Background: The coronavirus 2019 (COVID-19) pandemic has spread worldwide rapidly. Commonly reported symptoms, such as fever, cough, dyspnea, fatigue, and myalgia, are nonspecific, and the lack of testing in some European countries may make the diagnosis of COVID-19 challenging. However, 2 distinctive symptoms were identified recently: loss of smell and loss of taste (1). These symptoms were not reported extensively in initial studies (2) and might help in the clinical diagnosis of COVID-19.
Objective: To evaluate the prevalence and features of, as well as recovery from, smell dysfunction in European patients with mild to moderate COVID-19.
Methods: From 22 March to 23 April 2020, we identified 2153 consecutive ambulatory and hospitalized patients with positive results on reverse transcriptase polymerase chain reaction (RT-PCR) testing at 18 European hospitals. Patients had mild to moderate COVID-19, defined as an infection not requiring intensive care, and those who were hospitalized were discharged during the study period. Of the 2153 patients, 2013 (93.5%) provided informed consent and participated in the study (Figure), which was approved by the ethics committee at each institution.
Figure.
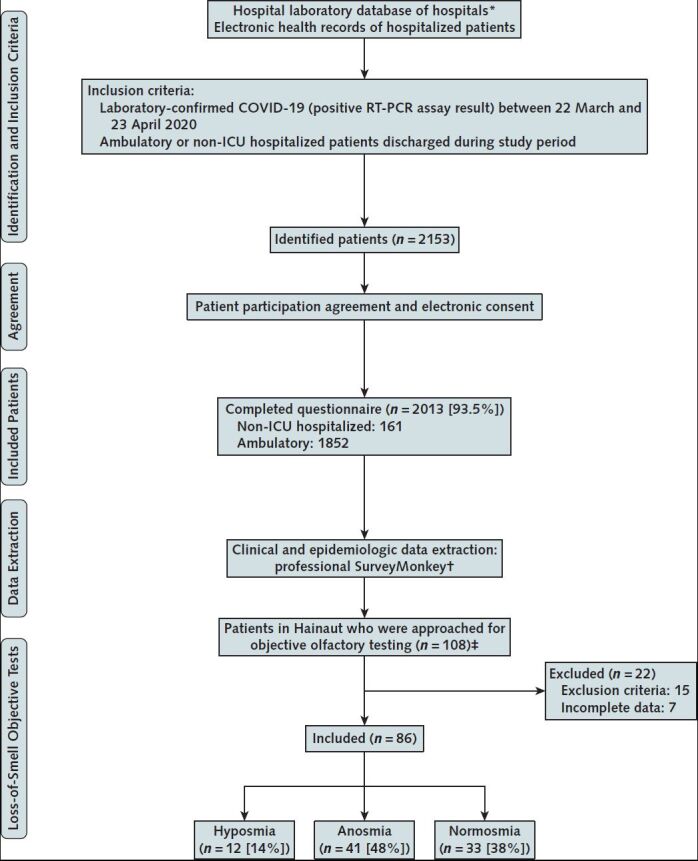
Flow chart of the study.
* The diagnostic tests of ambulatory and hospitalized patients were centralized in hospital laboratories, allowing identification of patients with positive results.
† For hospitalized patients, physicians entered information directly on an online questionnaire; home-managed (quarantined) patients completed the questionnaire at home.
‡ Among the patients with total loss of smell, those who came from Hainaut, Belgium, were contacted to be included in a second study to objectively assess the olfactory disorder. The following exclusion criteria were considered: history of nasal surgery, history of head or neck trauma, history of chronic rhinosinusitis or polyposis, degenerative neurologic disease, and history of chronic loss of smell before the epidemic. Patients who did not respond to the questionnaire were excluded (n = 7).
Using a standardized online questionnaire, we collected clinical and epidemiologic data from hospitalized patients on discharge day and from ambulatory patients after the resolution of key symptoms (such as cough, fever, dyspnea, headache, myalgia, and arthralgia) (Figure). We assessed general and otolaryngologic symptoms believed to be associated with COVID-19 (by using a 4-point scale, from 0 for no symptoms to 4 for severe symptoms). The olfactory and gustatory assessment was based on 8 questions on the smell and taste component of the National Health and Nutrition Examination Survey (3).
Objective evaluations for olfactory dysfunction were performed in a subset of patients who reported total loss of smell at 1 of the study sites (EpiCURA Hospital, Hainaut, Belgium) at the time of the questionnaire. We used a standard olfactory identification test (Sniffin' Sticks [MediSense]) (4), in which we presented 16 scented pens to each patient to smell every 30 seconds. Patients were asked to choose the best term among 4 options to describe the aroma. The test was scored on the basis of a 16-point total. According to the results, patients were classified as normosmic (12 to 16 points), hyposmic (9 to 11 points), or anosmic (<9 points).
