Abstract
Background:
SSRIs are commonly used to treat pediatric anxiety disorders, including generalized anxiety disorder (GAD); however, their efficacy and tolerability are difficult to predict. We evaluated the efficacy and tolerability of escitalopram in adolescents with GAD and the impact of CYP2C19 phenotype on escitalopram pharmacokinetics from February 2015 through November 2018.
Methods:
Patients were treated with escitalopram (forced titration to 15 mg/day, then flexible titration to 20 mg/day) (n=26, mean age: 14.8±1.7 years) or placebo (n=25 mean age: 14.9±1.6 years) for 8 weeks. Outcomes were the change in the Pediatric Anxiety Rating Scale (PARS) score and Clinical Global Impressions (CGI) scales as well as vital signs and adverse events. Plasma escitalopram and desmethylescitalopram AUC0-24 and CMAX were determined and compared across CYP2C19 phenotypes.
Results:
Escitalopram was superior to placebo for baseline-to-endpoint change in PARS (−8.65±1.2 vs. −3.52±1.1, p<0.001) and CGI and increasing CYP2C19 metabolism was associated with decreases in escitalopram CMAX and AUC0-24 (p<0.05). Vital signs, QTc and adverse events were similar in patients who received escitalopram and placebo.
Conclusions:
Escitalopram reduces anxiety symptoms and CYP2C19 phenotype influences the trajectory and magnitude of improvement. Variation in CYP2C19 metabolism accounts for significant differences in escitalopram pharmacokinetics, raising the possibility that CYP2C19 phenotype should be considered when dosing escitalopram.
ClinicalTrials.gov Identifier:
Keywords: adolescent, pharmacogenomics, pharmacokinetics, psychiatric, clinical trials
INTRODUCTION
Characterized by uncontrollable, diffuse anxiety and accompanied by functionally impairing somatic and cognitive symptoms, generalized anxiety disorder (GAD), is among the most common anxiety disorders in adolescents.1 Selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs) and cognitive behavioral therapy (CBT) reduce anxiety and improve functioning in many pediatric patients with GAD and related anxiety disorders.2-5 However, 40% of youth do not respond to these treatments2 or experience treatment-limiting side effects.6,7 Thus, there is an urgent need to develop and test additional interventions, to expand the treatment armamentarium and to identify which patients will respond to these treatments.
While SSRIs represent the first-line pharmacotherapy for anxious youth, including those with GAD,2,8 predicting treatment response is difficult. SSRI-related improvement varies considerably from patient to patient, often resulting in a trial-and-error process of medication selection and dosing. An additional shortcoming of SSRI treatment in pediatric anxiety disorders is the limited availability of data to aid clinicians in determining which patients will respond. In the Child/Adolescent Anxiety Multimodal Study (CAMS),2 which compared sertraline, sertraline + CBT, CBT and placebo, youth with severe anxiety required both an SSRI and CBT9 while youth with less anxiety at baseline and those who did not have social anxiety disorder were more likely to remit with all treatments.10 Despite studies of clinical and demographic factors that predict SSRI or (SSRI + CBT) response in anxious youth, examinations of biological (including pharmacogenetic) factors and SSRI response are rare in youth and virtually non-existent in pediatric anxiety disorders.11
Cytochrome P450 2C19 (CYP2C19) metabolizes multiple SSRIs,12,13 including citalopram, and its s-enantiomer, escitalopram.14 More than two dozen variants in the CYP2C19 gene produce substantial variation in metabolic activity and include loss-of-function alleles (e.g., no function alleles *2-*9) as well as alleles with increased activity (e.g., *17). In adults with reduced CYP2C19 metabolism, plasma escitalopram concentrations are higher compared to patients with normal CYP2C19 metabolism,15 while patients with faster CYP2C19 metabolism have lower plasma escitalopram concentrations. The influence of CYP2C19 metabolizer status on escitalopram pharmacokinetics is well described in adults.15 By contrast, only one small study (N=9) has examined this relationship in citalopram-treated youth and found “substantial correlation between CYP2C19 activity and s-citalopram concentration).”16
With these considerations in mind, we examined the efficacy and tolerability of escitalopram in adolescents with GAD as well as predictors of escitalopram-related improvement. Based on prior SSRI trials in youth and meta-analyses,17 we hypothesized that escitalopram would be superior to placebo in decreasing anxiety. Finally, based on studies in adults,14 we hypothesized that faster CYP2C19 metabolism would be associated with decreased maximum concentrations (CMAX), exposure (area under the curve [AUC0-24]) and ratios of escitalopram to its major metabolite, desmethylescitalopram.
METHODS
This Institutional Review Board-approved study was conducted in accordance with Good Clinical Practice guidelines at a single academic site in the United States from February 2015 (first patient visit) to November 2018 (last patient visit). Written, informed consent and assent were obtained from all legal guardians and patients, respectively prior to any research procedures being completed.
