Abstract
Nonalcoholic fatty liver disease (NAFLD) is estimated to afflict approximately 1 billion individuals worldwide. In a subset of NAFLD patients, who have the progressive form of NAFLD termed nonalcoholic steatohepatitis (NASH), it can progress to advanced fibrosis, cirrhosis, hepatocellular carcinoma, and liver-related morbidity and mortality. NASH is typically characterized by a specific pattern on liver histology, including steatosis, lobular inflammation, and ballooning with or without peri-sinusoidal fibrosis. Thus, key issues in NAFLD patients are the differentiation of NASH from simple steatosis and identification of advanced hepatic fibrosis. Until now, liver biopsy has been the gold standard for identifying these 2 critical end points, but has well-known limitations, including invasiveness; rare but potentially life-threatening complications; poor acceptability; sampling variability; and cost. Furthermore, due to the epidemic proportion of individuals with NAFLD worldwide, liver biopsy evaluation is impractical, and noninvasive assessment for the diagnosis of NASH and fibrosis is needed. Although much of the work remains to be done in establishing cost-effective strategies for screening for NASH, advanced fibrosis, and cirrhosis, in this review, we summarize the current state of the noninvasive assessment of liver disease in NAFLD, and we provide an expert synthesis of how these noninvasive tools could be utilized in clinical practice. Finally, we also list the key areas of research priorities in this area to move forward clinical practice.
Keywords: NAFLD, Steatosis, NASH, Fibrosis, Noninvasive, VCTE, CAP, MRI-PDFF, MRE, Serum Biomarkers, ARFI, SWE
Nonalcoholic fatty liver disease (NAFLD) affects around one-fourth of the general population worldwide.1 Nonalcoholic steatohepatitis (NASH), the active form of NAFLD, characterized by histological lobular inflammation and hepatocyte ballooning, is associated with faster fibrosis progression and affects around 1.5%–6.5% of the general population.1 NAFLD is frequently associated with metabolic comorbidities, such as obesity (51%; 95% confidence interval [CI], 41%–61%), type 2 diabetes (22%; 95% CI, 18%–28%), hyperlipidemia (69%; 95% CI, 50%–83%), hypertension (39%; 95% CI, 33%–%46), and metabolic syndrome (42%; 95% CI, 30%–56%).1 Although the most common cause of death in patients with NAFLD is cardiovascular disease, independent of other metabolic comorbidities, NAFLD is becoming a major cause of liver disease-related morbidity (eg, cirrhosis, end-stage liver disease, hepatocellular carcinoma, and liver transplantation), as well as mortality.2,3 NAFLD is expected in the next decade to become the leading indication for liver transplantation in the United States.4 It is estimated that liver-specific mortality and overall mortality among patients with NAFLD are 0.77 and 11.77 per 1000 person-years, whereas they are 15.44 and 25.56 per 1000 person-years among patients with NASH.1
The vast majority of NAFLD patients, however, will not progress, only a minority, namely those with NASH and advanced hepatic fibrosis, are at greatest risk of developing complications of chronic liver disease.5 Indeed, advanced fibrosis has been shown to be the major driver for long-term outcome and mortality.6–8 Thus, key issues in patients with NAFLD are the differentiation of NASH from simple steatosis and identification of advanced hepatic fibrosis. Given the huge number of at-risk patients, there is a substantial unmet need for efficient and cost-effective means for risk stratification of NAFLD patients for these 2 critical end points. Liver biopsy, the gold standard for identifying these 2 end points until now, appears unrealistic and unsuitable. In addition, it has well-known limitations, including invasiveness, poor acceptability, sampling variability, and cost. As a result, this has fueled the development of alternative noninvasive strategies, which have been an area of intensive research over the past decade.9
This review is aimed at discussing the performance, advantages, and limitations of noninvasive methods for the management of patients with NAFLD, including diagnosis and quantification of steatosis, differentation of NASH from simple steatosis, and identification of advanced hepatic fibrosis.
Currently Available Noninvasive Methods and Their Limitations
Noninvasive methods rely on 2 different approaches: a “biological” approach based on the quantification of biomarkers in serum samples or a “physical” approach based on the measurement of liver stiffness, using either ultrasound- or magnetic resonance-based elastography techniques. Although these approaches are complementary, they are based on different rationales. Liver stiffness corresponds to a genuine and intrinsic physical property of liver parenchyma, whereas serum biomarkers indicate several, not strictly liver-specific, clinical and serum parameters that have been associated with NASH or fibrosis stage, as assessed by liver biopsy.
Serum Biomarkers
Current serum biomarkers (summarized in Table 1) include predictive models for diagnosing or grading steatosis (such as the Fatty Liver Index) or staging fibrosis (eg, NAFLD Fibrosis Score), direct measures of hepatocellular damage (eg, circulating keratin 18 fragments) to differentiate patients with NASH from those with simple steatosis and direct measures of fibrosis (eg, PIIINP or Pro-C3) to discriminate patients with advanced fibrosis. Some are specific of NAFLD (eg, BARD and NAFLD fibrosis scores) whereas some have been initially designed in hepatitis C (aspartate transaminase [AST]/alanine transaminase [ALT] ratio, Aspartate Transaminase-to-Platelet Ratio Index [APRI], FIB-4). A few are proprietary formulas (FibroTest, Fibrometer, Hepascore, and Enhanced Liver Fibrosis [ELF] score) but most are nonpatented.
Table 1.
Available Serum Biomarkers for Diagnosing Steatosis or for Staging Fibrosis in Patients With Nonalcoholic Fatty Liver Disease
| Index (ref) | Items, n | Age | Sex | BMI | Diabetes | Platelet count | AST level | ALT level | AST/ALT ratio | GGT level | TG level | Other components |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steatosis | ||||||||||||
| FLI14 | 4 | X | X | X | Waist circumference | |||||||
| HSI15 | 3 | X | X | X | — | |||||||
| SteatoTest13 | 12 | X | X | X | X | X | X | A2M, ApoA1, haptoglobin, T bilirubin, cholesterol, and glucose | ||||
| LAP16 | 3 | X | X | Waist circumference | ||||||||
| ION17 | 3/4 | X | X | X | Waist-to-hip ratio (male yes; female no), and HOMA | |||||||
| NAFLD-LFS18 | 4 | X | X | Metabolic syndrome and insulin | ||||||||
| Fibrosis | ||||||||||||
| APRI160 | 2 | X | X | — | ||||||||
| FIB-4161 | 4 | X | X | X | X | — | ||||||
| FibroTest162 | 8 | X | X | X | X | A2M, ApoA1, haptoglobin, and total bilirubin | ||||||
| Fibrometer NAFLD163 | 7 | X | X | X | X | glucose, ferritin and body weight | ||||||
| ELF164 | 3 | Hyaluronic acid, PIIINP and TIMP-1 | ||||||||||
| Hepacore165 | 6 | X | X | X | A2M, hyaluronic acid and total bilirubin | |||||||
| BARD score166 | 3 | X | X | X | — | |||||||
| NFS167 | 6 | X | X | X | X | X | Albumin |
A2M, α2-macroglobulin; APOA1, apolipoprotein A1; FLI, fatty liver index; GGT, γ-glutamyltransferase; HSI, Hepatic Steatosis Index; ION, Index of NASH; LAP, lipid accumulation product; NAFLD-LFS, NAfLd liver fat score; NFS, NAFLD fibrosis score.
The practical advantages of analyzing serum biomarkers include their high applicability (>95%), their good interlaboratory reproducibility, and their potential widespread availability (nonpatented). However, none are liver-specific—their results can be influenced by comorbid conditions and they require critical interpretation of results.
Imaging Techniques
Elastography.
There are 2 different kinds of elastography techniques: ultrasound-based or magnetic resonance-based. The first one uses ultrasound to detect the velocity of the microdisplacements (shear waves) induced in the liver tissue, whereas the latter uses the magnetic resonance scanner. The shear wave’s velocity is then converted into a liver stiffness measurement (LSM), expressed in kilopascals (kPa) or in meters per second. Vibration-controlled transient elastography (TE) has been the pioneer ultrasound-based technique and is the most widely used worldwide, but newer elastography modalities like point shear wave elastography (pSWE), which includes acoustic radiation force impulse imaging (ARFI), or 2dimensional shear wave elastography (2D-SWE), integrated in conventional ultrasound systems, are emerging.10–12 Their main characteristics, advantages, and limitations are summarized in Table 2. TE and magnetic resonance elastography (MRE) provide additional information in patients with NAFLD. The same machine can be used to determine whether steatosis is present: controlled attenuation parameter (CAP) for TE and calculation of the proton-density fat traction (PDFF) for MRE. Comprehensive technical details can be found in the Supplementary Material.
Table 2.
Respective Characteristics, Advantages, and Limitations of the 4 Available Elastography Techniques for Liver Fibrosis Staging
| Techniques | Performed by | Units (range) | Steatosis grading | Quality criteria | Failure rate, % | Confounders | Evidence in NAFLD study patients | Cost | Point of care | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation | Obesity | Others | |||||||||
| TE | Hepatologist, trained | kPa (2–75) | Yes | Well-defined | 3–27 | ++ | ++ | Congestion steatosis? | n = 25 | $ | Yes |
| nurse or technician | CAP | IQR/M <30% | XL-probe | 3862 | |||||||
| MRE | Radiologist | kPaa (2–11) | Yes | Emerging QIBA | 0–2 | + | - | Congestion iron overload | n = 6 | $$$ | No |
| PDFF | consensus | + | 676 | ||||||||
| statement | + | ||||||||||
| pSWE/ARFI | Radiologist or | m/s (0.5–4.4) | No | Not well-defined | 2 | + ? | + ? | Similar to TE? | n = 8 | $$ | No |
| ultrasonographer | limited data | limited data | limited data | 834 | |||||||
| 2D-SWE | Radiologist or | kPa (2–150) | No | Not well-defined | 13 ? | + ? | + ? | Similar to TE? | n = 2 | $$ | No |
| ultrasonographer | limited data | limited data | limited data | 447 | |||||||
IQR/M, interquartile range/median; QIBA, Quantitative Imaging Biomarkers Alliance.
