Abstract
We describe the clinical, laboratory and radiological features of 3 critically ill patients with COVID-19 who developed severe encephalopathy. The first patient did not regain consciousness when sedation was removed at the end of 2 weeks of intensive care. He had received treatment with convalescent plasma. His clinical examination was remarkable for intact brainstem reflexes, roving eye movements, later transient ocular flutter; and then what appeared to be slow ocular dipping. He had no coherent volitional response to the environment. The second patient recovered with measurable cognitive deficits after a prolonged period of encephalopathy. He had received combination treatment with interferon beta 1b and lopinavir/ritonavir. The third patient remained in persistent, severe agitated delirium and died 3 months into his illness. The MRI of the 3 patients showed multifocal abnormalities predominantly in the cerebral white matter, with varying involvement of the grey matter, brainstem and spinal cord. Case 1's MRI changes were consistent with acute disseminated encephalomyelitis. The patients also displayed blood markers, to varying degree, of autoimmunity and hypercoagulability. We were not able to convincingly show, from microbiological as well as immunological evaluation, if the effects of COVID-19 on these patients' nervous system were a direct consequence of the virus, proinflammatory-thrombotic state or a combination. Patient 1 responded partially to empirical, albeit delayed, therapy with intravenous immunoglobulins. Patient 2 recovered with no specific treatment. These cases illustrate the need to understand the full spectrum of encephalopathy associated with COVID-19 so as to better guide its management.
Keywords: COVID-19, SARS-CoV-2 virus, Encephalitis, Parainfectious, Autoimmunity
Highlights
-
•
We describe three severe COVID-19 patients with encephalopathy.
-
•
We hypothesize possible underlying pathophysiology; viral, parainfectious or both.
-
•
We discuss the dilemma we faced in their diagnosis and management.
1. Introduction
Corona viruses have rarely been reported to cause encephalitis, most convincingly with the HCoV-OC43 virus [[1], [2], [3]]. There are rare reports of microbiologically proven central nervous system involvement by severe acute respiratory syndrome (SARS) virus, SARS-CoV-1 [[4], [5], [6]]. Viral genome sequences were detected in the brains of patients who died from SARS although it is unclear what neurological symptoms these patients had antemortem [7]. Most other neurological complications of SARS [5,8] and the closely related Middle Eastern Respiratory Syndrome (MERS) [9,10] appear to be indirect and consequential pathology rather than primary viral encephalitis.
We describe 3 patients with severe COVID-19, with what we believe is the spectrum of encephalopathy that can develop from SARS-CoV-2 infection. The definition of infective encephalitis as well as Acute Disseminated Encephalomyelitis (ADEM) was based on published criteria [[11], [12], [13]]. Likewise, the likelihood of these complications being related to COVID-19 was graded using guidelines from a recently published review by Ellul et al. [14].
2. Case 1 (Appendix, Fig. A)
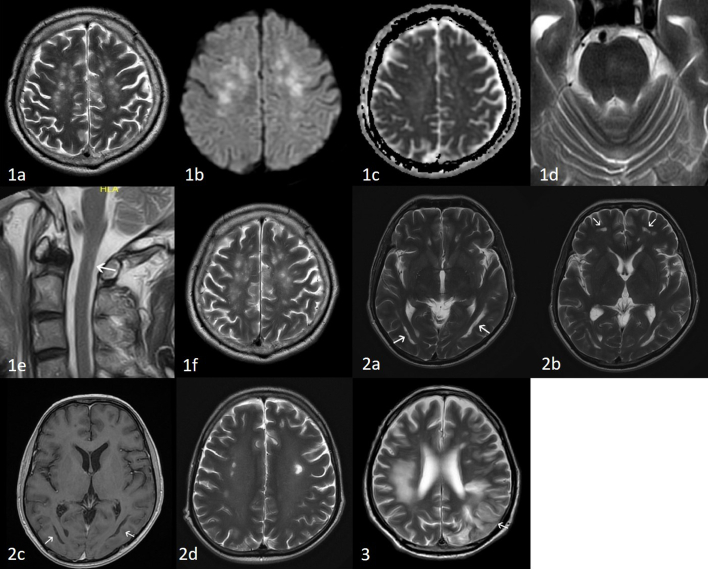
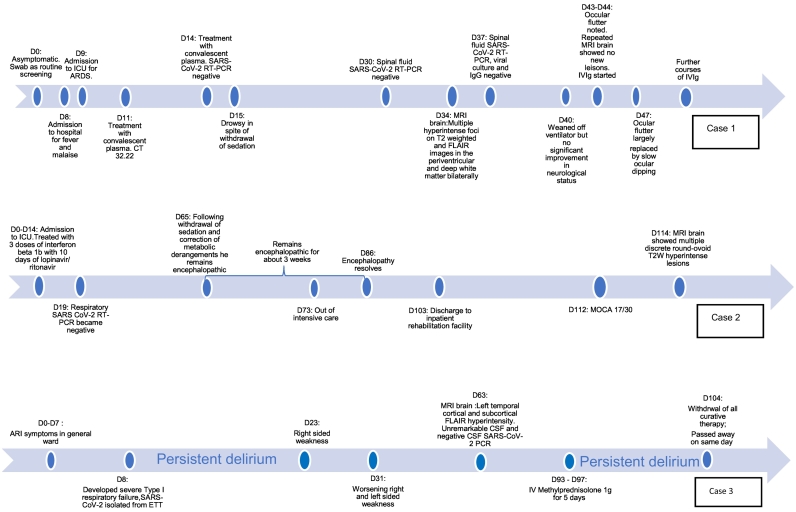
A 59-year old man, with a past history of chronic hypertension was diagnosed with COVID-19 by a positive nasopharyngeal swab for SARS-CoV-2 real-time polymerase chain reaction (RT-PCR). He was asymptomatic and was tested during routine screening of COVID-19 patient contacts. He only reported fever and malaise on day 8 of illness. He had no neurological symptoms, including anosmia and dysgeusia. He rapidly declined from day 9, developed severe acute respiratory distress syndrome (ARDS) and required inotropic support. Renal function worsened. Viraemia was documented on day 11 (cycle threshold value 32.22). He received convalescent plasma on day 11 and 14. Serum SARS-CoV-2 