Abstract
Our research team was asked to consider the relationship of the neuroscience of sensorimotor control to the language of emotions and feelings. Actions are the principal means for the communication of emotions and feelings in both humans and other animals, and the allostatic mechanisms controlling action also apply to the regulation of emotional states by the self and others. We consider how motor control of hierarchically organised, feedback-based, goal-directed action has evolved in humans, within a context of consciousness, appraisal and cultural learning, to serve emotions and feelings. In our linguistic analysis, we found that many emotion and feelings words could be assigned to stages in the sensorimotor learning process, but the assignment was often arbitrary. The embodied nature of emotional communication means that action words are frequently used, but that the meanings or senses of the word depend on its contextual use, just as the relationship of an action to an emotion is also contextually dependent.
Keywords: Emotion, Feeling, Action, Facial expression, motor, Sensorimotor, Planning, Linguistics, Emotion regulation, Cognitive appraisal, Embodied cognition, Mirror neurons
1. Introduction
This review on the neuroscience of action and affect is being undertaken as part of the ‘The Human Affectome Project’, a 2016 initiative organised by the non-profit organisation Neuroqualia. The project aims to produce a series of overarching reviews that can summarise much of what is currently known about affective neuroscience while simultaneously exploring the language that we use to convey feelings and emotions. The project is comprised of twelve teams organised into a taskforce focused on the development of a comprehensive and integrated model of affect that can serve as a common focal point for affective research in the future.
To that end, our team was specifically tasked to review the neuroscience research related to actions, the way that people communicate feelings that relate to actions, and whether or not the feelings terms that people convey in communication might inform the way we approach action-related neuroscience research.
The evolutionary origins of feelings and emotions lie in their critical role in regulating behaviour, and so consequently, emotions are tied closely to actions. One might consider these regulatory behaviours to be of two types. Firstly, there are those behaviours that serve to regulate an individual’s behaviour so that he or she can maintain a physiologically healthy state. Secondly, many animals live in social groups of varying size and complexity, and so additional behaviours are designed to influence the behaviour of others. Humans stand out as being different to other animal species in the way that actions relate to emotions, and this paper will review these differences. We consider that there may not be fundamental differences but that these are largely a matter of degree. Most obviously, whereas in other animals, actions are highly limited in their variability, in humans they are highly flexible and adaptive to variation in context. The exception occurs in states of mental disorder where action patterns may become stereotyped and repetitive.
Given that humans are a highly social species, living in large, socially complex societies, the action repertoire for social communication has become particularly extensive. In addition to language, we communicate our emotions to one another through actions such as body posture, speech (tone, volume or intonation), facial expression and hand gestures (Vaessen et al., 2018), and complex control systems have evolved to serve this function. In humans, emotional states are constructed through a self-awareness of actions, associated with contextual and cultural learning. Flexibility depends upon complex interaction between a subcortical network serving associative learning and cortical mechanisms allowing for contextual influence and sensorimotor learning. Potential conflicts between outcomes of intentions at varying levels within a hierarchical system of goals are managed, and systems constantly learn and become updated through evaluation and appraisal mechanisms.
In this review we aim to consider how the relationship between action and emotion has evolved in humans, examining how an enhanced capacity for flexible motor control and motor learning has resulted in a complex system for the communication and regulation of emotional expression and communication, involving cultural learning and consciousness. We consider what happens to this capacity in states of disorder and disease, and how, through embodied cognition, action words are often employed to describe emotions and feeling states.
Firstly though, we will consider two principles that underpin our review, the first of which is expressed through predictive models of perception, action, and cognition, which argue for an active inference account of the mind (Friston, 2010; Clark, 2013).
Within active inference accounts, the primary goal of the brain is to maintain allostasis. Allostasis is a term used to describe how the body maintains stability through change. It differs slightly from homeostasis in allowing learning and anticipatory responding to vary set-levels of parameters in order for the organism to adapt to its environment, rather than keeping predetermined levels constant (Ramsay et al., 2014). In the brain, allostasis is achieved primarily by comparing bottom-up sensory inputs from the world and body to top-down ‘predictions’ about the world and body (Clark, 2013). Mismatch between feedback and feedforward processes gives rise to ‘prediction errors’, presenting potential risks to stability. These prediction errors trigger action in order to address the cause of the error and restore equilibrium. Rather than perception being a blank canvas onto which the state of the world is painted, perception is the result of comparing predictions about the world to actual sensory inputs. When an organism detects a discrepancy between predicted and experience inputs, this brings key threats to homeostasis to attention, triggering action to address these issues.
These actions are usually intentional which means that they are enacted with a plan to achieve a specific goal. Therefore, all actions have a motivation or an emotional value, which result in the action being planned to achieve a desirable goal with an associated sensory feedback. This process has been learned from birth ever since the infant starts to act on his or her environment in an effort to achieve desirable ends. This learning process utilises feedback-feedforward processes. Internal sensorimotor models are encoded in the brain which associate internal models for action patterns with those that encode their sensory consequences and goal-achievement. Consequently, ideation of the action’s goal triggers an associated motor plan, which determines the selection, sequences, and power of muscular contractions that form actions, needed to achieve the goal. The action itself triggers a range of sensory consequences, occurring across all modalities, whether visual, tactile, vestibular or kinaesthetic, creating further feedback. This feedback is then compared to the predicted consequences of any planned action, actively inferring causes of any error and modifying the internal sensorimotor model, so that prediction error is decreased to an acceptable range, actions are coordinated to achieve goals in an optimal fashion, and allostasis is maintained.
The second principle is that internal models of goal-sensorimotor relationships are organised in hierarchies. Any action can be reduced to a set of specific muscular contractions which combine to form simple actions and then complex actions, which are enacted to serve short term plans, and finally longer-term plans. The longer-term plans may be so distal to the actions taken to achieve them that they are largely independent of these individual actions. Consider for example, the combination of muscle contractions required to grasp a door handle and open the door, which may be serving an immediate goal of leaving a room. This may be serving a higher goal of leaving a meeting, which could be an act which communicates an expression of a desire to leave a group. At each level wider contextual factors characterise the intention.
In this review we consider the relationship of sensorimotor control mechanisms to feelings and emotion, before considering whether feelings and emotion words can inform the way in which we approach research related to sensorimotor control. The principles are summarised in Fig. 1. One notable feature of this model is the omission of something specifically called “executive function” since the function of control is an integral aspect of the relationship between higher levels of the hierarchy of the sensorimotor-goal model and limbic associations. Similarly, we do not, at this stage, make a distinction between social and non-social actions, but assume that the strong motivational value of interpersonal function will mean that limbic associations will be particularly important in the development of those sensorimotor goal relationships. Finally, we do not discuss anatomical correlates at this point though these are considered in more detail in Section 4.
Fig. 1.
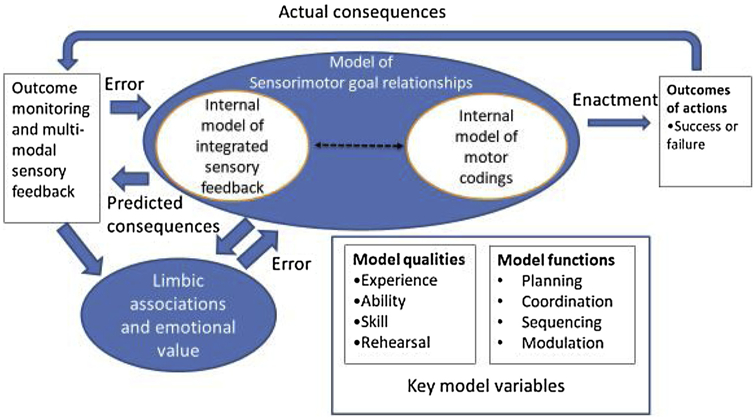
Schema of components of internal model of sensorimotor relationships involved in the encoding and enactment of actions with emotional values.
2. Comparative psychology of emotion expressed in action
The expression of emotion through action reflects the biological continuity of emotional communication with other animals. Behaviours can convey emotion explicitly through displays, such as when an animal shows aggression or courtship behaviour, or passively, such as when an animal reveals its distress. In many species, the emotional expressions of conspecifics affect observers’ actions.
Humans lie at the end of a continuum in their ability to vary their expressions of emotion, through a combination of intentional and automatic control of actions controlling body posture, manual gesture, facial movements or vocalisation. Apart from the vertebrates, most other animal species are relatively inflexible regarding their behavioural repertoire including motor patterns or vocalisations and rely on fixed, innate patterns of action. Most animals have fixed patterns of vocal expression that are largely innate (the chicken is a good example). However, many primates do adjust their actions or vocalisations to their observers, and some species may even flexibly mimic the vocalisations of other species including those of predators or for instance of human speech. The parrot is the most obvious example of that, but the phenomenon has been observed in other species too (e.g., seals producing speech-like sounds; Ralls et al., 1985). Vocal learning is observed in several distantly related mammalian species including bats, cetaceans, elephants and seals (Chakraborty and Jarvis, 2015). Some birds are comparable to humans in having a capacity for an extensive and constantly variable vocal repertoire (e.g., songbirds). Whether this extends to other aspects of action is a question hardly explored but research in raven and parrot species, shows that they adjust their social behaviours and related actions when knowing that they are being observed by conspecifics.
To facilitate emotion transmission, humans have evolved exceptionally communicative faces where all the expressive parts are enlarged and accentuated (Kobayashi and Kohshima, 1997; Kret, 2015; Kret and Tomonaga, 2016; Tomasello et al., 2007). As well as evolving a high level of intentional motor control over expression, human facial features are adapted to maximising expressiveness. For example, our visible eye-white, which facilitates emotion expression and gaze following, is larger than in other species, likewise lip colour and eyebrow size are emphasised. Using our unique collection of facial features, humans express their emotions explicitly through emotional signals that can be subject to intentional control (such as smiling). These emotional signals may also combine with other forms of expression that may be under a lesser degree of intentional control or awareness such as pupil dilation (Harrison et al., 2009; Kret et al., 2013a, b; Kret et al., 2014, 2015; Kret and De Dreu, 2017), blushing (Dij et al., 2009; Leary et al., 1992), and other subtle autonomic cues (Kret, 2015; Levenson and Gottmann, 1983; Reed et al., 2013).
Given the degree to which humans have evolved a capacity to express emotion so clearly, one might expect that the emotional signals would be unambiguous. However, the opposite is more often the case, and the emotion being conveyed by any particular form of expression depends on a variety of factors. For example, the smile is an expression seen across cultures, which participants in lab experiments are supposed to link with the label ‘happy’. However, oftentimes it is not happiness that is expressed in a smile, but something completely different (e.g., nervousness or contempt). For instance, when participants were shown the facial expression of an athlete winning a medal at the Olympics (thus a truly happy person), they were not able to say whether the athlete had won or lost the competition (Aviezer et al., 2012).
In this case, examination of the way that expressions are used by other species may throw a light on their adaptive function. To continue with the example of the smile, this expression stems from the so-called ‘bared teeth display’, an expression shared amongst primates (Van Hooff, 1976; Waller et al., 2006). It is shown when a primate is afraid, but also to signal subordination to a more dominant individual. The smile still has this function in humans – during interactions between a person high, and a person low in power, it is the latter smiling most (Hecht and LaFrance, 1998). In humans, the smile has become ritualised, and next to expressing nervousness or subordination, communicates affiliation, love and affection. Over our lifetime, we learn when to use or reciprocate this expression and when to inhibit it (Hess and Fischer, 2013).
Another example of a facial expression long-thought to be a universal expression of emotion is that of fear. However, there is no universal agreement on what constitutes a fearful expression. The expression associated with fear in Western stimuli (e.g., Ekman, 1993) is instead interpreted as threatening and aggressive in other societies such as in a case study of the people of the Trobriand Islands of Papua New Guinea (Crivelli et al., 2016). This expression is also a common image in apotropaic art featuring threat displays that are meant to ward off harm and deter evil (Kret and Straffon, 2018). Representations of this expression in apotropaic art generally show staring or bulging eyes, flared nostrils, open mouth, flaunted tongue, face distortions, and very often bared fangs or teeth (Emigh, 2011).
The lack of universal agreement on what constitutes a fearful expression is also signified in popular validated facial expression stimulus sets used in psychology, including the one used in the study by Crivelli et al. (2016), which intermix different facial expressions in the category ‘fear’. The faces of some of the actors in the set show widened eyes in combination with the display of upper and lower teeth, similar to the primate bared teeth display (Andersson, 1980; Waller et al., 2006), whilst others within the same face set show the typical expression epitomised by Edvard Munch’s painting ‘The Scream’ or the ‘Home Alone’ film poster; the eyes are enlarged and the mouth is wide open but the teeth do not show. Still other stimuli show a mixture of the two (Kret and Straffon, 2018). This example demonstrates why it is important to make a clear distinction between different negative expressions – they likely have different evolutionary origins and their meanings are context-dependent. The bared teeth face, like other threat displays, probably evolved from the ritualisation of attack or pre-fight movements or intentions, such as biting. In contrast, the gasping face most likely evolved as a fear display from screaming or calling behaviour (Andersson, 1980).
The study of facial expressions in our closest living relatives, the chimpanzees and bonobos, along with studies in more distantly related species such as macaques, can help resolve such ambiguities. Behavioural observations have demonstrated that non-human primate emotional expressions and human emotional expressions can play similar functional roles. For example, human infants use a pout face to solicit their mother’s attention, and a similar facial expression can be found in infant chimpanzees for the same bonding functions (Blurton Jones, 1971; van Lawick-Goodall, 1968). Therefore, cultural appropriation of expressions to serve conventional understandings of associated emotional states would seem to be one factor that has led to a difference in the emotion associated with an expression in some primate groups to that of humans. Notably, the exception of pouting occurs in infants who are the least exposed to cultural influences.
