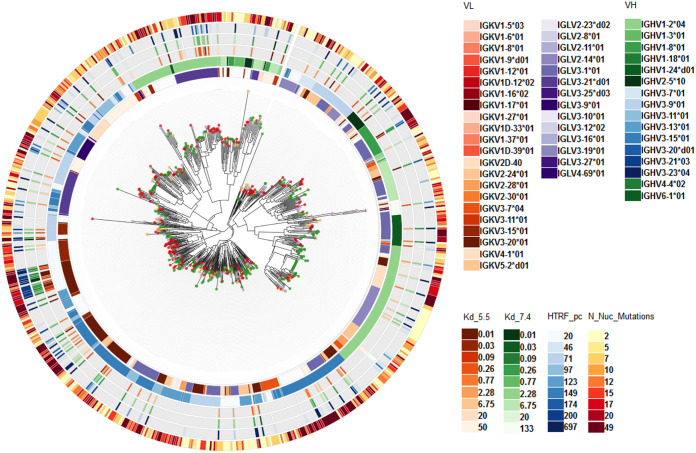
To generate human monoclonal antibodies (MAbs) directed to typhoid toxin, we used mice transgenic for the nonrearranged human immunoglobulin (Ig) loci. Specifically we used mice that are homozygous for both humanized heavy- and kappa light-chain alleles but with an unmodified but low-expressing mouse lambda light-chain locus or mice homozygous for human heavy- and lambda light-chain alleles and homozygous knockouts for the kappa locus (36). Immunization of these mice with a purified preparation of a genetically detoxified version of typhoid toxin carrying catalytic mutations in both of its active subunits, PltA (PltAE133A) and CdtB (CdtBH160Q) (19), resulted in a robust immune response to the toxin (endpoint titers measured by enzyme-linked immunosorbent assay [ELISA] of ≤1 to 5 × 10−5). Immunoglobulin paired heavy- and light-chain sequences were amplified by PCR from single-cell preparations of plasma cells from spleens and bone marrows, directly sequenced, and subsequently transferred to expression vectors for monoclonal antibody production and binding assay screening. From four different immunizations, a total of 153 96-well plates were obtained, yielding 13,464 single plasma cells. High-throughput MAb expression and primary screening yielded 1,743 (13%) MAbs able to bind typhoid toxin, of which 1,330 were validated in secondary MAb affinity screening by surface plasmon resonance (SPR). In parallel, immunoglobulin sequence data were obtained for 1,312 B cells, of which 648 (49%) were paired heavy- and light-chain sequences, and of those, 115 (18%) had matched binding data (Fig. 1). The plasma cell population within the immunized mice covered a range of IgHV and IgL kappa and lambda V gene usage (Fig. 1, IgL lambda), and the antigen-binding antibodies were distributed throughout the phylogeny, including antibodies with high numbers of mutations from inferred germ line IgH and L sequences, indicating a broad and vigorous adaptive immune response to typhoid toxin immunization. Affinity measurement of MAbs binding to toxin at pH 5.5 and 7.4, together with the degree of somatic hypermutation from germ line and IgV gene usage diversity were used to select 120 MAbs for production and further analyses.
FIG 1.
Antibody repertoire analysis from typhoid toxin immunized mice. The immunoglobulin heavy-and light-chain (IgH&L) phylogeny was built as a neighbor-joining tree from the single-cell sequencing of plasma cells. Colors on the tips of the phylogeny correspond to the individual mouse analyzed. The circular plot around the phylogeny shows antibody sequences and functional properties as per the color key. The inner circle corresponds to the sequence assigned IgL V gene, followed by IgH V gene, antibody binding affinity to typhoid toxin at pH 5.5 (dissociation constant [Kd] from 0.01 to 50 nM) or pH 7.4 (Kd from 0.01 to 133 nM), with the outer circle corresponding to IgH&L mutations from the predicted germ line sequence indicative of somatic hypermutation.
Typhoid toxin-neutralizing activity of human monoclonal antibodies.
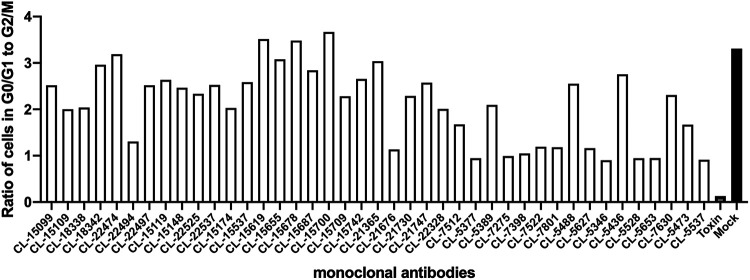
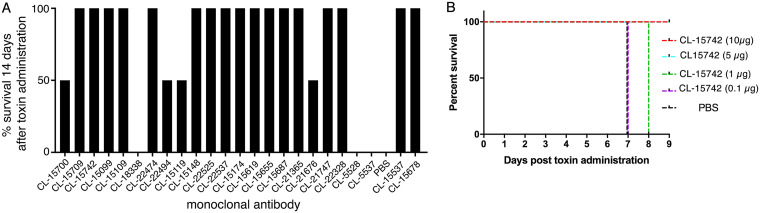
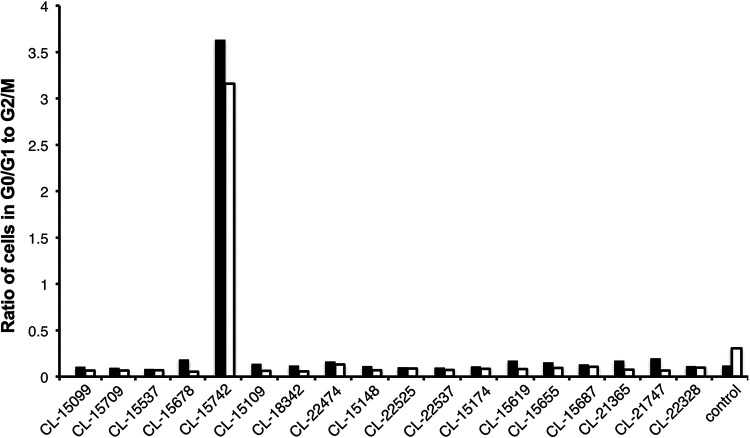
Application of typhoid toxin to cultured cells results in G2/M cell cycle arrest due to DNA damage caused by its CdtB subunit (18, 19). To evaluate the toxin-neutralizing activity of the monoclonal antibodies, we tested their ability to neutralize the typhoid toxin-dependent cell cycle arrest in cultured cells. We preincubated the different monoclonal antibodies with purified typhoid toxin, and the toxin/antibody mixture was subsequently applied to cultured epithelial Henle-407 cells. Sixty-eight hours after intoxication, the cells were analyzed for DNA content to determine their cell cycle state. We found that 41 of the 120 monoclonal antibodies in this assay were able to neutralize the toxin activity (Fig. 2). We then produced larger amounts of 34 of the antibodies that showed toxin-neutralizing activity and tested them for their ability to protect mice against toxin challenge. Mice were administered intraperitoneally 10 μg of monoclonal antibodies, and 24 h later, the treated mice were challenged with a lethal dose of typhoid toxin (2 μg). We found that half (17) of the antibodies were able to fully protect mice from typhoid toxin challenge (Fig. 3A). Protected mice showed no signs of intoxication and did not lose weight during the experiment. One monoclonal antibody (CL-15742) was further evaluated to find its minimal toxin neutralization concentration. Mice were administered 10, 5, 1, or 0.1 μg of the selected antibody, and 24 h afterward, treated mice were challenged with 2 μg of typhoid toxin. Mice receiving 5 and 10 μg of the antibody were fully protected against toxin challenge, while mice that received 1 and 0.1 μg succumbed to intoxication, although those receiving 1 μg died later after challenge (Fig. 3B). Taken together, these experiments identified several human sequence monoclonal antibodies that exhibit potent toxin-neutralizing activity both in vitro and in vivo and are therefore candidates for the development of potential antitoxin therapies.
FIG 2.
In vitro typhoid toxin-neutralizing activity of candidate human monoclonal antibodies. Neutralizing activity is depicted as a ratio of the number of cells in G0/G1 to G2/M of the cell cycle, as determined by their DNA content measured by flow cytometry. Controls include cells treated with toxin alone (toxin) or medium alone (mock).
FIG 3.
In vivo typhoid toxin-neutralizing activity of candidate human monoclonal antibodies assayed in mice. (A) The toxin-neutralizing activity was measured in mice by intraperitoneally administering equal amounts (10 μg) of each of the antibodies prior to the administration of a lethal dose (2 μg) of typhoid toxin. A minimum of 2 animals were used to assay each antibody. Data are expressed as the percentage of animals that survived toxin administration. (B) Titration of the neutralizing activity of a selected monoclonal antibody. The toxin-neutralizing activity was measured in mice as indicated above after administration of the indicated amounts of the monoclonal antibody. A minimum of 3 animals were used for each amount of antibody tested.
Toxin-neutralizing monoclonal antibodies recognize the typhoid toxin holotoxin.
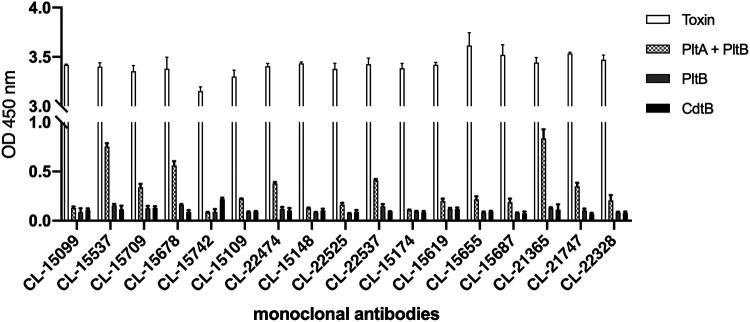
To gain insight into potential epitopes recognized by the typhoid toxin-neutralizing human monoclonal antibodies, we examined with ELISA the subset that showed protection in mice for their reactivity to the different subunits of typhoid toxin. We found that while all antibodies robustly recognized the holotoxin, they showed poor or no reactivity when the individual toxin subunits were used as antigens in the assay (Fig. 4). Furthermore, with the exception of one monoclonal antibody (CL-15742), which recognized CdtB, none of the antibodies recognized any of the individual typhoid toxin subunits in Western blot assays (see Fig. S1 in the supplemental material). These results suggest that the toxin-neutralizing monoclonal antibodies must recognize nonlinear, conformational epitopes that are best displayed in the fully assembled holotoxin. However, other hypothesis that could explain these results cannot be ruled out, such as differential binding of the different antigens to the assay plate.
FIG 4.
Reactivity of the monoclonal antibodies to different components of typhoid toxin measured by solid-phase ELISA. Numbers represent absorbance at 450 nm and are the mean ± standard deviation of 3 technical replicates. This experiment was conducted at least three independent times with equivalent results. OD, optical density.
Monoclonal antibodies neutralize typhoid toxin by blocking toxin receptor binding or toxin intracellular transport.