Participants
Outpatients aged 12–17 years who met DSM-IV-TR criteria for GAD were assessed using the Anxiety Disorders Interview Schedule (ADIS);18 had a Pediatric Anxiety Rating Scale (PARS) score ≥15 at screening and baseline; and had a Clinical Global Impression of Severity (CGI-Severity) (25) score ≥4 were eligible. Patients were required to be medically stable and provide a negative urine drug screen and a negative urine pregnancy test (for girls) at screening. Patients with secondary diagnoses of separation or social anxiety disorder or panic disorder and/or agoraphobia were enrolled, provided that GAD was the primary diagnosis; however, patients with current MDD or any history of bipolar disorder, psychotic disorder, obsessive compulsive disorder or post-traumatic stress disorder were excluded. Stable concomitant psychotherapy was allowed during the study. Antidepressant, antipsychotic, anticonvulsant, stimulant and benzodiazepine use was prohibited; although caffeine, non-opiate analgesics, non-sedating H1 and H2 antagonists and oral contraceptives were allowed.
Study Treatment
Randomization to escitalopram or placebo (1:1) was assigned, in blocks of 4, by investigational pharmacists and was stratified by sex, using a random number generator. Patients, caregivers, and investigational staff were blind to treatment assignment. Escitalopram was initiated at 5 mg daily for 2 days and titrated to 10 mg daily for 7 days and then 15 mg daily. At the week 4 and 6 visits, escitalopram could be titrated to 20 mg daily. The study incorporated a 1-week screening period and an 8-week double-blind treatment period.
Assessments
The primary efficacy outcome was change in PARS score from baseline to week 8 and change from baseline in CGI-S and CGI-I response (defined as a CGI-I score of 1 or 2). All efficacy assessments were administered at weeks 1, 2, 4, 6, and 8, and/or at early termination and were completed in an outpatient setting. Efficacy measures were administered by a blinded study physician who underwent training on the use of the instrument and met pre-determined inter-rater reliability criteria as previously described. Because of the relatively small sample and the single-site setting, the same rater evaluated each patient during the course of the trial.
Spontaneously reported adverse events (AEs), pulse, blood pressure, and weight were recorded at every visit. EKGs were collected at baseline and weeks 2 and 8 or early termination. The Columbia-Suicide Severity Rating Scale (C-SSRS) was completed at all visits and the Children’s Depression Rating Scale – Revised (CDRS-R)19 was used to evaluate concurrent depressive symptoms at each visit. AEs were classified using the Medical Dictionary for Regulatory Activities (MedDRA, v. 22.0, www.medra.org) system organ class and high level group terms and activation-cluster symptoms were categorized as previously described20 and included: activation; worsening insomnia; irritability; impulsivity; worsening anxiety. A physical examination was performed at screening and week 8. The Wechsler Abbreviated Scale Intelligence (WASI)21 was used to estimate IQ at screening.
Pharmacogenetic Testing
Genomic DNA was collected with a buccal swab using a commercially available pharmacogenomic test (Myriad Genetics, Mason, OH). Genotyping assessed nine allelic variations in CYP2C19. Clinical Pharmacogenetics Implementation Consortium guidelines were used to assign a CYP2C19 metabolizer phenotype from the alleles present for each patient.22 Poor metabolizers have two no function alleles; intermediate metabolizers have one no function and one normal or increased function allele (e.g., *1/*2, *2/*17); normal metabolizers have two normal function alleles (*1/*1), rapid metabolizers have one increased function allele and one normal function allele (*1/*17); and ultrarapid metabolizers have two increased function alleles (*17/*17).22 HTR2A and SLC6A4 were genotyped as previously described.23
Escitalopram and Desmethylescitalopram Plasma Measures
Escitalopram and desmethylescitalopram concentrations were determined using high performance liquid chromatography at endpoint (or early termination), as previously described.24 Then, based on the escitalopram dose, sampling time and compliance for each patient, plasma escitalopram concentrations were modeled using MwPharm (MediWare BV, version 3.82), which permitted allometric scaling and incorporated each patient’s weight, height and age at the time of PK sampling, as previously described.25 From these models, AUC0-24 and Cmax for each adolescent for which PK samples were available, were determined and compared across CYP2C19 phenotypes at a modeled oral escitalopram dose of 15 mg/day (the forced-titration dose in this study). Adherence was assessed by pill count at each visit.
Statistical Methods
Sample size consisted of 32 patients in the escitalopram group and 32 patients in the placebo group and 80% power was used to detect group differences of ≥0.7 (Cohen’s d). All analyses of continuous measures included randomized patients with both a baseline and at least one post-baseline value for the variable being analyzed. Imputation occurred via last observation carried forward (LOCF). For categorical outcomes (e.g., adverse events), treatments were compared with the use of Pearson’s X2 or beta posterior probabilities and logistic regression, as appropriate. For continuous outcomes, logarithmic mixed effect models were employed to determine predicted mean outcome values at weeks 0, 1, 2, 4, 6 and 8 (or early termination) to examine group differences. Mixed-effects models included indicator variables for week and treatment as fixed effects. Each model was created with a limited number of covariates (e.g., age, sex) and refined as previously described to obtain the most parsimonious response model.26,27 Change from baseline CGI-S and CGI-I were examined using the same approach.
For pharmacogenomic predictors of treatment response, Bayesian logistic regression models of categorical response (CGI-I ≤2) were utilized to evaluate the impact of the predictors. Average treatment response rate was assumed to be uniformly distributed between 0 and 1 (i.e., Beta(1,1)) for all analyses. For change in symptom severity, a logarithmic trajectory model was utilized. All model parameters for average change in symptoms were modeled with an uninformative Normal prior, i.e. normally distributed with mean zero and large variance, N(μ = 0, σ2 = 1000). Predictors included the CYP2C19 phenotype (intermediate vs. normal metabolizer), a logarithmic trend and age and sex. Model sets were refined, as previously described,27 based on the parametric fit in addition to p-values and the Bayesian and Akaike Information Criteria (BIC and AIC, respectively). Models were evaluated for omitted variables bias and for the inclusion of irrelevant variables. Then, for any statistically significant predictors of treatment response, models of the log trajectory response probability were developed.
