Abstract
Atrial fibrillation is a frequent complication among patients with severe coronavirus disease-2019 (COVID-19) infection. Both direct and indirect mechanisms through COVID-19 have been described to explain this relationship. COVID-19 infection increases the risk of developing both arterial and venous thrombotic complications through systemic coagulation activation, leading to increased mortality. Chronic oral anticoagulation is essential to reduce the thromboembolic risk among AF patients. Switching to low-molecular-weight heparin has been recommended during hospitalization for COVID-19 infection. Of note, at discharge, the prescription of direct oral anticoagulants may offer some advantages over vitamin K antagonists. However, oral anticoagulants should only be prescribed after the consideration of drug–drug interactions with antiviral therapies as well as of the risk of hepatotoxicity, which is common among individuals with severe COVID-19 pneumonia. Not all anticoagulants have the same risk of hepatotoxicity; dabigatran has shown a good efficacy and safety profile and could have a lower risk of hepatotoxicity. Furthermore, its metabolism by cytochrome P450 is absent and it has a specific reversal agent. Therefore, dabigatran may be considered as a first-line choice for oral anticoagulation at discharge after COVID-19 infection. In this review, the available information on the antithrombotic management of AF patients at discharge after COVID-19 infection is updated. In addition, a practical algorithm, considering renal and liver function, which facilitates the anticoagulation choice at discharge is presented.
Keywords: atrial fibrillation, COVID-19, dabigatran, direct oral anticoagulants, hepatotoxicity
Introduction
The severe acute respiratory syndrome-coronavirus-2 causing the coronavirus disease-2019 (COVID-19) has attained pandemic numbers since March 2020, worldwide.1 COVID-19 infection produces an acute and complex disorder that, in some cases, may lead to the development of severe interstitial pneumonia, acute respiratory distress syndrome, or death.2 Typical symptoms may include fever, cough, dyspnea, fatigue, hemoptysis, myalgia, headache, nausea, vomiting, diarrhea, and alterations of taste or smell, among others.3,4 Of note, COVID-19 has also been related with cardiovascular complications, including atrial fibrillation (AF).1 Thus, up to 10–30% of patients hospitalized due to COVID-19 have acute cardiac damage, including cardiac arrhythmias.3,4 In addition, patients who develop cardiac injury, mainly those with prior cardiovascular disease, have a worse prognosis.5
The aim of this narrative review was to update the available information about the antithrombotic management of patients with AF at discharge after COVID-19 infection and provide a practical algorithm, considering renal and liver functions, in order to facilitate the choice of anticoagulation therapy at discharge. For this purpose, a search on MEDLINE and EMBASE databases was performed until August 2020. The MEDLINE and EMBASE search was performed using both medical subject headings and keywords, including AF or dabigatran or direct oral anticoagulants or hepatotoxicity or renal failure and COVID-19. References of the retrieved articles were also screened for additional studies. There were no language restrictions.
Risk of AF and COVID-19 infection
AF is the most frequent sustained arrhythmia in routine practice,6 and is a common complication among individuals with severe COVID-19 infection, including those with severe pneumonia, acute respiratory distress syndrome, or sepsis.7 In a survey performed in 76 countries, approximately one-fifth of respondents reported cases of AF in hospitalized patients with COVID-19.8 In a study that analyzed 99 consecutive hospitalized subjects with COVID-19 pneumonia, 53 had a history of cardiac disease, of whom 40% had previous heart failure, 36% exhibited AF, and 30% had coronary artery disease. Of note, death rates and rates of thromboembolic events were higher in patients with cardiac disease (36% versus 15% and 23% versus 6%, respectively).9 Another study showed that 22.5% of non-surviving patients who had COVID-19 presented with a history of AF before COVID-19 infection.7 In a large urban population of 700 hospitalized patients with COVID-19 (mean age 50 years) over a 9-week period, there were 25 new cases of AF (3.6%). Furthermore, patients admitted to the intensive care unit exhibited a greater risk of new-onset AF, suggesting that AF is not only a direct consequence of COVID-19 infection but also the result of systemic illness.10 Moreover, in addition to the elderly and nursing-home residence, chronic respiratory and cardiac diseases, including AF, increase the risk of having COVID-19.11 On the other hand, it has been reported that, during the COVID-19 lockdown period, new-onset AF cases were underdiagnosed. During the COVID-19 pandemic, the risk of ischemic stroke and death among new cases of AF was higher compared with the corresponding period in 2019.12
With regard to the pathophysiology of the relationship between COVID-19 infection and AF, more studies are warranted to elucidate the possible direct and indirect mechanisms through which COVID-19 infection may increase the risk of AF.13 In patients with severe COVID-19 pneumonia, hypoxia, electrolyte abnormalities, dehydration, systemic inflammation metabolic dysfunction, and the activation of the sympathetic system that occurs may play a role in the onset of AF.13–15 Interleukin-6, a cytokine highly expressed in individuals with severe COVID-19 infection and a biomarker target for these patients, has been related to a greater risk of AF.13,16–18 In addition, leukocyte infiltration in the atrial tissue of patients with AF has been described.13,16–18 Moreover, reactive oxygen species, direct oxidized Ca2+/calmodulin-dependent protein kinase II, and enhanced cardiomyocyte NLRP3 inflammasome signaling pathways have been recognized as potential triggers for developing AF.19–21
Risk of thromboembolic complications and COVID-19 infection
