Abstract
Background:
The volume of regional denervated myocardium (D-M) on positron emission tomography has been recently suggested as a strong independent predictor of cause-specific mortality from sudden cardiac arrest (SCA) in chronic heart failure. We sought to evaluate whether ECG indices of global autonomic function predict risk of SCA to a similar degree as regional D-M.
Methods:
Subjects enrolled in the Prediction of Arrhythmic Events using Positron Emission Tomography (PAREPET) study were included in this study. Patients completed a 24-hour Holter ECG at enrollment and were followed up at 3-month intervals. SCA events were adjudicated by two board-certified cardiologists. Other cardiovascular death events were classified as nonsudden cardiac death (NSCD). Eight measures of heart rate variability were analyzed: SDNN, RMSSD, low-frequency (LF) and high-frequency (HF) power, heart rate turbulence onset and slope, and acceleration and deceleration capacity. We used competing risk regression to delineate cause-specific mortality from SCA versus NSCD.
Results:
Our sample included 127 patients (age 67 ± 12, 92% male). After a median follow-up of 4.1 years, there were 22 (17%) adjudicated SCA and 18 (14%) adjudicated NSCD events. In multivariate Cox-regression, LF power was the only HRV parameter to predict time-to-SCA. However, in competing risk analysis, reduced LF power was preferentially associated with NSCD rather than SCA (HR = 0.92 [0.85–0.98], p = 0.019).
Conclusion:
Depressed LF power might indicate impaired vagal reflex, which suggests that increasing vagal tone in these patients would have a protective effect against NSCD beyond that achieved by the mere slowing of heart rate using β-blockers.
Keywords: Heart rate variability, Heart rate turbulence, Deceleration capacity, Heart failure, Sudden cardiac arrest, Cardiovascular death
Introduction
Patients with ischemic heart disease and left ventricular dysfunction are at particularly high risk of sudden cardiac arrest (SCA). Non-invasive risk stratification to identify and manage such high-risk patients is a clinical priority [1,2]. A depressed left ventricular ejection fraction (i.e. LVEF ≤ 35%) remains the only risk stratification tool employed clinically to identify candidates of implantable-cardioverter defibrillation (ICD) therapy for the primary prevention of SCA. However, using LVEF to target ICD therapy has been found to be very inefficient [3,4]. Not only do most SCA events occur in people without an indication for an ICD, but only 25% of those with an ICD for the primary prevention of SCA will use their device within the next 5 years [5]. Therefore, more accurate risk stratification tools for cause-specific mortality from SCA constitute an unmet clinical need [6].
The analysis of heart rate variability (HRV) provides a non-invasive tool to characterize the sympathetic autonomic function, and has been shown to predict the risk of cardiovascular death in various clinical populations [7–9]. However, the majority of these studies have not evaluated cause-specific mortality from SCA and have primarily focused on populations at relatively low risk of SCA. In fact, it is still unknown if HRV plays any significant role in high-risk population (e.g., ischemic cardiomyopathy) due to the high prevalence of pacing and atrial fibrillation and the widespread clinical use of beta blockers in these patients [10].
In the PAREPET trial (Prediction of Arrhythmic Events using Positron Emission Tomography), we reported that the volume of regional denervated myocardium (D-M) is a predictor of cause-specific mortality from SCA in patients with ischemic cardiomyopathy [11]. The PAREPET dataset is unique since it has both PET data and Holter ECG data. As such, we sought to evaluate whether ECG indices of global autonomic function predict risk of SCA to a similar degree as regional D-M. Given that patients need to be stratified on optimal medical therapy (e.g., ICD), we used a competing risk regression approach to delineate cause-specific mortality from SCA versus mortality due to non-sudden cardiac death (NSCD).
