Abstract
Stimulant use disorders are both common and associated with suicidal ideation and attempts. The psychometric properties of the 12-item Concise Health Risk Tracking Scale Self-Report (CHRT-SR), a measure that was created to assess suicidal thinking and several factors associated with a propensity to act, has been established in persons with mood disorders. This is a secondary analysis to assess the CHRT-SR in 302 stimulant abusing patients that had participated in a clinical trial.
A confirmatory factor analysis (CFA) was conducted to assess the factor validity of the 12-item CHRT-SR model with a second-order Propensity factor. The CHRT-SR total score and 2 factor scores (Propensity and Suicidal Thoughts) demonstrated acceptable internal consistency and test-retest reliabilities. These two subscales and the total score were modestly but significantly associated with measures of depression and life satisfaction, demonstrating construct validity. Two additional items assessing Impulsivity were also analyzed, and demonstrated acceptable internal consistency, test-retest reliability, and construct validity. The CHRT-SR appears to be a reliable and valid tool to assess suicidality in persons with stimulant use disorder.
Keywords: Stimulant use disorder, Suicidality, CHRT-SR, Suicide risk
1. Introduction
Suicide is a leading public health priority with evidence of an increase in the prevalence of suicide attempts (Olfson et al., 2017). Over the past decade, the National Institute of Health and other agencies have emphasized the importance of suicidal assessment and monitoring. From 2007-2008, in response to a research funding announcement (RFA) from NIH, the Suicide Assessment Methodology Study was conducted, with the primary aim of identifying tools to assess change in symptoms of suicidality in patients with major depressive disorder. The Concise Health Risk Tracking Scale Self-Report (CHRT-SR) was developed as a self-report measure to assess not only suicidal ideation and intent, but also common risk factors associated with suicide, such as pessimism/hopelessness, self-worth, and lack of social support that increase suicidal propensity (Trivedi et al., 2011a,b). As a self-report, it may be a more practical measure of suicidal thoughts and risk factors in large research trials and clinical practice (as opposed to clinician-rated measures).
While there are several suicidality questionnaires and clinician-rated measures, many scales only assess suicidal ideation and past behaviors, and few assess associated symptoms within the same scale. As a self-report, the CHRT-SR may be especially useful for some patients who are likely to be more comfortable disclosing guarded information without being interviewed by the clinician (Trivedi et al., 2011b). The CHRT-SR has demonstrated excellent psychometric properties in samples with major depressive (Ostacher et al., 2015; Trivedi et al., 2011b) and bipolar disorders (Reilly-Harrington et al., 2016;Villegas et al., 2018). The CHRT-SR was originally developed to include 12 items reflecting the following domains: (1) hopelessness, (2) interpersonal attachment/social support, and (3) active suicidal ideation and behavior, with a principal component analysis identifying a 7-item version (Trivedi et al., 2011b). The 7-item and 12-item versions have demonstrated acceptable reliability (Cronbach's coefficient alpha of .78 in adults with major depressive disorder [MDD]; Cronbach's coefficient alpha of > .80 in adults with bipolar disorder and suicidal ideation) (Ostacher et al., 2015; Trivedi et al., 2011b). Two additional items were added to assess impulsivity in a sample of 482 adults with bipolar disorder, and the 14-item CHRT-SR had a Cronbach's coefficient alpha of .88 (Reilly-Harrington et al., 2016). This 14-item CHRT-SR was also recently evaluated in 271 adolescents at high risk for suicidal behaviors. The factor structure was modified to account for these new impulsivity items, with three factors identified: Propensity, Impulsivity, and Suicidal Thoughts (Mayes et al., in press).
The psychometric properties of the CHRT-SR have not been evaluated in other patient populations that are at risk of suicide, such as patients with substance use disorders. This report evaluated the psychometric properties in a convenience sample of adults with stimulant use disorder – participants in the multi-site trial Stimulant Reduction Intervention using Dosed Exercise (STRIDE) implemented through the National Institute on Drug Abuse (NIDA) National Drug Abuse Treatment Clinical Trials Network (CTN) (clinicaltrials.gov identifier NCT01141608). STRIDE compared two interventions (exercise and health education) as treatments for stimulant use disorder delivered initially in residential treatment settings (Trivedi et al., 2011a). Specifically, we aim to replicate the results already reported in prior studies to test the psychometric properties of the scale, including factor validity, internal consistency reliability, test-retest reliability, construct validity, and criterion-related validity of the 12-item CHRT-SR (CHRT-SR12). In addition, because impulsivity has also been shown as a risk factor for suicide attempts (Liu et al., 2017) and has been linked to suicidal ideation in adults with substance use disorders (Rodriguez-Cintas et al., 2017), two additional items to assess impulsivity were added to the CHRT, and will be reported on here as part of the 14-item scale (CHRT-SR14).