COVID-19 infection raises the risk of developing both arterial and venous thrombotic complications through systemic coagulation activation, leading to increased mortality.22 Thus, a scoping review showed that, among patients with COVID-19 infection, stroke and venous thromboembolism occurred in around 3% and 20% of patients, respectively, being more frequent as the severity of infection increased. Furthermore, thromboembolic risk was increased despite anticoagulant prophylaxis use.23 Of note, higher rates of thrombotic complications have been reported in patients with COVID-19 than in patients without COVID-19 but with acute respiratory distress syndrome.24
The European Society of Cardiology guidance for the management of cardiovascular disease during the COVID-19 pandemic recommends full therapeutic anticoagulation for the prevention of AF-related thromboembolic complications in men or women with a CHA2DS2-VASc score of ≥2/3, unless contraindicated, and anticoagulation should also be considered in men or women with a CHA2DS2-VASc score of 1/2.1 Despite anticoagulation with low-molecular-weight heparin (LMWH) decreasing the risk of death in severe COVID-19 patients with coagulopathy,25 many patients with acute respiratory distress syndrome still develop severe thrombotic complications,24 suggesting the need for full therapeutic-intensity anticoagulation in patients with severe illness or when anticoagulation is indicated (i.e. AF patients).26
On the other hand, it has been described that some patients with COVID-19 infection exhibit heparin resistance, requiring higher doses of heparin and leading to an increased risk of life-threatening hemorrhage. To reduce this risk, monitoring of the activity of unfractionated heparin therapy based on anti-Xa levels has been suggested.27
Efficacy and safety of direct oral anticoagulants in patients with AF
Overall, direct oral anticoagulants (DOACs) have shown a better benefit–risk profile than warfarin among individuals with non-valvular AF.28 Nevertheless, despite the varied clinical profile of patients included in the pivotal clinical trials and the fact that only indirect comparisons can be performed, there are some disparities in the main results of these studies.29–32
Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) was a phase III non-inferiority trial in which 18,113 AF patients at risk of stroke received, in a blinded fashion, dabigatran 110 mg or 150 mg bid, or, in an unblinded fashion, warfarin. Compared with warfarin, dabigatran 150 mg bid significantly reduced the risk of stroke or systemic embolism by 34% (RR 0.66, 95% CI 0.53–0.82, p<0.001 for superiority) and dabigatran 110 mg bid had a similar risk to warfarin. Of note, dabigatran 150 mg bid significantly decreased the risk of ischemic stroke by 24% (RR 0.76, 95% CI 0.60–0.98). By contrast, the rate of major bleeding was significantly reduced with dabigatran 110 mg bid (RR 0.80, 95% CI 0.69–0.93) but was similar to dabigatran 150 mg bid compared to warfarin. In addition, both doses of dabigatran significantly reduced the risk of intracranial bleeding and dabigatran 150 mg bid also reduced the risk of cardiovascular death (Table 1).29
Table 1.
Main results of pivotal clinical trials with direct oral anticoagulants.a
| RE-LY (CHA2DS2 2.1) | ROCKET-AF (CHA2DS2 3.5) | ARISTOTLE (CHA2DS2 2.1) | ENGAGE AF-TIMI 48 (CHA2DS2 2.8) | |
|---|---|---|---|---|
|
| ||||
| RR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
|
| ||||
| Stroke or systemic embolism | D150–W: 0.66 (0.53–0.82) | R–W: 0.88 (0.75–1.03)b | A–W: 0.79 (0.66–0.95) | E60–W: 0.87 (0.73–1.04)b,c |
| D110–W: 0.91 (0.74–1.11) | ||||
|
| ||||
| Ischemic stroke | D150–W: 0.76 (0.60–0.98) | R–W: 0.94 (0.75–1.17) | A–W: 0.92 (0.74–1.13) | E60–W: 1.00 (0.83–1.19) |
| D110–W: 1.11 (0.89–1.40) | ||||
|
| ||||
| Hemorrhagic stroke | D150–W: 0.26 (0.14–0.49) | R–W: 0.59 (0.37–0.93) | A–W: 0.51 (0.35–0.75) | E60–W: 0.54 (0.38–0.77) |
| D110–W: 0.31 (0.17–0.56) | ||||
|
| ||||
| Major bleeding | D150–W: 0.93 (0.81–1.07) | R–W: 1.04 (0.90–1.20) | A–W: 0.69 (0.60–0.80) | E60–W: 0.80 (0.71–0.91) |
| D110–W: 0.80 (0.69–0.93) | ||||
|
| ||||
| Intracranial bleeding | D150–W: 0.40 (0.27–0.60) | R–W: 0.67 (0.47–0.93) | A–W: 0.42 (0.30–0.58) | E60–W: 0.47 (0.34–0.63) |
| D110–W: 0.31 (0.20–0.47) | ||||
|
| ||||
| Cardiovascular death | D150–W: 0.85 (0.72–0.99) | R–W: 0.89 (0.73–1.10) | A–W: 0.89 (0.76–1.04) | E60–W: 0.86 (0.77–0.97) |
| D110–W: 0.90 (0.77–1.06) | ||||
Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) was a double-blind clinical trial in which 14,264 patients with non-valvular AF and a high risk of stroke were randomized to rivaroxaban (20 mg od; 15 mg od in patients with a creatinine clearance rate of 30–49 mL/min) or warfarin. In the intention-to-treat analysis, there was a trend toward a reduction in the risk of stroke or systemic embolism with rivaroxaban (HR 0.88, 95% CI 0.74–1.03). While the risk of major bleeding was similar in both groups, rivaroxaban significantly reduced the risks of death and of intracranial hemorrhage (Table 1).30
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) was a randomized, double-blind trial in which 18,201 patients with AF and ≥1 additional risk factor for stroke were randomized to apixaban (5 mg bid; 2.5 mg bid in case of ≥2 of the following criteria: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL) or warfarin. Compared with warfarin, apixaban significantly reduced the risk of stroke or systemic embolism by 21% (HR 0.79, 95% CI 0.66–0.95), the risk of major bleeding by 31% (HR 0.69, 95% CI 0.60–0.80), and the risk of intracranial bleeding by 58% (HR 0.42, 95% CI 0.30-0.58) (Table 1).31
Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) was a randomized, double-blind, double-dummy trial in which 21,105 patients with a moderate-to-high risk of AF were randomized to edoxaban 60/30 mg od, edoxaban 30/15 mg od, or warfarin. Compared with warfarin, high-dose edoxaban was associated with a similar risk of stroke or systemic embolism, although in the intention-to-treat analysis, there was a trend for superiority (HR 0.87, 97.5% CI 0.73–1.04, p=0.08). Major bleeding, intracranial bleeding, and cardiovascular death were also significantly reduced by high-dose edoxaban (Table 1).32
In summary, DOACs exhibit a greater benefit–risk profile compared with vitamin K antagonists (VKAs). Dabigatran 150 mg bid significantly reduced the risks of stroke or systemic embolism, ischemic stroke, intracranial hemorrhage, and cardiovascular death whereas dabigatran 110 mg bid reduced the risk of major bleeding and intracranial hemorrhage.