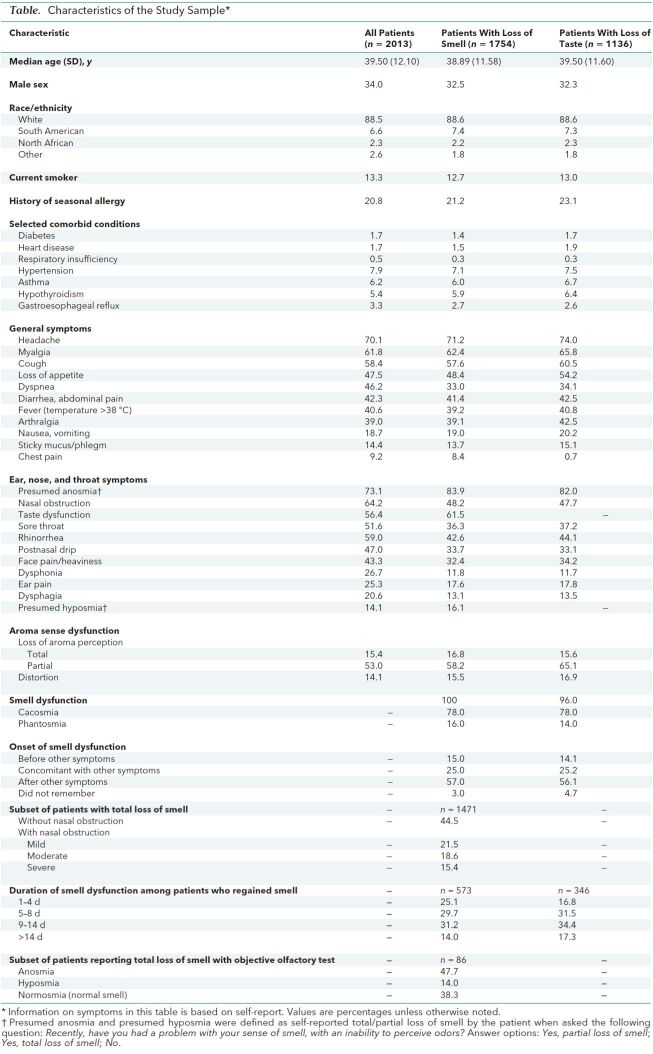
Findings: Of 2013 patients, 161 (8%) were hospitalized (Figure); the remainder were ambulatory patients not previously hospitalized for COVID-19. The Table presents characteristics of our study participants. Loss of smell and headache were the most prevalent symptoms. A total of 1754 patients (87%) reported loss of smell, whereas 1136 (56%) reported taste dysfunction. Mean time from the end of the disease to the evaluation was 7.8 days (SD, 6.8); mean duration of general symptoms (excluding loss of smell and taste) was 11.5 days (SD, 6.0). Most patients had loss of smell after other general and otolaryngologic symptoms (Table). At the time of evaluation, 573 of 1754 patients regained their sense of smell, 60.9% of them between 5 and 14 days after the onset of smell loss; mean duration of olfactory dysfunction was 8.4 days (SD, 5.1). In the subset of 93 patients who were eligible to have an objective olfactory evaluation, 86 (92.5%) completed the assessment; anosmia and hyposmia were confirmed in more than half the patients (Table), but more than a third showed no objective signs of dysfunction.
Table. Characteristics of the Study Sample*†.
Taste disorder, defined as partial or total loss of the 4 taste types—salty, sweet, bitter, and sour (3)—affected 56.4% of patients. Additional aroma disorder (3) occurred in 82.5% of patients. Characteristics of patients with loss of taste and smell are shown in the Table.
Two main categories of patients with total loss of smell were observed: those with and those without nasal obstruction (Table). No significant association was found between loss of smell and the otolaryngologic symptoms of nasal obstruction, rhinorrhea, and postnasal drip in the entire cohort or among subgroups with anosmia, hyposmia, or normosmia.
Discussion: The prevalence of self-reported smell and taste dysfunction in our study is higher than previously reported and may be characterized by different clinical forms. Our results suggest that anosmia may not be related to nasal obstruction or inflammation. Future studies are needed to understand the pathophysiologic mechanisms underlying loss of smell and taste in COVID-19, including potential viral spread through the olfactory neuroepithelium and invasion of the olfactory bulb and central nervous system (5). Our study has limitations. Hospitalized patients were assessed at discharge, which may have biased our assessment of symptom duration. The study population was limited to patients with mild to moderate symptoms. Because patients were asked about taste and smell after they received their diagnosis, they may have been influenced by news reports of smell and taste dysfunction in COVID-19 and overreported these symptoms: Only two thirds of patients reporting olfactory symptoms and who had objective olfactory testing had abnormal results. Nonetheless, these findings highlight the importance of considering loss of smell and taste in the diagnosis of mild to moderate COVID-19.
Biography
Note: Drs. Lechien and Chiesa-Estomba contributed equally to this article and share first authorship.
Acknowledgment: The authors thank the University of Mons and EpiCURA and other centers for the collaboration, as well as the physicians who collected the data.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-2428.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Lechien (e-mail, Jerome.lechien@umons.ac.be).
Corresponding Author: Jerome R. Lechien, MD, PhD, MS, Department of Otorhinolaryngology and Head and Neck Surgery, Foch Hospital, School of Medicine, UFR Simone Veil, Université Versailles Saint-Quentin-en-Yvelines (Paris Saclay University), Paris, France; e-mail, Jerome.Lechien@umons.ac.be
Footnotes
This article was published at Annals.org on 26 May 2020.
References
- 1. doi: 10.1111/joim.13089. Lechien JR, Chiesa-Estomba CM, Place S, et al; COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020. [PMID: 32352202] doi:10.1111/joim.13089. [DOI] [PMC free article] [PubMed]
- 2. doi: 10.1016/j.tmaid.2020.101623. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. [PMID: 32179124] doi:10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed]
- 3. doi: 10.1093/chemse/bjv057. Rawal S, Hoffman HJ, Bainbridge KE, et al. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 2016;41:69-76. [PMID: 26487703] doi:10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed]
- 4. doi: 10.1007/s00405-006-0173-0. Hummel T, Kobal G, Gudziol H, et al. Normative data for the “Sniffin' Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237-43. [PMID: 17021776] [DOI] [PubMed]
- 5. doi: 10.1126/sciadv.abc5801. Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Preprint. Posted online 9 April 2020. bioRxiv 009084. doi:10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed]