Bacterial biofilms are linked with chronic infections and have properties distinct from those of planktonic, single-celled bacteria. The virulence mechanisms associated with Staphylococcus aureus biofilms are becoming better understood. Human neutrophils are critical for the innate immune response to S. aureus infection. Here, we describe two virulence strategies that converge to promote the ability of S. aureus biofilms to evade killing by neutrophils.
KEYWORDS: NETs, S. aureus, biofilms, neutrophil, phagocytosis
ABSTRACT
Bacterial biofilms are linked with chronic infections and have properties distinct from those of planktonic, single-celled bacteria. The virulence mechanisms associated with Staphylococcus aureus biofilms are becoming better understood. Human neutrophils are critical for the innate immune response to S. aureus infection. Here, we describe two virulence strategies that converge to promote the ability of S. aureus biofilms to evade killing by neutrophils. Specifically, we show that while neutrophils exposed to S. aureus biofilms produce extracellular traps (NETs) and phagocytose bacteria, both mechanisms are inefficient in clearance of the biofilm biomass. This is attributed to the leukocidin LukAB, which promotes S. aureus survival during phagocytosis. We also show that the persistence of biofilm bacteria trapped in NETs is facilitated by S. aureus nuclease (Nuc)-mediated degradation of NET DNA. This study describes key aspects of the interaction between primary human neutrophils and S. aureus biofilms and provides insight into how S. aureus evades the neutrophil response to cause persistent infections.
INTRODUCTION
Staphylococcus aureus is a leading cause of multiple devastating chronic infections (1). This is attributed in part to a large repertoire of toxins produced by the pathogen, allowing rapid evasion of host immune defenses (2). In addition, S. aureus is proficient at forming biofilms, a characteristic that contributes greatly to its success as a pathogen (3). Bacterial biofilms are structured communities with properties that are distinct from those of planktonic populations (4); this often includes a shift in the virulence mechanisms utilized by the pathogen to survive host defenses (5–7). Neutrophils are the most abundant white blood cells in circulation and are crucial for the innate immune response to S. aureus (8). Some of the main mechanisms used by neutrophils to clear bacteria are phagocytosis, release of antimicrobial peptides, generation of antimicrobial reactive oxygen and nitrogen species, and the formation of antibacterial neutrophil extracellular traps (NETs) (9, 10). Understanding the responses that govern the interactions of S. aureus with neutrophils is vital to the development of interventions for these infections (11). While S. aureus biofilms are often associated with chronic infections, our knowledge of the response of biofilms to neutrophil defenses is somewhat limited (12, 13). Here, we describe two of these mechanisms and provide insights as to why neutrophils fail to eradicate S. aureus biofilms.
Leukocidins are a group of toxins that lyse leukocytes and red blood cells, thereby playing important roles in S. aureus pathogenesis (14). S. aureus strains can produce up to 5 leukocidins: Panton-Valentine leukocidin (PVL; LukSF-PV), LukAB (also known as LukGH), LukED, HlgCB, and HlgAB (14, 15). Each leukocidin is composed of an S subunit that recognizes a receptor on the host cell to recruit the corresponding F subunit, leading to the formation of an octameric pore in the cell membrane, ultimately causing cell death (14). Most studies have evaluated roles for leukocidins during planktonic S. aureus growth (16–19). Chronic infections, however, are commonly associated with aggregate or biofilm bacteria, which often utilize virulence mechanisms distinct from those used by planktonic populations (6, 20, 21). The roles of leukocidins during biofilm growth remain largely uncharacterized (12, 22). Recent work with planktonic S. aureus, by us and others, has shown that leukocidins can synergize or antagonize each other’s activities (23–25). We found that unlike necrotic cell death associated with planktonic populations, PVL and HlgAB released during growth of biofilms skew neutrophils toward NETosis (12). NET formation is the controlled release of neutrophil chromatin laced with antimicrobial proteins, designed to physically trap and kill bacteria, respectively (10). Interestingly, we found that NETs were not effective at clearing biofilms (12).
In the present study, we describe the virulence mechanisms that allow S. aureus biofilm survival during the induction of NETs by biofilm-released PVL and HlgAB. Specifically, we show that two distinct virulence strategies converge to facilitate S. aureus biofilm survival. First, LukAB enhances persistence of bacteria that are engulfed by neutrophils, since LukAB enhances survival of biofilm-grown S. aureus in the presence of neutrophils. This survival phenotype could be mitigated when phagocytosis was blocked, thus solidifying a role for LukAB during phagocytosis. Furthermore, we show that neutrophil lysis requires LukAB, independent of NETosis (26, 27).
Second, we describe a role for the nuclease Nuc in facilitating the breakdown of NET DNA and survival of trapped S. aureus. We found that a biofilm-grown nuc mutant showed reduced levels of survival, compared to the survival of wild-type S. aureus. However, when biofilms from a nuc mutant were exposed to neutrophils and treated with DNase I, this restored S. aureus survival. Lastly, we show that degradation of NET DNA by Nuc causes a dispersal event, potentially allowing dissemination of biofilm bacteria and promoting infection. Altogether, this study provides an understanding of some of the mechanisms by which biofilm-grown S. aureus survives, upon exposure to neutrophils.
RESULTS
Leukocidins are required for bacterial survival in biofilms exposed to neutrophils.
Our previous results show that incubation of wild-type S. aureus biofilms with neutrophils causes the release of antimicrobial NETs. These NETs were unable to clear the biofilm biomass after a 2-h incubation with neutrophils (12). To understand the mechanisms underlying the recalcitrance of S. aureus biofilms exposed to primary human neutrophils, we incubated the wild-type USA300 strain LAC (widespread community-acquired methicillin-resistant S. aureus [MRSA] strain; referred to here as USA300) (28) with Cell Tracker Blue (cytosolic stain)-labeled neutrophils for 2 h. Syto-9 was used to stain biofilm biomass, and ethidium homodimer-1 (EthHD-1) was used to evaluate the release of neutrophil chromatin material, which is associated with cell death via the release of extracellular traps (10). Similar to previous reports, we found that neutrophils penetrate the biofilm biomass but are unable to clear USA300 (Fig. 1A) (29). Intact neutrophils were found at the base of the biofilm, releasing DNA (Fig. 1B, arrow). Additionally, previous studies described the presence of NETosis-associated markers (citrullinated histone, myeloperoxidase, and neutrophil elastase) in EthHD-1-stained material that is released when neutrophils are exposed to biofilms, confirming the release of NETs (12).
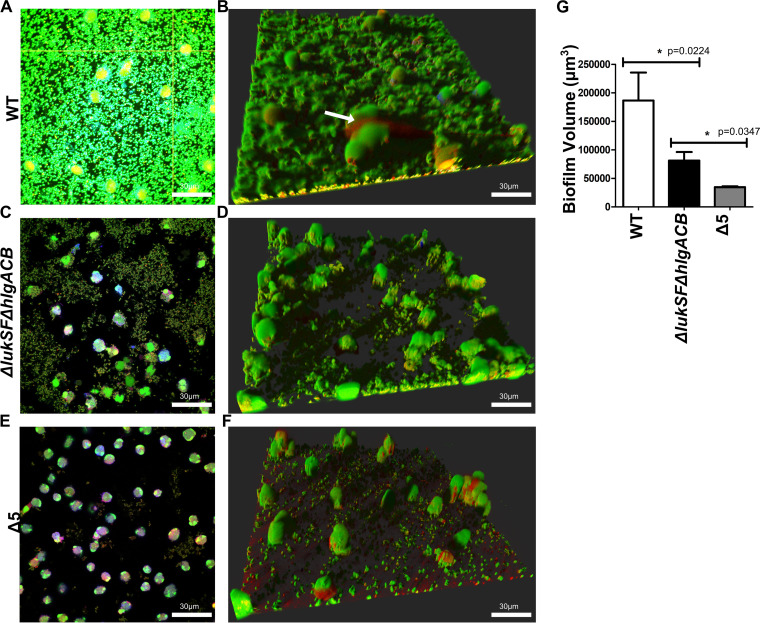
FIG 1.