Another factor that may be important in modifying the meaning of a facial expression is bodily expression. In fact, bodily expression of emotion may be just as important as facial expression. However the existing literature has largely focused on posed facial expressions (Adolphs, 2002; Dimberg, 1982; Ekman, 1993; Ekman and Rosenberg, 1997; Frijda, 2016; Hess and Bougeois, 2010), which may affect the ecological-validity of the findings (Kret, 2015). In fact, two of the most illustrious theoreticians of emotion, Darwin and James, discussed whole-body expressions at great length. Darwin famously included postural descriptions in ‘The expression of the emotions in man and animals’ (Darwin, 1872), and James (1890) investigated recognition of emotion with photographs of whole-body posture.
Faces and bodies are equally salient and familiar in daily life and often convey some of the same information; when they do not, it is oftentimes the body that reveals expressers’ genuine feelings (i.e., Aviezer et al., 2012; for a review, see de Gelder et al., 2010). In recent decades researchers have taken up the issue of bodily expression recognition, and results from several behavioural experiments using independent stimulus sets now allow us to conclude that recognition performance for bodily expressions is similar for face and body stimuli (Kret et al., 2013a, b; Kret et al., 2011; de Valk et al., 2015).
In conclusion, most other animals show relatively limited range of emotional expressions, and tight correlations between emotional state, body posture and facial expressions. However, the capacity for social learning and intentional control over expression results in a departure from these relationships. Such a departure can result in more flexible behaviours, such as the diverse repertoires of vocalisation in songbirds. In humans who have evolved an especially strong capacity for intentional control and social learning, facial expressions of emotion may be modified to convey varied and subtle meanings in the context of bodily expression, autonomic reactivity, and cultural convention.
3. Interoception and action in emotion
Modern theory increasingly recognises emotion as providing important influences upon action to make it serve an adaptive function, in much the same way as cognition and perception (Moors and Fischer, 2019). This stands in great contrast to the previous thinking of emotions as maladaptive disruptors of decision-making and action. In fact, it is increasingly recognised that the common principles of motor learning and control are likewise applicable to the awareness and regulation of emotions. More importantly, the ‘active inference’ principles of sensorimotor control discussed in the introduction can also be applied to emotion. Within active inference accounts of emotion, emotion represents the inferred causes of a ‘prediction error’ (Barrett et al., 2016), wherein emotions represent inference about why there is a discrepancy between the expected and experienced sensory input. This prediction error then arises as a readiness for action (Ridderinkhof, 2017). Emotions act as motivators for action, through facilitating ideation of goals (Moors et al., 2017), and thus are not separate from cognition and perception, but rely upon the same processes.
In daily life, emotional experiences are almost always accompanied by physical action, whether through unintentional facial expressions or intentional actions to either communicate the emotional state or address its causes. Evolutionary theory suggests that emotions are, at their core, adaptations that motivate action to benefit the organism (Damasio, 2018). This can be as simple as the fear that motivates an individual to run from danger, or as complex as the sympathy and guilt that motivates an individual to donate to charity. In this way, emotion is closely tied to action and perception.
Yet a major factor that makes human actions so complex and variable is the existence of subjective awareness – the ability to be consciously aware of one’s own emotional experiences. Subjective awareness allows humans to consciously control how emotions are expressed through action, resulting in greater variability of emotional expressions, as well as variability in how emotions influence actions and decision-making (Moors and Fischer, 2019). More nuanced awareness of one’s own emotions facilitates the more effective selection of appropriate emotional responses, but also more accurate perception of others' emotional actions. Yet this relationship is unlikely to be unidirectional – the imitation and production of emotional actions during development also seems to be related to developing more sophisticated and nuanced emotion concepts, leading to greater awareness of the emotions of the self and other.
It is increasingly argued that awareness of one’s own emotions is variable across individuals, and this variability has socioemotional and clinical implications (Smidt and Suvak, 2015). This variability is best understood through the constructed theory of emotion (Barrett, 2017), which posits that emotions are not categorical ‘natural kind’ experiences with limited associated action plans, but dimensional experiences constructed through consolidating bodily sensations with contextual information and prior learning. Subsequently, individual variation in emotional self-awareness can be attributed to sensitivity to bodily sensations, as well as individual and cultural learning. In this way, more fine-tuned interoception – the sense of the emotional state of the body – is associated with more nuanced awareness of one’s own emotions (Craig, 2003).
The association between interoception and emotional self-awareness has been demonstrated by empirical studies examining accuracy in perceiving one’s own heartbeats. More accurate heartbeat perception is associated with greater ability to identify and describe one’s own emotions in adults (Herbert et al., 2011) and children (Koch and Pollatos, 2014), as well as in people with autism spectrum disorder (Shah et al., 2016). This association has also been replicated with other measures of interoceptive sensitivity, such as the ability to discriminate between similar levels of muscular strain (Murphy et al., 2018). Intervention work targeting interoceptive abilities has also been found to improve emotional self-awareness in healthy participants (Bornemann and Singer, 2017), suggesting a causal nature to this relationship. Such findings illustrate both that emotional experiences are inherently embodied, and that degree of conscious awareness of these experiences is dependent upon sensitivity to physical sensation.
Given the importance of physical sensation to the conscious experience of emotion, it would follow that motor action and its kinaesthetic feedback are likewise important. Most of the work on action and emotion has focused on how motor actions relate to the perception of emotion in others. For instance, imitating viewed facial expressions facilitates faster and more accurate emotional recognition (Wood et al., 2016). Social learning models suggest that mimicking others’ actions allows us to share in their subjective experience, and mimicry early in life facilitates development of an understanding of how actions relate to subjective experiences (Decety and Meyer, 2008). In support of this argument, Niedenthal et al. (2012) report that pacifier use in male infants is associated with lower emotional intelligence later in life, through inhibiting infants’ abilities to mimic the facial expressions of others, thus limiting opportunities for social and emotional learning.
Cross-sectional research has likewise found associations between emotional self-awareness and emotional actions. Poorer emotional self-awareness is associated with diminished ability to imitate and spontaneously produce emotional facial expressions (Trinkler et al., 2017), as well as lower expressivity in social and non-social situations (Wagner and Lee, 2008). Such findings indicate how conscious awareness of one’s own emotions facilitates more diverse and effective emotional communication. This is likely as subjectivity facilitates more refined conscious control of emotional actions.
The association between subjective awareness of emotions and conscious control of emotions can be seen in emotional regulation research. Identifying emotions is considered a fundamental step in effective emotional regulation (Gross, 2015). Furthermore, more differentiated awareness of emotions is associated with more frequent emotional regulation (Barrett et al., 2001). Greater emotional awareness and regulation has also been found to be associated with greater social success (Kimhy et al., 2016), as this allows for individuals to select more appropriate emotional actions. Likewise, difficulties interpreting and imitating motor actions is associated with greater emotional self-awareness difficulties (Brezis et al., 2017).
The subjectivity of emotion facilitates the intentional cognitive control of emotional actions, which in turn regulates emotional experiences. Moreover, this subjectivity leads to a wide diversity in emotional actions and expressions – the same emotion can result in many different actions, and the same action may be associated with many different emotions. This variability is reflected in diversity of emotional language, particularly in the frequency and diversity of emotion terms relating to action.
The relationship between emotion and action can also be seen on the neural level. The next section further details how these relations can be seen in the brain, before further discussing its relation to emotional regulation and psychopathology.
4. Brain bases for emotional communication
Ample neuroscientific evidence in monkeys and in humans has shown that the cortical sensorimotor regions, specifically the premotor and parietal cortices, are involved in emotional communication (Sato et al., 2015; Trautmann-Lengsfeld et al., 2013). These studies were inspired by the discovery of mirror neurons in monkeys. Single-unit recording studies in monkeys revealed that specific neurons of the ventral premotor cortex discharge both when the monkey executes specific hand actions and when it observes experimenters performing similar actions (di Pellegrino et al., 1992). These neurons have been named mirror neurons (Gallese et al., 1996; Rizzolatti et al., 1996). Later, mirror neurons were also found in the parietal cortices (Fogassi et al., 2005). As the superior temporal sulcus (and its adjacent temporal regions) contains neurons that respond during the observation of actions (Perrett et al., 1985), this region is thought to provide input to the mirror neurons in the premotor and parietal regions. Some researchers have proposed that these regions constitute a functional network, as the mirror neuron system, and are involved in important social cognitive functions, such as imitation and intention understanding (e.g., Williams et al., 2001). Hamilton (2008) proposed that the superior temporal region, parietal region, and inferior frontal gyrus represent the visual, goal, and motor features, respectively.
Direct evidence from a single-unit recording study in monkeys revealed that the neurons in the ventral premotor cortex discharge during observation of emotional facial communication, such as lip smacking (Ferrari et al., 2003). Several neuroimaging studies using functional magnetic resonance imaging (fMRI) in humans have confirmed the involvement of the premotor or parietal cortex in the processing of dynamic emotional facial expressions (Arsalidou et al., 2011; LaBar et al., 2003; Sato et al., 2004; Schultz and Pilz, 2009; Trautmann et al., 2009).
For example, in one fMRI study (Sato et al., 2004), brain activity was measured during observation of dynamic facial expressions, static expressions, and dynamic mosaic images. The results revealed that certain regions in the mirror neuron system, including the inferior frontal gyrus (the human homologue of the ventral premotor cortex in the monkey; Rizzolatti and Arbib, 1998), inferior parietal lobule, and superior temporal sulcus, were more active in response to dynamic facial expressions than to static expressions and dynamic mosaics.
Some other studies showed that observation of dynamic bodily gestures also activated the premotor or parietal cortex (Grèzes et al., 2007; Kret et al., 2011). Electroencephalography and magnetoencephalography studies have supported the rapid activation of premotor and parietal regions in response to dynamic emotion expressions. For example, an electroencephalography study reported activation of the inferior frontal gyrus within 200–300 ms in response to dynamic emotional (happy and disgusted) facial expressions, compared with dynamic neutral expressions (Trautmann-Lengsfeld et al., 2013).
Neuroimaging studies have suggested that activity in the mirror neuron system regions during observation of dynamic emotional expressions is related to the matching of observation and execution of actions (Carr et al., 2003; Hennenlotter et al., 2005; Kircher et al., 2013; Leslie et al., 2004; Likowski et al., 2012; van der Gaag et al., 2007). For example, Likowski et al. (2012) measured facial electromyography and fMRI simultaneously during observation of dynamic emotional facial expressions and found a positive association between facial muscle activity and activity in certain brain regions, including the inferior frontal gyrus. Hennenlotter et al. (2005) evaluated common patterns among brain regions in the observation and execution of smiling facial expressions and found shared activation in brain regions, such as the inferior frontal gyrus
Theoretical and empirical studies have explored the functional networking patterns of brain regions in the mirror neuron system during emotional communication. Hamilton (2008) proposed that the superior temporal region, parietal region, and inferior frontal gyrus represent the visual, goal, and motor features, respectively. In this model, mimicry can be implemented by direct connectivity from the superior temporal gyrus region to the inferior frontal gyrus, and goal-directed imitation can be accomplished by connectivity among the superior temporal sulcus region, parietal region, and inferior frontal gyrus. Sato et al.’s (2012, 2015) fMRI and magnetoencephalography studies applied dynamic causal modelling analysis to brain activity data obtained during observation of dynamic facial expressions versus dynamic mosaic images and found that the optimal model accounting for the data involved bidirectional (feedforward and feedback) modulatory connectivity between the superior temporal sulcus region and inferior frontal gyrus, which was accomplished as early as 200 ms after stimulus onset. Engelen et al.’s (2018) combined stimulation and fMRI study revealed that that the inferior parietal lobule communicates with the premotor cortex, as well as a number of other regions, including the amygdala, when processing the emotional content of actions. In short, these data suggest that the premotor and parietal cortices are involved in emotional communication and are possibly responsible for the matching observations with execution of emotional actions.
Beyond these sensorimotor regions, substantial neuroscientific evidence indicates an extended cortical and subcortical network, including the amygdala, insula, anterior cingulate gyrus, and orbitofrontal cortex (OFC). The evidence further suggests that, as in the case of the cortical mirror neuron system, these regions can be activated by mirroring actions (see meta-analysis by Molenberghs et al., 2012a).
The amygdala has been consistently implicated in the recognition, and experience, of emotion from faces, voices and bodies (Schirmer and Adolphs, 2017) and forms part of a neural network enabling context-appropriate social behaviours (Adolphs, 2010). Mimicking smiles has been linked to activity in the amygdala, as well as the striatum (Lee et al., 2006; Schilbach et al., 2008) and the amygdala is a key component of the Simulation of Smiles model, in which embodied simulation can be used to understand different types of smiles (Niedenthal et al., 2010). In humans, the amygdala and motor-related areas are coactivated when perceiving emotions (e.g., Van den Stock et al., 2011). In addition, there are direct pathways between the amygdala and cortical motor areas, linked to emotion-related brain structures (such as the STS and OFC) involved in emotional communication (Grèzes et al., 2014).
The insula is activated both in the experience of disgust (as evoked by unpleasant odours) and the observation of disgusted facial expressions (Wicker et al., 2003). Weakened spontaneous expressions of disgust in response to odours (Hayes et al., 2009a), and reduced ability to voluntarily pose disgusted expressions (Hayes et al., 2009b) and imitate at least some basic facial expressions (Trinkler et al., 2011) has also been reported in patients with Huntington’s disease, which is associated with a loss of volume in key social network structures, including the amygdala and insular cortex (Kordsachia et al., 2017). More recently, Braadbaart et al. (2014) have argued that the insula plays an important role in learning facial expressions, which would make this structure sensitive to mismatches between observed and imitated facial expressions.
Empathy has been argued to be based on an action-perception mechanism, with ‘affective’ empathy, such as mimicry and emotional contagion (which is likely to be shared across species) likely to be associated with neural systems involved in sensation, movement and emotion (i.e., premotor-parietal, temporal and subcortical regions; Ferrari and Coudé, 2018). Meta-analysis has shown that the anterior insular cortex, along with medial and anterior cingulate cortex are involved in empathy for pain and the direct experience of pain (Lamm et al., 2011). Along similar lines, a recent study used fMRI to compare representations of self and others' expressions of pain and found selectively greater activity for ‘self’ pain-related stimuli in the anterior mid-cingulate cortex, a region critical for pain perception and recognition (Benuzzi et al., 2018). Interestingly, areas of the insula and amygdala were more active during emotional expressions from a mother’s own child than another child, and brain responses were correlated with an indirect measure of empathy (Lenzi et al., 2009).