Oral anticoagulation and COVID-19 infection
A number of authors have recommended switching from oral anticoagulation to LMWH in patients hospitalized for COVID-19 infection.33–36 In the case of VKAs, this is mainly related to the difficulties in achieving an adequate International Normalized Ratio (INR) control during hospitalization.37 In the case of DOACs, this recommendation is based on the risk of drug–drug interactions, leading to an increase/decrease of drug concentrations caused by significant pharmacological interferences.38 Thus, in a study performed in 12 individuals with concomitant treatment with DOACs and antiviral drugs, C-trough levels of DOACs increased up to six times during hospitalization due to drug–drug interactions.38 However, the risk of interactions between drugs differs with the type of DOAC, as there are relevant disparities between them (i.e. effects on CYP 450 isoenzyme or P-glycoprotein [P-gp]).13,39,40
Both in vitro and in vivo studies have not reported any (0%) inhibition or induction of the principal isoenzymes of cytochrome P450 with dabigatran (i.e. CYP 3A4 or CYP 2C9),39,41 indicating that drug–drug interactions with dabigatran are unlikely. As with other DOACs, dabigatran etexilate is a transporter P-gp substrate, and caution should be exercised with the concomitant use of strong P-gp inhibitors or inducers. Thus, the concomitant use of dabigatran with ketoconazole, dronedarone, itraconazole, cyclosporine, or glecaprevir/pibrentasvir is contraindicated, the concomitant use with tacrolimus is not recommended, and a dose reduction is required with verapamil. By contrast, concomitant coadministration of P-gp inducers is anticipated to decrease dabigatran concentrations and should be avoided.39,41
Rivaroxaban is a substrate for P-gp metabolized by CYP 3A4 (≈18%). The use of rivaroxaban is not recommended in patients receiving concomitant systemic treatment with potent CYP 3A4 and P-gp inhibitors, such as ritonavir, as they could increase the risk of bleeding. By contrast, drugs that strongly inhibit only CYP 3A4 or P-gp but not both are anticipated to exhibit a lower increase in rivaroxaban concentrations and attention should be paid in patients with a high risk of bleeding.39,42
Apixaban is a substrate for P-gp metabolized by CYP 3A4 (≈25%). The use of apixaban is not recommended for the concomitant treatment with potent CYP 3A4 and P-gp inhibitors, such as ritonavir, as there is a higher risk of bleeding. The concomitant use of apixaban with strong CYP 3A4 and P-gp inducers may lead to a significant reduction of apixaban concentrations and caution should be exercised.39,43
Edoxaban is a substrate for P-gp metabolized through hydrolysis (mediated by carboxylesterase 1), conjugation, or oxidation by CYP 3A4/5 (<10%) and is eliminated primarily as unchanged drug in urine. Concomitant treatment with P-gp inhibitors increases edoxaban plasma concentrations. Concomitant use of edoxaban with ciclosporin, dronedarone, erythromycin, or ketoconazole but not with quinidine, verapamil, or amiodarone requires an edoxaban dose reduction to 30 mg od. The concomitant use of edoxaban with HIV protease inhibitors (P-gp inhibitors) has not been analyzed. By contrast, edoxaban coadministration with P-gp inducers leads to reductions in edoxaban concentrations and should be used with caution.39,44
In the light of this evidence, it seems that dabigatran may be the DOAC with the lowest risk of interactions with COVID-19 drugs that are metabolized via cytochrome P450. However, no specific studies have been carried out in this setting and the recommendations given are based on studies performed between HIV protease inhibitors and some DOACs (i.e. dabigatran with ritonavir) as well as on the effects of COVID-19 drugs on P-gp and CYP 3A4.39–45 Thus, in a study performed in 14 individuals treated with dabigatran and antiretrovirals, no thromboembolic or bleeding complications occurred.46 Another study showed the successful coadministration of dabigatran 110 mg bid and ritonavir/lopinavir in a subject with AF undergoing ablation, with similar levels than those reported in the RE-LY trial.47 Other studies have shown that ritonavir-boosted protease inhibitors seem safe in patients taking dabigatran.48,49 A recent review indicates that the concomitant use of protease inhibitors is contraindicated or not recommended with apixaban, rivaroxaban, and edoxaban but, in the case of dabigatran, although there are limited data, no significant interaction is expected.50 Another recent review reported that no dose modification is required with the concomitant use of lopinavir/ritonavir and dabigatran, whereas a 50% dose reduction is necessary with apixaban and coadministration is not recommended for edoxaban and rivaroxaban.51 The European Society of Cardiology states that in patients taking antiretroviral drugs, apixaban and rivaroxaban should be avoided.1 Despite the report of a woman treated with tocilizumab and dabigatran experiencing mesenteric arterial thrombosis,52 no clinically significant interaction is expected between these drugs.45 The recommendations performed by the Liverpool Drug Interactions Group are summarized in Table 2.45
Table 2.