2. Methods
2.1. Design
The rationale and design for STRIDE have been previously published (Trivedi, Greer, 2011a). Conducted at nine geographically diverse, residential substance abuse treatment centers across the United States, the study was approved by the Institutional Review Board (IRB) at the University of Texas Southwestern Medical Center and each local IRB. All participants provided written informed consent. For the current study, baseline data included the CHRT-SR12, to which 2 items to assess impulsivity were added. This report focuses on the 12-item version, but we report as well on these additional 2 impulsivity items for the CHRT-SR14. CHRT-SR12 data from the baseline and Week 1 time-point assessment were analyzed to examine the test-retest reliability of the measure.
2.2. Study sample and procedures
The study sample and overall study outcomes have been reported previously (Trivedi et al., 2017). Participants were adult stimulant users (N = 302), aged 18–65, in residential substance abuse treatment. Participants had to meet DSM-IV criteria for stimulant abuse or dependence within the last 12 months, report illicit stimulant drug use within 30 days prior to admission to residential treatment, and be cleared to exercise by a protocol-defined stress test. Participants with opioid dependence, general medical conditions or medications that contraindicated exercise, pregnancy, and psychosis or other psychiatric conditions that posed a safety risk were excluded (Trivedi et al., 2011a).
Eligible participants were randomized (stratified based on site, the presence of depressive symptoms as defined by the 16-item Quick Inventory of Depressive Symptomatology – Clinician Rated (QIDS-C16), and severity of stimulant use as defined by number of days of substance use prior to admission) to either: 1) dosed exercised intervention (Exercise) or 2) health education intervention (Health Education). Both interventions included intervention and assessment visits weekly during the 3-month acute phase.
2.3. Measures
Stimulant use disorders were assessed with the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) (Version 2.1) (Robins et al., 1988; WHO, 1997). Drug, alcohol, and nicotine quantity and frequency were assessed for the 30 days prior to residential treatment admission using the Timeline Followback (TLFB) method (Sobell and Sobell, 1992).
The MINI International Neuropsychiatric Interview (MINI) (Sheehan et al., 1997) was used to obtain diagnostic information at baseline for current DSM-IV Axis I disorders, including depression, suicidality, generalized anxiety disorder, panic disorder, and posttraumatic stress disorder (PTSD). The MINI suicide module was used to assess suicide risk in the last month (yes/no) and to classify risk levels as low, moderate, and high. Symptoms of depression were assessed with the QIDS-C16 (range of 0–27; higher scores indicate more severe depressive symptoms) (Trivedi et al., 2004). The 16-item Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (Q-LES-Q-SF) (Endicott et al., 1993) assessed participants’ general quality of life based on their satisfaction with their physical health, feelings, work, household duties, school/course-work, leisure time activities, and social relations (scores range from 0-100; higher scores indicate greater life satisfaction and enjoyment).
Participants completed the CHRT-SR14 within a median of 10 days of admission into residential treatment (interquartile range: 8 to 14), and weekly thereafter. For the proposed analyses, we examined the CHRT-SR12 (derived from the CHRT-SR14) at baseline and week 1 (Time 2). Each item response ranges from 0 (strongly disagree) to 4 (strongly agree) (Trivedi et al., 2011b). The total score for the CHRT-SR12 ranges from 0-48. In addition, we report on the two additional Impulsivity items of the CHRT-SR14 with total scores ranging from 0-56.
2.4. Statistical analyses
Confirmatory factor analysis (CFA) was conducted to assess the fit of the data to the CHRT-SR12 model and tested using the factors and subscales (Fig. 1). Model fit was assessed using Model chi-square value (χ2) (Kline, 2011), model chi-square statistic per degrees of freedom (χ2/df) (Bollen, 1989), the root mean square error of approximation (RMSEA) (Kline, 2011) with a 90% confidence interval (90%CI), Bentler Comparative Fit Index (CFI) and Tucker-Lewis index (TLI) (Hu and Bentler, 1999; Kline, 2011), and weighted root-mean-square residual (WRMR) (Yu, 2002). CFA was conducted with Mplus 8.0 (Muthén and Muthén, 2012) using weighted least squares means and variance adjusted (WLSMV) estimation (Brown, 2006). WLSMV is a robust estimator not requiring normally distributed data, therefore optimal for skewed data as expected when meauring a construct such as suicidality.
Fig. 1.
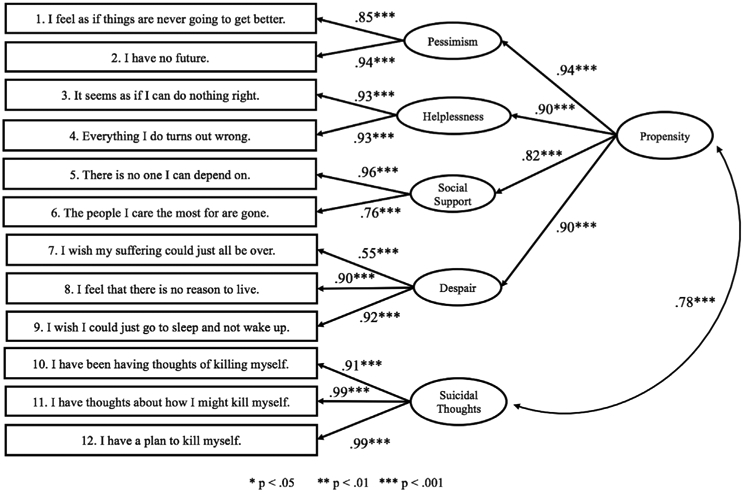
CHRT-SR confirmatory factor model.