The relationship between CYP2C19 phenotype and escitalopram AUC0-24 and CMAX at 15 mg/day as well as the relationship between CYP2C19 phenotype and escitalopram: desmethylescitalopram ratios were examined using ANOVA tests. Post-hoc explorations of medication exposure and clinical outcomes were limited to response and the presence of activation-cluster symptoms given that fluoxetine28 and fluvoxamine20 concentrations/exposure have been associated with activation in pediatric anxiety disorders. CMAX and AUC0-24 were compared between patients with and without activation and between responders and non-responders (at week 8/ET) with Student’s t-tests and examined in a multivariate logistic regression described above. All analyses were performed with R (version 3.3.1) and Julia (version 1.2.0)29 and findings were considered statistically significant at the 5% threshold.
RESULTS
Patients
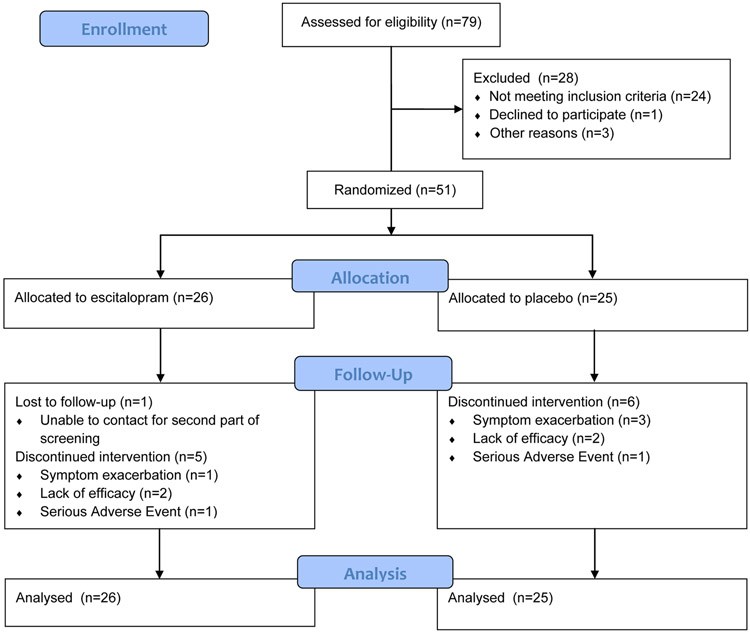
Patients were recruited between 2015 and 2018 and the last follow-up visit was completed in 2019. Figure 1 illustrates patient flow during the study. Baseline characteristics did not significantly differ between treatment groups (Table 1). Of 79 individuals screened, 51 were randomly assigned to treatment, took at least one dose of escitalopram (or placebo) and were included in the analyses (escitalopram n=26; placebo, n=25). The majority of patients, in both groups were girls (placebo: n=20, 77%; escitalopram n=19, 76%) and White (placebo: n=23, 88%; escitalopram n=20, 80%). Mean baseline PARS scores were 18±2 and 17±2 for patients randomized to escitalopram and placebo, respectively, and reflect moderate-to-severe anxiety. CDRS-R scores were similar between groups (escitalopram: 33±4; placebo: 32±6), although these scores largely reflect overlapping anxiety and depressive symptoms rather than symptoms of MDD, per se (Table 1). Finally, approximately three quarters of the sample was SSRI/SNRI-naïve (Table 1). The most common reasons for discontinuation were lack of efficacy or persistent/worsening anxiety and these rates did not differ between groups (escitalopram: n=3 [12%]; placebo: n=5 [20%], p=0.429). Nine percent of efficacy measures were imputed by LOCF and the frequency of missing data did not differ between patients receiving escitalopram and those who received placebo (p=0.619)
FIGURE 1. Study Flow.
During the study, all patients and study staff remained unaware of trial group assignments. Of 134 referrals, 79 were enrolled and 51 were randomized.
TABLE 1.
Demographic and Clinical Characteristics of Study Patients
| Baseline Characteristics | Escitalopram, n = 26 | Placebo, n = 25 |
|---|---|---|
| Age, years | 14.8±1.7 | 14.9±1.6 |
| Female, n (%) | 20 (77) | 19 (76) |
| Duke Tanner Stage, n (%) | ||
| Stage 3, female ∣ male | 6 (23) ∣ 3 (12) | 1 (4) ∣ 0 (0) |
| Stage 4, female ∣ male | 5 (19) ∣ 3 (12) | 10 (40) ∣ 5 (20) |
| Stage 5, female ∣ male | 9 (34) ∣ 0 (0) | 8 (32) ∣ 1 (4) |
| Race, n (%) | ||
| Asian | 0 (0) | 2 (8) |
| Black and African American | 1 (4) | 1 (4) |
| Caucasian | 23 (88) | 20 (80) |
| Other | 2 (8) | 2 (8) |
| Hispanic or Latino | 3 (12) | 0 (0) |
| Full scale IQ | 106±12 | 105±10 |
| PARS score | 18±2 | 17±2 |
| CGI-Severity score, median | 4 | 4 |
| CDRS-R score, mean | 33±4 | 32±6 |
| PSC score, mean | 23.12±3.15 | 30.76±4.99 |
| Co-occurring disorders, n (%) | ||
| Separation anxiety | 4 (15) | 5 (20) |
| Panic disorder | 13 (50) | 15 (60) |
| Agoraphobia | 7 (27) | 7 (28) |
| ADHD | 5 (19) | 4 (16) |
| Specific phobia | 9 (35) | 3 (12) |
| Prior SSRI/SNRI treatment, n (%) | 6 (23) | 7 (28) |
| Current psychotherapy treatment, n (%) | 3 (6) | 5 (10) |
| CYP2C19 genotype | ||
| *2/*2 | 1 (4) | |
| *1/*2 | 5 (19) | |
| *1/*1 | 10 (38) | |
| *1/*17 | 4 (15) | |
| *2/*17 | 3 (12) | |
| *4/*17 | 2 (8) 1 (4) |
|
| *17/*17 | 1 (4) | |
CGI-S, Clinical Global Impression Scale-Severity; CGI-I, Clinical Global Impression Scale-Improvement; CDRS-R, Children’s Depression Rating Scale-Revised; PARS, Pediatric Anxiety Rating Scale; ADHD, Attention/Deficit-Hyperactivity Disorder; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; PSC, Physical Symptom Checklist.
Efficacy
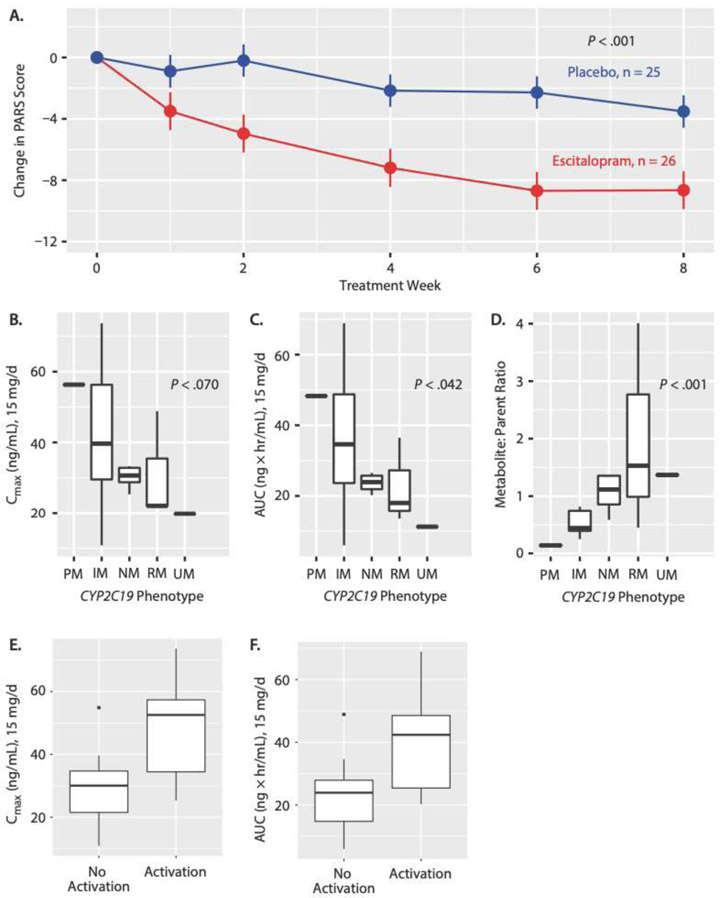
The trajectory of improvement over the 8-week trial and improvement at week 8 was significantly greater in escitalopram-treated patients compared to those receiving placebo (both p-values <0.001, Figure 2A). At week 8 (LOCF), the mean change in PARS score in escitalopram-treated patients was −8.65±1.31 compared to −3.52±1.06 in patients receiving placebo (95% CI: −8.57 to −1.70, p=0.005). The trajectory of improvement in anxiety (escitalopram vs. placebo) over time—as measured by the PARS score—was not associated with age (p=0.478), age-by-time (p=0.155), sex (p=0.870) or sex-by-time (p=0.708) but was associated with baseline PARS score (p<0.001). In the logistic response trajectory model, CGI-I response (i.e., CGI-I ≤2) was greater in escitalopram-treated patients compared to those receiving placebo (p<0.001) and was associated with age (p=0.041) with younger patients experiencing greater improvement. At week 8 (LOCF), 16/26 (62%) escitalopram-treated patients compared to 6/25 (24%) who received placebo had a CGI-I ≤2 (95% CI: 0.95 to 0.578, p=0.0039). Finally, the number needed to treat (NNT) for escitalopram was 3.
Figure 2:
(A) Change Over Time in PARS Score for Escitalopram Versus Placeboa; Change From Baseline to Endpoint by CYP2C19 Phenotype in (B) Maximum Concentration (Cmax), (C) Area Under the Curve (AUC), and (D) Metabolite:Parent Drug Ratiob; and Difference in (E) Cmax and (F) AUC Between Patients With and Without Activation Symptoms
aValues shown as mean ±SD.
bBoxplots for parts B, C, and D show median (IQR) and minimum and maximum values.
cBoxplots for parts E and F show median (IQR) and minimum and maximum values and outliers.
Abbreviations: CYP2C19=cytochrome P450 2C19, IM=intermediate metabolizer, IQR=interquartile range, NM=normal metabolizer, PM=poormetabolizer, RM=rapid metabolizer, UR=ultrarapaid metabolizer.
Mean improvements in CGI-S (treated as a continuous variable) was statistically significantly greater for escitalopram-treated patients compared to those receiving placebo (p<0.001). At week 8 (LOCF), the mean CGI-S score in escitalopram-treated patients was 2.8±0.277 compared to 3.6±0.199 in patients receiving placebo (p=0.032).
Adverse Events and Discontinuation
Rates and reasons for early discontinuation are shown in Figure 1 and did not significantly differ between escitalopram and placebo groups (escitalopram, n=5 (19%); placebo, n=6 (24%)). One escitalopram-treated patient and one patient who received placebo each experienced an SAE (placebo: hospitalization for verbal aggression and increased irritability; escitalopram, aborted suicide attempt). Compared to baseline, there were 6 events of CSSRS worsening in patients receiving escitalopram compared to 2 events in patients receiving placebo; one escitalopram-treated patient experienced an aborted suicide attempt. Two patients receiving escitalopram, compared to one patient receiving placebo, engaged in self-injurious behavior (superficial cutting, without penetration of subcutaneous tissue). The emergence/worsening of suicidality did not significantly differ between escitalopram-treated patients and those receiving placebo (p=0.449).
Spontaneously reported AEs are shown in Table 2 and did not differ between groups, with the exception of bruising which trended towards being more common in escitalopram-treated patients compared to those receiving placebo (4 vs. 0 patients, p=0.056). Finally, in the post-hoc exploration of activation and medication exposure, activation was associated with greater escitalopram CMAX (p=0.04) and AUC0-24 (p=0.04) in patients for whom PK sampling was completed (n=18).
TABLE 2.
Spontaneously-Reported Treatment-Emergent Adverse Events (AEs)
| System Organ Class (MedDRA) | Adverse event | Escitalopram, n (%) |
Placebo, n (%) |
|---|---|---|---|
| Ear and labyrinth | Otitis media | 0 (0) | 1 (4%) |
| Gastrointestinal | Abdominal pain | 10 (38%) | 9 (36%) |
| Constipation | 0 | 0 | |
| Diarrhea or loose bowel movements |
0 | 5 (20%) | |
| Dry mouth | 0 | 2 (8%) | |
| Gastroenteritis | 2 (8%) | 2 (8%) | |
| Gastroesophageal reflux | 1 (4%) | 0 | |
| Nausea | 8 (30%) | 11 (44%) | |
| Vomiting | 2 (8%) | 3 (12%) | |
| General | Appetite, decreased | 4 (15%) | 4 (16%) |
| Appetite, increased | 2 (8%) | 1 (4%) | |
| Chills | 0 | 0 | |
| Fever | 1 (4%) | 2 (8%) | |
| Flushing | 0 | 0 | |
| Hyperhidrosis | 1 (4%) | 2 (8%) | |
| Somnolence or fatigue | 9 (35%) | 5 (20%) | |
| Investigations | Weight gain | 1 (4%) | 0 |
| Weight loss | 1 (4%) | 1 (4%) | |
| Musculoskeletal and connective tissue | Arthralgias | 1 (4%) | 2 (8%) |
| Leg cramping | 0 | 2 (8%) | |
| Myalgias | 1 (4%) | 0 | |
| Nervous system | Blurred vision | 0 | 0 |
| Dizziness | 2 (8%) | 0 | |
| Headache | 15 (58%) | 12 (48%) | |
| Syncope | 0 | 1 (4%) | |
| Vivid or unusual dreams | 5 (19%) | 3 (12%) | |
| Psychiatric | Activation-cluster symptoms |
7 (27%) | 5 (20%) |
| Insomnia | 5 (19%) | 5 (20%) | |
| Reproductive system and breast | Dysmenorrhea | 4 (15%) | 2 (8%) |
| Respiratory, thoracic and mediastinal | Epistaxis | 1 (4%) | 0 |
| Nasal congestion | 5 (19%) | 2 (8%) | |
| Sinusitis | 1 (4%) | 0 | |
| Respiratory tract infection, pharyngitis |
10 (38%) | 5 (20%) | |
| Yawning | 0 | 0 | |
| Skin and subcutaneous tissue | Acne | 1 (4%) | 0 |
| Dry skin | 0 | 1 (4%) | |
| Hair loss | 0 | 1 (4%) | |
| Rash | 1 (4%) | 0 | |
| Vascular | Bruising | 4 (15%) | 0 |
Vital Signs and EKG
Escitalopram-treated patients did not significantly differ from those receiving placebo with regard to baseline-to-endpoint changes in pulse (escitalopram: 1.54±2.13; placebo: 1.59±2.92, p=0.99), systolic blood pressure (escitalopram: −1.20±2.09 mm Hg; placebo: 0.66±2.74 mm Hg, p=0.583), diastolic blood pressure (escitalopram: −0.066±1.73 mm Hg; placebo: −3.26±2.21 mm Hg, p=0.251). Similarly, no differences in change in weight were observed between groups (escitalopram: −0.47±0.35 kg; placebo: −0.67±0.44 kg, p=0.716). No patients in either group had prolonged QTc >500 msec at any time during the study and QTc changes were not significantly different between escitalopram (−6.63±5.05) and placebo (0.99±4.13, p=0.238).