MRE is reported as shear modulus, while ultrasound elastography techniques are reported in Young modulus. The Young modulus is 3 times the shear modulus.
Diagnosis and Grading of Steatosis
Serum Biomarkers
Several steatosis scores have been proposed for the detection of steatosis, including the SteatoTest,13 Fatty Liver Index,14 Hepatic Steatosis Index,15 lipid accumulation product,16 the Index of NASH,17 and the NAFLD Liver Fat Score.18 Their diagnostic performances have been summarized in a recent review.19 Although SteatoTest, Fatty Liver Index NAFLD Liver Fat Score, lipid accumulation product, and Hepatic Steatosis Index have been validated independently,20–23 their diagnostic performances are difficult to compare, as they have been designed and validated against different standards: liver biopsy, ultrasonography, or magnetic resonance spectroscopy. Nevertheless, when the Fatty Liver Index, NAFLD Liver Fat Score, and Hepatic Steatosis Index were retrospectively compared in the same cohort of 324 patients with suspected NAFLD and liver biopsy, their area under the receiver operating characteristic (AUROC) values for the diagnosis of steatosis (>5%) did not differ (0.83, 0.80, and 0.81, respectively).21 Further studies are needed, but it should be acknowledged that these scores have not gained much popularity, as they do not add much to the information provided by clinical, laboratory, and imaging studies done routinely in patients with suspected NAFLD.
Ultrasonography
Conventional ultrasonography is the most commonly used imaging method for the diagnosis of hepatic steatosis because it is widely available, well established, well tolerated, and cheap. Typical ultrasonography features are hyperechogenicity as compared to the right kidney parenchyma, distal attenuation, and the presence of areas of focal sparing.24 The degree of steatosis can be subjectively scored as mild, moderate, and severe, or as reported in some studies by using ordinal ultrasonography scores.25,26 In a large meta-analysis24 (n = 34 studies, 2815 patients with suspected or known liver diseases), pooled sensitivities and specificities of ultrasonography to distinguish moderate-to-severe fatty liver from the absence of steatosis, taking liver biopsy as the reference, were 85% (80%–89%) and 93% (87%–97%), respectively. However, in clinical practice, mainly the presence or absence of steatosis is recorded and ultrasonography has the limitation that it can only detect steatosis with >2.5%–20% liver fat content27 and, therefore, a relevant number of patients with steatosis starting at 5% liver fat content can be missed.28 In addition, the accuracy of ultrasonography for diagnosis of liver steatosis is reduced in patients with obesity and coexistent renal disease.29,30 Recent studies obtained better results using quantitative ultrasound.28,31 Nevertheless, European guidelines for the management of NAFLD recommend using ultrasonography as first-choice imaging in adults at risk for NAFLD.2
Controlled Attenuation Parameter
In the initial study assessing its performances in 115 patients with chronic liver diseases (15% with NAFLD only), CAP was able to accurately detect steatosis ≥11%, ≥33%, and ≥66% with AUROCs of 0.91, 0.95 and 0.89, respectively.32 Nevertheless, despite a good correlation with histological steatosis, overlapped results between different grades of steatosis suggest that CAP cannot differentiate adjacent grades of steatosis with good precision. A recent individual data meta-analysis33 based on 19 studies using the M-probe and having included 2735 patients (537 with NAFLD; 19.6%) has reported for steatosis ≥11%, ≥33%, and ≥66%, AUROCs of 0.82, 0.86, and 0.88, respectively, sensitivities of 0.69, 0.77, and 0.88 and specificities of 0.82, 0.81 and 0.78, respectively. The authors proposed optimal cutoffs of 248 dB/m (95 % CI, 237–261 dB/m), 268 dB/m (95 % CI, 257–284 dB/m), and 280 dB/m (95% CI, 268–294 dB/m), respectively. Interestingly, CAP values were influenced by several covariates, including NAFLD, diabetes, and body mass index (BMI). Other authors, using magnetic resonance imaging (MRI)-PDFF as reference have recently suggested 288 dB/m as an optimal cutoff for detection of ≥5% fat in the liver.34 Table 3 summarizes the results of CAP in NAFLD patients.35–45 Several comments can be made: most studies have been conducted in small sample size (<100 patients) and heterogeneous populations with variable BMI and diabetes prevalence; this may be an explanation for the differences in proposed cutoffs. However, the cutoff associated with significant steatosis (>33% of hepatocytes) is almost always >250 dB/m. Finally, most of these studies have been performed with the M-probe. In a recent US multicenter study, using the XL-probe, in 393 NAFLD patients, CAP had an AUROC of 0.76 for detecting steatosis >5% and a 96% positive predictive value at a cutoff of 263 dB/m.45 In contrast, the accuracy of CAP for separating steatosis ≥33% and ≥66% was suboptimal.
Table 3.
Performances of Controlled Attenuation Parameter for the Diagnosis and Grading of Steatosis in Patients With Nonalcoholic Liver Disease, Taking Liver Biopsy as Reference
| Authors | Year | Design | Patients, n | Male sex, % | Age, y | Diabetes, % | BMI, kg/m2 | Steatosis grading, % | Steatosis prevalence, % | CAP probe | Failure rate, % | AUC | CAP cutoff, dB/m | Se, % | Sp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Friedrich-Rust38 | 2012 | P | 57 | 53 | 45 ± 14 | 28.0 ± 5.5 | ≥33 | 74 | M | 19 | 0.78 | 245 | 97 | 67 | |
| ≥66 | 46 | 0.72 | 301 | 76 | 68 | ||||||||||
| Kumar41 | 2013 | P | 63 | 73 | 37a | — | 25.1 ± 2.0 | ≥33 | 59 | M | — | 0.79 | 258 | 78 | 73 |
| ≥66 | 11 | 0.77 | 283 | 71 | 68 | ||||||||||
| Chan35 | 2014 | P | 105 | 51 | 50 ± 11 | 52 | 29.4 ± 3.9 | ≥5 | 97 | M | 4 | 0.97 | 263 | 92 | 94 |
| ≥33 | 64 | 0.86 | 281 | 97 | 68 | ||||||||||
| ≥66 | 14 | 0.75 | 283 | 100 | 53 | ||||||||||
| Karlas40 | 2014 | P | 50 (11 C) | 50 | 55 ± 9 | 28 | 31.1 ± 4.2 | ≥5 | 100 | M | 8 | 0.93 | 233 | 93 | 87 |
| ≥33 | 62 | 0.94 | 268 | 97 | 81 | ||||||||||
| ≥66 | 24 | 0.82 | 301 | 82 | 76 | ||||||||||
| de Ledinghen37 | 2016 | R | 261 | 59 | 56 ± 12 | 59 | 30.2 ± 5.1 | >33 | 72 | M | 24 | 0.80 | 310 | 79 | 71 |
| >66 | 32 | 0.66 | 311 | 87 | 47 | ||||||||||
| Imajo39 | 2016 | P | 142 (10 C) | 57 | 57 ± 15 | 50 | 28.1 ± 4.6 | ≥5 | 83 | M | 11 | 0.88 | 236 | 82 | 91 |
| >33 | 58 | 0.73 | 270 | 78 | 80 | ||||||||||
| >66 | 17 | 0.70 | 302 | 64 | 74 | ||||||||||
| Park43 | 2017 | P | 104 | 43 | 51 ± 15 | 28 | 30.4 ± 5.2 | ≥5 | 91 | M | — | 0.85 | 261 | 72 | 86 |
| >33 | 43 | XL | 6.7 | 0.70 | 305 | 63 | 69 | ||||||||
| >66 | 15 | 0.73 | 312 | 64 | 70 | ||||||||||
| Runge44 | 2017 | P | 55 | 73 | 52a | — | 27.8a | ≥5 | 91 | M | 0 | 0.77 | 260 | 90 | 60 |
| >33 | 47 | 0.78 | 296 | 92 | 55 | ||||||||||
| >66 | 16 | 0.78 | 334 | 78 | 76 | ||||||||||
| Chan36 | 2017 | R | 57 (22 C) | 49 | 50 ± 10 | — | 30.2 ± 5.0 | ≥5 | 98 | M | 6 | 0.94 | 260 | 91 | 87 |
| >33 | 76 | XL | 2 | 0.80 | 266 | 91 | 87 | ||||||||
| >66 | 26 | 0.69 | 267 | 100 | 47 | ||||||||||
| Naveau42 | 2017 | R | 194 | 22 | 41 ± 01 | 18 | 44.0 ± 0.4 | ≥5 | 85 | XL | — | 0.85 | 308 | 68 | 69 |
| >33 | 59 | 0.59 | 335 | 65 | 79 | ||||||||||
| >66 | 39 | 0.39 | 341 | 74 | 74 | ||||||||||
| P | 123 | 26 | 40 ± 01 | 22 | 44.0 ± 0.6 | ≥5 | 81 | XL | — | 0.81 | 298 | 78 | 83 | ||
| >33 | 58 | 0.58 | 303 | 90 | 69 | ||||||||||
| >66 | 37 | 0.37 | 326 | 83 | 71 | ||||||||||
| Siddiqui45 | 2018 | P | 393 | 32 | 51 ± 11 | 43 | 34.4 ± 6.4 | ≥5 | 95 | M | 5.0 | 0.76 | 285 | 80 | 77 |
| >33 | 57 | XL | 0.70 | 311 | 77 | 57 | |||||||||
| >66 | 27 | 0.58 | 306 | 80 | 40 |
C, controls; P, prospective; R, retrospective; Se, sensitivity; Sp, specificity.
Median.
Thus far only 2 studies36,46 compared head to head the performance of CAP with M- and XL-probes, using liver biopsy as reference, with conflicting results. In one study (236 Western patients with chronic liver disease with a mean BMI 24.4 ± 6.3 kg/m2), the performances and cutoff values were similar,46 whereas in another study (57 NAFLD Chinese patients with a mean BMI 30.2 ± 5.0 kg/m2), the performances were similar, but cutoff values were higher with the XL-probe.36 Therefore, further studies are necessary before any firm conclusions can be drawn.
Only 2 studies have performed a head-to head comparison of CAP with ultrasonography, taking liver biopsy as reference: 1 in 72 patients with chronic liver disease47 and the other 1 in 366 patients with chronic hepatitis B.48 Both studies showed that the performance of CAP for detecting and grading liver steatosis was higher than that of ultrasonography, however, the rate of overestimation was significantly higher for CAP than for ultrasonography (30.5% vs 12.4%; P < .05).48 More studies are needed before any firm conclusion can be drawn.
In the 3 studies39,43,44 comparing CAP and PDFF magnetic resonance spectroscopy (MRS) for grading steatosis, using liver biopsy as reference, CAP was outperformed by MRI-PDFF. In a study in 78 American patients with NAFLD,43 MRI-PDFF performed better than CAP for diagnosing all grades of steatosis (AUROC 0.99 vs 0.85, respectively; P = .0091). Similarly, in a study in 127 Japanese patients with NAFLD,39 MRI-PDFF had better diagnostic accuracy than CAP, whatever the grade of steatosis. Finally, a study in 55 Dutch patients with NAFLD found similar results.44
Longitudinal studies are awaited. Recently, a study that followed up 4282 patients who had both a reliable LSM and ≥10 successful CAP measurements has shown that neither the presence nor the severity of hepatic steatosis predicted liver-related events, cancer, or cardiovascular events in the short term, while LSM and etiology independently predicted liver-related events.49 Subgroup analyses of viral hepatitis (hepatitis B: 37.0%; hepatitis C: 2.9%) and NAFLD patients (40.7% of the entire cohort) revealed similar results.
In summary, CAP is a promising point-of-care technique for rapid and standardized steatosis quantification, but needs to be better validated in patients with NAFLD with the XL-probe. CAP is outperformed by MRI-PDFF, but should to be compared to ultrasonography, which, despite its limitations, remains the most widely used tool for first-line steatosis assessment.
Magnetic Resonance Imaging Proton-Density Fat Traction
Cross-Sectional Utility of Magnetic Resonance Imaging Proton-Density Fat Traction.
MRS has been employed in several large epidemiologic studies, and now with the development of MRI-PDFF, it has been more widely utilized in epidemiologic studies to classify presence of hepatic steatosis as well as to quantify the amount of liver fat.50–53
Longitudinal Comparison With Histology and Magnetic Resonance Spectroscopy.
A series of single-center studies suggested the utility of MRI-PDFF in the longitudinal assessment of changes in liver fat content with paired assessment with MRS and liver histology over a 24-week period.54–56 These studies suggested that MRI-PDFF was more sensitive than liver histology in assessing changes in liver fat and has may be utilized in the setting of a clinical trial.57 These data have since been confirmed in multicenter studies in both adult and pediatric populations.58 These studies have shown that longitudinal change in MRI-PDFF robustly correlates with longitudinal change in MRS-PDFF (with correlation coefficients ranging from 0.96 to 0.99), when both the MRI and MRS measurements at each time points are meticulously colocalized.55,57
Role of Magnetic Resonance Imaging Proton-Density Fat Traction as an End Point in Early-Phase Nonalcoholic Steatohepatitis Trial.
Several early phase trials in NASH have adopted MRI-PDFF as an end point to examine efficacy of various drugs to assess treatment response. Le et al54 followed by the MOZART (Magnetic Resonance Imaging and Elastography in Ezetimibe Versus Placebo for the Assessment of Response to Treatment in NASH) trial proposed the need for co-localization of regions of interests before and after treatment, as the liver fat is heterogeneously distributed.54,55,57 MRI-PDFF is unable to assess liver inflammation, ballooning, or resolution of NASH or improvement in fibrosis.59
Clinical Utility of Amount of Decline in Liver Fat.
As the new trial data emerged, the experts started noticing a range of liver fat improvement in various trials. Using the paired MRI-PDFF and histology data from the MOZART trial, it appeared that, at a threshold of a relative 30% reduction in MRI-PDFF, one may start to appreciate significantly higher odds of a 2-point improvement in NAFLD Activity Score on liver histology.60 These data require further validation, which is ongoing in the multicenter setting.61 Higher liver fat content at baseline in patients without fibrosis has recently been shown to be associated with significantly higher odds of fibrosis progression than those patients who have lower liver fat content.62 These emerging data suggest that liver fat content may have prognostic significance, especially early on the fibrosis progression cascade, but need to be confirmed. MRI-PDFF estimation methods have been successfully implemented in the clinical setting as a tool for fat quantification. They are Food and Drug Administration-approved and commercially available on the several MRI vendors, including GE Healthcare, Siemens, and Philips, and are now more readily available on newer scanners.63
Diagnosis of Nonalcoholic Steatohepatitis
Serum Biomarkers
Many serum biomarkers have been investigated for the diagnosis of NASH 64 but cytokeratin (CK)-18 is by far the one that has been the most widely investigated. CK-18 fragments come from apoptosis of hepatocytes accomplished by the enzyme caspase 3 and can be measured in serum by immunoassay. The M30 enzyme-linked immunosorbent assay measures the caspase-cleaved K18 fragments and detects apoptosis, which is a hallmark of steatohepatitis, whereas the M65 enzyme-linked immunosorbent assay detects total cell death. Since the initial study by Feldstein et al,65 reporting circulating serum levels of CK-18 to be predictive of NASH in patients with NAFLD (with AUROC of 0.83 and sensitivity of 0.75 and specificity of 0.81 for a CK- 18 value of about 250 U/L), many studies66–75 have confirmed these results, though in rather small populations. In 2 subsequent meta-analyses,76,77 CK-18 had pooled AUROC of 0.82 (95% CI, 0.76–0.88) to predict NASH with a median sensitivity of 66%–78% and specificity of 82%–87%. There are however, several issues with CK-18: lack of a commercially available clinical test,78 limited sensitivity at the individual level79 and considerable variability in the suggested cutoffs and their respective diagnostic accuracy among studies, which makes choosing which threshold to use very difficult.64 These limitations have resulted in limited clinical utility in practice so far. To increase CK-18 sensitivity, some authors have combined it with other biological parameters, such as sFas levels,80 uric acid,81 adiponectin and resistin (NASH diagnostics),74,82 or ALT and presence of metabolic syndrome (Nice Model).83 Other predictive models, combining clinical and laboratory parameters, have been proposed for the diagnosis of NASH, including HAIR (hypertension, increased ALT, and insulin resistance),84 Palekar score (age, sex, AST, BMI, AST/ALT ratio, and hyaluronic acid),85 Gholam score (AST and diabetes mellitus),86 oxNASH (13-hydroxyl-octadecadienoic acid/linoleic acid ratio, age, BMI, and AST),87 NAFIC score (ferritin, insulin and type IV collagen 7s),88 and NashTest (Biopredictive, Paris, France), a proprietary formula including 12 variables (age, sex, height, weight, serum levels of triglycerides, cholesterol, α2-macroglobulin, apolipoprotein A1, haptoglobin, γ-glutamyltransferase, aminotransferases ALT, AST, and total bilirubin).89 In a meta-analysis from the developer, in 494 obese patients with a prevalence of NASH of 17.2%, the weighted AUROC of NashTest was 0.84.20 However, most of these models rely on small and highly selected populations (morbidly obese patients)83,90–92 and have not been externally validated.93 The diagnostic performances of these different models have been recently reviewed and are therefore not detailed in the present review.78,64 Recently, several approaches using genetic biomarkers have been proposed, including single nucleotide polymorphisms located in PNPLA3, such as the NASH Score (PNPLA3 genotype, AST, and fasting insulin)94 and the NASH ClinLipMet Score (glutamate, isoleucine, glycine, lysophosphatidylcholine 16:0, phosphoethanolamine 40:6, AST, fasting insulin, and PNPLA3 genotype),95 but also expression of noncoding RNAs, specifically microRNAs, such as miR-122.96,97 However, the information yielded has had moderate clinical utility.
In summary, none of the currently available serum marker are able to differentiate NASH from simple steatosis with high sensitivity and specificity, however, their diagnostic accuracy can be improved by combining different approaches.
Imaging Techniques
Studies on the ability of elastography to discriminate between isolated steatosis and NASH are limited to TE and MRE. The performances of MRE have been investigated in 5 studies39,43,98–100 and those of TE in 4.39,43,45,101 A wide range of AUROCS (0.35–0.93) and optimal cutoffs have been reported and likely depend on the prevalence of advanced fibrosis in the study population.102 In the 2 studies39,43 with head-to-head comparison, there was no difference between TE and MRE. Currently, neither modality can reliably discriminate NASH from simple steatosis, although MR-based modalities are showing promise, as discussed in the Other Magnetic Resonance-Based Methods section.
Staging of Liver Fibrosis
Serum Biomarkers
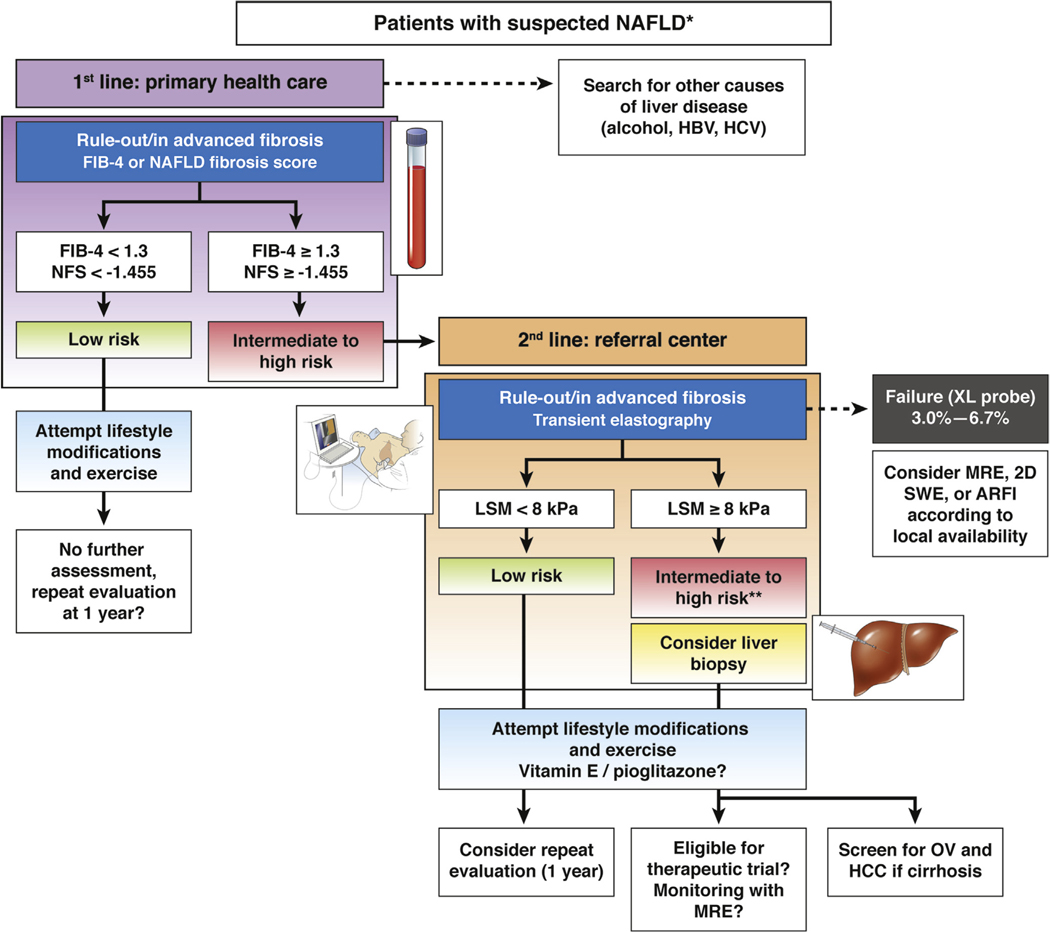
The diagnostic performances of serum biomarkers have already been summarized in several reviews78,64,103 and, therefore, will not be detailed here. Briefly, as for nonpatented tests, a recent meta-analysis (based on 64 studies in 13,046 NAFLD patients) comparing BARD, APRI, FIB-4, and NAFLD fibrosis score (NFS) for diagnosing advanced fibrosis reported summary AUROCS of 0.76, 0.77, 0.84, and 0.84, respectively.104 With an APRI threshold of 1.0 and 1.5, the sensitivities and specificities for advanced fibrosis were 50.0% and 84.0% and 18.3% and 96.1%, respectively. With a FIB-4 threshold of 2.67 and 3.25, the sensitivities and specificities for advanced fibrosis were 26.6% and 96.5% and 31.8% and 96.0%, respectively. The summary sensitivities and specificities of BARD score (threshold of 2), and NFS (threshold of −1.455) for advanced fibrosis were 0.76 and 0.61, 0.72, and 0.70, respectively. Among the 4 biomarkers, FIB-4 and NFS are the most accurate with high negative predictive values (>90%) for ruling out advanced fibrosis. They could therefore be used as first-line tools in primary health care setting to identify patients without advanced fibrosis who do not need further assessment. In that respect, FIB-4 may be more attractive to general practitioners, as it is based on widely available and simple parameters (age, transaminases, and platelets) and easier to calculate than NFS. There are, however, several limitations that should be acknowledged: first, performances of FIB-4 and NFS to rule in advanced fibrosis are rather inadequate, meaning that further assessment with another test is needed in case of positive results (Figure 1). Second, it is important to keep in mind that they have been mostly validated in liver clinics, where the prevalence of advanced fibrosis is much higher than in primary health care settings. Third, when using FIB-4 or NFS, a significant proportion of patients (around 30%) fall in the intermediate-risk cate- gory105 (Figure 1) and cannot be correctly classified. This may lead to unnecessary referral of these patients to liver clinics for further assessment. Finally, new age-adjusted cutoffs have been proposed recently to improve the diagnostic performance of NFS and FIB-4 for advanced fibrosis.106
Figure 1.
A suggested algorithm for the use of noninvasive tests for risk stratification of patients with suspected NAFLD in clinical practice. *Suspicion of NAFLD is based on the presence of steatosis on ultrasound or abnormal liver tests (transaminases/γ-glutamyltransferase) in patients with risk factors (obesity, type 2 diabetes, or metabolic syndrome). Significant alcohol consumption and secondary causes of steatosis should be excluded. The proposed algorithm is based on expert opinion. The choice of noninvasive tools should be sequential, guided by local availability and the context of use: in primary health care setting, simple inexpensive and widely available serum biomarkers, such as FIB-4 or NFS, with high negative predictive value (88%−95%) for ruling out advanced fibrosis should be used as first-line. Patients with low risk (FIB-4 <1.3 or NAFLD Fibrosis score < −1.455; 55 to 58% of cases) do not need further assessment. They should be offered lifestyle modifications and exercise. Those with intermediate (FIB-4 = 1.3 to 3.25 or NFS = −1.455 to 0.672; 30% of cases) and high risk (FIB-4 >3.25 or NFS >0.672; 12%–15% of cases, positive predictive value 75%–90%) of having advanced fibrosis should be addressed to a referral center for LSM, using TE, in fasting condition, using M-probe for patients with skin-liver capsule distance <25 mm otherwise with the XL-probe. Patients at low risk of having advanced fibrosis (LSM <8 kPa; NPV 94%–100%) should be considered for a repeat evaluation within 1 year. Those with intermediate (LSM = 8–10 kPa) or high risk (LSM ≥10 kPa, PPV 47%–70%) of having advanced fibrosis should be considered for liver biopsy. However, confounders for liver stiffness should be carefully excluded to minimize the risk of false positive results. **Also patented serum biomarkers (FibroTest, Fibrometer, or ELF) could be considered in patients with intermediate risk according to local availability. In case of TE failure, alternative such as SWE/ARFI, MRE (particularly when BMI >35 kg/m2) may be considered according to local availability. In any case, all patients should be offered lifestyle modifications and exercise. As recommended by recent European Association for the Study of the Liver or American Association for the Study of Liver Diseases clinical practice guidelines, vitamin E (in non-diabetics) and pioglitazone may be considered in these patients. Also patients with cirrhosis should be screened for esophageal varices (OV) and hepatocellular carcinoma (HCC). In those with a liver biopsy, follow-up during treatment of LSM, using MRE, is the most promising noninvasive approach but requires further validation. NPV, negative predictive value; PPV, positive predictive value.
As for patented tests, no independent meta-analysis is available. A recent French study107 from the developer comparing Fibrometer to other patented (FibroTest and Hepascore) and nonpatented (APRI, FIB-4, BARD, and NFS) serum biomarkers in 452 NAFLD patients, showed that Fibrometer (AUROC 0.82) outperformed all of the other tests for diagnosing advanced fibrosis. These results require further independent confirmation. Finally, novel markers, such as the PRO-C3, a commercially available assay that detects the synthesis of type III collagen, has been recently suggested to be superior to APRI, FIB-4, and NFS to identify patients with NAFLD and advanced fibrosis when combined with age, platelet, and diabetes.108 These promising results require further validation. Finally, despite slight improvement in diagnostic accuracy over nonpatented biomarkers, the limited availability of patented tests and their cost might limit their wider application.
In summary, among the different serum biomarkers studied, NFS and FIB-4 have been the most extensively studied and validated in different NAFLD populations and with consistent results. These tests perform best at excluding advanced fibrosis (with negative predictive values >90%) and could therefore be used as a first-line triage to identify patients at low risk of advanced fibrosis in settings where more sophisticated tests are unavailable.9
Ultrasound-Based Elastography
Transient Elastography.
Several meta-analysis, mostly performed in viral hepatitis patients, have reported good (88%–89%) and excellent (93%–96%), accuracies of TE for diagnosing advanced fibrosis and cirrhosis, respectively.11 Two meta-analysis performed in NAFLD patients have confirmed these results.76,104 The meta-analysis by Kwok, based on 9 studies (8 with the M-probe) including a total of 1047 NAFLD patients, reported summary sensitivities of 85% and 92% and specificities of 82% and 92% for diagnosing advanced fibrosis and cirrhosis, respectively.76 Table 4 summarizes the results of the most recent studies (since 2015),39,43,107,109–112 as prior studies have been already summarized.103 These results deserve several comments: 1) these studies have included heterogeneous populations with a rather limited number of cirrhotic patients (<20%) and wide range or BMI (27–40 kg/m2); 2) the failure or unreliable results is lower when the XL-probe is used. Also, as shown in the most recent meta-analysis based on 19 studies (4 using the XL-probe) including a total of 2495 NAFLD patients from different ethnic back-grounds, summary AUROCs of TE did not differ between M- and XL-probes for diagnosing advanced fibrosis (0.87 vs 0.86) and cirrhosis (0.92 vs 0.94), respectively104; 3) apart from the type of probe used, the uneven distribution of fibrosis stages between studies may likely be an explanation for the observed differences between proposed cutoffs for a given end point, known as the spectrum bias.113,114 Finally, it should be stressed that all of these studies have been conducted in tertiary referral centers, where the proportion of patients with advanced fibrosis is higher that in the general population, thus making it difficult to extrapolate the performance of TE if used to detect cirrhosis in large populations. Overall, these results suggest that TE could be of interest to exclude confidently advanced fibrosis and cirrhosis with high negative predictive value (around 90%) in these patients.9 For instance, at a cutoff <8 kPa, TE had a 94%–100% negative predictive value.112,115 Finally, TE is recommended in the current guidelines on management of NAFLD.2,9
Table 4.
