Abstract
IMPORTANCE
Metastatic colorectal cancer (mCRC) is heterogeneous, and primary tumors arising from different regions of the colon are clinically and molecularly distinct.
OBJECTIVE
To examine the prognostic and predictive value of primary tumor location in patients with RAS wild-type (wt) mCRC treated with first-line fluorouracil, leucovorin, and irinotecan (FOLFIRI) plus cetuximab in the Cetuximab Combined With Irinotecan in First-line Therapy for Metastatic Colorectal Cancer (CRYSTAL) trial and FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment For Patients With Metastatic Colorectal Cancer (FIRE-3) trial.
DESIGN, SETTING, AND PARTICIPANTS
In this retrospective analysis patients with RAS wt metastatic colorectal cancer from the CRYSTAL and FIRE-3 trials were classified as having left-sided or right-sided mCRC, defined, respectively, as patients whose tumors originated in the splenic flexure, descending colon, sigmoid colon, or rectum vs appendix, cecum, ascending colon, hepatic flexure, or transverse colon.
MAIN OUTCOMES AND MEASURES
Progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were assessed according to tumor location and treatment arm.
RESULTS
In the RAS wt populations of the CRYSTAL and FIRE-3 trials, patients with left-sided tumors (n = 142 and n = 157, respectively) had markedly superior PFS, OS, and ORR compared with patients with right-sided tumors (n = 33 and n = 38, respectively). Among CRYSTAL and FIRE-3 study patients with RAS wt left-sided tumors, FOLFIRI plus cetuximab significantly improved OS relative to the respective comparators (FOLFIRI and FOLFIRI plus bevacizumab); in contrast, in RAS wt patients with poor-prognosis right-sided tumors, limited efficacy benefits were observed upon the addition of cetuximab to FOLFIRI in CRYSTAL, and comparable outcomes were observed between the FOLFIRI plus cetuximab and FOLFIRI plus bevacizumab arms of FIRE-3. A significant interaction was observed between primary tumor location and treatment for OS (CRYSTAL: hazard ratio [HR], 1.95; 95% CI, 1.09–3.48 and FIRE-3: HR, 0.40; 95% CI, 0.23–0.70) within the RAS wt populations of both studies in multivariable models that also included sex, prior adjuvant therapy, and BRAF mutational status.
CONCLUSIONS AND RELEVANCE
In the RAS wt populations of CRYSTAL and FIRE-3, patients with left-sided tumors had a markedly better prognosis than those with right-sided tumors. First-line FOLFIRI plus cetuximab clearly benefitted patients with left-sided tumors (vs FOLFIRI or FOLFIRI plus bevacizumab, respectively), whereas patients with right-sided tumors derived limited benefit from standard treatments.
TRIAL REGISTRATION
clinicaltrials.gov Identifiers: CRYSTAL, NCT00154102, and FIRE-3, NCT00433927
The randomized phase 3 Cetuximab Combined With Irinotecan in First-line Therapy for Metastatic Colorectal Cancer (CRYSTAL) study demonstrated that adding the anti-epidermal growth factor receptor (EGFR) monoclonal antibody cetuximab to infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) significantly improved progression-free survival (PFS; the primary end point), overall survival (OS), and objective response rate (ORR) in the first-line treatment of patients with KRAS wild-type (wt) metastatic colorectal cancer (mCRC). A subsequent analysis revealed that expanded RAS testing (KRAS/NRAS, exons 2–4) resulted in even more pronounced treatment effects.1–3
The FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment For Patients With Metastatic Colorectal Cancer (FIRE-3) trial was a randomized phase 3 trial comparing first-line FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab in patients with KRAS wt mCRC. Objective response rate (the primary end point) and PFS were similar between treatment arms; however, OS was significantly improved in cetuximab-treated patients. Preplanned evaluation of expanded RAS status suggested an increased treatment effect in terms of the cetuximab-conferred OS benefit.4
Further underscoring the notion that mCRC is a heterogeneous disease, primary tumors arising from different regions of the colon are clinically and molecularly distinct. Left-sided tumors (those originating in the splenic flexure, descending colon, sigmoid colon, rectum, or one-third of the transverse colon; referred to as “distal tumors” elsewhere) derive from the embryonic hindgut; in contrast, right-sided tumors (those originating in the appendix, cecum, ascending colon, hepatic flexure, or two-thirds of the transverse colon; alternatively termed “proximal tumors”) derive from the embryonic midgut. As defined in the Methods, these fine embryological distinctions regarding the origin of the transverse colon cannot be fully taken into account in retrospective analyses; we will nonetheless hereafter refer to patients as having “left-sided mCRC” or “right-sided mCRC” for clarity.
