Our smartphone application–based analysis of 6 million menstrual cycles among Japanese females found age-dependent and seasonal changes of cycle length and basal body temperature.
Abstract
OBJECTIVE:
To evaluate the effects of age and season on menstrual cycle length and basal body temperature (BBT). We also examined the effects of climate on cycle length and BBT, taking into account Japanese geographic and social characteristics.
METHODS:
In this retrospective cohort study, we analyzed data from 6 million menstrual cycles entered into a smartphone application from 310,000 females from 2016 to 2017. Only those who entered more than 10 cycles in 2 years were included. Generalized estimation equations were used to adjust for confounding factors and for within-person correlations of multiple records. Multiple regression analysis was conducted, with age, external average temperature, precipitation amount, and sunshine hours as confounding factors.
RESULTS:
The mean menstrual cycle length increased from age 15–23 years, subsequently decreased up to age 45 years, and then increased again. Average follicular phase body temperature showed no significant age-dependent changes, but luteal phase body temperature gradually increased up to 29 years and then stabilized and started to decrease after age 42 years. A significant association between external temperature and body temperature (follicular and luteal phase) was observed, though menstrual cycle length did not show such an association.
CONCLUSION:
These results, derived from data self-entered into a smartphone application, revealed underrecognized age-dependent and seasonal changes in menstrual cycle length and BBT, which will contribute to a better understanding of female reproductive health in the modern world.
Current understanding of menstrual cycle length and basal body temperature (BBT) is largely based on knowledge that was drawn from small descriptive studies performed in the 1950s.1–3 However, women's eating habits, educational standards, and lifestyles have changed greatly in many countries since then, and the background against which these gynecologic findings were obtained has thus changed accordingly. In addition, cycle length and BBT may be affected by ethnic and social factors,4,5 and it is therefore not possible to make reliable comparisons among different countries.
Basal body temperature is controlled by the thermoregulatory center in the preoptic anterior hypothalamus and increases by 0.3–0.5°C after ovulation as a result of progesterone secretion from the ovaries.6,7 Basal body temperature can be measured easily to provide information on ovulation, which is, in turn, a useful index for pregnancy.8 Basal body temperature, as a deep-body temperature, is considered to remain stable and has recently been used as an indicator of healthy aging and longevity.4,9 Although BBT has been considered to be relatively unaffected by the environment, including external temperature, sunshine duration, and precipitation,10,11 the effect of regional environment and season on human health and longevity remain unknown. The existence of any seasonality in BBT, if present, would be expected to be very subtle, and its detection would thus require the analysis of a large sample.5,10
Japan comprises approximately 380,000 km2, with a population of 128 million people, mostly of the same ethnic group and with similar educational levels and lifestyles among the prefectures.12 Moreover, it is geographically long from north to south, with 2,500 km and 16.85° of latitude between the prefectures of Hokkaido and Okinawa. These northernmost and southernmost regions of Japan have subarctic and subtropical climates, respectively,13 providing a unique opportunity to examine the effects of climate differences on menstrual cycle length and BBT while excluding the effects of genetic background and socioeconomic factors.
The use of big data has recently attracted attention in the medical field.14,15 Luna Luna is a smartphone application, provided by MTI Ltd., that aims to predict menstrual cycle and ovulation under normal physical conditions. The basic functions of this application are freely available on the Android and iOS platforms, and it is currently downloaded by more than 14 million females as the most popular female health care application in Japan. This application is used for both pregnancy planning and contraception planning and for general health management, thus reflecting the Japanese female population in general.16 We therefore aimed to test the effects of age and season on menstrual cycle length and BBT by analyzing data from 6 million anonymized menstrual cycles reported in this application. We also examined the effect of climate on cycle length and BBT by taking into account Japanese geographic and social characteristics.
METHODS
The study protocol was approved by the ethics committee of the National Center for Child Health and Development (ethical approved number: 1900). We provided all current application users with in-application notifications about the outline of the research plan, how data collected from the application would be used, and the required steps to opt out of the study. For former users, MTI Ltd. published a press release about the study, together with information on how to opt out of the study.