RT-PCR turned negative on day 14. He remained drowsy in spite of withdrawal of sedation and adequate renal replacement therapy. The brain stem reflexes, namely pupillary, doll's and corneal reflexes were intact. He had no limb movements to pain. Deep tendon reflexes, jaw jerk and pout reflexes were brisk. Babinski's reflex was absent. Initially slow conjugate ping-pong movements were noted. Subsequently, the eyes drifted continuously and conjugately in vertical and horizontal directions; often remaining in right and downward positions for up to a minute. Spinal tap done twice on days 30 and 37 was remarkable for only borderline low glucose, slight pleocytosis, and raised protein (Table 1). Spinal fluid SARS-CoV-2 RT-PCR, IgG and viral culture were negative. EEG showed continuous diffuse slow activity (1–3 Hz, 20 -40uV) intermixed with theta activity. Plain and contrasted CT brain did not reveal any abnormalities to explain his condition. MRI brain was only done on day 34 when endotracheal tube SARS-CoV-2 RT-PCR became negative (Fig. 1a–d). It showed multiple discrete hyperintense foci on T2 weighted (T2W) and FLAIR images in the periventricular and deep white matter bilaterally, with foci in the temporal region, subcortical white matter as well as the forceps minor. T2W and FLAIR hyperintense foci were also present bilaterally in the cerebral peduncles, pons, middle cerebellar peduncles and cerebellar white matter. These were hyperintense on diffusion weighted imaging (DWI) without corresponding restricted diffusion on the apparent diffusion coefficient (ADC) maps. The lesions in the cerebral white matter were centrally more T2W hyperintense, corresponding to low signal on T1 weighted images (T1WI), and were ill defined and fluffy peripherally. Susceptibility weighted images (SWI) showed 2 foci of microhaemorrhage in the left cerebellar white matter and left middle cerebellar peduncle. Equivocal FLAIR signal was seen in the olfactory nerves and bulbs. Chronic lacunar infarcts in the right caudate head and right thalamus were attributed to background small vessel disease. Magnetic Resonance Angiogram (MRA) was unremarkable. There were several subtle foci of intramedullary hyperintense signal on T2W images (T2WI) within the spinal cord at C1, T9-T11 and the conus (Fig. 1e). Gadolinium could not be given because of his renal failure. The combination of white matter, brain stem and spinal cord changes were suggestive of ADEM [13]. His blood investigations (Table 1) indicated an inflammatory and prothrombotic state. He was given low molecular weight heparin prophylaxis on day 25 which was stopped subsequently because of unexplained decrease in hemoglobulin. At day 40 he was weaned-off the ventilator. On day 43 ocular flutter was noted. As ocular flutter and its extension opsoclonus are associated with para-infectious autoimmunity, serum anti-Ri antibody was requested, and he was given a course of intravenous immunoglobulin (IVIg). Follow up MRI brain performed on day 44 showed no new lesions but some, particularly on left side of the pons, became more discrete. Diffuse confluent background T2W and FLAIR hyperintensity in the cerebral white matter was also more apparent (Fig. 1f). By day 47 the ocular flutter was largely replaced by slow ocular dipping. Although the anti-Ri antibody returned negative, we extended the IVIg for a total of 7 days because of the suggestion of positive response. At the end of the treatment, the ocular dipping and roving eye movements improved. Further 2 courses of 2 g/kg of IVIg was given at 3 weekly intervals. At 3.5 months of illness he was able to open eyes spontaneously, track visually and smile meaningfully. However, he had no coherent volitional motor and verbal response to the environment. His lower limbs showed disproportional spastic weakness compared to upper limbs, corresponding to the periventricular changes on brain MRI. No interval changes were noted on spinal follow-up images.