One way to recognise emotions in others might be to internally simulate the emotional state (e.g., Decety and Chaminade, 2003; Gallese, 2003; Goldman and Sripada, 2005; Keysers and Gazzola, 2007; Niedenthal et al., 2010; Winkielman, 2010). Simulation could occur relatively automatically and involve neural substrates that were activated for both recognition and experience (Heberlein and Atkinson, 2009). Simulation might be more important for understanding dynamic, ambiguous expressions than prototypical ones (Niedenthal and Ric, 2017; Rychlowska et al., 2014; Sato and Yoshikawa, 2007) and might also be more effective for some people than others (Hess and Fischer, 2014; Niedenthal and Ric, 2017). Heberlein and Atkinson (2009) suggest that evidence is consistent with shared substrates for emotion recognition and experience in the amygdala and OFC, but less clearly consistent with a simulation model (c.f. somatosensory cortices). However, by prioritising and enhancing processing emotional information (at least visual, but perhaps also auditory) the amygdala and OFC could be influencing simulation processes in other parts of the network (Heberlein and Atkinson, 2009). Finally, Williams (2013) suggests that systems for goal-directed action are connected with amygdala-orbitofrontal circuits central to emotional learning.
5. The hierarchical organization of motor control and emotional regulation
For animals with a limited range of stereotyped behavioural responses to either rewarding or aversive conditions, the relationship between conditioning and sensorimotor systems can be made relatively easily. However, in humans, the expression and communication of emotional responses is highly flexible and ever-evolving in relation to cultural demands. This requires another level of control over action execution which is intentional and self-aware. In this section we consider two actions. First, we consider a monkey in a motor learning experiment, moving a robotic arm to guide a cursor onto a target on the computer screen to acquire a juice reward. Secondly, we consider a parent witnessing her child stepping dangerously out into the road. Her initial reaction is to generate and exhibit fear, but she realises this reaction might scare the child and make things worse. Therefore, some cognitive control involving self-awareness is likely to moderate the initial sensorimotor response.
As discussed in the introduction, for any action, we plan (goal setting), execute the plan (action), and adjust the action according to feedback (error detection and learning) until the desired outcome is achieved. In the motor learning experiment, where macaque monkeys move a robotic arm to acquire a juice reward, the monkeys manage this easily, achieving optimal performance within just a few tens of trials of training. A clockwise force field is then turned on, and the reach trajectory is perturbed in the force direction such that the monkeys fail to reach the target in time. Sensing the change, the monkeys learn to adapt to the force field and again in a few tens of trials, can accomplish the task – the movement trajectory becomes straight and dynamics reflects an optimal profile. The monkeys appear to have learned to reset the movement strategy to accomplish the goal. If at this point the force field is removed, movement trajectories are once again deviated, and the monkeys start another adaptation cycle to accommodate the perturbation.
Neuronal activities have been recorded from motor cortical structures in these experiments (Li et al., 2001; Padoa-Schioppa et al., 2002, 2004). In the motor cortex, ensemble neuronal activities encode the target direction and movement synergy and these activities are aligned when no force field is present. When a force field is turned on, the ensemble activities initially align with the movement kinematics (target location) but gradually change to reflect the dynamics rather than the desired kinematics of the upcoming movement. Thus, the neuronal activities reflect a sensorimotor or kinematics-dynamics transformation to meet the desired goal. This simple example highlights the core component processes of motor control: goal (to reach the target; specified in kinematics), movement (muscle synergy required to execute the movement or dynamics), error detection and post-error learning behavioural adjustment.
There are a few issues worth considering from these motor learning studies. First, movement control is hierarchical only in the sense that the sequence of events unfolds in time but not that the processes of control are unamenable to change. Second, when the environment is stable, hierarchical interaction is established in favour of goal to action translation rather than outcome monitoring and goal resetting. As goal action translation becomes most expedient, a habit is formed and the behavioural contingency may transpire without awareness. Third, much of neuroscience research have focused on understanding the neural processes subserving the “linear” chain of command and less is known about the mechanisms serving how the outcome resets the goal.
In this example of motor control the goal is clear – monkeys must reach the target in order to obtain juice reward. Whereas this applies to many of the actions one routinely performs, social and emotional communication is a different matter. Then there are conflicting goals and behaviour needs to be optimised to meet these goals. If we now consider the example in which a parent whose child is about to step dangerously out into the road, she faces a conflict between exhibiting a prepotent response and an anticipation of the effects of her behaviour on that of another person, which conflicts with the prepotent response. Therefore, and additional level of cognitive control is employed to moderate the initial reaction.
5.1. Conflict control
Cognitive control facilitates decision making in a changing environment. One of the cardinal features of cognitive control is the ability to learn from the outcome of our actions and revise our knowledge of world and action plans accordingly. This ability to learn and change is supported by a brain system that integrates moment-to-moment information into our behavioural repertoire. By exploring the changing environment, individuals strengthen behavioural routines that lead to positive outcomes and revise those in association with negative consequences. Cognitive control is particularly critical in the face of conflicting goals.
In the laboratory, investigators have combined brain imaging or electrophysiological recording and a variety of behavioural tests (e,g., go/no-go; Simon, Stroop flankers; stop signal task) to examine the neural processes of cognitive control. Here we use recent studies of the stop signal task (SST) as an example to highlight the neural circuits of cognitive control and the interactive nature of regional processes to support optimal performance in the face of conflicting goals.
In the SST, a frequent “go” signal instructs participants to quickly respond (by pressing a button) and an occasional “stop” signal (1/3 or 1/4 in frequency) instructs participants to withhold the response. The stop signal follows the go signal and the time interval – stop signal delay (SSD) – determines how difficult it is for participants to withhold the response. With a long SSD, the motor command to respond has likely been relayed to the muscle and reached a “point of no return” (Logan, 2015), and a stop error ensues. In a typical SST experiment, the SSD is adjusted stop-trial by stop-trial either pseudo-randomly or following a staircase procedure, so that participants achieve success in only half of the stop-trials. There are two main reasons in manipulating this variable in the SST. First, it would allow the computation of the stop signal reaction time – the time needed to stop a motor response – using the race model (Logan et al., 1984). Second, there will be a sufficient number of error trials, so one can examine the neural processes underlying error detection and post-error behavioural adjustment (Chang et al., 2014; Ide and Li, 2011; Li et al., 2008a, b). Participants are confronted with two conflicting goals in the SST – a speeded response to the go-signal to meet a time window and a cautious act so the response can be withheld when the stop-signal appears. As a result of these conflicting goals, participants typically fluctuate in go-trial reaction time (RT) and slow down after committing a stop-error – an observation termed post-error slowing. That is, compared to a go-trial that follows another go-trial, the go-trial that follows a stop-error (or stop success) trial is prolonged in RT. There are different accounts of why participants slow down following a conflict (stop-trial), including diversion of attention (Van der Borght et al., 2016) and conflict-elicited control. Without going into the details of the debate, here we elaborate on the behavioural and neural evidence in support of conflict-elicited cognitive control.
In a series of studies, investigators posited that, because stop-trial occurs randomly but influences go-trial RT, it is possible that individuals track the occurrences of stop-trial and slow down in response when they anticipate a stop signal. Using a Bayesian model, Yu and colleagues estimated the trial by trial likelihood of stop signal or P (Stop) and showed that a higher P(Stop) is associated with prolonged go-trial RT – an observation termed “sequential effect.” (Yu and Cohen, 2008). That is, participants proactively prolong the response if they anticipate that a stop signal will occur. This provides a strategy to negotiate the conflicting goals between speedy and cautious go-responses.
Combining fMRI and the Bayesian model of SST performance, studies have delineated the neural correlates of conflict anticipation, RT slowing, and unsigned prediction error or the absolute discrepancy between anticipated and actual outcome – a surprise signal. Regional activities in response to P(Stop) are located in the anterior pre-supplementary motor area (pre-SMA) and bilateral inferior parietal cortices (Hu et al., 2015). RT slowing engages the posterior pre-SMA and bilateral anterior insula, the latter of which has an acknowledged role in conflict awareness (Ullsperger et al., 2010). Importantly, using a Granger causality analysis, investigators are able to demonstrate directional influence of P(Stop) on RT activities. An event-related potential study in combination with source reconstruction confirmed these findings (Chang et al., 2017). Thus, these studies together support proactive control of motor response in the SST. Further, a distinct area of the medial prefrontal cortex (mPFC) – in the dorsal anterior cingulate cortex – responds to unsigned prediction error, highlighting the functional heterogeneity of the mPFC (Hu et al., 2015; Ide et al., 2013). An important question pertaining to the control hierarchy concerns the roles of prediction error signal in driving SST performance and remains unanswered.
The aforementioned studies describe how we proactively control motor response in anticipation of conflicting goals. A complementary process of cognitive control is the reaction evoked by an infrequent, behaviourally relevant stimulus. In the SST, the stop signal appears infrequently and is highly relevant, as it instructs an interruption of the motor command. In imaging studies of the SST this issue is commonly addressed by computing the stop signal reaction time (SSRT), as estimated from the race model, and identifying its neural correlates on the basis of between-subject analyses. A circuit involving right inferior frontal cortex, anterior pre-SMA and subcortical structures including the caudate nucleus has been identified in supporting reactive response inhibition (Cai et al., 2017; Duann et al., 2009; Li et al., 2006). Brain regions within this circuit interact to respond to the stop signal and interrupt the motor action. RT slowing following an error can therefore also be conceived in terms of a reactive process. That is, error signals may engage cerebral processes of control and prolongs RT in the next trial.
Earlier imaging studies demonstrated that a cortical-thalamic-cerebellar-cortical circuit, in congruence with known anatomical connectivity, supports the reactive response of post-error slowing (Hendrick et al., 2010; Ide and Li, 2011; Li et al., 2008b). Thus, there are both reactive and proactive processes that subserve the hierarchy of cognitive control. In contrast with motor control, which we illustrate with a force field learning experiment in macaque monkeys, cognitive control requires constant outcome monitoring and resolving conflicting goals, and engages multiple loops of reactive and proactive control circuits. The hierarchical nature of cognitive control can only be meaningfully considered with this complexity in mind.
A few issues can be considered in contrasting cognitive and motor control (though we might equally refer to high level and low level motor control). First, motor control often comes with a clearly set goal whereas cognitive control is demanded in situations where multiple goals are in place and often in conflict. Second, with a set goal, motor control may become a routine with repeated practice or a habit that is “closed-loop” and expressed without concomitant awareness. Cognitive control, in contrast, is often engaged to override a habit and requires active monitoring (and awareness) of performance to be effective. Finally, these control mechanisms involve very distinct circuits even when the same sensory (input) and motor (output) modalities are engaged. These mechanisms exist across primate species and similar mechanism located in the dorsal aspect of the anterior cingulate and prefrontal cortex, show many similarities in serving cognitive control (Mansouri et al., 2017).
5.2. Hierarchical control of complex actions
One of the other differences between the monkey and the parent in our examples is the involvement of other psychological processes such as the recall of several behavioural rules and conventions the mother may have learnt. An important aspect of motor control is the way that a set of actions and rules can be integrated to determine a coherent response, by incorporating abstract concepts into the organisation of response. Importantly, studies have converged to suggest a hierarchical organization or rostrocaudal gradient in the frontal cortex with the rostral and caudal regions respectively supporting more abstract and concrete representations (Azuar et al., 2014; Badre and D’Esposito, 2009; Badre et al., 2008; Koechlin et al., 2003). It is suggested that more rostral regions may be critical for progressively later stages of perception and action (Fuster and Bressler, 2012).
This complexity of cognitive functional organisation has also been construed in terms of the time scale of activities. It is posited that the frontal cortex embodies a rostro-caudal hierarchy that is sensitive to different time scales of environmental dynamics, with caudal and rostral regions each engaging faster (shorter time scale) and slower dynamics (Badre, 2008; Botvinick, 2008; Fuster, 2004; Koechlin and Hyafil, 2007; Koechlin et al., 2003; see, however, Zhang and Rowe, 2015). The time scale of activities has also been explored for neural circuits beyond the prefrontal cortex. For instance, by creating distinct narratives with word changes while preserving the grammatical structure across stories, investigators reported different neural responses between the stories that gradually increased along the hierarchy of processing timescales (Yeshurun et al., 2017). In early perceptual auditory cortex the differences in neural responses between stories were relatively small. In contrast, in areas with the longest integration windows, such as the precuneus, temporal parietal junction, and medial frontal cortices, there were large differences in neural responses between stories. Further, this gradual increase in neural differences between the stories was correlated with an area's ability to integrate information over time. These findings suggest that hierarchical control of complex mental act may unfold according to the temporal scales at which component processes take place.
5.3. Valence
Whether actions are favoured or discouraged depends upon learning systems which attribute a valence to their outcomes. Encountered environmental stimuli are encoded with positive or negative valence and mediate behavioural changes accordingly, with changes being encoded in hypothalamic nuclei, which also mediate neuroendocrine stress responses (Kim et al., 2019). According to traditional learning theory, when valence is attributed to environmental stimuli, associated behaviours are either reinforced or punished, meaning that they either increase or decrease. A range of psychological models propose systems that explain how these systems might work in humans. (e.g., Davidson, 1992, 2000; Davidson et al., 2002; Gray, 1982, 1987; Lang and Bradley, 2010). Neurologically based approach and avoidance systems are thought to mediate emotional sensitivity, personality, positive and negative affective experiences, and goal- directed behaviour (e.g., Davidson, 1998; Fowles, 1988; Lang and Bradley, 2010; Laricchiuta, 2015; McNaughton and Corr, 2014).