Consistent with these differences in embryological origin, left-sided and right-sided tumors possess unique gene expression profiles. Notably, right-sided tumors are more frequently characterized by a host of adverse prognostic factors, including BRAF mutation positivity, microsatellite instability (prognostic in stage IV disease), hypermutation, serrated pathway signature positivity, and mucinous histology; conversely, left-sided tumors more frequently possess gene expression profiles characteristic of an EGFR inhibitor-sensitive phenotype (ie, EGFR/ERBB2 [formerly HER2 or HER2/neu] amplified, epiregulin high, and possessing classic chromosomal instability).5–8 These molecular differences manifest as differential clinical behavior, with right-sided tumors typically displaying worse prognosis.5,7–18 Nevertheless, primary tumor location has not traditionally been included as a stratification criterion in clinical trials, and the influence of tumor location on responsiveness to particular therapies remains incompletely understood.
Of interest, a retrospective analysis of the NCIC CTG CO.17 trial19 recently reported that tumor location was predictive of treatment benefit. In this population of chemotherapy-refractory patients with KRAS wt mCRC, adding cetuximab to best supportive care significantly benefitted patients with left-sided tumors, but had limited benefit in patients with right-sided tumors; furthermore, a significant interaction was observed between tumor location and treatment for PFS. Similarly, Wang et al20 recently reported that adding cetuximab to first-line or second-line chemotherapy significantly improved ORR, PFS, and OS inpatients with left-sided mCRC, but had limited benefit in patients with right-sided tumors. Furthermore, it has been reported that the EGFR pathway is not comparably activated in left-sided vs right-sided tumors, and an EGFR inhibitor-sensitive phenotype appears to be more prevalent in left-sided tumors, leading to the hypothesis that EGFR inhibitors may exhibit differential activity based on primary tumor location.5,21
Prompted by these observations, we examined the potential prognostic and predictive value of primary tumor location in patients with RAS wt mCRC treated with first-line FOLFIRI plus cetuximab in 2 large international randomized clinical trials (CRYSTAL and FIRE-3).
Methods
Study Design and Patients
The CRYSTAL and FIRE-3 study designs, treatment parameters, and eligibility criteria have been previously reported.1–4 Progression-free survival, as assessed by an independent review committee, was the primary end point of CRYSTAL; the primary end point of FIRE-3 was ORR. Both studies were approved by independent ethics committees for each trial center and were carried out in accordance with the Declaration of Helsinki. All patients provided informed consent prior to their participation.
As part of CRYSTAL trial enrollment, information regarding ethnic origin was collected from all study participants; this information was captured by the investigator as defined in the clinical study protocol in 2004. Options were prespecified in the case report form (Caucasian/white, black, Asian, Hispanic, or other). Since the FIRE-3 study was performed in Germany and Austria, the assumption appears to be justified that all or nearly all patients in FIRE-3 were white. In this manuscript, ethnic origin is summarized as either white or nonwhite. This information was of no relevance to this manuscript as no respective analyses were performed.
Only patients with RAS wt (KRAS and NRAS, exons 2–4) tumors were included in the present analysis.
Categorization of Primary Tumor Location
Primary tumors originating in the splenic flexure, descending colon, sigmoid colon, or rectum were classified as left-sided mCRC. Primary tumors originating in the appendix, cecum, ascending colon, hepatic flexure, or transverse colon were classified as right-sided mCRC. If tumors in an individual patient were sited in both left-sided and right-sided locations and the origin could not be ascribed to either side, the patient was excluded from the present analysis and the patient was classified as having tumors of indeterminate origin.
Statistical Analysis
This was not a pooled analysis: owing to the differing control arms, data from the CRYSTAL and FIRE-3 trials were analyzed separately. Objective response rate was analyzed using a logistic regression model, while PFS and OS analyses were carried out by employing Cox regression models (univariable and multivariable). For the multivariable regression models, covariates included treatment and primary tumor location, as well as their interaction term (an interaction term was included only for treatment and primary tumor location; no other interactions were tested), and the following baseline characteristics: sex, prior adjuvant therapy, and BRAF mutational status. All data was analyzed using SAS software (SAS Institute Inc). Although these are post hoc analyses, P less than 0.05 was defined as indicative of notable differences and considered significant.
Results
Study Populations and Baseline Characteristics
Of CRYSTAL study patients with RAS wt mCRC, 280 (76%) had left-sided tumors and 84 (23%) had right-sided tumors. The remaining 1% of patients (n = 3) had tumors sited on both the left and right sides whose origin could not be determined; these patients were excluded from the present analysis (eFigure, A in the Supplement).
In the final RAS wt population of FIRE-3 (n = 400), 306 RAS wt patients had left-sided tumors (76.5%), 88 had right-sided tumors (22%), and 6 had tumors whose origin could not be determined (1.5%) (eFigure, B in the Supplement).
Several differences in baseline characteristics and subsequent therapy received were noted between the tumor location subgroups within the RAS wt populations of CRYSTAL and FIRE-3 (eTables 1 and 2 in the Supplement). Likely reflecting the biological and prognostic differences known to exist between left-sided and right-sided tumors, a higher proportion of patients with right-sided tumors were women and had multiple metastatic sites; patients with left-sided tumors more frequently had liver-only metastases.