Interactions of direct oral anticoagulants with potential COVID-19 therapies.a
| Co-administration is not recommended | ||
|---|---|---|
|
| ||
| DOAC | COVID-19 therapy | Commentary |
|
| ||
| Apixaban | Atazanavir | Atazanavir (potent CYP 3A4 and P-gp inhibitor): Potential increase of apixaban concentration |
|
|
|
|
| Dabigatran | Atazanavir (potent P-gp inhibitor): An increase of dabigatran concentration is expected | |
|
|
|
|
| Rivaroxaban | Atazanavir (potent CYP 3A4 and P-gp inhibitor): Potential increase of rivaroxaban concentration | |
|
| ||
| Apixaban | Lopinavir/ritonavir | Lopinavir/ritonavir (potent CYP 3A4 and P-gp inhibitor): Potential increase of apixaban concentration |
|
|
|
|
| Rivaroxaban | Lopinavir/ritonavir (potent CYP 3A4 and P-gp inhibitor): Potential increase of rivaroxaban concentration | |
|
| ||
| Potential clinically significant interaction (may require additional monitoring, dose adjustment, modification of timing of administration) | ||
|
| ||
| Edoxaban | Atazanavir | Atazanavir (potent P-gp inhibitor): Consider edoxaban dose reduction |
|
| ||
| Dabigatran | Lopinavir/ritonavir | Lopinavir/ritonavir (potent P-gp inhibitor): Close monitoring, mainly if renal insufficiency |
|
|
|
|
| Edoxaban | Lopinavir/ritonavir (potent P-gp inhibitor): Consider edoxaban dose reduction | |
|
| ||
| Dabigatran | Chloroquine | Chloroquine (P-gp inhibitor): Consider dabigatran dose reduction |
|
|
|
|
| Edoxaban | Chloroquine (P-gp inhibitor): Consider edoxaban dose reduction | |
|
| ||
| Dabigatran | Hydroxychloroquine | Hydroxychloroquine (P-gp inhibitor): Consider dabigatran dose reduction |
|
|
|
|
| Edoxaban | Chloroquine (P-gp inhibitor): Consider edoxaban dose reduction | |
|
| ||
| Dabigatran | Ruxolitinib | Ruxolitinib (P-gp inhibitor): Caution with concomitant use with dabigatran |
|
|
|
|
| Edoxaban | Ruxolitinib (P-gp inhibitor): Caution with concomitant use with edoxaban | |
|
| ||
| Potential weak interaction (no additional action may be required) | ||
|
| ||
| Apixaban | Tocilizumab | Unlikely that apixaban dose should be modified |
|
|
|
|
| Rivaroxaban | Unlikely that rivaroxaban dose should be modified | |
|
| ||
| Apixaban | Chloroquine | Chloroquine (P-gp and CYP 2C8 inhibitor): Modest impact on apixaban concentration |
|
|
|
|
| Rivaroxaban | Chloroquine (P-gp inhibitor): Modest impact on rivaroxaban concentration | |
|
| ||
| Apixaban | Hydroxychloroquine | Chloroquine (P-gp and CYP 2C8 inhibitor): Modest impact on apixaban concentration |
|
|
|
|
| Rivaroxaban | Chloroquine (P-gp inhibitor): Modest impact on rivaroxaban concentration | |
|
| ||
| Apixaban | Anakinra | Unlikely that apixaban dose should be modified |
|
|
|
|
| Rivaroxaban | Unlikely that rivaroxaban dose should be modified | |
|
| ||
| Apixaban | Sarilumab | Unlikely that apixaban dose should be modified |
|
|
|
|
| Rivaroxaban | Unlikely that rivaroxaban dose should be modified | |
|
| ||
| Apixaban | Azithromycin | Azithromycin (P-gp inhibitor): Modest impact on apixaban concentration |
|
|
|
|
| Dabigatran | Azithromycin (P-gp inhibitor): Modest impact on dabigatran concentration | |
|
|
|
|
| Edoxaban | Azithromycin (P-gp inhibitor): Modest impact on edoxaban concentration | |
|
|
|
|
| Rivaroxaban | Azithromycin (P-gp inhibitor): Modest impact on rivaroxaban concentration | |
|
| ||
| Unlikely clinically significant interaction | ||
|
| ||
| Apixaban | Baricitinib | |
| Dabigatran | Favipiravir | |
| Edoxaban | Interferon beta | |
| Rivaroxaban | Nitazoxanide | |
| Remdesivir | ||
| Ribavirin | ||
| Sofosbuvir | ||
|
| ||
| Dabigatran | Tocilizumab | |
|
| ||
| Edoxaban | ||
|
| ||
| Dabigatran | Anakinra | |
|
| ||
| Edoxaban | ||
|
| ||
| Dabigatran | Sarilumab | |
|
| ||
| Edoxaban | ||
|
| ||
| Apixaban | Ruxolitinib | |
|
| ||
| Rivaroxaban | ||
Data retrieved from Liverpool Drug Interactions Group.45
DOACs, direct oral anticoagulants; P-gp, P-glycoprotein.
Of note, dabigatran and apixaban are taken twice daily whereas edoxaban and rivaroxaban are taken once daily. Although some authors (though not all) have observed that a once-daily dosing regimen leads to better adherence, missing a once-daily dose may have a greater impact on anticoagulation.53 In addition, the impact of drug–drug interactions (i.e. reduction of efficacy or increase of bleeding risk) may be more relevant with once-daily regimens.