Predictors of Treatment Response to Escitalopram
For the change in PARS score in escitalopram-treated patients, greater improvement over time was associated with being an intermediate CYP2C19 metabolizer (p<0.016).
For response (CGI-I ≤2) over time, a logistic regression including age, sex, time, 2C19 phenotype (normal or intermediate), greater response was significantly associated with having at least one long allele of the SLC6A4 (p=0.005), being an intermediate CYP2C19 metabolizer (p=0.015).
Plasma escitalopram and desmethylescitalopram concentrations were determined in 18 youth (70% of escitalopram treated patients), including poor (n=1), intermediate (n=7), normal (n=6), rapid (n=3) and ultrarapid (n=1) metabolizers. Escitalopram AUC0-24 significantly decreased with increased CYP2C19 metabolism at 15 mg/day (ANOVA test for linear trend, p=0.042, Figure 2B). CMAX trended towards being higher in slower metabolizers, relative to faster metabolizers, at the 15 mg/day (ANOVA test for trend, p=0.070, Figure 2C). Desmethylescitalopram: escitalopram ratios were increased in patients with faster CYP2C19 metabolism relative to those with slower metabolism (p<0.001, Figure 2D).
Escitalopram exposure (AUC0-24) at the 15 mg/day dose did not differ between 8 week (ET) responders (n=11, 26.98±10.8) and non-responders (n=7, 32.45±20.1, p=0.490). Similarly, escitalopram:desmethylescitalopram ratios did not differ between responders 1.07±0.727 and non-responders (1.15±1.319, p=0.876). In the post hoc logistic regression of pharmacokinetic parameters (i.e., CMAX, AUC0-24), age, CYP2C19 phenotype and response (CGI-I ≤2), higher CMAX, AUC0-24 were not statistically significantly associated with response (p=0.067 and 0.062, respectively). Models incorporating escitalopram:desmethylescitalopram ratios did not suggest an association with response (p=0.342). Finally, patients who experienced activation symptoms had higher CMAX (p=0.040) and AUC0-24 (p=0.040) compared to those who did not (Figure 2E,F).
DISCUSSION
To our knowledge, this is the first double-blind, placebo-controlled trial of escitalopram in pediatric patients with GAD. Our findings demonstrate the superiority of escitalopram relative to placebo both in magnitude and trajectory of improvement. This study suggests the potential predictive utility of pharmacogenetic markers of treatment response and, for the first time in anxious youth, links escitalopram pharmacokinetics with CYP2C19 metabolizer status and side effects.
Consistent with most studies of SSRIs in pediatric anxiety disorders,2,3 escitalopram was superior to placebo in reducing anxiety symptoms. In fact, the NNT in this study is similar to other studies of SSRIs in pediatric anxiety disorders,2 and placebo response was relatively low (24%) which is similar to most trials of SSRIs in pediatric anxiety disorders.2,3 Similar to other studies of SSRIs in pediatric anxiety disorders, escitalopram was well-tolerated.30 The side effects observed herein were consistent with larger trials, although the sample size and duration of the trial decreased our ability to explore longer-term side effects (e.g., weight gain) which has been reported in pediatric patients with depressive and anxiety disorders, particularly those who are slower CYP2C19 metabolizers.31 Additionally, with regard to acute side effects, we observed a relationship between activation-related symptoms and both CMAX and AUC0-24 in the PK cohort which is consistent with two other reports of SSRI exposure-related activation.20,28 Thus, in anxious youth, activation has been demonstrated to be associated with higher exposure to fluoxetine, fluvoxamine and now escitalopram, although it has not been associated with dose in any of these studies. These findings remind clinicians to consider medication exposure rather than just dose in assessing SSRI-related side effects.
The findings that (1) CYP2C19 phenotype predicts escitalopram exposure as well as response trajectory (and magnitude) and, (2) in our post-hoc analyses, escitalopram levels are associated with response (at a 6% significance threshold) and activation suggests that we might re-consider the role of therapeutic drug monitoring in psychiatry. Guidelines suggest “target doses” for SSRIs that are often based on large RCTs in pediatric patients. However, a “target dose” fails to acknowledge variability in the actual exposure to the medication that may be dependent on individual differences in metabolism. Additionally, the exposure-response relationship suggested herein is likely confounded by AEs. Patients with higher levels may be more likely to respond, but may also be more likely to discontinue medication.