Among CRYSTAL study patients with right-sided tumors, there were multiple imbalances between treatment arms that appeared to favor the FOLFIRI arm, including that patients more frequently had Eastern Cooperative Oncology Group performance status (ECOG PS) 0, shorter index lesions, and less frequently received prior adjuvant therapy. Furthermore, patients with right-sided tumors treated with FOLFIRI plus cetuximab less frequently received subsequent follow-up therapy compared with patients with right-sided tumors treated with FOLFIRI. Importantly, however, baseline characteristics and subsequent therapy received were relatively balanced between treatment arms among patients with left-sided tumors (eTable 1 in the Supplement).
Numerical differences in several baseline characteristics and subsequent therapy received were also apparent between treatment arms in the tumor location subgroups of the FIRE-3 RAS wt population. Among patients with right-sided tumors, ECOG PS 0 was more frequent in the FOLFIRI plus bevacizumab arm, whereas patients treated with FOLFIRI plus cetuximab more frequently did not receive subsequent follow-up therapy. Baseline characteristics and subsequent therapy received were relatively balanced between treatment arms among patients with left-sided tumors (eTable 2 in the Supplement).
Relevant Prognostic Value of Primary Tumor Location
Data concerning the potential prognostic value of primary tumor location in CRYSTAL and FIRE-3 study patients with RAS wt mCRC are summarized in Table 1.
Table 1.
Efficacy Results for RAS Wild-Type CRYSTAL and FIRE-3 Study Patients, Stratified Based on Treatment Arm
| Parameter | CRYSTAL | FIRE-3 | ||||||
|---|---|---|---|---|---|---|---|---|
| FOLFIRI + Cetuximab | FOLFIRI | FOLFIRI + Cetuximab | FOLFIRI + Bevacizumab | |||||
| Right-Sided Tumors (n = 33) | Left-Sided Tumors (n = 142) | Right-Sided Tumors (n = 51) | Left-Sided Tumors (n = 138) | Right-Sided Tumors (n = 38) | Left-Sided Tumors (n = 157) | Right-Sided Tumors (n = 50) | Left-Sided Tumors (n = 149) | |
| ORR | ||||||||
| Rate, % | 42.4 | 72.5 | 33.3 | 40.6 | 52.6 | 68.8 | 50.0 | 61.7 |
| Odds ratio (95% CI) | 3.55 (1.63–7.75) | 1.39 (0.70–2.76) | 1.98 (0.97–4.08) | 1.61 (0.85–3.08) | ||||
| P value | <.001 | .34 | .09 | .18 | ||||
| PFS | ||||||||
| Median, mo | 8.1 | 12.0 | 7.1 | 8.9 | 7.6 | 10.7 | 9.0 | 10.7 |
| HR (95% CI) | 1.77 (1.08–2.91) | 1.54 (0.96–2.46) | 2.00 (1.36–2.93) | 1.38 (0.99–1.94) | ||||
| P value | .02 | .07 | <.001 | .06 | ||||
| OS | ||||||||
| Median, mo | 18.5 | 28.7 | 15.0 | 21.7 | 18.3 | 38.3 | 23.0 | 28.0 |
| HR (95% CI) | 1.93 (1.24–2.99) | 1.35 (0.93–1.97) | 2.84 (1.86–4.33) | 1.48 (1.02–2.16) | ||||
| P value | .003 | .11 | <.001 | .04 | ||||
Abbreviations: FOLFIRI, fluorouracil, leucovorin, and irinotecan; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Progression-free survival, OS, and ORR were significantly greater in left-sided vs right-sided tumors among RAS wt CRYSTAL study patients treated with FOLFIRI plus cetuximab. Furthermore, median PFS, median OS, and ORR were numerically superior in FOLFIRI-treated CRYSTAL study patients with left-sided tumors compared with patients with right-sided tumors.
Similarly, among FOLFIRI plus cetuximab-treated RAS wt FIRE-3 study patients, those with left-sided tumors had superior investigator-assessed ORR, PFS, and OS, relative to patients with right-sided tumors receiving FOLFIRI plus cetuximab. Although less pronounced, this effect was also observed in the FOLFIRI plus bevacizumab treatment arm of FIRE-3 for ORR, PFS, and OS.
