Abstract
Background:
Manganese (Mn) in drinking water may increase the risk of several neurodevelopmental outcomes, including attention-deficit hyperactivity disorder (ADHD). Earlier epidemiological studies on associations between Mn exposure and ADHD-related outcomes had small sample sizes, lacked spatiotemporal exposure assessment, and relied on questionnaire data (not diagnoses)—shortcomings that we address here.
Objective:
Our objective was to assess the association between exposure to Mn in drinking water during childhood and later development of ADHD.
Methods:
In a nationwide population-based registry study in Denmark, we followed a cohort of 643,401 children born 1992–2007 for clinical diagnoses of ADHD. In subanalyses, we classified cases into ADHD-Inattentive and ADHD-Combined subtypes based on hierarchical categorization of International Classification of Diseases (ICD)-10 codes. We obtained Mn measurements from 82,574 drinking water samples to estimate longitudinal exposure during the first 5 y of life with high spatiotemporal resolution. We modeled exposure as both peak concentration and time-weighted average. We estimated sex-specific hazard ratios (HRs) in Cox proportional hazards models adjusted for age, birth year, socioeconomic status (SES), and urbanicity.
Results:
We found that exposure to increasing levels of Mn in drinking water was associated with an increased risk of ADHD-Inattentive subtype, but not ADHD-Combined subtype. After adjusting for age, birth year, and SES, females exposed to high levels of Mn (i.e., ) at least once during their first 5 y of life had an HR for ADHD-Inattentive subtype of 1.51 [95% confidence interval (CI): 1.18, 1.93] and males of 1.20 (95% CI: 1.01, 1.42) when compared with same-sex individuals exposed to . When modeling exposure as a time-weighted average, sex differences were no longer present.
Discussion:
Mn in drinking water was associated with ADHD, specifically the ADHD-Inattentive subtype. Our results support earlier studies suggesting a need for a formal health-based drinking water guideline value for Mn. Future Mn-studies should examine ADHD subtype-specific associations and utilize direct subtype measurements rather than relying on ICD-10 codes alone. https://doi.org/10.1289/EHP6391
Introduction
Attention-deficit hyperactivity disorder (ADHD) is one of the most common mental disorders in children and, although it is highly heritable, environmental risk factors of ADHD may be important (Banerjee et al. 2007; Faraone and Larsson 2019). There is increasing evidence of neurotoxic effects of oral manganese (Mn) intake, especially in children (Bjørklund et al. 2017; Ljung and Vahter 2007). Despite Mn being an essential element, exposure to high concentrations has long been known to cause manganism, a disorder with parkinsonian symptoms (Couper 1837; Racette 2014).
Mn occurs naturally in groundwater, with concentration levels varying with geographical location and geochemical conditions in the aquifers (WHO 2011). The scientific validity of the World Health Organization’s (WHO) health-based drinking water guideline value of has been questioned, because it is “based partly on debatable assumptions, where information from previous reports has been used without revisiting original scientific articles” (Ljung and Vahter 2007). The WHO has not established a formal drinking water guideline value but, rather, only states that Mn is “not of health concern at levels normally causing acceptability problems in drinking water” (WHO 2017). Addressing these consumer acceptability problems, many countries have set a lower, cosmetic-based drinking water standard in order to achieve an acceptable taste and to avoid water discoloring and staining of laundry—for example, in Denmark and the United States (Ministry of Environment and Food of Denmark 2018; U.S. EPA 2018).
The Danish drinking water supply is highly decentralized, with approximately 2,600 waterworks supplying approximately 5.7 million users (Schullehner and Hansen 2014). Approximately 3% of Danish households obtain their drinking water from a private water well, and the remaining 97% from public waterworks (Schullehner et al. 2017). Groundwater is the sole source of all Danish drinking water, typically undergoing only simple treatment. Under aeration at the waterworks, Mn is oxidized, and subsequently precipitates in sand filters. At a well-functioning waterworks, the drinking water Mn concentrations at the consumers’ taps are typically below the analytical detection limit of . However, the efficiency of Mn removal at the waterworks depends on the biogeochemistry of the groundwater and, hence, varies with geographical location. More important, functioning of a filter may suddenly be disrupted due to a number of reasons, including mechanical filter breaches, filter media replacements, and start-up of new filters. During such episodes of disrupted filtering, Mn concentrations in drinking water sharply increase, typically between 10- and 100-fold, and stay elevated for long periods, from several months to more than a year, before the removal of Mn is reestablished (Breda et al. 2017; Bruins et al. 2014; Gouzinis et al. 1998; Ramsay et al. 2018). Exposure to high levels of Mn through drinking water supplies based on simple-treated groundwater is therefore mainly governed by episodic water treatment failure.
Children appear to be especially vulnerable to environmental Mn exposure because, compared with adults, their Mn homeostasis is not fully developed and their central nervous system is not yet matured and hence is more vulnerable to neurotoxins (Ljung and Vahter 2007). Mn accumulates in the brain regions with dopaminergic pathways, potentially leading to dopaminergic dysfunction (Chen et al. 2014; Nitin and Bowman 2018; Tuschl et al. 2016). In children, exposure to Mn in drinking water has been shown to correlate with higher levels of Mn measured in biomarkers such as hair and toenails (Bouchard et al. 2007; Ntihabose et al. 2018). Both exposure to Mn in drinking water and high levels of Mn in biomarkers have been associated with a higher risk of adverse neurodevelopmental outcomes (Lucchini et al. 2018). Evidence of sex differences in neurotoxic susceptibility to environmental Mn exposure has been reported in several studies (Broberg et al. 2019; Kullar et al. 2019; Lucchini et al. 2018) and age-specific incidence rate patterns for ADHD differed between boys and girls in a comprehensive Danish study (Dalsgaard et al. 2019).
Observational studies in humans have found associations between Mn in drinking water and different neurodevelopmental end points, including lower intelligence quotient (IQ), cognitive difficulties, inattention, hyperactivity, and impulsivity (Bouchard et al. 2007, 2011; He et al. 1994; Khan et al. 2011; Kullar et al. 2019; Lucchini et al. 2018; Rodríguez-Barranco et al. 2013; Wasserman et al. 2006; Woolf et al. 2002) and, in rare cases, also more serious detrimental end points, such as infant death (Hafeman et al. 2007). However, results were not always unequivocal; a study from Bangladesh found, for example, early life exposure to Mn in drinking water to adversely affect children’s behavior but to be positively associated with cognitive function in girls (Rahman et al. 2017). Drinking water Mn levels in these studies varied from below the regulatory limit () up to several milligrams per liter.
Previous epidemiological studies on the association between Mn in drinking water and ADHD or ADHD-related symptoms are few, often of cross-sectional design, comprising small population sizes and relying on questionnaire data (Bouchard et al. 2007; Khan et al. 2011; Oulhote et al. 2014a). Furthermore, these studies rely on unvalidated exposure data, often from drinking water samples collected only once, at the end of study. Prior studies did not have the required time series of exposure data to assess whether there is a difference in associated ADHD risk between duration of exposure to moderately increased levels of Mn and brief periods with high Mn exposure. Given the specific circumstances of exposure fluctuations due to treatment failure at the waterworks, modeling Mn exposure with a high spatial and temporal resolution is likely crucial—an issue neglected in earlier studies. In this study, we address this by taking advantage of a comprehensive drinking water quality database to model spatiotemporal exposure to Mn in drinking water in a large, nationwide population-based cohort study of children followed for ADHD.
Methods
Study Population
The study population consisted of all singletons born in Denmark between 1 January 1992 and 31 December 2007 whose parents were born in Denmark. Cohort members had to be alive and residents of Denmark on their fifth birthday, and their mothers had to be residents of Denmark 9 months before giving birth (). The study population was identified from the Civil Registration System (CRS), which includes all residents of Denmark since 1968 and their assigned unique personal identification numbers (Pedersen et al. 2006). Complete residential history of the entire study population was obtained from the CRS along with information on sex, date and place of birth, vital status, date of death (if relevant), and personal identification numbers of the parents.