While this is the first double-blind palcebo-controlled study of escitalopram in pediatric anxiety disorders and the first pediatric study to prospectively examine the impact of pharmacodynamic and pharmacokinetic genes on treatment response, as well as the relationship between metabolizer status and medication exposure, several important limitations warrant additional discussion. First, the sample was small, racially homogeneous (>80% white), relatively treatment naïve (~75% without prior SSRI/SNRI treatment), primarily female and was recruited from a single site. While this homogeneity potentially reduced outcome variability, it limited our ability to examine some predictors of differential outcomes. The racial homogeneity also obscured our ability to examine differences in CYP2C19 alleles. The “hypermetabolic” *17 allele is twice as common in African Americans (19%) and US Caucasians (18%) compared to Hispanics which could account for differences in tolerability or efficacy across demographics.32 Second, the forced-flexible escitalopram titration that potentially increased response, decreased treatment dose variability (i.e., patients received either 15 mg or 20 mg/day). Our ability to examine dose was limited; however, it is noteworthy that there was substantial variability in medication exposure (i.e., AUC0-24). Third, while we examined escitalopram exposure and response, tolerability (which may also relate to response) confounds these models and there is endogeneity among variables. Fourth, we included patients with severe anxiety and significant co-morbidity (e.g., more than half of the sample had panic disorder and nearly one third had agoraphobia) which may have amplified the medication-placebo difference and may limit generalizability. Prior studies suggest that less severe pathology is associated with a greater placebo response. Fourth, patients were not systematically followed after completion of this acute treatment trial. Fifth, we have focused on a single developmental period (adolescence) and a singular diagnosis (GAD); however, anxiety disorders often emerge prior to adolescence.33 Many,2,34,35 but not all 3,4 studies of pharmacotherapy in anxious youth focus on generalized, separation and social anxiety disorders as they commonly co-occur, share risk factors and respond similarly to psychotherapy and pharmacotherapy.36 However, we believe that given the degree of overlap with other separation and social anxiety disorders and our use of a global measure of anxiety (PARS), our findings could be extrapolated to separation and social anxiety. Sixth, while we focused on adolescents to minimize pharmacokinetic confounds and to be consistent with the FDA labeled age range for escitalopram, this limits extrapolation of these findings to younger patients. Last, our small sample precluded analysis of other pharmacokinetic genes (e.g., CYP2D6 and CYP3A4), so only the primary metabolizing enzyme (CYP2C19) was analyzed; the extreme phenotypes of CYP2D6 and CYP3A4 were too infrequent in this study sample.
This study has many implications for clinicians treating adolescents with GAD and provides preliminary answers to important questions: (1) Is escitalopram effective in treating you with anxiety? (2) How quickly will patients respond? and (3) Does CYP2C19 metabolism affect escitalopram pharmacokinetics in youth? Finally, the answers to these questions, although incomplete, provide a scaffold for larger studies of pharmacodynamic and pharmacokinetic predictors of treatment response and studies that leverage variability in exposure to inform medication dosing and selection.
CLINICAL POINTS.
Escitalopram reduces anxiety in adolescents with generalized anxiety disorder and is well tolerated.
CYP2C19 genotype influences the trajectory and magnitude of improvement as well as escitalopram pharmacokinetics in anxious adolescents.
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Mental Health (K23 MH106037). We also thank the patients and their families who participated in this study as well as the members of the data safety monitoring board for this study.
FUNDING: This study was supported by the National Institute of Mental Health (K23 MH106037).
Footnotes
ROLE OF THE FUNDING AGENCY: The NIMH had no role in the design or implementation of this study nor analyses of these data or the decision to submit to J Clin Psychiatry.
CONFLICT OF INTEREST: Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS) as well as Allergan, Neuronetics and Otsuka. He has received material support from and provided consultation to Myriad Genetics and receives royalties from the publication of two texts (Springer) and serves as an author for UpToDate and an Associate Editor for Current Psychiatry. Drs. Strawn and Mills receive research support from the Yung Family Foundation. Dr. Desta reports no biomedical conflicts of interest. Dr. Ramsey has received research support from the National Institutes of Health (NICHD) and BTG, International Ltd. Kim Cecil receives research support from the NIH. Dr. DelBello receives research support from NIH, PCORI, Acadia, Allergan, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, and Sunovion. She is also a consultant, on the advisory board, or has received honoraria for speaking for Alkermes, Allergan, Assurex, CMEology, Janssen, Johnson and Johnson, Lundbeck, Myriad, Neuronetics, Otsuka, Pfizer, Sunovion, and Supernus. Ethan Poweleit reports no biomedical conflicts of interest. Heidi Schroeder reports no biomedical conflicts of interest. Sarah Mossman reports no biomedical conflicts of interest. Sara Varney reports no biomedical conflicts of interest.