Performances of Transient Elastography for the Diagnosis of Advanced Fibrosis and Cirrhosis in Adult Patients With Nonalcoholic Fatty Liver Disease, Taking Liver Biopsy as Reference
| Authors | Year | Design | Patients, n | Age, y | BMI, kg/m2 | Scoring system | End point | Prevalence, % | Cutoff, kPa | AUC | Se, % | Sp, % | Failure or unreliable, % | Probe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Petta111 | 2015 | R | 179 | 45 ± 13 | 29.3 ± 4.1 | Kleiner | F3-F4 | 23 | 7.9/9.6 | 0.86 | 85 | 81 | 13.0 | M |
| 142 | 44 ± 12 | 27.4 ± 3.7 | F3-F4 | 20 | 7.9/9.6 | 0.85 | 68 | 86 | 23.0 | M | ||||
| Cassinotto109 | 2016 | P | 291 | 57 ± 12 | 32.1 ± 6.0 | NASH-CRN | F3-F4 | 43 | 8.2/12.5 | 0.86 | 90/57 | 61/90 | 22.0 | M |
| F4 | 17 | 9.5/16.1 | 0.87 | 92/65 | 62/90 | |||||||||
| Tapper112 | 2016 | P | 164 | 51 ± 13 | 32.2a | Brunt | F3-F4 | 18 | 9.9 | 0.93 | 95 | 77 | 26.8 | M |
| F4 | — | |||||||||||||
| Boursier107 | 2016 | R | 452 | 56 ± 12 | 31.1 ± 5.2 | NASH-CRN | F3-F4 | 38 | 8.7 | 0.83 | 88 | 63 | 14.1 | M |
| F4 | 13 | — | 0.87 | — | — | |||||||||
| Imajo39 | 2016 | P | 142 (10 C) | 57 ± 15 | 28.1 ± 4.6 | Brunt | F3-F4 | 32 | 11.4 | 0.88 | 86 | 84 | 11.0 | M |
| F4 | 8 | 14.0 | 0.92 | 100 | 76 | |||||||||
| Petta115 | 2017 | R | 324 | 54 ± 13 | — | Kleiner | F3-F4 | 35 | 10.1 | 0.86 | 78 | 78 | — | M |
| Park43 | 2017 | P | 104 | 51 ± 15 | 30.4 ± 5.2 | NASH-CRN | F3-F4 | 20 | 7.3 | 0.80 | 78 | 72 | — | M |
| F4 | 8 | 6.9 | 0.69 | 63 | 66 | 6.7 | XL | |||||||
| Chen110 | 2017 | P | 111 | 48a | 40.3 | Brunt | F3-F4 | 20 | 7.6 | 0.87 | 84 | 64 | — | M |
| F4 | 10 | 14.6 | 0.92 | 82 | 92 | 18.7 | XL | |||||||
| Petta168 | 2017 | R | 761 | 51 ± 13 | 29.6 ± 4.9 | Kleiner | F3-F4 | 31 | 7.9/9.6 | 0.86 | 90/74 | 65/81 | — | M |
| Siddiqui45 | 2018 | P | 393 | 51 ± 11 | 34.4 ± 6.4 | NASH-CRN | F3-F4 | 32 | 8.6 | 0.83 | 80 | 74 | — | M |
| F4 | 9 | 13.1 | 0.93 | 89 | 86 | 5.0 | XL |
AUC, area under the curve; C, controls; NASH-CRN, nonalcoholic steatohepatitis clinical research network; P, prospective; R, retrospective; Se, sensitivity; Sp, specificity.
Median.
Acoustic Radiation Force Imaging.
Meta-analyses of pSWE using ARFI imaging in patients with chronic liver disease reported diagnostic accuracies of 89%–91% for advanced fibrosis and 92%–93% for cirrhosis, with cutoffs ranging from 1.55–1.61 m/s for advanced fibrosis and 1.80–1.87 m/s for cirrhosis, respectively.116,117 Other pSWE systems show comparable results to ARFI. However, different cutoffs are recommended for different systems.10,118 Only a few studies have evaluated pSWE using ARFI in patients with NAFLD with diagnostic accuracies of 84%–98% for advanced fibrosis.38,109,119–123 A systematic review of 7 studies having included 723 NAFLD patients, reported a summary diagnostic accuracy, sensitivity and specificity of 90%, 80% and 85%, respectively, for the detection of significant fibrosis.124 However, significant fibrosis is not the most relevant end point and no data are available to date for advanced fibrosis and cirrhosis. Similarly, no data are available for the follow-up of NAFLD patients using pSWE. Therefore, pSWE is not included in the current guidelines on management of NAFLD.
Shear Wave Elastography.
A retrospective meta-analysis, evaluating 2D-SWE in 1340 patients with chronic liver diseases from 13 centers worldwide, reported diagnostic accuracies of 91% and 95% for advanced fibrosis and cirrhosis, and optimal cutoffs of 9.2, and 13.5 kPa, respectively.125 In the subgroup of 172 NAFLD patients, diagnostic accuracies were 93% and 92% for advanced fibrosis and cirrhosis, respectively, with the same optimal cutoffs as for the overall group. When 2D-SWE was compared to TE in a subgroup of 91 NAFLD patients with reliable TE-values, 2D-SWE performed significantly better for diagnosing advanced fibrosis (AUROC difference of 12%; P = .003). In another study in 291 NAFLD patients, 2D-SWE had diagnostic accuracies of 89% and 88% for detecting advanced fibrosis and cirrhosis, respectively.109 The cutoff values with sensitivity >90% were 8.3 kPa and 10.5 kPa for advanced fibrosis and cirrhosis, respectively. Interestingly, 2D-SWE outperformed TE and ARFI only for significant fibrosis. No data are available for the follow-up of NAFLD patients using 2D-SWE. Therefore, 2D-SWE is not included in the current guidelines on management of NAFLD.
Magnetic Resonance Elastography
2D-MRE has been shown in a prospective cohort of 117 patients with biopsy-proven NAFLD to have a high diagnostic accuracy for the detection of advanced fibrosis.99 The AUROCs for the detection of any fibrosis, advanced fibrosis, and cirrhosis were 0.84, 0.92, and 0.89, respectively, with an optimal cutoff for advanced fibrosis of 3.64 kPa. These results have been confirmed in a meta-analysis based on 9 studies and 232 NAFLD patients.126 In another recent meta-analysis based on 5 studies and 628 NAFLD patients, the pooled AUROC of 2D-MRE for advanced fibrosis was 0.96.104
In a head-to-head comparison between 3D-MRE vs 2D-MRE, 3D-MRE at 40 Hz was superior to 2D-MRE at 60 Hz with an AUROC for the detection for advanced fibrosis of 0.98 (3D-MRE) vs 0.92 (2D-MRE).100 However, processing of 3D-MRE takes a much longer time and has yet not been applied in multicenter studies. 3D-MRE appears to be an extremely promising tool for longitudinal changes in fibrosis assessment. Further studies are needed to determine its role in fibrosis assessment in routine clinical practice.
Other Magnetic Resonance-Based Methods.
A novel method called LiverMultiScan (Perspectum Diagnostics) has been proposed recently as a noninvasive, imaging-based biomarker to measure liver fat and correlate it with liver iron content, fibrosis, and inflammation. The 3 parameters included in the proprietary algorithm are liver fat assessment, T2*, and corrected T1 decay on advanced MRI.127 A pilot proof-of-concept study has shown promising data, and further larger validation of these parameters in patients with biopsy-proven NAFLD, and its utility in assessment of treatment response in NASH trials is being actively assessed.127
Recent novel data suggest that addition of damping ratio in addition to 2D-MRE and perhaps MRI-PDFF may help further advance the assessment of both inflammation and fibrotic components of disease activity and severity of NAFLD.128 Further studies are needed to examine the exact utility and applicability of these approaches in assessment of NAFLD severity.
Comparison and Combination of Approaches
Among clinical available modalities, MRE has the highest diagnostic accuracy in the detection of advanced fibrosis in NAFLD,129 but the evidence is based on a limited number of selected patients (n < 700) in highly specialized tertiary centers. In a head-to-head comparison with 7 serum fibrosis markers, including FIB-4, in 102 patients with NAFLD, 2D- MRE performed better than all serum markers for the detection of advanced fibrosis.130 When compared head to head with serum markers (FIB-4, NFS, APRI, BARD, Fibr- ometer, and FibroTest) in large cohorts of NAFLD patients (n = 452 and n = 761), TE outperformed all other serum markers,115 apart from Fibrometer.107 Some authors have proposed strategies combining TE with FIB-4 or NFS, either in a paired or in a serial fashion.111,115 Such serial strategy, however, increased the diagnostic performance with an accuracy around 70%, but at the price of an uncertainty area (around 20%) and a 10% rate of misclassified patients.115 MRE and TE have been compared head to head in patients with biopsy-proven NAFLD in 3 studies39,43,110 with conflicting results. Chen et al110 have shown in 111 patients with morbid obesity (mean BMI, 40.3 kg/m2) that MRE performed better than TE for detecting advanced fibrosis in intention to diagnose, but not in per-protocol, analysis. In 2 other studies, 1 in 142 Japanese patients (mean BMI, 28.1 kg/m2), using only the M-probe39 and 1 in 104 American patients (mean BMI, 30.4 kg/m2), using both M and XL-probes,43 there was no statistical difference between MRE and TE for the detection of advanced fibrosis. Differences in the studied populations might account for this discrepancy. Recent data suggest that, when staging fibrosis taking liver biopsy as reference, BMI is significantly associated with discordance of findings between MRE and TE, the degree of discordancy increasing with BMI.131 Finally, a recent individual patient meta-analysis (based on 230 patients) found that MRE had a statistically significantly higher diagnostic accuracy than vibration-controlled TE in assessing each stage of fibrosis in patients with biopsy-proven NAFLD.132 Therefore, further studies are needed before any firm conclusion can be drawn. As for comparison with pSWE using ARFI, 2D-MRE has been shown to be superior to pSWE for the detection of any fibrosis, but not for advanced fibrosis.119 In addition, pSWE underperformed in the setting of obesity and higher liver fat content.
In summary, all of these modalities have a role in clinical practice and understanding the caveats associated with their utility (summarized in Table 2) are helpful in optimal clinical use of these tools.