Hepatotoxicity, COVID-19 infection, and anticoagulation
COVID-19 causes a respiratory infection as well as damage in multiple organs, with the liver being one of the most relevant. In a study performed in 552 hospitals in China including 1,099 patients (median age 47 years), despite only 2.1% of patients having had prior hepatitis B infection, 21.3% and 22.2% of patients presented significant increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), respectively.54 Of note, liver damage is more common in patients with severe or critical disease.55 Different mechanisms have been proposed to explain this damage, including direct viral-induced cellular injury, hepatotoxicity secondary to COVID-19 therapies and concomitant medications, hyperinflammatory reactions as a response to COVID-19 infection, and the exacerbation of previous chronic liver disease during the COVID-19 infection.55 The most frequent pathological findings when liver damage occurs are mild increases in sinusoidal lymphocytic infiltration and sinusoidal dilatation, whereas moderate steatosis and multifocal hepatic necrosis are less common.55
The increase in transaminase levels follows a dynamic temporal pattern. Thus, a retrospective study performed in 5,771 adults with COVID-19 pneumonia showed that AST levels increased first followed by ALT levels in patients with severe disease, without important changes in alkaline phosphatase or total bilirubin levels. Of note, AST alterations were associated with higher mortality. As a result, it has been recommended that these laboratory parameters should be monitored during COVID-19 hospitalization.56
Liver toxicity associated to COVID-19 treatment is common in clinical practice. In a retrospective study performed in 217 individuals hospitalized for COVID-19, up to 38% of patients presented adverse drug reactions (gastrointestinal disorders 23%; liver system disorders 14%). The adverse drug reactions were mainly related to the use of lopinavir/ritonavir and umifenovir (64% and 18%, respectively). Severe adverse drug reactions were more common in patients with liver injury. The great majority of adverse drug reactions (97%) occurred within 14 days of hospitalization. Length of stay, polymedication, and comorbidities (many of them included in CHA2DS2-VASc) were independently associated with the development of adverse drug reactions57; this is of particular relevance as polymedication is highly prevalent in the AF population.58 In addition, a recent meta-analysis showed that the lopinavir/ritonavir-based combination had superior virologic eradication rates than other anti-COVID-19 agents and that the increase in transaminases is more frequent in patients hospitalized for COVID-19.59
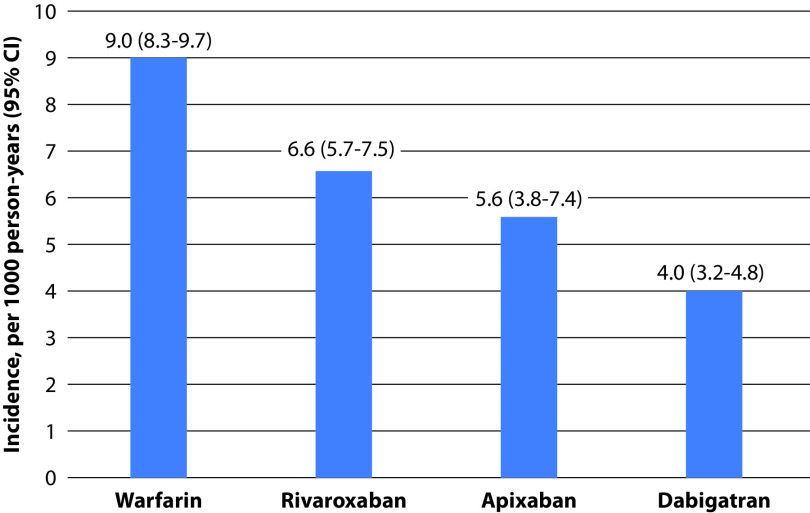
As most patients with AF require oral anticoagulation to reduce thromboembolic complications,6 it is recommendable to consider the risk of hepatotoxicity among individuals with AF and COVID-19 infection. A study that aimed to assess the risk of hospitalization due to liver injury in 113,717 patients with AF after starting oral anticoagulants (VKAs, dabigatran, rivaroxaban, and apixaban) showed that, after 12 months of treatment, dabigatran had the lowest rates of risk of hospitalization for liver injury (warfarin 9.0; rivaroxaban 6.6; apixaban 5.6; dabigatran 4.0 per 1000 person-years). Liver damage hospitalization rates were lower with DOACs versus with warfarin (HR 0.57, 95% CI 0.46–0.71) and, among DOACs, dabigatran had the lowest risk (Table 3 and Figure 1).60 This is relevant, as some antiviral drugs, such as remdesivir or tocilizumab, which have been shown to be beneficial in the treatment of severe COVID-19 pneumonia, may increase the risk of hepatotoxicity; therefore, the use of drugs with a lower risk is preferable, not only for drug–drug interactions but also for liver injury.61,62 With regard to edoxaban and liver damage, data from hospitalized individuals with COVID-19 infection are lacking. However, a substudy of the ENGAGE AF-TIMI 48 trial showed that in patients with a history of liver disease, bleeding rates but not thromboembolic outcomes were augmented. Although no significant differences were found between both drugs, drug-induced liver injury was reported in 2 (0.03%) patients receiving high-dose edoxaban, in 1 (0.01%) receiving low-dose edoxaban, and in no patients receiving warfarin.63
Table 3.
Incidence and predictors of hospitalization due to liver injury.a
| Incidence, 1000 person-years (95% CI) | Warfarin: 9.0 (8.3–9.7) | |
| Rivaroxaban: 6.6 (5.7–7.5) | ||
| Apixaban: 5.6 (3.8–7.4) | ||
| Dabigatran: 4.0 (3.2–4.8) | ||
|
| ||
| Predictors of liver injury hospitalization (DOACs versus warfarin) | ||
|
| ||
| Derivation sample | Validation sample | |
| HR (95% CI) | HR (95% CI) | |
|
| ||
| Dabigatran | 0.57 (0.44–0.73) | 0.47 (0.31–0.69) |
|
| ||
| Rivaroxaban | 0.84 (0.69–1.02) | 0.78 (0.59–1.02) |
|
| ||
| Apixaban | 0.74 (0.50–1.08) | 0.49 (0.26–0.93) |
Data retrieved from Alonso et al.60
DOACs, direct oral anticoagulants.
Figure 1.
Incidence of hospitalization due to liver injury by type of oral anticoagulant.a
aFigure constructed using data retrieved from Alonso et al.60
Although an optimal anticoagulation strategy for patients with AF who have liver disease remains unclear,64 it seems that DOACs, particularly dabigatran, may provide an added value.