In this retrospective cohort study, a total of 4.5 million application users entered their menstrual cycle data using the smartphone application for at least 10 cycles each from January 1, 2016, to December 31, 2017. To examine the effects of age and season on menstrual cycle length and BBT, we extracted cycle data based on the following criteria: 1) data derived from users with birthdate information, 2) data obtained at age 15.0–54.9 years, and 3) data derived from users who lived in Japan with residence information (ie, prefecture). As a result, data from 5,958,110 cycles derived from 310,000 users fulfilled the criteria. We excluded outliers for height shorter than 130 cm or taller than 200 cm (540 cycles), weight less than 30 kg or greater than 120 kg (594 cycles), and having more than five children (89 cycles), because this was considered unreliable compared with the general Japanese female population. The remaining 5,956,886 cycles from 310,668 users were analyzed. The collected data included age at the start of the menstruation cycle (all participants), residence of the user (all participants), length of the menstruation cycle (5,956,886 cycles), average follicular phase temperature (696,784 cycles), average luteal phase temperature (646,412 cycles), purpose of application usage (ie, contraception or pregnancy) (644,823 cycles), number of children (458,395 cycles), marital status (144,352 cycles), partner status (ie, present or absent) (149,679 cycles), weight (176,876 cycles), and height (167,202 cycles). We also excluded cycles from the analysis if they were too short (7 days or less; 25,286 cycles) or too long (more than 90 days; 38,715 cycles), because it was highly likely that the data were entered incorrectly or that the user became pregnant.
In this study, average follicular phase temperature was defined as the average BBT over the 10-day period from the start date of menstruation and average luteal phase temperature as the average BBT over the 10 days before the start date of the subsequent menstruation. The difference between the luteal and follicular phase temperatures was defined as ΔBBT. Cycles with ΔBBT of at least 0.2 were defined as biphasic phase temperature, and those with ΔBBT 0.1 or less were defined as monophasic body temperature. Prefectures and regions were classified according to the Statistics Bureau of the Ministry of Internal Affairs and Communications.12 Spring, summer, autumn, and winter (March–May, June–August, September–November, December–February, respectively) were defined according to the classification of the Japan Meteorological Agency.13 The external average temperature, precipitation amount, and sunshine hours in Hokkaido and Okinawa were extracted from the reports of the Japan Meteorological Agency17,18 (summarized in Appendix 1, available online at http://links.lww.com/AOG/B904).
First, we examined the distribution of the data characteristics, which included residence, age, marital or partner status, distribution of menstrual cycle length, and BBT. Because menstrual cycle length days did not follow a normal distribution, the mean and SD were calculated after Box-Cox transformation, a commonly used technique that chooses and fits a power transformation to change the distribution of a (nonnormal) variable closest to normal.19 To confirm our results, we also conducted a subanalysis in which we restricted cycles to one cycle per woman to eliminate the bias related to repeated sampling. Next, generalized estimating equations using the identity-link function to take into account within-person correlations of multiple records,20,21 assuming normal distribution of the outcome and adjusting for confounding factors, were conducted to estimate the effect of age, season, and year reported on menstrual cycle length and BBT according to our previous reports.22,23 To minimize bias related to residence, such as external temperature, precipitation amount, and sunshine hours, we restricted data to 798,589 cycles in Tokyo for this analysis. Third, we examined the effect of climate on menstrual cycle length and BBT using data obtained in Hokkaido and Okinawa, as representative subarctic and subtropical climates, respectively (Appendix 1, http://links.lww.com/AOG/B904). For this we conducted multiple regression analysis with age, external average temperature, precipitation amount, and sunshine hours as confounding factors included by forced input method. From this analysis, Pearson's correlation and standardized partial regression coefficients were calculated. Finally, we repeated all analyses on menstrual cycle length, restricting the data to those for cycles with 15–45 days, because mean menstrual cycle length is generally considered to be about 15–45 days.24 In all analyses, a two-tailed P value was assessed using χ2 or t test, and P<.05 was considered statistically significant. Statistical analysis was carried out using SPSS 25, graphs were drawn using GraphPad Prism 8, and images were adjusted using Adobe Illustrator CC.
