Abstract
Aims
To evaluate whether subjects with diabetes hospitalized for Coronavirus disease-19 (Covid-19) represent a subgroup of patients with high-risk clinical features compared to patients with diabetes without Covid-19.
Methods
In this case-control study 79 patients with type 2 diabetes out of 354 adults hospitalized for Covid-19 and 158 controls with type 2 diabetes but without Covid-19, matched for age and gender, were enrolled. Medical history and concomitant therapies were retrieved from medical charts and compared between cases and controls, controlling for confounders.
Results
Fully-adjusted multivariate logistic regression model showed that previous CVD history did not differ between patients with and without Covid-19 (odds ratio 1.40, 95% confidence interval [CI]: 0.59–3.32, p = 0.45). A higher prevalence of chronic obstructive pulmonary disease (COPD) (OR 3.72, 95%CI: 1.42–9.72, p = 0.007) and of chronic kidney disease (CKD) (OR 3.08, 95%CI: 1.18–8.06, p = 0.022) and a lower prevalence of ever smokers (OR 0.30, 95%CI: 0.13–0.67, p = 0.003), of users of lipid lowering agents (OR 0.26, 95%CI: 0.12–0.54, p < 0.001), and of anti-hypertensive drugs (OR 0.39, 95%CI: 0.16–0.93, p = 0.033) were found among cases.
Conclusions
CVD prevalence does not differ between people with diabetes with and without Covid-19 requiring hospitalization. An increased prevalence of COPD and of CKD in Covid-19 patients with type 2 diabetes is suggested. These findings aid to clarify the relationship between underlying conditions and SARS-CoV-2 infection in the high-risk group of patients with diabetes.
Keywords: Covid-19, Diabetes, SARS-CoV-2
1. Introduction
At the end of 2019 the beta-coronavirus SARS-CoV-2 has spread in Wuhan, China, causing coronavirus disease 2019 (Covid-19). The infection outbreak has rapidly reached the dimension of a pandemic and thousands of people are dying around the word [1]. Elderly with underlying conditions, especially cardiometabolic comorbidities, seems more vulnerable to Covid-19. Age > 65 years, hypertension, diabetes and history of cardiovascular events are the most prevalent conditions among patients hospitalized for Covid-19 [2], [3], [4], [5], and they are even more frequently described among those patients requiring intensive care or dying for this disease, irrespective of the geographical variation in both Covid-19 and comorbidities prevalence [6], [7], [8]. The proinflammatory and hypercoagulable states characterizing these conditions have been hypothesized to contribute to the deadly interaction between cardiometabolic disorders and SARS-CoV-2 infection [9], [10], [11]. However, it is not known if the coexistence of comorbidities potentially acting on similar pathways to increase patients’ vulnerability to Covid-19, such as diabetes and cardiovascular disease, results in a stepwise increased risk. Indeed, while it has been shown that both diabetes and cardiovascular disease may exacerbate Covid-19 in the general population [12], [13], [14], whether and at what extent the presence of overt cardiovascular disease or of other underlying conditions provide an incremental risk for Covid-19 in patients with type 2 diabetes is unknown.
We hypothesized that patients with type 2 diabetes hospitalized for Covid-19 represent a group of patients with high risk clinical features, such as history of cardiovascular events or presence of other relevant comorbidities.
Therefore, in order to identify, quantify and further explore the risk profile of patients with type 2 diabetes hospitalized for Covid-19, we compared their clinical features to those of patients with type 2 diabetes without signs or symptoms of SARS-CoV-2 infection.