Use in Clinical Practice
In patients with suspected NAFLD (presence of steatosis on ultrasound or abnormal liver tests [transaminases/γ-glutamyltransferase] in patients with risk factors such as obesity, type 2 diabetes, or metabolic syndrome), noninvasive tests can be used in clinical practice for risk stratification. Whatever the approach, serum biomarkers or elastography, each modality is most reliable in excluding the presence of advanced fibrosis. As shown in Figure 1, the choice of noninvasive tools to be used should be guided by local availability and context of use. In primary health care setting, simple inexpensive and widely available serum biomarkers, such as FIB-4 or NAFLD fibrosis scores, with high negative predictive value (>90%) for ruling out advanced fibrosis should be used as first-line. Patients with low risk (FIB-4 <1.3 or NAFLD Fibrosis score < −1.455; 55% to 58% of cases) of having advanced fibrosis do not need further assessment. They should be offered lifestyle modifications and exercise. Those with intermediate (FIB-4 = 1.3 to 3.25 or NFS = −1.455 to 0.672; 30% of cases) and high risk (FIB-4 >3.25 or NFS >0.672; 12% to 15% of cases, positive predictive value 75% to 90%) should be sent to a referral center for further assessment. Patented serum biomarkers (FibroTest, Fibrometer, or ELF) could be considered in patients with intermediate risk according to local availability. Otherwise TE, as the most widely available and best evaluated point-of-care technique, appears to be the tool of choice, although ARFI and SWE are becoming increasingly available. XL-probe should be used in patients with skin-liver capsule distance >25 mm in order to minimize the TE failure rate (<7%). Patients at low risk of having advanced fibrosis (LSM <8 kPa; negative predictive value 94%–100%) should be offered lifestyle modifications and reevaluation after 1 year. For those with intermediate (LSM = 8–10 kPa) or high risk (LSM ≥10 kPa, positive predictive value 47%–70%) of having advanced fibrosis should be considered for liver biopsy. However, confounders should be carefully excluded to minimize the risk of false positive. In case of TE failure despite the use of XL-probe or high BMI (≥35 kg/m2), alternative techniques such as MRE or SWE/ARFI may be considered according to local availability. However, although SWE and ARFI seem to be as promising as TE, data are currently limited for these modalities regarding the determination of advanced fibrosis in NAFLD. As for MRE, despite its high accuracy, cost and limited availability are limitations to its use in practice. Its role as a surrogate of fibrosis improvement in therapeutic trials remains to be demonstrated. In any case, these patients should be offered lifestyle modifications and exercise and vitamin E (in nondiabetics) and pioglitazone may be considered, as recommended by recent European Association for the Study of the Liver or American Association for the Study of Liver Diseases clinical practice guidelines.2,3 Finally, patients identified as having advanced fibrosis or cirrhosis should be screened for portal hypertension and liver cancer, given the increased risk of this disease in these individuals.
Special Populations and Controversies
Patients with type 2 diabetes mellitus are known to be at increased risk for NAFLD and advanced fibrosis. Noninvasive screening strategies for NAFLD, NASH, or advanced fibrosis have been proposed in diabetic patients, including the use of routinely available clinical variables,133 TE,134,135 MRE,52 or combination of TE and ELF.136 It is noteworthy that most studies on noninvasive tests in NAFLD patients have not been stratified for the presence of diabetes. Several recent studies suggested that noninvasive tests, which were developed and validated in nondiabetic cohorts, underperformed when applied to diabetic patients.137–139 Thus, caution is requested when extrapolating results of noninvasive tests from nondiabetic populations to patients with diabetes. Also the role of ethnicity may be important to take into account,140,141 as most available studies have been done in Caucasians. Further studies are needed to address these issues.
Prognosis
Several recent studies have shown the ability of liver stiffness, measured using TE,107 or serum biomarkers107,142,143 to predict clinical decompensation as well as survival in patients with NAFLD. A meta-analysis144 based on 17 studies in 7058 patients with chronic liver diseases (mainly related to viral hepatitis) has shown that baseline liver stiffness, measured using TE, was associated significantly with risk of hepatic decompensation (6 studies; relative risk [RR], 1.07; 95% CI, 1.03–1.11), hepatocellular carcinoma (9 studies; RR, 1.11; 95% CI, 1.05–1.18), death (5 studies; RR, 1.22; 95% CI, 1.05–1.43), or a composite of these outcomes (7 studies; RR, 1.32; 95% CI, 1.16–1.51). In a nationwide study (National Health and Nutrition Examination Survey cohort)143 in 11,154 participants (34% with NAFLD) with a median follow-up of 14.5 years, those with a high probability of advanced fibrosis using APRI (>1.5), NFS (>0.676), or FIB-4 (>2.67), had a 69% increase in mortality compared to subjects without fibrosis (for NFS: hazard ratio, 1.69; 95% CI, 1.09–2.63; for APRI: hazard ratio, 1.85; 95% CI, 1.02>3.37; for FIB-4: hazard ratio, 1.66; 95% CI, 0.98>2.82) after adjustment for other known predictors of mortality.
Future Directions
There is a wealth of data that are informing clinicians regarding the utility and limitations of each of the diagnostic modalities in the assessment of NAFLD. However, further advances are needed to refine clinical management and more accurate identification of patients at risk for fibrosis progression and those who need to be treated in the setting of a clinical trial without subjecting them to a liver biopsy evaluation. The key research priorities in the field are listed in Table 5. Addressing these gaps in knowledge would greatly impact the field.
Table 5.
Research Priorities and Unmet Needs in the Field
| Cut point for each modality with the context of use needs to be determined (eg, screening in primary care or screening in a diabetes clinic) |
| Validation of quality criteria for each modality |
| Cost-effectiveness of sequential use of clinical prediction rules (eg, FIB-4) followed by TE/SWE/ARFI followed by MRE |
| Clinically meaningful increase/decrease in liver stiffness that is linked to a clinical outcome in NAFLD |
| Clinically meaningful increase in liver stiffness that is associated with a 1-stage increase in liver fibrosis |
| Clinically meaningful decrease in liver stiffness that is associated with a 1-stage decrease in liver fibrosis |
| Cut point for liver stiffness for each modality that is associated with a need to treat varices in patients with NAFLD |
| Clinically meaningful decrease in liver stiffness that is linked to a clinical outcome in NAFLD |
| Does reduction in liver stiffness in cirrhosis is associated with reduction in the risk of liver decompensation despite no change in fibrosis stage |
Recently, efforts concentrating on “omics” approaches (lipidomics, proteomics, and metabolomics) using high- throughput technologies have shown promising results to identify novel biomarkers of NAFLD, NASH, and advanced fibrosis.145 For instance, several studies based on lipidomics approaches have shown circulating oxidized fatty acids and products of arachidonic acid metabolism to be predictive of NASH.146–148 Similarly, proteomics have been used to identify NAFLD patients with active fibrosis, by measuring extracellular matrix remodeling rates in tissue and blood.149 Using a metabolomic approach,150 subtypes of NAFLD with specific serum metabolomic profiles that differentiate steatosis from NASH in each subtype could be identified and might be used to monitor disease progression and identify therapeutic targets for patients. Finally, omics technologies have been used for the profiling of gut microbiota and identification of fecal-microbiome–derived metagenomic signatures associated with NASH and fibrosis in several human studies.151,152 It should be stressed, however, that these findings rely on small cross-sectional studies with a lack of external validation. In addition, the complicated methodology involved in omics platforms as well as reproducibility between centers and stability of samples and high cost prevent far widespread application in clinical practice.
Finally, given the high prevalence of NAFLD in the general population, noninvasive tests could be used as screening tools to identify patients with NAFLD at high risk of progression.153 Recently, several studies have screened systematically for liver fibrosis, using either serum biomarkers154 or TE,155–157 the general population or at-risk populations,158 or diabetics or those with a family history of NAFLD cirrhosis.51,134 Their results suggest an alarmingly high prevalence of chronic liver diseases, mainly related to NAFLD, ranging between 5% and 8% in the general adult population and between 18% and 27% among individuals with risk factors.159 Thus, screening programs for liver fibrosis in the general population, using TE for identifying patients with presymptomatic chronic liver diseases susceptible to interventions should be further assessed.
Conclusions
Significant progress has been made regarding the noninvasive assessment of liver disease in patients with NAFLD. Use of noninvasive tests should be tailored according to the setting (primary heath care, tertiary referral center, trial) and clinical needs (screening, staging of fibrosis, follow-up). Regarding detection and grading of steatosis, MRI-PDFF is the most accurate method but appears better suited for assessment and follow-up of selected patients in clinical trials, whereas conventional ultrasound, and if no steatosis is shown, CAP, as a point of care technique, could be used as triage in large unselected populations. Regarding NASH, no highly sensitive and specific blood tests are available to differentiate NASH from simple steatosis. Neither imaging modality can reliably discriminate NASH from simple steatosis, although MR-based modalities are showing promise. As for the identification of advanced fibrosis, MRE, TE, as well as FIB-4 and NFS are the most accurate and validated methods. FIB-4 and NFS are best suited as first-line tools in primary health care setting to confidently exclude advanced fibrosis, whereas TE and MRE are more suited for referral centers to select the patients who require a liver biopsy. Finally, there is increasing evidence that serum markers and liver stiffness, measured using TE, accurately identify the subgroup of patients with NAFLD at a higher risk to reach the outcome of liver-related complications and death/liver transplantation.
Supplementary Material
Acknowledgments
Author contributions: Laurent Castera contributed to drafting and writing of the manuscript and to critical revision for important intellectual content. Mireen Friedrich-Rust contributed to drafting and writing of the manuscript and to critical revision for important intellectual content. Rohit Loomba contributed to drafting and writing of the manuscript and to critical revision for important intellectual content.
Funding
Rohit Loomba is supported in part by grant R01-DK106419–01 from the National Institute of Diabetes and Digestive and Kidney Diseases National Institute of Health. Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institute of Health under Award P42ES010337.