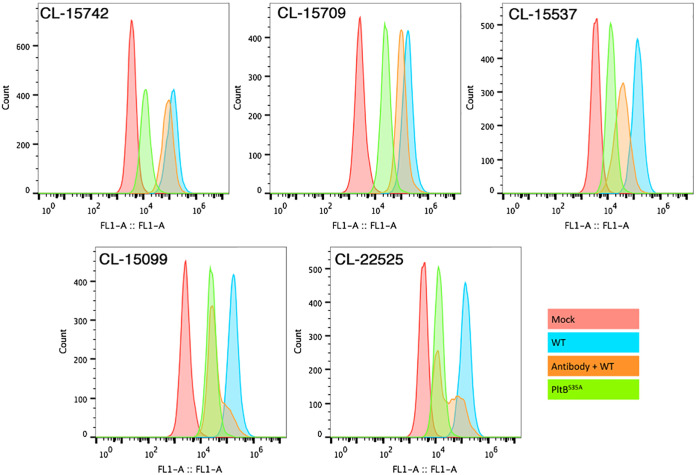
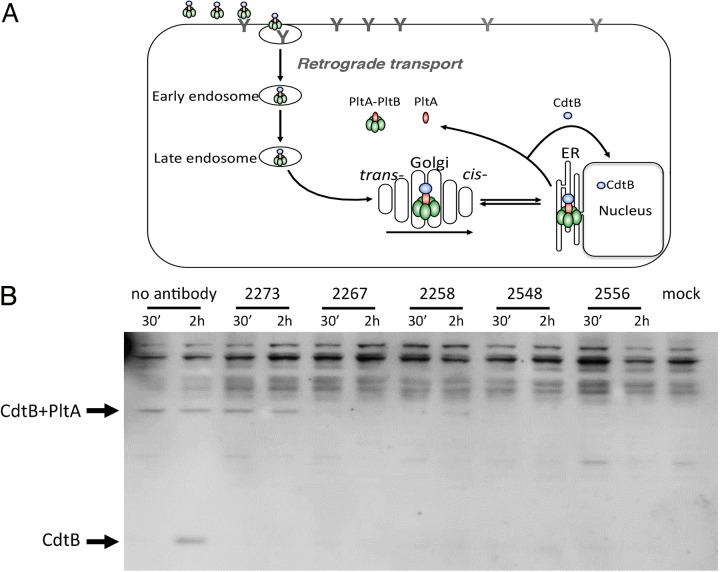
Antibody-mediated toxin neutralization often occurs by preventing toxin uptake (37–40). However, studies have also shown that toxin neutralization can occur without interfering with toxin uptake (38, 41). To gain insight into potential mechanisms of toxin neutralization, we used a flow cytometric assay to investigate the ability of a subset of the typhoid toxin-neutralizing monoclonal antibodies to block fluorescently labeled toxin binding to cultured epithelial cells. We found that in this assay, 3 (CL-15537, SL-15099, and CL-22525) out of the 5 antibodies tested blocked toxin binding, as shown by the decreased toxin-associated fluorescence (Fig. 5). One antibody (CL-15742), which recognizes CdtB (Fig. S1), had no effect on the levels of toxin-associated fluorescence, suggesting that its neutralizing activity must be exerted by a mechanism different from blocking toxin binding. The interpretation of the results obtained with antibody CL-15709 were complicated by residual direct binding of the antibody to culture cells, as shown by the enhanced binding of the PltBS35A typhoid toxin mutant in the presence of this antibody under these assay conditions. However, under more stringent conditions (see below), this was not the case. We then examined the potential neutralization mechanism of the different monoclonal antibodies with an assay that probes the ability of the toxin to traffic within the cell (42, 43). More specifically, this assay uses the cleavage of the disulfide bond that links the two A subunits of typhoid toxin, PltA and CdtB (19), as a measure of toxin arrival to the endoplasmic reticulum since previous studies have shown that the disassembly of the PltA-CdtB covalent complex occurs in this cellular compartment (42). We found that, consistent with the flow cytometric analysis, monoclonal antibodies CL-15709, CL-15537, CL-15099, and CL-22525 blocked toxin binding, which was shown by the inability to detect typhoid toxin in cells that had received typhoid toxin preincubated with these antibodies (Fig. 6). In contrast, typhoid toxin was readily detected after its neutralization with antibody CL-15742, further demonstrating that this antibody does not block toxin binding and internalization into the cell. However, the disassembly of the CdtB-PltA covalent complex was blocked, indicating that this antibody interferes with the retrograde transport of typhoid toxin to the endoplasmic reticulum. Taken together, these results revealed at least two independent mechanisms of toxin neutralization exerted by different human monoclonal antibodies.
FIG 5.
Inhibition of toxin binding by different monoclonal antibodies. Cells were treated with fluorescently labeled typhoid toxin or a mutant unable to bind its cellular receptor due to a mutation in its PltB subunit (PltBS35A), which had been preincubated with the indicated monoclonal antibodies. Toxin binding was evaluated by flow cytometry. This experiment was carried out at least three times with equivalent results. WT, wild type.
FIG 6.
Inhibition of holotoxin disassembly by the different monoclonal antibodies. (A) Diagram of typhoid toxin intracellular transport. (B) Cells were treated with typhoid toxin that had been preincubated with the indicated monoclonal antibodies. At the indicated times, cells were lysed and analyzed by western immunoblotting with an antibody directed to the CdtB subunit of typhoid toxin. Indicated are the expected migration positions of CdtB and the CdtB/PltA complex. This experiment was repeated three times with equivalent results. ER, endoplasmic reticulum.
Identification of a monoclonal antibody that neutralizes both the PltC and PltB versions of typhoid toxin.
As discussed above, both S. Typhi and S. Paratyphi A assemble two alternative, functionally distinct versions of typhoid toxin, with either PltB or PltC as its B subunit component (22). Although the monoclonal antibodies were generated after immunization with the PltB version of typhoid toxin, we investigated the ability of a subset of monoclonal antibodies to neutralize the activity of the PltC-typhoid toxin. We found that only one (CL-15742) of the five monoclonal antibodies tested was able to neutralize PltC-typhoid toxin (Fig. 7). This result is consistent with the observation that CL-15742 recognizes CdtB, which is present in both toxins. Although the epitopes recognized by the monoclonal antibodies that are unable to neutralize PltC-typhoid toxin are not known, the observation that they preferentially interact with the holotoxin suggests that the epitope they recognize must depend on the presence of PltB. The identification of a monoclonal antibody that can neutralize both versions of typhoid toxin has implications for the development of anti-typhoid toxin therapeutic strategies.
FIG 7.
