Salmonella causes grave systemic infections in humans and other animals and provides a paradigm for other diseases in which the bacteria have both intracellular and extracellular lifestyles. New generations of vaccines rely on the essential contribution of the antibody responses for their protection. The quality, antigen specificity, and functions associated with antibody responses to this pathogen have been elusive for a long time. Recent approaches that combine studies in humans and genetically manipulated experimental models and that exploit awareness of the location and within-host life cycle of the pathogen are shedding light on how humoral immunity to Salmonella operates.
KEYWORDS: Antibodies, IgM, IgA, IgG, systemic infections, Salmonella, vaccines
ABSTRACT
Salmonella causes grave systemic infections in humans and other animals and provides a paradigm for other diseases in which the bacteria have both intracellular and extracellular lifestyles. New generations of vaccines rely on the essential contribution of the antibody responses for their protection. The quality, antigen specificity, and functions associated with antibody responses to this pathogen have been elusive for a long time. Recent approaches that combine studies in humans and genetically manipulated experimental models and that exploit awareness of the location and within-host life cycle of the pathogen are shedding light on how humoral immunity to Salmonella operates. However, this area of research remains full of controversy and discrepancies. The overall scenario indicates that antibodies are essential for resistance against systemic Salmonella infections and can express the highest protective function when operating in conjunction with cell-mediated immunity. Antigen specificity, isotype profile, Fc-gamma receptor usage, and complement activation are all intertwined factors that still arcanely influence antibody-mediated protection to Salmonella.
INTRODUCTION
Several serovars of Salmonella enterica cause systemic diseases in humans and other animals. The global estimated burden of typhoid fever (Salmonella enterica subsp. enterica serovar Typhi) is over 21 million illnesses and 200,000 deaths, with sustained high incidence in Southeast Asia and endemic/epidemic occurrence increasingly reported in Africa (1–6). Paratyphoid fever (serovars Paratyphi A, B, and C) has an estimated 5.4 million illnesses worldwide (3). Invasive nontyphoidal Salmonella (iNTS) serovars (e.g., Salmonella enterica serovars Typhimuriun and Enteritidis) are a leading cause of lethal sepsis and severe relapsing infections in young children and immunocompromised individuals, especially in countries of the sub-Saharan African region (6–12), with an estimated 3.8 million illnesses leading to 680,000 deaths annually and very high case fatality ratios (20%) (7, 11). Antimicrobial resistance is an increasing problem in tackling many bacteria, including Salmonella (7, 11, 13–15).
WHY IS IT IMPORTANT TO GAIN A BETTER UNDERSTANDING OF ANTIBODY-MEDIATED IMMUNITY TO SALMONELLA?
There is extensive international consensus on the urgent need for better and affordable vaccines against systemic Salmonella infections. Vaccination has the potential for a high economic and health impact in fighting antimicrobial-resistant infections (16–19). Several classes of vaccines against systemic Salmonella disease have been considered in the past decades (20, 21). These differ in their ability to induce protective cell-mediated and humoral immunity with, broadly speaking, live attenuated vaccines being more efficient than nonliving preparations at eliciting Th1 type T-cell immunity known to contribute to host resistance to this bacterium (22, 23).
Despite the superior protective activity shown in animal models, live Salmonella vaccines can cause lethal infections in immunocompromised hosts (24–27). Mutants of Salmonella, including those that have been considered so far as vaccine candidates, retain virulence and can rapidly kill immune-suppressed mice (24–27). Mice that lack T-cell functions (25, 27) and mice coinfected with malaria are very susceptible to infection with live Salmonella, including with mutants that have been considered as vaccine candidates (28, 29). Efforts to identify single-gene mutations for the development of live vaccine candidates that would be completely safe (totally unable to grow to high numbers) in severely immune-deficient animals have been unsuccessful (24). This raises some concerns for the use of live vaccines in areas where Salmonella is endemic and that have a higher incidence of immune-suppressive conditions. For example, HIV and malaria are comorbidities that make humans more susceptible to systemic Salmonella infection, leading to severe, often fatal disease, and which therefore pose dangers to the use of live vaccines (30, 31). These comorbidities are widespread and epidemiologically colocalize with areas of the developing world where there is a high incidence of epidemic or endemic systemic salmonelloses and that are therefore where anti-Salmonella vaccines are most needed (9, 20, 31, 32).
Mainly for safety reasons, nonliving vaccines are currently being considered prime candidates for immunization against Salmonella diseases. The protective ability of these vaccines relies largely on the induction of antibodies. If we are to use nonliving vaccines as tools against systemic salmonelloses, it is therefore essential to rationally optimize the antibody responses induced by these preparations. This knowledge would also be immensely useful to understand how those comorbidities that impair antibody-mediated functions increase susceptibility to disease and to design vaccine strategies that can at least in part reverse these immune-suppressing conditions. This will need to be based on a clearer understanding of the qualitative and functional features of the protective antibody response against Salmonella.
This article will briefly outline the factors that influence the protective efficacy of the antibody response to systemic Salmonella infections and will embed antibody functions in the context of the location and spread of the bacteria during the infection process. This minireview will also highlight the interactions and dual requirement of T cell- and B cell-mediated immunity, both in the engenderment of antibody and T-cell responses and in the expression of in vivo resistance to the pathogen.
HOW CAN ANTIBODIES PROTECT AGAINST A PATHOGEN THAT HAS AN INTRACELLULAR LIFESTYLE?
The relative importance of antibodies and cellular immunity in host resistance to systemic Salmonella infections has been a matter of debate for a long time.
The controversy initially originated from uncertainties in the location of the bacteria within the tissues of an infected host. It is undisputable that in many animal species, including humans, Salmonella resides inside phagocytes and grows within these cells, using an armory of genes and effectors to foster its replication and evade intracellular killing (24, 33–40). Evidence from mouse models on the role of the phagocyte innate resistance gene Ity (now known as Scla11a1) in the control of bacterial division in vitro and in vivo, as well as studies where the bacteria could be seen to grow within cultured phagocytic cells, has corroborated these views (41–44). However, evidence for extracellular growth and lack of intracellular replication had also been produced, albeit based on electron microscopy studies where infections were allowed to progress to very high premortem bacterial numbers in the tissues. In these studies, intracellular bacteria were seen to undergo various stages of degradation inside macrophages and granulocytes and it was therefore inferred that Salmonella was more likely to be an extracellular pathogen and its virulence directly related to its antiphagocytic property (45).
The debate was further fueled by immunological studies in both experimental animals and humans. On the one hand, evidence was provided for the protective role of antibodies being limited to the very early stages of parenteral experimental infections, when the bacteria have not yet reached an intracellular location, with no detectable effect on their later growth in the tissues (46, 47); on the other hand, the protective effect of whole-cell nonliving typhoid vaccines, which induce mainly humoral immunity, and Vi vaccines that do not directly contain Salmonella-specific T-cell antigens was undeniable (48–53). However, protection by passive transfer of antibodies or T cells alone and by nonliving vaccines was seen only in host-pathogen interactions where the infection is naturally mild and sublethal, such as when challenging resistant mice with either virulent or weakly virulent strains or susceptible mice with weakly virulent strains (46, 50).
These discrepant views on the relative importance of humoral and cellular immunity to Salmonella were largely reconciled by studies showing that passive transfer of both antibodies and T cells, but neither alone, could protect recipient innately susceptible animals against fatal systemic Salmonella disease (54).
More recently, the essential role of both antibodies and T cells in host resistance to Salmonella has been corroborated by additional evidence obtained from human studies. For example, clinical observations indicate that lack of antibodies at young age correlates with the incidence of iNTS in African children (55, 56), despite these children acquiring Salmonella-specific CD4 Th1 cell immunity very early in life; only when antibodies are developed in addition to T-cell immunity is full protection achieved (57). This resistance is then abrogated by comorbidities that can affect either cellular or antibody-mediated functions (29, 58–62). For example, HIV, which has a profound effect on CD4-positive (CD4+) T-cell immunity, and malaria, which impairs phagocyte functions, increase susceptibility to systemic Salmonella infections such as iNTS. A similar increase in susceptibility is seen in patients with deficiencies in cytokines such as gamma interferon (IFN-γ) and interleukin 12 (IL-12) and in their receptors, leading to impaired activation of phagocytes and likely also an impairment of T-cell immunity (9, 27, 63–67).
SALMONELLA: A BACTERIUM WITH COMPLEX INTRA- AND EXTRACELLULAR PATHOGENESIS
Where do antibodies target Salmonella?