Abbreviations:
- ALT
alanine transaminase
- APRI
Aspartate Transaminase-to-Platelet Ratio Index
- ARFI
acoustic radiation force imaging
- AST
aspartate transaminase
- AUROC
area under the receiver operating characteristic
- BMI
body mass index
- CAP
controlled attenuation parameter
- CI
confidence interval
- CK
cytokeratin
- 2D-SWE
2-dimensional shear wave elastography
- ELF
Enhanced Liver Fibrosis
- kPa
kilopascals
- LSM
liver stiffness measurement
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- MRE
magnetic resonance elastography
- NFS
nonalcoholic fatty liver disease fibrosis score
- PDFF
proton density fat fraction
- pSWE
point shear wave elastography
- RR
relative risk
- SWE
shear wave elastography
- TE
transient elastography
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2018.12.036.
Conflicts of interest
The authors disclose the following: Laurent Castera: speaker bureau of AbbVie, Echosens, Intercept, Gilead, and Sirtex. Advisory boards for Allergan, Gilead, MSD, Pfizer, and Servier. Mireen Friedrich-Rust: speaker honorarium from Echosens, Siemens. Advisory board for Toshiba. Research support for Echosens, Supersonic, and Siemens. Rohit Loomba: grants from Allergan, BMS, Boehringer Ingleheim, Eli Lily, Galectin, Galmed, GE, Genfit, Gilead, Intercept, Janssen, Madrigal, NGM, Prometheus, Siemens, Shire, Pfizer, advisory committees for Arrowhead Research, Conatus, Galmed, Gemphire, Gilead, Intercept, NGM, and Cirius. Consultant for Bird Rock Bio, BMS, Coh Bar, Celgene, Civi Bio, Conatus, Enanta, Gilead, GRI Bio, Ionis, Metacrine, NGM, Receptos, Sanofi, Salix, Kowa, and Median technologies. Co-founder of Liponexus Inc.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090–1099 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–654 e1-e9; quiz e39-e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with longterm outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017; 65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265–1273. [DOI] [PubMed] [Google Scholar]
- 9.European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL- ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–264. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich C, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, Update 2017 (Long Version). Eur J Ultrasound 2017;38:e16–e47. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol 2016; 13:402–411. [DOI] [PubMed] [Google Scholar]
- 12.Ferraioli G, Wong VW, Castera L, et al. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med Biol 2018;44: 2419–2440. [DOI] [PubMed] [Google Scholar]
- 13.Poynard T, Ratziu V, Naveau S, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol 2005;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503–508. [DOI] [PubMed] [Google Scholar]
- 16.Bedogni G, Kahn HS, Bellentani S, et al. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol 2010;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otgonsuren M, Estep MJ, Hossain N, et al. Single noninvasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J Gastroenterol Hepatol 2014;29:2006–2013. [DOI] [PubMed] [Google Scholar]
- 18.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009; 137:865–872. [DOI] [PubMed] [Google Scholar]
- 19.Stern C, Castera L. Non-invasive diagnosis of hepatic steatosis. Hepatol Int 2017;11:70–78. [DOI] [PubMed] [Google Scholar]
- 20.Poynard T, Lassailly G, Diaz E, et al. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS One 2012;7:e30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedchuk L, Nascimbeni F, Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2014;40:1209–1222. [DOI] [PubMed] [Google Scholar]
- 22.Cuthbertson DJ, Weickert MO, Lythgoe D, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol 2014;171:561–569. [DOI] [PubMed] [Google Scholar]
- 23.Calori G, Lattuada G, Ragogna F, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology 2011;54:145–152. [DOI] [PubMed] [Google Scholar]
- 24.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballestri S, Lonardo A, Romagnoli D, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int 2012;32:1242–1252. [DOI] [PubMed] [Google Scholar]
- 26.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102:2708–2715. [DOI] [PubMed] [Google Scholar]
- 27.Bril F, Ortiz-Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015;35:2139–2146. [DOI] [PubMed] [Google Scholar]
- 28.Paige JS, Bernstein GS, Heba E, et al. A Pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol 2017;208:W168–W177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Moura Almeida A, Cotrim HP, Barbosa DBV, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol 2008;14:1415–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 2004;14:635–637. [DOI] [PubMed] [Google Scholar]
- 31.Lin SC, Heba E, Wolfson T, et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol 2015;13:1337–1345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010; 36:1825–1835. [DOI] [PubMed] [Google Scholar]
- 33.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017; 66:1022–1030. [DOI] [PubMed] [Google Scholar]
- 34.Caussy C, Alquiraish MH, Nguyen P, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018;67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2014;29:1470–1476. [DOI] [PubMed] [Google Scholar]
- 36.Chan WK, Nik Mustapha NR, Wong GL, et al. Controlled attenuation parameter using the FibroScan(R) XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J 2017;5:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Ledinghen V, Wong GL, Vergniol J, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2016;31:848–855. [DOI] [PubMed] [Google Scholar]
- 38.Friedrich-Rust M, Romen D, Vermehren J, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol 2012;81:e325–e331. [DOI] [PubMed] [Google Scholar]
- 39.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150:626–637 e7. [DOI] [PubMed] [Google Scholar]
- 40.Karlas T, Petroff D, Garnov N, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One 2014;9:e91987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar M, Rastogi A, Singh T, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol 2013;28:1194–1201. [DOI] [PubMed] [Google Scholar]
- 42.Naveau S, Voican CS, Lebrun A, et al. Controlled attenuation parameter for diagnosing steatosis in bariatric surgery candidates with suspected nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2017; 29:1022–1030. [DOI] [PubMed] [Google Scholar]
- 43.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017; 152:598–607 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Runge JH, Smits LP, Verheij J, et al. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology 2017:162931. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019. 17:156–163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Ledinghen V, Hiriart JB, Vergniol J, et al. Controlled Attenuation Parameter (CAP) with the XL Probe of the Fibroscan®. A Comparative Study with the M Probe and Liver Biopsy. Dig Dis Sci 2017;62:2569–2577. [DOI] [PubMed] [Google Scholar]
- 47.de Ledinghen V, Vergniol J, Foucher J, et al. Noninvasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012;32:911–998. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Lu W, Li P, et al. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis 2017;49:910–917. [DOI] [PubMed] [Google Scholar]
- 49.Liu K, Wong VW, Lau K, et al. Prognostic value of controlled attenuation parameter by transient elastography. Am J Gastroenterol 2017;112:1812–1823. [DOI] [PubMed] [Google Scholar]
- 50.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caussy C, Soni M, Cui J, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest 2017;127:2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loomba R, Schork N, Chen CH, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 2015;149:1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le TA, Chen J, Changchien C, et al. Effect of colese- velam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012;56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016;65:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Middleton MS, Heba ER, Hooker CA, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017; 153:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caussy C, Reeder SB, Sirlin CB, et al. Non-invasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018;68:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016;9:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol 2019;70:133–141. [DOI] [PubMed] [Google Scholar]
- 62.Ajmera V, Park CC, Caussy C, et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2018; 155:307–310.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loomba R Role of imaging-based biomarkers in NAFLD: Recent advances in clinical application and future research directions. J Hepatol 2018;68:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol 2018; 68:305–315. [DOI] [PubMed] [Google Scholar]
- 65.Feldstein AE, Wieckowska A, Lopez AR, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009;50:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diab DL, Yerian L, Schauer P, et al. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol 2008;6:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grigorescu M, Crisan D, Radu C, et al. A novel pathophysiological-based panel of biomarkers for the diagnosis of nonalcoholic steatohepatitis. J Physiol Pharmacol 2012;63:347–353. [PubMed] [Google Scholar]
- 68.Joka D, Wahl K, Moeller S, et al. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology 2012;55:455–464. [DOI] [PubMed] [Google Scholar]
- 69.Musso G, Cassader M, De Michieli F, et al. Effect of lectin-like oxidized LDL receptor-1 polymorphism on liver disease, glucose homeostasis, and postprandial lipoprotein metabolism in nonalcoholic steatohepatitis. Am J Clin Nutr 2011;94:1033–1042. [DOI] [PubMed] [Google Scholar]
- 70.Papatheodoridis GV, Hadziyannis E, Tsochatzis E, et al. Serum apoptotic caspase activity in chronic hepatitis C and nonalcoholic Fatty liver disease. J Clin Gastroenterol 2010;44:e87–e95. [DOI] [PubMed] [Google Scholar]
- 71.Pirvulescu I, Gheorghe L, Csiki I, et al. Noninvasive clinical model for the diagnosis of nonalcoholic steato- hepatitis in overweight and morbidly obese patients undergoing bariatric surgery. Chirurgia (Bucur) 2012; 107:772–779. [PubMed] [Google Scholar]
- 72.Wieckowska A, Zein NN, Yerian LM, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006;44:27–33. [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz Y, Dolar E, Ulukaya E, et al. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol 2007;13:837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Younossi ZM, Jarrar M, Nugent C, et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes Surg 2008; 18:1430–1437. [DOI] [PubMed] [Google Scholar]
- 75.Shen J, Chan HL, Wong GL, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol 2012;56:1363–1370. [DOI] [PubMed] [Google Scholar]
- 76.Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of nonalcoholic fatty liver disease—the role of transient elas- tography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther 2014;39:254–269. [DOI] [PubMed] [Google Scholar]
- 77.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–649. [DOI] [PubMed] [Google Scholar]
- 78.Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) and associated fibrosis. Hepatology 2018;68:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cusi K, Chang Z, Harrison S, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014;60:167–174. [DOI] [PubMed] [Google Scholar]
- 80.Tamimi TI, Elgouhari HM, Alkhouri N, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol 2011;54:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang JF, Yeh ML, Huang CF, et al. Cytokeratin-18 and uric acid predicts disease severity in Taiwanese nonalcoholic steatohepatitis patients. PLoS One 2017; 12:e0174394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Younossi ZM, Page S, Rafiq N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg 2011;21:431–439. [DOI] [PubMed] [Google Scholar]
- 83.Anty R, Iannelli A, Patouraux S, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of nonalcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther 2010;32:1315–1322. [DOI] [PubMed] [Google Scholar]
- 84.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91–100. [DOI] [PubMed] [Google Scholar]