Exposure Assessment
During the study period, Mn was measured in 82,574 drinking water samples from 3,509 active public waterworks and registered in the national geodatabase Jupiter (Hansen and Pjetursson 2011). Mn concentrations in drinking water and sampling dates at the waterworks level were linked to water supply areas (Schullehner and Hansen 2014) and updated in each water supply area every time a new Mn measurement was obtained. Concentrations of Mn were assigned to all cohort members based on longitudinal spatial linkage of their geocoded residential history and water supply areas (Pedersen 2011; Schullehner et al. 2017). The analytical detection limit for Mn in water samples was .
Private wells that provide drinking water to fewer than 10 households have very limited Mn monitoring and, in general, poorer water quality for a range of parameters (Schullehner and Hansen 2014). Hence, private well users were excluded from the analysis. In addition, we excluded children with of Mn exposure information available before 5 years of age (i.e., those living outside a public water supply area with Mn monitoring) and children for whom we had of information on maternal Mn exposure during pregnancy (excluded in total ).
Prior studies did not have data on time series of Mn exposure, which is required to investigate whether longer exposure to moderately increased levels of Mn is comparable to brief exposure to highly increased Mn levels in terms of risk of developing ADHD. To capitalize on the level of detail in temporal changes in our exposure data, we modeled Mn exposure using two different definitions.
First, we modeled exposure as the highest level of Mn in drinking water each individual was exposed to during the first 5 y of life. We modeled this both as a categorical and a continuous variable on the log-scale using natural cubic splines with knots set at the breaks of the exposure categories. Approximately 20% of the study population was never exposed to Mn levels above the detection limit, which was defined as the reference category. The remaining categories’ cut points were calculated as quintiles in the study population, rounded to the nearest microgram per liter, which resulted in five exposure categories of similar size (, 5–19, , , ). In the continuous exposure variable model, we imputed Mn concentrations using robust regression on order statistics for the approximately 20% of the study population with a highest level of Mn below the analytical detection limit.
Second, to estimate the effects of long-term exposure, we computed a time-weighted average Mn concentration, where we modeled the exposure weighted by the number of days exposed to different levels of Mn during the first 5 y of life. Here, we categorized exposure into four groups, using individuals exposed to Mn as the reference group (constituting 70% of all cohort members’ exposure duration) and divided the rest into three exposure categories (5–10, , ), each with approximately 10% of the study population.
Outcome Assessment
Clinical diagnoses of ADHD were obtained from the Danish Psychiatric Central Research Register (DPCRR) and the Danish National Patient Register (DNPR). The DPCRR includes all contacts to hospital-based mental health services in Denmark, with data available for all in- and out-patient contacts during the follow-up period (Mors et al. 2011). Similarly, during the follow-up period, the DNPR holds data on contacts to nonpsychiatric hospital-based health services, with information on in-patient admissions, out-patient contacts, and emergency department visits. Both DPCRR and DNPR hold diagnostic information according to the ICD Classification of Mental and Behavioural Disorders, Diagnostic Criteria for Research, 10th edition (ICD-10-DCR; WHO 1993).
A diagnosis of ADHD-Overall was based on the ICD-10-DCR codes F90.x (hyperkinetic disorders) or F98.8 (attention deficit disorder without hyperactivity). ICD-10-DCR code F90.x covered codes F90.0 (disturbance of activity and attention), F90.1 (hyperkinetic conduct disorder), F90.8 (other hyperkinetic disorders) and F90.9 (hyperkinetic disorder, unspecified).
For analyses of different subtypes of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision), 4th edition (DSM-IV-TR; APA 2000), we created hierarchical categories of ADHD. Individuals with a contact with an ICD-10-DCR code F98.8 (inattention without hyperactivity) and no contact with ICD-10-DCR code F90.x (hyperkinetic disorders) previously, or within 2 y after the contact with ICD-10-DCR code F98.8, were defined as ADHD-Inattentive subtype. Individuals having a contact with ICD-10-DCR code F90.x were defined as ADHD-Combined subtype, regardless of any contact with ICD-10-DCR code F98.8. The ICD-10 holds no specific diagnosis for the ADHD-Hyperactive/impulsive subtype. In the analyses of ADHD subtypes, follow-up ended 2 y before the end of the study (see details in the section “Statistical Analyses”), that is, 31 December 2014, to allow sufficient time to distinguish cases with the ADHD-Inattentive subtype from cases that might be reclassified as ADHD-Combined based on a subsequent indication of a hyperkinetic disorder.
The date of first diagnosis of mental disorder was defined as the date of first contact with the diagnosis. Individuals with a first diagnosis of ADHD before their fifth birthday were excluded (), given that the validity of ADHD diagnoses before 5 years of age is lower than in school-age children (Overgaard et al. 2019) and only very few preschool-age children are diagnosed with ADHD in Denmark (Dalsgaard et al. 2019). This resulted in a final study population of 643,401 persons.
Covariates
All covariates were accessed for the year the cohort member was born. Low parental level of education (Dalsgaard et al. 2015) and income (Larsson et al. 2014) are known to be associated with ADHD in offspring. We obtained information on these parental socioeconomic status (SES) variables from Statistics Denmark’s Education Register and Income Register. We included data on maternal educational level and paternal income. We did not use maternal income because its estimate the year the child is born is more biased than paternal income, due to maternity leave. The mother’s highest attained education was included in the following categories: a) primary school only; b) shorter education (high school and short vocational training); c) medium-long education (vocational training and bachelor degrees); and d) long education (university graduates). The father’s income was included as annual quintiles.
Geographical differences in the incidence of ADHD diagnoses have been observed in Denmark (Madsen et al. 2015). We therefore included the degree of urbanicity, defined as residing in a) the capital; b) suburbs of the capital; c) municipalities where the largest city has more than 100,000 inhabitants; d) municipalities where the largest city has between 10,000 and 100,000 inhabitants; and e) municipalities with largest towns having fewer than 10,000 inhabitants.
Statistical Analyses
A total of 643,401 individuals were followed for outcomes from their fifth birthday until date of diagnosis of ADHD, death, emigration, or the end of the study (31 December 2016), whichever came first. In the subtype analyses, follow-up ended 2 y earlier and individuals were censored, where required, 2 y before a ICD-10-DCR code F90.x diagnosis in the analyses for the ADHD-Inattentive subtype.
Cox proportional hazards models were used to estimate hazard ratios (HRs). Age was used as the underlying time scale for all Cox regression models. Because age-specific incidence rates for ADHD have two distinct patterns for males and females (Dalsgaard et al. 2019) and because evidence of sex differences in neurotoxic susceptibility to Mn exposure has been reported in several studies (Broberg et al. 2019; Kullar et al. 2019; Lucchini et al. 2018), all analyses were stratified by sex. The base model was adjusted for birth year in four 4-y bands. In a second model, we adjusted additionally for parental SES. As a sensitivity analyses, we adjusted additionally for degree of urbanicity. We calculated for trend () by assigning each exposure category an equally spaced integer value from 0, modeled as a continuous variable.
When covariate data was not available, a separate missing category was included. The proportional hazards assumption was checked for each of the models by assessing the null hypothesis of a zero slope of the Schoenfeld residuals on time and was not violated in any of the presented models. Statistical analyses were done in R (version 3.4; R Developmental Core Team).
Ethics
The Danish Data Protection Agency and the Danish Health Data Authority approved this study. Informed consent is not required for large-scale, registry-based studies in Denmark (Ludvigsson et al. 2015).
Results
Table 1 shows the characteristics of the study population. Of the 643,401 individuals followed for ADHD-Overall between 1997 and 2016 during 7,367,911 person-years at risk, a total of 22,730 children were diagnosed with ADHD, of which 30.6% were females and 69.4% were males. A total of 1,928 females and 2,863 males had their first ADHD diagnosis after 31 December 2014, corresponding respectively to 27.7% and 18.2% of cases in the study population and were consequently not subtyped. Fifty-four females and 109 males were assigned both subtypes, resulting in 2,672 ADHD-Inattentive subtype cases and 15,430 ADHD-Combined subtype cases. A total of 477 females and 1,954 males received a ICD-10-DCR code F90.x diagnosis during the first 2 y of follow-up, that is, before their seventh birthday, and were consequently excluded from the ADHD-Inattentive subtype analyses.
Table 1.