Table 1.
Summary of relevant investigations.
| Case 1 | Case 3 | ||
|---|---|---|---|
| Spinal fluid analysis | |||
| Day 30 | Day 37 | Day 67 | |
| Cell Count (cells/uL) | RBC -22 | RBC -9 | RBC -1 |
| Nucleated cells - 6 | Nucleated cells – 6 | Nucleated cells <1 | |
| Neutrophils 0% | Neutrophils 0% | ||
| Lymphocytes 41% | Lymphocytes 57% | ||
| Monocytes 59% | Monocytes 42% | ||
| Protein (0.10–0.40 g/L) | 0.56 | 0.76 | 0.49 |
| Glucose (2.5–5.5 mmol/L) | 2.7 | 3.8 | 5.3 |
| Microscopy | Clear and colourless | Clear and colourless | Clear and colourless |
| SARS-CoV RT-PCR | Not detected | Not detected | |
| SARS-CoV viral culture | Not detected | ||
| SARS-CoV IgG | Not detected | ||
| HSV DNA PCR | Not detected | Not detected | |
| CMV DNA PCR | Not detected | Not detected | |
| VZV DNA PCR | Not detected | Not detected | |
| VDRL | Not done | Negative | |
| Oligoclonal bands | Negative | Not done | |
| Autoimmune encephalitis panel (CSF) | Negative | Not done | |
| |||
| |||
| |||
| |||
| |||
| |||
| Blood tests | |||
| Syphilis IgG and RPR | Negative | Negative | |
| Autoimmune encephalitis panel (Serum) | Negative | Negative | |
| |||
| |||
| |||
| |||
| |||
| |||
| MOG and AQP4 antibodies | Negative | Not done | |
| Paraneoplastic panel | All Negative | Not done | |
| (Hu, Yo, Ri, CV2, Amphiphysin, PNMA2/Ta, recoverin, SOX1, titin, zic4, GAD65 and Tr (DNER) | |||
| Systemic autoimmune markers | |||
| Anti-nuclear antibodies (ANA) | Not done | 320 titre, Nucleolar pattern | |
| (Normal <80) | |||
| Anti-ds-DNA (0–25 IU/mL) | Normal | 40 | |
| Anti-Ro (RU/mL) | Negative | Negative | |
| Anti-La (RU/mL) | Negative | Negative | |
| Anti-Sm (RU/mL) | Negative | Negative | |
| Anti-RNP (RU/mL) | Not done | Negative | |
| Anti-Jo1 (RU/mL) | Not done | Negative | |
| Anti-Scl 70 (RU/mL) | Not done | Negative | |
| C3 (0.80–1.60) | 1.14 | 0.67 (low) | |
| C4 (0.17–0.60) | 0.15 (low) | 0.14 (low) | |
| Anti TPO and antithyroglobulin | Negative | Negative | |
| Pro-thrombotic markers | |||
| Lupus anticoagulant | Inconclusive | Weakly positive | |
| Anti-cardiolipin IgM | Negative | Negative | |
| Anti-cardiolipin IgG (<20 GPL units) | Negative | 25 | |
| Anti beta2 glycoprotein Ig M | Negative | Negative | |
| Anti beta2 glycoprotein Ig G | Negative | Negative | |
| Protein C, Protein S, Anti thrombin III | Normal | Normal | |
| VWF Antigen (56–160%) | 286% | 362% | |
| Factor VIII (60–150%) | 405% | 357% | |
| PT (11.7–14 s) | 15 s | 14.2 | |
| APTT (27–37 s) | 32.9 s | 39.6 | |
| D-Dimer (<0.5 μg/mL) | >4 | >4 | |
| Fibrinogen (1.8–4.5 g/L) | 5.7 | 5.1 | |
Figs. 1–3.