Relevant Predictive Value of Primary Tumor Location
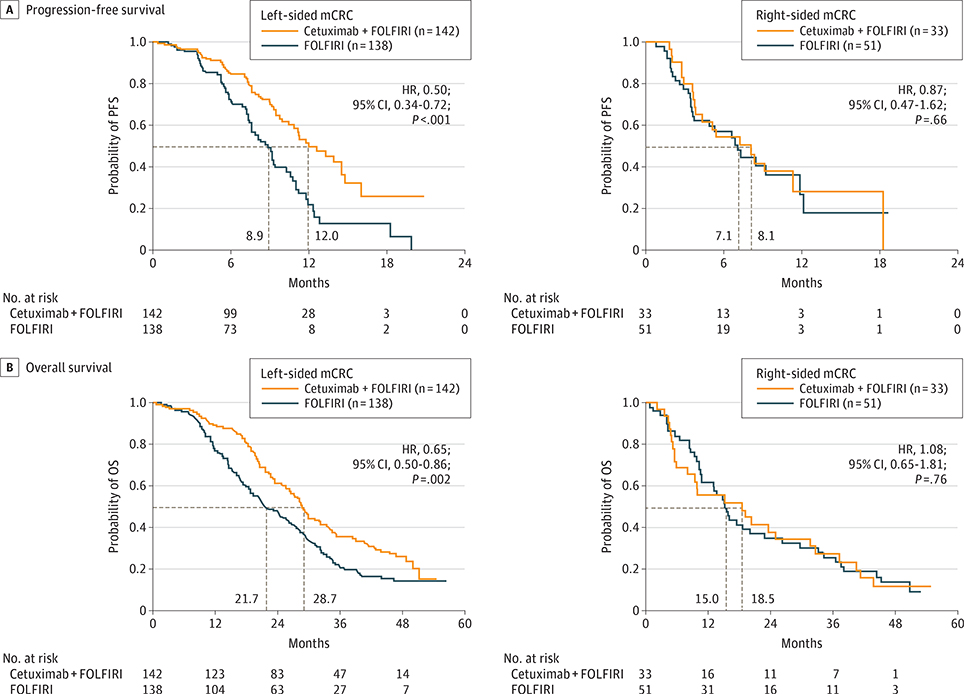
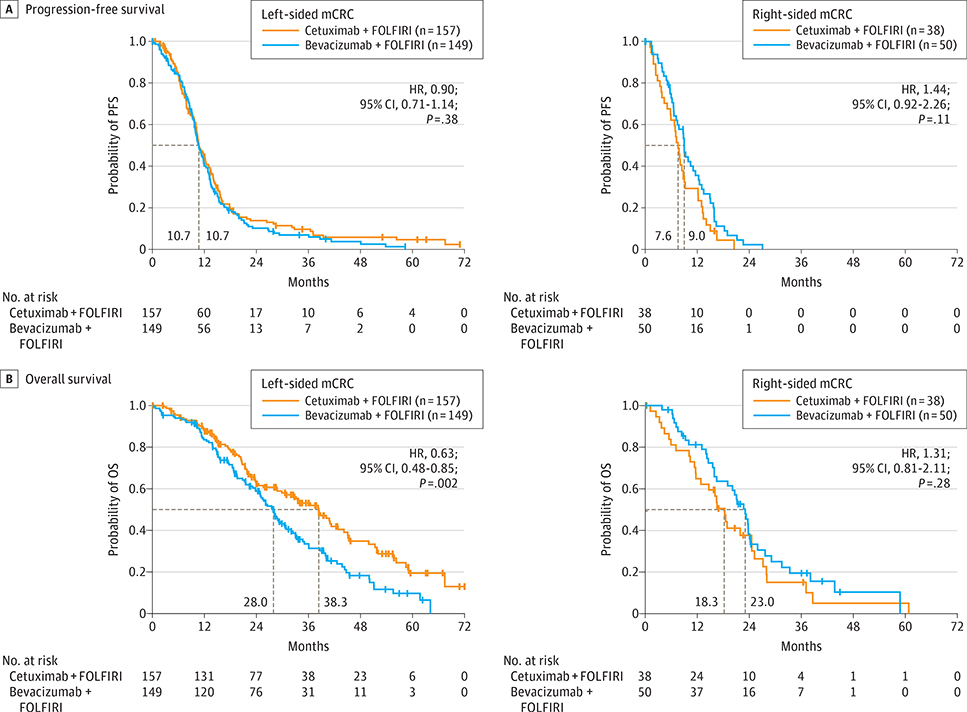
Of interest, primary tumor location was associated with differential treatment effects in CRYSTAL and FIRE-3 study patients with RAS wt mCRC (Figure 1 and Figure 2 and Table 2).
Figure 1. Survival Characteristics of CRYSTAL Study Patients.
A, Progression-free survival (PFS) and (B) overall survival (OS) for RAS wild-type (wt) CRYSTAL study patients, stratified based on tumor location. P values derive from a log-rank test, stratified by region and Eastern Cooperative Oncology Group performance status. FOLFIRI indicates fluorouracil, leucovorin, and irinotecan; HR, hazard ratio; mCRC, metastatic colorectal cancer.
Figure 2. Survival Characteristics of FIRE-3 Study Patients.
A, Progression-free survival (PFS) and (B) overall survival (OS) for RAS wild-type (wt) FIRE-3 study patients, stratified based on tumor location. P values derive from a log-rank test, stratified by region and Eastern Cooperative Oncology Group performance status. FOLFIRI indicates fluorouracil, leucovorin, and irinotecan; HR, hazard ratio; mCRC, metastatic colorectal cancer.
Table 2.