In vitro PltC-typhoid toxin-neutralizing activity of candidate human monoclonal antibodies. Neutralizing activity is depicted as a ratio of the number of cells in G0/G1 to G2/M of the cell cycle, as determined by their DNA content measured by flow cytometry. The results of two independent experiments (open and closed bars) are shown. Cells treated with toxin that had been incubated with buffer are indicated as “control.”
DISCUSSION
Typhoid and paratyphoid fevers continue to be a major global health challenge, with an estimated 30 million annual cases (3–5). The case fatality rate for patients infected with S. Typhi and S. Paratyphi is relatively high (∼2%), even in infections with drug-susceptible bacteria (33). Fatalities are due to a variety of complications that are associated with severe forms of the disease (31–34). The quick emergence of multiple drug-resistant S. Typhi and S. Paratyphi bacteria is likely to complicate therapy even more (8, 9). The ongoing deployment of the typhoid conjugate vaccine (TCV) in areas of endemicity is likely to help mitigate some of these issues. However, the vaccine confers no protection against Salmonella Paratyphi A, which currently accounts for ∼20% of all cases of typhoid fever, a proportion likely to rise as the vaccine deployment expands (14–16). Consequently, there is an urgent need to develop novel therapeutic and prevention strategies that can address this urgent public health issue. Typhoid toxin, a virulence factor encoded by both Salmonella Typhi and Salmonella Paratyphi A, offers a unique opportunity for therapeutic intervention (20). Compelling data strongly suggest that typhoid toxin is responsible for the generation of some of the symptoms of severe typhoid fever since those symptoms can be reproduced when the toxin is applied to susceptible animals (19, 22, 29). Furthermore, the toxin is present in all the serovars associated with typhoid fever (S. Typhi and S. Paratyphi A), but it is absent from serovars that induce self-limiting gastroenteritis (e.g., Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Enteritidis) (20). Close homologs of typhoid toxin have also been detected in other serovars, which are rarely associated with disease in humans (23). However, when some of these serovars (e.g., Salmonella enterica subsp. arizonae) infect humans, the clinical presentation is often indistinguishable from typhoid fever (24). A recent controlled human volunteer study compared infections with wild-type S. Typhi or a S. Typhi mutant carrying a deletion in the typhoid toxin locus. This study found no difference in the onset and severity of disease associated with infection with either of these strains (30). However, in this model, infection is interrupted immediately upon the appearance of early symptoms (i.e., fever), which would prevent typhoid toxin from exerting its effect. Consequently, the human infection volunteer study could not address the contribution of typhoid toxin to the development of severe typhoid fever, which in turn is directly associated with fatalities. In areas of endemicity, the average length between the onset of fever and admission to a health care facility is ∼10 days, thus leading to a different manifestation of typhoid fever and the occurrence of severe typhoid (31, 32, 35). In fact, the occurrence of severe typhoid is directly correlated with the duration of illness prior to admission to a hospital. Consequently, although the human volunteer study suggested that typhoid toxin might not be important for initial infection in a controlled setting, it did not address the potential role of typhoid toxin in later stages of disease and the development of severe typhoid fever. As fatalities are associated with severe manifestations of typhoid fever, targeting typhoid toxin could provide the bases for the development of potentially life-saving therapeutic interventions.
In this study, we aimed to develop typhoid toxin-neutralizing human monoclonal antibodies that could be deployed in areas of endemicity to treat severe cases of typhoid fever. We used genetically engineered mice that have a full set of human immunoglobulin variable region genes, which has been shown to be a powerful platform for generating a full repertoire of human antibodies (36). We identified several monoclonal antibodies with strong toxin-neutralizing activity both in vitro and in a mouse model of intoxication. With the exception of one monoclonal antibody that can recognize an epitope in the active subunit CdtB, all other antibodies appear to recognize nonlinear, conformational epitopes in the holotoxin. Consequently, precise identification of the specific epitope(s) recognized by these antibodies most likely will require the solution of the atomic structures of the toxin-antibody complexes.
We investigated the potential mechanisms of action of a subset of the toxin-neutralizing antibodies and found at least two different mechanisms of action. Four monoclonal antibodies prevented the binding of typhoid toxin to target cells. Although the epitope(s) recognized by these monoclonal antibodies have not been identified, they all showed a strong preference for binding to the holotoxin and did not bind to any of the toxin subunits in a Western blot assay. These observations suggest that these monoclonal antibodies may bind nonlinear conformational epitopes on the holotoxin and may prevent toxin binding by steric hindrance. One of the monoclonal antibodies did not block toxin binding but instead blocked toxin retrograde transport to the endoplasmic reticulum. This antibody most likely recognizes a linear epitope on CdtB, as it was the only antibody that showed reactivity in Western blot assays. However, as some degree of protein renaturalization does occur during Western blotting transfer, more experiments will be required to ascertain this hypothesis. The location of CdtB away from the PltB subunit of the holotoxin is consistent with the inability of this monoclonal antibody to block toxin binding.
We have recently discovered that S. Typhi and S. Paratyphi A produce an alternative form of typhoid toxin assembled with a different B subunit, PltC (22). This alternative form of typhoid toxin exhibits different biological properties. When administered to mice, the PltC form of typhoid toxin causes a more profound leukopenia but much reduced neurological symptoms. Therefore, by diversifying its B subunits, Salmonella sp. can presumably broaden the cellular and tissue target range of typhoid toxin. For therapeutic purposes, it would be highly desirable to block both forms of typhoid toxin. We therefore tested a subset of the typhoid toxin-neutralizing antibodies raised against the PltB version of the toxin for their ability to neutralize the PltC form of typhoid toxin. We found one antibody, CL-15742, which was capable of effectively neutralizing the PltC-typhoid toxin, both in vitro and in vivo. The broad neutralizing activity of this monoclonal antibody is consistent with the observation that it targets CdtB, which is a common subunit to both forms of typhoid toxin. The other monoclonal antibodies tested failed to neutralize PltC-typhoid toxin, which is also consistent with the notion that these antibodies recognize nonlinear conformation epitopes available only in the fully assembled toxin.