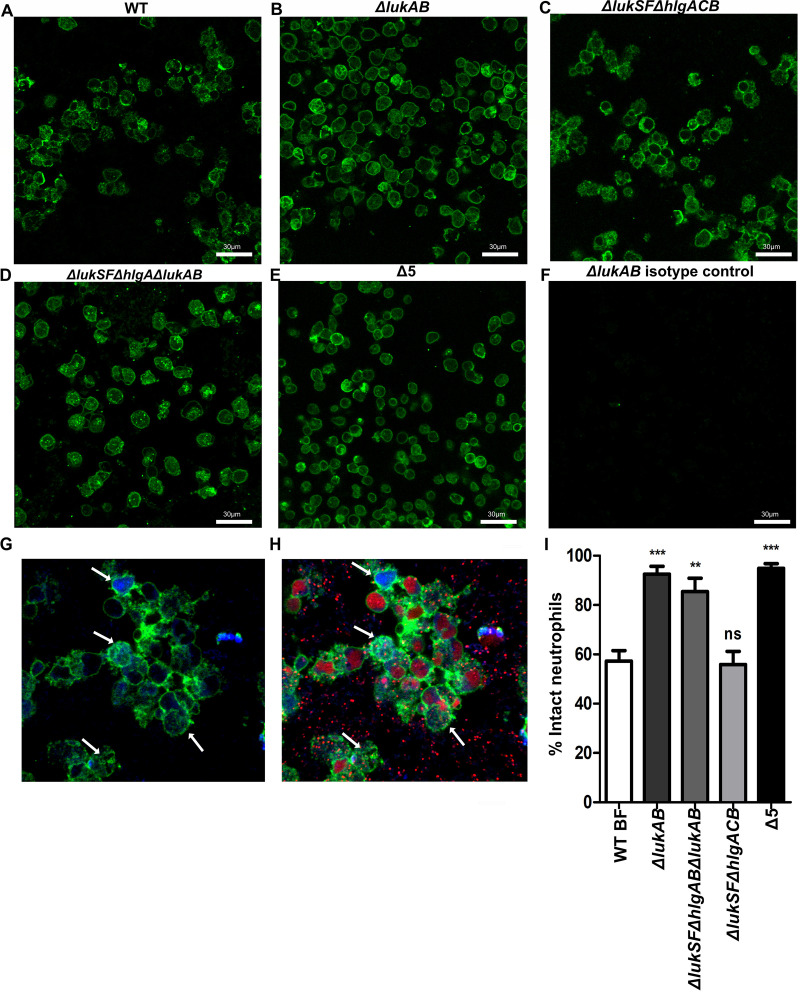
Leukocidins are required for survival of biofilms exposed to neutrophils. Wild-type USA300 biofilms were incubated with Cell Tracker Blue-labeled primary human neutrophils for 2 h, stained with Syto-9 (viable bacterial cells; green) and ethidium homodimer-1 (DNA of dead mammalian cells; red), and imaged using confocal laser scanning microscopy. (A) Image of a section taken close to the base of the biofilm. (B) Three-dimensional image showing a wild-type USA300 biofilm after a 2-h incubation with primary human neutrophils. The arrow indicates DNA of a dying neutrophil. (C and D) Experiments similar to those for panels A and B, performed with biofilms of a ΔlukSF ΔhlgACB USA300 strain. (E and F) Experiments similar to those for panels A and B, performed with biofilms of a Δ5 USA300 strain, lacking all leukocidin proteins. (G) Volume quantification of WT, ΔlukSF ΔhlgACB, and Δ5 USA300 biofilms after treatment with neutrophils for 2 h. Biofilms were grown in μ-slides and captured at a ×600 magnification. Images represent the majority population phenotype seen in six independent experiments performed in triplicate. Student t tests were performed for pairwise comparisons.
When similar experiments were performed with a ΔlukSF ΔhlgACB strain, this mutant was found to have an impaired ability to induce NETs, with bacterial survival and biofilm thickness being greatly diminished (Fig. 1C and D). Biofilms from a USA300 strain unable to express the 5 leukocidins (Δ5 strain) (Fig. 1E and F) showed a significantly lower biomass than the ΔlukSF ΔhlgACB strain upon exposure to neutrophils (Fig. 1G). These results confirmed our previous findings (12) and also established that leukocidins independent of PVL and HlgACB play a role in facilitating the survival of S. aureus biofilms exposed to primary human neutrophils.
LukAB contributes to the evasion of phagocytosis-mediated killing of biofilm bacteria.
LukAB has been reported to play a role in the survival of biofilms incubated with macrophages (22). Additionally, the expression of lukAB is increased in planktonic S. aureus exposed to neutrophils and facilitates survival of bacteria in phagosomes (18, 26, 30–32). To understand if LukAB contributed to the differences in biofilm biomass and bacterial survival observed between the ΔlukSF ΔhlgACB and Δ5 strains (Fig. 1G), we compared the survival of wild-type USA300 and isogenic ΔlukAB biofilms when they were exposed to primary human neutrophils for 1 and 2 h. Wild-type (WT) biofilm bacteria stained with Syto-9 were found to be internalized by neutrophils (Fig. 2A, inset), but the neutrophils were unable to clear the biomass after a 2-h incubation period (Fig. 2B). In comparison, neutrophils incubated with a ΔlukAB biofilm showed large areas of clearing around neutrophils (Fig. 2C, inset). However, some biofilm biomass remained 2 h after exposure to neutrophils (Fig. 2D). Nevertheless, ΔlukAB biofilms treated with neutrophils retained significantly lower levels of biomass than the wild-type strain (Fig. 2E). Levels of biomass were found to be comparable to WT levels when lukAB was complemented with a chromosomally encoded allele (see Fig. S1A in the supplemental material). This was independent of the biofilm-forming capacity of these strains, since untreated wild-type and ΔlukAB biofilms were equally proficient at biofilm formation (Fig. S1B and C; Fig. S2A).
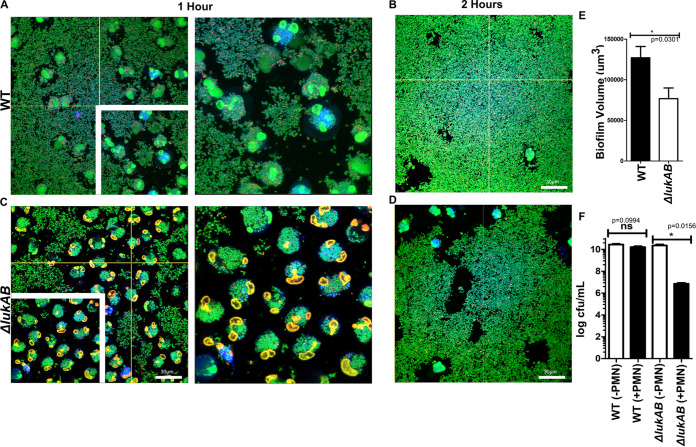
FIG 2.
LukAB contributes to bacterial survival when biofilms are in contact with neutrophils. (A and B) Wild-type USA300 biofilms were incubated with Cell Tracker Blue-labeled primary human neutrophils for 1 (A) and 2 (B) h, stained with Syto-9 (viable bacteria), and imaged using confocal laser scanning microscopy. Representative images of sections taken close to the base of biofilms. Insets show a ×1.2 optical zoom of the area outlined in white. (C and D) Experiments similar to those described for panels A and B, performed with an isogenic ΔlukAB USA300 strain. (E) Biofilm biomass measured at 1 h after incubation of wild-type (WT) and ΔlukAB USA300 biofilms with neutrophils for 1 h. (F) Total CFU per milliliter of biofilms from indicated strains, incubated with (+PMN) and without (−PMN) neutrophils for 1 h. Biofilms were grown in μ-slides and captured at a ×600 total magnification. Results are averages of six independent experiments performed in triplicate, with standard errors of the means (SEM). Images and measurements of volume were taken using Imaris software version X 6.4. Enumerations of CFU per milliliter were done independently, using biofilms grown in silicone tubing. Student t tests were performed for pairwise comparisons.