Methods
Setting and subjects
This analysis was based on subjects enrolled in the Prediction of ARrhythmic Events with Positron Emission Tomography (PAREPET) study. PAREPET was a prospective observational study sponsored by the National Heart Lung and Blood Institute designed to determine whether denervated and/or hibernating myocardium as quantified with PET could predict SCA among subjects with ischemic cardiomyopathy who were eligible for a primary prevention ICD [2]. Eligible subjects had coronary artery disease, pre-enrollment LVEF ≤ 35%, and NYHA Class I–III heart failure symptoms. The IRB-approved study design and methods have been reported in detail [2,12] and are summarized here. After informed consent, eligible patients underwent echocardiography, PET scans, and 24-hour ambulatory ECG monitoring. Subjects were followed at 3-month intervals for the development of cardiac events (defined below). LVEF and LV end-diastolic volume index (LVEDVI) were quantified by biplane transthoracic echocardiography [2].
PET data and endpoints determination
Our analysis included subjects from PAREPET (n = 127) after exclusion of: [1] those with persistent pacing (n = 43) or atrial fibrillation (n = 20); and [2] those without high-fidelity ECG recordings (n = 14). Total volume (% of LV) of D-M, viable D-M, and infarcted myocardium (infarcted and denervated) were quantified with PET, as previously described [2].
The primary endpoint was SCA. This included [1] arrhythmic death using modified Hinkle-Thaler criteria [13,14] or [2] documented ICD discharge for ventricular fibrillation or rapid ventricular tachycardia (N>240 beats/min), which approximates the reduction in SCA in primary prevention ICD trials [15]. Therapies for ventricular tachycardia at lower rates were excluded since they substantially overestimate the benefit of an ICD and are frequently self-terminating [16]. Any other cardiovascular death but not due to SCA was classified as NSCD. Endpoints were independently adjudicated by two board-certified cardiologists. Disagreements were resolved by consensus with a third cardiologist.
Electrocardiography
Continuous 24-hour ambulatory ECG monitoring was performed using H12+ Holter recorders (V3.12, Mortara Instrument) on all subjects at baseline. High-frequency recordings (1000 Hz) using the Mason-Likar configuration (i.e., limb leads on body) were preprocessed using H-Scribe v 5.11 (Mortara Instrument). After the automated pre-processing, ECG streams were manually annotated (noise and artifacts deleted) by a blinded reviewer to ensure that ECG quality was adequate for subsequent analysis. Non-sinus beats were manually labeled for exclusion. This resulted in 23 ± 3 h of monitoring per eligible patient. All measures were computed according to the methods recommended by the European Society of Cardiology [17].
The annotated ECG streams were then analyzed using Super-ECG (Mortara Instrument), which provided global HRV estimates for time-domain (i.e., SDNN, RMSSD) and frequency-domain (i.e., normalized low- and high-frequency power) measures. Measures of heart rate turbulence (i.e., onset and slope) and acceleration/deceleration-related modulations of heart rate were computed using a custom-written algorithm as previously described [18]. HRT onset was computed as the percentage difference between the heart rate immediately following and immediately preceding a PVC. HRT slope was computed as the steepest regression slope for each sequence of five consecutive sinus rhythm R-R intervals within the first 15 sinus rhythm R-R intervals after a PVC. Acceleration capacity (AC) and deceleration capacity (DC) of the heart rate were quantified using phase rectified signal averaging (PRSA) as previously described [7]. Computer processing of heart period sequences generates the PRSA curve and the center deflection of this curve characterizes the average capacity of the heart rate to accelerate or decelerate from one beat to the next.
Statistical analysis
Values are reported as mean ± SD or as n (%). All analyses were conducted using STATA v 14.0 for Windows, with p < 0.05 considered statistically significant. Demographic and clinical characteristics of subgroups were compared using ANOVA with Tukey posthoc for continuous variables and chi-square for categorical variables. The independent relationship between predictors and time-to-event data was evaluated using Cox proportional-hazards regression models. Variables significant at p < 0.1 in the bivariate analysis were entered in the multivariate models with backward selection method. The goodness-of-fit of the final model was assessed using the simple test previously reported by O’Quigley and Moreau [19]. Then, a competing risks analysis was then performed to simultaneously assess factors associated with SCA vs. NSCD [20]. The variables that resulted from the multivariable Cox-regression analyses of the individual end points (SCA or NSCD) were included in this competing risks analysis. Sub-hazard ratio was computed for the predictors significant at the competing risk model.