RESULTS
The data were analyzed among users ranging in age from 15.0 to 54.9 years, with most participants being in their mid-30s (Appendix 2A, available online at http://links.lww.com/AOG/B904). The distribution of the participants' residences was similar to the distribution of the general Japanese population reported by the Ministry of Internal Affairs and Communications12 (Pearson's correlation, r=0.984, P<.001; Appendix 2B, http://links.lww.com/AOG/B904), suggesting that the data reflected the Japanese female population in general. There was no clear relationship between residence and cycle length distribution by age (Appendix 2C and D, http://links.lww.com/AOG/B904). We confirmed that the cycle data were derived from participants using the application for pregnancy planning and for contraception (Appendix 3, available online at http://links.lww.com/AOG/B904). The number of children was significantly correlated with age (r=0.739, P=.153). Married participants or those with partners were older than unmarried participants or those without partners (mean age 30.8 vs 25.5, 29.9 vs 27.7, respectively). The mean body mass index (BMI calculated as weight in kilograms divided by height in meters squared) of the participants was 22.8±4.1, and there was no relationship between cycle length and height (Appendix 4A, available online at http://links.lww.com/AOG/B904) or weight (Appendix 4B, http://links.lww.com/AOG/B904).
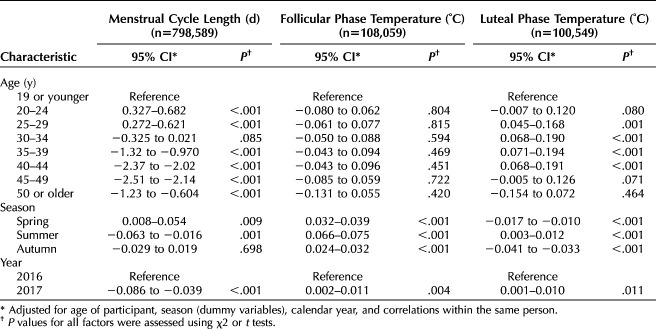
Menstrual cycle length data were aggregated by age at the start of each cycle, and the mean and SD values were calculated (Fig. 1A). An increase in menstrual cycle length was observed from age 15 years to the early 20s. The longest mean menstrual cycle length of 30.7 days was observed at age 23 years; this then decreased to 27.3 days at age 45 years and then increased. The variance in cycle length was relatively large among participants younger than age 20 years or older than 45 years, with the smallest variance in participants aged 41 years.
Fig. 1. Age-dependent changes of menstrual cycle length (A), follicular phase temperature (B), luteal phase temperature (C) and Δ basal body temperature (D). Five percent trimmed means (lines) and SDs (shaded area) are shown.
Tatsumi. Changes in Menstrual Cycle Length and Body Temperature. Obstet Gynecol 2020.
Regarding BBT, average follicular phase temperature showed no significant age-dependent changes, with the greatest variance among teenagers (Fig. 1B). However, luteal phase temperature showed an age-dependent change, with values gradually increasing up to the late 20s, peaking at 36.71°C at age 29 years, and then stabilizing and starting to decrease after age 42 years (Fig. 1C). The variance in luteal phase temperature was similar to that in follicular phase temperature. The mean ΔBBT showed similar age-dependent changes to luteal phase temperature, being stable from 27 to 42 years, with a maximum of +0.301°C at age 33 years (Fig. 1D). A subanalysis that restricted cycles to one cycle per participant to eliminate the bias related to repeated sampling showed results similar to those obtained from all-cycle data (Appendix 5A–C, available online at http://links.lww.com/AOG/B904). The proportion of cycles with biphasic body temperature varied depending on the age group: 15–19 years, 67%; 20–24 years, 78%; 25–29 years, 88%; 30–34 years, 91%; 35–39 years, 91%; 40–44 years, 89%; 45–50 years, 81%; and 50–54 years, 69%.