- 85.Palekar NA, Naus R, Larson SP, et al. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int 2006;26:151–156. [DOI] [PubMed] [Google Scholar]
- 86.Gholam PM, Flancbaum L, Machan JT, et al. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol 2007;102:399–408. [DOI] [PubMed] [Google Scholar]
- 87.Feldstein AE, Lopez R, Tamimi TA, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res 2010;51:3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sumida Y, Yoneda M, Hyogo H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol 2011; 46:257–268. [DOI] [PubMed] [Google Scholar]
- 89.Poynard T, Ratziu V, Charlotte F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campos GM, Bambha K, Vittinghoff E, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology 2008; 47:1916–1923. [DOI] [PubMed] [Google Scholar]
- 91.Ulitsky A, Ananthakrishnan AN, Komorowski R, et al. A noninvasive clinical scoring model predicts risk of nonalcoholic steatohepatitis in morbidly obese patients. Obes Surg 2010;20:685–691. [DOI] [PubMed] [Google Scholar]
- 92.Lassailly G, Caiazzo R, Hollebecque A, et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol 2011; 23:499–506. [DOI] [PubMed] [Google Scholar]
- 93.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 2013;58:1007–1019. [DOI] [PubMed] [Google Scholar]
- 94.Hyysalo J, Mannisto VT, Zhou Y, et al. A population-based study on the prevalence of NASH using scores validated against liver histology. J Hepatol 2014; 60:839–846. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Y, Oresic M, Leivonen M, et al. Noninvasive detection of nonalcoholic steatohepatitis using clinical markers and circulating levels of lipids and metabolites. Clin Gastroenterol Hepatol 2016;14:1463–1472 e6. [DOI] [PubMed] [Google Scholar]
- 96.Becker PP, Rau M, Schmitt J, et al. Performance of Serum microRNAs −122, −192 and −21 as biomarkers in patients with non-alcoholic steatohepatitis. PLoS One 2015;10:e0142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pirola CJ, Fernandez Gianotti T, Castano GO, et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut 2015; 64:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 2011;259:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60:1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loomba R, Cui J, Wolfson T, et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am J Gastroenterol 2016;111:986–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee CM, Jeong WK, Lim S, et al. Diagnosis of clinically significant portal hypertension in patients with cirrhosis: splenic arterial resistive index versus liver stiffness measurement. Ultrasound Med Biol 2016; 42:1312–1320. [DOI] [PubMed] [Google Scholar]
- 102.Ajmera V, Loomba R. Can elastography differentiate isolated fatty liver from nonalcoholic steatohepatitis? Semin Liver Dis 2018;38:14–20. [DOI] [PubMed] [Google Scholar]
- 103.Castera L Noninvasive evaluation of nonalcoholic fatty liver disease. Semin Liver Dis 2015;35:291–303. [DOI] [PubMed] [Google Scholar]
- 104.Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017; 66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 105.Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med 2018;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017; 112:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol 2016;65:570–578. [DOI] [PubMed] [Google Scholar]
- 108.Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: an algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology 2018. July 16 10.1002/hep.30163. [DOI] [PubMed] [Google Scholar]
- 109.Cassinotto C, Boursier J, de Ledinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016;63:1817–1827. [DOI] [PubMed] [Google Scholar]
- 110.Chen J, Yin M, Talwalkar JA, et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 2017;283:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petta S, Vanni E, Bugianesi E, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2015;35:1566–1573. [DOI] [PubMed] [Google Scholar]
- 112.Tapper EB, Challies T, Nasser I, et al. The performance of vibration controlled transient elastography in a us cohort of patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2016;111:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 1978;299:926–930. [DOI] [PubMed] [Google Scholar]
- 114.Poynard T, Halfon P, Castera L, et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem 2007;53:1615–1622. [DOI] [PubMed] [Google Scholar]
- 115.Petta S, Wong VW, Camma C, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther 2017;46:617–627. [DOI] [PubMed] [Google Scholar]
- 116.Friedrich-Rust M, Nierhoff J, Lupsor M, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat 2012;19:e212–e219. [DOI] [PubMed] [Google Scholar]
- 117.Nierhoff J, Chavez Ortiz AA, Herrmann E, et al. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol 2013;23:3040–3053. [DOI] [PubMed] [Google Scholar]
- 118.Sporea I, Bota S, Gradinaru-Tascau O, et al. Comparative study between two point shear wave elastographic techniques: acoustic radiation force impulse (ARFI) elastography and ElastPQ. Med Ultrason 2014;16:309–314. [DOI] [PubMed] [Google Scholar]
- 119.Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016;63:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fierbinteanu Braticevici C, Sporea I, Panaitescu E, et al. Value of acoustic radiation force impulse imaging elastography for non-invasive evaluation of patients with nonalcoholic fatty liver disease. Ultrasound Med Biol 2013;39:1942–1950. [DOI] [PubMed] [Google Scholar]
- 121.Guzman-Aroca F, Frutos-Bernal MD, Bas A, et al. Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging. Eur Radiol 2012;22:2525–2532. [DOI] [PubMed] [Google Scholar]
- 122.Palmeri ML, Wang MH, Rouze NC, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol 2011;55:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010;256:640–647. [DOI] [PubMed] [Google Scholar]
- 124.Liu H, Fu J, Hong R, et al. Acoustic radiation force impulse elastography for the non-invasive evaluation of hepatic fibrosis in non-alcoholic fatty liver disease patients: a systematic review & meta-analysis. PLoS One 2015;10:e0127782-e0127782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herrmann E, de Ledinghen V, Cassinotto C, et al. Assessment of biopsy-proven liver fibrosis by twodimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology 2018; 67:260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 2015;13:440–451 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pavlides M, Banerjee R, Tunnicliffe EM, et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int 2017;37:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between hepatic inflammation and fibrosis with MR elastography. Radiology 2017;284:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for noninvasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016;65:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther 2015;41:1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caussy C, Chen J, Alquiraish MH, et al. Association between obesity and discordance in fibrosis stage determination by magnetic resonance vs transient elastography in patients with nonalcoholic liver disease. Clin Gastroenterol Hepatol 2018;16:1974–1982.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2019;17:630–637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care 2015;38: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016; 65:1359–1368. [DOI] [PubMed] [Google Scholar]
- 135.Roulot D, Roudot-Thoraval F, G NK, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int 2017; 37:1897–1906. [DOI] [PubMed] [Google Scholar]
- 136.Patel P, Hossain F, Horsfall LU, et al. A pragmatic approach identifies a high rate of nonalcoholic fatty liver disease with advanced fibrosis in diabetes clinics and at- risk populations in primary care. Hepatol Commun 2018; 2:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bril F, McPhaul MJ, Caulfield MP, et al. Performance of the SteatoTest, ActiTest, NashTest and FibroTest in a multiethnic cohort of patients with type 2 diabetes mellitus. J Investig Med 2019;67:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bril F, Millan L, Kalavalapalli S, et al. Use of a metabolomic approach to non-invasively diagnose non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2018;20:1702–1709. [DOI] [PubMed] [Google Scholar]
- 139.Chang YH, Lin HC, Hwu DW, et al. Elevated serum cytokeratin-18 concentration in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Ann Clin Biochem 2018:4563218796259. [DOI] [PubMed] [Google Scholar]
- 140.Bril F, Portillo-Sanchez P, Liu IC, et al. Clinical and histologic characterization of nonalcoholic steatohepatitis in African American patients. Diabetes Care 2018; 41:187–192. [DOI] [PubMed] [Google Scholar]
- 141.Xia MF, Yki-Jarvinen H, Bian H, et al. Influence of ethnicity on the accuracy of non-invasive scores predicting non-alcoholic fatty liver disease. PLoS One 2016; 11:e0160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782–789 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1573–1584 e1-e2; quiz e88-e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pirola CJ, Sookoian S. Multiomics biomarkers for the prediction of nonalcoholic fatty liver disease severity. World J Gastroenterol 2018;24:1601–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gorden DL, Myers DS, Ivanova PT, et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res 2015;56:722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Loomba R, Quehenberger O, Armando A, et al. Poly-unsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res 2015;56:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Puri P, Wiest MM, Cheung O, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009;50:1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Decaris ML, Li KW, Emson CL, et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology 2017;65:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alonso C, Fernandez-Ramos D, Varela-Rey M, et al. Metabolomic identification of subtypes of nonalcoholic steatohepatitis. Gastroenterology 2017;152:1449–1461 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loomba R, Seguritan V, Li W, et al. Gut Microbiome- based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054–1062 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Castera L Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: non-invasive tests are enough. Liver Int 2018;38(Suppl 1):67–70. [DOI] [PubMed] [Google Scholar]
- 154.Poynard T, Lebray P, Ingiliz P, et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol 2010;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 2016;63:138–147. [DOI] [PubMed] [Google Scholar]
- 156.Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011;60:977–984. [DOI] [PubMed] [Google Scholar]
- 157.Wong VW, Chu WC, Wong GL, et al. Prevalence of nonalcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using protonmagnetic resonance spectroscopy and transient elastography. Gut 2012;61:409–415. [DOI] [PubMed] [Google Scholar]
- 158.Harman DJ, Ryder SD, James MW, et al. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross-sectional diagnostic study utilising transient elastography. BMJ Open 2015;5:e007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gines P, Graupera I, Lammert F, et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol 2016;1:256–260. [DOI] [PubMed] [Google Scholar]
- 160.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–526. [DOI] [PubMed] [Google Scholar]
- 161.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 162.Imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001;357:1069–1075. [DOI] [PubMed] [Google Scholar]
- 163.Cales P, Laine F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009;50:165–173. [DOI] [PubMed] [Google Scholar]
- 164.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:17041713. [DOI] [PubMed] [Google Scholar]
- 165.Adams LA, George J, Bugianesi E, et al. Complex noninvasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:1536–1543. [DOI] [PubMed] [Google Scholar]
- 166.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology 2000;118: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 167.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 168.Petta S, Wong VW-S, Camma C, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 2017;65:1145–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.