2. Materials and methods
2.1. Study design and population
The Covid-19 & Diabetes (CoViDiab) Study is a collaborative research project involving 4 different Covid academic centers in Rome (Umberto I “Policlinico” General hospital, Campus Bio-Medico hospital, Sant’Andrea hospital) and Latina (Santa Maria Goretti hospital), aimed to evaluate Covid-19 features and progression in people with diabetes. The present case-control study was designed to primarily test whether the prevalence of cardiovascular disease, and secondarily of other underlying conditions, differ between patients with type 2 diabetes hospitalized for Covid-19 (cases), compared to patients with type 2 diabetes without signs or symptoms of SARS-CoV-2 infection (controls). Cases were identified among adult patients (>18 years old) hospitalized for Covid-19 in one of the four study centers and enrolled in the CoViDiab Study no later than May 15th, 2020. Controls matched for age and sex with cases were identified among patients with type 2 diabetes referring to the Diabetes Unit of Policlinico Umberto I General hospital and enrolled at a case-control ratio of 1:2. Briefly, medical charts of patients aged > 18 years and with a diagnosis of type 2 diabetes attending the clinic up to May 15th, 2020 were screened backwards and data were retrieved from patients fulfilling the inclusion/exclusion criteria, according to the matching strategy fully described in the Supplementary material. Inclusion criteria for this study were: being aged > 18 years old and having a diagnosis of type 2 diabetes, defined as at least one random blood glucose value > 200 mg/dl, or fasting blood glucose > 126 mg/dl, or HbA1c > 6.5% (48 mmol/mol), or self-reported history of diabetes. A diagnosis of type 1 diabetes or of monogenic diabetes were considered exclusion criteria. All controls were screened for signs and symptoms of SARS-CoV-2 infection and patients hospitalized for any respiratory infection or with any of the following signs or symptoms experienced in the 30 days before enrolment were excluded: fever, cough, chill, chest tightness, worsening dispnoea, conjunctivitis, nausea, vomiting, diarrhea.
2.2. Data collection strategy
The following data were retrieved from medical records of both cases and controls: age, gender, smoking habits, height, weight, body mass index (calculated as weight in kilograms divided by the square of height in meters), serum creatinine, concomitant lipid lowering and anti-hypertensive therapies, previous history of major adverse cardiovascular events (MACE: any among myocardial infarction, stroke, percutaneous coronary revascularization, coronary artery by-pass graft), of heart failure, of chronic obstructive pulmonary disease (COPD). Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease (CKD) Epidemiology Collaboration formula [15] and the percentage of participants in CKD stage IIIb (eGFR < 45 ml/min/1.73 m2) was calculated. Data retrieved for cases are those collected at the time of hospitalization, while data retrieved for controls are those collected during the last follow-up visit attended at the outpatient diabetes clinic.
Data about anti-diabetes therapeutic regimens were retrieved from the web-based reimbursement system of Lazio region (WebCare Lazio) and therefore reflect the therapeutic regimens as reported in the system: euglycemic agents (EuGlA: metformin, dipeptidyl peptidase 4 inhibitors [DPP4i], glucagon-like peptide 1 receptor agonists [GLP1RA], sodium-glucose co-transporter 2 inhibitors [SGLT2i] and/or pioglitazone); oral hypoglycemic agents (OHA: sulphonylureas or glinides); basal insulin (alone or in combination with EuGlA or OHA); multiple daily insulin injections (MDI: ≥ 3 insulin injections per day). Data about the use of each specific anti-diabetes drug class were available for all controls and for a subsample of cases (n = 61).
2.3. Statistical analysis
Continuous variables are presented as median [25th-75th percentile] and categorical variables as number (%). Kruskal-Wallis test was used to evaluate differences in continuous variables between groups. Chi-squared test or Fisher exact test were used to test differences of the distribution of categorical variables among groups.
Logistic regression was used for multivariate analyses with hospitalization for symptomatic Covid-19 as dichotomous outcome and positive history of MACE as main exposure. Based on the literature showing a significant association between hypertension and Covid-19 [5], [6], we pre-specified use of anti-hypertensive drugs as confounder to be forced in the model irrespective of its level of association with the outcome. Further variables found to differ between subjects with and without Covid-19 at a nominal p-value < 0.1 were also tested in the multivariate logistic regression model with the main exposure and the outcome. Results of the logistic regression models are expressed as odds ratio (OR) with 95% confidence intervals (CI). Since eGFR values were missing for 26 cases and for 3 controls, predictive mean matching imputation was used to impute CKD categories before being tested in the model.
The sample size of this study provided > 80% power to detect a hypothesized 2-times higher prevalence of MACE among Covid-19 cases compared to controls at a two-sided alpha level of 0.05. Two-sided tests at the 0.05 level of significance were used for all statistical comparisons. Stata/IC 12.1 software used for data analysis and Prism 8.4 Software for graphical representations.
2.4. Ethics
The study complies with the principle of Helsinki Declaration and was approved by the Ethical Committee of Umberto I “Policlinico” General hospital. Because of the study’s retrospective design, verbal informed consent was obtained from participants who were not able to reach the study center due to the lockdown restrictions, while informed consent was waived in cases of impossibility of contact with patients and in case of exitus. The privacy and anonymity of the data collected was guaranteed in accordance with current regulations.