Gray’s (1982) early two-system model of motivation proposed a behaviour inhibition system (BIS) and a behaviour activation system (BAS). BIS activation is sensitive to anticipation of threatening stimuli and inhibiting aversive outcomes, and responsible for regulating negative feelings such as anxiety and fear. The BAS is sensitive to anticipation of reward and approaching appetitive experiences, and responsible for regulating positive feelings such as hope, elation, happiness. Within Gray’s model, positive affect (PA) and negative affect (NA) are viewed as state manifestations of underlying regulatory reward-driven and punishment-driven motivational systems. The BIS is thought to primarily involve serotonergic and noradrenergic pathways (Gray, 1994), whereas, the BAS is thought to be mediated by dopaminergic pathways (Depue and Iacono, 1989).
Self-report motivational scales designed to assess motivational systems such as the BIS/BAS are not direct measures of motivation or underlying neurophysiological activation. Ongoing integrative research investigating neurological activation, explicit (effortful, awareness) and implicit (autonomous, spontaneous) motivational, cognitive and affective processes is required to better understand motivated action. A more recent theoretical development by McNaughton and Corr (2014) distinguishes underlying independent motivational systems from more surface level behaviours based on approach and avoidance interactions that may lead to the activation of approach-avoidance conflicts. Their model purports that more surface level behaviour may be determined interactively, even when the underlying approach and avoidance motivational systems are independent (Corr, 2013).
5.4. Self-regulation and being regulated by others
Further models place the valence systems within a context of emotion regulation. Higgin’s Regulatory Focus Theory (RFT; Higgins, 2000) and Carver and Scheier’s (1998, 2001) self-regulatory models provide a theoretical framework for investigating the interface between motivational, cognitive and affective systems involved in goal-directed action and emotion (Higgins, 2000; Carver and Scheier, 1998, 2001).
Rooted in self-regulatory motivational sensitivities, approach and avoidance goal striving actions represent sustained activity towards desirable outcomes and away from undesirable outcomes, respectively. Goal-directed action is guided by the process of ongoing self-regulation that modulates an individual’s thoughts, affect and attention (e.g., Dickson et al., 2017; Winch et al., 2015). In sum, a two-system view of motivation has persisted over time, even though different labels have been put forward to define approach and avoidance systems. Approach-and avoidance-oriented actions and emotional sensitivities in response to rewarding or threatening stimuli are seen as rooted in specific neurological brain systems (Gentry et al., 2016; Steinman et al., 2018). Laricchiuta (2015) posits that brain networks are implicated in instigating approach and avoidance behaviours in reaction to salient stimuli. Such networks include cerebral nodes interconnected as prefrontal cortex, amygdala, hypothalamus, striatum and cerebellum.
There is also evidence that the dopaminergic system and interconnected brain regions process positive and negative stimuli to reinforce approach and avoidance behaviours (Gentry et al., 2018). Although sensorimotor reactions to appetitive or aversive stimuli are typically spontaneous and automatic, goal-directed conflict, lack of goal progress or unpleasant emotions may stimulate reflective awareness, goal planning and more effortful cognitive control. McNaughton and Corr (2014) draw an important distinction between underlying orthogonal motivational systems and possible approach and avoidance interactive surface level behavioural conflicts.
A key aspect to emotional regulation is the capacity to be regulated by others, whether during childhood by adults or by peers. This requires bridges to be built between codings for one’s own emotion-action states and those of others. We are able to do this by generating sensory changes in our own body state to identify how someone else is feeling (Craig, 2003; Seth, 2013), a process controlled by the somatosensory and prefrontal cortices (Adolphs et al., 2000; de Gelder, 2006; Hornak et al., 2003; Radice-Neumann et al., 2007). Although these internally generated emotional responses generally lack intentional control and awareness, they significantly impact our recognition of nonverbal emotion cues (Naranjo et al., 2011; Neumann et al., 2014). They also reflect our desired outcome for the social interaction we are engaged in and thus modulate our emotional experiences in response to these cues (Naranjo et al., 2011; Soussignan, 2002). Our interoceptive response, desired outcome and ultimate interpretation of the emotional experience are influenced by gender, social roles and culture (Chaplin et al., 2005; Fischer et al., 2004). As outlined in the embodied-contextual model of emotion, our interpretation of others’ feelings is further mediated by previous experience and the environmental context in which the interaction took place (Barrett, 2017; Eder, 2017).
The influence of context becomes more apparent as we develop and gain more sophisticated cognitive skills. With increased cognition, we learn that an emotion expression may have multiple (and often conflicting) meanings depending on the context in which it is produced. In response, we learn to rely upon our prior experience and memories to accurately interpret and respond to the emotion expressions of others (Boone and Cunningham, 1998; Buck, 1991; de Gelder, 2006). Thus, recognising and appropriately responding to emotion operates as part of a feedback system, one in which our analysis of the actions and movements of others as well as our own internally generated sensory changes, leads to learning. The responses we receive during social interactions provide feedback and guide our future behaviour – if the response is a rewarding one, we are more likely to behave similarly in future social interactions, but if the response is a punishing one, we will learn to adjust our behaviour to pursue a more positive emotional outcome (Baumeister et al., 2007; Gendolla, 2017). We are constantly appraising the meaning of the interaction and modifying our emotional actions in response (Ridderinkhof, 2017). We then enact cognitive and motor control to guide future responses in similar contexts through goal striving actions that result in an (usually desirable) outcome (Griffiths et al., 2014; Higgins, 2000). This learning should ultimately contribute to conscious adaptation that leads us to choose actions that are appropriate within a social context (Baumeister et al., 2007).
It is therefore evidence that the 'regulation' of emotion is directly concerned with learning patterns of behavioural responses to environmental stimuli such that they minimise the experience of negative valence and maximise the positive. This requires ongoing and iterative motor learning and conditioning. Emotion regulation for humans, involves constant appraisal and reappraisal through explicit or implicit regulatory processes (Braunstein et al., 2017). People draw from a large number of different strategies in the service of regulating their emotions (Heiy and Cheavens, 2014), but the neural correlates of emotion regulation have been studied primarily through fMRI studies of reappraisal (the cognitive reinterpretation of emotionally evocative events), and sometimes distraction or expressive suppression (Etkin et al., 2015; Frank et al., 2014).
Broadly speaking, explicit emotion regulation through reappraisal recruits frontal cognitive control regions of the brain, including regions involved in sensorimotor control, with concomitant changes in subcortical regions, including the amygdala and ventral striatum (Ochsner et al., 2012). A consistent finding across meta-analyses is that down-regulation of emotions (particularly through reappraisal) recruits the dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, and anterior cingulate cortex (Buhle et al., 2014; Etkin et al., 2015; Frank et al., 2014; Kohn et al., 2014).
These findings mesh with psychological models of the process of emotion regulation whereby reappraisal involves working memory and selective attention to generate and maintain the reappraisal representation, inhibition to prevent prepotent responses, and monitoring to assess the effectiveness of the reappraisal response (e.g., Ochsner et al., 2012). For example, Kohn et al. (2014) note that activation of the ventrolateral prefrontal cortex also occurs during emotion generation and appraisal, and, as such may reflect emotional salience as well as the operation of regulatory processes like inhibition. In addition, the anterior middle cingulate cortex has been described as a limbic motor control region, involved in controlling motor responses in situations of reward and punishment (Kohn et al., 2014). In tandem with these regions of activation, explicit down-regulation of negative emotions involves reduced activity in the amygdala, known for signalling the presence of emotionally-arousing stimuli, and the ventral striatum, known for representing the reward value of stimuli. Other regions may also be implicated in explicit emotion regulation. For example, the supplementary motor area, which also is active during emotional mimicry tasks and mental imagery studies, and plays a role in preparatory motor movement, is noted to be active in up-regulation and down-regulation of emotions (Etkin et al., 2015; Frank et al., 2014; Kohn et al., 2014).
6. Development, psychopathology and disordered states
Due to its deep evolutionary roots, our ability to perceive emotion begins early in life and is thought to be an automatic and spontaneous component of social interaction (Boone and Cunningham, 1998) connected to early mimicry (Decety and Meyer, 2008). A recent study showed that newborns appear to be sensitive to dynamic faces expressing emotions at birth (Addabbo et al., 2018). Infants between five and seven months of age start to preferentially attend to fearful faces rather than happy faces, and disengage attention less readily from fearful faces, than from happy or neutral faces (Hoehl, 2014). The various neurological structures (e.g., prefrontal cortex, amygdala) that control our capacity to recognise and express emotions continue to develop throughout childhood and adolescence, allowing us to perceive more nuanced and subtle differences in the emotions expressed by others (Herba and Phillips, 2004; Thomas et al., 2007). Hence, the accuracy in which we can differentiate between emotions is mediated by both age and gender, with female children and adolescents showing more accurate perception than males (Herba et al., 2006; Lawrence et al., 2015; McClure, 2000). Cognition is also strongly associated with recognising how someone else is feeling (Lawrence et al., 2015; Thomas et al., 2007), likely because accurate perception requires simultaneous processing and integration of many different cues.
Our choice of actions within the emotional regulation system can be significantly affected by experience. For instance, studies with infants and children who have been abused have shown that this aberrant social experience alters their perception of facial and bodily movements indicative of anger (Pollak et al., 2000; Pollak and Kistler, 2002; Pollak and Sinha, 2002). Specifically, Pollak and his colleagues found children who were abused to be more in-tune with nonverbal expressions of anger in their environment. Although this may be an adaptive response reflecting a desire to avoid punishing responses in future interactions, results of these studies additionally indicate that children who are abused may attribute anger to expressions intended to elicit a more sympathetic or positive response. These results indicate that children who are abused may view even rewarding responses as punishing ones, and thus respond negatively or withdraw from the interaction, thereby minimising the frequency and range of social interactions they have with others.
Breakdowns in the feedback system are seen in many neurological populations where the neuroanatomical circuitry necessary for recognising, analysing, and responding to the facial and bodily movements of others has been damaged. For instance, people with traumatic brain injury (TBI) who commonly experience damage to the prefrontal cortex, limbic system, and parietal cortex, have been shown to have poor social outcomes due to the difficulty they have understanding and identifying their own emotions (i.e., alexithymia; Henry et al., 2006; Neumann et al., 2014; Williams and Wood, 2010) as well as difficulties in recognising, interpreting, and accurately responding to the nonverbal emotional expressions of others (Babbage et al., 2011; McDonald, 2005; Milders et al., 2003; Neumann et al., 2012; Zupan et al., 2014, 2016). Patients with Parkinson’s Disease, resulting in damage to the basal ganglia, show impaired facial expression recognition, which is linked with voluntary control of facial muscles (Gray and Tickle-Degnen, 2010; Marneweck et al., 2014). Disruption of amygdala-cortical pathways, such as in autism spectrum disorder (Gotts et al., 2012) or amyotrophic lateral sclerosis (ALS, Passamonti et al., 2013), may also affect emotional perception and social interaction.
Another way that brain disorder impacts upon the action-emotion relationship is to diminish flexibility. The diversity, flexibility and range of action seems to be diminished in psychopathological conditions like schizophrenia, autism spectrum disorder and obsessive compulsive disorder, where behaviours are often quite inflexible and stereotyped, and the outward expression of emotion quite fixed.
Schizophrenia for example, is well characterised by negative symptoms including flat or blunted affect, emotional withdrawal and apathy. Reduced emotional expressivity in the context of intact subjective emotional experience (Kring and Moran, 2008) has led some to conceptualise the symptom of blunted affect in schizophrenia as reflecting or mirroring abnormality, given the previously described role of the motor system in the physical action of emotion expression and the simulation of others’ emotive states (Gaebel and Wolwer, 1992). Several different studies show mirror neuron disturbances in schizophrenia (Enticott et al., 2008a; Mehta et al., 2014a), which directly correlate with negative symptoms such as affective blunting, anhedonia, avolition and alogia; as well performance on facial emotion processing tasks (Kohler et al., 2003, 2010; Lee et al., 2014; Turetsky et al., 2007).
Deficits in facial affect processing are also core to the social cognitive profile of schizophrenia (Kring and Elis, 2013), and are consistently associated with reduced recruitment of a neural network encompassing limbic and prefrontal areas including the mirror neuron enriched inferior frontal gyrus, as well as regions in the occipital and temporal cortex (Gur et al., 2007, 2002; Kilner et al., 2009; Leitman et al., 2011; Taylor et al., 2012). These widespread neural abnormalities and associated behavioural deficits appear to reflect disruption in the activity and integration of several systems involved in general face perception, motor behaviour and emotional states (Eimer et al., 2011; McCleery et al., 2015; Rossell et al., 2014; Taylor et al., 2012; Van Rheenen et al., 2017). Relevantly, mirror neuron-related motor system abnormalities in schizophrenia may result in an inability to adequately mimic and recognise the emotional expressions of others, and thus the extent to which their emotional state can be internally simulated (Enticott et al., 2008b; Haker and Rössler, 2009).
Mirror neuron disturbances have been directly linked to poor theory of mind in schizophrenia (Mehta et al., 2014b). However, it is possible that this relationship is somehow mediated by other top-down abnormalities as the mirror neuron system is likely a predictive system that is activated not only by the visual representation of an action but by its goal or intention (Kilner et al., 2007a; Umiltá et al., 2001). Indeed, mirror neuron activity appears to be moderated by the context in which an action occurs (Liepelt et al., 2009), as well as the biases of the observer (Liepelt and Brass, 2010; Molenberghs et al., 2012b). Thus, intentions inferred by observing actions are biased by prior knowledge, reflecting the outcome of the brain’s attempt to minimise differences between what is observed and what is expected (i.e., a prediction error) (Kilner et al., 2007b; Maranesi et al., 2014; Miall, 2003).