Cronbach's χ (internal consistency reliability) was calculated for the total CHRT-SR12 and the factors and subscales. Intraclass correlation coefficients (ICC) (Hallgren, 2012) and Standard Error of Measurement (SEM) were used to examine for test-retest reliability (Weir, 2005). The SEM scores were calculated as follows:
When testing for SEM, Hopkins (2000) suggested the use of the mean square error (MSe) calculated within a repeated-measures ANOVA using scores from both time points as a within-subjects factor. This procedure was used for each of the CHRT-SR12 total, factor, and subscale scores.
Spearman's rho rank order correlations were used to test the association among the CHRT-SR12 total, factor, and subscales with both the QIDS-C and Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (Q-LES-Q-SF). To test criterion-related validity, Kruskal-Wallis ANOVA (H test) and Mann-Whitney U tests were used to test for differences on the CHRT-SR total and subscale scores for groups of participants diagnosed with major depression, generalized anxiety disorder, panic disorder, suicidality, and/or posttraumatic stress disorder based on the MINI. The significance level was set at p < .05.
We then examined the additional 2 impulsivity items and the CHRT-SR14 total score for the above analyses. All analyses were performed using SPSS 25.0.
3. Results
A total of 302 participants enrolled in the study. Table 1 provides the sample characteristics.
Table 1.
Demographic and clinical characteristics.
| Total Sample (n = 302) |
|
|---|---|
| Age | 39.0 ± 10.8 |
| Gender (Female) | 40.1% (121) |
| Ethnicity | |
| Hispanic | 10.3% (31) |
| Non-Hispanic | 89.4% (270) |
| Unknown | 0.3% (1) |
| Race | |
| Caucasian | 49.0% (148) |
| African American | 45.7% (138) |
| Other | 7.9% (24) |
| Unknown | 1.3% (4) |
| Years of Education | 12.4 ± 2.0 |
| Any Prior Inpatient Hospitalization | 66.9% (202) |
| Current Major Depressive Episode | 22.8% (69) |
| Suicidality | 38.4% (116) |
| Baseline QIDS-C16 Total Score | 5.4 ± 3.1 |
| None | 57.3% (173) |
| Mild | 37.4% (113) |
| Moderate | 4.3% (13) |
| Severe | 1.0% (3) |
| Baseline CHRT-SR12 | 18.3 ± 6.0 |
| Baseline CHRT-SR14 | 22.9 ± 7.4 |
Abbreviations: QIDS-C16: Quick Inventory of Depressive Symptomatology – Clinician (16 item); CHRT-SR: Concise Health Risk Tracking Self Report.
3.1. CHRT-SR12
Factor validity.
The CFA performed supported the CHRT-SR12 model with two factors (or subscales) – Propensity and Suicidal Thoughts, as well as four domains within the Propensity subscale (Pessimism, Helplessness, Social Support and Despair). See Fig. 1. The the χ2 model test was significant (χ2 = 188.40, df = 49, p < .001), and the χ2/df = 3.84, which is near the cutoff of 3.0 thus indicating model fit (Bollen, 1989). Model fit was further supported with a RMSEA value of 0.097 (90%CI of 0.083 to .112; CFI = 0.988, TLI = 0.984), and WRMR score near 1.0 (WRMR = 1.099).
Internal consistency.
Using the CHRT-SR12 model, the CHRT-SR12 total, subscales, and Propensity domain scores were calculated (see Table 2). Overall, the CHRT-SR12 subscales and total score demonstrated acceptable internal consistency (Cronbach's alpha greater than 0.7), although the Despair domain was lower (χ = 0.507). The CHRT-SR12 total had an obtained χ = 0.844, with Propensity χ = .838 and Suicidal Thoughts χ = 0.892. At Time 2 (approximately one week after baseline), comparable Cronbach's χ coefficients were obtained. Table 3 provides the inter-correlations among the CHRT-SR12 total and subscale scores. Inter-subscale scores ranged from moderate to strong (χ = 0.319 to 0.990, all p < .001). Among the items indicating Propensity (Pessimism, Helplessness, Social Support, and Despair), inter-correlations ranged from 0.428 to 0.713 (all p < .001) and all were strongly correlated with the Propensity score (χ = 0.583 to 0.802, all p < .001).
Table 2.