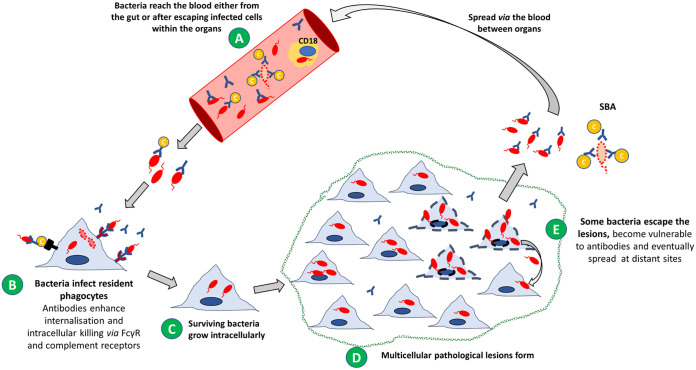
Salmonella infections are normally acquired by the oral route and reach the blood after invading the gastrointestinal tract (68). In the blood, the bacteria can be found either as extracellular bacteria or associated with CD18+ cells (69) (Fig. 1A). Extracellular Salmonella in the blood can be targeted by antibodies that enhance their uptake and killing by resident phagocytes of the spleen and liver (46, 70, 71) and can potentially lyse them via activation of the complement classical pathway (56). From the blood, Salmonella reach an intracellular location within phagocytes of the liver, spleen, and bone marrow (70, 72, 73) (Fig. 1B). Early in infection Salmonella reaches mainly splenic red pulp (F4/80+, MSR-Alow) and marginal zone macrophages (Macrophage Scavenger Receptor A [MSR-A+]) (74). In the liver, Salmonella localizes preferentially in the resident Kupffer cells. During the infection, bacteria can also be found in dendritic cells or B cells (36, 74–77). As the infection progresses and bacteria reside and possibly grow intracellularly (Fig. 1C), inflammatory cells infiltrate the initial unicellular foci of infection to form multicellular pathological lesions surrounded by normal tissue (Fig. 1D). These lesions contain polymorphonuclear phagocytes (PMNs) during the first few days of infection, which are later replaced by inflammatory macrophages (36, 78).
FIG 1.
The complex pathogenesis of Salmonella consists of an intracellular antibody-refractive growth phase and an extracellular antibody-susceptible phase of spread. (A) After invading the gut, Salmonella bacteria reach the blood, where can be targeted by antibodies. Some bacteria in the blood are lysed by antibody- and complement-dependent serum bactericidal activity (SBA). (B) Bacteria opsonized by antibodies are engulfed and killed more efficiently by resident phagocytes as soon as they reach the tissues (e.g., spleen, liver, bone marrow). (C) Those bacteria that resist killing establish unicellular initial infection foci (infected phagocytes). Surviving bacteria grow mainly in an intracellular location. (D) The initial single-cell infection foci become spatially separated multicellular pathological lesions due to the infiltration of polymorphonuclear phagocytes (PMNs) and later mononuclear cells. In this intracellular location, bacteria are inaccessible to antibodies. Bacteria grow intracellularly within the multicellular infection foci, but their numbers within each phagocyte remain low due to the spread of the infection to infection of new host cells. (E) Some bacteria escape the lesions, become vulnerable to antibodies, and eventually spread at distant sites. When the bacteria are released from infected cells, they might undergo three different fates, as follows: (i) being targeted by antibodies and killed via complement-mediated SBA or opsonophagocytosis, (ii) rapidly infecting neighboring cells within the same lesion, or (iii) traveling to distant sites in the body to establish new unicellular infection foci.
Once Salmonella bacteria have homed inside host cells, their intracellular location would render the bacteria inaccessible to antibodies, making it difficult to envisage a role of humoral immunity in protection. Detailed analysis of intracellular bacterial densities over the course of experimental infections in mice has shed light on how and where the bacteria can become vulnerable to antibodies. In fact, microscopic observation of immune-labeled and/or fluorochrome-expressing intracellular bacteria in the spleen and liver revealed that the majority of infected phagocytes contain very low intracellular numbers at any time of the infection, irrespective of the overall net growth of the bacteria in the organs (77). The infection process is underpinned both by intracellular growth/survival and by an increase in the number of infected cells and multicellular pathological lesions, but not by increases in the number of visible intracellular bacterial per phagocytes (24, 77, 79) (Fig. 1D). This is due to the continuous redistribution of the bacteria from infected host cells to uninfected ones and the spread of individual microorganisms to new infection foci (Fig. 1E), mediated by the Salmonella type three secretion system (T3SS) encoded by the Salmonella pathogenicity island 2 (SPI-2) (80, 81). Salmonella infections are therefore dispersive processes in which bacteria have an intracellular phase of growth and an extracellular one of spread.
Is the dispersiveness of the infection a plausible explanation for the role of antibodies in protection against Salmonella?
It would be reasonable to postulate that in a dispersive infection process, cell-mediated immunity enhances the antimicrobial functions of phagocytes, therefore affecting the fate of the intracellular bacteria (82–86); antibodies would opsonize the extracellular bacteria in transit between cells and target them to activating cellular receptors, thus increasing the antimicrobial activity of otherwise naive phagocytes at new infection foci (87, 88). Antibodies would therefore be expected to have a major impact on the process.
However, we know from a large body of data that antibodies alone, in the absence of cell-mediated immunity, are unable to modify the net growth rate of Salmonella in the spleen and liver of an infected experimental animal (21, 46, 50). For example, adoptive transfer of Salmonella-specific immunoglobulins or immunization with nonliving vaccines do not, surprisingly, affect the growth curve of the bacteria in vivo. An effect of antibodies is visible only in the first few hours of the infection, when the initial kill of the inoculum is enhanced (46). This early protective effect of antibodies is more evident when experimental animals are challenged via the intraperitoneal route, where antibodies most likely accelerate capture of the bacteria by peritoneal phagocytes and therefore abort extracellular growth in the peritoneal cavity. The use of molecularly tagged and therefore individually traceable Salmonella populations, combined with mathematical modeling and statistical analysis of data, has further confirmed the inability of antibodies to significantly affect the growth and spread of bacteria in the body. This research approach has confirmed that, in the early stages of the infection, bacteria spread from cell to cell within each organ, later followed by systemic spread of bacterial populations between distant body sites, coinciding with the appearance of bacteria in the blood (89). Using this system, it became clear that only live vaccines (which induce both humoral and cell-mediated immunity) would be able to control bacteremia and restrain growth and spread of the bacteria in the body. Conversely, mice where antibodies were the only vaccine-mediated effector mechanisms (i.e., mice immunized with killed vaccines or depleted of T cells after immunization) would not be able to control the spread of the bacteria or abort bacteremia (71).
ANTIGEN SPECIFICITY AND PROTECTION
Salmonella infections induce antibody responses against a large array of surface, periplasmic, and cytoplasmic antigens in humans and other infected animals. Immunoreactive antigens have been detected using enzyme-limited immunosorbent assay (ELISA), immunoblotting, and protein microarrays (90–94). These include lipopolysaccharide (LPS) antigens, porins, lipoproteins, fimbriae, flagella, heat shock proteins, and in some serovars, the Vi surface polysaccharide antigen (95, 96). Several antigens have been identified as targets of the protective antibody response. The O antigen, which consists of sugar repeats exposed on the bacterial surface, is a prime target of protective antibodies, with some epitopes being more protective than others. Immunodominant serovar-specific O antigens (e.g., O:4 and O:9) determine to a large extent the specificity of protection between serovars and induce antibodies that are more protective than the ones directed against O antigens shared among different Salmonella serogroups (e.g., O:12) (97–100). The Vi antigen is immunogenic and was shown to confer protective immunity in human volunteers and in field trials, both as a native polysaccharide and as a protein conjugate (49, 51, 53, 101–103).
The functional role of the antibody response to porins is still not fully clarified. Antibody responses against some porins, but not others, are protective in animal models (104, 105). For example, antibodies to the trimeric OmpD porin, but not to the monomeric and abundant OmpA protein, protect mice against parenteral challenge. Furthermore, OmpD is a candidate antigen for iNTS vaccines (20, 105, 106). The reasons for the different protective abilities of OmpA and OmpD have been elegantly postulated to be due to different accessibility of antibodies to OmpD at the bacterial surface. Both OmpA and OmpD are located in the outer membrane of Salmonella in a position potentially shielded by the LPS O side chain. However, OmpD creates a larger footprint than OmpA in the O-antigen layer, sufficient for a single IgG to gain access to the most-exposed surface loop epitopes of OmpD (105). It remains unclear how antibodies to OmpD mediate serum bactericidal activity (SBA), given that at least two Ig need to come together for activation of the classical pathway of the complement. The conclusion that OmpA is poorly accessible by IgG also clashed with data showing its binding by human recombinant IgG specific for a mimotope inserted in OmpA (107).