In schizophrenia, aberrant predictive coding may contribute to symptoms by reducing precision for prior expectations, leading to abnormal attentional control over sensory information and altered integration of top-down and bottom-up input (Adams et al., 2013; Stephan et al., 2009; Tschacher et al., 2017). Several studies show abnormal cognitive control of bottom-up emotional experience in schizophrenia, indicating deficient emotion regulation by lateral prefrontal control regions that are consistently hypoactive in the presence of emotionally evocative stimuli, with this hypoactivation perpetuated in patients with affectively relevant negative symptoms, such as alogia, avolition and blunted affect (Anticevic et al., 2012; Dichter et al., 2008; Potkin et al., 2002; Vai et al., 2015). Further, ventrolateral-orbitofrontal cortex activation during emotion processing does not appear to be modulated by context in schizophrenia as it is in healthy individuals, suggesting that patients with schizophrenia do not adequately integrate prefrontal representations of existing knowledge into their evaluations of social stimuli (Leitman et al., 2011).
Indeed, it has been shown that negative symptoms in schizophrenia are associated with an increased tendency to over-weight sensory information relative to prior expectations when making inferences about the social actions of others, which results in an inability to accurately predict others’ intentions (Chambon et al., 2011). On the contrary, when inferring intent during interactions between others and meaningless objects, patients with schizophrenia over-weigh prior expectations over visual (sensory) information, and this top-down bias correlates with the severity of positive symptoms (Chambon et al., 2011). Thus, it appears that non-social situations in schizophrenia invoke heightened conviction in prior beliefs even in the face of contradictory external evidence – a mismatch of which would normally give rise to a prediction error that allows for the readjustment of one’s worldview. This over-reliance on (potentially aberrant) prior beliefs fits with arguments that schizophrenia reflects an impaired separation of one’s own intentions from that of others, resulting in a disconnect between action and free will that gives rise to positive symptoms involving passivity experiences (the misattribution of intentions to non-agents) or paranoia (attributing intent in the absence of any) (Chambon et al., 2011; Frith, 1987).
7. Linguistics
With this understanding of the current state of action research as a backdrop, our team was specifically tasked to review the language that people use to express feelings related to action. To better understand, the range of verbally articulated feelings that are expressed in the English language, a small task team within the Human Affectome Project led a computational linguistics research effort to identify feeling words (Siddharthan et al., 2018).
Results were extracted from the Google n-gram corpus (Younes and Reips, 2019), which includes roughly 8 million books and then manually annotated by more than one hundred researchers from this project. This resulted in 9 proposed categories of feelings and a new affective dataset that identifies 3664 word senses as feelings. Of relevance to this review is a category related to Actions and “Prospects”, which was defined as follows:
“Feelings related to goals, tasks and actions (e.g. purpose, inspired), including feelings related to planning of actions or goals (e.g., ambitious), feelings related to readiness and capacity of planned actions (e.g. ready, daunted), feelings related to levels of arousal, typically involving changes to heart rate, blood pressure, alertness, etc., physical and mental states of calmness and excitement (e.g. relaxed, excited, etc.), feelings related to a person’s approach, progress or unfolding circumstances as it relates to tasks/ goals within the context of the surrounding environment (e.g. organised, overwhelmed, surprised, cautious, etc.), feelings related to prospects (e.g. afraid, anxious, hopeful, tense, etc.).“
This subset of the results included about 1137 feeling words, including 130 words that were judged by individual raters to express AF within the context of planning (251 words). About 48 anticipatory feeling words were exclusively related to feelings of fear and anxiety, whereas about 54 words expressed feelings of optimism, while three smaller clusters of about 10–15 words expressed feelings of hope, suspicion or suspense (see word corpus of the Human Affectome Project). Although it was not within the scope of this effort to undertake a formal analysis of this dataset, we reviewed these feelings words and we attempted to roughly organize the words into discernable categories. The individual word senses and this sorting attempt can be found in the supplemental data accompanying this review. However, a degree of caution should be exercised in the interpretation of this sorting effort, as it was created only to give us an initial sense of how feeling words related to the various stages of sensorimotor function, as shown in Fig. 2.
Fig. 2.
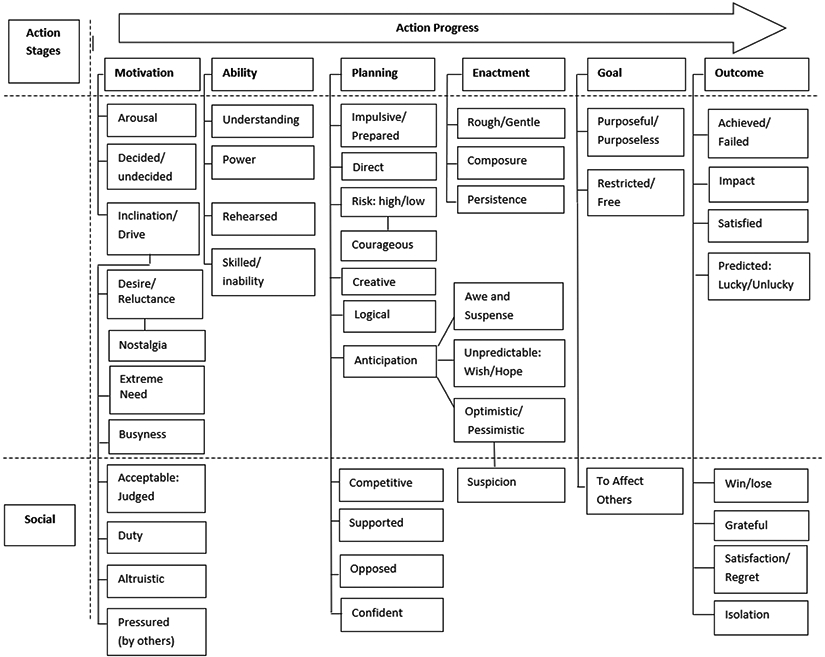
Schema of how feeling words may relate to stages of sensorimotor function. Emotion word categories are considered sub-categories of action stages (see Fig. 1) which may have a social or non-social dimension. In the case of inclination and anticipation, further sub-categories are listed.
From our perspective, these feeling words are interesting, relevant and warrant further study. A significant number of words simply described general levels of arousal (e.g., calm, aroused), but many feeling words were very specific and reflected different aspects of action-related thought. For example, feelings related to the Hierarchy of Goals included having a sense of purpose, immediate physiological needs, social/moral obligations, external influences (e.g., social prodding), the acquisition of resources, competitiveness, sentimentality, and even fate/superstition. In this area, recent research in monkeys has provided new insights about the role of the frontopolar cortex in monitoring the significance of current and alternative goals (Mansouri et al., 2017). Current goal-management models involve arbitration processes between exploitation and exploration behaviours (Donoso et al., 2014) and additional research is needed to determine whether humans may have additional cognitive capacities for the directed exploration of concurrent alternative strategies (Mansouri et al., 2017). So, this roster of articulated feelings which appears to help us better understand the range of goal priorities in humans will be useful when formulating future research.
Other feelings that we reviewed related to Planning and Coordination. This included competing priorities, the degree of creativity needed/employed, decision speed, risk involved, readiness, optimism/pessimism about prospective outcomes, the degree of rationality (e.g. irrational, rational), inclination towards action (e.g., reluctant, undecided, inclined), the degree of caution to be exercised, the level of aggressiveness employed, and assessments of persistence (e.g., resistless, persistent). In neuroscience research, the prefrontal cortex is the primary focus when it comes to planning, executive attention, decision-making, and inhibitory controls (Fuster, 2019), and many of aspects of planning and coordination have been already been subjected to a considerable degree of research. However, the full scope of PFC function is still not well known (see Burgess and Stuss, 2017, for a historical review), so just having an initial inventory of articulated feelings in this area is helpful.
Feelings related to the outcomes of actions, assessed the degree of success, external assistance, luck involved, the predictability of the result and overall acceptability of the outcome. While feelings of suspense reflected unresolved circumstances. Finally, feelings related to outcomes from a personal perspective additionally related freedom, composure, understanding, skill level and power.
A principal observation made during this categorisation exercise was that there are many categorical overlaps. Many words will fit into more than one category because the stages are interdependent to a large degree. A single word sense may describe the goal of an action as well as its motivation, whilst motivation is also dependent upon ability (e.g., knowing that you can do something successfully is a prerequisite to having the drive to do it). Given the hierarchical, feedback-dependent nature of motor control, one may always argue that it is the outcome that is really the goal of on action. For example, feelings of courage in the execution of an action may suggest careful judgment of risk in its planning. A person’s judgement of risk would impact upon motivation. Alternatively, feelings of courage may be considered to reflect that person’s ability as a character trait, or an attribution that is dependent upon judgement by others. Indeed, it may be the goal of the person undertaking these actions to be to be considered courageous by others, and this may subsequently be the outcome of the action.
Similarly, affordance learning theory argues that the properties of an object will shape the action that is enacted towards it. Social affordance theory similarly (Marsh et al., 2009) argues that sensorimotor states are determined by expectations of the consequences of an action. Therefore, a word used to describe the goal of an action may also describe the action’s expected consequences on others, as well as the way that action is performed.
Also, because of the very nature of embodied cognition, action words often reflect emotions or feelings being expressed metaphorically. This is particularly the case for words used to describe how one person might affect another person. Therefore, words that can be used to describe a simple action can also be used to describe the nature of one person’s behaviour towards another. In this respect, words such as ‘snare’ or ‘stifle’, which are used to describe the goal of an action as to have a restricting effect, can also describe social oppositionality.
Another way of explaining this is to consider that some words have evolved to serve actions at a high level of the action organisational hierarchy. As such they have more metaphorical properties and will serve a range of actions, whether they have literal, selfish or social properties. A word such as emancipate would seem to be free from any specific action form but describes the relationship between an action and its consequences.
Nonetheless, we do think that this inventory of English feeling words may have some utility. Fig. 2 is not intended as a final model, and it has not been tied to corresponding neuroanatomy. Rather, it is intended only as an illustration of the ways in which articulated feelings might be related sensorimotor control. Additional research in each of these areas will be needed to determine whether our feelings can be closely tied to these areas of neural function.
8. Conclusions
In the first part of our paper we reviewed the relationship between emotion and the sensorimotor system. We showed that the sensorimotor system is intrinsic to the communication of emotional states between individuals, and how the nature of this communication develops and evolves from expression of simple emotional states to much more complex communication at a more abstract level and higher hierarchical level of organisation. Sensorimotor control mechanisms regulating emotional states have evolved from serving simple allostatic functions through selection of stereotyped actions and optimised motor performance in most non-human animals, to having a capacity for highly flexible, novel and creative responses, drawing upon a wide range of cultural and physical influences, operating across differing temporal scales, to satisfy conflicting social and appetitive goals. The sensorimotor system of representation is key as part of appraisal and reappraisal systems that modulate action and emotional awareness. In the second part of this paper, we reviewed the process creating a linguistic framework that categorises feeling words within the context of these appraisal and reappraisal systems.
Considering the more granular array of feeling words that were generated in this project, a large proportion of the words in the original list were considered to describe feelings related to actions. This is unsurprising given that embodied cognition theory argues that feelings and emotions are intrinsic to the sensorimotor states that are used to communicate them. We found that further categorisation of words according to action stage was an exercise with limited value with respect to obtaining a neat and reliable classification but was of more value for learning the reasons why this was the case. It was often found that words applied to multiple categories, which is unsurprising given that the meaning of a word is dependent upon its context. This was especially so where words described action properties at a relatively high level within the action progress hierarchy. At these high levels, categorisation would depend upon the tense which is applied, whether it is considered as being done by the person or to the person, or whether there is a social context. Multiple classifications of the same word also seemed dependent on the integrated nature of sensorimotor control, by which we mean that even allocating aspects of sensorimotor control to action stages is arbitrary to some degree. For example, the desire for a successful outcome is inherent to motivation, which is at least partially determined by ability and skill. Nevertheless, despite these reservations, we can conclude that a sensorimotor action framework can throw a useful light on the classification of emotions and feelings.
Supplementary Material
Acknowledgments
JHGW and CFH are supported by the Northwood Trust. TEVR was supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088785). RP and MW were supported by the the Australian Research Council (ARC) Centre of Excellence for Cognition and its Disorders (CE110001021)
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2020.02.014.