Efficacy Results for RAS Wild-Type CRYSTAL and FIRE-3 Study Patients, Stratified Based on Tumor Location
| Parameter | CRYSTAL | FIRE-3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alla | Left-Sided Tumors | Right-Sided Tumors | Allb | Left-Sided Tumors | Right-Sided Tumors | |||||||
| FOLFIRI + Cetuximab (n = 178) | FOLFIRI (n = 189) | FOLFIRI + Cetuximab (n = 142) | FOLFIRI (n = 138) | FOLFIRI + Cetuximab (n = 33) | FOLFIRI (n = 51) | FOLFIRI + Cetuximab (n = 199) | FOLFIRI + Bevacizumab (n = 201) | FOLFIRI + Cetuximab (n = 157) | FOLFIRI + Bevacizumab (n = 149) | FOLFIRI + Cetuximab (n = 38) | FOLFIRI + Bevacizumab (n = 50) | |
| ORR | ||||||||||||
| Rate, % | 66.3 | 38.6 | 72.5 | 40.6 | 42.4 | 33.3 | 65.3 | 58.7 | 68.8 | 61.7 | 52.6 | 50.0 |
| Odds ratio (95% CI) | 3.11 (2.03–4.78) | 3.99 (2.40–6.62) | 1.45 (0.58–3.64) | 1.33 (0.88–1.99) | 1.37 (0.85–2.19) | 1.11 (0.48–2.59) | ||||||
| P value | <.001 | <.001 | .43 | .18 | .23 | .83 | ||||||
| P value for interaction | NA | .07 | NA | .68 | ||||||||
| PFS | ||||||||||||
| Median, mo | 11.4 | 8.4 | 12.0 | 8.9 | 8.1 | 7.1 | 10.3 | 10.2 | 10.7 | 10.7 | 7.6 | 9.0 |
| HR (95% CI) | 0.56 (0.41–0.76) | 0.50 (0.34–0.72) | 0.87 (0.47–1.62) | 0.97 (0.78–1.20) | 0.90 (0.71–1.14) | 1.44 (0.92–2.26) | ||||||
| P value | <.001 | <.001 | .66 | .77 | .38 | .11 | ||||||
| P value for interaction | NA | .11 | NA | .09 | ||||||||
| OS | ||||||||||||
| Median, mo | 28.4 | 20.2 | 28.7 | 21.7 | 18.5 | 15.0 | 33.1 | 25.0 | 38.3 | 28.0 | 18.3 | 23.0 |
| HR (95% CI) | 0.69 (0.54–0.88) | 0.65 (0.50–0.86) | 1.08 (0.65–1.81) | 0.70 (0.54–0.90) | 0.63 (0.48–0.85) | 1.31 (0.81–2.11) | ||||||
| P value | .002 | .002 | .76 | .006 | .002 | .28 | ||||||
| P value for interaction | NA | .17 | NA | .009 | ||||||||
Abbreviations: FOLFIRI, fluorouracil, leucovorin, and irinotecan; HR, hazard ratio; NA, not applicable; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Includes 3 patients with primary tumors sited on both the left and right sides whose origin could not be determined.
Includes 6 patients with primary tumors sited on both the left and right sides whose origin could not be determined.
Among CRYSTAL study patients with RAS wt left-sided tumors, the addition of cetuximab to FOLFIRI significantly improved PFS, OS, and ORR, as expected based on the overall findings in the RAS wt population of the CRYSTAL study.3 However, although cautious interpretation of these data is required due to small sample sizes, limited benefit in terms of PFS, OS, and ORR was observed upon the addition of cetuximab to FOLFIRI in CRYSTAL study patients with right-sided tumors.
Comparable findings were obtained from the RAS wt population of FIRE-3. Among RAS wt FIRE-3 study patients with left-sided tumors, those treated with FOLFIRI plus cetuximab had significantly longer OS than patients receiving FOLFIRI plus bevacizumab in the absence of a significant difference in ORR or PFS, in consonance with the overall findings of FIRE-3.4 In contrast, among FIRE-3 study patients with right-sided tumors, although interpretation is again limited by small sample sizes, there were no significant differences in ORR, PFS, or OS with FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab.
Multivariable Models Investigating the Possible Interaction Between Primary Tumor Location and Treatment
Further exploring its potential predictive value via post hoc statistical modeling, a significant interaction was observed between primary tumor location and treatment for PFS and OS, but not for ORR, upon multivariable analysis of the CRYSTAL RAS wt population. This multivariable model also revealed that sex was prognostic for PFS but not OS, while treatment and BRAF mutational status were associated with both PFS and OS (eTable 3A-C in the Supplement).
Similarly, via post hoc statistical modeling, there was a significant interaction between primary tumor location and treatment for OS, but not for ORR or PFS upon multivariable analysis of the FIRE-3 RAS wt population. This analysis also showed that primary tumor location and BRAF mutational status were prognostic factors for both PFS and OS, sex was prognostic for PFS but not OS, and treatment was associated with OS but not PFS (eTable 3D-F in the Supplement).
Discussion
In this retrospective analysis, we assessed the potential prognostic and predictive relevance of primary tumor location in patients with RAS wt mCRC treated with first-line FOLFIRI–either alone or in conjunction with cetuximab or bevacizumab–in the CRYSTAL and FIRE-3 studies.