Since ΔlukAB biofilms treated with neutrophils showed higher levels of biomass clearance than wild-type biofilms under similar conditions, we reasoned that LukAB might play a role in the survival of S. aureus during phagocytosis. To evaluate this, wild-type and ΔlukAB biofilms were grown in silicone tubing, and the numbers of viable bacteria retained before and after incubation with neutrophils were measured by scraping biofilm biomass and plating on tryptic soy agar to enumerate CFU. While there was no significant decrease in wild-type biofilm bacteria in comparison to an untreated control (Fig. 2F), there was an ∼4-log decrease in ΔlukAB biofilms that were treated with neutrophils, compared to a similar control (Fig. 2F), indicating that LukAB plays a role in protecting the biofilm biomass and bacterial survival.
To confirm the contribution of LukAB to the ability of USA300 biofilms to survive the phagocytic response, we evaluated the survival of ΔlukAB biofilms when neutrophil phagocytosis was inhibited (Fig. 3A and B). Neutrophils were treated with cytochalasin D to block phagocytosis before incubation with ΔlukAB biofilms (Fig. 3C and D) (33). While addition of cytochalasin D had no significant effect on WT biomass (Fig. S1D), the biomass of ΔlukAB biofilms treated similarly was restored to levels comparable to those of WT biofilms. This increase was not observed with neutrophils that were capable of phagocytosis (no cytochalasin D) (Fig. 3E). Enumeration of biofilm bacteria grown in silicone tubing (similar to Fig. 2F) showed that there was a significant increase (∼2 log units) in the total population of surviving bacteria from ΔlukAB biofilms incubated with neutrophils in the presence of cytochalasin D in comparison to an untreated control (Fig. 3F). The results in Fig. 2 and 3 collectively suggest that while LukAB is important for the survival of USA300 during phagocytosis, it likely does not play a major role in the ability of biofilms to survive neutrophil killing mechanisms that are independent of phagocytosis (e.g., NET-mediated killing).
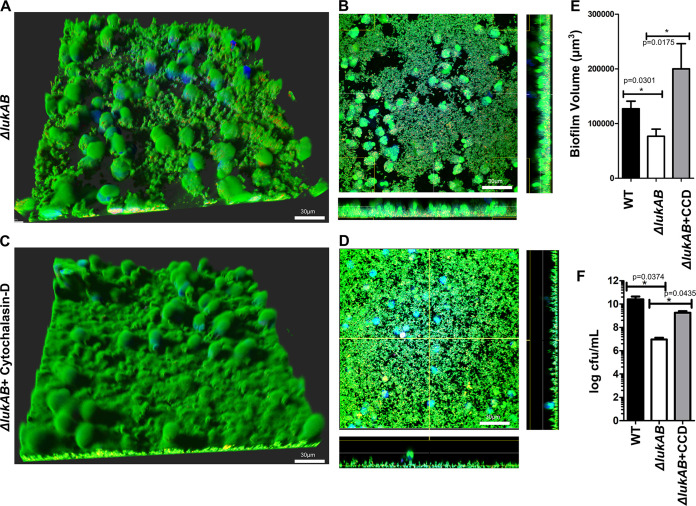
FIG 3.
LukAB facilitates survival of biofilm bacteria in a phagocytosis-dependent manner. (A) Biofilms from a ΔlukAB USA300 strain incubated with Cell Tracker Blue-labeled primary human neutrophils for 1 h and stained with Syto-9 (live bacteria) and ethidium homodimer-1 (dead/dying neutrophils). (B) Representative image of a section taken close to the base of the biofilm. (C and D) Cell Tracker Blue-labeled primary human neutrophils were incubated with cytochalasin D for 1 h before incubation with ΔlukAB USA300 biofilms for 1 h and stained as described for panels A and B. (E) Biofilm biomass measured at 1 h after incubation with neutrophils for the ΔlukAB USA300 strain treated with cytochalasin D (+CCD). (F) Numbers of CFU per milliliter, calculated for biomass retrieved from biofilms of the ΔlukAB USA300 strain without and with cytochalasin D (+CCD). Images were captured at a total magnification of ×600, and measurements were taken using Imaris software version X 6.4. Results are averages from six independent experiments performed in triplicate, with SEM. Enumerations of CFU per milliliter were done independently, using biofilms grown in silicone tubing. Student t tests were performed for pairwise comparisons.
Results from Fig. 1 revealed that leukocidin(s) in addition to PVL and HlgACB was required for survival of biofilm-grown S. aureus. Moreover, we found that LukAB contributed to the evasion of phagocytosis-mediated killing of biofilm bacteria. To determine if PVL, HlgAB, and LukAB were sufficient to promote survival of biofilm-grown S. aureus when exposed to neutrophils, we evaluated the ability of a mutant unable to express PVL, HlgA, and LukAB (ΔlukSF ΔhlgA ΔlukAB) to survive neutrophil killing. Biofilms of this strain and the isogenic wild type were incubated with neutrophils and imaged as described above. In comparison to a wild-type strain (Fig. 4A and B), biofilms of the ΔlukSF ΔhlgA ΔlukAB mutant were efficiently cleared by neutrophils (Fig. 4C and D) at a level comparable to that seen with the Δ5 strain (Fig. 4E and F), with most of the biomass being removed by neutrophils at 1 h postincubation (Fig. 4G). This was corroborated by quantification of the total numbers of surviving bacteria (Fig. 4H). A significant increase in biomass was observed when lukAB was introduced as a single copy on the chromosome of the ΔlukSF ΔhlgA ΔlukAB strain (Fig. S1E). Additionally, the observed differences in bacterial survival were independent of initial biofilm volume, since all strains tested were found to be comparable in biofilm formation (Fig. S2A and B). These results indicate that the combined activity of the leukocidins PVL, HlgAB, and LukAB was required for survival of USA300 biofilms that were exposed to neutrophils.
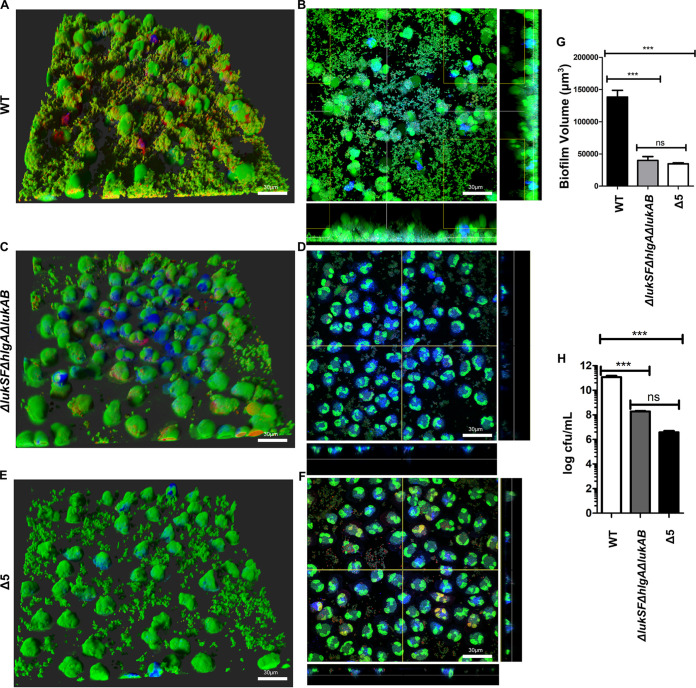
FIG 4.
PVL, HlgAB, and LukAB are required for biofilms to evade neutrophil killing. (A) Neutrophils were incubated with wild type biofilms and imaged after 1 h. Staining of neutrophils and bacteria was done as described for Fig. 1. (B) Representative image of section taken close to the base of wild-type biofilms and imaged as described for panel A. (C and D) Experiments performed similar to those for panel A, with an isogenic ΔlukSF ΔhlgA ΔlukAB mutant of USA300. (E and F) Experiments performed similar to those for panel A, with an isogenic mutant lacking all 5 leukocidins (Δ5 USA300). (G) Biofilm volume quantified after a 1-h incubation of biofilms from the indicated strains with neutrophils. (H) Measurements of total bacteria (CFU per milliliter) isolated from biofilms of indicated strains, after a 1-h incubation with neutrophils. Results are averages from six independent experiments performed in triplicate, with SEM. Enumerations of CFU per milliliter were done independently, using biofilms grown in silicone tubing. Multiple comparisons were done using one-way analysis of variance and Tukey’s post hoc analysis where appropriate. **, P < 0.01; ***, P < 0.001; ns, not significant. Images were taken using Imaris software version X 6.4. MFI calculations were performed using ImageJ.
LukAB and not NETosis causes lysis of neutrophils exposed to S. aureus biofilms.
Previously, we showed that neutrophils incubated with wild-type USA300 biofilms release NETs (12). Additionally, the process of NET release can occur without cell membrane rupture, with neutrophils being able to retain membrane integrity post-NETosis (nonlytic NETosis) (34). Subsequently, anuclear neutrophils can continue to phagocytose bacteria, after NET release (34–36). Similar to these observations, our studies indicate that neutrophils incubated with wild-type USA300 biofilms remain intact after a 1-h incubation, since they retain the cytosolic dye Cell Tracker Blue (Fig. S2C). We therefore reasoned that neutrophils exposed to wild-type biofilms could undergo the process of nonlytic NETosis (34). WT biofilms were incubated with neutrophils for 1 h and stained with an anti-CD45 antibody, which recognizes the membrane surface protein CD45 (Fig. 5A) (34). Lower levels of cell debris were observed when neutrophils were incubated with a ΔlukAB strain, in comparison to the ΔlukSF ΔhlgACB strain (no NET formation) (Fig. 5B and C). The cell membrane integrity of neutrophils treated with ΔlukAB biofilms was comparable to that seen with the ΔlukSF ΔhlgA ΔlukAB and Δ5 strains (Fig. 5D and E). Indeed, when neutrophils were coincubated with wild type biofilms, a population of cells simultaneously retained Cell Tracker Blue and stained with an anti-CD45 antibody (Fig. 5G, arrows). However, these cells lost staining for ethidium homodimer-1 (Fig. 5H, arrows). This staining pattern indicates the presence of cells with intact membranes (blue and green) that have undergone NETosis (blue and green, no red).
FIG 5.
LukAB and not NETosis induces neutrophil lysis. (A to E) Biofilms of the indicated USA300 strains were exposed to neutrophils for 1 h. Neutrophils were then labeled with an anti-CD45 antibody and stained with an Alexa Fluor 488 secondary antibody (green). (F) An anti-IgG antibody was used as an isotype control. (G and H) Wild-type biofilms incubated with Cell Tracker Blue-labeled primary human neutrophils (blue) for 1 h, stained with ethidium homodimer-1 (red, dead/dying neutrophils) and an FITC-labeled anti-CD45 antibody (green). Arrows indicate cells that retained staining with Cell Tracker Blue and anti-CD45 antibody but not ethidium homodimer-1. (I) Percent intact neutrophils undergoing NETosis, calculated for the indicated strains by counting the number of neutrophils that stained with anti-CD45+ Cell Tracker Blue as a percentage of the total number of cells per field (anti-CD45 plus Cell Tracker Blue plus EthHD-1), after a 1-h incubation with the respective strains, for 12 fields per strain, from 6 independent experimental replicates. Results are averages from six independent experiments. Images were taken at a total magnification of ×600. Digital zoom (×1.2) was applied to images in G and H, using Imaris software version X 6.4.
Enumeration of intact neutrophil populations showed that while there was no significant difference in numbers between neutrophils incubated with the WT and the ΔlukSF ΔhlgACB strains (unable to induce NETs), there was a significantly higher number of intact neutrophils after a 1-h incubation with biofilms of the ΔlukAB strain (Fig. 5I). This was similar to the numbers of intact neutrophils observed with both the ΔlukSF ΔhlgA ΔlukAB strain and the Δ5 strain. Collectively, these results provide evidence that USA300-mediated lysis of primary human neutrophils occurs due to the activity of LukAB and not NETosis.
Nuc contributes to the survival of biofilm bacteria associated with NETs.
Our observations indicate a role for LukAB in aiding the survival of biofilm bacteria during phagocytosis. As described above, we found that although there was a significant reduction in survival of neutrophil-associated bacteria (∼4 log) when a ΔlukAB biofilm was incubated with neutrophils, this strain was able to retain biofilm biomass after a 2-h treatment with neutrophils (Fig. 2D). This indicated that while LukAB plays a role in protecting the biofilm bacteria during phagocytosis, other factors are involved in the survival of extracellular bacterial populations. We therefore hypothesized that these factors contribute to the ability of S. aureus to escape killing mediated by the antimicrobial properties of NETs (10, 12, 36, 37). Planktonic S. aureus releases the nuclease Nuc, which can cleave NET DNA, resulting in a loss of NET antimicrobial properties and allowing bacterial survival during NETosis (33, 35). However, the function of Nuc in evasion of NET-mediated killing of S. aureus biofilms is not fully understood (38).
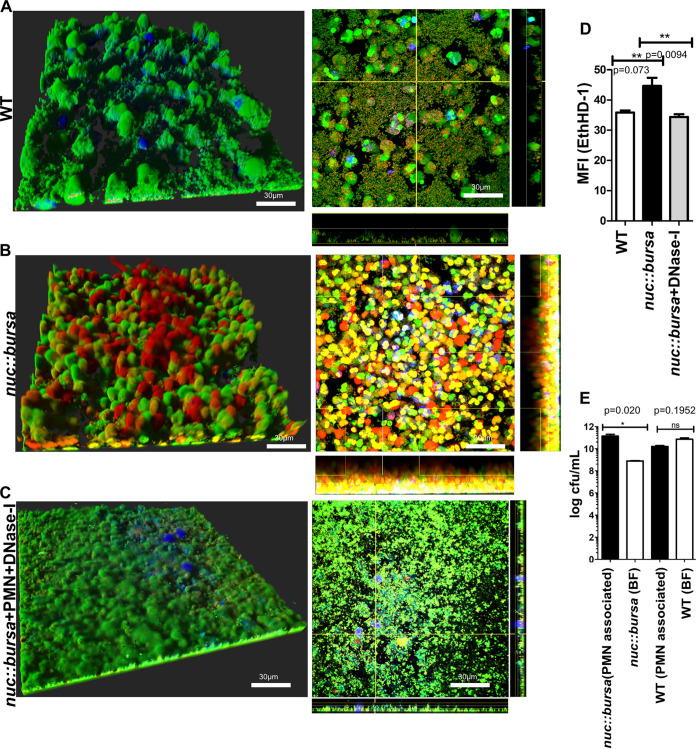
We tested the effect of neutrophils on biofilms of parental USA300 and an isogenic nuc::bursa strain (no nuclease production, bursa aurealis insertion in nuc) (39, 40). Indeed, in comparison to a wild-type strain (Fig. 6A), biofilms of the nuc::bursa strain showed a larger number of NETs released 1 h after incubation with neutrophils (Fig. 6B). To confirm that this DNA was not derived from extracellular DNA associated with the biofilm matrix (40, 41), untreated nuc::bursa biofilms were stained with EthHD-1 and imaged after a 1-h incubation. Levels of EthHD-1 staining were found to be negligible in comparison to those seen with biofilms that were treated with neutrophils, confirming the presence of neutrophil-derived chromatin material (Fig. S2D).