3. Results
Medical charts of 354 adults enrolled in the CoViDiab study and with known diabetes status were screened. After exclusion of 2 patients with type 1 diabetes, 79 patients with type 2 diabetes hospitalized for Covid-19 were enrolled. According to the prespecified enrolment plan and matching strategy (Supplementary material), 158 controls matched for age and sex with the 79 Covid-19 cases were enrolled.
When this analysis was carried out, 9 out of the 79 cases (11.4%) died 20 [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24] days after hospitalization, 38 (48.1%) were discharged after 23 [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32] days and 32 (40.5%) were still hospitalized. Admission to an intensive care unit was required in 21 cases (26.6%).
Features of cases and controls are summarized in table 1 . Cases and controls had comparable age (76 [66–83] vs 74 [65–82] years, p = 0.88), gender distribution (63.9% vs 63.3% males, p = 0.92) and body mass index. Cases were less likely ever smokers than controls (22.8% vs 45.6%, p = 0.001.)
Table 1.
Clinical features of patients with diabetes with and without Covid-19.
| Without Covid-19 n = 158 |
With Covid-19 n = 79 |
p-value | |
|---|---|---|---|
| Age, years | 74 [65–82] | 76 [66–83] | 0.88 |
| Gender | 0.92 | ||
| - Males | 101 (63.9%) | 50 (63.3%) | |
| - Females | 57 (36.1%) | 29 (36.7%) | |
| Smoke | 0.001 | ||
| - Never | 86 (54.4%) | 61 (77.2%) | |
| - Ever | 72 (45.6%) | 18 (22.8%) | |
| - Ex | 37 (23.4%) | 17 (21.5%) | |
| - Current | 35 (22.2%) | 1 (1.3%) | |
| Body mass index, Kg/m2 | 28.0 [24.6–31.2] | 26.5 [24.8–29.4] | 0.24 |
| eGFR < 45 ml/min/1.73 m2, n (%)* | 18 (11.6%) | 20 (37.4%) | <0.001 |
| Chronic obstructive pulmonary disease, n (%) | 14 (8.9%) | 17 (21.5%) | 0.006 |
| Major adverse cardiovascular events, n (%) | 43 (27.2%) | 13 (16.5%) | 0.066 |
| Heart failure, n (%) | 21 (13.3%) | 10 (12.8%) | 0.92 |
| Anti-hypertensive drugs, n (%) | 134 (84.8%) | 54 (68.4%) | 0.003 |
| - ACEi | 47 (29.8%) | 19 (24.0%) | 0.45 |
| - ARB | 57 (36.1%) | 14 (17.7%) | 0.006 |
| Lipid lowering agents, n (%) | 121 (76.6%) | 29 (36.7%) | <0.001 |
| Anti-diabetes therapeutic regimen** | <0.001 | ||
| - Diet alone, n (%) | 6 (3.8%) | 8 (10.8%) | |
| - EuGlA, n (%) | 84 (53.2%) | 32 (43.2%) | |
| - OHA, alone or in combination with EuGlA, n (%) | 0 (0.0%) | 10 (13.5%) | |
| - Basal insulin, alone or in combination with EuGlA or OHA, n (%) | 47 (29.8%) | 5 (6.8%) | |
| - MDI, n (%) | 21 (13.3%) | 19 (25.7%) |
Abbreviations: eGFR, estimated Glomerular Filtration Rate, ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; EuGlA, Euglycemic agents (metformin, DPP4i, GLP1RA, SGLT2i and/or pioglitazone); OHA, oral hypoglycemic agents (sulphonylureas or glinides); MDI, multiple daily insulin injections. *CKD data available for 155 controls and for 53 controls; **Data available for 74 cases and for all controls.
Previous history of MACE was present in 13 (16.5%) cases and in 43 (27.2%) controls (p = 0.066), COPD in 17 (21.5%) cases and 14 (8.9%) controls (p = 0.006) and heart failure in 10 (12.8%) cases and 21 (13.3%) controls (p = 0.92). Among participants with available eGFR data, CKD stage IIIb was present in 37.7% cases and in 11.6% controls (p < 0.001).
Anti-hypertensive agents, lipid lowering drugs, EuGlA and basal insulin alone or in combination were more frequently used in controls than in cases, while OHA and MDI were more frequently observed among Covid-19 cases (p ≤ 0.01 for all, table 1). When considering the single anti-diabetes drug-classes, a less frequent use of all EuGlA was confirmed in the subsample of 61 Covid-19 cases with available data compared to controls (supplementary table S1).