CHRT-SR12 test-retest reliability and internal consistency reliability.
| Measure | Baseline M ± SD | Baseline Cronbach's χ | Time 2aM ± SD | Time 2 Cronbach's χ | ICC | SEM |
|---|---|---|---|---|---|---|
| CHRT-SR12 Total (range 0–48) | 18.28 ± 6.01 | .844 | 17.61 ± 5.89 | .847 | .702 | 2.198 |
| CHRT-SR12 Propensity (range 0–36) | 14.90 ± 5.51 | .838 | 14.20 ± 5.30 | .832 | .707 | 1.964 |
| CHRT-SR12Pessimism (range 0–8) | 3.08 ± 1.53 | .763 | 2.92 ± 1.45 | .683 | .650 | 0.637 |
| CHRT-SR12 Helplessness (range 0–8) | 3.26 ± 1.63 | .869 | 3.01 ± 1.51 | .858 | .657 | 0.662 |
| CHRT-SR12 Social Support (range 0–8) | 3.42 ± 1.78 | .723 | 3.36 ± 1.71 | .738 | .720 | 0.609 |
| CHRT-SR12 Despair (range 0–12) | 5.14 ± 2.04 | .507 | 4.91 ± 2.05 | .507 | .602 | 0.972 |
| CHRT-SR12 Suicidal Thoughts (range 0–12) | 3.47 ± 1.16 | .892 | 3.41 ± 1.06 | .906 | .542 | 0.589 |
Abbreviations: CHRT-SR: Concise Health Risk Tracking Self Report; M: Mean; SD: standard deviation; ICC: Intraclass correlation coefficients; SEM: Standard Error of Measurement.
Time 2 occurred approximately one week after baseline.
Table 3.
Inter-subscale correlations for CHRT-SR12 (ρ, Spearman's rho rank order coefficient test)a.
| CHRT-SR12 total, subscales, and item scores |
Total | Propensity | Pessimism | Helplessness | Social Support | Despair | Suicidal Thoughts |
|---|---|---|---|---|---|---|---|
| Total | 1.00 | – | – | – | – | – | – |
| Propensity | .990*** | 1.00 | – | – | – | – | – |
| Pessimism | .782*** | .798*** | 1.00 | – | – | – | – |
| Helplessness | .820*** | .832*** | .713*** | 1.00 | – | – | – |
| Social Support | .772*** | .782*** | .564*** | .595*** | 1.00 | – | – |
| Despair | .802*** | .795*** | .505*** | .535*** | .428*** | 1.00 | – |
| Suicidal Thoughts | .501*** | .392*** | .341*** | .319*** | .319*** | .411*** | 1.00 |
| Item 1 | .740*** | .757*** | .965*** | – | – | – | – |
| Item 2 | .728*** | .734*** | .838*** | – | – | – | – |
| Item 3 | .782*** | .795*** | – | .946*** | – | – | – |
| Item 4 | .793*** | .802*** | – | .942*** | – | – | – |
| Item 5 | .758*** | .761*** | – | – | .831*** | – | – |
| Item 6 | .698*** | .708*** | – | – | .945*** | – | – |
| Item 7 | .673*** | .678*** | – | – | – | .925*** | – |
| Item 8 | .603*** | .587*** | – | – | – | .590*** | – |
| Item 9 | .597*** | .583*** | – | – | – | .606*** | – |
| Item 10 | .489*** | – | – | – | – | – | .996*** |
| Item 11 | .501*** | – | – | – | – | – | .929*** |
| Item 12 | .486*** | – | – | – | – | – | .844*** |
p < .05
p < .01
p < .001.
Abbreviations: CHRT-SR: Concise Health Risk Tracking Self Report.
Spearman's rho rank order coefficients were not provided when redundant or when the items did not belong to a subscale of the CHRT.
Test-retest reliability.
The CHRT-SR12 total score demonstrated acceptable test-retest reliability between the baseline and Week 1 time-point (ICC = 0.702), as did the Propensity subscale (ICC = 0.707). The Suicidal Thoughts subscale (ICC = 0.542) had a lower test-retest reliability score. Table 2 shows the SEM scores for the CHRT-SR12 total and subscale scores.
Construct validity.
The relationships between depression severity (QIDS-C16 total score) and CHRT-SR12 total and subscale scores were significant but modest (ρ = 0.208 to 0.308, all p < .001). The CHRT-SR12 Suicidal Thoughts subscale score and the QIDS-C16 depression severity total score were unrelated (ρ = 0.066, p = .263). However, the relationship between the Suicidal Thoughts subscale and the QIDS-C16 suicide item was significant, though modest (ρ = 0.207, p < .001). Q-LES-Q-SF total scores were weakly to moderately correlated with all subscale and CHRT-SR12 total scale scores (ρ = −0.185 to −0.455, all p < .001), with greater scores reported on the Q-LES-Q-SF associated with lower scores on the CHRT-SR12 (Table 4).
Table 4.