Fig. 1; case 1: Brain MRI done at day 34 and 44 of illness showing combination of white matter, brain stem and spinal cord changes suggestive of acute disseminated encephalomyelitis. Fig. 1a: Axial T2WI of the brain showing discrete hyperintense foci in the deep and subcortical white matter. Fig. 1b DWI and Fig. 1c: ADC, shows DWI hyperintensity of the lesions without restricted diffusion on the ADC maps. Fig. 1d: Axial T2WI showing subtle hyperintensity in the left side of the pons. Fig. 1e: Sagittal T2WI of the cervical spine with a small linear lesion on the right side of the spinal cord at C1 (white arrow). Fig. 1f: Follow-up MRI, axial T2WI showed no new white matter lesions, however, there is increase in the surrounding and background diffuse white matter hyperintensity. Fig. 2; case 2: Brain MRI done at day 114 deemed possible sequelae of hypoxia-ischaemia or inflammatory demyelination; corresponding to the contemporaneous neuropsychological assessment. Fig. 2a & b: Axial T2WI showing a) bilateral curivilinear high signal paralleling the ventricles and b) in the inferior frontal white matter (white arrows). Fig. 2c: Axial T1WI showing low signal in the white matter lesions (white arrows). Fig. 2d: Axial T2WI showing the discrete hyperintense lesions in the deep white matter on a background diffuse leuokoencephalopathy. Fig. 3; case 3: Brain MRI done at nine weeks of illness; the changes were attributable to para-infectious autoimmune coagulopathy and corresponding to his clinical state of sequential hemiplegia and persistent delirium. Fig. 3: Axial T2WI showing T2W hyperintensity in the right frontal and parietal deep and subcortical white matter; and evolved previous infarct in the left frontal and parietal lobes. There is cortical swelling and hyperintensity in the left parietotemporal region (arrow).
3. Case 2 (Appendix, Fig. A)
A 39-year -old man spent 73 days in intensive care unit from complications arising from severe COVID-19 infection. He had severe respiratory failure requiring prolonged intubation with episodes of metabolic and respiratory acidosis, refractory hypotension and acute renal failure needing prolonged renal replacement therapy. He had 4 episodes of nosocomial infections, line-related and pneumonia, and multiple episodes of systemic inflammatory response syndrome. Serum D-Dimer (>4 ul/L) and fibrinogen (7.6 g/L) were markedly raised, pointing to an inflammatory and prothrombotic state. He had bilateral pneumothoraces, pancreatitis, presumed cytomegalovirus colitis and terminal ileitis. During the first 2 weeks of intensive care, he received 3 doses of interferon beta 1b and 10 days of lopinavir/ ritonavir. Respiratory SARS CoV-2 RT-PCR was negative on day 19 of illness. He was paralysed and sedated till day 63 of ICU. Following withdrawal of sedation and correction of metabolic derangements he was persistently encephalopathic for 3 weeks from day 65 of illness. He had no focal neurological signs. He was eventually discharged after 103 days to a rehabilitation hospital. The Montreal Cognitive Assessment score on day 112 was 17/30. Formal neuropsychological assessment showed mild impairment in working memory, processing speed, visuospatial-perceptual abilities and planning. This prompted a brain MRI on day 114 of illness. It showed bilateral symmetrical curvilinear non-enhancing lesions in the temporal and occipital deep-subcortical white matter that paralleled the ventricles, with intervening normal appearing periventricular white matter. They were hyperintense on T2W, suppressed on FLAIR and hypointense on T1WI with no restricted diffusion. Similar and smaller lesions were present in the inferior frontal white matter bilaterally (Fig. 2a–c). Multiple discrete round-ovoid T2W hyperintense lesions, some corresponding to hypointensity in T1WI, were also present in the deep and subcortical white matter bilaterally. There was also a background of subtle diffuse T2 prolongation in the centrum semiovale (Fig. 2d). A few scattered microhemorrhages were present in the deep and subcortical white matter. Overall, the lesions were chronic and deemed to be possible sequelae of hypoxia-ischaemia, or alternatively inflammatory demyelination; and could explain the patient's cognitive deficits.