Antibodies to flagellin are detectable in infected individuals and targeting this antigen can mediate opsonophagocytosis (87, 93).
WHICH IS THE MAIN PROTECTIVE MECHANISM OF ANTI-SALMONELLA ANTIBODIES?
Following their binding to the bacterial surface, antibodies can exert antibacterial efficacy mainly in two ways: either by increasing phagocytosis of bacteria via targeting the bacteria to specific receptors on the surface of immune cells, or by direct bactericidal activity, mediated by the activation of the classical complement pathway (serum bactericidal activity [SBA]). Both of these mechanisms are likely to operate in Salmonella infections. Early studies showed that clearance of Salmonella from the blood of mice is dramatically accelerated by immune serum, suggesting a role for antibodies in the enhancement of phagocytosis (70). More recently, evidence for an enhancement of opsonophagocytosis by Salmonella antibodies has been corroborated by in vitro systems. Sera from humans vaccinated with live attenuated Salmonella enterica subsp. enterica serovar Typhi enhance opsonophagocytosis in vitro, with IgG playing a major role (108). Engagement of opsonized bacteria with the activating Fc-gamma receptors on the surface of murine or human phagocytes results in increased uptake of the bacteria, increased production of reactive oxygen intermediates (ROI), and enhanced antibacterial functions of the infected cells (87, 88, 107). Opsonization drives increases in both the number of phagocytes that ingest bacteria and in the efficiency of each individual cell in ingesting Salmonella, as indicated by the higher intracellular numbers per phagocyte of opsonized versus nonopsonized bacteria (88, 107). Antibodies have also shown to be essential for optimal phagocytosis of iNTS strains by peripheral blood cells from Africans (55).
Antibody-dependent, complement-mediated SBA correlates with susceptibility to iNTS in African children (56), and therefore SBA has been often taken as an indication of the functional activity of antibodies when testing new vaccines against iNTS (109). However, the role of SBA as a true mechanism of antibody-mediated protection must be evaluated with caution. Studies that used human blood and sera to compare the kinetics of SBA and the phagocytosis of iNTS clinical isolates show that phagocytosis allows bacteria to escape the blood and establish intracellular infection before they are killed by the complement membrane attack complex (110). This would indicate that opsonophagocytosis is likely to be the prevailing antimicrobial mechanism mediated by anti-Salmonella antibodies. Furthermore, no correlation was found between protection afforded by live vaccination and by SBA in a controlled human typhoid challenge model (111).
COMPLEMENT OR Fc-GAMMA RECEPTORS?
The complement system and Fc-gamma receptors (FcγRs) can potentially play a crucial role in antibody-mediated immunity against Salmonella diseases. However, their relative importance is unclear.
Complement-mediated SBA can result in bacterial killing, but, as discussed above, it is unclear whether this mechanism is relevant for immunity to Salmonella. Complement appears to be essential for antibody-dependent opsonophagocytosis of iNTS strains by human blood phagocytes and for the production of ROI and bacterial killing (55). However, binding of antibody-opsonized Salmonella to FcγR on human and murine cells in the absence of complement also enhances phagocytosis and ROI-mediated bacterial killing, with the activating FcγRI playing a major role (88, 107).
In vivo studies also provide evidence for a role for both complement and FcγR, with their relevance depending on the experimental model used. When mice lacking FcγRI, -II, -III, and -IV (FcγR knockout [KO]), or mice lacking the complement C3 component (C3 KO), or lacking all four FcγR and C3 (FcγR/C3 KO), were passively immunized with anti‐LPS O4 IgG2a monoclonal antibodies and subsequently infected parenterally with Salmonella Typhimurium, only FcγR KO animals showed a significant reduction in the bacterial loads in the liver, spleen, and mesenteric lymph nodes (112), at a level similar to those observed in wild-type control animals. In this model, therefore, the role of complement prevails on that of FcγRs. However, when mice lacking FcγRI, -II, and -III and mice lacking C3 were immunized with a live attenuated vaccine and later challenged orally with virulent Salmonella, only the FcγR-deficient mice succumbed to the infection (113), indicating a prevailing role of FcγR.
In summary, evidence for a role of both FcγR and complement has been provided, but firm conclusions on their relative importance are difficult to draw due to discrepancies between experimental conditions, models, and host species.
DOES QUALITY OF THE ANTIBODY RESPONSE MATTER?
The qualitative traits of the antibody response have a great effect on its function and potency. The isotype profile has effects on the binding of antibodies to FcγR receptors and on efficiency of complement activation. This in turn has effects on SBA, opsonophagocytosis, and on the enhancement of the intracellular antibacterial mechanisms of phagocytes.
Virtually all classes of Ig are produced in response to infection with Salmonella. As expected, IgM appear early after infection and are usually followed by IgG and IgA (92, 95, 114–117). Different isotype profiles are seen following natural infection or vaccination with live or nonliving vaccines (22, 118). Some nonliving vaccines can induce isotype-switched responses to Salmonella polysaccharide antigens, indicating that the T-cell responses induced by these preparations may not be able to mediate the suppression of bacterial growth in the tissues, but are sufficient to support isotype switching of the Ig response. For example, IgG responses are detected following vaccination with Vi-conjugate vaccines (51, 119, 120); interestingly, outer membrane vesicle vaccines (OMV) are capable of inducing highly effective IgG2a and IgG2b in mice (109); live vaccines induce high titers of all IgG subclasses, including higher levels of IgG2a (22).
The isotype profile of the antibody response impacts on its protective function. The efficacy of individual subclasses has been studied in murine and human systems, and this area of research is not devoid of controversy. In murine studies, polyclonal and monoclonal IgM, as well as IgA to Salmonella polysaccharide antigens, were found to be highly protective and in some cases more protective than IgG (100, 121, 122). Conversely, opsonization of Salmonella with O4-specific IgA, IgG1, IgG2a, or IgG2b, but not with IgM monoclonal antibodies, resulted in cell-dependent bacterial killing in vitro. In in vivo passive immunization studies, IgG2a and IgG2b O4-specific monoclonal antibodies provided higher functional activity than IgA, IgM, and IgG1 by decreasing the bacterial load in the blood and tissues (123).
A role for IgA in protection against Salmonella is shown by the increased susceptibility to infection of polymeric immunoglobulin receptor (pIgR−/−) knockout mice, which are unable to bind and actively transport dimeric IgA to the mucosae (124), and by the protective ability of IgA monoclonal antibodies in vivo (125).
The relative potency of individual isotypes can be dependent on the antigen that is targeted. In fact, unlike what is seen in the case of antibodies against the LPS-O antigen, it has been shown that mice lacking IgG1, but not lacking IgG2a, are substantially less protected after immunization with the OmpD porin than wild-type controls. Immunization with OmpD was maintained in T bet-deficient mice that do not produce IgG2a. This is consistent with IgG1 having an important role for protection after immunization with OmpD, but IgG2a being less important (126).
The relative potency of human IgG was studied in an in vitro system in which OmpA and flagella were tagged with a foreign CD52 mimotope (TSSPSAD). The bacteria were opsonized with a panel of humanized recombinant CD52 antibodies that share the same antigen-binding V region but have constant regions of different subclasses. This work revealed that, although opsonization with all IgG subclasses increases Salmonella uptake by human phagocytes, differences in potency can easily be revealed. IgG3 resulted in the highest level of bacterial uptake and the highest average bacterial load per infected cell, which was closely followed by those of IgG1, then IgG4, and lastly IgG2. Phagocytosis mediated by IgG1, IgG3, and IgG4 had a higher dependency on FcγRI than on FcγRIIA, whereas IgG2-mediated phagocytosis required FcγRIIA more than FcγRI (87, 107). Therefore, both the subclass of human IgG and the type of FcγR that is available for antibody binding affects the function of anti-Salmonella antibodies.
Some IgG subclasses can be detrimental to the antimicrobial function of the antibody response. In fact, the inability of blood from HIV patients to kill Salmonella is due to an inherent inhibitory effect of anti-LPS antibodies. This inhibition is dependent on high concentrations of antibodies and is strongly associated with IgA and IgG2 anti-LPS antibodies, possibly related to the poor ability of IgA and IgG2 to activate complement and to deposition of complement at sites where it cannot insert in the bacterial membrane (127).