In summary, we have generated a panel of human monoclonal antibodies with the ability to neutralize both versions of typhoid toxin by either preventing its binding to target cells or interfering with its intracellular transport. These antibodies could provide the basis for the development of much needed therapeutic strategies for the treatment of typhoid fever.
MATERIALS AND METHODS
Typhoid toxin purification.
PltB- or PltC-typhoid toxin, the genetically detoxified derivative (PltB/PltAE133A/CdtBH160Q), PltA/PltB, or CdtB were purified as previously described (19, 22).
Generation of antibodies.
Genetically engineered mice that have a full set of human immunoglobulin variable region genes were immunized with detoxified mutant typhoid toxin (PltB/PltAE133A/CdtBH160Q) to generate anti-typhoid toxin antibodies. All mice were maintained and all procedures carried out under United Kingdom Home Office PPL 80/2432 and with the approval of the Sanger Institute Animal Welfare and Ethical Review Body. The mice were immunized with up to 25 μg of intraperitoneal typhoid toxoid and were boosted between one and three times during the study. The final boost was given intravenously. Sera were collected for analysis, and mice were periodically assessed for their immune response by ELISA. Following sacrifice, spleens and bone marrows were harvested and processed to a single-cell suspension for sorting by fluorescence-activated cell sorter (FACS).
Recovery of antigen-specific monoclonal antibodies by B cell technology.
Following sacrifice, spleens and bone marrows from the immunized mice were harvested and processed to a single-cell suspension. To obtain single-cell plasmablasts and plasma cells, the cell suspensions were sorted by FACS using the sorting markers CD138high, IgM−, IgD−, IgA−, CD3−, and B220. Ninety-eight 96-well plates were sorted from 16 immunized mice, and B cells were processed by PCR to amplify IgH and IgL chain sequences for sequencing and monoclonal antibody expression. The sequences of the antibody V region were recovered by reverse transcriptase PCR (RT-PCR) and two rounds of PCRs. Single-cell sorted plates stored at –80°C were thawed on ice and briefly centrifuged before use. Plates were incubated in the thermal cycler at 65°C for 5 min and indefinitely at 4°C. A 6-μl mixture of primers, Superscript III, deoxynucleoside triphosphate (dNTP), and RNase inhibitor and buffer were added into each well and mixed by pipetting. Plates were briefly centrifuged and incubated at 50°C for 60 min. Constant region-specific primers for heavy chain and light chain were used to generate cDNAs from single cells. Gene-specific reverse primers were used to amplify the kappa (kappa RT1), lambda (lambda RT3), and gamma (gamma RT1) chains. The first round of PCR was performed with forward V gene-specific primers with a human cytomegalovirus (hCMV) promoter fragment at the 5′ end and reverse constant region-specific primers. All of the products from the RT-PCR were used as the templates for the 1st PCR, which yielded the variable immunoglobulin region and part of the constant region. In the second round of PCR, a generic forward primer that annealed to the hCMV tag was used with a reverse nested primer for the constant region. For antibody expression in mammalian cells, the amplified products were bridged with a linear Ig-cassette containing a 5′ PiggyBac (PB) long terminal repeat (LTR)-CMV promoter and a constant region-poly(A) signal-3′ PB LTR. The Ig-cassette contains all essential elements for expression of the antibody, including the CMV promoter, the immunoglobulin chain constant region, and the poly(A) signal. The bridge step allows all the expression elements and PB LTRs to be brought together to form the PB transposon with heavy-chain and light-chain expression genes. Ig sequences were then transfected into HEK293 cells (Expi293F cells, catalog number A14635; Gibco) for antibody production. The Ig sequences were then binned into separate files (for IgG1, Igκ, and Igλ) by constant region using the Kymab seq-utils program. The alignment of the V(D)J sequence was performed using IMGT/V-QUEST software (www.imgt.org) that compares an antibody chain sequence to a database of known alleles of germ line sequences and predicts the germ line alleles used in the antibody, compares how the germ line sequences were recombined, and compares mutations in the antibody relative to the germ line. The variable immunoglobulin region comprises a VDJ region of an immunoglobulin nucleotide sequence for heavy genes and a VJ region of an immunoglobulin nucleotide sequence for Igκ and Igλ. A clonal family is generally defined by the use of related immunoglobulin heavy-chain and/or light-chain V(D)J sequences by 2 or more samples. Related immunoglobulin heavy-chain V(D)J sequences can be identified by their shared usage of V(D)J gene segments present in the genome.
Screening supernatants containing mouse IgGs by HTRF.
Antibody supernatant collected on day 8 after transfection was screened for binding to typhoid toxoid by homogeneous time-resolved fluorescence (HTRF), as previously described (44). A control anti-His antibody was diluted in Expi293 expression medium (Gibco) from a starting working concentration of up to 120 nM to 2e-3 nM (5e-4 nM final concentration), over 11-point titration. Titrations of 5 μl of the antibody preparation were added to a 384-well white-walled assay plate (Greiner Bio-One). Negative-control wells only received 5 μl of Expi293 expression medium. Five microliters of test supernatants (undetermined concentration) were added to each well of the 384-well plate. This procedure was carried out in duplicate, and to each well, either 5 μl of typhoid toxoid conjugated to Alexa 647 (Lightning-Link; Innova Bioscience) (15 nM final concentration) or 5 μl of irrelevant His-tagged protein conjugated to Alexa 647 was added, except for the negative-control wells, which instead received 5 μl Expi293 expression medium. Finally, 10 μl of anti-mouse IgG donor MAb (Southern Biotech) labeled with europium cryptate (Cis Bio) (1:4,000 final concentration) was added to each well, and the assay was incubated at room temperature in the dark for 2 h. After incubation, the assay was read on an Envision plate reader (Perkin Elmer) using a standard HTRF protocol (44). The values for the 620-nm and 665-nm channels were exported to Excel (Microsoft), and Delta-F calculations were performed. Percent effect values were calculated relative to Delta-F values for control antibody A at 6.66 nM. All antibodies which had a percent effect of 10% to 20% or greater (and were specific for typhoid toxoid only) were used for further screening. The Delta-F calculation (665/620 nm ratio for ratio metric data reduction) is as follows:
where signal negative control is the average of the minimum signal ratio.