Renal failure and DOAC use in the COVID-19 pandemic
Acute kidney injury in patients hospitalized for COVID-19 infection is frequent, with an incidence of about 3–15% that increases up to 50% in most severe patients such as those admitted in intensive care units.65 Although the pathophysiology is multifactorial, systemic inflammatory cytokine release plays a key role. To reduce the risk of acute kidney injury, an accurate volume correction and avoiding nephrotoxic agents are mandatory.66
With regard to anticoagulation, overall, the primary efficacy and safety endpoints of all DOACs compared with warfarin seem to be irrespective of renal function.39,67 On the other hand, while dabigatran is contraindicated among patients with a creatinine clearance rate of <30 mL/min, caution should be taken when using rivaroxaban, apixaban, and edoxaban in patients with a creatinine clearance rate of 15–29 mL/min as data are lacking in this population.39 Of note, the DOAC dosage should be performed according to the clinical profile of patients. Therefore, a patient’s advanced age or renal insufficiency should not discourage physicians from initiating or maintaining chronic oral anticoagulation with DOACs in patients with AF.68
On the other hand, a decline in renal function has been reported in patients taking warfarin, particularly in those with a poor INR control (‘warfarin nephropathy’). This decline in renal function has been associated with more adverse outcomes. However, it seems that, overall, DOACs exhibit a lower decline of renal function compared with VKA.69 In an analysis of the RE-LY trial, the decline in renal function was higher with warfarin than with dabigatran. Furthermore, the decline in renal function with warfarin was greater in patients with a poor INR control, diabetics, and in those who had previous VKA use.70 However, not all DOACs exhibit the same effects on renal parameters. Thus, in a study that compared renal outcomes in patients taking apixaban, dabigatran, rivaroxaban, and warfarin, patients treated with dabigatran and rivaroxaban but not with apixaban had a lower risk of adverse renal outcomes compared to treatment with warfarin.71
The ANIBAL protocol to improve oral anticoagulation in individuals with AF and COVID-19 infection
During the pandemic due to COVID-19, many patients with cardiac symptoms were reluctant to attend hospital, leading to delays in seeking care.72,73 This also occurred in patients with AF.12 Additionally, poor anticoagulation control among patients taking VKA is associated with higher rates of ischemia and bleeding and with higher mortality.74 Remarkably, in this setting (i.e. lockdown period), patients with life-threatening bleeding may delay medical attention with catastrophic consequences. In these cases, anticoagulants with a specific reversal agent, such as dabigatran, may provide an additional and relevant benefit.75
Switching to LMWH has been recommended during hospitalization for COVID-19 infection mainly due to the difficulties in attaining an adequate INR control with VKAs as well as due to the possibilities of drug–drug interactions between DOACs and antivirals and concomitant treatment during hospitalization for COVID-19 infection.33–36 However, moving to DOACs at discharge may be more beneficial than VKA administration as DOACs have a better benefits–risk profile.28,76 In addition, a reduction in mortality of elderly patients with COVID-19 pneumonia has been reported for those chronically treated with DOACs.77 Additionally, some authors have recommended switching from VKA to DOACs to reduce the number of needed laboratory tests and thus reduce unnecessary exposition to COVID-19.78,79 However, some case reports have been published of thrombotic complications during current treatment with DOACs, such as rivaroxaban or apixaban.80,81 Therefore, some factors for initiating oral anticoagulation for the prevention of thromboembolic events in patients with non-valvular AF after discharge for COVID-19 infection should be considered, including safety, efficacy, drug-induced hepatotoxicity risk, liver and renal function, simplicity of use, drug–drug interactions, or the risk of bleeding (i.e. importance of the availability of a specific reversal agents) (Box 1). Considering all these factors, dabigatran could be deemed a first-line choice for oral anticoagulation at discharge.
Box 1: Patients with non-valvular atrial fibrillation after discharge for COVID-19 infection: considerations for anticoagulation therapy.
|
DOACs, direct oral anticoagulants; VKA, vitamin K antagonists.
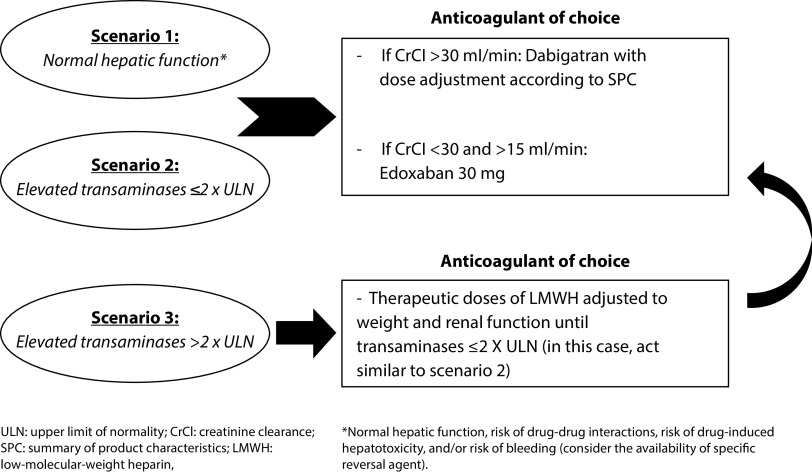
The Anticoagulation at discharge with Non-VKA after COVID-19 pNeumonIa and Based on Abnormalities of Liver’s parameters (ANIBAL) protocol is a simple approach that considers liver and renal function as well as the product label of DOACs in order to facilitate the choice of anticoagulation therapy at discharge after hospitalization for COVID-19 (Figure 2). Thus, in patients with a normal liver function and a creatinine clearance rate of >30 mL/min, dabigatran is recommended in order to reduce the risk of drug–drug interactions, drug-induced hepatotoxicity, and bleeding. In case with a creatinine clearance rate between 15 and 30 mL/min, edoxaban 30 mg should be preferred. The same recommendations apply for patients with elevated transaminases at ≤2 times upper limit of normal (ULN); by contrast, in patients with elevated transaminases at >2 × ULN, LMWH is recommended until transaminases decrease to ≤2 × ULN (in this case, act as previously recommended).
Figure 2.
Algorithm approach for patients with non-valvular atrial fibrillation at discharge after COVID-19 infection – the ANIBAL protocol.
LMWH, low-molecular-weight heparin; ULN, upper limit of normal.
Conclusions
Patients with COVID-19 infection have a high risk of arterial and venous thrombotic complications. On the other hand, the risk of AF is increased in these patients. Switching to LMWH has been recommended during hospitalization for COVID-19 infection. However, at discharge, the prescription of DOACs may offer some advantages over VKAs. Considering that dabigatran has shown a good efficacy and safety profile, seems to have a low risk of hepatotoxicity, is not metabolized by cytochrome P450, and has a specific reversal agent, it may be considered as a first-line choice for oral anticoagulation at discharge after COVID-19 infection.
Acknowledgements
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain).
Footnotes
The name of this great Carthaginian military general has been chosen for the protocol as a tribute by the authors, as they carry out their healthcare work in Cartagena
Contributions: All the authors were fully responsible for all the content and editorial decisions, involved in the design, algorithm proposal and preliminary bibliography research. All authors have reviewed, commented on, and approved the final version of the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest related to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/09/dic.2020-8-3-COI.pdf
Funding declaration: Writing and editorial assistance was supported financially by Boehringer Ingelheim.