Construct Validity for CHRT-SR12 (ρ, Spearman's rho rank order coefficient test).
| Measure | QIDS-C16 Total |
Q-LES-Q-SF Total |
QIDS-C16 Suicidal Ideation Item |
|---|---|---|---|
| CHRT-SR12 Total | .296*** | −.445*** | .111 + |
| CHRT-SR12 Propensity | 308*** | −.451*** | .118* |
| CHRT-SR12 Pessimism | .240*** | −.396*** | .089 |
| CHRT-SR12 Helplessness | .265*** | −.455*** | .074 |
| CHRT-SR12 Social Support |
.208*** | −.376*** | .126* |
| CHRT-SR12 Despair | .254*** | −.295*** | .134* |
| CHRT-SR12 Suicidal Thoughts |
.066 | −.185*** | .207*** |
p < .10
p < .05
p < .01
p < .001.
Abbreviations: CHRT-SR: Concise Health Risk Tracking Self Report; QIDS-C16: Quick Inventory of Depressive Symptoms-Clinician Rated; Q-LES-Q: Quality of Life Enjoyment and Satisfaction Questionnaire Short Form.
Criterion-related known groups validity.
Table 5 shows the relationships between diagnostic information obtained by the MINI and the CHRT-SR12 total and subscales. Diagnostic groups were used to evaluate criterion-related validity of the CHRT-SR12 and the Total, Propensity and Suicidal Thoughts scores were all significantly higher in those participants experiencing a current major depressive episode than in those not in a current episode. Only the CHRT-SR12 Suicidal Thoughts subscale was significantly related to the clinical assessment of the presence of suicidality. Specifically, of those assessed as suicidal by clinicians (n = 116) with the MINI suicide scale, CHRT-SR12 scores did not significantly differ among participants rated as low, medium, or high suicidal risk (all p > .05). However, there were so few participants in the sample with moderate to high risk (14.9%; n = 45), that this is not unexpected. Both total and factor CHRT-SR12 scores were higher in most cases when Panic Disorder or Posttraumatic Stress Disorder were present (Table 5).
Table 5.
Criterion-related validity tests for CHRT-SR12 and MINI diagnoses (non-parametric tests).
| Measure | n (%) | CHRT-SR12, M ± sd | ||
|---|---|---|---|---|
| Total | Propensity | Suicidal Thoughts | ||
| Major depressive episode, current | U = 9392.50*** | U = 10006.50** | U = 8342.00* | |
| Yes | 69 (22.8%) | 20.15 ± 4.72 | 16.51 ± 5.42 | 3.79 ± 1.54 |
| No | 233 (77.2%) | 17.73 ± 5.93 | 14.42 ± 5.45 | 3.38 ± 1.01 |
| Number of depressive episodesa | 12.06 (13.70) | −.017 | −.028 | −.125 |
| Suicidalityb | U = 11140.00 + | U = 11774.50 | U = 11310.50** | |
| Yes | 116 (38.4%) | 19.13 ± 6.52 | 15.53 ± 5.95 | 3.74 ± 1.47 |
| No | 186 (61.6%) | 17.77 ± 5.63 | 14.50 ± 5.18 | 3.31 ± 0.90 |
| Suicide Risk Severity, currentc, d | H = 1.72 | H = 1.55 | H = 1.97 | |
| Low | 71 (61.2%) | 18.47 ± 6.03 | 14.93 ± 5.37 | 3.54 ± 1.14 |
| Moderate | 20 (17.2%) | 20.84 ± 7.95 | 17.45 ± 7.74 | 4.16 ± 1.92 |
| High | 25 (21.6%) | 19.65 ± 6.66 | 15.72 ± 5.88 | 4.00 ± 1.83 |
| Generalized Anxiety Disorder | U = 6379.00+ | U = 7366.00* | U = 5309.00 | |
| Yes | 47 (15.6%) | 19.70 ± 6.26 | 16.72 ± 5.83 | 3.54 ± 1.55 |
| No | 255 (84.4%) | 18.03 ± 5.94 | 14.56 ± 5.39 | 3.46 ± 1.08 |
| Panic Disorder, current | U = 3268.00** | U = 3636.50** | U = 2653.50 | |
| Yes | 18 (6.0%) | 22.53 ± 6.89 | 18.89 ± 5.94 | 3.94 ± 1.52 |
| No | 269 (89.1%) | 18.05 ± 5.88 | 14.67 ± 5.40 | 3.45 ± 1.14 |
| Panic Disorder, lifetime | U = 6195.50*** | U = 6891.50*** | U = 5084.00* | |
| Yes | 37 (12.3%) | 22.18 ± 6.49 | 19.28 ± 7.91 | 3.94 ± 1.50 |
| No | 265 (87.7%) | 17.77 ± 5.76 | 14.42 ± 5.28 | 3.41 ± 1.10 |
| Posttraumatic Stress Disorder, current | U = 4978.00** | U = 5442.50** | U = 4424.50** | |
| Yes | 29 (9.6%) | 23.26 ± 8.76 | 19.28 ± 7.91 | 3.94 ± 1.50 |
| No | 273 (90.4%) | 17.77 ± 5.42 | 14.43 ± 4.98 | 3.41 ± 1.10 |
p < .05
p < .01
p < .001
p < .10
Abbreviations: CHRT-SR: Concise Health Risk Tracking Self Report; MINI: International Neuropsychiatric Interview; H: Kruskal-Wallis ANOVA (H test); U: Mann-Whitney U test.