SPR analysis using the ProteOn XPR array system.
An anti-mouse IgG capture (product number BR100838; GE Healthcare) surface on a GLM biosensor chip (1765012; Bio-Rad) was created using primary amine coupling. Test antibodies were captured on the anti-mouse surface, and typhoid toxoid (at 200, 40, 12.5, 3.125, and 0.78 mg/ml) was used as the analyte passed over the captured antibodies to generate binding sensograms. Double referencing was carried out with a buffer injection (i.e., 0 nM), and the data were analyzed using the 1:1 binding model inherent to the ProteOn analysis software (Bio-Rad). HBS-EP running buffer was altered to between pH 5.5 or 7.4 to provide alternative analysis. In the case of human antibodies, an anti-human IgG capture surface using a cocktail of three anti-human IgG antibodies (109-005-008, 109-006-008, and 309-006-008; Jackson Laboratory) on a GLC biosensor chip (1765011; Bio-Rad) was created using primary amine coupling. In both cases, antibodies were considered to bind the toxin if binding, as detected by SPR, was at least 20% of the theoretical Rmax.
Indirect ELISA for antibody screening for reactivity to the different typhoid toxin subunits.
Purified preparations of typhoid toxin holotoxin, PltA/PltB, PltB, or CdtB (1 μm/ml in Tris-buffered saline [TBS]) were used to coat 96-well ELISA plates at room temperature for 8 h. After blocking, the different monoclonal antibodies were added to the coated plates and incubated at room temperature for 3 h. Plates were washed (4 times) with TBS buffer, and horseradish peroxidase (HRP)-conjugated anti-human antibody was added and incubated at room temperature for 1 h. The HRP substrate was subsequently added to the plates and incubated until the development of a color reaction (∼15 min), the enzymatic reaction was stopped by the addition of 2 M H2SO4, and the absorbance at 450 nm was read on a plate reader.
Antibody-mediated toxin neutralization.
The ability of the different antibodies to neutralize the typhoid toxin activity was evaluated as follows. Purified typhoid toxin (6 nM) was incubated with the different antibodies (90 nM) for 30 min at room temperature and applied to cultured cells for 68 h. Treated cells were trypsinized, collected, washed, and fixed overnight at –20°C in 70% ethanol and phosphate-buffered saline (PBS). The fixed cells were resuspended in 500 μl of PBS containing 50 μg ml−1 propidium iodide, 0.1 mg ml−1 RNase A, and 0.05% Triton X-100. After incubation for 40 min at 37°C, cells were washed with PBS, resuspended in 500 μl PBS, filtered, and analyzed by flow cytometry. The DNA content was determined using FlowJo (Treestar). The threshold for antibody neutralization activity was determined using an irrelevant antibody as a negative control.
Antibody-mediated inhibition of toxin binding to cultured mammalian cells.
The ability of the different monoclonal antibodies to inhibit toxin binding to cultured Henle-407 human epithelial cells was evaluated as follows. Cells were seeded in 12-well plates at a density of ∼2.5 × 105 cells per well and treated with 1 μg of purified Oregon Green-488 (Invitrogen)-labeled typhoid toxin that had been previously incubated with 10 μg of the different antibodies at room temperature for 30 min. Treated cells were incubated at room temperature for 30 min, washed three times with PBS, detached from the culture dishes after addition of 400 μl of 15 mM EDTA per well, and incubated again for 30 min at 37°C. Detached cells were washed in PBS, fixed in 2% paraformaldehyde (PFA), and analyzed by flow cytometry as previously described (19).
Typhoid toxin disassembly assay.
The effect of the different antibodies on the disassembly of typhoid toxin after treatment of cultured cells was evaluated as previously described (42). Briefly, Henle-407 cells seeded on 10-cm dishes were treated with purified typhoid toxin that had been preincubated (30 min at room temperature) with the different antibodies. Treated cells were washed in Dulbecco’s PBS (DPBS), incubated in media containing 10% FBS for the indicated times, lysed in lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.4], 0.5% Triton-100, and 1× protease inhibitor cocktail [Roche]) for 30 min on ice, and centrifuged at 14,000 rpm for 15 min. Typhoid toxin from the soluble fractions was recovered by affinity chromatography through a nickel resin (Qiagen) after overnight incubation at 4°C and subsequent elution in 30 μl of an elution buffer containing 200 mM imidazole and 0.15 M Tris-HCl (pH 6.8) for 20 min at room temperature. The protein eluates were analyzed by Western blot with a rabbit anti-typhoid toxin antibody and a secondary HRP-conjugated goat anti-rabbit antibody in the presence or absence of dithiothreitol (DTT).
Animal intoxication experiments.