Results
After excluding patients with pacing or atrial fibrillation (33%), the final sample included 127 subjects (age 67 ± 12 years, 92% male). The majority of patients were in NYHA heart failure classes II and III (83%), and the majority were on β-blockers (96%) and angiotensin inhibition therapy (93%). After a median follow-up of 4.1 years (range 2.5–7.2 years), there were 22 (17%) adjudicated SCA cases and 18 (14%) adjudicated NSCD cases. Table 1 compares the clinical and ECG characteristics between patients with endpoints and survivors.
Table 1.
Demographic and clinical characteristics of study sample.
| Parameter | All patients (n = 127) | Endpoint | ||
|---|---|---|---|---|
| SCA (n = 22,17%) | NSCD (n = 18,14%) | Survivors (n = 87, 69%) | ||
| Clinical parameters | ||||
| Age (years) | 66.6 ± 12.0 | 65 ± 8 | 73 ± 10* | 66 ± 13 |
| Sex (male) | 117 (92%) | 22 (100%) | 17(94%) | 78 (90%) |
| LV ejection fraction (%) | 28±9 | 26 ± 8 | 25 ± 11 | 29 ± 9 |
| LVEDV index (ml/m2) | 90 ± 31 | 114±20*** | 96 ± 34 | 82 ± 29 |
| Total D-M (% of LV) | 27 ± 12 | 32 ± 10* | 27 ± 13 | 0.26 ± 0.11 |
| Viable D-M (%of LV) | 7±5 | 9±5 | 8±6 | 0.07 ± 0.05 |
| Infarcted myocardium (% of LV) | 20 ± 9 | 21 ± 7 | 19±8 | 0.21 ± 0.10 |
| ECG parameters | ||||
| Average heart rate (bpm) | 71 ± 10 | 74 ± 11 | 68 ± 7 | 71 ± 10 |
| SDNN (ms) | 45.6 ± 22.1 | 48 ± 22 | 47 ± 32 | 45 ± 20 |
| RMSSD (ms) | 64.4 ± 47.2 | 83 ± 42** | 79 ± 64 | 57 ± 43 |
| LF power (nu) | 25.8 ± 5.8 | 23 ± 5** | 23 ± 6* | 27 ± 5 |
| HF power (nu) | 38.8 ± 7.2 | 40 ± 7 | 40 ± 8 | 38 ± 7 |
| HRT onset (%) | 0.00 ± 0.02 | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.02 |
| HRT slope (ms) | 3.1 ± 3.6 | 2.2 ± 2.0 | 2.8 ± 4.6 | 3.5 ± 3.5 |
| AC (ms) | −6.9 ± 4.0 | −6.2 ± 2.7 | −6.6 ± 2.6 | −6.3 ± 2.5 |
| DC (ms) | 3.0 ± 3.3 | 1.7 ± 2.5** | 1.7 ± 3.5* | 3.7 ± 2.6 |
Values are mean ± standard deviation (SD), or n (%).
SCA: Sudden Cardiac Arrest, D-M: Denervated Myocardium, LV: left ventricle, LVEDV: left ventricle end-diastolic volume SDNN: standard deviation of normal-to-normal RR intervals, RMSSD: root mean square of the standard deviation of RR intervals, LF: low frequency, HF: high frequency, HRT: heart rate turbulence, AC: acceleration capacity, DC: deceleration capacity.
p < 0.05 against survivors using post-hoc Tukey test.
p < 0.01 against survivors using post-hoc Tukey test.
p < 0.001 against survivors using post-hoc Tukey test.
Table 2 shows the univariate and multivariate predictors of time-to-event data. At the univariate level, left ventricular end-diastolic volume (LVEDV), regional D-M, RMSSD, LF power, and DC were associated with time-to-SCA; while age, LF power, and DC were associated with time-to-NSCD. At the multivariate level, LVEDV, regional D-M, and LF power predicted time-to-SCA; and only age and LF power predicted time-to-NSCD.