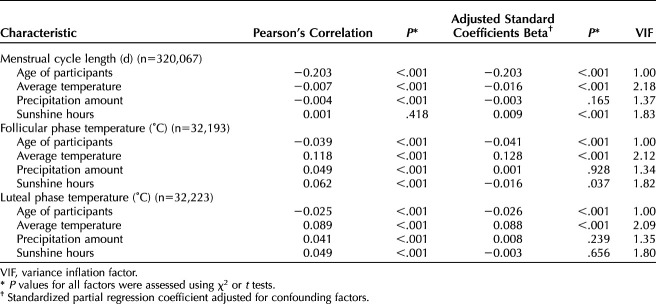
We investigated the effects of season on menstrual cycle length and BBT. There was no correlation between menstrual cycle length and season (Fig. 2A). In contrast, BBT showed clear seasonality. Both follicular and luteal phase temperatures were higher in summer and lower in winter (Fig. 2B and C), indicating that BBT was associated with environmental factors. Generalized estimating equation analysis also confirmed the significant effect of age on cycle length and luteal phase temperature, as well as showed the presence of seasonality in both follicular and luteal phase temperature; no such seasonality was seen in cycle length (Table 1).
Fig. 2. Seasonality of menstrual cycle length (A), follicular phase temperature (B), and luteal phase temperature (C). Probability densities were calculated for each month, and their distributions are shown in the heat maps.
Tatsumi. Changes in Menstrual Cycle Length and Body Temperature. Obstet Gynecol 2020.
Table 1.
Effects of Age, Season, and Year on Menstrual Cycle Length and Basal Body Temperature, Adjusting for Correlations Within the Same Participant Using Generalized Estimating Equations
Visualization of the external average temperature of Hokkaido and Okinawa and the average follicle phase body temperature of females living in these prefectures both showed a similar seasonality (Appendix 6A and B, available online at http://links.lww.com/AOG/B904). The correlation between climate and menstrual cycle length and BBT estimated using multiple regression analysis adjusted for age is shown in Table 2. There was little association between climactic factors and cycle length. External average temperature was weakly correlated with BBT (beta=0.128 and 0.088 in the follicular and luteal phases, respectively) and cycle length (beta= −0.016), and sunshine hours were weakly correlated with follicular phase temperature (beta= −0.016) and cycle length (0.009); there were no significant correlations between BBT and precipitation amount.
Table 2.
Effects of Climate and Participant Age on Menstrual Cycle Length and Basal Body Temperature, Multiple Regression Analysis
A subanalysis focusing on cycles with 15–45 days showed results similar to those from all data with cycles with 7–90 days. Age-dependent changes in menstrual cycle length were comparable with those calculated from all data (Appendix 7A, available online at http://links.lww.com/AOG/B904). Results from the generalized estimating equation analysis (corresponding to Table 1, Appendix 8, available online at http://links.lww.com/AOG/B904) and multiple regression analysis (corresponding to Table 2, Appendix 9, available online at http://links.lww.com/AOG/B904) were also similar. Interestingly, histograms on ΔBBT showed similar distributions, with a peak around 0.4°C (Appendix 7B and C, http://links.lww.com/AOG/B904), suggesting that most females with cycle lengths of 45–90 days probably have delayed ovulation (oligo-ovulation) but not anovulation.
DISCUSSION
We observed in our present study that menstrual cycle length changed with age, but it was not associated with season or other climatic factors. Luteal, but not follicular, phase temperature changed with age, and both follicular and luteal phase BBTs were associated with environmental temperature. We analyzed data from 6 million menstrual cycles derived from 310,000 active application users to add to current knowledge concerning characteristics associated with cycle length and BBT.