Correct attribution: Copyright © 2020 Iturbe-Hernandez T, García de Guadiana Romualdo L, Gil Ortega I, Martínez Francés A, Meca Birlanga O, Cerezo-Manchado JJ. https://doi.org/10.7573/dic.2020-8-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 17 August 2020
Drugs in Context is published by BioExcel Publishing Ltd Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. [Accessed August 31, 2020]. https://www.escardio.org/static_file/Escardio/Education-General/Topic%20pages/Covid-19/ESC%20Guidance%20Document/ESC-Guidance-COVID-19-Pandemic.pdf. Last updated on June 10, 2020.
- 2.Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020 doi: 10.1055/s-0040-1712097. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of auricular fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 Mar 23; doi: 10.1001/jama.2020.4683. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Gopinathannair R, Merchant FM, Lakkireddy DR, et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020:1–8. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.016. S1547-5271(20)30594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vila-Córcoles Á, Ochoa-Gondar O, Torrente-Fraga C, et al. Evaluation of incidence and risk profile for suffering Covid-19 infection by underlying conditions among middle-aged and older adults in Tarragona. Rev Esp Salud Publica. 2020;94:e202006065. https://pubmed.ncbi.nlm.nih.gov/32588837/ [PubMed] [Google Scholar]
- 12.Holt A, Gislason GH, Schou M, et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J. 2020:ehaa494. doi: 10.1093/eurheartj/ehaa494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo V, Rago A, Carbone A, et al. Atrial fibrillation in COVID-19: from epidemiological association to pharmacological implications. J Cardiovasc Pharmacol. 2020 doi: 10.1097/FJC.0000000000000854. doi: 10.1097/FJC.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 14.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for Cardiac Electrophysiology During the Coronavirus (COVID-19) Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.028. S1547-5271(20)30289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020:cvaa106. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Khiali S, Khani E, Entezari-Maleki T. A comprehensive review on tocilizumab in COVID-19 acute respiratory distress syndrome. J Clin Pharmacol. 2020 doi: 10.1002/jcph.1693. doi: 10.1002/jcph.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Dudley SC., Jr Evidence for inflammation as a driver of atrial fibrillation. Front Cardiovasc Med. 2020;7:62. doi: 10.3389/fcvm.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purohit A, Rokita AG, Guan X, et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao C, Veleva T, Scott L, Jr, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattar Y, Ullah W, Rauf H, et al. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. Int J Cardiol Heart Vasc. 2020;29:100589. doi: 10.1016/j.ijcha.2020.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42(Suppl 1):19–20. doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 29.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 30.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 31.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 32.Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 33.Poli D, Tosetto A, Palareti G, et al. Managing anticoagulation in the COVID-19 era between lockdown and reopening phases. Intern Emerg Med. 2020;15:783–786. doi: 10.1007/s11739-020-02391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Testa S, Paoletti O, Giorgi-Pierfranceschi M, Pan A. Switch from oral anticoagulants to parenteral heparin in SARS-CoV-2 hospitalized patients. Intern Emerg Med. 2020;15:751–753. doi: 10.1007/s11739-020-02331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivas D, Roldán V, Esteve-Pastor MA, et al. Recommendations on antithrombotic treatment during the COVID-19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Rev Esp Cardiol. 2020;73(9):749–757. doi: 10.1016/j.recesp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva PGMBE, Sznejder H, Vasconcellos R, et al. Anticoagulation therapy in patients with non-valvular atrial fibrillation in a private setting in Brazil: a real-world study. Arq Bras Cardiol. 2020;114:457–466. doi: 10.36660/abc.20180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18:1320–1323. doi: 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 40.Rattanawong P, Shen W, El Masry H, et al. Guidance on Acute Management of Atrial Fibrillation in COVID-19. J Am Heart Assoc. 2020:e017529. doi: 10.1161/JAHA.120.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.European Medicines Agency (EMA) Pradaxa®, Summary of product characteristics. [Accessed August 31, 2020]. https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf.
- 42.European Medicines Agency (EMA) Xarelto®, Summary of product characteristics. [Accessed August 31, 2020]. https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf.
- 43.European Medicines Agency (EMA) Eliquis®, Summary of product characteristics. [Accessed August 31, 2020]. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf.
- 44.European Medicines Agency (EMA) Lixiana®, Summary of product characteristics. [Accessed August 31, 2020]. https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_en.pdf.
- 45.Liverpool Drug Interactions Group. University of Liverpool. Interaction report. [Accessed July 3, 2020]. https://www.covid19-druginteractions.org/downloads/interaction_reports.pdf?interaction_ids%5B%5D=1886.