Number of depressive episodes are tested with Spearman's rho with Mean ± Standard Deviation given.
Suicidality was defined by the clinician rating on the MINI Suicidality Module; if any questions about suicidal thoughts or behaviors were rated positive, a rating of “Yes” was given.
Suicide risk severity was defined by the clinician rating on the MINI Suicidality Module; if Suicidality was rated as “Yes,” the clinician added the total points for answers B1-19 checked “yes”; Severity is rated as Low (1–8 points), Moderate (9–16 points), or High (≥17 points).
Kruskal-Wallis Test was used to test for differences between the three suicide risk categories.
3.2. CHRT-SR14
The CHRT-SR14 model was less supported after CFA analyses with three subscales (Propensity, Impulsivity, and Suicidal Thoughts). The χ2 model test was significant and greater than the obtained value from the CHRT-SR12 (χ2 = 213.98, df = 70, p < .001, χ2/df = 3.05). The CHRT-SR14 RMSEA value was 0.083 (90%CI of 0.070 to .095; CFI = 0.988, TLI = 0.984), and WRMR score near 1.0 (WRMR = 1.020). The CHRT-SR14 total internal consistency was 0.856 with an Impulsivity reliability score of 0.769. The test-retest reliability of the CHRT-SR14 was acceptable (ICC = 0.722) with the Impulsivity subscale demonstrating moderate scores (ICC = 0.666). Impulsivity scores were significantly correlated with QIDS-C16 Total (χ = 0.269, p < .001), Q-LES-Q-SF Total (χ = −0.339, p < .001), but not the QIDS-C16 Suicidal Ideation item (χ = 0.039, p = .501). Impulsivity scores significantly varied between those with current major depressive disorder (5.45 ± 2.27 vs. 4.45 ± 2.12, U = 10088.00, p < .001), but were not associated with MINI Suicidality (p = .325) or Suicide Risk (p = .400).
4. Discussion
The psychometric properties of the CHRT-SR12 were in the acceptable to excellent range in a sample of 302 stimulant users recruited from residential treatment. The factor validity analysis indicated two factors (subscales): Propensity and Suicidal Thoughts. The scale demonstrated acceptable internal consistency reliability and test-retest reliability. Furthermore, the measure demonstrated construct validity with significant correlations with depression and quality of life measures, and criterion-related known groups validity, supporting findings previously reported in patients with major depressive disorder and bipolar disorder (Ostacher et al., 2015; Reilly-Harrington et al., 2016; Trivedi et al., 2011b).
While the total scale and subscales had acceptable internal reliability (Cronbach's coefficient alpha greater than 0.70), the Despair domain within the Propensity subscale had lower internal reliability across both time points. With Cronbach's χ considered a lower bound of reliability and a more precise indicator of reliability with fewer items (Cronbach, 1951), these results further support the internal consistency reliability of the CHRT-SR12.
At baseline, CHRT-SR12 scores positively correlated with depression scores and negatively correlated with quality of life. Specifically, higher QIDS-C16 total scores were correlated with higher CHRT-SR12 total score and Propensity subscale, but not with the Suicidal Thoughts subscale. On the other hand, the Suicidal Thoughts subscale was highly correlated with the suicide item on the QIDS-C16. In addition, participants evaluated by a clinician as currently suicidal had significantly higher CHRT-SR12 Suicidal Thoughts subscale scores, and that relationship was found regardless of the level of clinical risk of suicide. Additionally, higher quality of life scores was correlated with lower suicidality. CHRT-SR12 scores were also associated with the MINI ratings of depression, anxiety, panic disorder and PTSD.
The addition of the two impulsivity items did not improve the psychometric properties of the scale, and in fact, led to slightly lower significance scores. The two impulsivity items created a third subscale as the items loaded together, but the added value of these two items has yet to be substantiated. Whether “impulsivity” predicts future suicide attempts is unknown.
This study furthers the findings of the previously established strong psychometric properties of the CHRT-SR in both the 12- and 14-item versions (Mayes et al., in press; Ostacher et al., 2015; Reilly-Harrington et al., 2016; Trivedi et al., 2011b). In this sample, only two factors (or subscales) were identified in the CHRT-SR12 – Propensity and Suicidal Thoughts. In earlier reports, the three subscales were identified: Perceived Lack of Social Support, Hopelessness, and Suicidal Thoughts. In this sample, all items in the Perceived Lack of Social Support and Hopelessness subscales loaded onto the Propensity subscale.