References
- Adams R, Stephan K, Brown H, Frith C, Friston K, 2013. The computational anatomy of psychosis. Front. Psychiatry 4 (47). 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addabbo M, Longhi E, Marchis IC, Tagliabue P, Turati C, 2018. Dynamic facial expressions of emotions are discriminated at birth. PLoS One 13 (3), eOl93868. 10.1371/journal.pone.0193868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, 2002. Recognizing emotions from facial expressions: psychological and neurological mechanisms. Behav. Cogn. Neurosci. Rev 1, 21–62. 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Adolphs R, 2010. Conceptual challenges and directions for social neuroscience. Neuron 65, 752–767. 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR, 2000. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neurosci 20 (7), 2683–2690. 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, 1980. Why are there so many threat displays? J. Theor. Biol 86, 773–781. 10.1016/0022.5193(80)90310-0. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM, 2012. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr. Bull 38 (5), 967–980. 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Morris D, Taylor MJ, 2011. Converging evidence for the advantage of dynamic facial expressions. Brain Topogr. 24 (2), 149–163. 10.1007/s10548-011-0171-4. [DOI] [PubMed] [Google Scholar]
- Aviezer H, Trope Y, Todorov A, 2012. Body cues, not facial expressions, discriminate between intense positive and negative emotions. Science 338, 1225–1229. 10.1126/science.1224313338/6111/1225. [DOI] [PubMed] [Google Scholar]
- Azuar C, Reyes P, Slachevsky A, Voile E, Kinkingnehun S, Kouneiher F, et al. , 2014. Testing the model of caudo-rostral organization of cognitive control in the human with frontal lesions. Neuroimage 84, 1053–1060. https://doi.Org/10.1016/j.neuroimage.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Babbage DR, Yim J, Zupan B, Neumann D, Tomita MR, Wilier B, 2011. Meta-analysis of facial affect recognition difficulties after traumatic brain injury. Neuropsychology 25 (3), 277–285. 10.1037/a0021908. [DOI] [PubMed] [Google Scholar]
- Badre D, 2008. Cognitive control, hierarchy, and the rostro-causal organization of the frontal lobes. Trends Cogn. Sci 12, 193–200. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M, 2009. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci 10, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Hoffman J, Cooney JW, D’Esposito M, 2008. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat. Neurosci 12, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, 2017. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci 12 (1), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M, 2001. Knowing what you’re feeling and knowing what to do about it: mapping the relation between emotion differentiation and emotion regulation. Cogn. Emot 15 (6). 10.1080/02699930143000239. [DOI] [Google Scholar]
- Barrett LF, Quigley KS, Hamilton P, 2016. An active inference theory of allostasis and interoception in depression. Phil. Trans. R. Soc. B 371, 20160011. 10.1098/rstb.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, DeWall CN, Zhang L, 2007. How emotion shapes behaviour: feedback, anticipation, and reflection, rather than direct causation. Pers. Soc. Psychol. Rev 11 (2), 167–203. 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Benuzzi F, Ballotta D, Handjaras G, Leo A, Papale P, Zucchelli M, et al. , 2018. Eight weddings and six funerals: an fMRI study on autobiographical memories. Front. Behav. Neurosci 12, 212. 10.3389/fnbeh.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton Jones NG, 1971. Criteria for use in describing facial expression in children. Hum. Biol 41, 365–413. [PubMed] [Google Scholar]
- Boone RT, Cunningham JG, 1998. Children’s decoding of emotion in expressive body movements: the development of cue attunement. Dev. Psychol 34 (5), 1007–1016. 10.1037/0012-1649.34.5.1007. [DOI] [PubMed] [Google Scholar]
- Bornemann B, Singer T, 2017. Taking time to feel our body: steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology 54 (3), 469–482. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, 2008. Hierarchical models of behavior and prefrontal function. Trends Cogn. Sci 12 208–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braadbaart L, de Grauw H, Perrett DI, Waiter GD, Williams JHG, 2014. The shared neural basis of empathy and facial imitation accuracy. Neuroimage 84, 367–375. 10.1016/j.neuroimage.2013.08.061. [DOI] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, Oschner KN, 2017. Explicit and implicit emotion regulation: a multi-level framework. Soc. Cogn. Affect. Neurosci 12 (10), 1545–1557. 10.1093/scan/nsx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis RS, Noy L, Alony T, Gotlieb R, Cohen R, Golland Y, Levit-Binnun N, 2017. Patterns of joint improvisation in adults with autism spectrum disorder. Front. Psychol 8. 10.3389/fpsyg.2017.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck R, 1991. Motivation, emotion, and cognition: a developmental-interactionalist view. In: In: Strongman KT (Ed.), International Review of Studies on Emotion Vol. 1. Wiley, New York, pp. 101–142. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. , 2014. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex 24 (11), 2981–2990. 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Stuss DT, 2017. Fifty years of prefrontal cortex research: impact on assessment. J. Int. Neuropsychol. Soc 23 (9–10), 755–767. 10.1017/S1355617717000704. [DOI] [PubMed] [Google Scholar]
- Cai W, Chen T, Ide JS, Li CR, Menon V, 2017. Dissociable fronto-operculum-insula control signals for anticipation and detection of inhibitory sensory cue. Cereb. Cortex 27, 4073–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL, 2003. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. U. S. A 100 (9), 5497–5502. 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, 1998. On the Self-regulation of Behavior. Cambridge University Press, New York, NY, US. [Google Scholar]
- Carver CS, Scheier MF, 2001. Optimism, pessimism, and self-regulation. In: Chang EC (Ed.), Optimism & Pessimism: Implications for Theory, Research, and Practice. American Psychological Association, Washington, DC, US, pp. 31–51. [Google Scholar]
- Chakraborty M, Jarvis ED, 2015. Brain evolution by brain pathway duplication. Philos. Trans. R. Soc. B 370, 20150056. 10.1098/rstb.2015.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon V, Pacherie E, Barbalat G, Jacquet P, Franck N, Farrer C, 2011. Mentalizing under influence: abnormal dependence on prior expectations in patients with schizophrenia. Brain 134 (12), 3728–3741. [DOI] [PubMed] [Google Scholar]
- Chang A, Chen CC, Li HH, Li CS, 2014. Event-related potentials for post-error and post-conflict slowing. PLoS One 9 (6), e99909. 10.1371/journal.pone.0099909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Ide JS, Li HH, Chen CC, Li CR, 2017. Proactive control: neural oscillatory correlates of conflict anticipation and response slowing. eNeuro 4 (3). 10.1523/ENEURO.0061-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Cole PM, Zahn-Waxler C, 2005. Parental socialization of emotion expression: gender differences and relations to child adjustment. Emotion 5 (1). 10.1037/1528-3542.5.1.80. [DOI] [PubMed] [Google Scholar]
- Clark A, 2013. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci 36 (3), 181–204. 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Corr PJ, 2013. Approach and avoidance behaviour: multiple systems and their interactions. Emot. Rev 5, 286–291. [Google Scholar]
- Craig ADB, 2003. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol 13 (4), 500–505. [DOI] [PubMed] [Google Scholar]
- Crivelli C, Russell JA, Jarillo S, Fernández-Dols J, 2016. The fear gasping face as a threat display in Melanesian society. Proc. Nat. Acad. Sci. U. S. A 113 (44), 12403–12407. 10.1073/pnas.1611622113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, 2018. The Strange Order of Things: Life, Feeling, and the Making of Cultrues. Pantheon Books, New York. [Google Scholar]
- Darwin C, 1872. The Expression of the Emotions in Man and Animals, 1 ed. John Murray, London. [Google Scholar]
- Davidson RJ, 1992. Emotion and affective style: hemispheric substrates. Psychol. Sci 3 (1) , 39–43. 10.1111/j.1467-9280.1992.tb00254.x. [DOI] [Google Scholar]
- Davidson RJ, 1998. Affective style and affective disorders: perspectives from affective neuroscience. Cogn. Emot 12, 307–330. [Google Scholar]
- Davidson RJ, 2000. Affective style, mood and anxiety disorders. In: Davidson RJ (Ed.), Anxiety, Depression and Emotion. Oxford University Press, Oxford, pp. 88–107. [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K, M, 2002. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol 53, 545–574. [DOI] [PubMed] [Google Scholar]
- de Gelder B, 2006. Towards the neurobiology of emotional body language. Nat. Rev. Neurosci 7 (3), 242–249. 10.1038/nm1872. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Van den Stock J, Meeren HK, Sinke CB, Kret ME, Tamietto M, 2010. Standing up for the body. Recent progress in uncovering the networks involved in the perception of bodies and bodily expressions. Neurosci. Biobehav. Rev 34, 513–527. 10.1016/j.neubiorev.2009.10.008. [DOI] [PubMed] [Google Scholar]
- de Valk JM, Wijnen JG, Kret ME, 2015. Anger fosters action. Fast responses in a motor task involving approach movements towards angry faces and bodies. Front. Psychol 6, 1240. 10.3389/fpsyg.2015.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chaminade T, 2003. Neural correlates of feeling sympathy. Neuropsychologia 41, 127–138. 10.1016/S0028-3932(02)00143-4. [DOI] [PubMed] [Google Scholar]
- Decety J, Meyer M, 2008. From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev. Psychopathol 20 (4), 1053–1080. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG, 1989. Neurobehavioral aspects of affective disorders. Annu. Rev. Psychol 40, 457–492. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G, 1992. Understanding motor events: a neurophysiological study. Exp. Brain Res 91 (1), 176–180 doi: 0014-4819. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Bellion C, Casp M, Belger A, 2008. Impaired modulation of attention and emotion in schizophrenia. Schizophr. Bull 36 (3), 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson JM, Johnson S, Huntley CD, Peckham A, Taylor PJ, 2017. An integrative study of motivation and goal regulation processes in subclinical anxiety, depression and hypomania. Psychiatry Res. 256, 6–12. 10.1016/j.psychres.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Dijk C, de Jong PJ, Peters ML, 2009. The remedial value of blushing in the context of transgressions and mishaps. Emotion 9, 287–291. 10.1037/a00150812009-04472-016. [DOI] [PubMed] [Google Scholar]
- Dimberg U, 1982. Facial reactions to facial expressions. Psychophysiology 19, 643–647. [DOI] [PubMed] [Google Scholar]
- Donoso M, Collins AG, Koechlin E, 2014. Human cognition. Foundations of human reasoning in the prefrontal cortex. Science 344 (6191), 1481–1486. 10.1126/science.1252254. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS, 2009. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci 29, 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder AB, 2017. From boxology to scientific theories: on the emerging field of emotional action sciences. Emot. Rev 9 (4), 343–355. [Google Scholar]
- Eimer M, Gosling A, Nicholas S, Kiss M, 2011. The N170 component and its links to configural face processing: a rapid neural adaptation study. Brain Res. 1376, 76–87. 10.1016/j.brainres.2010.12.046. [DOI] [PubMed] [Google Scholar]
- Ekman P, 1993. Facial expression and emotion. Am. Psychol 48, 384–392. [DOI] [PubMed] [Google Scholar]
- Ekman P, Rosenberg EL, 1997. What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System (FACS). Oxford University Press. [Google Scholar]
- Emigh J, 2011. Minding bodies: demons, masks, archetypes, and the limits of culture. J. Dram. Theory Crit 25, 125–139. [Google Scholar]
- Engelen T, Zhan M, Sack AT, de Gelder B, 2018. Dynamic interactions between emotion perception and action preparation for reacting to social threat: a combined cTBS-fMRI study. eNeuro 5 (3). 10.1523/ENEURO.0408-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Johnston PJ, Herring SE, Hoy KE, Fitzgerald PB, 2008a. Mirror neuron activation is associated with facial emotion processing. Neuropsychologia 46 (11) , 2851–2854. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB, 2008b. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit? Schizophr. Res 102 (1), 116–121. 10.1016/j.schres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Etkin A, Buechel C, Gross JJ, 2015. The neural bases of emotion regulation. Nat. Rev. Neurosci 16 (11), 693–700. 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Coudé G, 2018. Mirror neurons, embodied emotions, and empathy. In: Meyza KZ, Knapska E (Eds.), Neuronal Correlates of Empathy: From Rodent to Human. Elsevier Academic Press, San Diego, CA, pp. 67–77. [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L, 2003. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci 17 (8), 1703–1714 doi: 0953–816X. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Rodriguez-Mosquera PM, van Vianen AE, Manstead AS, 2004. Gender and culture differences in emotion. Emotion 4 (1), 87–94. 10.1037/1528-3542.4.1.87. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G, 2005. Parietal lobe: from action organization to intention understanding. Science 308 (5722), 662–667. 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fowles DC, 1988. Psychophysiology and psychopathology: a motivation approach. Psychophysiology 25, 373–391. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, et al. , 2014. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev 45, 202–211. 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Frijda NH, 2016. The evolutionary emergence of what we call “emotions”. Cogn. Emot 30, 609–620. 10.1080/02699931.2016.1145106. [DOI] [PubMed] [Google Scholar]
- Friston K, 2010. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci 11, 127–138. 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Frith CD, 1987. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol. Med 17 (3), 631–648. [DOI] [PubMed] [Google Scholar]
- Fuster JM, 2004. Upper processing stages of the perception-action cycle. Trends Cogn. Sci 8, 143–145. [DOI] [PubMed] [Google Scholar]
- Fuster JM, 2019. The prefrontal cortex in the neurology clinic. Handb. Clin. Neurol 163, 3–15. 10.1016/B978-0-12-804281-6.00001-X. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bressler SL, 2012. Cognit activation: a mechanism enabling temporal integration in working memory. Trends Cogn. Sci 16, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebel W, Wolwer W, 1992. Facial expression and emotional face recognition in schizophrenia and depression. Eur. Arch. Psychiatry Clin. Neurosci 242 (1), 46–52. 10.1007/bf02190342. [DOI] [PubMed] [Google Scholar]
- Gallese V, 2003. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 36, 171–180. 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G, 1996. Action recognition in the premotor cortex. Brain 119, 593–609. [DOI] [PubMed] [Google Scholar]
- Gendolla GHE, 2017. Comment: do emotions influence action? – of course, they are hypo-phenomena of motivation. Emot. Rev 9 (4), 348–350. 10.1177/1754073916673211. [DOI] [Google Scholar]
- Gentry RN, Lee B, Roesch MR, 2016. Phasic dopamine release in the rat nucleus accumbens predicts approach and avoidance performance. Nat. Commun 7. 10.1038/ncomms13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry RN, Schuweiler DR, Roesch MR, 2018. Dopamine signals related to appetitive and aversive events in paradigms that manipulate reward and avoidability. Brain Res. 10.1016/j.brainres.2018.10.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AI, Sripada CS, 2005. Simulationist models of face-based emotion recognition. Cognition 94, 193–213. 10.1016/j.cognition.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A, 2012. Fractionation of social brain circuits in autism spectrum disorders. Brain 135. 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, 1982. The Neuropsychology of Anxiety: an Enquiry Into the Functions of the Septo-hippocampal System. Oxford University Press, Oxford. [Google Scholar]