All animal experiments followed the ethical regulations and were conducted according to protocols approved by Yale University’s Institutional Animal Care and Use Committee. C57BL/6 mice were anesthetized with 30% (wt/vol) isoflurane in propylene glycol, and 100 μl of a purified toxin (2 μg) solution that had been pretreated (60 min at room temperature) with the indicated amounts of the different antibodies was administered via the retro-orbital route. Changes in behavior and weight and the survival of the toxin-injected mice were closely monitored during the experiment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Gates Foundation grant number OPP1112035 and National Institute of Allergy and Infectious Diseases grant number AI079022 (to J.E.G.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Dougan G, Baker S. 2014. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu Rev Microbiol 68:317–336. doi: 10.1146/annurev-micro-091313-103739. [DOI] [PubMed] [Google Scholar]
- 2.Parry C, Hien TT, Dougan G, White N, Farrar J. 2002. Typhoid fever. N Engl J Med 347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 3.Buckle G, Walker C, Black R. 2012. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2:010401. doi: 10.7189/jogh.01.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogasale V, Maskery B, Ochiai R, Lee J, Mogasale V, Ramani E, Kim Y, Park J, Wierzba T. 2014. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2:e570–e580. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Mogasale V, Im J, Ramani E, Marks F. 2017. Updated estimates of typhoid fever burden in sub-Saharan Africa. Lancet Glob Health 5:e969. doi: 10.1016/S2214-109X(17)30328-5. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta Z. 1996. Impact of age and drug resistance on mortality in typhoid fever. Arch Dis Child 75:214–217. doi: 10.1136/adc.75.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler T, Islam A, Kabir I, Jones P. 1991. Patterns of morbidity and mortality in typhoid fever dependent on age and gender: review of 552 hospitalised patients with diarrhoea. Rev Infect Dis 13:85–90. doi: 10.1093/clinids/13.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Ochiai R, Acosta C, Danovaro-Holliday M, Baiqing D, Bhattacharya S, Actinic M, Bhutta Z, Cahn D, Ali M, Shin S, Wain J, Page A, Albert M, Farrar J, Abu-Elated R, Pang T, Galindo C, von Seidlein L, Clemens J. 2008. DOMI study typhoid group. A study of typhoid fever in five Asian countries: disease burden and implications for control. Bull World Health Organ 86:260–268. doi: 10.2471/blt.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau T, Campbell J, Galindo C, Hoang N, Diep T, Nga T, Vinh Chau N, Tuan P, Page A, Ochiai L, Schultsz C, Wain J, Bhutta Z, Parry C, Bhattacharya S, Dutta S, Agtini M, Dong B, Honghui Y, Anh D, Canh D, Naheed A, Albert M, Phetsouvanh R, Newton P, Basnyat B, Arjyal A, La T, Rang N, Phuong L, Bay P, von Seidlein L, Dougan G, Clemens J, Vinh H, Hien T, Chinh N, Acosta C, Farrar J, Dolecek C. 2007. Antimicrobial drug resistance of Salmonella enteric serovar Typhi in Asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob Agents Chemother 51:4315–4323. doi: 10.1128/AAC.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milligan R, Paul M, Richardson M, Neuberger A. 2018. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 5:CD001261. doi: 10.1002/14651858.CD001261.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuckerman J, Hatz C, Kantele A. 2017. Review of current typhoid fever vaccines, cross-protection against paratyphoid fever, and the European guidelines. Expert Rev Vaccines 16:1029–1043. doi: 10.1080/14760584.2017.1374861. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. 2018. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec 93:153–172. [Google Scholar]
- 13.Mohan V, Varanasi V, Singh A, Pasetti M, Levine M, Venkatesan R, Ella K. 2015. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis 61:393–402. doi: 10.1093/cid/civ295. [DOI] [PubMed] [Google Scholar]
- 14.Teh CSJ, Chua KH, Thong KL. 2014. Paratyphoid fever: splicing the global analyses. Int J Med Sci 11:732–741. doi: 10.7150/ijms.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker S, Karkey A, Parry C. 2014. Are we adequately prepared for the emergence of Salmonella enterica serovar Paratyphi A? Lancet Glob Health 2:e195–e196. doi: 10.1016/S2214-109X(14)70009-9. [DOI] [PubMed] [Google Scholar]
- 16.Sahastrabuddhe S, Carbis R, Wierzba T, Ochiai R. 2013. Increasing rates of Salmonella Paratyphi A and the current status of its vaccine development. Expert Rev Vaccines 12:1021–1031. doi: 10.1586/14760584.2013.825450. [DOI] [PubMed] [Google Scholar]
- 17.Haghjoo E, Galán JE. 2004. Salmonella Typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A 101:4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spano S, Ugalde JE, Galán JE. 2008. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 3:30–38. doi: 10.1016/j.chom.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Gao X, Galán JE. 2013. Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature 499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galán J. 2016. Typhoid toxin provides a window into typhoid fever and the biology of Salmonella Typhi. Proc Natl Acad Sci U S A 113:6338–6344. doi: 10.1073/pnas.1606335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler C, Galán J. 2018. Decoding a Salmonella Typhi regulatory network that controls typhoid toxin expression within human cells. Cell Host Microbe 23:65–76.e6. doi: 10.1016/j.chom.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler CC, Stack G, Jiao X, Lara-Tejero M, Galán JE. 2019. Alternate subunit assembly diversifies the function of a bacterial toxin. Nat Commun 10:3684. doi: 10.1038/s41467-019-11592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng R, Wiedmann M. 2019. The ADP-ribosylating toxins of Salmonella. Toxins 11:416. doi: 10.3390/toxins11070416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akinyemi KO, Coker AO, Olukoya DK, Oyefolu AO, Amorighoye EP, Omonigbehin EO. 2000. Prevalence of multi-drug resistant Salmonella Typhi among clinically diagnosed typhoid fever patients in Lagos, Nigeria. Z Naturforsch C J Biosci 55:489–493. doi: 10.1515/znc-2000-5-630. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Deng L, Stack G, Yu H, Chen X, Naito-Matsui Y, Varki A, Galán J. 2017. Evolution of host adaptation in the Salmonella typhoid toxin. Nat Microbiol 2:1592–1599. doi: 10.1038/s41564-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng L, Song J, Gao X, Wang J, Yu H, Chen X, Varki N, Naito-Matsui Y, Galán J, Varki A. 2014. Host adaptation of a bacterial toxin from the human pathogen Salmonella Typhi. Cell 159:1290–1299. doi: 10.1016/j.cell.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varki N, Strobert E, Dick EJ, Benirschke K, Varki A. 2011. Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of sialic acid biology. Annu Rev Pathol 6:365–393. doi: 10.1146/annurev-pathol-011110-130315. [DOI] [PubMed] [Google Scholar]