Characteristics of the study population for ADHD-Overall and ADHD subtypes, stratified by sex. Data were complete for all variables unless otherwise stated.
| Characteristics | ADHD-Overall | ADHD-Inattentive subtype | ADHD-Combined subtype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | |||||||||||||
| Cases | Person-years at risk | Cases | Person-years at risk | Cases | Person-years at risk | Cases | Person-years at risk | Cases | Person-years at risk | Cases | Person-years at risk | |||||||
| Total | 313,413 | 6,964 | 3,615,736 | 329,988 | 15,766 | 3,752,175 | 312,936 | 973 | 2,995,010 | 328,034 | 1,699 | 3,101,128 | 313,413 | 4,117 | 3,007,820 | 329,988 | 11,313 | 3,132,771 |
| Mn in drinking water (highest exposure) () | ||||||||||||||||||
| 60,501 | 1,082 | 535,831 | 63,144 | 2,856 | 548,913 | 60,376 | 94 | 415,652 | 62,629 | 228 | 424,048 | 60,501 | 588 | 417,229 | 63,144 | 1,936 | 428,884 | |
| 69,352 | 1,404 | 735,445 | 73,341 | 3,301 | 765,728 | 69,227 | 179 | 598,006 | 72,902 | 341 | 620,923 | 69,352 | 842 | 600,407 | 73,341 | 2,320 | 627,191 | |
| 58,403 | 1,355 | 695,611 | 61,454 | 2,977 | 723,183 | 58,320 | 196 | 579,962 | 61,100 | 290 | 601,931 | 58,403 | 821 | 582,529 | 61,454 | 2,197 | 607,904 | |
| 63,505 | 1,581 | 838,680 | 66,900 | 3,464 | 867,976 | 63,444 | 256 | 713,082 | 66,571 | 424 | 736,243 | 63,505 | 941 | 716,261 | 66,900 | 2,563 | 743,861 | |
| 61,652 | 1,542 | 810,171 | 65,149 | 3,168 | 846,375 | 61,569 | 248 | 688,308 | 64,832 | 416 | 717,984 | 61,652 | 925 | 691,393 | 65,149 | 2,297 | 724,931 | |
| Mn in drinking water (time-weighted average exposure) () | ||||||||||||||||||
| 220,043 | 4,648 | 2,343,484 | 231,312 | 10,878 | 2,427,506 | 219,668 | 587 | 1,907,404 | 229,820 | 1,066 | 1,970,827 | 220,043 | 2,683 | 1,915,402 | 231,312 | 7,703 | 1,991,475 | |
| 31,832 | 754 | 417,242 | 33,631 | 1,635 | 434,162 | 31,795 | 120 | 354,254 | 33,473 | 197 | 367,850 | 31,832 | 470 | 355,770 | 33,631 | 1,196 | 371,450 | |
| 31,211 | 775 | 420,688 | 33,112 | 1,654 | 439,435 | 31,181 | 130 | 358,957 | 32,953 | 213 | 374,214 | 31,211 | 472 | 360,573 | 33,112 | 1,234 | 377,905 | |
| 30,327 | 787 | 434,323 | 31,933 | 1,599 | 451,072 | 30,292 | 136 | 374,395 | 31,788 | 223 | 388,238 | 30,327 | 492 | 376,074 | 31,933 | 1,180 | 391,940 | |
| Year of birth | ||||||||||||||||||
| 1992–1995 | 71,628 | 1,835 | 1,271,927 | 75,840 | 3,503 | 1,327,303 | 71,595 | 349 | 1,130,800 | 75,665 | 553 | 1,178,367 | 71,628 | 1,284 | 1,135,472 | 75,840 | 2,739 | 1,188,082 |
| 1996–1999 | 75,198 | 2,131 | 1,039,476 | 79,478 | 4,095 | 1,080,748 | 75,133 | 363 | 890,795 | 79,164 | 523 | 923,955 | 75,198 | 1,229 | 894,681 | 79,478 | 3,183 | 932,905 |
| 2000–2003 | 79,784 | 1,812 | 787,684 | 83,547 | 4,472 | 808,679 | 79,627 | 173 | 629,398 | 82,862 | 420 | 643,818 | 79,784 | 1,023 | 632,125 | 83,547 | 3,384 | 651,762 |
| 2004–2007 | 86,803 | 1,186 | 516,649 | 91,123 | 3,696 | 535,445 | 86,581 | 88 | 344,018 | 90,343 | 203 | 354,988 | 86,803 | 581 | 345,542 | 91,123 | 2,007 | 360,022 |
| Maternal highest education | ||||||||||||||||||
| Primary school | 59,729 | 2,662 | 747,666 | 63,321 | 5,536 | 771,757 | 59,554 | 336 | 629,844 | 62,663 | 511 | 647,505 | 59,729 | 1,634 | 634,773 | 63,321 | 4,123 | 658,516 |
| Shorter education | 156,546 | 3,189 | 1,832,406 | 163,693 | 7,433 | 1,891,610 | 156,335 | 458 | 1,522,170 | 162,768 | 843 | 1,568,520 | 156,546 | 1,879 | 1,528,039 | 163,693 | 5,334 | 1,583,751 |
| Medium-long education | 71,855 | 886 | 778,483 | 76,547 | 2,265 | 820,542 | 71,783 | 145 | 635,878 | 76,251 | 281 | 669,027 | 71,855 | 481 | 637,455 | 76,547 | 1,492 | 673,426 |
| Long education | 24,436 | 197 | 246,565 | 25,552 | 441 | 257,579 | 24,420 | 34 | 198,165 | 25,483 | 64 | 207,076 | 24,436 | 104 | 198,541 | 25,552 | 292 | 207,898 |
| Missing | 847 | 30 | 10,617 | 875 | 91 | 10,687 | 844 | a | 8,954 | 869 | a | 9,000 | 847 | 19 | 9,012 | 875 | 72 | 9,180 |
| Paternal income | ||||||||||||||||||
| Q1 | 4,920 | 154 | 44,639 | 5,076 | 307 | 44,720 | 4,895 | 13 | 34,953 | 5,023 | 19 | 34,780 | 4,920 | 99 | 35,230 | 5,076 | 217 | 35,299 |
| Q2 | 13,488 | 533 | 147,487 | 14,393 | 1,133 | 153,252 | 13,446 | 78 | 120,918 | 14,216 | 97 | 125,062 | 13,488 | 327 | 121,903 | 14,393 | 837 | 127,183 |
| Q3 | 32,826 | 1,203 | 361,814 | 34,331 | 2,465 | 372,926 | 32,739 | 142 | 296,928 | 33,992 | 239 | 305,493 | 32,826 | 719 | 299,060 | 34,331 | 1,762 | 310,242 |
| Q4 | 99,578 | 2,513 | 1,141,639 | 104,742 | 5,761 | 1,177,012 | 99,397 | 322 | 944,185 | 104,058 | 622 | 970,235 | 99,578 | 1,497 | 948,699 | 104,742 | 4,141 | 981,863 |
| Q5 | 162,498 | 2,561 | 1,919,065 | 171,313 | 6,090 | 2,002,978 | 162,356 | 418 | 1,597,126 | 170,612 | 722 | 1,664,526 | 162,498 | 1,475 | 1,602,015 | 171,313 | 4,356 | 1,677,133 |
| Missing | 103 | a | 1,093 | 133 | 10 | 1,287 | 103 | a | 900 | 133 | a | 1,033 | 103 | a | 914 | 133 | a | 1,052 |
| Degree of urbanicity | ||||||||||||||||||
| Capital | 46,175 | 934 | 533,383 | 48,818 | 2,085 | 555,201 | 46,097 | 96 | 442,109 | 48,545 | 208 | 459,066 | 46,175 | 654 | 443,944 | 48,818 | 1,596 | 463,363 |
| Capital suburb | 45,235 | 1,114 | 548,569 | 47,697 | 2,602 | 566,317 | 45,166 | 123 | 459,061 | 47,358 | 262 | 472,415 | 45,235 | 702 | 461,099 | 47,697 | 1,975 | 477,801 |
| Municipalities with largest town inhabitants | 39,811 | 815 | 453,935 | 41,931 | 1,586 | 474,005 | 39,764 | 169 | 375,157 | 41,756 | 227 | 391,182 | 39,811 | 438 | 376,748 | 41,931 | 1,089 | 394,578 |
| Municipalities with largest town 10,000 –100,000 inhabitants | 86,858 | 2,083 | 1,000,660 | 91,477 | 4,596 | 1,039,119 | 86,717 | 295 | 828,583 | 90,892 | 491 | 858,608 | 86,858 | 1,224 | 832,320 | 91,477 | 3,241 | 867,667 |
| Other municipalities (largest town inhabitants) | 95,254 | 2,018 | 1,078,346 | 99,980 | 4,897 | 1,116,689 | 95,112 | 290 | 889,395 | 99,398 | 511 | 919,169 | 95,254 | 1,099 | 893,003 | 99,980 | 3,412 | 928,652 |
| Missing | 80 | a | 845 | 85 | a | 844 | 80 | a | 704 | 85 | a | 690 | 80 | a | 706 | 85 | a | 710 |
Note: Subtypes were classified using hierarchical categories of ICD-10-DCR diagnosis codes (see the “Methods” section). Due to the reclassification period for subtype analyses, and person-years at risk vary for the different analyses and cases of subtypes do not add up to total cases of ADHD-Overall (see the “Results” section). ADHD, attention-deficit hyperactivity disorder; ICD-10-DCR, ICD Classification of Mental and Behavioural Disorders, Diagnostic Criteria for Research, 10th edition; Mn, manganese; Q, quintile.