4. Case 3 (Appendix, Fig. A)
Case 3, a 73-year-old man, was in prolonged critical care and suffered multiple complications, including ARDS, septic shock, and renal failure. He received interferon beta 1b and lopinavir/ ritonavir. He had persistent hyperactive delirium 2 months into illness, punctuated by two episodes of right and left sided weakness on days 23 and 31. Examination revealed spastic quadriparesis; no volitional eye and limb movements. He remained ventilated via tracheostomy. MRI brain at this point showed evolved infarcts with evidence of haemorrhage and cortical laminar necrosis. There was a large area of non-enhancing confluent T2W hyperintensity with restricted diffusion and a few microhaemorrhages in the right frontal and parietal deep and subcortical white matter, sparing the cortex (Fig. 3). Additionally, extensive cortical T2W/FLAIR hyperintensity and oedema were noted within the left temporal lobe, left middle, inferior frontal gyri and insula, with relative sparing of the adjacent white matter, no associated restricted diffusion, susceptibility or pathological enhancement. MRA showed chronic occlusion of distal left vertebral artery and attenuated flow in the left posterior cerebral artery. The spinal fluid was normal. D Dimer, fibrinogen, antiphopholipid and DsDNA antibodies were raised, while complement was low (Table 1). Although the strokes could be attributed to his co-morbidities (hypertension, atrial fibrillation) the rest of the radiological pathology was believed to be related to para-infectious autoimmune coagulopathy. He could not receive anticoagulation or immunotherapy and remained in poor neurological state. The agitation and restlessness required constant sedation and restraints. He had no volitional response to external stimulus. After 93 days he was given an empirical trial of high dose steroids, with the intention of improving his mental state and quality of life. His condition did not change. On day 104 all curative therapy was withdrawn, and he passed away.
5. Discussion
We describe the clinical, laboratory and radiological features of 3 critically ill patients with COVID-19 who developed severe encephalopathy. Case 1 satisfied criteria for ADEM while case 2 and 3 “possible encephalitis” [[11], [12], [13]]. Using case definition of Ellul et al. [14], we graded the association with COVID-19 as “probable” for all 3 cases. Although it was difficult to accurately date the onset of illness, the median interval from onset of respiratory symptoms to the point when encephalopathy was suspected was 23 days. All 3 patients were critically ill with multiple and severe organ dysfunction. They demonstrated to varying degrees, inflammatory and prothrombotic markers that COVID-19 has been associated with, the so-called “cytokine storm” [18]. We discuss the key clinical dilemmas we faced in the evaluation and management of these 3 patients.
Firstly, it was difficult to delineate the cause of the patients' altered mental state, considering the combined and interacting effects of their multiple co-morbidities. For instance in case 1, uremic encephalopathy and the prolonged sedative effects of midazolam and morphine, that are not efficiently removed via dialysis, were initially thought be the cause of his coma [[15], [16], [17]] These drugs had to be used because of the worldwide shortage of intensive care drugs. Further investigations were largely prompted by the patient's lack of recovery after adequate renal replacement and switching the sedatives to dexmedetomidine and remifentanil. Case 2 was initially not thought to have any central nervous system pathology. We found significant cognitive impairment when he was recuperating, which corresponded with the changes seen on MRI brain. However, it was difficult to decide if these lesions were due to a direct viral effect, possible hypoxia-ischaemia or inflammatory demyelination. This segues to our second dilemma.
We struggled to understand the underlying pathophysiology of our patients' encephalopathy. Were they suffering from direct viral infection of the central nervous system or from the spectrum of parainfectious and prothrombotic processes related to COVID-19? The experience with other infections such as tick-borne encephalitis suggests that there is no correlation between viral load, timing of viraemia and clinical severity. Not surprising diagnostic criteria for encephalitides do not mandate the demonstration of viral particles in spinal fluid or blood [11,12,19,20]. We have summarized the pointers that led to our clinical equipoise, with regards to direct viral effect versus parainfectious pathology for cases 1 and 3 in Table 2.
Table 2.
Pointers that supported direct viral encephalitis versus parainfectious process for the patients' encephalopathy.
| For direct viral invasion | For parainfectious process | |
|---|---|---|
| Case 1 | Patient developed illness close to the time of his documented viremia (Viraemia, at cycle threshold value of 32.22 was documented on day 11; patient noted to be drowsy day 15) | Patient did not improve in spite of clearing virus (ETT SARS-CoV-2 RT-PCR negative 32 and 33 of illness) |
| Most serological markers of autoimmunity were negative (anti ds DNA, anti-Ro, anti La, Anti-Sm, Anti TPO Ab, Anti thyroglobulin, anti-cardiolipin IgG and IgM) | Some serological markers of autoimmunity were positive (transient lupus anticoagulant positive mildly low C4) | |
| Equivocal olfactory nerve, bulb hyperintensity | MRI features suggestive of acute disseminated encephalomyelitis | |
| Spinal cord MRI changes only subtle | ||
| RT-PCR and COVID-19 IgG have not been validated in spinal fluid | Absence of SARS-CoV-RT- PCR and IgG in CSF. | |
| Spinal fluid: no oligoclonal bands | Spinal fluid: Only mild increase in cells, protein and borderline low glucose | |
| Case 3 | No neurological symptoms during viremia period | Persistent delirium 2 months into illness although SARS CoV-2 RT-PCR negative in ETT on day 12 and stool day 21 |
| Serological markers of autoimmunity, ANA, DsDNA, anticardiolipin IgG and Lupus anticoagulant were positive; C3/C4 low | ||
| MRI lesions suggestive of autoimmune coagulopathy | ||
| SARS-CoV RT-PCR and serology has not been validated in spinal fluid | Absence of SARS-CoV-RT- PCR in CSF | |
| Spinal fluid: Normal except for mild increase in protein |
To date there have only been a few reports of COVID-19 patients with positive SARS-CoV-2 RT-PCR in CSF [23,24] or post mortem evidence of viral antigens in CNS [25]. In the latter report, the patient's primary neurological manifestation was agitation and delirium [25]. Most reports did not support a direct neurotropism of the virus. In a series describing neurological complications associated with hospitalized COVID-19 patients, two were encephalopathic. Both had normal MRI. One case had normal spinal fluid including negative RT-PCR for SARS-CoV2; the other case did not have spinal fluid analysed [26]. In an autopsy series of 18 patients, all of whom were noted to be drowsy or confused antemortem, the main pathological findings were hypoxic changes, with no specific features of encephalitis. There were also no viral inclusions. SARS-CoV-2 RT-PCR were positive at low levels in only 5 patients [27]. Of the 578 spinal fluid samples from a cohort of 555 patients (neurological symptoms unclear) only 2 were positive for SARS-CoV-2 RT-PCR, at CT values of 32 and 35. Both were suspected to be contamination by blood as the brain biopsy samples were negative. This study suggested that although SARS-CoV-2 can replicate in neuronal cells in vitro, demonstration in CSF is uncommon [28]. On the other hand, most reports of COVID-19 related encephalopathy were a result of indirect CNS complications such as stroke [[29], [30], [31], [32], [33]] and para-infectious, inflammatory conditions such as acute hemorrhagic necrotizing encephalopathy [[34], [35], [36]] and ADEM. A 40-year-old lady with dysarthria, dysphagia, facial weakness and gaze presence had imaging consistent with ADEM. Her CSF was normal. She had some improvement with IVIg treatment [37]. Another patient with similar MRI findings but presenting with seizures and drowsiness responded well to high dose dexamethasone [38]. A 51-year-old with coma and ADEM-like MRI changes responded to high dose steroids and IVIg [39]. A 71-year-old man passed away from severe COVID-19 related respiratory failure and hyperinflammatory state. His post-mortem showed brain findings consistent with, ischaemia, acute hemorrhagic leukoencephalitis and ADEM. The patient's antemortem neurological manifestations were however unclear [40].
Finally, what was the best treatment for our patients, beyond what they received during the acute respiratory illness and the supportive intensive care? Remdesivir has shown some efficacy in COVID-19. In one case of Ebola virus related meningoencephalitis, intravenous remdesivir together with high dose dexamethasone was associated with reduction in viral load and the patient improved [21]. Did Remdesivir have a role in our patients, notwithstanding the renal failure, its poor spinal fluid penetration (brain:plasma ratio < 5%, [22]) and the relatively late presentation into illness when viral clearance was already demonstrated in a combination of sputum, serum, spinal fluid and stool? If the pathology was parainfectious and autoimmune, should they have received immunosuppressants? In our patients, the decision to start immunomodulators empirically was confounded by recurrent and severe nosocomial infections. For instance, case 1 had Pseudomonas aeruginosa and Enterococcus faecium when the ocular flutter was noted. Hence, the decision to try IVIg with appropriate antibiotic cover rather than corticosteroids. Without good evidence of its efficacy it was also difficult to justify the substantial undertaking of plasma exchange. Increasing reports of the efficacy of immune therapy [25,[37], [38], [39]] and the findings of the RECOVERY study [41], showing lower mortality among critically ill COVID-19 patient receiving dexamethasone, have raised the possibility that earlier administration of corticosteroids, or gentler immunotherapy such as IVIg, could have improved the neurological outcome for Cases 1 and 3.