The human menstrual cycle is regulated by the hypothalamus-pituitary-gonad axis.25 Menstruation is irregular in girls in their early teens because secretion of gonadotropins (ie, luteinizing hormone and follicular stimulating hormone) from the pituitary is suppressed owing to the immature gonadotropin-releasing hormone pulse from the hypothalamus.26 Pulsatile gonadotropin-releasing hormone secretion matures during adolescence, sex steroid secretion increases, and the menstrual cycle becomes stabilized. The increase in menstrual cycle length in teenagers likely reflects the maturation of the hypothalamus-pituitary-gonad axis. Polycystic ovarian syndrome (PCOS), with an estimated prevalence of 5–20% among reproductive-aged females, causes defective ovulation, resulting in elongation of the menstrual cycle, which stabilizes when women are in their 30s.27,28 This age-dependent change helps to explain the shortened mean cycle length for women in their 30s. Another possible explanation is an increase in early-onset primary ovarian insufficiency in women in their 30s. This disease occurs in 1% of women in their 30s, with increasing frequency throughout the 30s,29 and could be associated with shortening of the menstrual cycle. Women in their 40s have fewer ovarian follicles, and their cycles therefore tend to be shortened.25 Collectively, we demonstrated age-dependent change in menstrual cycle length, from relatively short in the teens, stable in the 20s, shortened in the 30s and early 40s, and patchy in the 50s. These findings are in agreement with current knowledge on physiology and pathology involving the menstrual cycle. Interestingly, the mean menstrual cycle length peaked at 23 years, which was consistent with the peak age of fecundability (22–23 years)30 and the dip age for risk of miscarriage (23 years).31 This similarity implies the presence of common biological factors affecting menstrual cycle, fecundability, and rate of miscarriage.
Basal body temperature can be self-measured. Basal body temperature is known to increase by 0.3–0.5°C as a result of the action of progesterone on the hypothalamic body temperature center.6,7 Because BBT remains stable, it may be possible to predict the date of ovulation by daily monitoring.7,8 Although the American Society for Reproductive Medicine reports that the use of BBT should be discouraged because of its inconvenience and because it was not useful for determining luteal phase deficiency,32 the biphasic nature of the menstrual cycle is a useful indicator for women who want to become pregnant. However, there is currently no clear age-specific standard data, such as means and SDs, for BBT changes.5,9 The current results revealed that follicular phase temperature did not show any significant age-dependent changes but that luteal phase temperature gradually increased from the teens, stabilized from the late 20s to the early 40s, and then gradually decreased.
Information on the effects of the environmental factors on the menstrual cycle and BBT is lacking.10,11 The present study shows that BBT increased during warm periods and decreased during cold periods and suggests that BBT may differ by climate. The climate is mainly characterized by external temperature, precipitation amount, and sunshine hours.33,34 One strength of our study is that Japan has wide geographical characteristics and uniquely consists of islands with both subarctic and subtropical climates13 while also having relatively uniform eating habits, educational standards, and lifestyle habits throughout the country, thus making it easier to study the effect of climate. Hokkaido, the northernmost region, has a subarctic climate, with large temperature differences from month to month and lower annual precipitation and ultraviolet irradiation; Okinawa, as the southernmost region, has a subtropical, always warm climate, with fewer temperature differences throughout the year and higher annual precipitation and ultraviolet irradiation.17,18 Confirmation of our findings is anticipated in other populations, though it may be difficult to find participants living in a large variety of climates maintaining similar living habits. Although our study suggests only an association between climate and women's health, a recent study reports that a lower BBT can support a slower rate of aging.4 Such findings raise an alarm that recent global warming and environmental changes throughout the world may have effects on human health and longevity.
Several smartphone application–based studies have recently reported descriptive statistics for menstrual cycle length and BBT.35–37 However, these studies had major limitations in terms of relatively small numbers of cycle data and limited numbers of cycles per person (2.5–5 cycles per person). Application users in these studies were also biased to women who wanted to become pregnant, resulting in very limited generalizability. The nature of the application used in the current study, which has been used by the Japanese female population for both contraception and to become pregnant, allowed us to use data that were less prone to selection bias.
This study had some limitations. First, there could be selection bias associated with being an active application user. It was expected that females who were self-motivated and could input details into the application and those who were sexually active would be more likely to use this application. Second, most cycle data in our application lacked information on body size (ie, height and weight), resulting in decreased power to detect potential effects of body size on cycle length and BBT. Although larger studies are warranted to confirm our result that there is no relationship between body size and cycle length, this could be true considering the fact that height and weight are not involved in irregular menstrual cycles in PCOS.27 Third, we did not have data on ovulation; thus, our sample may have included cycles with anovulation as seen in females with PCOS, hypothalamic amenorrhea, or hyperprolactinemia. However, our subanalyses using cycle data restricted to 15–45 days also showed age-dependent changes in menstrual cycle length and the association between external temperature and BBT; thus, it is unlikely our principal findings were driven by such outliers. Similarly, it is possible that our sample included cycle data obtained from those taking oral contraceptive pills (OCPs). However, considering the very low proportion of OCP usage for contraception in the Japanese female population (1.0%),38 we believe the effect of data derived from OCP users would be minimal. Fourth, this study was conducted in a single country (Japan) with relatively uniform genetic background. Although it is suggested that age-dependent changes in BBT do not depend on ethnic variations,4 and age-dependent changes in menstrual cycle length similar to those in our study have been reported in a recent study that enrolled Caucasian participants,36 menstrual cycle length and BBT are known to vary by ethnicity.39,40 Thus, our findings on BBT should be tested in other areas and in other ethnicities to draw reliable conclusions.