To protect confidentiality, for presentation in this table alone, the absolute number of cases in this cell was added to the cell above.
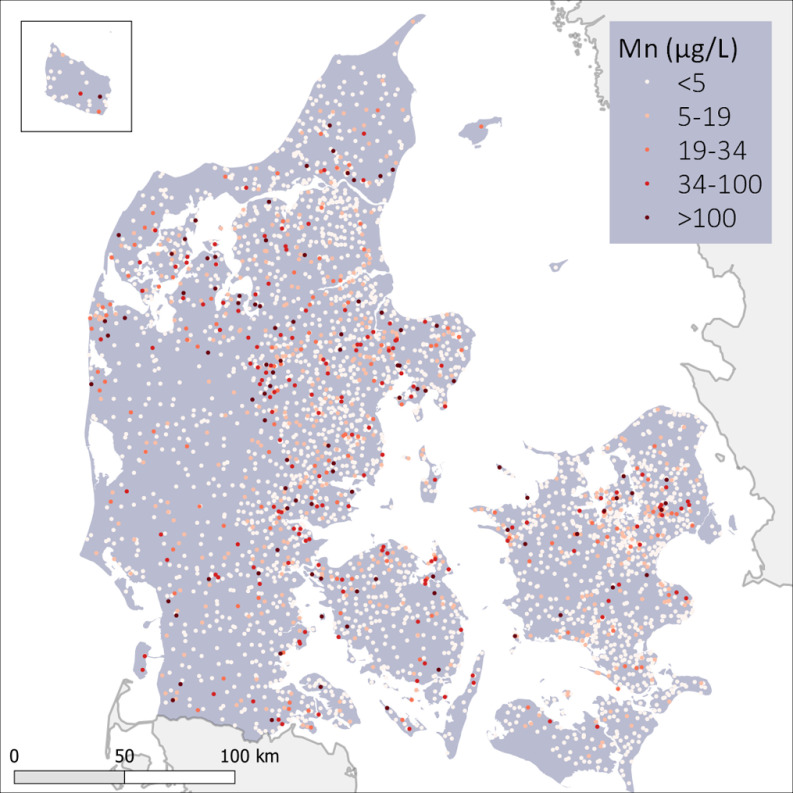
The geographical distribution of Mn concentrations across Denmark in 2012 is shown in Figure 1. More important than this snapshot are the spatiotemporal variations in Mn concentrations during the entire exposure period from 1992 to 2012, which are shown in Video S1. Of all 82,574 water samples, 67% were below the analytical detection limit. However, the vast majority (90%) of waterworks providing data for this study had at least one episode with Mn concentrations above the detection limit () during the exposure period. In the total study population, 20% of the children were exposed to more than Mn at some point during their first 5 y of life. The distribution of number of individuals and cases in each of the exposure categories and covariates is shown in Table 1.
Figure 1.
Geographical distribution of manganese concentrations in public drinking water across Denmark. Each dot represents one of the 3,509 waterworks. Most recent sample at each waterworks by end of exposure period (December 2012). The island of Bornholm is shown in the top left inset. For temporal changes refer to Video S1.
Table 2 shows the results for associations between exposure modeled as highest level of Mn in drinking water during the first 5 y of life and the ADHD-Overall and ADHD subtypes. Exposure to the highest category () was associated with a significantly higher risk of ADHD-Overall in females [ 1.09 [95% confidence interval (CI): 1.00, 1.18], ], but not males [ 0.98 (95% CI: 0.93, 1.03), ] when compared with those exposed to the lowest category (). In both females and males, HRs for the ADHD-Combined subtype, which accounted for 81% and 87% of the subtyped cases in girls and boys, respectively, were similar to those for ADHD-Overall but less precise and not significant for any exposure category after adjusting for SES. In contrast, associations with the ADHD-Inattentive subtype were stronger and statistically significant in both sexes before and after adjusting for parental SES, with larger HRs for the highest vs. lowest exposure category in females [ of 1.51 (95% CI: 1.18, 1.93) than males ( 1.20 (95% CI: 1.01, 1.42)]. Although trend tests were significant for both groups, HRs increased monotonically with exposure in females, but not in males.
Table 2.
Hazard ratios (95% confidence intervals) for the association between highest drinking water Mn exposure during the first 5 y of life (as exposure categories, with of the study population in each category) and ADHD-Overall and ADHD subtypes, stratified by sex.
| Highest Mn exposure [] | ADHD-Overall | ADHD-Inattentive subtype | ADHD-Combined subtype | |||
|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | |
| Cases | 6,964 | 15,766 | 973 | 1,699 | 4,117 | 11,313 |
| Person-years | 3,615,736 | 3,752,175 | 2,995,010 | 3,101,128 | 3,007,820 | 3,132,771 |
| Base adjustmenta | ||||||
| (Ref) | 1 | 1 | 1 | 1 | 1 | 1 |
| 5–19 | 1.03 (0.95, 1.12) | 0.98 (0.93, 1.03) | 1.30 (1.01, 1.67) | 1.09 (0.92, 1.29) | 1.08 (0.97, 1.21) | 0.96 (0.91, 1.02) |
| 1.10 (1.02, 1.20) | 1.03 (0.98, 1.08) | 1.46 (1.13, 1.87) | 0.99 (0.83, 1.18) | 1.14 (1.02, 1.27) | 1.03 (0.97, 1.09) | |
| 1.12 (1.03, 1.22) | 1.09 (1.04, 1.15) | 1.54 (1.21, 1.97) | 1.21 (1.03, 1.44) | 1.10 (0.99, 1.23) | 1.06 (1.00, 1.13) | |
| 1.15 (1.06, 1.25) | 1.03 (0.98, 1.09) | 1.57 (1.23, 2.01) | 1.23 (1.04, 1.46) | 1.13 (1.02, 1.26) | 0.99 (0.93, 1.06) | |
| 0.0001 | 0.0037 | 0.0002 | 0.0034 | 0.046 | 0.27 | |
| SES adjustmentb | ||||||
| (Ref) | 1 | 1 | 1 | 1 | 1 | 1 |
| 5–19 | 1.00 (0.92, 1.08) | 0.96 (0.91, 1.00) | 1.26 (0.98, 1.62) | 1.07 (0.90, 1.27) | 1.04 (0.94, 1.16) | 0.94 (0.88, 1.00) |
| 1.07 (0.98, 1.16) | 1.01 (0.96, 1.06) | 1.41 (1.10, 1.82) | 0.97 (0.82, 1.16) | 1.10 (0.98, 1.22) | 1.01 (0.95, 1.07) | |
| 1.06 (0.98, 1.15) | 1.05 (1.00, 1.11) | 1.48 (1.16, 1.89) | 1.18 (1.00, 1.39) | 1.04 (0.93, 1.16) | 1.02 (0.96, 1.08) | |
| 1.09 (1.00, 1.18) | 0.98 (0.93, 1.03) | 1.51 (1.18, 1.93) | 1.20 (1.01, 1.42) | 1.07 (0.96, 1.19) | 0.94 (0.88, 1.00) | |
| 0.011 | 0.32 | 0.0007 | 0.011 | 0.34 | 0.62 | |
| SES and urbanicity adjustmentc | ||||||
| (Ref) | 1 | 1 | 1 | 1 | 1 | 1 |
| 5–19 | 0.99 (0.91, 1.07) | 0.95 (0.90, 1.00) | 1.28 (1.00, 1.65) | 1.08 (0.90, 1.27) | 1.01 (0.91, 1.13) | 0.93 (0.87, 0.99) |
| 1.05 (0.97, 1.14) | 1.00 (0.95, 1.05) | 1.50 (1.16, 1.93) | 0.99 (0.83, 1.18) | 1.04 (0.93, 1.16) | 0.98 (0.92, 1.05) | |
| 1.04 (0.96, 1.13) | 1.02 (0.97, 1.08) | 1.55 (1.21, 1.99) | 1.19 (1.00, 1.40) | 0.99 (0.89, 1.11) | 0.99 (0.93, 1.05) | |
| 1.06 (0.97, 1.15) | 0.96 (0.91, 1.01) | 1.53 (1.19, 1.96) | 1.20 (1.01, 1.43) | 1.03 (0.92, 1.16) | 0.92 (0.87, 0.99) | |
| 0.079 | 0.97 | 0.0008 | 0.013 | 0.77 | 0.24 | |
Note: Adjustment models for the main analyses (base adjustment and SES) and sensitivity analyses (additional adjustment for urbanicity). ADHD, attention-deficit hyperactivity disorder; Mn, manganese; Ref, reference; SES socioeconomic status.