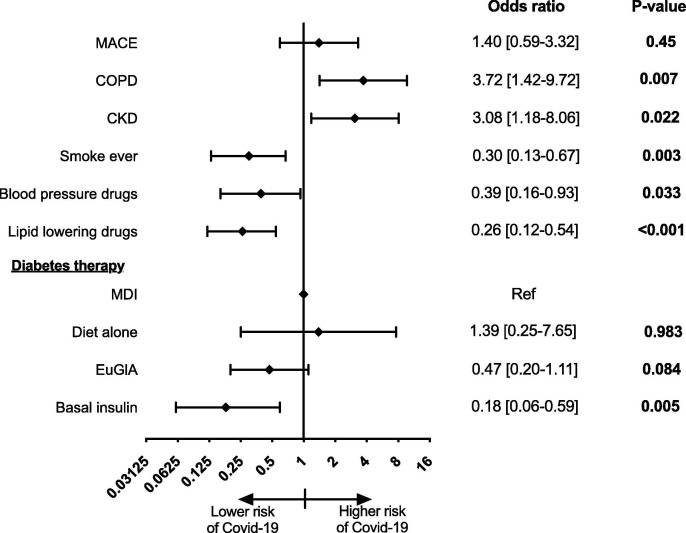
The multivariate regression model showed no differences in previous history of MACE between cases and controls (OR 1.40, 95%CI: 0.59–3.32, p = 0.45), while COPD (OR 3.72, 95%CI: 1.42–9.72, p = 0.007), CKD stage IIIb (OR 3.08, 95%CI: 1.18–8.06, p = 0.022), lower prevalence of smoking habits (OR 0.30, 95%CI: 0.13–0.67, p = 0.003) and less frequent use of lipid lowering agents (OR 0.26, 95%CI: 0.12–0.54, p < 0.001), of anti-hypertensive drugs (OR 0.39, 95%CI: 0.16–0.93, p = 0.033), and of basal insulin (OR 0.18, 95%CI: 0.06–0.59, p = 0.005), all remained significantly associated with Covid-19 (Fig. 1 ).
Fig. 1.
Factors associated to Covid-19 in diabetes. This forest plot is a graphical representation of the final multivariate logistic regression model with main effect (previous history of major adverse cardiovascular events, MACE) and the outcome (evidence of symptomatic Covid-19 infection requiring hospitalization), adjusted for factors associated with the outcome at the bivariate analysis at a p-value < 0.1. Oral hypoglycemic agents (sulphonylureas or glinides) not represented because perfectly predict the outcome.
4. Discussion
This study shows that previous history of cardiovascular events is similarly prevalent among patients with type 2 diabetes with and without Covid-19. However, we found that, after adjustment for confounders, patients with type 2 diabetes and hospitalized for Covid-19 were more likely affected by COPD and less likely on lipid lowering treatment and smokers compared to controls. Furthermore, EuGlA and basal inulin (alone or in combination) were more frequently used in controls than in cases.
Recently, two large meta-analyses showed an increased risk of hospitalization after infection with SARS-CoV-2 in patients with cardiovascular disease [13], [14]. The presence of underlying cardiovascular conditions also increases the risk of in-hospital death for Covid-19 [16], further suggesting an intimate relationship between cardiovascular disease and SARS-CoV-2 infection. Differently from previous studies, here we investigated the impact of cardiovascular disease in a population affected by type 2 diabetes, often considered a “coronary heart disease equivalent” [17]. In this population we failed to find differences in the prevalence of cardiovascular disease between patients with and without Covid-19. These results suggest that mechanisms through which type 2 diabetes and cardiovascular disease increase vulnerability to Covid-19 may overlap and are not additive. Although we did not investigate for the impact of cardiovascular disease on Covid-19 progression among inpatients, our data are in line with other studies which did not find independent associations between prior history of cardiovascular events and outcomes among patients with diabetes hospitalized for Covid-19 [18], [19], [20]. This topic needs, however further investigations, being still debated as contrasting data are reported depending on the chosen outcome [20].