Our observations suggested that primary tumor location is a prognostic factor: patients with right-sided tumors have worse prognosis than those with left-sided tumors. This prognostic difference was independent of the first-line regimen, suggesting that there is a significant unmet clinical need for developing new treatment strategies for patients with right-sided mCRC.22
Furthermore, in both CRYSTAL and FIRE-3, FOLFIRI plus cetuximab had higher treatment effects in left-sided vs right-sided tumors. These observations underscore that mCRC is a heterogeneous disease. Prior work has uncovered that an EGFR inhibitor-sensitive phenotype appears to be more prevalent in left-sided tumors, whereas right-sided tumors appear to be more heterogeneous and only a subset of them are EGFR sensitive.5,7 According to the present multivariable analyses, heterogeneous responses to FOLFIRI plus cetuximab based on tumor location are not driven by BRAF mutational status, which represents an independent prognostic factor. Of note, however, prior studies have revealed that even BRAF wt tumors may possess a BRAF-mutant-like gene expression signature, and this is most often present in right-sided mCRC.5,7,8 Sex-related factors may help explain some of the heterogeneity that exists among right-sided tumors, which could be driven by biological differences or imbalances in baseline characteristics.
These issues highlight the need for future research to understand the biology of right-sided mCRC and develop more effective therapeutic strategies. It is critical to improve our understanding of the biology of tumor location (eg, biomarker signatures), which may help to better anticipate treatment benefit from cetuximab, bevacizumab, other targeted agents, and even conventional chemotherapy. This will require the identification of biomarkers beyond RAS and BRAF; indeed, it is conceivable that differential treatment effects could persist within individual molecular subtypes,7,8 even after stratifying patients on the basis of tumor location. Furthermore, despite preliminary observations from the oxaliplatin with fluorouracil and folinic acid chemotherapy (FOLFOX) subgroup of CALGB/SWOG 80405,23 we cannot exclude the possibility that our observations are attributable to the chemotherapy backbone deployed in CRYSTAL and FIRE-3 (FOLFIRI).
As is the case for all retrospective studies, potential limitations of our work include imbalances in certain baseline characteristics between treatment arms and the relatively modest number of patients in some subgroups; these imbalances may have actually favored the control arms. It should also be noted that, given the different comparators used in CRYSTAL (FOLFIRI) and FIRE-3 (FOLFIRI plus bevacizumab), comparisons between the 2 trials are challenging, and it would be informative to also have data comparing FOLFIRI vs FOLFIRI plus bevacizumab.
Our observations support the notion that primary tumor location potentially possesses both relevant prognostic and predictive value in patients with RAS wt mCRC. This suggests that patient stratification based on primary tumor location should be considered in the design of future trials in mCRC; these trials should also take mutational and gene expression signatures into account. Further clarity may be afforded by future prospective studies and retrospective analyses of already-published trials.
Our observations are broadly consistent with previous reports.5,7–20,24 Active signaling via the EGFR pathway is more frequent in patients with left-sided tumors, ostensibly rendering them more responsive to EGFR inhibitor-based therapy.5,21 Patients with right-sided tumors have poor prognosis, which arises independent of BRAF status and does not appear to be successfully salvaged by any approved first-line regimen.5,7–18 Also in general consonance with the present findings, retrospective analysis of the NCIC CTG CO.1719 trial reported that adding cetuximab to best supportive care in patients with KRAS wt chemotherapy-refractory mCRC significantly benefitted individuals with left-sided tumors but had limited benefit for those with right-sided tumors; furthermore, there was a significant interaction between primary tumor location and treatment for PFS. Similarly, Wang et al20 found that adding cetuximab to first-line or second-line chemotherapy significantly improved ORR, PFS, and OS in patients with left-sided mCRC but had limited benefit in patients with right-sided tumors. Tumor location subgroup data from CALGB/SWOG 8040523 were presented at the 2016 annual meeting of the American Society of Clinical Oncology: these data were similar to our observations regarding the prognostic and predictive impact of tumor location; notably, these data indicate that OS was significantly longer in KRAS wt patients with left-sided tumors treated with first-line chemotherapy (FOLFOX or FOLFIRI) plus cetuximab vs those receiving chemotherapy (FOLFOX or FOLFIRI) plus bevacizumab, whereas there was no significant difference between treatment arms among patients with right-sided tumors.23
In contrast, analysis of the PICCOLO trial15 reported that adding panitumumab to irinotecan as second-line or third-line therapy resulted in a PFS benefit in both left-sided and right-sided tumors; however, these observations may be attributable to patient selection during assessment of later-line treatment outcomes. Additionally, whereas data from the AVF2107g, AGITG MAX, and NO16966 trials suggest that both patients with right-sided tumors and those with left-sided tumors benefit from the addition of bevacizumab to chemotherapy,11,16 2 other analyses concluded that adding bevacizumab to chemotherapy principally benefits patients with left-sided mCRC.12,25 Data from the MAVERICC trial24 suggest that PFS was longer in patients with left-sided mCRC receiving first-line FOLFIRI plus bevacizumab vs FOLFOX plus bevacizumab; however, no PFS difference between arms was observed among patients with right-sided tumors.