Base: Cox proportional hazards model with age as underlying time scale and additional adjustment for birth year (4-y categories).
SES: Base model adjusted for maternal highest attained education and paternal income.
SES and urbanicity: Base model adjusted for maternal highest attained education, paternal income, and degree of urbanicity.
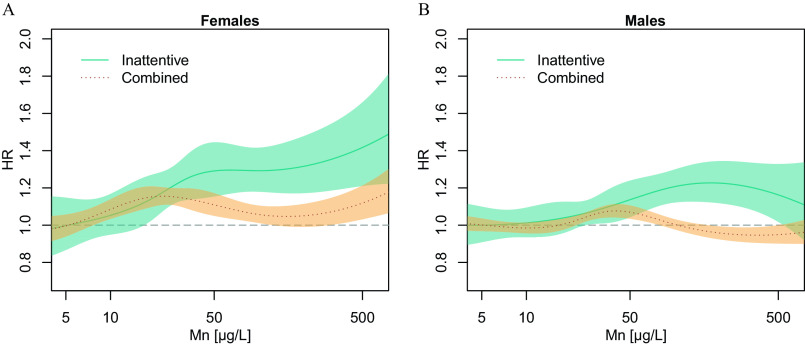
The suggested exposure–response relationship for the ADHD-Inattentive subtype in females and males seen in exposure categories can also be examined on a continuous scale in Figure 2. In females (Figure 2A), exposure to increasing levels of Mn in early childhood is associated with an increased risk of ADHD-Inattentive subtype, with a clear exposure–response pattern. In males (Figure 2B), the association with the ADHD-Inattentive subtype is less clear compared with females, following a flatter exposure–response pattern, with a dip at the very high exposure levels. No clear exposure–response pattern is observed in the association with ADHD-Combined subtype in either sex.
Figure 2.
Hazard ratios (HRs) for the association between highest exposure to manganese in drinking water during the first 5 y of life and ADHD by subtype for (A) females and (B) males. Smoothed HRs with 95% CIs. Cox proportional hazards model with age as underlying time scale and additionally adjusted for birth year and SES (maternal highest attained education and paternal income). Values below detection limit () were imputed using robust regression on order statistics. Given that exposure is skewed to the right and CIs increase along with fewer study population members in the high end of exposure, only exposures until (99% percentile of the study population) are shown in this figure for better visualization. Note: ADHD, attention-deficit hyperactivity disorder; CI, confidence interval; SES, socioeconomic status.
Modeling exposure as a time-weighted average gave similar results (Table 3). After adjusting for SES, Mn in drinking water was significantly associated only with ADHD-Inattentive subtype. In contrast with estimates based on the highest exposure, HRs for time-weighted exposures were similar between females and males and increased monotonically with exposure in males, but not females (, SES-adjusted, females: 0.042, males: 0.0011). Results were robust in the sensitivity analyses, when adjusting additionally for degree of urbanicity (Tables 2 and 3).
Table 3.
Hazard ratios (95% confidence intervals) for the association between time-weighted average drinking water Mn exposure during the first 5 y of life and ADHD-Overall and ADHD subtypes, stratified by sex.
| Time-weighted average Mn exposure [] | ADHD-Overall | ADHD-Inattentive subtype | ADHD-Combined subtype | |||
|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | |
| Cases | 6,964 | 15,766 | 973 | 1,699 | 4,117 | 11,313 |
| Person-years | 3,615,736 | 3,752,175 | 2,995,010 | 3,101,128 | 3,007,820 | 3,132,771 |
| Base adjustmenta | ||||||
| 1 | 1 | 1 | 1 | 1 | 1 | |
| 5–10 | 1.05 (0.90, 1.22) | 1.06 (0.96, 1.18) | 1.29 (0.88, 1.89) | 1.16 (0.86, 1.57) | 1.12 (0.92, 1.36) | 1.02 (0.90, 1.15) |
| 1.12 (0.98, 1.29) | 1.12 (1.02, 1.23) | 1.49 (1.06, 2.09) | 1.37 (1.05, 1.79) | 1.06 (0.89, 1.27) | 1.12 (1.00, 1.25) | |
| 1.18 (1.04, 1.33) | 1.11 (1.02, 1.21) | 1.42 (1.06, 1.93) | 1.44 (1.14, 1.82) | 1.16 (0.99, 1.36) | 1.03 (0.93, 1.14) | |
| 0.0022 | 0.0008 | 0.0014 | 0.0001 | 0.045 | 0.12 | |
| SES adjustmentb | ||||||
| 1 | 1 | 1 | 1 | 1 | 1 | |
| 5–10 | 0.96 (0.82, 1.12) | 0.98 (0.88, 1.09) | 1.22 (0.83, 1.80) | 1.09 (0.81, 1.48) | 1.03 (0.84, 1.25) | 0.93 (0.82, 1.05) |
| 1.08 (0.94, 1.25) | 1.08 (0.98, 1.19) | 1.47 (1.04, 2.06) | 1.33 (1.02, 1.74) | 1.02 (0.85, 1.23) | 1.08 (0.97, 1.21) | |
| 1.09 (0.96, 1.23) | 1.02 (0.94, 1.12) | 1.37 (1.01, 1.85) | 1.36 (1.07, 1.71) | 1.07 (0.92, 1.25) | 0.95 (0.85, 1.05) | |
| 0.11 | 0.22 | 0.042 | 0.0011 | 0.38 | 0.76 | |
| SES and urbanicity adjustmentc | ||||||
| 1 | 1 | 1 | 1 | 1 | 1 | |
| 5–10 | 0.94 (0.80, 1.10) | 0.96 (0.86, 1.06) | 1.16 (0.79, 1.72) | 1.06 (0.78, 1.43) | 1.06 (0.87, 1.29) | 0.92 (0.81, 1.04) |
| 1.11 (0.96, 1.28) | 1.07 (0.97, 1.18) | 1.53 (1.08, 2.17) | 1.40 (1.06, 1.83) | 1.09 (0.90, 1.32) | 1.09 (0.97, 1.22) | |
| 1.11 (0.98, 1.27) | 1.02 (0.93, 1.12) | 1.31 (0.96, 1.79) | 1.38 (1.08, 1.76) | 1.19 (1.01, 1.40) | 0.97 (0.88, 1.08) | |
| 0.055 | 0.33 | 0.011 | 0.0009 | 0.028 | 0.86 | |
Note: Adjustment models for the main analyses (base adjustment and SES) and sensitivity analyses (additional adjustment for urbanicity). ADHD, attention-deficit hyperactivity disorder; Mn, manganese; SES socioeconomic status.