- 46.Perram J, O’Dwyer E, Holloway C. Use of dabigatran with antiretrovirals. HIV Med. 2019;20:344–346. doi: 10.1111/hiv.12722. [DOI] [PubMed] [Google Scholar]
- 47.Barco S, Coppens M, van den Dool EJ, et al. Successful co-administration of dabigatran etexilate and protease inhibitors ritonavir/lopinavir in a patient with atrial fibrillation. Thromb Haemost. 2014;112:836–838. doi: 10.1160/TH14-03-0214. [DOI] [PubMed] [Google Scholar]
- 48.Kakadiya PP, Higginson RT, Fulco PP. Ritonavir-Boosted protease inhibitors but not cobicistat appear safe in HIV-positive patients ingesting dabigatran. Antimicrob Agents Chemother. 2018;62:e02275–17. doi: 10.1128/AAC.02275-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West TA, Perram J, Holloway CJ. Use of direct oral anticoagulants for treatment of atrial fibrillation in patients with HIV: a review. Curr Opin HIV AIDS. 2017;12:554–560. doi: 10.1097/COH.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 50.Pastori D, Mezzaroma I, Pignatelli P, Violi F, Lip GYH. Atrial fibrillation and human immunodeficiency virus type-1 infection: a systematic review. Implications for anticoagulant and antiarrhythmic therapy. Br J Clin Pharmacol. 2019;85:508–515. doi: 10.1111/bcp.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiggins BS, Dixon DL, Neyens RR, Page RL, 2nd, Gluckman TJ. Select drug-drug interactions with direct oral anticoagulants: JACC review topic of the week. J Am Coll Cardiol. 2020;75:1341–1350. doi: 10.1016/j.jacc.2019.12.068. [DOI] [PubMed] [Google Scholar]
- 52.Clarivet B, Robin P, Pers YM, et al. Tocilizumab and mesenteric arterial thrombosis: drug-drug interaction with anticoagulants metabolized by CYP 450 and/or by P-glycoprotein. Eur J Clin Pharmacol. 2016;72:1413–1414. doi: 10.1007/s00228-016-2107-0. [DOI] [PubMed] [Google Scholar]
- 53.Ageno W, Beyer-Westendorf J, Rubboli A. Once- versus twice-daily direct oral anticoagulants in non-valvular atrial fibrillation. Expert Opin Pharmacother. 2017;18:1325–1332. doi: 10.1080/14656566.2017.1361405. [DOI] [PubMed] [Google Scholar]
- 54.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92(9):1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 56.Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72(2):389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun J, Deng X, Chen X, et al. Incidence of adverse drug reactions in COVID-19 patients in China: an active monitoring study by hospital pharmacovigilance system. Clin Pharmacol Ther. doi: 10.1002/cpt.1866. Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallagher C, Nyfort-Hansen K, Rowett D, et al. Polypharmacy and health outcomes in atrial fibrillation: a systematic review and meta-analysis. Open Heart. 2020;7:e001257. doi: 10.1136/openhrt-2020-001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res. 2020;157:104872. doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso A, MacLehose RF, Chen LY, et al. Prospective study of oral anticoagulants and risk of liver injury in patients with atrial fibrillation. Heart. 2017;103:834–839. doi: 10.1136/heartjnl-2016-310586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leegwater E, Strik A, Wilms EB, et al. Drug-induced liver injury in a COVID-19 patient: potential interaction of remdesivir with P-glycoprotein inhibitors. Clin Infect Dis. 2020:ciaa883. doi: 10.1093/cid/ciaa883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muhović D, Bojović J, Bulatović A, et al. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40(8):1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qamar A, Antman EM, Ruff CT, et al. Edoxaban versus warfarin in patients with atrial fibrillation and history of liver disease. J Am Coll Cardiol. 2019;74:179–189. doi: 10.1016/j.jacc.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 64.Qamar A, Vaduganathan M, Greenberger NJ, et al. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71:2162–2175. doi: 10.1016/j.jacc.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Adapa S, Aeddula NR, Konala VM, et al. COVID-19 and renal failure: challenges in the delivery of renal replacement therapy. J Clin Med Res. 2020;12:276–285. doi: 10.14740/jocmr4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raza A, Estepa A, Chan V, Jafar MS. Acute renal failure in critically ill COVID-19 patients with a focus on the role of renal replacement therapy: a review of what we know so far. Cureus. 2020;12:e8429. doi: 10.7759/cureus.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961–970. doi: 10.1161/CIRCULATIONAHA.113.003628. [DOI] [PubMed] [Google Scholar]
- 68.Molteni M, Bo M, Di Minno G, et al. Dabigatran etexilate: appropriate use in patients with chronic kidney disease and in the elderly patients. Intern Emerg Med. 2017;12:425–435. doi: 10.1007/s11739-017-1660-6. [DOI] [PubMed] [Google Scholar]
- 69.Pastori D, Ettorre E, Lip GYH, et al. Association of different oral anticoagulants use with renal function worsening in patients with atrial fibrillation: a multicentre cohort study. Br J Clin Pharmacol. doi: 10.1111/bcp.14350. Published online May 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. 2015;65:2481–2493. doi: 10.1016/j.jacc.2015.03.577. [DOI] [PubMed] [Google Scholar]
- 71.Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–2632. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 72.Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-Segment–Elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, et al. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC Interv Cardiol. 2020;2:82–89. doi: 10.24875/RECIC.M20000120. [DOI] [Google Scholar]
- 74.Haas S, Ten Cate H, Accetta G, et al. Quality of vitamin k antagonist control and 1-year outcomes in patients with atrial fibrillation: a global perspective from the GARFIELD-AF registry. PLoS One. 2016;11:e0164076. doi: 10.1371/journal.pone.0164076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arellano-Rodrigo E, Fernandez-Gallego V, López-Vilchez I, et al. Idarucizumab, but not procoagulant concentrates, fully restores dabigatran-altered platelet and fibrin components of hemostasis. Transfusion. 2019;59:2436–2445. doi: 10.1111/trf.15259. [DOI] [PubMed] [Google Scholar]
- 76.Badreldin H. Hospital length of stay in patients initiated on direct oral anticoagulants versus warfarin for venous thromboembolism: a real-world single-center study. J Thromb Thrombolysis. 2018;46:16–21. doi: 10.1007/s11239-018-1661-y. [DOI] [PubMed] [Google Scholar]
- 77.Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID-19 era. Eur J Intern Med. 2020;77:158–160. doi: 10.1016/j.ejim.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thachil J, Tang N, Gando S, et al. DOACs and “newer” hemophilia therapies in COVID-19: reply. J Thromb Haemost. 2020;18:1795–1796. doi: 10.1111/jth.14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hermans C, Lambert C. Impact of the COVID-19 pandemic on therapeutic choices in thrombosis-hemostasis. J Thromb Haemost. 2020;18:1794–1795. doi: 10.1111/jth.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Tano G, Moschini L, Loffi M, Testa S, Danzi GB. Late pulmonary embolism after COVID-19 pneumonia despite adequate rivaroxaban treatment. Eur J Case Rep Intern Med. 2020;7:001790. doi: 10.12890/2020_001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dumitrascu OM, Volod O, Bose S, Wang Y, Biousse V, Lyden PD. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. J Stroke Cerebrovasc Dis. 2020;29:104982. doi: 10.1016/j.jstrokecerebrovasdis.2020.104982. [DOI] [PMC free article] [PubMed] [Google Scholar]