REFERENCES
- 1.Merikangas KR, He J-P, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry. 2001;158(12):2008–2014. [DOI] [PubMed] [Google Scholar]
- 4.Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61(11):1153–1162. doi: 10.1001/archpsyc.61.11.1153 [DOI] [PubMed] [Google Scholar]
- 5.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–293. doi: 10.1016/j.jaac.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 6.Luft MJ, Lamy M, DelBello MP, McNamara RK, Strawn JR. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):50–62. doi: 10.1016/j.cppeds.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilens TE, Biederman J, Kwon A, et al. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13(2):143–152. [DOI] [PubMed] [Google Scholar]
- 8.Strawn JR, Geracioti L, Rajdev N, Clemenza K, Levine A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057–1070. doi: 10.1080/14656566.2018.1491966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor JH, Lebowitz ER, Jakubovski E, Coughlin CG, Silverman WK, Bloch MH. Monotherapy Insufficient in Severe Anxiety? Predictors and Moderators in the Child/Adolescent Anxiety Multimodal Study. J Clin Child Adolesc Psychol. Published online 2018. doi: 10.1080/15374416.2017.1371028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsburg GS, Kendall PC, Sakolsky D, et al. Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. J Consult Clin Psychol. 2011;79(6):806–813. doi: 10.1037/a0025933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strawn J, Levine A. Treatment Response Biomarkers in Anxiety Disorders: From Neuroimaging to Neuronally-Derived Extracellular Vesicles and Beyond. Biomarkers in Neuropsychiatry. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JH, Liu ZQ, Wang W, et al. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;70(1):42–47. doi: 10.1067/mcp.2001.116513 [DOI] [PubMed] [Google Scholar]
- 13.Steere B, Baker JAR, Hall SD, Guo Y. Prediction of in vivo clearance and associated variability of CYP2C19 substrates by genotypes in populations utilizing a pharmacogenetics-based mechanistic model. Drug Metab Dispos. 2015;43(6):870–883. doi: 10.1124/dmd.114.061523 [DOI] [PubMed] [Google Scholar]
- 14.Chang M, Tybring G, Dahl ML, Lindh JD. Impact of Cytochrome P450 2C19 Polymorphisms on Citalopram/Escitalopram Exposure: A Systematic Review and Meta-Analysis. Clin Pharmacokinet. 2014;53(9):801–811. doi: 10.1007/s40262-014-0162-1 [DOI] [PubMed] [Google Scholar]
- 15.Jukić MM, Haslemo T, Molden E, Ingelman-Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am J Psychiatry. Published online 2018. doi: 10.1176/appi.ajp.2017.17050550 [DOI] [PubMed] [Google Scholar]
- 16.Findling RL, McNamara NK, Stansbrey RJ, et al. The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J Child Adolesc Psychopharmacol. 2006;16(1–2):131–145. doi: 10.1089/cap.2006.16.131 [DOI] [PubMed] [Google Scholar]
- 17.Strawn JR, Mills JA, Sauley BA, Welge JA. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 2018;57(4):235–244.e2. doi: 10.1016/j.jaac.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albano AM, Silverman, Wendy K. Anxiety Disorders Interview Schedule for DSM-IV-Child Version: Clinician Manual. Psychological Corporation; 1996. [Google Scholar]
- 19.Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. J Child Adolesc Psychopharmacol. 2010;20(6):513–516. doi: 10.1089/cap.2010.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–126. doi: 10.1089/cap.2008.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCrimmon AW, Smith AD. Review of the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). J Psychoeduc Assess. Published online 2013. doi: 10.1177/0734282912467756 [DOI] [Google Scholar]
- 22.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19(2):215–223. doi: 10.1038/gim.2016.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greden JF, Parikh S V., Rothschild AJ et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. Published online 2019. doi: 10.1016/j.jpsychires.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 24.Ji Y, Schaid DJ, Desta Z, et al. Citalopram and escitalopram plasma drug and metabolite concentrations: Genome-wide associations. Br J Clin Pharmacol. 2014;78(2):373–383. doi: 10.1111/bcp.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strawn JR, Poweleit EA, Ramsey LB. CYP2C19-Guided Escitalopram and Sertraline Dosing in Pediatric Patients: A Pharmacokinetic Modeling Study. J Child Adolesc Psychopharmacol. Published online 2019. doi: 10.1089/cap.2018.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills JA, Prasad K. A comparison of model selection criteria. Econom Rev. 1992;11(2):201–233. [Google Scholar]
- 27.Strawn JR, Dobson ET, Mills JA, et al. Placebo Response in Pediatric Anxiety Disorders: Results from the Child/Adolescent Anxiety Multimodal Study. J Child Adolesc Psychopharmacol. 2017;27(6):501–508. doi: 10.1089/cap.2016.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birmaher B, Axelson D a, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F [DOI] [PubMed] [Google Scholar]
- 29.Bezanson J, Edelman A, Karpinski S, Shah VB. Julia: A Fresh Approach to Numerical Computing. 2014;59(1):65–98. [Google Scholar]
- 30.Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–729. doi: 10.1097/CHI.0b013e3181a2b304 [DOI] [PubMed] [Google Scholar]
- 31.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 metabolizer status on escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front Pharmacol. Published online 2019. doi: 10.3389/fphar.2019.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strom CM, Goos D, Crossley B, et al. Testing for variants in CYP2C19: Population frequencies and testing experience in a clinical laboratory. Genet Med. Published online 2012. doi: 10.1038/gim.0b013e3182329870 [DOI] [PubMed] [Google Scholar]
- 33.Beesdo K, Pine DS, Lieb R, Wittchen H-U. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67(1):47–57. doi: 10.1001/archgenpsychiatry.2009.177 [DOI] [PubMed] [Google Scholar]
- 34.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703 [DOI] [PubMed] [Google Scholar]
- 35.Strawn JR, Compton SN, Robertson B, Albano AM, Hamdani M, Rynn MA. Extended Release Guanfacine in Pediatric Anxiety Disorders: A Pilot, Randomized, Placebo-Controlled Trial. J Child Adolesc Psychopharmacol. 2017;27(1):29–37. doi: 10.1089/cap.2016.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strawn JR, Lu L, Peris TS, Levine A, Walkup JT. Research Review: Peadiatric Anxiety Disorders: What have we learnt in the last 10 years? J Child Psychol Psychiatry. Published online 2020. doi: 10.1111/JCPP.13262 [DOI] [PMC free article] [PubMed] [Google Scholar]