Table 2.
Bivariate and multivariate Cox-regression of time-to-event data.
| Predictors | Time-to-SCA | Time-to-NSCD | ||
|---|---|---|---|---|
| Bivariate | Multivariate¥ | Bivariate | Multivariate£ | |
| Clinical parameters | ||||
| Age (per 10 years) | NS | - | p = 0.013 | p = 0.02 |
| Sex (male) | NS | - | NS | - |
| LV ejection fraction (%) | NS | - | NS | - |
| LVEDV index (per 10 ml/m2) | p = 0.001 | p = 0.001 | NS | - |
| Total D-M (per 1% ofLV) | p = 0.018 | p = 0.05 | NS | - |
| Viable D-M (% ofLV) | NS | - | NS | - |
| Infarcted myocardium (% of LV) | NS | - | NS | - |
| ECG parameters | ||||
| Average heart rate (bpm) | NS | - | NS | |
| SDNN (ms) | NS | - | NS | - |
| RMSSD (ms) | p = 0.022 | p = 0.07 | NS | - |
| LF power (nu) | p = 0.001 | p = 0.03 | p = 0.003 | p = 0.02 |
| HF power (nu) | NS | - | NS | - |
| HRT onset (%) | NS | - | NS | - |
| HRT slope (ms) | NS | - | NS | - |
| AC (ms) | NS | - | NS | - |
| DC (ms) | p = 0.001 | NS | p = 0.001 | NS |
SCA: sudden cardiac arrest, NSCD: nonsudden cardiac death; D-M: denervated myocardium, LV: left ventricle, LVEDV: left ventricle end-diastolic volume SDNN: standard deviation of normal-to-normal RR intervals, RMSSD: root mean square of the standard deviation of RR intervals, LF: low frequency, HF: high frequency, HRT: heart rate turbulence, AC: acceleration capacity, DC: deceleration capacity.
Bold indicates the variable remained significant in the final multivariate model.
Goodness-of-fit Chi-square = 6.121, p = 0.1903.
Goodness-of-fit Chi-square = 5.024, p = 0.2848.
Table 3 shows the results of the competing risk analysis for predicting cause-specific mortality from SCA versus NSCD. In this competing risk model, we used variables significant at p <0.10 at the multivariate Cox-regression. Overall, we found that LVEDV and regional D-M were preferentially associated with SCA, while age and LF power were preferentially associated with NSCD. For each 1% increase in LF power, the risk of NSCD decreased by 8%. Fig. 1 shows the Kaplan-Meier events probability curves of NSCD for tertiles of LF power in this sample.
Table 3.
Competing risk model for SCA versus NSCD.
| Variable | SCA | NSCD | ||
|---|---|---|---|---|
| Sub-hazard ratio [95% CI] | p value | Sub-hazard ratio [95% CI] | p value | |
| Age (per 10 years) | - | - | 1.82 [1.05–7.70] | 0.034 |
| LVEDV (per 10 ml/m2) | 1.29 [1.14–1.45] | <0.001 | - | |
| Denervated myocardium (per 1%) | 1.03 [1.00–1.07] | 0.05 | - | |
| RMSSD | NS | 0.108 | - | |
| Low-frequency power (per 1%) | NS | 0.107 | 0.92 [0.85–0.98] | 0.019 |
SCA: sudden cardiac arrest, NSCD: nonsudden cardiac death; LVEDV: left ventricle end-diastolic volume RMSSD: root mean square of the standard deviation of RR intervals.
Bold indicates the variable remained significant in the final multivariate model.
Fig. 1.