On the contrary, our finding of a possible additional risk conferred by COPD, may suggest this condition is associated to SARS-CoV-2 infection through different pathways than type 2 diabetes. The prevalence of COPD in our control population is in line with previous reports showing about 10% of patients with diabetes are also affected by COPD [21]. However, we found a 2-times higher prevalence of COPD among type 2 diabetes patients with Covid-19 requiring hospitalization. Of note, large case series from Asia, Europe and North America showed a relatively low prevalence of COPD in patients hospitalized for Covid-19, ranging from 1.5% in China to 5.4% in New York City [5], [6], [22]. We are instead describing a 20% prevalence of COPD in a selected population with concomitant type 2 diabetes, suggesting that type 2 diabetes may facilitate the interaction between COPD and SARS-CoV-2 infection. In this regard, Interleukin-6 (IL6) is a key cytokine of systemic inflammation in patients with COPD, particularly increased in those with concomitant insulin resistance and metabolic syndrome [23], [24]. Of note, IL6 has emerged as a possible target for anti-Covid-19 therapies [25], [26] and has been described increased in hospitalized patients with Covid-19 and type 2 diabetes [27]. A possible role for IL6 as a mediator of the interaction between type 2 diabetes and COPD in Covid-19 patients may therefore be investigated in future studies.
In our study, the prevalence of patients currently smoking among those with Covid-19 was lower compared to patients without Covid-19. While surprising, this result is consistent with evidence gathered so far from large case series of patients with Covid-19 from several countries [5], [16], [22], [28]. It has been hypothesized that nicotine may mediate the protective effect of smoking by modulating angiotensin converting enzyme 2 (ACE2) or by inhibiting the cytokine storm responsible of the most severe Covid-19 cases [29]. Nevertheless, contrasting results on the impact of smoking in Covid-19 progression and severity have also been described [30], [31].
While a lower use of lipid lowering agents and of anti-hypertensive drugs, and a higher use of MDI and of OHA were found among Covid-19 patients, these results should be interpreted with caution and no cause-effect relationship may be inferred from our study. We acknowledge that these associations may indeed be biased by the fact that controls have been sampled from a population of patients with type 2 diabetes referring to a high-specialty diabetes clinic, with a high compliance to the current standards of care for the management of type 2 diabetes. Nevertheless, in our Covid-19 cohort the percentages of patients using lipid-lowering agents and on MDI are also largely lower and largely higher than the respective percentages registered in the general Italian population with type 2 diabetes [32]. While statin use has been recently associated to lower mortality in Covid-19 [16], this finding may also suggest that the cohort of patients with type 2 diabetes hospitalized for Covid-19 represent a group with sub-optimally managed type 2 diabetes. The higher use of MDI and of OHA, as well as the higher prevalence of CKD, also goes in the same direction, suggesting that patients with type 2 diabetes hospitalized for Covid-19 are more frequently those with advanced diabetes requiring third and fourth line therapies or those not benefitting from the newer anti-diabetes drugs [16]. Unfortunately, HbA1c and diabetes duration data were not available for the majority of Covid-19 cases.
No differences in BMI between cases and controls were found, and BMI was not retained in our final multivariate regression model at the pre-specified nominal p-value < 0.1. Differently from previous reports showing that obesity may worsen Covid-19 outcomes among hospitalized patients [5], [20], our study was aimed to describe the risk profile of patients with type 2 diabetes and Covid-19 and not to describe risk factors for poor Covid-19 prognosis among hospitalized patients. Furthermore, recent evidence suggests that high visceral adiposity, more than high BMI per se, may be associated with Covid-19 progression [33], [34]. Since measures of visceral adiposity were not available in our population, ad-hoc case-control studies in people with type 2 diabetes with and without Covid-19 should be conducted to clarify this topic.
Finally, coherently with the aim of this study to evaluate factors associated with symptomatic Covid-19 requiring hospitalization, we did not enroll patients with SARS-CoV-2 infection not requiring hospitalization. Therefore, based on our data we cannot establish whether the factors we identified are associated to the infection or to the progression of cases towards hospitalization. In this regard, we also cannot exclude asymptomatic infection (not causing Covid-19) among controls.
In conclusion, to the best of our knowledge, this is the first case-control study to compare clinical features of patients with type 2 diabetes with and without Covid-19, showing no difference in positive history of established cardiovascular disease. Conversely, an increased prevalence of COPD and of CKD in Covid-19 patients with type 2 diabetes is suggested. These findings clarify the relationship between different underlying conditions and SARS-CoV-2 infection in the high-risk group of patients with diabetes.
Declaration of Competing Interest
E.M. reports research support from scientific societies with unrestricted grants from Lilly and from AstraZeneca and personal fees from Merck Serono, AstraZeneca, Abbott, PikDare.; C.M. has received speaker fees from AstraZeneca; G.L. has received honoraria from NovoNordisk, Lilly, AstraZeneca, Sanofi; P.P. has received research support from Eli Lilly and Company and serves on the speaker bureau for Sanofi‐Aventis; R.B. has received honoraria or consulting fees from Sanofi, Eli Lilly, Abbott, and AstraZeneca.