- 28.Chou H, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. 2002. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci U S A 99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y-A, Lee S, Zhao J, Thompson AJ, McBride R, Tsogtbaatar B, Paulson JC, Nussinov R, Deng L, Song J. 2018. In vivo tropism of Salmonella Typhi toxin to cells expressing a multiantennal glycan receptor. Nat Microbiol 3:155–163. doi: 10.1038/s41564-017-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibani M, Jones E, Barton A, Jin C, Meek J, Camara S, Galal U, Heinz E, Rosenberg-Hasson Y, Obermoser G, Jones C, Campbell D, Black C, Thomaides-Brears H, Darlow C, Dold C, Silva-Reyes L, Blackwell L, Lara-Tejero M, Jiao X, Stack G, Blohmke C, Hill J, Angus B, Dougan G, Galán J, Pollard A. 2019. Investigation of the role of typhoid toxin in acute typhoid fever in a human challenge model. Nat Med 25:1082–1088. doi: 10.1038/s41591-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bano-Zaidi M, Aguayo-Romero M, Campos F, Colome-Ruiz J, Gonzalez M, Piste I, Magaña C, Gamboa M. 2018. Typhoid fever outbreak with severe complications in Yucatan, Mexico. Lancet Glob Health 6:e1062–e1063. doi: 10.1016/S2214-109X(18)30312-7. [DOI] [PubMed] [Google Scholar]
- 32.Cruz Espinoza L, McCreedy E, Holm M, Im J, Mogeni O, Parajulee P, Panzner U, Park S, Toy T, Haselbeck A, Seo H, Jeon H, Kim J, Kwon S, Kim J, Parry C, Marks F. 2019. Occurrence of typhoid fever complications and their relation to duration of illness preceding hospitalization: a systematic literature review and meta-analysis. Clin Infect Dis 69:S435–S448. doi: 10.1093/cid/ciz477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieters Z, Saad N, Antillón M, Pitzer V, Bilcke J. 2018. Case fatality rate of enteric fever in endemic countries: a systematic review and meta-analysis. Clin Infect Dis 67:628–638. doi: 10.1093/cid/ciy190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad Hatib N, Chong C, Thoon K, Tee N, Krishnamoorthy S, Tan N. 2016. Enteric fever in a tertiary paediatric hospital: a retrospective six-year review. Ann Acad Med Singapore 45:297–302. [PubMed] [Google Scholar]
- 35.Parry C, Thompson C, Vinh H, Chinh N, Phuong lT, Ho V, Hien T, Wain J, Farrar J, Baker S. 2014. Risk factors for the development of severe typhoid fever in Vietnam. BMC Infect Dis 14:73. doi: 10.1186/1471-2334-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E, Liang Q, Ali H, Bayliss L, Beasley A, Bloomfield-Gerdes T, Bonoli L, Brown R, Campbell J, Carpenter A, Chalk S, Davis A, England N, Fane-Dremucheva A, Franz B, Germaschewski V, Holmes H, Holmes S, Kirby I, Kosmac M, Legent A, Lui H, Manin A, O'Leary S, Paterson J, Sciarrillo R, Speak A, Spensberger D, Tuffery L, Waddell N, Wang W, Wells S, Wong V, Wood A, Owen M, Friedrich G, Bradley A. 2014. Complete humanization of the mouse immunoglobulin loci enables efficient therapeutic antibody discovery. Nat Biotechnol 32:356–363. doi: 10.1038/nbt.2825. [DOI] [PubMed] [Google Scholar]
- 37.Orth P, Xiao L, Hernandez L, Reichert P, Sheth P, Beaumont M, Yang X, Murgolo N, Ermakov G, DiNunzio E, Racine F, Karczewski J, Secore S, Ingram R, Mayhood T, Strickland C, Therien A. 2014. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem 289:18008–18021. doi: 10.1074/jbc.M114.560748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acquaye-Seedah E, Huang Y, Sutherland J, DiVenere A, Maynard J. 2018. Humanised monoclonal antibodies neutralise pertussis toxin by receptor blockade and reduced retrograde trafficking. Cell Microbiol 20:e12948. doi: 10.1111/cmi.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oganesyan V, Peng L, Damschroder M, Cheng L, Sadowska A, Tkaczyk C, Sellman B, Wu H, Dall'Acqua W. 2014. Mechanisms of neutralization of a human anti-α-toxin antibody. J Biol Chem 289:29874–29880. doi: 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez L, Kroh H, Hsieh E, Yang X, Beaumont M, Sheth P, DiNunzio E, Rutherford S, Ohi M, Ermakov G, Xiao L, Secore S, Karczewski J, Racine F, Mayhood T, Fischer P, Sher X, Gupta P, Lacy D, Therien A. 2017. Epitopes and mechanism of action of the Clostridium difficile toxin A-neutralizing antibody actoxumab. J Mol Biol 429:1030–1044. doi: 10.1016/j.jmb.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yermakova A, Klokk T, Cole R, Sandvig K, Mantis N. 2014. Antibody-mediated inhibition of ricin toxin retrograde transport. mBio 5:e00995-13. doi: 10.1128/mBio.00995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang S, Jin S, Jiao X, Galán J. 2019. Unique features in the intracellular transport of typhoid toxin revealed by a genome-wide screen. PLoS Pathog 15:e1007704. doi: 10.1371/journal.ppat.1007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroh H, Chandrasekaran R, Zhang Z, Rosenthal K, Woods R, Jin X, Nyborg A, Rainey G, Warrener P, Melnyk R, Spiller B, Lacy D. 2018. A neutralizing antibody that blocks delivery of the enzymatic cargo of Clostridium difficile toxin TcdB into host cells. J Biol Chem 293:941–952. doi: 10.1074/jbc.M117.813428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scally SW, McLeod B, Bosch A, Miura K, Liang Q, Carroll S, Reponen S, Nguyen N, Giladi E, Rämisch S, Yusibov V, Bradley A, Lemiale F, Schief WR, Emerling D, Kellam P, King CR, Julien J-P. 2017. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat Commun 8:1568. doi: 10.1038/s41467-017-01924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.