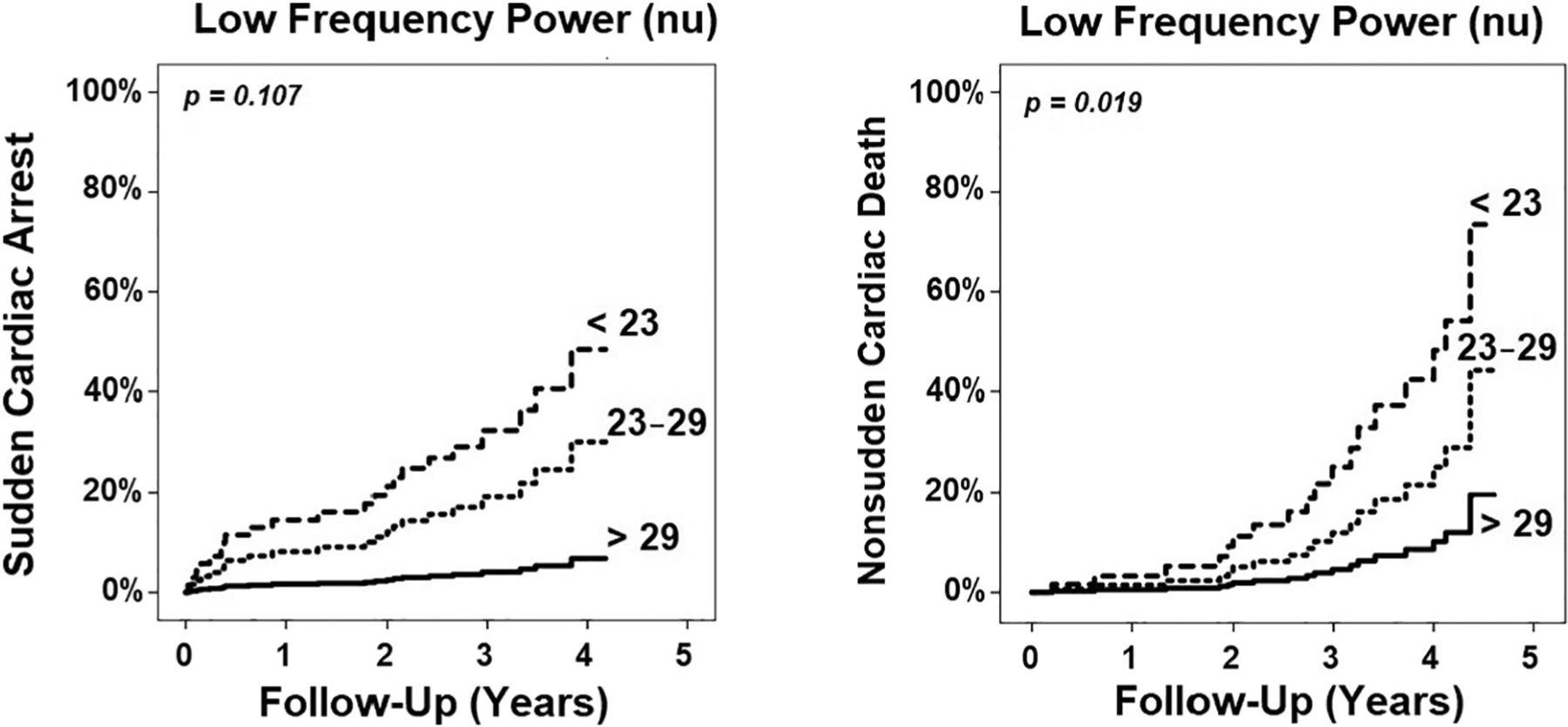
Kaplan-Meier events probability curve. This figure illustrates that tertiles of LF power are preferentially associated with non-sudden cardiac death but not sudden cardiac arrest (p values based on competing risk analysis). Those with depressed LF power have the highest rate of events during follow up period.
Finally, to better understand the relationship between total D-M and autonomic dysfunction by means of HRV, we explored whether a model of autonomic markers could adequately estimate the amount of D-M (Table 4). We found that HRV correlates with viable, but not infarcted, myocardium, with LF/HF power components being the only independent correlates of D-M.
Table 4.