Our study found that menstrual cycle length and BBT, both of which were previously considered to be stable, are associated with age and external environment. Additionally, our study shows how recording of serial health data may not only help the individual, but lead to better understanding of female reproductive health.
Footnotes
This work was partly supported by a grant from AMED-WISE (Project for Whole Implementation to Support and Ensure the Female Life; project code 19gk0210023s0401) to Naho Morisaki.
Financial Disclosure The authors did not report any potential conflicts of interest.
The authors thank Susan Furness, PhD, from Edanz Group for editing a draft of the manuscript.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B905.
Figure.
No available caption
REFERENCES
- 1.Buxton CL, Atkinson WB. Hormonal factors involved in the regulation of basal body temperature during the menstrual cycle and pregnancy. J Clin Endocrinol Metab 1948;8:544–9. [DOI] [PubMed] [Google Scholar]
- 2.Whitelaw MJ. Hormonal control of the basal body temperature pattern. Fertil Steril 1952;3:230–1. [DOI] [PubMed] [Google Scholar]
- 3.Siegler SL, Siegler AM. Evaluation of the basal body temperature; an analysis of 1012 basal body temperature recordings. Fertil Steril 1951;2:287–301. [DOI] [PubMed] [Google Scholar]
- 4.Simonsick EM, Meier HCS, Shaffer NC, Studenski SA, Ferrucci L. Basal body temperature as a biomarker of healthy aging. Age 2016;38:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly G. Body temperature variability (Part 1): a review of the history of body temperature and its variability due to site selection, biological rhythms, fitness, and aging. Altern Med Rev 2006;11:278–93. [PubMed] [Google Scholar]
- 6.Charkoudian N, Hart ECJ, Barnes JN, Joyner MJ. Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res 2017;27:149–55. [DOI] [PubMed] [Google Scholar]
- 7.de Mouzon J, Testart J, Lefevre B, Pouly JL, Frydman R. Time relationships between basal body temperature and ovulation or plasma progestins. Fertil Steril 1984;41:254–9. [DOI] [PubMed] [Google Scholar]
- 8.Moghissi KS. Accuracy of basal body temperature for ovulation detection. Fertil Steril 1976;27:1415–21. [PubMed] [Google Scholar]
- 9.Weinert D. Circadian temperature variation and ageing. Ageing Res Rev 2010;9:51–60. [DOI] [PubMed] [Google Scholar]
- 10.Horne JA, Coyne I. Seasonal changes in the circadian variation of oral temperature during wakefulness. Experientia 1975;31:1296–8. [DOI] [PubMed] [Google Scholar]
- 11.Cisse F, Martineaud R, Martineaud JP. Circadian cycles of central temperature in hot climate in man. Arch Int Physiol Biochim Biophys 1991;99:155–9. [DOI] [PubMed] [Google Scholar]
- 12.Statistics Bureau of Japan. Statistical handbook of Japan, 2018. Tokyo, Japan: Statistics Bureau, Ministry of Internal Affairs and Communications; 2018. [Google Scholar]
- 13.Japan Meteorological Agency. General information on climate of Japan. Tokyo, Japan: Japan Meteorological Agency; 2019. [Google Scholar]
- 14.Takeshima K, Saito H, Nakaza A, Kuwahara A, Ishihara O, Irahara M, et al. Efficacy, safety, and trends in assisted reproductive technology in Japan-analysis of four-year data from the national registry system. J Assist Reprod Genet 2014;31:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones KH, Ford DV. Population data science: advancing the safe use of population data for public benefit. Epidemiol Health 2018;40:e2018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohda S, Suzuki K, Igari I. Relationship between the menstrual cycle and timing of ovulation revealed by new protocols: analysis of data from a self-tracking health app. J Med Internet Res 2017;19:e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japan Meteorological Agency. Climate change monitoring report, 2016. Tokyo, Japan: Japan Meteorological Agency; 2016. [Google Scholar]