The value of a brief self-report measure of suicidal risk with promising psychometric properties, that are consistent with clinical measures of depression and quality of life, has strong implications for practice and for clinical trials. While previous studies have confirmed its accuracy, the MINI requires extensive clinician training and time to administer. The CHRT-SR is straightforward, requiring minimal clinician time to train and administer (Reilly-Harrington et al., 2016). It offers particular utility as a simple and quick assessment of helplessness and pessimism, symptoms common among people with chronic stimulant use, a population who should be closely monitored for suicide risk (Marshall et al., 2011; Zullig and Divin, 2012).
4.1. Limitations
The study has several limitations. Generalizability is limited to the participants who met eligibility criteria for STRIDE and to residential patients with stimulant use disorder. STRIDE was a behavioral intervention study with specific eligibility criteria that excluded at-risk individuals with conditions that might contraindicate exercise. The study had fewer participants than expected with heavy stimulant use at baseline (i.e., > 18 days in the 30 days prior to residential treatment entry), or with significant depressive symptoms (QIDS-C16 > 10), and therefore may have enrolled a less severe group of individuals compared to other individuals in residential treatment. In addition, patients with significant risk for suicide were excluded from participation and the study sample as a whole improved dramatically during the study (Trivedi et al., 2017). Furthermore, suicidality often wanes quickly upon admission to an inpatient unit, which may have resulted in the low incidence of suicidal thoughts for the sample.
5. Conclusion
The CHRT-SR12 (as well as the CHRT-SR14) is a brief self-report with acceptable psychometric properties in a substance disordered population that can be used to monitor ongoing changes in suicidal thoughts and associated risk factors.
Acknowledgements
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number U10DA020024 and UG1DA020024 (PI: Trivedi). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
Dr. Rush has received consulting fees from Akili, Brain Resource Inc., Compass Inc., Curbstone Consultant LLC, Eli Lilly, Emmes Corp, Liva-Nova, Mind Linc., Sunovion, Takeda USA, Taj Medical; speaking fees from Liva-Nova and Sing-Health; and royalties from Guilford Press and the University of Texas Southwestern Medical Center, Dallas, TX. (for the Inventory of Depressive Symptoms and its derivatives). He is also named co-inventor on two patents: U.S. Patent No. 7,795,033: Methods to Predict the Outcome of Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S, Wilson AS and U.S. Patent No. 7,906,283: Methods to Identify Patients at Risk of Developing Adverse Events During Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S. Dr. Trivedi has received consulting fees from or has served on the advisory boards of Alkeremes Inc., Akili Interactive, Allergan Pharmaceuticals, Arcadia Pharmaceuticals, Avanir Pharmaceuticals, Brintellix Global, Bristol Myers Squibb, Caudex, Cerecor, Forest Pharmaceuticals, Global Medical Education Inc, Health Research Associates, Insys, Johnson & Johnson Pharmaceutical Research & Development, Lilly Research Laboratories, Lundbeck Research USA, Medscape, Merck & Co. Inc, Mitsubishi Pharma, MSI Methylation Sciences – Pamlab Inc., Navitor, Otsuka America Pharmaceutical Inc., One Carbon Therapeutics, Otsuka America Pharmaceutical Inc., Pfizer Inc, and Takeda Global Research; royalties from Janssen Research and Development LLC; honoraria from the American Psychiatric Association; grants from the Agency for Healthcare Research and Quality (AHRQ), Cancer Prevention and Research Institute of Texas (CPRIT) (PP160121), National Institute of Mental Health (NIMH) (U01 MH092221, R25 MH101078), National Institute of Drug Abuse (NIDA) (U10 DA020024), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01 DK085512), National Center for Advancing Translational Sciences (NCATS) (UL1 TR001105), Johnson & Johnson, PCORI; and holds author agreements with Janssen Asia Pacific and Oxford University Press. Drs. Trombello currently owns stock in Merck and Gilead Sciences and within the past 36 months previously owned stock in Johnson & Johnson. Dr. Walker's research is funded by National Institute ofDrug Abuse (NIDA) (U10 DA020024), and Alkermes, Inc. donated medication for a NIDA-funded study (NCT03078075) unrelated to the current manuscript. Dr. Greer is a paid consultant for H Lundbeck A/S. Drs. Killian, Lindblad, and Carmody, Mrs. Mayes, and Mr. Grannemann have no conflicts to report.