Exploratory analysis of the relationship between denervated myocardium and autonomic dysfunction.
| Predictors | Viable denervated myocardium | Infarcted myocardium | ||
|---|---|---|---|---|
| Bivariate | Multivariate¥ | Bivariate | Multivariate | |
| ECG parameters | ||||
| SDNN (ms) | NS | - | NS | - |
| RMSSD (ms) | p = 0.011 | NS | NS | - |
| LF power (nu) | p = 0.033 | p = 0.008 | NS | - |
| HF power (nu) | p = 0.100 | p = 0.010 | NS | - |
| HRT onset (%) | p = 0.097 | NS | NS | - |
| HRT slope (ms) | NS | - | NS | - |
| AC (ms) | NS | - | p = 0.065 | NS |
| DC (ms) | p = 0.091 | NS | NS | - |
SDNN: standard deviation of normal-to-normal RR intervals, RMSSD: root mean square of the standard deviation of RR intervals, LF: low frequency, HF: high frequency, HRT: heart rate turbulence, AC: acceleration capacity, DC: deceleration capacity.
Bold indicates the variable remained significant in the final multivariate model.
Simple linear regression model: r = 0.296, R2 = 0.09; F = 5.394, p = 0.006.
Discussion
In this study, we evaluated whether ECG indices of global autonomic function predict risk of SCA to a similar degree as regional D-M. Despite the limitations induced by our small sample size, we found that LF power was the only HRV parameter in multivariate analysis to predict time-to-SCA. This finding suggests that LF power possesses some prognostic information not covered by total D-M; especially given that power analysis of HRV could only explain 9% of the variability observed in viable D-M in this study. However, in competing risk analysis, reduced LF power was preferentially associated with NSCD rather than SCA. This is the first study to evaluate the role of ECG indices of global autonomic function in predicting cause-specific mortality in patients at very high-risk of SCA.
Cause-specific mortality from SCA
Despite controversy in the literature, the prognostic value of HRV has been repeatedly emphasized in heart failure patients. Nolan et al. [21] evaluated time-domain HRV parameters in 433 heart failure patients and found that SDNN was the most powerful predictor of the risk of death due to progressive heart failure. La Rovere et al. [22] evaluated time- and frequency-domain HRV parameters in 444 heart failure patients and found that LF power was an independent predictor of SCA. Similar results were also obtained by Galinier et al. [23] who found that LF is an independent predictor of SCA in 190 heart failure patients. Yet, Tamaki et al. [24] evaluated time- and frequency-domain HRV parameters in 106 heart failure patients, but found none to be predictive of SCA.
These studies have one limitation in common; they either did not evaluate both SCA and NSCD endpoints in the same study, or they did not simultaneously evaluate both endpoints in a competing risk model. Competing risk analysis accounts for participants who experience one outcome during follow up period before they reach a study endpoint. Using this approach is critical to accurately determine level of risk to best inform clinical decision-making [20]. In our study, we found that LF power is preferentially associated with NSCD rather than SCA in heart failure patients. This finding is interesting and suggests that simple frequency-domain HRV analysis can help target specific therapies to those at the greatest risk of non-arrhythmic heart failure death.
Clinical significance of LF power
The sympathetic and parasympathetic systems work in an opposing yet complementary fashion. The interaction between both systems is complex, ranging from centrally-mediated baroreceptors to local neuronal interactions [25]. While sympathetic activation is known to be proarrhythmic, parasympathetic activation is not. The LF oscillation signal in heart rate behavior was historically thought to represent the cardiac sympathetic tone, which explains the association between reduced LF power and SCA in some previous studies [22,23]. However, recent literature suggests that LF power is not a measure of cardiac sympathetic tone but rather a measure of parasympathetic tone and baroreceptor reflex function [26,27]. This new paradigm for understanding the LF power component of HRV can explain two interesting observations in the current study. First, LF power was preferentially associated with NSCD rather than SCA. This can be explained by the well-known relationship between baroreceptor-heart rate reflex sensitivity and the severity of heart failure progression (NYHA class) [28]. Second, the majority (96%) of patients in this study were on β-blockers, which are known to blunt the sympathetic function and limit the value of HRV parameters, yet, LF power remained a significant prognostic variable in predicting mortality in this study. This can be explained by the fact that baroreceptor-heart rate reflex sensitivity possesses a prognostic value independent from the modification of sympathetic dysfunction brought about by β-blockers [29].
This study has some important clinical implications. Depressed LF power remains a strong predictor of NSCD in our current beta blockade era. Given that depressed LF power could indicate an impaired vagal reflex, then it follows that increasing the vagal activity would have a protective effect against cardiovascular death beyond that achieved by the mere slowing of heart rate using β-blockers [28]. Any additional sympathetic inhibition produced by vagal stimulation would add an incremental protective effect against cardiovascular death [30].
Although the direct electrical stimulation of the vagal nerve was thought to improve baroreceptor sensitivity, recent findings from the INNOVATE-HF trial (Increase of Vagal Tone in Heart Failure) have shown that vagal nerve stimulation does not reduce the rate of death or heart failure events [31]. Alternatively, exercise training has been shown to reduce 10-year cardiovascular mortality, provided that it is associated with a clear shift of the autonomic balance toward an increase in baroreceptor sensitivity [32]. This means that exercise training remains the only clinically plausible mechanism to increase vagal activity in heart failure.
Limitations
This paper has few limitations. First, HRV cannot be analyzed in patients with pacing and atrial fibrillation which are prevalent in ischemic cardiomyopathy [33]. As a result, we excluded nearly one third of subjects in the original PAREPET cohort (n = 63/204, 31%). While this could introduce selection bias into the findings reported in this paper, the prior multivariate parameters we identified for predicting SCA including volume of denervated myocardium and left ventricular end diastolic volume were retained in the competing risks analysis. Second, our sample size was very small. Out of the 127 patients included in this study, only 22 reached the primary endpoint. As such, our results should be viewed as suggestive rather than conclusive. Finally, this analysis was based on patients with chronic heart failure due to ischemic cardiomyopathy, so our findings may not apply to those with non-ischemic cardiomyopathy.
Conclusions
This study demonstrates that depressed LF power of HRV is preferentially associated with NSCD in ischemic cardiomyopathy. Depressed LF power might indicate impaired vagal reflex, which suggests that increasing vagal tone in these patients would have a protective effect against cardiovascular death beyond that achieved by the mere slowing of heart rate using β-blockers.
Funding sources
Supported by the National Institutes of Health (grants K23 NR-009716 — MGC; R01 HL-076252 — JMC & JAF). The authors have no relationships whatsoever with any interests of any kind in business or industry related to the planning, execution, and/or publication of this study.
References
- [1].Goldberger JJ, Cain ME, Hohnloser SH, et al. AHA/ACC/HRS scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation 2008;118:1497–518. [PubMed] [Google Scholar]
- [2].Fallavollita JA, Heavey BM, Luisi AJ Jr, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 2014;63:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol 2007;50:1150–7. [DOI] [PubMed] [Google Scholar]
- [4].Anderson KP. Sudden cardiac death unresponsive to implantable defibrillator therapy: an urgent target for clinicians, industry and government. J Interv Card Electrophysiol 2005;14:71–8. [DOI] [PubMed] [Google Scholar]
- [5].Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- [6].Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society workshop. Circulation 2010;122:2335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bauer A, Kantelhardt JW, Barthel P, et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet 2006;367: 1674–81. [DOI] [PubMed] [Google Scholar]
- [8].Lombardi F, Mäkikallio TH, Myerburg RJ, Huikuri HV. Sudden cardiac death: role of heart rate variability to identify patients at risk. Cardiovasc Res 2001;50:210–7. [DOI] [PubMed] [Google Scholar]
- [9].Cygankiewicz I, Zareba W, Vazquez R, et al. Risk stratification of mortality in patients with heart failure and left ventricular ejection fraction N35%. Am J Cardiol 2009;103: 1003–10. [DOI] [PubMed] [Google Scholar]
- [10].Al-Zaiti SS, Fallavollita JA, Canty JM Jr, Carey MG. Electrocardiographic predictors of sudden and non-sudden cardiac death in patients with ischemic cardiomyopathy. Heart Lung 2014;43:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fallavollita JA, Dare JD, Carter RL, Baldwa S, Canty JM Jr. Denervated myocardium is preferentially associated with sudden cardiac arrest in ischemic cardiomyopathy: a pilot competing risks analysis of cause-specific mortality. Circ Cardiovasc Imaging 2017;10:e006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fallavollita JA, Luisi AJ Jr, Michalek SM, et al. Prediction of ARrhythmic Events with Positron Emission Tomography: PAREPET study design and methods. Contemp Clin Trials 2006;27:374–88. [DOI] [PubMed] [Google Scholar]
- [13].Hinkle LE, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65: 457–64. [DOI] [PubMed] [Google Scholar]
- [14].Buxton AE, Lee KL, Dicarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med 2000;342: 1937–45. [DOI] [PubMed] [Google Scholar]
- [15].Daubert J, Wilber D, Lin A, et al. ICD therapy for fast ventricular tachycardia or ventricular fibrillation is a surrogate endpoint for mortality in MADIT II. Circulation 2003;108:385. [Google Scholar]
- [16].Sedláček K, Ruwald A-C, Kutyifa V, et al. The effect of ICD programming on inappropriate and appropriate ICD therapies in ischemic and nonischemic cardiomyopathy: the MADIT-RIT trial. J Cardiovasc Electrophysiol 2015;26:424–33. [DOI] [PubMed] [Google Scholar]
- [17].Malik M, Bigger JT, Camm AJ, et al. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996;17:354–81. [PubMed] [Google Scholar]
- [18].Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999; 353:1390–6. [DOI] [PubMed] [Google Scholar]
- [19].O’Quigley J, Moreau T. Cox’s regression model: computing a goodness of fit statistic. Comput Methods Programs Biomed 1986;22:253–6. [DOI] [PubMed] [Google Scholar]
- [20].Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 2010;58:783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK-HEART). Circulation 1998;98:1510–6. [DOI] [PubMed] [Google Scholar]
- [22].La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003; 107:565–70. [DOI] [PubMed] [Google Scholar]
- [23].Galinier M, Pathak A, Fourcade J, et al. Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J 2000;21:475–82. [DOI] [PubMed] [Google Scholar]
- [24].Tamaki S, Yamada T, Okuyama Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction. Results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol 2009;53:426–35. [DOI] [PubMed] [Google Scholar]
- [25].Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 2008;50:404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goldstein DS, Bentho O, Park M-Y, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 2011;96: 1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reyes del Paso GA, Langewitz W, Mulder LJM, Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 2013;50: 477–87. [DOI] [PubMed] [Google Scholar]
- [28].Schwartz PJ, De Ferrari GM. Sympathetic–parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev 2011;16:101–7. [DOI] [PubMed] [Google Scholar]
- [29].La Rovere MT, Pinna GD, Maestri R, et al. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol 2009;53: 193–9. [DOI] [PubMed] [Google Scholar]
- [30].Schwartz PJ, Pagani M, Lombardi F, Malliani A, Brown AM. A cardiocardiac sympathovagal reflex in the cat. Circ Res 1973;32:215–20. [DOI] [PubMed] [Google Scholar]
- [31].Gold MR, Van Veldhuisen DJ, Hauptman PJ, et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol 2016;68:149–58. [DOI] [PubMed] [Google Scholar]
- [32].La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 2002;106:945–9. [DOI] [PubMed] [Google Scholar]
- [33].Carey MG, Al-Zaiti SS, Canty JM, Fallavollita JA. High-risk electrocardiographic parameters are ubiquitous in patients with ischemic cardiomyopathy. Ann Noninvasive Electrocardiol 2012;17:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