Acknowledgments
Funding. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108454.
Appendix A. Investigators of the CoViDiab study group (by study center, in alphabetical order).
Umberto I “Policlinico” General Hospital:
Camilla Ajassa, Rugova Alban, Francesco Alessandri, Federica Alessi, Raissa Aronica, Valeria Belvisi, Raffaella Buzzetti, Matteo Candy, Alessandra Caputi, Anna Carrara, Elena Casali, Eugenio Nelson Cavallari, Giancarlo Ceccarelli, Luigi Celani, Maria Rosa Ciardi, Lucia Coraggio, Ambrogio Curtolo, Claudia D’Agostino, Gabriella D’Ettorre, Luca D’Onofrio, Francesca De Giorgi, Gabriella De Girolamo, Valeria Filippi, Lucio Gnessi, Cecilia Luordi, Ernesto Maddaloni, Claudio Maria Mastroianni, Ivano Mezzaroma, Carmen Mignogna, Chiara Moretti, Francesco Pugliese, Gregorio Recchia, Marco Ridolfi, Francesco Eugenio Romani, Gianluca Russo, Franco Ruberto, Giulia Savelloni, Guido Siccardi, Antonio Siena, Sara Sterpetti, Serena Valeri, Mauro Vera, Lorenzo Volpicelli, Mikiko Watanabe.
Santa Maria Goretti Hospital:
Massimo Aiuti, Giuseppe Campagna, Cosmo Del Borgo, Laura Fondaco, Blerta Kertusha, Frida Leonetti, Gaetano Leto, Miriam Lichtner, Raffaella Marocco, Renato Masala, Paola Zuccalà.
Campus Bio-Medico University:
Felice Eugenio Agrò, Giulia Nonnis, Giuseppe Pascarella, Paolo Pozzilli, Alessandra Rigoli, Alessandro Strumia
Sant’Andrea Hospital:
Daniela Alampi, Monica Rocco.
Appendix B. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020 doi: 10.1111/joim.13091. joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020 doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3321. e33213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020 doi: 10.1055/S-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth.14850. jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8 e35. [PMC free article] [PubMed] [Google Scholar]
- 14.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007621. NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Juutilainen A., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Type 2 diabetes as a “Coronary Heart Disease Equivalent”: An 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901–2907. doi: 10.2337/diacare.28.12.2901. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: A two-center, retrospective study. Diabetes Care. 2020 doi: 10.2337/dc20-0598. dc200598. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y., Yang D., Cheng B. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose- lowering medication. Diabetes Care. 2020:1–9. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 20.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-salameh A. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes : the CORONADO study. Diabetologia. 2020 doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzola M., Rogliani P., Calzetta L., Lauro D., Page C., Matera M.G. Targeting mechanisms linking COPD to type 2 diabetes mellitus. Trends Pharmacol Sci. 2017;38:940–951. doi: 10.1016/j.tips.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J. 2020;2000547 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton C.E., Evans M., Ionescu A.A., Edwards S.M., Morris R.H.K., Dunseath G., et al. Insulin resistance and inflammation—a further systemic complication of COPD. COPD J Chronic Obstr Pulm Dis. 2007;4:121–126. doi: 10.1080/15412550701341053. [DOI] [PubMed] [Google Scholar]
- 24.Watz H., Waschki B., Kirsten A., Müller K.-C., Kretschmar G., Meyer T., et al. The metabolic syndrome in patients with chronic bronchitis and COPD. Chest. 2009;136:1039–1046. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;202005615 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossato M., Russo L., Mazzocut S., Di Vincenzo A., Fioretto P., Vettor R. Current smoking is not associated with COVID-19. Eur Respir J. 2020;2001290 doi: 10.1183/13993003.01290-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farsalinos K., Niaura R., Le Houezec J., Barbouni A., Tsatsakis A., Kouretas D., et al. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Reports. 2020 doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F.R. Smoking links to the severity of Covid-19: An update of a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25967. jmv.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., et al. The impact of COPD and smoking history on the severity of Covid-19: A systemic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osservatorio ARNO. Osservatorio ARNO Diabete Il profilo assistenziale della popolazione con diabete. vol. XXXI. Bologna: 2019.
- 33.Battisti S., Pedone C., Napoli N., Russo E., Agnoletti V., Nigra S.G., et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020 doi: 10.2337/dc20-1333. dc201333. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M., et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111 doi: 10.1016/j.metabol.2020.154319. 154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.