FIG 6.
Nuc facilitates survival of biofilm S. aureus from NETosis-mediated killing. (A and B) Cell Tracker Blue-labeled neutrophils were incubated with biofilms of the wild type (WT) or an isogenic nuc::bursa USA300 strain for 1 h and stained as described for Fig. 1. Sections taken close to the base of the biofilm are shown in the panels on the right. (C) Images similar to those in A andB, with nuc::bursa USA300 biofilms that were treated with DNase I for 1 h after incubation with neutrophils. (D) Mean fluorescence intensity measurements of ethidium homodimer-1 staining, comparing wild-type (WT) biofilms treated with neutrophils to biofilms of nuc::bursa strains or nuc::bursa strains plus DNase I, similarly treated. (E) Numbers of CFU per milliliter calculated for biofilms grown in silicone tubing and treated with neutrophils for 1 h. Neutrophil-associated bacteria were enumerated and compared to biofilm-associated extracellular populations (BF) for wild-type and nuc::bursa USA300 strains. Results are averages from six independent experiments performed in triplicate, with SEM. Enumerations of CFU per milliliter were done independently, using biofilms grown in silicone tubing (as described in Materials and Methods). Student t tests were performed for pairwise comparisons. Multiple comparisons were done using one-way analysis of variance and a Tukey’s post hoc analysis where appropriate. **, P < 0.01; ns, not significant. Images were taken using Imaris software version X 6.4. MFI calculations were performed using ImageJ.
While DNase I treatment had negligible effects on WT biofilm biomass (Fig. S2E), the levels of NETs observed were reduced when nuc::bursa biofilms were incubated with neutrophils in the presence of DNase I (Fig. 6C). These observations were corroborated by measuring the mean fluorescence intensities for EthHD-1, to stain for DNA of dead or dying neutrophils. Indeed, nuc::bursa biofilms incubated with neutrophils showed significantly higher levels of EthHD-1 staining than those under wild-type and nuc::bursa+DNase I conditions (Fig. 6D). Last, to understand whether the breakdown of NET DNA by Nuc provided biofilms with a survival advantage, neutrophils were separated from biofilm bacteria after a 1-h incubation and bacterial survival was assessed in each population. We found that in nuc::bursa biofilms (grown as described for Fig. 2F), there was a significant reduction in the numbers of nonphagocytosed (extracellular) biofilm bacteria in comparison to neutrophil-associated bacterial populations (Fig. 6E). This was not observed in the wild-type strain, indicating that Nuc is required for the extracellular or phagocytosis-independent survival of biofilm bacteria.
Nuc-mediated breakdown of NET DNA induces biofilm dispersal.
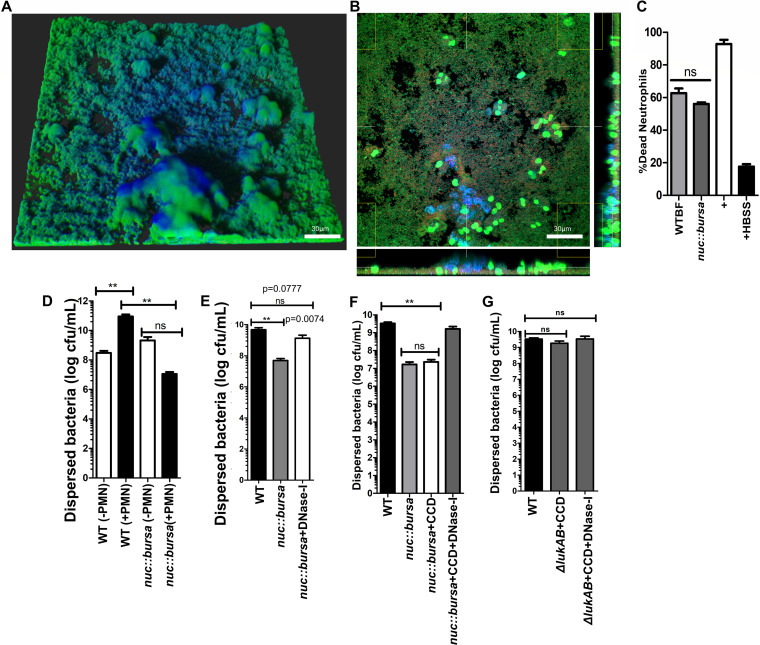
In the absence of immune cells, S. aureus biofilms release Nuc to cleave matrix-associated bacterial DNA. This results in the dispersal of a subpopulation of cells (40, 41). Indeed when nuc::bursa biofilms were incubated with supernatants from WT biofilms, we observed a reduction in NETs, similar to WT biofilms treated with DNase I (Fig. 7A and B). Additionally, spent media collected from wild-type and nuc::bursa biofilms showed no significant differences in their ability to kill neutrophils, indicating that these results were not attributed to an increased expression of the NET-inducing toxins PVL and HlgAB from nuc::bursa biofilms (Fig. 7C). Whether this function can contribute to the dispersal of S. aureus biofilms trapped in host DNA (such as NETs) has not previously been evaluated. To test whether cleavage of NET DNA by Nuc could cause dispersion, we quantified bacterial numbers in the effluent collected after a 1-h incubation of neutrophils with either wild-type or nuc::bursa biofilms grown in silicone tubing, using methods similar to those described for Fig. 2F. Contact of neutrophils with wild-type biofilms resulted in a significantly higher number of dispersed bacteria than in a corresponding untreated (no polymorphonuclear leukocytes [PMN]) control (Fig. 7D). Comparison of a nuc::bursa biofilm before and after incubation with neutrophils did not show significant differences in numbers of dispersed bacteria. Rather, numbers of dispersed populations were significantly lower (∼4 log) in nuc::bursa biofilms treated with neutrophils than in wild-type biofilms that were similarly treated (Fig. 7D, black bars). This indicated that Nuc did indeed contribute to the dispersal of USA300 biofilms treated with neutrophils. Furthermore, we found that this dispersal could be abrogated by treating nuc::bursa biofilms with DNase I after a 1-h incubation with neutrophils (Fig. 7E).
FIG 7.
Nuc-mediated breakdown of NET DNA contributes to biofilm bacterial dispersal. (A) nuc::bursa USA300 biofilms were treated with neutrophils for 1 h and subsequently with wild-type (WT) biofilm supernatant for an additional hour. Staining was done as described for Fig. 1. (B) Cross section of biofilm from panel A, incubated with neutrophils taken at the bottom of the biofilm. (C) Neutrophil killing activity of spent media collected from wild-type and nuc::bursa USA300 biofilms. Neutrophils were incubated with spent media for 30 min. LIVE-DEAD measurements show a ratio of Syto-9 (live) to propidium iodide fluorescence calculated as a percentage against a positive control (neutrophils plus 0.1% SDS). Neutrophils treated with Hanks balanced salt solution (HBSS) were used as a negative control. (D) Comparisons of dispersed bacteria before (−PMN) and after (+PMN) treatment of wild-type (WT) or nuc::bursa USA300 biofilms with neutrophils for 1 h. (E) Enumerations similar to those in panel D, comparing neutrophil-treated wild-type biofilms with nuc::bursa biofilms with and without DNase I treatment subsequent to incubation with neutrophils. (F) Counts (CFU per milliliter) of dispersed bacterial populations collected after a 60-min incubation of wild-type and nuc::bursa USA300 biofilms with primary human neutrophils, before and after treatment with cytochalasin D with and without DNase I. (G) Enumerations similar to those in panel F, performed with neutrophils treated with WT and ΔlukAB USA300 biofilms. Measurements of CFU per milliliter were done using biofilms grown in silicone tubing. Results are averages from six independent experiments performed in triplicate, with SEM. **, P < 0.01, using one-way analysis of variance and Tukey’s post hoc analysis; ns, not significant.