6. Conclusion
The medical ethos of nonmaleficence, otherwise known as the “Do No Harm” principle; and the recent experience with hydroyxchloroquine in treatment of COVID-19 should guard us against our bias to “try something” in the absence of evidence [42]. On the other hand, “absence of evidence” does not equate to “no evidence”. A better understanding of the relationship between the effect of the SARS-CoV-2 and associated parainfectious autoimmunity is urgently needed [43,44]. We hope our cases generate discussions, and prompt other clinicians to report similar cases. We expect this will lead to derivation of best practices for the diagnosis and management of COVID-19 related encephalopathy. In this regard recent initiatives to set-up international databases of COVID-19 associated neurological complications is timely [45,46].
Addendum
Five months into the illness, Case 1 continues to make gradual recovery. He has regained useful function of his arms, but has residual spastic paraparesis. He is no longer dependent on nasogastric feeding and is able to take soft diet. He is able to speak, albeit incoherent in content. Repeat brain and spine imaging shows no radiological signs of active lesions; but spinal fluid protein is still raised (1.64g/L). In view of continued improvement we decided to give another dose of 1g/kg IVIG empirically, 7 weeks after the last dose. Our experience with cases 1 and 2 suggests that rehabilitation efforts, and possibly immunotherapy, should be sustained as recovery may be delayed.
Disclosures
1) Ethics approval: CIRB 2020/2014; A Survey of the Neurological Manifestations of COVID-19.
2) The 3 patients described in this paper have been included in the comprehensive survey of COVID-19 neurological manifestations in Singapore: Neurology of COVID-19 in Singapore, https://doi.org/10.1016/j.jns.2020.117118
Author contributions
-
•
Conceptualization; Writing and Reviewing; Data curation: TU, YWY
-
•
Radiology images: YWY, WMJQ
-
•
Writing; Formatting of manuscript and figures; Data curation: WMJQ, JMY; HSWK
-
•
Data curation: YYM, CYJC, LML
Acknowledgements
James J. Sejvar, MD National Center for Emerging and Zoonotic Diseases, U.S. Centers for Disease Control and Prevention for critical comments of manuscript.
Dr. Mathew Tay Rong Jie and Dr. Andrea Sng Jiaying for help with Fig. A.
Appendix
Fig. A.
Timelines for cases 1, 2 and 3.
References
- 1.Ann Yeh E., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113 doi: 10.1542/peds.113.1.e73. e73 LP-e76. [DOI] [PubMed] [Google Scholar]
- 2.Morfopoulou S., Brown J.R., Davies E.G., Anderson G., Virasami A., Qasim W., Chong W.K., Hubank M., Plagnol V., Desforges M., Jacques T.S., Talbot P.J., Breuer J. Human coronavirus OC43 associated with fatal encephalitis. N. Engl. J. Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 3.Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung E.C.W., Chim S.S.C., Chan P.K.S., Tong Y.K., Ng E.K.O., Chiu R.W.K., Leung C.-B., Sung J.J.Y., Tam J.S., Lo Y.M.D. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau K., Yu W., Chu C., Lau S., Sheng B. Infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10:2–4. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., Ding Y., Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umapathi T., Kor A.C., Venketasubramanian N., Lim C.C.T., Pang B.C., Yeo T.T., Lee C.C., Lim P.L., Ponnudurai K., Chuah K.L., Tan P.H., Tai D.Y.H., Ang S.P.B. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J. Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., Saeed B.T., Wahbi A., Saedy A., AlDabbagh T., Okaili R., Sadat M., Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., Stahl J.P., Mailles A., Drebot M., Rupprecht C.E., Yoder J., Cope J.R., Wilson M.R., Whitley R.J., Sullivan J., Granerod J., Jones C., Eastwood K., Ward K.N., Durrheim D.N., Solbrig M.V., Guo-Dong L., Glaser C.A., Sheriff H., Brown D., Farnon E., Messenger S., Paterson B., Soldatos A., Roy S., Visvesvara G., Beach M., Nasci R., Pertowski C., Schmid S., Rascoe L., Montgomery J., Tong S., Breiman R., Franka R., Keuhnert M., Angulo F., Cherry J. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the international encephalitis consortium. Clin. Infect. Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granerod J., Ambrose H.E., Davies N.W.S., Clewley J.P., Walsh A.L., Morgan D., Cunningham R., Zuckerman M., Mutton K.J., Solomon T., Ward K.N., Lunn M.P.T., Irani S.R., Vincent A., Brown D.W.G., Crowcroft N.S. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect. Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 13.Sejvar J.J., Kohl K.S., Bilynsky R., Blumberg D., Cvetkovich T., Galama J., Gidudu J., Katikaneni L., Khuri-Bulos N., Oleske J., Tapiainen T., Wiznitzer M. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 14.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;4422:2–3. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reves J.G., Fragen R.J., Vinik H.R., Greenblatt D.J. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–324. [PubMed] [Google Scholar]