CROSSTALK BETWEEN B CELLS AND T CELLS AND QUALITY TRAITS OF THE ANTIBODY RESPONSE
T-cell responses can be detected in animals and humans following infection with live Salmonella (128–130). Isotype switching and production of antiprotein antibodies require the presence of T cells (27, 118); for example, T cell-deficient athymic mice produce mainly IgG3 and IgM antibodies to lipopolysaccharide, whereas euthymic mice can produce IgM, IgG1, IgG2a, IgG2b, and IgG3 anti-LPS and anti-Salmonella protein antibodies (27). Similarly, some classes of nonliving vaccines only elicit a restricted isotype repertoire (22). The reciprocal interaction between T cells and B cells is essential for the development of both humoral and cell-mediated immunity to Salmonella (Fig. 2). In the absence of functional B cells, the onset of T-cell immunity is impaired, albeit not abrogated, in mice (131–134). Cytokine production and antigen presentation via major histocompatibility complex (MHC) class II molecules from B cells are essential for activation of Th1 and Th17 responses to Salmonella, as shown by studies in bone marrow chimera mice where only B cells are deficient in selected immunological functions (135). Interestingly, B cells can engender T-cell responses via engagement of innate immune receptors early in infection and via specific activation of the antigen-specific B-cell receptor later in the disease. These functions instigate a loop where T-cell activation in turn contributes to the isotype profile of the response. In fact, antigen-specific IgG2c primary responses are dependent on MyD88 signaling to B cells, while other Ig classes are not (IgG1 and IgG3) or are much less so (IgG2b and IgA). Lack of MyD88 signaling in B cells of chimeric mice results in impairment of development of IFN-γ effector T cells, a likely contributory factor in the lack of IgG2c (136).
FIG 2.
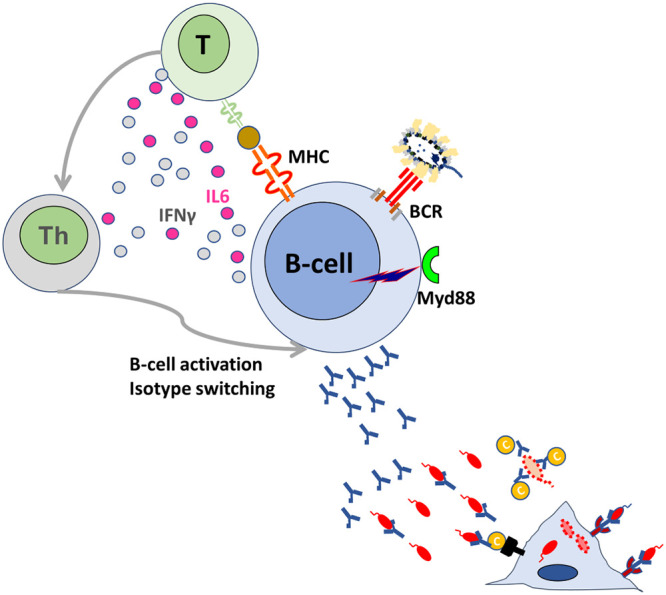
B-cell and T-cell cross talk impacts quality of the antibody response. MyD88 signaling and recognition of bacterial antigens via the B-cell receptor (BcR) leads to B-cell cell activation and antigen presentation to naive T-cells via MHC class II molecules. Interleukin 6 (IL-6) and gamma interferon (IFN-γ) from B cells mediate the development of Th immunity. Th immunity in turn triggers activation and maturation of B cells and induces isotype switching of the antibody response.
KEY CONSIDERATIONS FOR THE DEVELOPMENT OF SALMONELLA VACCINES
Antibodies are an essential component of the protective immune response to Salmonella. An ideal vaccine would therefore be the one able to induce both antibody responses and protective cellular immunity.
The induction of some level of T-cell immunity is essential whichever the choice of vaccine. T-cell immunity induced by natural infection or live vaccines is sufficient to support the isotype switching and affinity maturation of the antibody response, but also to mediate the suppression of the growth of virulent bacteria, when either resistant or susceptible animals are reinfected. In contrast, T-cell immunity induced by some nonliving vaccines is sufficient to support isotype switching and antiprotein responses (109, 118), but not protective cellular responses. Why this level or type of T-cell immunity is unable to activate phagocytes and curtail bacterial net growth in the tissues still remains one of the main unanswered questions in bacterial vaccinology.
Antibodies alone can have an impact on the very early stages of the infection, when they enhance bacterial killing before Salmonella bacteria have reached an intracellular location within phagocytes (46, 71); however, they have no effect on the net growth of bacteria in the tissues, or surprisingly, on the control of bacteremia (9, 46, 60, 71, 127, 137). Therefore, it is likely that in individuals who do not have specific Th1 memory, vaccine-induced antibody responses protect by preventing the establishment of the infection following transmission of the pathogen. The situation would be different in populations where background cellular immunity is present. For example, those vaccines that induce mainly antibody responses would be more effective in areas where disease is endemic, where a background of cellular immunity is already present in the population, likely due to low grade preexposure to the pathogen and/or to cross-reactive antigens of other microorganisms. Interestingly, young African children develop Th1 immunity to Salmonella very early in life, but remain susceptible to iNTS until they acquire Salmonella-specific antibodies (57); the aim of an effective iNTS vaccine for children would therefore be the induction of antibodies early in life. A lower protective efficacy of Vi vaccines against typhoid fever has been detected in volunteers in a controlled human challenge study compared to that in field studies, further suggesting the possibility that a different immunological exposure background due to the geographical area may affect vaccine efficacy (102, 103, 138).
The development of both safer live attenuated vaccines and nonliving vaccines would be desirable; the former would be more suitable for travelers, where elicitation de novo of both T-cell and B-cell immunity is required; the latter vaccines would be suitable for use in areas where disease is endemic, where, as discussed earlier, increased safety is a prerequisite and induction of antibodies would suffice.
Optimization of the immune response is important especially for nonliving vaccines, the efficacy of which is likely to be entirely based on antibodies. The importance of the isotype profile in relation to antigen specificity and function has been touched upon above with some representative examples.
Greater efforts are needed to optimize antibody responses induced by vaccines to be used in areas where immune suppressive comorbidities colocalize geographically with the target disease. An example is provided by malaria. This disease has a dual effect on humoral immunity. First, malaria can suppress the acquisition of anti-Salmonella antibodies (59, 139). Second, malaria can impair complement levels (61) and suppress the antimicrobial functions of phagocytes (28, 58, 140), thereby potentially not allowing the expression of antibody-mediated resistance. More research is needed to identify vaccine solutions that can overcome these problems. In fact, iNTS vaccines for Africa must induce immune responses optimized to confer resistance in the presence of multiple and various underlying comorbidities.
The optimal choice of antigens is still debatable, and several single-antigen vaccines are being developed and/or are in use. For example, the vaccines based on the Vi polysaccharide are immunogenic and induce protective serum responses. However, these vaccines include a single antigen that is not essential for the virulence of the bacterium (Vi-negative variants of S. Typhi are capable of causing disease [141]) and is downregulated within the tissues soon after infection (142). Multiantigen vaccines would probably be a wiser choice than single-antigen ones and would also offer the possibility of inducing antibody responses that can potentially protect against a large number of Salmonella serovars. For example, low-reactogenicity outer membrane vesicle-based vaccines that contain multiple structural antigens are very immunogenic and elicit highly functional isotype-switched antibody responses against a variety of polysaccharide and protein determinants (109, 143).
CONCLUSIONS
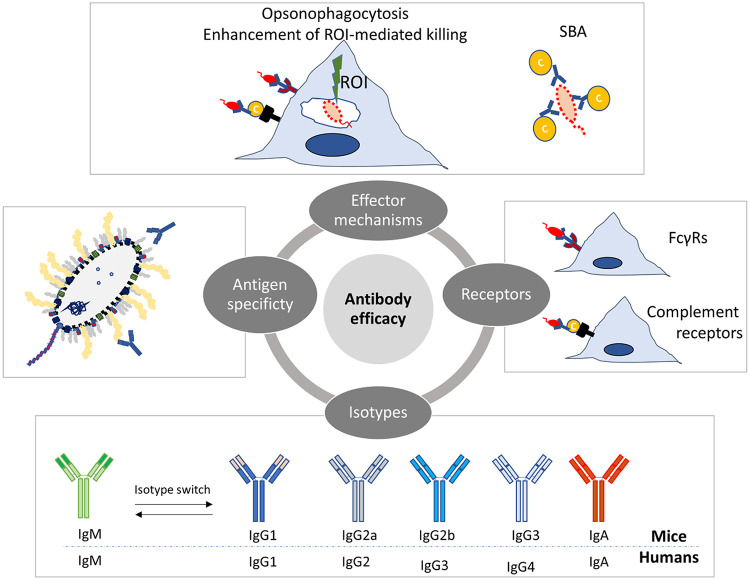
The overall scenario that emerges from decades of research in experimental animals and in humans indicates that antibodies are certainly essential for resistance against systemic Salmonella infections and can express the highest level of protective functions when operating in conjunction with T cell-mediated immunity. Antigen specificity, isotype profile, FcγR receptor usage, and complement activation (Fig. 3) are intertwined factors that have great influence on antibody-mediated protection to Salmonella.