- Gray JA, 1987. The Psychology of Fear and Stress Vol. 5 CUP Archive. [Google Scholar]
- Gray J, 1994. A framework for a taxonomy of psychiatric disorder. In: Goozen SHMV, Poll NEV, Nanne E (Eds.), Emotions: Essay on Emotion Theory. Lawrence Erlbaum Associates, New Jersey, pp. 25–59. [Google Scholar]
- Gray HM, Tickle-Degnen L, 2010. A meta-analysis of performance on emotion recognition tasks in Parkinson’s disease. Neuropsychology 24, 176–191. 10.1037/a0018104. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Pichon S, de Gelder B, 2007. Perceiving fear in dynamic body expressions. Neuroimage 35 (2), 959–967. 10.1016/j.neuroimage.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Valabrègue R, Gholipour B, Chevallier C, 2014. A direct amygdala-motor pathway for emotional displays to influence action: a diffusion tensor imaging study. Hum. Brain Mapp 35 (12), 5974–5983. 10.1002/hbm.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths KR, Morris RW, Balleine BW, 2014. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front. Syst. Neurosci 8, 101. 10.3389/fnsys.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, 2015. Emotion regulation: current status and future prospects. Psychol. Inq 26 (1). 10.1080/1047840X.2014.940781. [DOI] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, et al. , 2002. An fMRI study of facial emotion processing in patients with schizophrenia. Am. J. Psychiatry 159 (12), 1992–1999. 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. , 2007. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch. Gen. Psychiatry 64 (12), 1356–1366. [DOI] [PubMed] [Google Scholar]
- Haker H, Rössler W, 2009. Empathy in schizophrenia: impaired resonance. Eur. Arch. Psychiatry Clin. Neurosci 259 (6), 352–361. 10.1007/s00406-009-0007-3. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, 2008. Emulation and mimicry for social interaction: a theoretical approach to imitation in autism. Q. J. Exp. Psychol 61 (1), 101–115. 10.1080/17470210701508798. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Critchley HD, 2009. Dynamic pupillary exchange engages brain regions encoding social salience. Soc. Neurosci 4, 233–243. 10.1080/17470910802553508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CJ, Stevenson RJ, Coltheart M, 2009a. The processing of emotion in patients with Huntington’s disease: variability and differential deficits in disgust. Cogn. Behav. Neurol 22, 249–257. 10.1097/WNN.0b013e3181c124af. [DOI] [PubMed] [Google Scholar]
- Hayes CJ, Stevenson RJ, Coltheart M, 2009b. Production of spontaneous and posed facial expressions in patients with Huntington’s disease: impaired communication of disgust. Cogn. Emot 23, 118–134. 10.1080/02699930801949090. [DOI] [Google Scholar]
- Heberlein AS, Atkinson AP, 2009. Neuroscientific evidence for simulation and shared substrates in emotion recognition: beyond faces. Emot. Rev 1,162–177. 10.1177/1754073908100441. [DOI] [Google Scholar]
- Hecht MA, LaFrance M, 1998. License or obligation to smile: the effect of power and sex on amount and type of smiling. Pers. Soc. Psychol. Bull 24, 1332–1342. [Google Scholar]
- Heiy JE, Cheavens JS, 2014. Back to basics: a naturalistic assessment of the experience and regulation of emotion. Emotion 14 (5), 878–891. 10.1037/a0037231. [DOI] [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo X, Li CS, 2010. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS One 5 (10), e13155. 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, et al. , 2005. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage 26 (2). 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Theodorou G, Summers F, 2006. Cognitive and psychosocial correlates of alexithymia following traumatic brain injury. Neuropsychologia 44 (1), 62–72. 10.1016/j.neuropsychologia.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Herba C, Phillips M, 2004. Annotation: development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. J. Child Psychol. Psychiatry Allied Disciplines 45 (7). 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Herba CM, Landau S, Russell T, Ecker C, Phillips ML, 2006. The development of emotion-processing in children: effects of age, emotion, and intensity. J. Child Psychol. Psychiatry 47 (11). 10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Herbert C, Pollatos O, 2011. On the relationship between interoception awareness and alexithymia: is interoceptive awareness related to emotional awareness? J. Pers 79 (5), 1149–1175. [DOI] [PubMed] [Google Scholar]
- Hess U, Bourgeois P, 2010. You smile-I smile: emotion expression in social interaction. Biol. Psychol 84, 514–520. 10.1016/j.biopsycho.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Hess U, Fischer A, 2013. Emotional mimicry as social regulation. Personal. Soc. Psychol. Rev 17, 142–157. 10.1177/1088868312472607. [DOI] [PubMed] [Google Scholar]
- Hess U, Fischer A, 2014. Emotional mimicry: why and when we mimic emotions. Soc. Personal. Psychol. Compass 8 (2), 45–57. 10.1111/spc3.12083. [DOI] [Google Scholar]
- Higgins ET, 2000. Making a good decision: value from fit. Am. Psychol 55 (11), 1230–1243. 10.1037//0003-066X.55.11.1230. [DOI] [PubMed] [Google Scholar]
- Hoehl S, 2014. Emotion processing in infancy. In: Lagattuta KH (Ed.), Children and Emotion: New Insights into Developmental Affective Sciences. Karger, pp. 1–12. [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris EG, O’Doherty J, Bullock PR, Polkey CE, 2003. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 126 (7), 1691–1712. 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Li CS, 2015. Anticipating conflict: neural correlates of a Bayesian belief and its motor consequence. Neuroimage 119, 286–295. 10.1016/j.neuroimage.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS, 2011. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage 54 (1), 455–464. 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Shenoy P, Yu AJ, Li CS, 2013. Bayesian prediction and evaluation in the anterior cingulate cortex. J. Neurosci 33, 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W, 1890. The Principles of Psychology. H. Holt and Company, New York. [Google Scholar]
- Keysers C, Gazzola V, 2007. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn. Sci 11 (5), 194–196. 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD, 2007a. The mirror-neuron system: a Bayesian perspective. Neuroreport 18 (6), 619–623. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD, 2007b. Predictive coding: an account of the mirror neuron system. Cogn. Process 8 (3), 159–166. 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD, 2009. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci 29 (32), 10153–10159. 10.1523/jneurosci.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Fang Y, Shin A, Park S, Hashikawa K, et al. , 2019. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci 22. 10.1038/s41593-019-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Gill KE, Brucato G, Vakhrusheva J, Arndt L, Gross JJ, Girgis RR, 2016. The impact of emotion awareness and regulation on social functioning in individuals at clinical high risk for psychosis. Psychol. Med 46 (14). 10.1017/S0033291716000490. [DOI] [PubMed] [Google Scholar]
- Kircher T, Pohl A, Krach S, Thimm M, Schulte-Rüther M, Anders S, Mathiak K, 2013. Affect-specific activation of shared networks for perception and execution of facial expressions. Soc. Cogn. Affect. Neurosci 8 (4), 370–377. 10.1093/scan/nss008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kohshima S, 1997. Unique morphology of the human eye. Nature 387, 767–768. 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- Koch A, Pollatos O, 2014. Cardiac sensitivity in children: sex differences and its relationship to parameters of emotional processing. Psychophysiology 51 (9), 932–941. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A, 2007. Anterior prefrontal function and the limits of human decision-making. Science 318 (5850), 594–598. 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F, 2003. The architecture of cognitive control in the human prefrontal cortex. Science 302 (5648), 1181–1185. 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. , 2003. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am. J. Psychiatry 160 (10), 1768–1774. 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ, 2010. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull 36 (5), 1009–1019. 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U, 2014. Neural network of cognitive emotion regulation – an ALE meta-analysis and MACM analysis. Neuroimage 87, 345–355. 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordsachia CC, Labuschagne I, Stout JC, 2017. Beyond emotion recognition deficits: a theory guided analysis of emotion processing in Huntington’s disease. Neurosci. Biobehav. Rev 73, 276–292. 10.1016/j.neubiorev.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Kret ME, 2015. Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Front. Psychol 6 doi: ARTN 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, De Dreu CK, 2017. Pupil-mimicry conditions trust in partners: moderation by oxytocin and group membership. Proc. R. Soc. B: Biol. Sci 284. 10.1098/rspb.2016.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Straffon L, 2018. Reply to Crivelli et al. The bared teeth face is a universal expression of fear, submission and threat. Evidence from art across times and cultures. J. Hum. Evol 125, 193–197. 10.1016/j.jhevol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Kret ME, Tomonaga M, 2016. Getting to the bottom of face processing. Species-specific inversion effects for faces and behinds in humans and chimpanzees (Pan Troglodytes). PLoS One 11, e0165357. 10.1371/journal.pone.0165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Pichon S, Grezes J, de Gelder B, 2011. Similarities and differences in perceiving threat from dynamic faces and bodies. An fMRI study. Neuroimage 54, 1755–1762. 10.1016/j.neuroimage.2010.08.012. S1053–8119(10) 01079–7. [DOI] [PubMed] [Google Scholar]
- Kret ME, Roelofs K, Stekelenburg JJ, de Gelder B, 2013a. Emotional signals from faces, bodies and scenes influence observers’ face expressions, fixations and pupil-size. Front. Hum. Neurosci 7. 10.3389/fnhum.2013.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Stekelenburg JJ, Roelofs K, de Gelder B, 2013b. Perception of face and body expressions using electromyography, pupillometry and gaze measures. Front. Psychol 4. 10.3389/fpsyg.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Tomonaga M, Matsuzawa T, 2014. Chimpanzees and humans mimic the pupil-size of conspecifics. PLoS One 9, e104886. 10.1371/journal.pone.0104886PONE-D-14-21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret ME, Fischer A, De Dreu CKW, 2015. Pupil-mimicry correlates with trust in ingroup partners with dilating pupils. Psychol. Sci 26, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Kring AM, Elis O, 2013. Emotion deficits in people with schizophrenia. Annu. Rev. Clin. Psychol 9, 409–433. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK, 2008. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull 34 (5), 819–834. 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G, 2003. Dynamic perception of facial affect and identity in the human brain. Cereb. Cortex 13 (10), 1023–1033. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T, 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502. 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, 2010. Emotion and motivational brain. Biol. Psychol 84 (3), 437–450. 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laricchiuta D, 2015. Editorial: individual differences: from neurobiological bases to new insight on approach and avoidance behaviour. Front. Syst. Neurosci 9, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence K, Campbell R, Skuse D, 2015. Age, gender, and puberty influence the development of facial emotion recognition. Front. Psychol 6. 10.3389/fpsyg.2015.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary MR, Britt TW, Cutlip WD, Templeton JL, 1992. Social blushing. Psychol. Bull 112, 446–460. [DOI] [PubMed] [Google Scholar]
- Lee T-W, Josephs O, Dolan RJ, Critchley HD, 2006. Imitating expressions: emotion-specific neural substrates in facial mimicry. Soc. Cogn. Affect. Neurosci 1, 122–135. 10.1093/scan/ns1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Chun JW, Yoon SY, Park H-J, Kim J-J, 2014. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr. Res 152 (1), 268–274. 10.1016/j.schres.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Loughead J, Valdez JN, Kohler CG, Brensinger C, et al. , 2011. Ventrolateral prefrontal cortex and the effects of task demand context on facial affect appraisal in schizophrenia. Soc. Cogn. Affect. Neurosci 6 (1), 66–73. 10.1093/scan/nsq18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M, 2009. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb. Cortex 19, 1124–1133. 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST, 2004. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage 21 (2), 601–607. 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM, 1983. Marital interaction: physiological linkage and affective change. J. Pers. Soc. Psychol 45, 587–597. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E, 2001. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron 30 (2), 593–607. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R, 2006. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J. Neurosci 26, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R, 2008a. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J. Cogn. Neurosci 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Chao HH, Sinha R, Paliwal P, Constable RT, et al. , 2008b. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience 155, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepelt R, Brass M, 2010. Top-down modulation of motor priming by belief about animacy. Exp. Psychol 57 (3), 221. [DOI] [PubMed] [Google Scholar]
- Liepelt R, Ullsperger M, Obst K, Spengler S, von Cramon DY, Brass M, 2009. Contextual movement constraints of others modulate motor preparation in the observer. Neuropsychologia 47 (1), 268–275. [DOI] [PubMed] [Google Scholar]
- Likowski KU, Mühlberger A, Gerdes AB, Wieser MJ, Pauli P, Weyers P, 2012. Facial mimicry and the mirror neuron system: simultaneous acquisition of facial electromyography and functional magnetic resonance imaging. Front. Hum. Neurosci 6, 214. 10.3389/fnhum.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, 2015. The point of no return: a fundamental link on the ability to control thought and action. Q. J. Exp. Psychol 68 (5), 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA, 1984. On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform 10, 276–291. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Koechlin E, Rosa MGP, Bucklet MJ, 2017. Managing competing goals – a key role for the frontopolar cortex. Nat. Rev. Neurosci 18 (11), 645–657. 10.1038/nm.2017.111. [DOI] [PubMed] [Google Scholar]
- Maranesi M, Livi A, Fogassi L, Rizzolatti G, Bonini L, 2014. Mirror neuron activation prior to action observation in a predictable context. J. Neurosci 34 (45), 14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneweck M, Palermo R, Hammond G, 2014. Discrimination and recognition of facial expressions of emotion and their links with voluntary control of facial musculature in Parkinson’s disease. Neuropsychology 28, 917–928. 10.1037/neu0000106. [DOI] [PubMed] [Google Scholar]
- Marsh KL, Richardson MJ, Schmidt RC, 2009. Social connection through joint action and interpersonal coordination. Top. Cogn. Sci 1 (2). 10.1111/j.1756-8765.2009.01022.X. [DOI] [PubMed] [Google Scholar]
- McCleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF, 2015. Meta-analysis of face processing event-related potentials in schizophrenia. Biol. Psychiatry 77 (2), 116–126. 10.1016/j.biopsych.2014.04.015. [DOI] [PubMed] [Google Scholar]
- McClure EB, 2000. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol. Bull 126 (3). [DOI] [PubMed] [Google Scholar]