Base: Cox proportional hazards model with age as underlying time scale and additional adjustment for birth year (4-y categories).
SES: Base model adjusted for maternal highest attained education and paternal income.
SES and urbanicity: Base model adjusted for maternal highest attained education, paternal income, and degree of urbanicity.
Discussion
In this large nationwide follow-up study of 643,401 individuals, we found that increasing levels of Mn in drinking water were associated with an increasing risk of the ADHD-Inattentive subtype. The results were consistent for different ways of modeling exposure and different adjustment models and were seen in both sexes, with evidence of stronger associations with the highest drinking water Mn exposure in females compared with males, but no clear differences by sex for time-weighted exposure estimates. We did not find consistent evidence for associations between Mn in drinking water and the ADHD-Overall or ADHD-Combined subtypes.
Exposure to of Mn at any one time during the first 5 y of life was associated with a 51% (95% CI: 18%, 93%) increased risk of ADHD-Inattentive subtype in females and a 20% (1–42%) increased risk in males, when compared with those exposed only to the lowest level of Mn (SES-adjusted). Importantly, approximately 20% of the children in the total study population were exposed to these high levels of Mn () at least once during the 5-y exposure window. Modeling exposure as a time-weighted average, being exposed to the highest levels () was associated with a 37% (1–85%) increased risk of ADHD-Inattentive subtype in females and a 36% (7–71%) increased risk in males, when compared with those exposed to the lowest levels (SES-adjusted).
Earlier studies on the association between Mn in drinking water and ADHD are few and limited by small sample sizes and imprecise exposure estimations. A cross-sectional study of 201 children in Bangladesh found an association between Mn levels in household wells (median exposure level of ), sampled at the time of the study, and attention problems (Khan et al. 2011). In a pilot study of 46 children from Canada, Bouchard et al. (2007) examined Mn concentrations in two drinking water supply areas, one with Mn levels ranging from and the other with levels of . They found correlations between Mn levels in drinking water and Mn levels in hair. Subsequently, they also found that Mn levels in hair were associated with teacher-rated behavioral symptoms of opposition and hyperactivity in children. Another cross-sectional study of 375 children from Canada found an association between Mn levels in hair and lower memory and attention functions, and exposure to high Mn levels in drinking water (up to ) were also associated with lower memory function (Oulhote et al. 2014a). Drinking water was sampled once, at the time of study, thus neither of these studies included longitudinal information on Mn levels in drinking water. However, hair Mn levels may be a suitable proxy for longer-term exposure to Mn. In our study, Mn exposures before 5 years of age, at levels that were lower than in previous studies, were associated with an increase in the risk of ADHD after 5 years of age.
In our study, associations between Mn and ADHD were more pronounced in females than in males when exposure was based on the highest level in drinking water, whereas associations were similar for females and males when based on time-weighted average exposures. Kullar et al. (2019) emphasized that this is consistent with numerous earlier studies on environmental Mn exposure and adverse neurodevelopmental outcomes that, when reporting sex-differences, either found stronger associations in girls or associations only in girls and not in boys (Bauer et al. 2017; Bouchard et al. 2018; Chiu et al. 2017; Hernández-Bonilla et al. 2016; Kullar et al. 2019; Riojas-Rodríguez et al. 2010; Torres-Agustín et al. 2013). Even though some studies also reported stronger associations in boys (Claus Henn et al. 2018; Rahman et al. 2017), collectively, these earlier studies suggested that girls may be more susceptible to Mn neurotoxicity. Furthermore, a recent study on Mn exposure from soil and neurobehavioral outcomes found that girls, who were genetically less efficient at regulating Mn, may be a particularly vulnerable group (Broberg et al. 2019). Although the mechanisms that may explain potential differences in effects by sex are not fully understood, it has been shown that females have significantly higher blood Mn levels than males (Oulhote et al. 2014a). As a possible explanation, sex-related metabolic differences have been suggested (Bouchard et al. 2018; Dion et al. 2018; Oulhote et al. 2014b).
Dopaminergic dysfunction has been suggested as a possible mechanism that could explain an association between drinking water Mn exposure and increased risk of ADHD given that Mn accumulates primarily in the brain regions involved in dopaminergic function (Nitin and Bowman 2018). In rats, early postnatal Mn exposure altered catecholamine function in the same brain regions in which children with ADHD show hypofunctioning of catecholaminergic systems and caused lasting attentional dysfunction (Beaudin et al. 2017). Mn exposure impaired selective and focused attention, arousal regulation, and fine motor function, without altering impulse control, corresponding to an inattentive presentation of ADHD (Beaudin et al. 2017). This explanation supports our findings of a stronger association with the ADHD-Inattentive subtype.
Our study has a number of methodological strengths. First, the nationwide, register-based cohort design allowed us to enroll by far the largest study population on this topic to date while also avoiding selection bias. Data, both on exposure and outcome, were collected prospectively at the time of sampling and registered in centralized databases, minimizing information and recall bias. Our outcome was defined as a diagnosis of ADHD in health registers (for ADHD-Overall) or a combination of ADHD-related diagnoses (for ADHD subtypes). The diagnoses in the health registers are the result of a thorough clinical assessment by a team of mental health professionals, including child psychiatrists. Validation studies of ADHD diagnoses in the DPCRR concluded that risk of misclassification was low (Dalsgaard et al. 2001; Linnet et al. 2009). A validation study of hyperkinetic disorders showed that 87% of those with a ICD-10-DCR code F90.x diagnosis in the DPCRR indeed fulfilled the Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (DSM-5; APA 2013) criteria for ADHD (Mohr-Jensen et al. 2016). Furthermore, in our study, Mn exposure was estimated using longitudinal data with a high spatial and temporal resolution. This is crucial when estimating exposure to Mn in drinking water in groundwater-based supply systems with conventional treatment, where Mn oxidizes and subsequently precipitates in sand filters. Concentrations of Mn in drinking water are mainly determined by the filter efficiency at the individual waterworks. Depending on water flow rates, groundwater chemistry, filter media replacements, or start-ups of bio-coating, removal may suddenly become insufficient. Such sudden treatment breakdowns result in sharp increases of Mn in the drinking water, which may remain elevated for several months, before efficient Mn removal is reestablished (Breda et al. 2017; Bruins et al. 2014; Gouzinis et al. 1998); this can also be observed in Video S1. Because drinking water samples in the national geodatabase are registered with the date of sampling, and residential history in the CRS is registered with a resolution of 1 d, it was possible to assign each cohort member their corresponding exposure to drinking water Mn with a resolution of 1 d. Exposure was modeled based on the actual drinking water supply areas, thus no spatial interpolation was necessary, which would have been inappropriate given the distinct pattern of Mn exposure.
Given its design, our study has several limitations. We lacked information on other Mn exposure sources than drinking water. Mn is found in many foods, such as cereals, seafood, vegetables and nuts (Bouchard et al. 2007; Wenlock et al. 1979). Even though oral intake of Mn from drinking water is usually small compared with intake through foods, drinking water may be a crucial exposure path. For example, an earlier study found Mn concentrations in children’s hair were correlated with drinking water Mn intake but not with dietary Mn intake, suggesting that Mn from drinking water is metabolized differently than Mn from diet (Bouchard et al. 2011). Interaction between Mn and iron may also be important to consider when investigating the relation between Mn exposure and neurodevelopment. Several studies reported that iron-deficient individuals might have a higher blood Mn concentration and more severe signs of neurotoxicity following occupational or environmental exposure to Mn (Bjørklund et al. 2020). Information on iron status level was not available in our study and we were not able to account for the joint effect of low iron status and exposure to Mn. Likewise, we lacked data to take the potential effect of other drinking water contaminants into account. However, metallic contamination of different sources, such as leaching from pipes or installations, including lead, are unlikely to be correlated with naturally occurring Mn and are hence not expected to threaten the validity of the reported associations between Mn and ADHD. Although we controlled for several important variables such as age, sex, parental SES, and degree of urbanicity, we cannot exclude residual confounding by unobserved variables. We did not assess whether the number of peaks with elevated Mn levels in drinking water was associated with ADHD given that this would require uniform sampling frequencies at the waterworks.