Treatment of nuc::bursa biofilms with cytochalasin D to block phagocytosis did not affect the dispersal of bacteria (Fig. 7F). Treatment of neutrophils with both cytochalasin D and DNase I, however, showed significantly higher levels of nuc::bursa biofilm dispersal, comparable to those of wild-type biofilms (Fig. 7F). These results further indicated a role for Nuc-mediated dispersal of S. aureus biofilms exposed to neutrophils. Last, when ΔlukAB biofilms were similarly incubated with cytochalasin D alone or in combination with DNase I, we observed no change in the numbers of dispersed bacteria, indicating that the observed reduction in bacterial numbers was not affected by phagocytosis (LukAB activity) and was indeed attributed to the activity of Nuc (Fig. 7G).
Collectively, these data indicate that Nuc assists the survival of S. aureus biofilms in the presence of neutrophils via two distinct mechanisms. First, Nuc degrades NET DNA that is released due to the activity of PVL and HlgAB released from biofilms (12, 33). Second, this degradation causes a dispersal event in the biofilm, potentially allowing the bacteria to disseminate and colonize new surfaces (41).
DISCUSSION
S. aureus is a versatile pathogen that utilizes multiple virulence factors to evade host immune responses during infection (2, 42). Recent studies make it evident that delineating the collective response of this array of factors to a single-infection environment is necessary for a proper understanding of S. aureus virulence mechanisms (12, 22, 24, 25, 43). Most studies on S. aureus pathogenesis have focused on planktonic bacterial populations (16, 18, 44). While these have proved crucial for establishing the fundamentals of S. aureus virulence, planktonic bacteria may not represent populations found during persistent infections (45, 46). These bacteria are found as aggregates or biofilms, with properties that are often distinct from those of single-celled populations (5, 6, 21). Studies by us and others have emphasized the importance of evaluating the unique response of biofilms to host defense mechanisms (12, 22). Specifically, studies show that while S. aureus biofilms secrete virulence factors similar to those secreted during planktonic growth, the mechanisms used for immune evasion by biofilms are markedly different from those used by the same factors during planktonic growth (12).
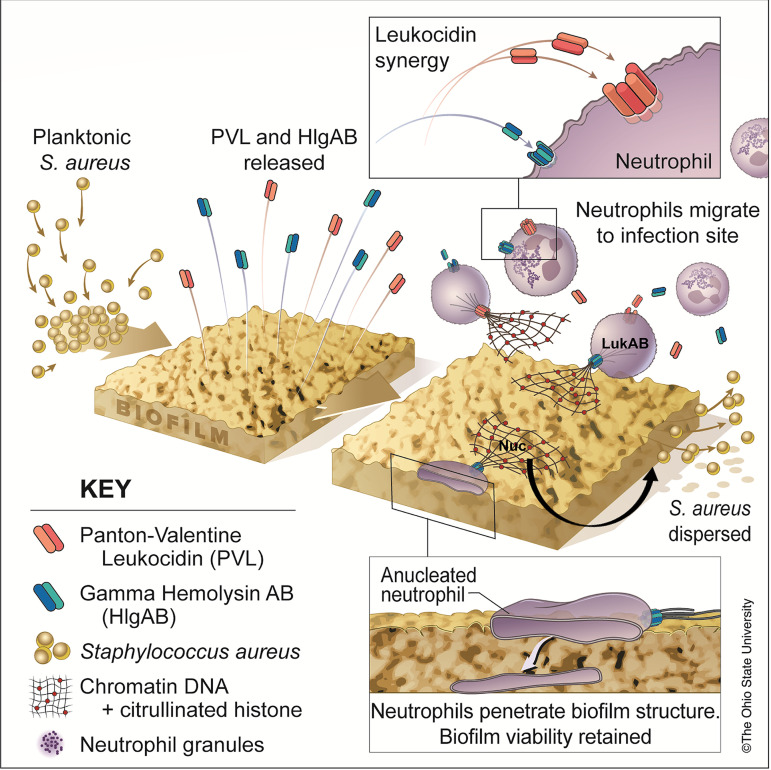
While neutrophils play a crucial role in eliminating bacterial infections, very little is known about their response during chronic biofilms infections (9, 47). Here, we reveal some novel mechanisms that facilitate the evasion of neutrophil antimicrobial responses by S. aureus biofilms (Fig. 8). These studies elaborate on our previous findings that describe the role of PVL and HlgAB released by S. aureus biofilms to induce NETosis during biofilm-associated infections (12). Consistent with in vivo studies, we postulate that neutrophils are functional during the process of biofilm-triggered NETosis and can efficiently phagocytose bacteria (34, 37). These reports describe a function for nonlytic NETosis postphagocytosis, as a mechanism for controlling the spread of bacteria (34, 36), similar to the work described here. We show that neutrophils are indeed capable of responding to biofilms via the antimicrobial processes of NETosis and phagocytosis-mediated killing; however, neither of these mechanisms is effective at preventing USA300 biofilm persistence. When genes encoding both PVL and HlgAB are deleted, NETosis is impeded and phagocytosis of biofilm bacteria remains unaffected (Fig. 1). While Nuc allows the escape of bacteria trapped in NETs (Fig. 6), we show that LukAB facilitates the survival of biofilm bacteria during phagocytosis by primary human neutrophils (Fig. 2 and 3).
FIG 8.
Summary of results. The activity of leukocidins PVL and gamma hemolysin AB (HlgAB) released from biofilms results in the induction of neutrophil extracellular traps (NETosis). While anuclear, neutrophils are still capable of penetrating the biofilm structure and phagocytosing bacteria; the antimicrobial activity of NETs is insufficient for clearing S. aureus biofilms. The leukocidin LukAB allows survival of bacteria during phagocytosis, and NET DNA is broken down by the nuclease (Nuc), resulting in dispersal of bacteria and thus perpetuating chronic infection. (Copyright The Ohio State University.)
LukAB is expressed when planktonic S. aureus organisms are exposed to neutrophils, and it facilitates bacterial survival within phagosomes (26). Phenol-soluble modulins (PSMs) are also considered an important virulence factor mediating survival of bacteria after phagocytosis by neutrophils (48, 49). Which bacterial proteins play the pivotal role depends in part on the nutrients and bacterial stressors of the infection environment (19). Similarly, after phagocytosis of S. aureus by macrophages, the concerted effect of LukAB and alpha-toxin contributes to bacterial survival (22, 43). Therefore, leukocidin-independent mechanisms could also contribute to survival of biofilms in vivo. However, results from multiple studies, including ours, indicate that LukAB is a key contributor to S. aureus survival upon exposure to primary human phagocytes (18, 26, 30, 50) (Fig. 2 and 3). Here, we show that LukAB is responsible for ultimately lysing neutrophils that are exposed to USA300 biofilms, consistent with studies on planktonic S. aureus (18, 26, 30, 51) (Fig. 5). Biofilm bacteria often adapt and evolve in response to environmental stresses (52, 53). The intracellular environment of neutrophils may therefore play a role in increased expression and production of LukAB from phagocytosed bacteria, and this could contribute to the role of LukAB in biofilm-mediated neutrophil killing.