- McDonald S, 2005. Are you crying or laughing? Emotion recognition deficits after severe traumatic brain injury. Brain Impair. 6 (1), 56–67. 10.1375/brim.6.1.56.65481. [DOI] [Google Scholar]
- McNaughton N, Corr PJ, 2014. Approach, avoidance, and their conflict: the problem of anchoring. Front. Syst. Neurosci 8, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta UM, Thirthalli J, Aneelraj D, Jadhav P, Gangadhar BN, Keshavan MS, 2014a. Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophr. Res 160 (1), 9–19. 10.1016/j.schres.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN, Pascual-Leone A, 2014b. Reduced mirror neuron activity in schizophrenia and its association with theory of mind deficits: evidence from a transcranial magnetic stimulation study. Schizophr. Bull 40 (5), 1083–1094. 10.1093/schbul/sbt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, 2003. Connecting mirror neurons and forward models. Neuroreport 14 (17), 2135–2137. [DOI] [PubMed] [Google Scholar]
- Milders M, Fuchs S, Crawford JR, 2003. Neuropsychological impairments and changes in emotional and social behaviour following severe traumatic brain injury. J. Clin. Exp. Neuropsychol 25 (2), 157–172. 10.1076/jcen.25.2.157.13642. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB, 2012a. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev 36, 341–349. 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Hayward L, Mattingley JB, Cunnington R, 2012b. Activation patterns during action observation are modulated by context in mirror system areas. Neuroimage 59 (1), 608–615. [DOI] [PubMed] [Google Scholar]
- Moors A, Fischer M, 2019. Demystifying the role of emotion in behaviour: towards a goal-directed account. Cogn. Emot 33 (1), 94–100. 10.1080/02699931.2018.1510381. [DOI] [PubMed] [Google Scholar]
- Moors A, Boddez Y, De Houwer J, 2017. The power of goal-directed processes in the causation of emotional and other actions. Emot. Rev 9 (4), 310–318. 10.1177/1754073916669595. [DOI] [Google Scholar]
- Murphy J, Catmur C, Bird G, 2018. Alexithymia is associated with a multidomain, multidimensional failure of interoception: evidence from novel tests. Exp. Psychol. Gen 147 (3). 10.1037/xge0000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo C, Kornreich C, Campanella S, Noel X, Vandriette Y, Gillain B, et al. , 2011. Major depression is associated with impaired processing in music as well as facial and vocal stimuli. J. Affect. Disord 128, 243–251. 10.1016/j.jad.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Neumann D, Zupan B, Babbage DR, Radnovich AJ, Tomita MR, Hammond F, Wilier B, 2012. Affect recognition, empathy, and dysosmia after traumatic brain injury. Arch. Phys. Med. Rehabil 93 (8), 1414–1420. 10.1016/j.apmr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Neumann D, Zupan B, Malec JF, Hammond F, 2014. Relationships between alexithymia, affect recognition, and empathy after traumatic brain injury. J. Head Trauma Rehabil 29 (1), E18–27. 10.1097/HTR.0b013e31827fb0b5. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Ric F, 2017. Psychology of Emotion, 2nd edition. Psychology Press, New York. [Google Scholar]
- Niedenthal PM, Mermillod M, Maringer M, Hess U, 2010. The simulation of smiles (SIMS) model: embodied simulation and the meaning of facial expression. Behav. Brain Sci 33, 417–433. 10.1017/S0140525X10000865. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Augustinova M, Rychlowska M, Droit-Volet S, Zinner L, Knafo A, Brauer M, 2012. Negative relations between pacifier use and emotional competence. Basic Appl. Soc. Psychol 34, 387–394. [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT, 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci 1251 (1), El–24. 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E, 2002. Neuronal correlates of kinematics-to-dynamics transformation in the supplementary motor area. Neuron 36 (4), 751–765. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E, 2004. Neuronal activity in the supplementary motor area of monkeys adapting to a new dynamic environment. J. Neurophysiol 91, 449–473. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Tessitore A, Russo A, Cerasa A, Gioia CM, et al. , 2013. Dysfunctions within limbic-motor networks in amyotrophic lateral sclerosis. Neurobiol. Aging 34 (11), 2499–2509. 10.1016/j.neurobiolaging.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Mistlin AJ, Chitty AJ, Head AS, Potter DD, et al. , 1985. Visual analysis of body movements by neurones in the temporal cortex of the macaque monkey: a preliminary report. Behav. Brain Res 16 (2–3), 153–170. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ, 2002. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc. Natl. Acad. Sci. U. S. A 99 (13), 9072–9076. 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Sinha P, 2002. Effects of early experience on children’s recognition of facial displays of emotion. Dev. Psychol 38 (5), 784–791. 10.1037/0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A, 2000. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol 36 (5), 679–688. 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Alva G, Fleming K, Anand R, Keator D, Carreon D, et al. , 2002. A PET study of the pathophysiology of negative symptoms in schizophrenia. Am. J. Psychiatry 159 (2), 227–237. [DOI] [PubMed] [Google Scholar]
- Radice-Neumann D, Zupan B, Babbage DR, Wilier B, 2007. Overview of impaired facial affect recognition in persons with traumatic brain injury. Brain Inj. 21 (8), 807–816. 10.1080/02699050701504281. [DOI] [PubMed] [Google Scholar]
- Ralls K, Fiorelli P, Gish S, 1985. Vocalizations and vocal mimicry in captive harbor seals, Phoca vitulina. Can. J. Zool 63 (5), 1050–1056. 10.1139/z85-157. [DOI] [Google Scholar]
- Ramsay DS, Woods S, C, 2014. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev 121 (2), 225–247. 10.1037/a0035942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RG, Randall AK, Post JH, Butler EA, 2013. Partner influence and in-phase versus anti-phase physiological linkage in romantic couples. Int. J. Psychophysiol 88, 309–316. https://doi.Org/10.1016/j.ijpsycho.2012.08.009. S0167-8760(12) 00581-8 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, 2017. Emotion in action: a predictive processing perspective and theoretical synthesis. Emot. Rev 9 (4), 319–325. 10.1177/1754073916661765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA, 1998. Language within our grasp. Trends Neurosci. 21 (5), 188–194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L, 1996. Premotor cortex and the recognition of motor actions. Cogn. Brain Res 3 (2), 131–141. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Van Rheenen TE, Joshua NR, O’Regan A, Gogos A, 2014. Investigating facial affect processing in psychosis: a study using the Comprehensive Affective Testing System. Schizophr. Res 157, 55–59. [DOI] [PubMed] [Google Scholar]
- Rychlowska M, Cañadas E, Wood A, Krumhuber EG, Fischer A, Niedenthal PM, 2014. Blocking mimicry makes true and false smiles look the same. PLoS One 9, e90876. 10.1371/journal.pone.0090876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Yoshikawa S, 2007. Spontaneous facial mimicry in response to dynamic facial expressions. Cognition 104, 1–18. 10.1016/j.cognition.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M, 2004. Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Cogn. Brain Res 20 (1), 81–91. 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sato W, Toichi M, Uono S, Kochiyama T, 2012. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neurosci. 13, 99. 10.1186/1471-2202-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, 2015. Spatiotemporal neural network dynamics for the processing of dynamic facial expressions. Sci. Rep 5, 12432. 10.1038/srep12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Mijzisch A, Vogeley K, 2008. What’s in a smile? Neural correlates of facial embodiment during social interaction. Soc. Neurosci 3 (1), 37–50. 10.1080/17470910701563228. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Adolphs R, 2017. Emotion perception from face, voice, and touch: comparisons and convergence. Trends Cogn. Sci 21, 216–228. 10.1016/j.tics.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Pilz KS, 2009. Natural facial motion enhances cortical responses to faces. Exp. Brain Res 194 (3), 465–475. 10.1007/s00221-009-1721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, 2013. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci 17 (11), 565–573. [DOI] [PubMed] [Google Scholar]
- Shah P, Hall R, Catmur C, Bird G, 2016. Alexithymia, not autism, is associated with impaired interoception. Cortex 81, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddharthan A, Cherbuin N, Eslinger PJ, Kozlowska K, Murphy NA, Lowe L, 2018. WordNet-feelings: A Linguistic Categorisation of Human Feelings, eprint, arXiv:1811.02435. pp. 1–22. [Google Scholar]
- Smidt KE, Suvak MK, 2015. A brief, but nuanced, review of emotional granularity and emotion differentiation research. Curr. Opin. Psychol 3, 48–51. [Google Scholar]
- Soussignan R, 2002. Duchenne smile, emotional experience, and automatic reactivity: a test of the facial feedback hypothesis. Emotion 2 (1), 52–74. 10.1037//1528-3542.2.1.52. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Trainor BC, 2018. Complementary neural circuits for divergent effects of oxytocin: social approach versus social anxiety. Biol. Psychiatry 10.1016/j.biopsych.2018.10.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD, 2009. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull 35 (3), 509–527. 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD, 2012. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry 71 (2), 136–145. 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, De Beilis MD, Graham R, LaBar KS, 2007. Development of emotional facial recognition in late childhood and adolescence. Dev. Sci 10 (5). 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Lehmann H, Call J, 2007. Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. J. Hum. Evol 52 (3), 314–420. 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Trautmann SA, Fehr T, Herrman M, 2009. Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain Res. 1285, 100–115. 10.1016/j.brainres.2009.05.075. [DOI] [PubMed] [Google Scholar]
- Trautmann-Lengsfeld SA, Domíngue-Borrás J, Escera C, Herrman M, Fehr T, 2013. The perception of dynamic and static facial expressions of happiness and disgust investigated by ERPs and fMRI constrained source analysis. PLoS One 8 (6), e66997. 10.1371/journal.pone.0066997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkler I, Cleret de Langavant L, Bachoud-Levi AC, 2011. Joint recognition-expression impairment of facial emotions in Huntington’s disease despite intact understanding of feelings. Cortex 49, 549–558. 10.1016/j.cortex.2011.12.003Get. [DOI] [PubMed] [Google Scholar]
- Trinkler I, Devignevielle S, Achaibou A, Ligneul RV, Brugières P, de Langavant LC, De Gelder B, et al. , 2017. Embodied emotion impairment in Huntington’s disease. Cortex 92, 44–56. [DOI] [PubMed] [Google Scholar]
- Tschacher W, Giersch A, Friston K, 2017. Embodiment and schizophrenia: a review of implications and applications. Schizophr. Bull 43 (4), 745–753. 10.1093/schbul/sbw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC, 2007. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr. Res 94 (1–3), 253–263. 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR, 2010. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct 214 (5–6), 629–643. 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G, 2001. I know what you are doing: a neurophysiological study. Neuron 31 (1), 155–165. 10.1016/S0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Vaessen MJ, Abassi E, Manicini M, Camurri A, de Gelder B, 2018. Computational feature analysis of body movements reveals hierarchical brain organization. Cereb. Cortex 29 (8), 3551–3560. 10.1093/cercor/bhy228. [DOI] [PubMed] [Google Scholar]
- Vai B, Sferrazza Papa G, Poletti S, Radaelli D, Donnici E, Bollettini I, et al. , 2015. Abnormal cortico-limbic connectivity during emotional processing correlates with symptom severity in schizophrenia. Eur. Psychiatry 30 (5), 590–597. 10.1016/j.eurpsy.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Van den Stock J, Tamietto M, Sorger B, Pichon S, Grézes J, de Gelder B, 2011. Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proc. Natl. Acad. Sci. U. S. A 108 (39), 16188–16193. 10.1073/pnas.1107214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght L, Schevernels H, Burle B, Notebaert W, 2016. Errors disrupt subsequent early attentional processes. PLoS One 11 (4), e0151843. 10.1371/journal.pone.0151843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa R, Keysers C, 2007. Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci 2, 179–222. 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Van Hooff J, 1976. The comparison of facial expressions in man and higher primates. Methods Inference Anim. Hum. Behav 3, 165. [Google Scholar]
- van Lawick-Godall J, 1968. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr 1, 161–311. [Google Scholar]
- Van Rheenen TE, Joshua N, Castle DJ, Rossell SL, 2017. Configural and featural face processing influences on emotion recognition in schizophrenia and bipolar disorder. J. Int. Neuropsychol. Soc 23 (3), 287–291. [DOI] [PubMed] [Google Scholar]
- Wagner H, Lee V, 2008. Alexithymia and individual differences in emotional expression. J. Res. Pers 42 (1), 83–95. [Google Scholar]
- Waller BM, Vick S, Parr LA, Bard KA, Pasqualini MCS, Gothard KM, Fuglevand AJ, 2006. Intramuscular electrical stimulation of facial muscles in humans and chimpanzees: duchenne revisited and extended. Emotion 6, 367–382. 10.1037/1528-3542.6.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G, 2003. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655–664. 10.1016/S0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams JHG, 2013. The mirror or portrait neuron system – time for a more organic model of action-coding? Cortex 49, 2962–2963. 10.1016/j.cortex.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Williams C, Wood RL, 2010. Alexithymia and emotional empathy following traumatic brain injury. J. Clin. Exp. Neuropsychol 32 (3), 259–267. 10.1080/13803390902976940. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI, 2001. Imitation, mirror neurons and autism. Neurosci. Biobehav. Rev 25 (4), 287–295. [DOI] [PubMed] [Google Scholar]
- Winch A, Moberly NJ, Dickson JM, 2015. Unique associations between anxiety, depression and motives for approach and avoidance goal pursuit. Cogn. Emot 29 (7), 1295–1305. 10.1080/02699931.2014.976544. [DOI] [PubMed] [Google Scholar]
- Winkielman P, 2010. Embodied and disembodied processing of emotional expressions: insights from autism spectrum disorders. Behav. Brain Sci 33, 463–464. 10.1017/S0140525X10001640. [DOI] [Google Scholar]
- Wood A, Rychlowska M, Korb S, Niedenthal P, 2016. Fashioning the face: sensorimotor simulation contributes to facial expression recognition. Trends Cogn. Sci 20 (3), 227–240. 10.1016/j.tics.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Nguyen M, Hasson U, 2017. Amplification of local changes along the timescale processing hierarchy. Proc. Natl. Acad. Sci 114, 9475–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes N, Reips U, 2019. Guideline for improving the reliability of Google Ngram studies: evidence from religious terms. PLoS One 14 (3), e0213554. 10.1371/journal.pone.0213554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Cohen JD, 2008. Sequential effects: superstition or rational behavior? Adv. Neural Inf. Process. Syst 21, 1873–1880. [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rowe JB, 2015. The neural signature of information regularity in temporally extended event sequences. Neuroimage 107, 266–276. 10.1016/j.neuroimage.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan B, Babbage DR, Neumann D, Wilier B, 2014. Recognition of facial and vocal affect following traumatic brain injury. Brain Inj. 28 (8), 1087–1095. 10.3109/02699052.2014.901560. [DOI] [PubMed] [Google Scholar]
- Zupan B, Babbage DR, Neumann D, Wilier B, 2016. Sex differences in emotion recognition and emotional inferencing following severe traumatic brain injury. Brain Impair. 18 (1), 36–48 doi: 10.1017.BrImp.2016.22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