- 16.Bolon M., Bastien O., Flamens C., Paulus S., Boulieu R. Midazolam disposition in patients undergoing continuous venovenous hemodialysis. J. Clin. Pharmacol. 2001;41:959–962. doi: 10.1177/00912700122010933. [DOI] [PubMed] [Google Scholar]
- 17.Jodoin K. 4th edition. 2016. The Renal Drug Handbook: The Ultimate Prescribing Guide for Renal Practitioners. [DOI] [Google Scholar]
- 18.Sinha P., Matthay M., Calfee C. Is a “cytokine storm” relevant to COVID-19? JAMA Intern. Med. 2020:9–11. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 19.Saksida A., Jakopin N., Jelovšek M., Knap N., Fajs L., Lusa L., Lotrič-Furlan S., Bogovič P., Arnež M., Strle F., Avšič-Županc T. Virus RNA load in patients with tick-borne encephalitis, Slovenia. Emerg. Infect. Dis. J. 2018;24:1315. doi: 10.3201/eid2407.180059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis. 2011;2:2–15. doi: 10.1016/j.ttbdis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs M., Rodger A., Bell D.J., Bhagani S., Cropley I., Filipe A., Gifford R.J., Hopkins S., Hughes J., Jabeen F., Johannessen I., Karageorgopoulos D., Lackenby A., Lester R., Liu R.S.N., MacConnachie A., Mahungu T., Martin D., Marshall N., Mepham S., Orton R., Palmarini M., Patel M., Perry C., Peters S.E., Porter D., Ritchie D., Ritchie N.D., Seaton R.A., Sreenu V.B., Templeton K., Warren S., Wilkie G.S., Zambon M., Gopal R., Thomson E.C. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388:498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang P., Xu X.M., Gao L.L., Wang H.Z., Xiong H.F., Li R.H. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv. 2020;202003:15. [Google Scholar]
- 25.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus −2 (SARS-CoV-2) J. Med. Virol. 2020;2:0–3. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Destras G., Bal A., Escuret V., Morfin F., Lina B., Josset L. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;0:8–11. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Zhang M.D., Meng Xiao M.D., Sc M., Zhang Shulan, Xia M.D. Peng, Cao M.D. Wei, Jiang M.D. Wei, Chen M.D. Huan, Ding M.D. Xin, Zhao M.D. Hua, Zhang M.D. Hongmin. Correspondence coagulopathy and antiphospholipid antibodies in patients with Covid-19. Nejm. 2020;38:1–3. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D., Luqmani A., Jenkins I.H., Nicholas R., Jones B., Everitt A. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:1–8. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M.K., Kadir M., Frick J., Lindeberg J., Olivero-Reinius H., Ryttlefors M., Cunningham J.L., Wikström J., Grabowska A., Bondeson K., Bergquist J., Zetterberg H., Rostami E. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020 doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T., Rodricks M.B., Hirsh E. COVID-19-associated acute disseminated encephalomyelitis: a case report. MedRxiv. 2020 [Google Scholar]
- 38.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., Fontanella M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020:1–4. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020:3–6. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020:1–11. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 42.No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19 — RECOVERY Trial, (n.d.). https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19 (accessed June 12, 2020).
- 43.Costello F., Dalakas M.C. Cranial neuropathies and COVID-19: neurotropism and autoimmunity. Neurology. 2020:4–11. doi: 10.1212/WNL.0000000000009921. [DOI] [PubMed] [Google Scholar]
- 44.Koralnik I.J., Tyler K.L. COVID-19: a global threat to the nervous system. Ann. Neurol. 2020 doi: 10.1002/ana.25807. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkler A.S., Knauss S., Schmutzhard E., Leonardi M., Padovani A., Abd-Allah F., Charway-Felli A., Emmerich J.V., Umapathi T., Satishchandra P., Hoo F.K., Dalmau J., Oreja-Guevara C., Ferreira L.B., Pfausler B., Michael B., Tagliavini F., Höglinger G., Endres M., Klein C., Hemmer B., Correll W., Sejvar J., Solomon T. A call for a global COVID-19 neuro research coalition. Lancet Neurol. 2020;19:482–484. doi: 10.1016/S1474-4422(20)30150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Román G.C., Reis J., Spencer P.S., Buguet A., Öztürk S., Wasay M., El Alaoui Faris M., Katrak S.M., Láinez M., Medina M.T., Meshram C., Mizusawa H. COVID-19 international neurological registries. Lancet Neurol. 2020;19:484–485. doi: 10.1016/S1474-4422(20)30148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]