References
- Bollen KA, 1989. Structural Equations with Latent Variables. Wiley, New York. [Google Scholar]
- Brown T, 2006. Confirmatory Factor Analysis for Applied Research. Guildford, New York. [Google Scholar]
- Cronbach LJ, 1951. Coefficient Alpha and the internal structure of tests. Psychometrika 16, 297–334. [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R, 1993. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol. Bull 29, 321–326. [PubMed] [Google Scholar]
- Hallgren KA, 2012. Computing inter-rater reliability for observational data: an overview and tutorial. Tutorials in Quantitative Methods for Psychology 8, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WG, 2000. Measures of reliability in sports medicine and science. Sports Med. 30, 1–15. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM, 1999. Cutoff Criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model.: A Multidiscip. J 6, 1–55. [Google Scholar]
- Kline RB, 2011. Principles and Practice of Structural Equation Modeling, third ed. Guildford Press, New York. [Google Scholar]
- Liu RT, Trout ZM, Hernandez EM, Cheek SM, Gerlus N, 2017. A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: meta-analysis and recommendations for future research. Neurosci. Biobehav. Rev 83, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BDL, Galea S, Wood E, Kerr T, 2011. Injection methamphetamine use is associated with an increased risk of attempted suicide: a prospective cohort study. Drug Alcohol Depend. 119, 134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes TL, Kennard BD, Killian M, Carmody T, Grannemann B, Rush AJ, Jha M, Hughes J, Emslie GJ, Trivedi MH, Psychometric properties of the Concise Health Risk Tracking (CHRT) in adolescents with suicidality, J. Affect. Dis, In press. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 2012. Statistical Analysis with Latent Variables Using Mplus, seventh ed. Muthén & Muthén, Los Angeles. [Google Scholar]
- Olfson M, Blanco C, Wall M, Liu SM, Saha TD, Pickering RP, Grant BL, 2017. National trends in suicide attempts among adults in the United States. JAMA Psychiatr. 74, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostacher MJ, Nierenberg AA, Rabideau D, Reilly-Harrington NA, Sylvia LG, Gold AK, et al. , 2015. A clinical measure of suicidal ideation, suicidal behavior, and associated symptoms in bipolar disorder: psychometric properties of the Concise Health Risk Tracking Self-Report (CHRT-SR). J. Psychiatr. Res 71, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly-Harrington NA, Shelton RC, Kamali M, Rabideau DJ, Shesler LW, Trivedi MH, et al. , 2016. A tool to predict suicidal ideation and behavior in bipolar disorder: the Concise Health Risk Tracking Self-Report. J. Affect. Disord 192, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. , 1988. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch. Gen. Psychiatr 45, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cintas L, Daigre C, Braguehais MD, Palma-Alvarez RF, Grau-Lopez L, Ros Cucurull E, Rodriguez-Martoz, Abad AC., Roncero C, 2017. Factors associated with lifetime suicidal ideation and suicide attempts in outpatients with substance use disorders. Psychiatr. Res http://dx.doi.Org/10.1016/j.psychres.2017.09.021. pii: S0165–1781 (17)30426-2. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Keskiner A, et al. , 1997. Reliability and validity of the MINI international neuropsychiatric Interview (M.I.N.I.): according to the SCID-P. Eur. Psychiatr 12, 232–241. [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-back: a technique for assessing self-reported ethanol consumption In: Allen J, Litten RZ (Eds.), Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Somoza E, Blair SN, et al. , 2011a. Stimulant reduction intervention using dosed exercise (STRIDE) - CTN 0037: study protocol for a randomized controlled trial. Trials 12, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Rethorst CD, Carmody T, Grannemann BD, Walker R, Warden D, Shores-Wilson K, Stoutenberg M, Oden N, Silverstein M, Hodgkins C, Love L, Seamans C, Stotts A, Causey T, Szucs-Reed RP, Rinaldi P, Myrick H, Straus M, Liu D, Lindblad R, Church T, Blair SN, Nunes EV, 2017. Randomized controlled trial comparing exercise to health education for stimulant use disorder: Results from the CTN-0037 Stimulant Reduction Intervention using Dosed Exercise (SSTRIDE) study. J. Clin. Psychiatr 78, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. , 2004. The inventory of depressive Symptomatology, clinician rating (IDS-C) and self-report (IDS-SR), and the quick inventory of depressive Symptomatology, clinician rating (QIDS-C) and self-report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol. Med 34, 73–82. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Wisniewski SR, Morris DW, Fava M, Gollan JK, Warden D, et al. , 2011b. Concise health risk tracking scale: a brief self-report and clinician rating of suicidal risk. J. Clin. Psychiatr 72, 757–764. [DOI] [PubMed] [Google Scholar]
- Villegas AC, DuBois CM, Celano CM, Beale EE, Mastromauro CA, Stewart JG, Auerback RP, Huffman JC, Hoeppner BB, 2018. A longitudinal investigation of the Concise Health Risk Tracking Self-Report (CHRT-SR) in suicidal patients during and after hospitalization. Psychiatr. Res 262, 558–565. [DOI] [PubMed] [Google Scholar]
- Weir JP, 2005. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Condit Res 19, 231–240. [DOI] [PubMed] [Google Scholar]
- WHO, 1997. Composite International Diagnostic Interview, Version 2.1. World Health Organization, Geneva. [Google Scholar]
- Yu C, 2002. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models with Binary and Continuous Outcomes. University of California, Los Angeles. [Google Scholar]
- Zullig KJ, Divin AL, 2012. The association between non-medical prescription drug use, depressive symptoms, and suicidality among college students. Addict. Behav 37, 890–899. [DOI] [PubMed] [Google Scholar]