FIG 3.
Antibody-mediated protection to Salmonella is underpinned by a complex interplay between qualitative traits and effector mechanisms. Antigen specificity, isotype profile, FcγR receptor usage, complement activation, and different effector mechanisms (opsonophagocytosis, killing via reactive oxygen intermediates [ROI] and serum bactericidal activity [SBA]) are intertwined factors that influence antibody-mediated protection to Salmonella.
There is still a great deal of discrepancy between findings and conclusions from different studies over several decades, and this makes rational vaccine design very difficult.
To improve current vaccines and design new ones in the future, it will be necessary to shed light on the mechanisms that underpin both the development of antibody responses and their effector functions. It will also be necessary to learn how to tailor antibody responses to situations in which other components of the immune system might be impaired, as often seen in areas where disease is endemic and where immune-suppressive comorbidities geographically coincide with systemic Salmonella infections. Different vaccines and antibody responses may be needed for travelers and residents in areas where salmonellosis is endemic.
Biographies

Pietro Mastroeni obtained a degree in medicine and surgery from the University of Messina, Italy, in 1990, and a Ph.D. in immunology from the University of Cambridge, United Kingdom, in 1994, where he was also awarded a doctor of science degree in 2017. He has worked at the University of Newcastle and Imperial College, London, and is now based at the University of Cambridge. His work has contributed to the fields of microbiology, immunology, and vaccine development for the last three decades. He is a Fellow of the Royal Society of Biology and of the American Academy of Microbiology.

Omar Rossi holds a master of science degree in molecular biology and received a Ph.D. in biotechnology at the Novartis Vaccine Institute for Global Health (NVGH) in 2014. After postdoctoral studies at NVGH and being a visiting scientist at the Wellcome Trust Sanger Institute, he was appointed as research associate at the University of Cambridge, working on pathogenesis of Salmonella disease. He is currently a senior scientist in the immunoassay group at the GSK Vaccines Institute for Global Health, working on development of vaccines, including ones against iNTS, for low- and middle-income countries. With over ten years of experience in the field of vaccinology, Dr. Rossi's interest is focused on the better understanding of mechanisms underpinning infections and on development of vaccines and functional assays to understand the action of antibodies in disease.
REFERENCES
- 1.Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW, Burke H, Williamson J, Oundo JO, Mintz ED, Feikin DR. 2012. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 7:e29119. doi: 10.1371/journal.pone.0029119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutterloh E, Likaka A, Sejvar J, Manda R, Naiene J, Monroe SS, Khaila T, Chilima B, Mallewa M, Kampondeni SD, Lowther SA, Capewell L, Date K, Townes D, Redwood Y, Schier JG, Nygren B, Tippett Barr B, Demby A, Phiri A, Lungu R, Kaphiyo J, Humphrys M, Talkington D, Joyce K, Stockman LJ, Armstrong GL, Mintz E. 2012. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis 54:1100–1106. doi: 10.1093/cid/cis012. [DOI] [PubMed] [Google Scholar]
- 5.Neil KP, Sodha SV, Lukwago L, S OT, Mikoleit M, Simington SD, Mukobi P, Balinandi S, Majalija S, Ayers J, Kagirita A, Wefula E, Asiimwe F, Kweyamba V, Talkington D, Shieh WJ, Adem P, Batten BC, Zaki SR, Mintz E. 2012. A large outbreak of typhoid fever associated with a high rate of intestinal perforation in Kasese District, Uganda, 2008–2009. Clin Infect Dis 54:1091–1099. doi: 10.1093/cid/cis025. [DOI] [PubMed] [Google Scholar]
- 6.Crump JA, Heyderman RS. 2014. Invasive Salmonella infections in Africa. Trans R Soc Trop Med Hyg 108:673–675. doi: 10.1093/trstmh/tru152. [DOI] [PubMed] [Google Scholar]
- 7.Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon MA. 2008. Salmonella infections in immunocompromised adults. J Infect 56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Zijlstra EE, Heyderman RS, Hart CA, Molyneux ME. 2008. Epidemics of invasive Salmonella enterica serovar Enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis 46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 11.Kariuki S, Gordon MA, Feasey N, Parry CM. 2015. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 33(Suppl 3):C21–C29. doi: 10.1016/j.vaccine.2015.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy EA, Shaw AV, Crump JA. 2010. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke FJ, Wain J. 2004. The emergence of antibiotic resistance in typhoid fever. Travel Med Infect Dis 2:67–74. doi: 10.1016/j.tmaid.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Harish BN, Menezes GA. 2011. Antimicrobial resistance in typhoidal salmonellae. Indian J Med Microbiol 29:223–229. doi: 10.4103/0255-0857.83904. [DOI] [PubMed] [Google Scholar]
- 15.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong VK, Dallman TJ, Nair S, Baker S, Shaheen G, Qureshi S, Yousafzai MT, Saleem MK, Hasan Z, Dougan G, Hasan R. 2018. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 9:e00105-18. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker SJ, Payne DJ, Rappuoli R, De Gregorio E. 2018. Technologies to address antimicrobial resistance. Proc Natl Acad Sci U S A 115:12887–12895. doi: 10.1073/pnas.1717160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloom DE, Black S, Salisbury D, Rappuoli R. 2018. Antimicrobial resistance and the role of vaccines. Proc Natl Acad Sci U S A 115:12868–12871. doi: 10.1073/pnas.1717157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugman KP, Black S. 2018. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci U S A 115:12896–12901. doi: 10.1073/pnas.1721095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tagliabue A, Leite LCC, Leroy OY, Rappuoli R. 2019. Editorial: A global perspective on vaccines: priorities, challenges and online information. Front Immunol 10:2556. doi: 10.3389/fimmu.2019.02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLennan CA, Martin LB, Micoli F. 2014. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother 10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. 2001. Salmonella: immune responses and vaccines. Vet J 161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 22.Harrison JA, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche CE. 1997. Correlates of protection induced by live Aro− Salmonella Typhimurium vaccines in the murine typhoid model. Immunology 90:618–625. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotlarski I, Pope M, Doherty K, Attridge SR. 1989. The in vitro proliferative response of lymphoid cells of mice infected with Salmonella Enteritidis 11RX. Immunol Cell Biol 67:19–29. doi: 10.1038/icb.1989.3. [DOI] [PubMed] [Google Scholar]
- 24.Grant AJ, Oshota O, Chaudhuri RR, Mayho M, Peters SE, Clare S, Maskell DJ, Mastroeni P. 2016. Genes required for the fitness of Salmonella enterica serovar Typhimurium during infection of immunodeficient gp91−/− phox mice. Infect Immun 84:989–997. doi: 10.1128/IAI.01423-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess J, Ladel C, Miko D, Kaufmann SH. 1996. Salmonella Typhimurium aroA− infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol 156:3321–3326. [PubMed] [Google Scholar]
- 26.Muotiala A. 1992. Anti-IFN-gamma-treated mice—a model for testing safety of live Salmonella vaccines. Vaccine 10:243–246. doi: 10.1016/0264-410x(92)90159-h. [DOI] [PubMed] [Google Scholar]
- 27.Sinha K, Mastroeni P, Harrison J, de Hormaeche RD, Hormaeche CE. 1997. Salmonella Typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun 65:1566–1569. doi: 10.1128/IAI.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lokken KL, Mooney JP, Butler BP, Xavier MN, Chau JY, Schaltenberg N, Begum RH, Muller W, Luckhart S, Tsolis RM. 2014. Malaria parasite infection compromises control of concurrent systemic non-typhoidal Salmonella infection via IL-10-mediated alteration of myeloid cell function. PLoS Pathog 10:e1004049. doi: 10.1371/journal.ppat.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux CM, Butler BP, Chau JY, Paixao TA, Cheung KW, Santos RL, Luckhart S, Tsolis RM. 2010. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to nontyphoidal Salmonella enterica serovar Typhimurium infection in mice. Infect Immun 78:1520–1527. doi: 10.1128/IAI.00887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feasey NA, Everett D, Faragher EB, Roca-Feltrer A, Kang’ombe A, Denis B, Kerac M, Molyneux E, Molyneux M, Jahn A, Gordon MA, Heyderman RS. 2015. Modelling the contributions of malaria, HIV, malnutrition and rainfall to the decline in paediatric invasive non-typhoidal Salmonella disease in Malawi. PLoS Negl Trop Dis 9:e0003979. doi: 10.1371/journal.pntd.0003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon MA. 2011. Invasive nontyphoidal Salmonella disease: epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis 24:484–489. doi: 10.1097/QCO.0b013e32834a9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JA, Berkley JA, Mwangi I, Ochola L, Uyoga S, Macharia A, Ndila C, Lowe BS, Mwarumba S, Bauni E, Marsh K, Williams TN. 2011. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet 378:1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garvis SG, Beuzon CR, Holden DW. 2001. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella -containing vacuoles in macrophages. Cell Microbiol 3:731–744. doi: 10.1046/j.1462-5822.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- 34.Helaine S, Thompson JA, Watson KG, Liu M, Boyle C, Holden DW. 2010. Dynamics of intracellular bacterial replication at the single cell level. Proc Natl Acad Sci U S A 107:3746–3751. doi: 10.1073/pnas.1000041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shea JE, Beuzon CR, Gleeson C, Mundy R, Holden DW. 1999. Influence of the Salmonella Typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun 67:213–219. doi: 10.1128/IAI.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter-Dahlfors A, Buchan AMJ, Finlay BB. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella Typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med 186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 38.Fields PI, Swanson RV, Haidaris CG, Heffron F. 1986. Mutants of Salmonella Typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A 83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon MA, Gordon SB, Musaya L, Zijlstra EE, Molyneux ME, Read RC. 2007. Primary macrophages from HIV-infected adults show dysregulated cytokine responses to Salmonella, but normal internalization and killing. AIDS 21:2399–2408. doi: 10.1097/QAD.0b013e3282f25107. [DOI] [PubMed] [Google Scholar]
- 40.Gordon MA, Kankwatira AM, Mwafulirwa G, Walsh AL, Hopkins MJ, Parry CM, Faragher EB, Zijlstra EE, Heyderman RS, Molyneux ME. 2010. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis 50:953–962. doi: 10.1086/651080. [DOI] [PubMed] [Google Scholar]
- 41.Harrington KA, Hormaeche CE. 1986. Expression of the innate resistance gene Ity in mouse Kupffer cells infected with Salmonella Typhimurium in vitro. Microb Pathog 1:269–274. doi: 10.1016/0882-4010(86)90051-3. [DOI] [PubMed] [Google Scholar]
- 42.Hormaeche CE. 1979. The natural resistance of radiation chimeras to S. Typhimurium C5. Immunology 37:329–332. [PMC free article] [PubMed] [Google Scholar]
- 43.Hormaeche CE. 1979. Genetics of natural resistance to salmonellae in mice. Immunology 37:319–327. [PMC free article] [PubMed] [Google Scholar]
- 44.Hormaeche CE. 1979. Natural resistance to Salmonella Typhimurium in different inbred mouse strains. Immunology 37:311–318. [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu HS. 1989. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev 53:390–409. doi: 10.1128/MMBR.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins FM. 1974. Vaccines and cell-mediated immunity. Bacteriol Rev 38:371–402. doi: 10.1128/MMBR.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackaness GB, Blanden RV, Collins FM. 1966. Host-parasite relations in mouse typhoid. J Exp Med 124:573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M, Schulz D, Armand J, Bryla DA, Trollfors B. 1987. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella Typhi: a preliminary report. N Engl J Med 317:1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 49.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. 1989. Progress in vaccines against typhoid fever. Rev Infect Dis 11(Suppl 3):S552–S567. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- 50.Eisenstein TK, Killar LM, Sultzer BM. 1984. Immunity to infection with Salmonella Typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis 150:425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 51.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. 1987. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever: preparation, characterization, and immunogenicity in laboratory animals. J Exp Med 166:1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thiem VD, Lin FY, Canh DG, Son NH, Anh DD, Mao ND, Chu C, Hunt SW, Robbins JB, Schneerson R, Szu SC. 2011. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol 18:730–735. doi: 10.1128/CVI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szu SC. 2013. Development of Vi conjugate—a new generation of typhoid vaccine. Expert Rev Vaccines 12:1273–1286. doi: 10.1586/14760584.2013.845529. [DOI] [PubMed] [Google Scholar]
- 54.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. 1993. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun 61:3981–3984. doi: 10.1128/IAI.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. 2010. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A 107:3070–3075. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, Hart CA, Molyneux ME, Drayson MT. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest 118:1553–1562. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nyirenda TS, Gilchrist JJ, Feasey NA, Glennie SJ, Bar-Zeev N, Gordon MA, MacLennan CA, Mandala WL, Heyderman RS. 2014. Sequential acquisition of T cells and antibodies to nontyphoidal Salmonella in Malawian children. J Infect Dis 210:56–64. doi: 10.1093/infdis/jiu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunnington AJ, de Souza JB, Walther M, Riley EM. 2011. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med 18:120–127. doi: 10.1038/nm.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunnington AJ, Riley EM. 2010. Suppression of vaccine responses by malaria: insignificant or overlooked? Expert Rev Vaccines 9:409–429. doi: 10.1586/erv.10.16. [DOI] [PubMed] [Google Scholar]
- 60.Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. 2002. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 61.Greenwood BM, Brueton MJ. 1974. Complement activation in children with acute malaria. Clin Exp Immunol 18:267–272. [PMC free article] [PubMed] [Google Scholar]
- 62.Mooney JP, Lee SJ, Lokken KL, Nanton MR, Nuccio SP, McSorley SJ, Tsolis RM. 2015. Transient loss of protection afforded by a live attenuated non-typhoidal Salmonella vaccine in mice co-infected with malaria. PLoS Negl Trop Dis 9:e0004027. doi: 10.1371/journal.pntd.0004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klemm EJ, Gkrania-Klotsas E, Hadfield J, Forbester JL, Harris SR, Hale C, Heath JN, Wileman T, Clare S, Kane L, Goulding D, Otto TD, Kay S, Doffinger R, Cooke FJ, Carmichael A, Lever AM, Parkhill J, MacLennan CA, Kumararatne D, Dougan G, Kingsley RA. 2016. Emergence of host-adapted Salmonella Enteritidis through rapid evolution in an immunocompromised host. Nat Microbiol 1:15023. doi: 10.1038/nmicrobiol.2015.23. [DOI] [PubMed] [Google Scholar]
- 64.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 65.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol 11:346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 66.MacLennan C, Fieschi C, Lammas DA, Picard C, Dorman SE, Sanal O, MacLennan JM, Holland SM, Ottenhoff TH, Casanova JL, Kumararatne DS. 2004. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis 190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 67.Picard C, Fieschi C, Altare F, Al-Jumaah S, Al-Hajjar S, Feinberg J, Dupuis S, Soudais C, Al-Mohsen IZ, Genin E, Lammas D, Kumararatne DS, Leclerc T, Rafii A, Frayha H, Murugasu B, Wah LB, Sinniah R, Loubser M, Okamoto E, Al-Ghonaium A, Tufenkeji H, Abel L, Casanova JL. 2002. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet 70:336–348. doi: 10.1086/338625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carter PB, Collins FM. 1974. The route of enteric infection in normal mice. J Exp Med 139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 70.Biozzi G, Howard JG, Halpern BN, Stiffel C, Mouton D. 1960. The kinetics of blood clearance of isotopically labelled Salmonella Enteritidis by the reticuloendothelial system in mice. Immunology 3:74–89. [PMC free article] [PubMed] [Google Scholar]
- 71.Coward C, Restif O, Dybowski R, Grant AJ, Maskell DJ, Mastroeni P. 2014. The effects of vaccination and immunity on bacterial infection dynamics in vivo. PLoS Pathog 10:e1004359. doi: 10.1371/journal.ppat.1004359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warren J, Mastroeni P, Dougan G, Noursadeghi M, Cohen J, Walport MJ, Botto M. 2002. Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar Typhimurium infection. Infect Immun 70:551–557. doi: 10.1128/iai.70.2.551-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunlap NE, Benjamin WH Jr, McCall RD Jr, Tilden AB, Briles DE. 1991. A ‘safe-site’ for Salmonella Typhimurium is within splenic cells during the early phase of infection in mice. Microb Pathog 10:297–310. doi: 10.1016/0882-4010(91)90013-Z. [DOI] [PubMed] [Google Scholar]
- 74.Salcedo SP, Noursadeghi M, Cohen J, Holden DW. 2001. Intracellular replication of Salmonella Typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol 3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 75.Dunlap NE, Benjamin WH Jr, Berry AK, Eldridge JH, Briles DE. 1992. A ‘safe-site’ for Salmonella Typhimurium is within splenic polymorphonuclear cells. Microb Pathog 13:181–190. doi: 10.1016/0882-4010(92)90019-K. [DOI] [PubMed] [Google Scholar]
- 76.Yrlid U, Svensson M, Hakansson A, Chambers BJ, Ljunggren HG, Wick MJ. 2001. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect Immun 69:5726–5735. doi: 10.1128/iai.69.9.5726-5735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheppard M, Webb C, Heath F, Mallows V, Emilianus R, Maskell D, Mastroeni P. 2003. Dynamics of bacterial growth and distribution within the liver during Salmonella infection. Cell Microbiol 5:593–600. doi: 10.1046/j.1462-5822.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 78.Mastroeni P, Skepper JN, Hormaeche CE. 1995. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect Immun 63:3674–3682. doi: 10.1128/IAI.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shea JE, Hensel M, Gleeson C, Holden DW. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella Typhimurium. Proc Natl Acad Sci U S A 93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown SP, Cornell SJ, Sheppard M, Grant AJ, Maskell DJ, Grenfell BT, Mastroeni P. 2006. Intracellular demography and the dynamics of Salmonella enterica infections. PLoS Biol 4:e349. doi: 10.1371/journal.pbio.0040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant AJ, Morgan FJ, McKinley TJ, Foster GL, Maskell DJ, Mastroeni P. 2012. Attenuated Salmonella Typhimurium lacking the pathogenicity island-2 type 3 secretion system grow to high bacterial numbers inside phagocytes in mice. PLoS Pathog 8:e1003070. doi: 10.1371/journal.ppat.1003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mastroeni P, Arena A, Costa GB, Liberto MC, Bonina L, Hormaeche CE. 1991. Serum TNF alpha in mouse typhoid and enhancement of a Salmonella infection by anti-TNF alpha antibodies. Microb Pathog 11:33–38. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- 83.Mastroeni P, Clare S, Khan S, Harrison JA, Hormaeche CE, Okamura H, Kurimoto M, Dougan G. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella Typhimurium. Infect Immun 67:478–483. doi: 10.1128/IAI.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mastroeni P, Harrison JA, Chabalgoity JA, Hormaeche CE. 1996. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun 64:189–196. doi: 10.1128/IAI.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. 1992. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro− Salmonella vaccines. Microb Pathog 13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 86.Muotiala A, Makela PH. 1990. The role of IFN-gamma in murine Salmonella Typhimurium infection. Microb Pathog 8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 87.Goh YS, Armour KL, Clark MR, Grant AJ, Mastroeni P. 2016. IgG subclasses targeting the flagella of Salmonella enterica serovar Typhimurium can mediate phagocytosis and bacterial killing. J Vaccines Vaccin 7:322. doi: 10.4172/2157-7560.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uppington H, Menager N, Boross P, Wood J, Sheppard M, Verbeek S, Mastroeni P. 2006. Effect of immune serum and role of individual Fcγ receptors on the intracellular distribution and survival of Salmonella enterica serovar Typhimurium in murine macrophages. Immunology 119:147–158. doi: 10.1111/j.1365-2567.2006.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grant AJ, Restif O, McKinley TJ, Sheppard M, Maskell DJ, Mastroeni P. 2008. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol 6:e74. doi: 10.1371/journal.pbio.0060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown A, Hormaeche CE, Demarco-de-Hormaeche R, Winther M, Dougan G, Maskell DJ, Stocker BA. 1987. An attenuated aroA Salmonella Typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis 155:86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- 91.Liang L, Juarez S, Nga TV, Dunstan S, Nakajima-Sasaki R, Davies DH, McSorley S, Baker S, Felgner PL. 2013. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep 3:1043. doi: 10.1038/srep01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villarreal-Ramos B, Manser J, Collins RA, Dougan G, Chatfield SN, Howard CJ. 1998. Immune responses in calves immunised orally or subcutaneously with a live Salmonella Typhimurium aro vaccine. Vaccine 16:45–54. doi: 10.1016/s0264-410x(97)00156-4. [DOI] [PubMed] [Google Scholar]
- 93.Brown A, Hormaeche CE. 1989. The antibody response to salmonellae in mice and humans studied by immunoblots and ELISA. Microb Pathog 6:445–454. doi: 10.1016/0882-4010(89)90086-7. [DOI] [PubMed] [Google Scholar]
- 94.Tran TH, Nguyen TD, Nguyen TT, Ninh TT, Tran NB, Nguyen VM, Tran TT, Cao TT, Pham VM, Nguyen TC, Tran TD, Pham VT, To SD, Campbell JI, Stockwell E, Schultsz C, Simmons CP, Glover C, Lam W, Marques F, May JP, Upton A, Budhram R, Dougan G, Farrar J, Nguyen VV, Dolecek C. 2010. A randomised trial evaluating the safety and immunogenicity of the novel single oral dose typhoid vaccine M01ZH09 in healthy Vietnamese children. PLoS One 5:e11778. doi: 10.1371/journal.pone.0011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy JR, Baqar S, Munoz C, Schlesinger L, Ferreccio C, Lindberg AA, Svenson S, Losonsky G, Koster F, Levine MM. 1987. Characteristics of humoral and cellular immunity to Salmonella Typhi in residents of typhoid-endemic and typhoid-free regions. J Infect Dis 156:1005–1009. doi: 10.1093/infdis/156.6.1005. [DOI] [PubMed] [Google Scholar]
- 96.Sztein MB. 2007. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis 45(Suppl 1):S15–S19. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 97.Hormaeche CE, Mastroeni P, Harrison JA, Demarco de Hormaeche R, Svenson S, Stocker BA. 1996. Protection against oral challenge three months after i.v. immunization of BALB/c mice with live Aro Salmonella Typhimurium and Salmonella Enteritidis vaccines is serotype (species)-dependent and only partially determined by the main LPS O antigen. Vaccine 14:251–259. doi: 10.1016/0264-410x(95)00249-z. [DOI] [PubMed] [Google Scholar]
- 98.Segall T, Lindberg AA. 1993. Oral vaccination of calves with an aromatic-dependent Salmonella Dublin (O9,12) hybrid expressing O4,12 protects against S. Dublin (O9,12) but not against Salmonella Typhimurium (O4,5,12). Infect Immun 61:1222–1231. doi: 10.1128/IAI.61.4.1222-1231.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Svenson SB, Lindberg AA. 1981. Artificial Salmonella vaccines: Salmonella Typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun 32:490–496. doi: 10.1128/IAI.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carlin NI, Svenson SB, Lindberg AA. 1987. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog 2:171–183. doi: 10.1016/0882-4010(87)90019-2. [DOI] [PubMed] [Google Scholar]
- 101.Hale C, Bowe F, Pickard D, Clare S, Haeuw JF, Powers U, Menager N, Mastroeni P, Dougan G. 2006. Evaluation of a novel Vi conjugate vaccine in a murine model of salmonellosis. Vaccine 24:4312–4320. doi: 10.1016/j.vaccine.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, Harris V, Gardner J, Nebykova A, Kerridge SA, Hill J, Thomaides-Brears H, Blohmke CJ, Yu LM, Angus B, Pollard AJ. 2017. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet 390:2472–2480. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, Liu X, Tonks S, Mazur O, Farooq YG, Clarke J, Hill J, Adhikari A, Dongol S, Karkey A, Bajracharya B, Kelly S, Gurung M, Baker S, Neuzil KM, Shrestha S, Basnyat B, Pollard AJ, TyVAC Nepal Study Team. 2019. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med 381:2209–2218. doi: 10.1056/NEJMoa1905047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuusi N, Nurminen M, Saxen H, Valtonen M, Makela PH. 1979. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun 25:857–862. doi: 10.1128/IAI.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Domínguez-Medina CC, Pérez-Toledo M, Schager AE, Marshall JL, Cook CN, Bobat S, Hwang H, Chun BJ, Logan E, Bryant JA, Channell WM, Morris FC, Jossi SE, Alshayea A, Rossiter AE, Barrow PA, Horsnell WG, MacLennan CA, Henderson IR, Lakey JH, Gumbart JC, López-Macías C, Bavro VN, Cunningham AF. 2020. Outer membrane protein size and LPS O-antigen define protective antibody targeting to the Salmonella surface. Nat Commun 11:851. doi: 10.1038/s41467-020-14655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, Buckley CD, Dougan G, MacLennan ICM, López-Macías C, Cunningham AF. 2009. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A 106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goh YS, Grant AJ, Restif O, McKinley TJ, Armour KL, Clark MR, Mastroeni P. 2011. Human IgG isotypes and activating Fcγ receptors in the interaction of Salmonella enterica serovar Typhimurium with phagocytic cells. Immunology 133:74–83. doi: 10.1111/j.1365-2567.2011.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. 2014. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol 21:427–434. doi: 10.1128/CVI.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Micoli F, Rondini S, Alfini R, Lanzilao L, Necchi F, Negrea A, Rossi O, Brandt C, Clare S, Mastroeni P, Rappuoli R, Saul A, MacLennan CA. 2018. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc Natl Acad Sci U S A 115:10428–10433. doi: 10.1073/pnas.1807655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siggins MK, O'Shaughnessy CM, Pravin J, Cunningham AF, Henderson IR, Drayson MT, MacLennan CA. 2014. Differential timing of antibody-mediated phagocytosis and cell-free killing of invasive African Salmonella allows immune evasion. Eur J Immunol 44:1093–1098. doi: 10.1002/eji.201343529. [DOI] [PubMed] [Google Scholar]
- 111.Juel HB, Thomaides-Brears HB, Darton TC, Jones C, Jones E, Shrestha S, Sie R, Eustace A, Galal U, Kurupati P, Van TT, Thieu NTV, Baker S, Blohmke CJ, Pollard AJ. 2017. Salmonella Typhi bactericidal antibodies reduce disease severity but do not protect against typhoid fever in a controlled human infection model. Front Immunol 8:1916. doi: 10.3389/fimmu.2017.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rossi O, Coward C, Goh YS, Claassens JWC, MacLennan CA, Verbeek SJ, Mastroeni P. 2019. The essential role of complement in antibody-mediated resistance to Salmonella. Immunology 156:69–73. doi: 10.1111/imm.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Menager N, Foster G, Ugrinovic S, Uppington H, Verbeek S, Mastroeni P. 2007. Fcγ receptors are crucial for the expression of acquired resistance to virulent Salmonella enterica serovar Typhimurium in vivo but are not required for the induction of humoral or T-cell-mediated immunity. Immunology 120:424–432. doi: 10.1111/j.1365-2567.2006.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindberg AA, Segall T, Weintraub A, Stocker BA. 1993. Antibody response and protection against challenge in mice vaccinated intraperitoneally with a live aroA O4-O9 hybrid Salmonella Dublin strain. Infect Immun 61:1211–1221. doi: 10.1128/IAI.61.4.1211-1221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Metcalf ES, O'Brien AD. 1981. Characterization of murine antibody response to Salmonella Typhimurium by a class-specific solid-phase radioimmunoassay. Infect Immun 31:33–41. doi: 10.1128/IAI.31.1.33-41.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Forrest BD, LaBrooy JT, Beyer L, Dearlove CE, Shearman DJ. 1991. The human humoral immune response to Salmonella Typhi Ty21a. J Infect Dis 163:336–345. doi: 10.1093/infdis/163.2.336. [DOI] [PubMed] [Google Scholar]
- 117.Tagliabue A, Villa L, De Magistris MT, Romano M, Silvestri S, Boraschi D, Nencioni L. 1986. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J Immunol 137:1504–1510. [PubMed] [Google Scholar]
- 118.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. 2007. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol 178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 119.Szu SC, Klugman KP, Hunt S. 2014. Re-examination of immune response and estimation of anti-Vi IgG protective threshold against typhoid fever-based on the efficacy trial of Vi conjugate in young children. Vaccine 32:2359–2363. doi: 10.1016/j.vaccine.2014.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dahora LC, Jin C, Spreng RL, Feely F, Mathura R, Seaton KE, Zhang L, Hill J, Jones E, Alam SM, Dennison SM, Pollard AJ, Tomaras GD. 2019. IgA and IgG1 specific to Vi polysaccharide of Salmonella Typhi Correlate with protection status in a typhoid fever controlled human infection model. Front Immunol 10:2582. doi: 10.3389/fimmu.2019.02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saxen H, Makela O. 1982. The protective capacity of immune sera in experimental mouse salmonellosis is mainly due to IgM antibodies. Immunol Lett 5:267–272. doi: 10.1016/0165-2478(82)90110-9. [DOI] [PubMed] [Google Scholar]
- 122.Saxen H, Makela O, Svenson SB. 1984. Isotype of protective anti-Salmonella antibodies in experimental mouse salmonellosis. Infect Immun 44:633–636. doi: 10.1128/IAI.44.3.633-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goh YS, Clare S, Micoli F, Saul A, Mastroeni P, MacLennan CA. 2015. Monoclonal antibodies of a diverse isotype induced by an O-antigen glycoconjugate vaccine mediate in vitro and in vivo killing of African invasive nontyphoidal Salmonella. Infect Immun 83:3722–3731. doi: 10.1128/IAI.00547-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. 2006. Innate secretory antibodies protect against natural Salmonella Typhimurium infection. J Exp Med 203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella Typhimurium. Infect Immun 60:1786–1792. doi: 10.1128/IAI.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Dominguez-Medina C, Cumley NJ, Heath JN, Essex SJ, Bobat S, Schager A, Goodall M, Kracker S, Buckley CD, May RC, Kingsley RA, MacLennan CA, López-Macías C, Cunningham AF, Toellner K-M. 2017. IgG1 is required for optimal protection after immunization with the purified porin OmpD from Salmonella Typhimurium. J Immunol 199:4103–4109. doi: 10.4049/jimmunol.1700952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goh YS, Necchi F, O’Shaughnessy CM, Micoli F, Gavini M, Young SP, Msefula CL, Gondwe EN, Mandala WL, Gordon MA, Saul AJ, MacLennan CA. 2016. Bactericidal immunity to Salmonella in Africans and mechanisms causing its failure in HIV infection. PLoS Negl Trop Dis 10:e0004604. doi: 10.1371/journal.pntd.0004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salerno-Goncalves R, Pasetti MF, Sztein MB. 2002. Characterization of CD8+ effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 169:2196–2203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 129.Salerno-Gonçalves R, Wyant TL, Pasetti MF, Fernandez-Viña M, Tacket CO, Levine MM, Sztein MB. 2003. Concomitant induction of CD4+and CD8+ T Cell Responses in Volunteers Immunized with Salmonella enterica Serovar Typhi strain CVD 908-htrA. J Immunol 170:2734–2741. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 130.Villarreal B, Mastroeni P, de Hormaeche RD, Hormaeche CE. 1992. Proliferative and T-cell specific interleukin (IL-2/IL-4) production responses in spleen cells from mice vaccinated with aroA live attenuated Salmonella vaccines. Microb Pathog 13:305–315. doi: 10.1016/0882-4010(92)90040-u. [DOI] [PubMed] [Google Scholar]
- 131.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. 2000. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun 68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ugrinovic S, Menager N, Goh N, Mastroeni P. 2003. Characterization and development of T-cell immune responses in B-cell-deficient (Igh-6−/−) mice with Salmonella enterica serovar Typhimurium infection. Infect Immun 71:6808–6819. doi: 10.1128/iai.71.12.6808-6819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. 2000. Cutting Edge: Role of B lymphocytes in protective immunity against Salmonella Typhimurium infection. J Immunol 164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 134.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. 2012. Cutting Edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol 189:5503–5507. doi: 10.4049/jimmunol.1201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barr TA, Brown S, Mastroeni P, Gray D. 2010. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol 185:2783–2789. doi: 10.4049/jimmunol.1001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barr TA, Brown S, Mastroeni P, Gray D. 2009. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol 183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, Leyton DL, Marshall JL, Gondwe EN, Bobat S, Lopez-Macias C, Doffinger R, Henderson IR, Zijlstra EE, Dougan G, Drayson MT, MacLennan IC, Molyneux ME. 2010. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328:508–512. doi: 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin FYC, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, Schneerson R, Szu SC, Lanh MN, Hunt S, Trinh L, Kaufman JB. 2001. The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 344:1263–1269. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 139.Greenwood BM, Bradley-Moore AM, Bryceson AD, Palit A. 1972. Immunosuppression in children with malaria. Lancet 1:169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- 140.Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M. 2012. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol 189:5336–5346. doi: 10.4049/jimmunol.1201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. 1970. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 142.Janis C, Grant AJ, McKinley TJ, Morgan FJ, John VF, Houghton J, Kingsley RA, Dougan G, Mastroeni P. 2011. In vivo regulation of the Vi antigen in Salmonella and induction of immune responses with an in vivo-inducible promoter. Infect Immun 79:2481–2488. doi: 10.1128/IAI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rossi O, Caboni M, Negrea A, Necchi F, Alfini R, Micoli F, Saul A, MacLennan CA, Rondini S, Gerke C. 2016. Toll-like receptor activation by generalized modules for membrane antigens from lipid A mutants of Salmonella enterica serovars Typhimurium and Enteritidis. Clin Vaccine Immunol 23:304–314. doi: 10.1128/CVI.00023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]