Discrepancies between the present findings and prior reports may be attributable to differences in the line of therapy assessed, the identity of the agent administered, analytical limitations imposed by relatively small patient numbers, and the retrospective character of these observations. However, the preponderance of available evidence suggests that primary tumor location impacts responsiveness to most therapies in mCRC (cetuximab, bevacizumab, other targeted agents, and even conventional chemotherapy).
Conclusions
Our findings from this retrospective analysis of the RAS wt populations of the first-line CRYSTAL and FIRE-3 trials confirm the prognostic role of primary tumor location. These data further suggest a site and treatment interaction, with poor-prognosis right-sided tumors not significantly benefitting from the addition of cetuximab but with a profound benefit of cetuximab for left-sided tumors, more than what was appreciated before splitting patient populations by site. The FIRE-3 trial analyzed by site indicated that FOLFIRI and cetuximab would be the preferred option for RAS wt left-sided tumors in terms of OS, while right-sided tumors remain tumors of poor prognosis with any of the studied regimens. The data collected to date therefore suggest that primary tumor location should be included in the stratification criteria for future mCRC trials, particularly those involving EGFR inhibitors, as an EGFR inhibitor-sensitive phenotype appears to be more prevalent in left-sided tumors. Additional research is necessary to elucidate the subset of patients with RAS wt right-sided mCRC who may derive benefit from cetuximab.
Supplementary Material
Key Points.
Question
What is the prognostic and predictive relevance of primary tumor location in patients with RAS wild-type (wt) metastatic colorectal cancer (mCRC)?
Findings
In the RAS wt populations of the CRYSTAL and FIRE-3 trials, patients with left-sided tumors had a markedly better prognosis than those with right-sided tumors. First-line fluorouracil, leucovorin, and irinotecan (FOLFIRI) plus cetuximab clearly benefitted patients with left-sided tumors (vs FOLFIRI or FOLFIRI plus bevacizumab), whereas patients with right-sided tumors derived limited benefit from standard treatments (FOLFIRI, FOLFIRI plus bevacizumab, and FOLFIRI plus cetuximab).
Meaning
Primary tumor location should be included in the stratification criteria for future trials in patients with mCRC, particularly those involving epidermal growth factor receptor inhibitors.
Acknowledgments
Funding/Support: The CRYSTAL study was funded and sponsored by Merck KGaA, Darmstadt, Germany. The FIRE-3 study was an investigator-sponsored trial funded by Merck KGaA and Pfizer.
Role of the Funder/Sponsor: For the CRYSTAL trial, the funder of the study played a role in study design, data collection, data analysis and data interpretation. For the FIRE-3 trial, the funder of the study had no role in study design, data collection, data analysis, or data interpretation; data analysis was performed by ClinAssess GmbH. For this manuscript, the funder, Merck KGaA, provided funding support permitting medical writing services. The funder, Merck KGaA, reviewed and approved the manuscript before journal submission.
Footnotes
Conflict of Interest Disclosures: Dr Tejpar has received honoraria from Merck KGaA, Bayer, Sanofi, and Roche; provided consulting for Merck KGaA, Bayer, Sanofi, Boehringer, and Roche; participated in speakers bureaus for Roche, Merck KGaA, and Sanofi; and received research funding from Sanofi and Bayer. Dr Stintzing has received honoraria from Amgen, Roche, Bayer, Merck KGaA, and Sanofi; provided consulting for Amgen, Roche, Bayer, Merck KGaA, and Sanofi; and received travel accommodations from Amgen, Roche, Bayer, Merck KGaA, and Sanofi. Dr Ciardiello has provided consulting for Merck KGaA, Bayer, Lilly, Roche, and AstraZeneca; and received research funding from Bayer and Merck KGaA. Dr Tabernero has provided consulting for Amgen, Boehringer Ingelheim, Celgene, Chugia, Imclone, Lilly, Merck, Merck KGaA, Millennium, Novartis, Roche, Sanofi, Symphogen, and Tahio. Dr Van Cutsem has received research funding from Amgen, Bayer, Boehringer, Celgene, Ipsen, Merck, Roche, and Sanofi. Mr Beier is an employee of Merck KGaA. Ms Esser is an employee of Merck KGaA and reports stock ownership. Dr Lenz has received honoraria from Merck KGaA and provided consulting for Merck KGaA. Dr Heinemann has received honoraria from Merck, Amgen, Roche, Sanofi, Baxalta, and SIRTEX; provided consulting for Merck, Amgen, Roche, Sanofi, Baxalta, Servier, and SIRTEX; participated in speakers bureaus for Merck, Amgen, Roche, Sanofi, Baxalta, and SIRTEX; received research funding from Merck, Amgen, Roche, Pfizer, and Sanofi; and received travel accommodations from Merck, Roche, Baxalta, and SIRTEX. No other disclosures are reported.
Correction: This article was corrected on October 26,2017, to fix an erroneous hazard ratio in Figure 2.