In the absence of immune cells, Nuc plays a role in mediating the dispersal of biofilm bacteria via its activity on biofilm matrix-associated DNA (40, 54). Consequently, a nuc::bursa strain forms a significantly thicker biofilm than its corresponding wild-type parent strain (41). Nuc also degrades DNA derived from NETs (33). Here, we show that, consistent with observations on planktonic S. aureus (33), when biofilms are exposed to primary human neutrophils, Nuc cleaves NET DNA, and this increases dispersion of bacteria (Fig. 7). This dispersal event could contribute to bacterial dissemination during infection. Of note, release of nuclease by S. aureus has been shown to contribute to the dispersal of bacteria independent of neutrophil exposure. This likely also plays a role in the observed phenotype (40, 54). Additionally, other virulence factors such as PSMs are also involved in the dispersal of S. aureus biofilms and might play additional roles, especially at later time points, such as those observed with chronic infections in vivo (3, 12).
While our studies describe a role for LukAB in subverting the phagocytic response to USA300 biofilms, additional extracellular factors likely contribute to the ability of bacteria to survive the neutrophil response, when phagocytosis is blocked (Fig. 3F). Studies with planktonic populations indicate a strong antimicrobial activity of NET-associated histone H4 proteins toward S. aureus (55). Biofilms, however, show enhanced tolerance to antimicrobials (56). Therefore, while we show that Nuc allows cleavage of NET DNA and dispersal of bacteria, the concentrations of antimicrobial NET components such as histones and granular proteins might significantly affect the viability of biofilm bacteria (55). A recent study described a loss in antimicrobial activity of NETs when DNA is cleaved (35). This could contribute to the persistence of S. aureus biofilms observed here. Nevertheless, our studies unravel some of the mechanisms used by S. aureus biofilms to evade the neutrophil antimicrobial response, greatly adding to our knowledge of biofilm-neutrophil interactions (Fig. 8). More importantly, our studies highlight the value of considering the contributions of multiple virulence factors to the outcome of bacterial infections and further potentiate the significance of leukocidins for evasion of neutrophil killing by S. aureus biofilms.
MATERIALS AND METHODS
Ethics statement.
Primary human neutrophils were obtained from healthy adult donors according to the protocol approved by The Ohio State University Biomedical Sciences Institutional Review Board (2009H0314), and informed written consent was obtained from all donors.
Bacterial strains and biofilm growth.
Unless otherwise specified, all studies were performed in the USA300 strain LAC background (AH1263, provided by Alex Horswill, University of Colorado, Denver, CO). Bacterial cultures and biofilms were grown using previously published methods (12, 57). Briefly, cultures were grown in tryptic soy broth plus dextrose for 16 to 18 h with shaking (37°C, 200 rpm), diluted, and allowed to reach exponential phase (optical density at 600 nm [OD600], 0.5 to 0.7). These cultures were diluted to an OD of 0.1 and seeded into chambers of a 6-channel μ-slide (ibidi) for biofilm formation. μ-slides were incubated overnight at 37°C under static, humidified conditions, and biofilms were allowed to form for 16 to 18 h. Biofilms were washed with Roswell Park Memorial Institute medium (RPMI) without phenol red before neutrophil interaction studies were performed. For details about the various mutants used in these studies, see Table S1.
Transposon mutants used in these studies were obtained from the Network for Antimicrobial Research in Staphylococcus aureus at the University of Nebraska Medical Center (39). The presence of transposon insertions for these mutants was verified by plating onto tryptic soy agar (TSA) plus 5 μg/ml erythromycin before use and performing PCRs with primers specific for the transposon as well as the respective genes (39).
Biofilm-neutrophil interaction imaging.
To understand the role of biofilm released leukocidins during interaction with neutrophils, biofilms grown in 6-channel μ-slides (ibidi) as described above were incubated with 4 × 106 PMN/ml and incubated for either 1 or 2 h, as required (37°C, with CO2). To visualize intact neutrophils, cells were incubated with 100 μM Cell Tracker Blue (Thermo Fisher) for 30 min before interaction with biofilms. After incubation, flow cells were washed with RPMI (without phenol red) to remove planktonic bacteria and fixed with 2% paraformaldehyde. Syto-9 (Invitrogen) was used to stain live cells, while EthHD-1 (Invitrogen) stained for DNA of dead or damaged cells. Images were captured using a FluoView filter confocal microscopy at a total magnification of ×600, and 3-dimensional images were analyzed with Imaris software (version x64 8.4.1; Bitplane, Concord, MA).
Silicone tube biofilms and CFU enumerations.
For studies describing CFU collected from biofilms treated with neutrophils, cultures were grown as described above, and 2 × 108 cells (2 ml) were seeded into 10-cm-long peroxide-cured silicone tubing (0.5-cm inner diameter), plugged at both ends. Tubes were incubated for 16 to 18 h at 37°C under static conditions to allow biofilm formation. Neutrophils (4 × 106/ml; 2 ml) were stained with Cell Tracker Blue (15 min) for microscopy (or used unstained for analyses of numbers of CFU per milliliter) and incubated with biofilms for 1 h. Dispersed populations of bacteria and unattached neutrophils were collected by unplugging one end of the tubing after the 1-h incubation period. Tubes were then cut longitudinally using a sterile metal scalpel (Bard-Parker), and biofilms were scraped off aseptically using a metal spatula and 2 ml of phosphate-buffered saline. Suspensions containing biofilms and attached neutrophils were lightly vortexed to break bacterial aggregates and centrifuged at 233 × g for 3 min at 4°C to pellet intact neutrophils, leaving extracellular bacterial populations in the supernatant. Extracellular populations were then pelleted by spinning supernatants at 20,000 × g.
Intracellular populations were enumerated from pelleted neutrophils by centrifuging at 20,000 × g. Neutrophils lyse at this speed, allowing intracellular bacteria to be collected from the pellet. Pellets were imaged before and after centrifugation to validate the separation of neutrophils from bacterial populations, using the LIVE/DEAD procedure describe above (Fig. S3).
Neutrophil isolation.
Neutrophils were isolated from whole blood using previously published methods (58). Briefly, heparinized blood derived from healthy human donors by venipuncture was collected in saline and layered into a Ficoll-Paque gradient. Tubes were then centrifuged at 404 × g for 40 min at 23°C, after which pellets were allowed to sediment in a 1:1 solution of 3% dextran and 0.9% saline. Spent medium was collected and centrifuged at 335 × g for 10 min. Red blood cells (RBCs) in pellets were then lysed with sterile water, and 1.8% sodium chloride used to restore isotonicity. Tubes were centrifuged at 233 × g for 3 min. Pellets containing 95 to 97% neutrophils were resuspended in RPMI without phenol red and counted in a hemocytometer chamber, using trypan blue exclusion (59). To block phagocytosis, neutrophils were simultaneously treated with 10 μg/ml cytochalasin D (Sigma-Aldrich) and 100 μM Cell Tracker Blue for 30 min before incubation with biofilms for 1 or 2 h. DNase I treatments were performed similarly, using a concentration of 0.5 μg/ml of neutrophils as described previously (33).
CD45 labeling of neutrophils.
Neutrophils were incubated with biofilms in the 6-channel μ-slide (ibidi) model described above. Biofilms were then blocked with 1% bovine serum albumin overnight at 4°C. Neutrophils were stained using a fluorescein isothiocyanate (FITC)-labeled anti-CD45 antibody (no. 368507; BioLegend) (34) and ethidium homodimer-1. Anti-mouse IgG(κ) was used as a negative isotype control (no. 400109; BioLegend). Cells were visualized with confocal laser scanning microscopy at a total magnification of ×600. The percentage of intact neutrophils that underwent NETosis was calculated by counting the number of neutrophils that stained with anti-CD45+ Cell Tracker Blue as a percentage of the total number of cells per field (anti-CD45 plus Cell Tracker Blue plus EthHD-1), after a 1-h incubation with respective strains, for 12 fields per strain, from 6 independent experimental replicates.
Quantification and statistical analysis.