We were restricted by the ICD-10-DCR codes used in the Danish registers, making it impossible to assess predominantly hyperactive/impulsive cases as a separate subgroup of ADHD. Consequently, children not classified as ADHD-Inattentive subtype were classified in the broader ADHD-Combined subgroup. To identify the subtypes, we applied a hierarchical classification method with a 2-y reclassification period at the end of the study in order to avoid misclassification of subtypes and conditioning on the future. This led to the exclusion of the most recent cases (i.e., those diagnosed in 2015 and 2016). Because there is an increasing trend in ADHD diagnosis over time (Atladottir et al. 2015) and the incidence rate of ADHD peaks later in females than in males (Dalsgaard et al. 2019), a relatively large proportion of cases was excluded in the subtype analyses, more so for females. As we cannot be certain that the excluded cases were a random sample of all cases, this study could be repeated in a few years to avoid excluding a significant proportion of cases even when applying a reclassification period. Because of differences in diagnostic criteria between ICD-10 and DSM, the group we classified as ADHD-Combined subtype may include some misclassified cases of ADHD-Inattentive subtype according to DSM. Preferably, future studies not constrained by ICD-10-DCR should make direct use of ADHD subtype classifications according to DSM-IV-TR or DSM-5, when available.
We found an increased risk for ADHD at Mn levels below the WHO’s health-based drinking water guideline value of , whose scientific foundation has previously been questioned (Ljung and Vahter 2007). This adds to other recent studies suggesting the need for a formal drinking water guideline value for Mn. For example, a recent study estimated drinking water Mn benchmark concentrations for a 1% decrease of the Performance IQ score to be for boys and for girls (Kullar et al. 2019).
To the best of our knowledge, this is the largest population-based cohort study on the association between exposure to Mn in drinking water and ADHD and the only study with a detailed spatiotemporal exposure assessment. Our study adds to the body of evidence suggesting an association between elevated Mn in drinking water and negative health outcomes in children at comparatively low levels. Waterworks in Denmark are typically efficient at removing Mn to levels , and techniques exist to mitigate Mn peaks in drinking water or minimize their durations after episodes of filter malfunctioning (Breda et al. 2017; Bruins et al. 2014; Gouzinis et al. 1998). If confirmed, this study shows that public waterworks not only have an incentive for providing efficient Mn removal for cosmetic reasons but may indeed have a valid public health argument for doing so.
In conclusion, we report novel findings indicating that exposure to increasing levels of Mn in drinking water early in life may be associated with increased risk of ADHD, especially of the inattentive subtype. Our exposure assessment also suggests that high resolution in exposure time series is crucial for studies of Mn in drinking water given that water supply systems are characterized by long periods of low Mn levels with episodic peaks. Further studies are needed to confirm our findings in other settings and should include assessing co-exposure with other drinking water compounds and assess whether children are especially susceptible to Mn toxicity at specific ages. Gene–environment interaction effects in subgroups with genetic susceptibility of poor Mn regulation should also be studied as well as associations with other neurodevelopmental and neurotoxic outcomes.
Supplementary Material
Acknowledgments
J.S. and S.D. were the principle investigators. J.S., C.B.P., and S.D. conceived study design. J.S. and M.T. performed data collection and management. J.S. carried out exposure estimation, statistical analysis and presentation of results in figures and tables. J.S., C.B.P., and S.D. contributed to data interpretation. J.S. wrote drafts and final version of the report, B.H. and S.D. commented on first draft. S.M.K. and B.H. contributed to exposure design and interpretation. All authors contributed to preparation of the final report and have approved the final version.
The authors thank engineer I.L. Breda from Skanderborg Forsyningsvirksomhed A/S for her valuable input on current research on manganese water treatment at the Danish waterworks. This study was funded by a grant from the Aarhus University Research Foundation (AUFF-E-2015-FLS-8-61). The research of S.D. is supported by grants from the Lundbeck Foundation (iPSYCH grant R102-A9118, R155-2014-1724, and R248-2017-2003), National Institutes of Health (R01, grant ES026993), Novo Nordisk Foundation (grant 22018), the European Commission (Horizon 2020, grant 667302), and Helsefonden (grant 19-8-0260).
References
- APA (American Psychiatric Association). 2000. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision). 4th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- APA. 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed Arlington, VA: American Psychiatric Association. [Google Scholar]
- Atladottir HO, Gyllenberg D, Langridge A, Sandin S, Hansen SN, Leonard H, et al. 2015. The increasing prevalence of reported diagnoses of childhood psychiatric disorders: a descriptive multinational comparison. Eur Child Adolesc Psychiatry 24(2):173–183, PMID: 24796725, 10.1007/s00787-014-0553-8. [DOI] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV. 2007. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 96(9):1269–1274, PMID: 17718779, 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. 2017. Manganese in teeth and neurobehavior: sex-specific windows of susceptibility. Environ Int 108:299–308, PMID: 28941415, 10.1016/j.envint.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin SA, Strupp BJ, Strawderman M, Smith DR. 2017. Early postnatal manganese exposure causes lasting impairment of selective and focused attention and arousal regulation in adult rats. Environ Health Perspect 125(2):230–237, PMID: 27384154, 10.1289/EHP258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G, Chartrand MS, Aaseth J. 2017. Manganese exposure and neurotoxic effects in children. Environ Res 155:380–384, PMID: 28282629, 10.1016/j.envres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Bjørklund G, Dadar M, Peana M, Rahaman MS, Aaseth J. 2020. Interactions between iron and manganese in neurotoxicity. Arch Toxicol 94(3):725–734, PMID: 32180038, 10.1007/s00204-020-02652-2. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. 2007. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect 115(1):122–127, PMID: 17366831, 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur M-È, Bouffard T, et al. 2011. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119(1):138–143, PMID: 20855239, 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Surette C, Cormier P, Foucher D. 2018. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology 64:110–117, PMID: 28716743, 10.1016/j.neuro.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Breda IL, Ramsay L, Roslev P. 2017. Manganese oxidation and bacterial diversity on different filter media coatings during the start-up of drinking water biofilters. J Water Supply Res Technol 66(8):641–650, 10.2166/aqua.2017.084. [DOI] [Google Scholar]
- Broberg K, Taj T, Guazzetti S, Peli M, Cagna G, Pineda D, et al. 2019. Manganese transporter genetics and sex modify the association between environmental manganese exposure and neurobehavioral outcomes in children. Environ Int 130:104908, PMID: 31233999, 10.1016/j.envint.2019.104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins JH, Vries D, Petrusevski B, Slokar YM, Kennedy MD. 2014. Assessment of manganese removal from over 100 groundwater treatment plants. J Water Supply Res Technol 63(4):268–280, 10.2166/aqua.2013.086. [DOI] [Google Scholar]
- Chen P, Parmalee N, Aschner M. 2014. Genetic factors and manganese-induced neurotoxicity. Front Genet 5:254, PMID: 25136353, 10.3389/fgene.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-HM, Claus Henn B, Hsu H-HL, Pendo MP, Coull BA, Austin C, et al. 2017. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ Res 159:458–465, PMID: 28858760, 10.1016/j.envres.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Austin C, Coull BA, Schnaas L, Gennings C, Horton MK, et al. 2018. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res 161:588–598, PMID: 29247915, 10.1016/j.envres.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper J. 1837. Sur les effets du peroxide de manganèse. [In French.] J Chim Méd Pharm Toxicol 3:223–225. [Google Scholar]