Contributor Information
Sabine Tejpar, Molecular Digestive Oncology Unit, University Hospital Gasthuisberg, Leuven, Belgium.
Sebastian Stintzing, Department of Hematology and Oncology, University of Munich (Ludwig-Maximilians-Universität), Munich, Germany.
Fortunato Ciardiello, Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale, Seconda Università di Napoli, Naples, Italy.
Josep Tabernero, Medical Oncology Department, Vall d’Hebron University Hospital and Institute of Oncology (VHIO), Universitat Autònoma de Barcelona, Barcelona, Spain.
Eric Van Cutsem, Digestive Oncology, University Hospitals Leuven and KULeuven, Leuven, Belgium.
Frank Beier, Merck KGaA, Darmstadt, Germany.
Regina Esser, Merck KGaA, Darmstadt, Germany.
Heinz-Josef Lenz, Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA.
Volker Heinemann, Department of Hematology and Oncology, University of Munich (Ludwig-Maximilians-Universität), Munich, Germany.
REFERENCES
- 1.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. [DOI] [PubMed] [Google Scholar]
- 5.Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014; 25(10):1995–2001. [DOI] [PubMed] [Google Scholar]
- 6.LaPointe LC, Dunne R, Brown GS, et al. Map of differential transcript expression in the normal human large intestine. Physiol Genomics. 2008;33 (1):50–64. [DOI] [PubMed] [Google Scholar]
- 7.Dienstmann R, Guinney J, Delorenzi M, et al. Colorectal cancer subtyping consortium (CRCSC) identification of a consensus of molecular subtypes. ASCO Meeting Abstracts 2014: abstr3511. [Google Scholar]
- 8.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modest DP, Schulz C, von Weikersthal LF, et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anticancer Drugs. 2014;25(2):212–218. [DOI] [PubMed] [Google Scholar]
- 10.von Einem JC, Heinemann V, von Weikersthal LF, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140(9):1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price TJ, Buizen L, Hardingham J, et al. Molecular subgroups from the AGITG MAX trial; right or left primary site of colorectal cancer and outcomes for metastatic colorectal cancer (mCRC). Ann Oncol. 2014;25(suppl 4):iv167–iv209. [DOI] [PubMed] [Google Scholar]
- 12.Boisen MK, Johansen JS, Dehlendorff C, et al. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann Oncol. 2013;24(10):2554–2559. [DOI] [PubMed] [Google Scholar]
- 13.Loupakis F, Yang D, Zhang W et al. Primary side of origin affects the outcome of mCRC patients treated with first-line FOLFIRI plus bevacizumab independently of BRAF status and mucinous histology. J Clin Oncol 30 2012;suppl: abstr 3549. [Google Scholar]
- 14.Sinicrope FA, Mahoney MR, Yoon HH, et al. ; Alliance for Clinical Trials in Oncology. Analysis of molecular markers by anatomic tumor site in stage III colon carcinomas from adjuvant chemotherapy trial NCCTG N0147(Alliance). Clin Cancer Res. 2015; 21(23):5294–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seligmann JF. Primary Tumour Location (PTL) as a prognostic and predictive factor in advanced colorectal cancer: data from 2075 patients in randomised trials. Ann Oncol. 2014;25(suppl 4):iv172. [Google Scholar]
- 16.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3): dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Ma J, Zhang S, et al. A prognostic analysis of 895 cases of stage III colon cancer in different colon subsites. Int J Colorectal Dis. 2015; 30(9):1173–1183. [DOI] [PubMed] [Google Scholar]
- 18.Sunakawa Y, Ichikawa W, Tsuji A, et al. Prognostic impact of primary tumor location on survival time in patients (pts) with metastatic colorectal cancer (mCRC) treated with cetuximab plus oxaliplatin-based chemotherapy: a subgroup analysis of the JACCRO CC-05/06. J Clin Oncol 34 2016;suppl 4S:abstr 613. [DOI] [PubMed] [Google Scholar]
- 19.Brulé SY, Jonker DJ, Karapetis CS, et al. Location ofcolon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51(11):1405–1414. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Bai L, Liu TS, et al. Right-sided colon cancer and left-sided colorectal cancers respond differently to cetuximab. Chin J Cancer. 2015;34(9):384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell AE, Wang Y, Li Y, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venook AP Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405(Alliance). J Clin Oncol 34 2016;suppl 4S:abstr 3504. [Google Scholar]
- 24.Lenz HJ, Lee FC, Yau L, et al. MAVERICC, a phase 2 study of mFOLFOX6-bevacizumab (BV) vs FOLFIRI-BV with biomarker stratification as first-line (1L) chemotherapy (CT) in patients (pts) with metastatic colorectal cancer(mCRC). J Clin Oncol 34 2016;suppl4S:abstr493. [Google Scholar]
- 25.He WZ, Yang Q, Jiang C et al. Bevacizumab effectiveness and primary tumor location in metastatic colorectal cancer patients. J Clin Oncol 34 2016;suppl 4S:abstr 683. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.