All statistical analysis was carried out using GraphPad Prism version 5.0b. One-way analysis of variance with Tukey’s least significant difference test was a post hoc test where appropriate. Bartlett’s test was used to determine the extent of variability between sample populations. Variance between groups was found to be similar. Results for all experiments represent triplicate conditions performed in at least 3 independent experiments. Sample sizes for neutrophil killing assays were chosen based on the standard deviation of a pilot experiment performed in triplicate to achieve approximately a specified half width of the 95% confidence interval (α = 0.05). For calculation of mean fluorescence intensities (MFI), ImageJ software version 5.3 was used to set the same threshold for all conditions, and MFI was calculated from 6 independent experiments.
Supplementary Material
ACKNOWLEDGMENTS
Images presented in this report were generated using the instruments and services at the Campus Microscopy and Imaging Facility, The Ohio State University. This facility is supported in part by grant P30 CA016058, National Cancer Institute, Bethesda, MD. We acknowledge Sara Cole for helpful guidance for the confocal laser scanning microscopy studies.
V.J.T. is an inventor on patents and patent applications filed by New York University School of Medicine, which are currently under commercial license to Janssen Biotech Inc.
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, R01AI134895 and R01AI143916 and a Cystic Fibrosis Foundation award (WOZNIA16GO) to D.J.W. and National Institute of Allergy and Infectious Diseases award numbers R01AI099394 and R01AI105129 to V.J.T. E.T.M.B. was supported by a Rubicon fellowship from the Netherlands Organization for Scientific Research. V.J.T. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Conceptualization, M.B., D.J.W., and V.J.T.; Experiments, M.B. and P.J.H.; Construction and validation of leukocidin mutants – E.T.M.B., R.C., and X.Z.; Writing – Original Draft, M.B. and D.J.W.; Review & Editing, M.B., X.Z., V.J.T., and D.J.W.; Funding Acquisition, D.J.W. and V.J.T.; Resources, D.J.W. and V.J.T.; Supervision, D.J.W. and V.J.T.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus Infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Chapman JR, Balasubramanian D, Tam K, Askenazi M, Copin R, Shopsin B, Torres VJ, Ueberheide BM. 2017. Using quantitative spectrometry to understand the influence of genetics and nutritional perturbations on the virulence potential of Staphylococcus aureus. Mol Cell Proteomics 16:S15–S28. doi: 10.1074/mcp.O116.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Periasamy S, Joo H-S, Duong AC, Bach T-H, Tan VY, Chatterjee SS, Cheung GYC, Otto M. 2012. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A 109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton JW, Geesey GG, Cheng KJ. 1978. How bacteria stick. Sci Am 238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 5.Ammons MCB, Tripet BP, Carlson RP, Kirker KR, Gross MA, Stanisich JJ, Copié V. 2014. Quantitative NMR metabolite profiling of methicillin-resistant and methicillin-susceptible Staphylococcus aureus discriminates between biofilm and planktonic phenotypes. J Proteome Res 3:2973–2985. doi: 10.1021/pr500120c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resch A, Leicht S, Saric M, Pásztor L, Jakob A, Götz F, Nordheim A. 2006. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6:1867–1877. doi: 10.1002/pmic.200500531. [DOI] [PubMed] [Google Scholar]
- 7.Brady RA, Mocca CP, Plaut RD, Takeda K, Burns DL. 2018. Comparison of the immune response during acute and chronic Staphylococcus aureus infection. PLoS One 13:e0195342. doi: 10.1371/journal.pone.0195342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayadas TN, Cullere X, Lowell CA. 2014. The multifaceted functions of neutrophils. Annu Rev Pathol 9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. 2012. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. 2015. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther 13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya M, Berends ETM, Chan R, Schwab E, Roy S, Sen CK, Torres VJ, Wozniak DJ. 2018. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci U S A 115:7416–7421. doi: 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PM, Kielian T. 2013. Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infect Immun 81:4363–4376. doi: 10.1128/IAI.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonzo F, Torres VJ. 2014. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA, Broglie PM, Marketon K, Austermann J, Vogl T, Foell D, Niemann S, Peters G, Roth J, Löffler B. 2012. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol 92:1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balasubramanian D, Ohneck EA, Chapman J, Weiss A, Kim MK, Reyes-Robles T, Zhong J, Shaw LN, Lun DS, Ueberheide B, Shopsin B, Torres VJ. 2016. Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. mBio 7:e00818-16. doi: 10.1128/mBio.00818-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resch A, Rosenstein R, Nerz C, Götz F. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71:2663–2676. doi: 10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. 2011. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol 11:143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherr TD, Hanke ML, Huang O, James DBA, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T. 2015. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. mBio 6:e01021-15. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Münzenmayer L, Geiger T, Daiber E, Schulte B, Autenrieth SE, Fraunholz M, Wolz C. 2016. Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol 18:1172–1183. doi: 10.1111/cmi.12577. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Robles T, Lubkin A, Alonzo F, Lacy DB, Torres VJ. 2016. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep 17:428–440. doi: 10.15252/embr.201540994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoong P, Torres VJ. 2015. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat Commun 6:8125. doi: 10.1038/ncomms9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuMont AL, Yoong P, Surewaard BGJ, Benson MA, Nijland R, van Strijp JAG, Torres VJ. 2013. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun 81:1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, Whitney AR, Sturdevant DE, Dorward DW, Holland SM, Kreiswirth BN, Musser JM, DeLeo FR. 2010. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2:560–575. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun 70:6339–6345. doi: 10.1128/iai.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuMont AL, Yoong P, Day CJ, Alonzo F, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Saïd-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 32.Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. 2010. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis 201:241–254. doi: 10.1086/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun 2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. 2012. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FHY, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 38.Sultan AR, Hoppenbrouwers T, Lemmens-Den Toom NA, Snijders SV, Van Neck JW, Verbon A, De Maat MPM, Van Wamel W. 2019. During the early stages of staphylococcus aureus biofilm formation, induced neutrophil extracellular traps are degraded by autologous thermonuclease. Infect Immun 87:e00605-19. doi: 10.1128/IAI.00605-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fey PD, Endres JL, Yajjala VK, Yajjala K, Widhelm TJ, Boissy RJ, Bose JL, Bayles W. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moormeier DE, Bose JL, Horswill AR, Bayles KW. 2014. Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio 5:e01341-14. doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 43.Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, Foster T, Potempa J. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 3:e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surewaard BGJ, de Haas CJC, Vervoort F, Rigby KM, DeLeo FR, Otto M, van Strijp JAG, Nijland R. 2013. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol 15:1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. 1987. Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 46.Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol 13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Segal AW. 2005. How neutrophils kill microbes. Annu Rev Immunol 23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. 2012. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kretschmer D, Gleske A-K, Rautenberg M, Wang R, Köberle M, Bohn E, Schöneberg T, Rabiet M-J, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. 2010. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melehani JH, James DBA, DuMont AL, Torres VJ, Duncan JA. 2015. Staphylococcus aureus Leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog 11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. 2010. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman LR, Déziel E, D'Argenio DA, Lépine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 103:19890–19895. doi: 10.1073/pnas.0606756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boles BR, Singh PK. 2008. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad Sci U S A 105:12503–12508. doi: 10.1073/pnas.0801499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Attia AS, Cassat JE, Aranmolate SO, Zimmerman LJ, Boyd KL, Skaar EP. 2013. Analysis of the Staphylococcus aureus abscess proteome identifies antimicrobial host proteins and bacterial stress responses at the host-pathogen interface. Pathog Dis 69:36–48. doi: 10.1111/2049-632X.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P. 2015. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 59:111–120. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shanks RMQ, Donegan NP, Graber ML, Buckingham SE, Zegans ME, Cheung AL, O'Toole GA. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect Immun 73:4596–4606. doi: 10.1128/IAI.73.8.4596-4606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nauseef WM. 2007. Isolation of human neutrophils from venous blood. Methods Mol Biol 412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- 59.Strober W. 2001. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 111:A.3B.1–A.3B.2. doi: 10.1002/0471142735.ima03bs111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.