- Dalsgaard S, Hansen N, Mortensen PB, Damm D, Thomsen PH. 2001. Reassessment of ADHD in a historical cohort of children treated with stimulants in the period 1969–1989. Eur Child Adolesc Psychiatry 10(4):230–239, PMID: 11794548, 10.1007/s007870170012. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. 2015. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2(8):702–709, PMID: 26249301, 10.1016/S2215-0366(15)00271-0. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Thorsteinsson E, Trabjerg BB, Schullehner J, Plana-Ripoll O, Brikell I, et al. 2019. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry 77(2):155–164, PMID: 31746968, 10.1001/jamapsychiatry.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion L-A, Saint-Amour D, Sauvé S, Barbeau B, Mergler D, Bouchard MF. 2018. Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water. Neurotoxicology 64:118–125, PMID: 28870865, 10.1016/j.neuro.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Larsson H. 2019. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry 24(4):562–575, PMID: 29892054, 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzinis A, Kosmidis N, Vayenas DV, Lyberatos G. 1998. Removal of Mn and simultaneous removal of NH3, Fe and Mn from potable water using a trickling filter. Water Res 32(8):2442–2450, 10.1016/S0043-1354(97)00471-5. [DOI] [Google Scholar]
- Hafeman D, Factor-Litvak P, Cheng Z, van Geen A, Ahsan H. 2007. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ Health Perspect 115(7):1107–1112, PMID: 17637930, 10.1289/ehp.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Pjetursson B. 2011. Free, online Danish shallow geological data. GEUS Bull 23:53–56, 10.34194/geusb.v23.4842. [DOI] [Google Scholar]
- He P, Liu DH, Zhang GQ. 1994. Effects of high-level-manganese sewage irrigation on children’s neurobehavior. [In Chinese.] Zhonghua Yu Fang Yi Xue Za Zhi. 28(4):216–218, PMID: 7842882. [PubMed] [Google Scholar]
- Hernández-Bonilla D, Escamilla-Núñez C, Mergler D, Rodríguez-Dozal S, Cortez-Lugo M, Montes S, et al. 2016. Effects of manganese exposure on visuoperception and visual memory in schoolchildren. Neurotoxicology 57:230–240, PMID: 27737811, 10.1016/j.neuro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F, et al. 2011. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect 119(10):1501–1506, PMID: 21493178, 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullar SS, Shao K, Surette C, Foucher D, Mergler D, Cormier P, et al. 2019. A benchmark concentration analysis for manganese in drinking water and IQ deficits in children. Environ Int 130:104889, PMID: 31200154, 10.1016/j.envint.2019.05.083. [DOI] [PubMed] [Google Scholar]
- Larsson H, Sariaslan A, Långström N, D’Onofrio B, Lichtenstein P. 2014. Family income in early childhood and subsequent attention deficit/hyperactivity disorder: a quasi-experimental study. J Child Psychol Psychiatry 55(5):428–435, PMID: 24111650, 10.1111/jcpp.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Secher NJ, Thomsen PH, Obel C, Dalsgaard S, et al. 2009. Coffee consumption during pregnancy and the risk of hyperkinetic disorder and ADHD: a prospective cohort study. Acta Paediatr 98(1):173–179, PMID: 18764862, 10.1111/j.1651-2227.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- Ljung K, Vahter M. 2007. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect 115(11):1533–1538, PMID: 18007980, 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Aschner M, Landrigan PJ, Cranmer JM. 2018. Neurotoxicity of manganese: indications for future research and public health intervention from the Manganese 2016 conference. Neurotoxicology 64:1–4, PMID: 29429640, 10.1016/j.neuro.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Håberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. 2015. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 7:491–508, PMID: 26648756, 10.2147/CLEP.S90589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KB, Ersbøll AK, Olsen J, Parner E, Obel C. 2015. Geographic analysis of the variation in the incidence of ADHD in a country with free access to healthcare: a Danish cohort study. Int J Health Geogr 14:24, PMID: 26297014, 10.1186/s12942-015-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Environment and Food of Denmark. 2018. Ministerial Order on Drinking Water Quality. [In Danish.] BEK nr 1068 af 23/08/2018. https://www.retsinformation.dk/api/pdf/202768 [accessed 8 September 2020].
- Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, Steinhausen H-C. 2016. The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. Eur Psychiatry 35:16–24, PMID: 27061373, 10.1016/j.eurpsy.2016.01.2427. [DOI] [PubMed] [Google Scholar]
- Mors O, Perto GP, Mortensen PB. 2011. The Danish Psychiatric Central Research Register. Scand J Public Health 39(suppl 7):54–57, PMID: 21775352, 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- Nitin R, Bowman AB. 2018. Connections between manganese neurotoxicity and neurological disease. Adv Neurotoxicol 2:87–113, 10.1016/bs.ant.2018.03.001. [DOI] [Google Scholar]
- Ntihabose R, Surette C, Foucher D, Clarisse O, Bouchard MF. 2018. Assessment of saliva, hair and toenails as biomarkers of low level exposure to manganese from drinking water in children. Neurotoxicology 64:126–133, PMID: 28867366, 10.1016/j.neuro.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur M-È, et al. 2014a. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect 122(12):1343–1350, PMID: 25260096, 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Bouchard MF. 2014b. Sex-and age-differences in blood manganese levels in the U.S. general population: National Health and Nutrition Examination Survey 2011–2012. Environ Health 13:87, PMID: 25342305, 10.1186/1476-069X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard KR, Oerbeck B, Friis S, Biele G, Pripp AH, Aase H, et al. 2019. Screening with an ADHD-specific rating scale in preschoolers: a cross-cultural comparison of the Early Childhood Inventory-4. Psychol Assess 31(8):985–994, PMID: 30958025, 10.1037/pas0000722. [DOI] [PubMed] [Google Scholar]
- Pedersen CB. 2011. The Danish Civil Registration System. Scand J Public Health 39(suppl 7):22–25, PMID: 21775345, 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Gøtzsche H, Møller JØ, Mortensen PB. 2006. The Danish Civil Registration: a cohort of eight million persons. Dan Med Bull 53(4):441–449, PMID: 17150149. [PubMed] [Google Scholar]
- Racette BA. 2014. Manganism in the 21st century: the Hanninen lecture. Neurotoxicology 45:201–207, PMID: 24148923, 10.1016/j.neuro.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SM, Kippler M, Tofail F, Bölte S, Hamadani JD, Vahter M. 2017. Manganese in drinking water and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Environ Health Perspect 125(5):057003, PMID: 28564632, 10.1289/EHP631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay L, Breda IL, Søborg DA. 2018. Comprehensive analysis of the start-up period of a full-scale drinking water biofilter provides guidance for optimization. Drink Water Eng Sci 11(2):87–100, 10.5194/dwes-11-87-2018. [DOI] [Google Scholar]
- Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, et al. 2010. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect 118(10):1465–1470, PMID: 20936744, 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, et al. 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454–455:562–577, PMID: 23570911, 10.1016/j.scitotenv.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Schullehner J, Hansen B. 2014. Nitrate exposure from drinking water in Denmark over the last 35 years. Environ Res Lett 9:095001, 10.1088/1748-9326/9/9/095001. [DOI] [Google Scholar]
- Schullehner J, Jensen NL, Thygesen M, Hansen B, Sigsgaard T. 2017. Drinking water nitrate estimation at household-level in Danish population-based long-term epidemiologic studies. J Geochem Explor 183(pt B):178–186, 10.1016/j.gexplo.2017.03.006. [DOI] [Google Scholar]
- Torres-Agustín R, Rodríguez-Agudelo Y, Schilmann A, Solís-Vivanco R, Montes S, Riojas-Rodríguez H, et al. 2013. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res 121:39–44, PMID: 23141434, 10.1016/j.envres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, et al. 2016. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism–dystonia. Nat Commun 7:11601, PMID: 27231142, 10.1038/ncomms11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2018. Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals [accessed 11 September 2018].
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. 2006. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 114(1):124–129, PMID: 16393669, 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenlock RW, Buss DH, Dixon EJ. 1979. Trace nutrients: 2. Manganese in British food. Br J Nutr 41(2):253–261, PMID: 427078, 10.1079/bjn19790034. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1993. ICD Classification of Mental and Behavioural Disorders, Diagnostic Criteria for Research, 10th ed Geneva, Switzerland: WHO; https://www.who.int/classifications/icd/en/GRNBOOK.pdf [accessed 8 September 2020]. [Google Scholar]
- WHO. 2011. Manganese in Drinking-water: Background Document for Development of WHO Guidelines for Drinking-water Quality. WHO/SDE/WSH/03.04/104/Rev/1. https://www.who.int/water_sanitation_health/dwq/chemicals/manganese.pdf [accessed 8 September 2020].
- WHO. 2017. Guidelines for Drinking-water Quality. 4th ed Geneva, Switzerland: WHO. [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. 2002. A child with chronic manganese exposure from drinking water. Environ Health Perspect 110(6):613–616, PMID: 12055054, 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.