- 18.Japan Meteorological Agency. Climate change monitoring report, 2017.Tokyo, Japan: Japan Meteorological Agency; 2017. [Google Scholar]
- 19.Box GE, Cox D. An analysis of transformations. J R Stat Soc Ser B 1964;26:211–52. [Google Scholar]
- 20.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157:364–75. [DOI] [PubMed] [Google Scholar]
- 21.Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010;21:467–74. [DOI] [PubMed] [Google Scholar]
- 22.Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. Pregnancy and neonatal outcomes following letrozole use in frozen-thawed single embryo transfer cycles. Hum Reprod 2017;32:1244–8. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi T, Ishida E, Tatsumi K, Okada Y, Saito T, Kubota T, et al. Advanced paternal age alone does not adversely affect pregnancy or live-birth rates or sperm parameters following intrauterine insemination. Reprod Med Biol 2018;17:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiazze L Jr, Brayer FT, Macisco JJ Jr, Parker MP, Duffy BJ. The length and variability of the human menstrual cycle. JAMA 1968;203:377–80. [PubMed] [Google Scholar]
- 25.Chabbert Buffet N, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Front Neuroendocrinol 1998;19:151–86. [DOI] [PubMed] [Google Scholar]
- 26.Hickey M, Balen A. Menstrual disorders in adolescence: investigation and management. Hum Reprod Update 2003;9:493–504. [DOI] [PubMed] [Google Scholar]
- 27.Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 2000;15:24–8. [DOI] [PubMed] [Google Scholar]
- 28.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–84. [DOI] [PubMed] [Google Scholar]
- 29.Rudnicka E, Kruszewska J, Klicka K, Kowalczyk J, Grymowicz M, Skorska J, et al. Premature ovarian insufficiency—aetiopathology, epidemiology, and diagnostic evaluation. Menopause Rev 2018;17:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor KA, Holman DJ, Wood JW. Declining fecundity and ovarian ageing in natural fertility populations. Maturitas 1998;30:127–36. [DOI] [PubMed] [Google Scholar]
- 31.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000;320:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Practice Committee of the American Society for Reproductive Medicine. Current clinical irrelevance of luteal phase deficiency: a committee opinion. Fertil Steril 2015;103:e27–32. [DOI] [PubMed] [Google Scholar]
- 33.Ishigooka Y, Fukui S, Hasegawa T, Kuwagata T, Nishimori M, Kondo M. Large-scale evaluation of the effects of adaptation to climate change by shifting transplanting date on rice production and quality in Japan. J Agric Meteorol 2017;73:156–73. [Google Scholar]
- 34.Zhang Y, Li L, Wang H, Zhang Y, Wang N, Chen J. Land surface phenology of Northeast China during 2000-2015: temporal changes and relationships with climate changes. Environ Monit Assess 2017;189:531. [DOI] [PubMed] [Google Scholar]
- 35.Faust L, Bradley D, Landau E, Noddin K, Farland LV, Baron A, et al. Findings from a mobile application-based cohort are consistent with established knowledge of the menstrual cycle, fertile window, and conception. Fertil Steril 2019;112:450–7.e3. [DOI] [PubMed] [Google Scholar]
- 36.Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med 2019;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shilaih M, Goodale BM, Falco L, Kubler F, De Clerck V, Leeners B. Modern fertility awareness methods: wrist wearables capture the changes in temperature associated with the menstrual cycle. Biosci Rep 2018;38:BSR20171279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Sakamoto H, Leslie A, Takahashi O, Tsuboi S, Kitamura K. Contraception in Japan: current trends. Contraception 2016;93:475–7. [DOI] [PubMed] [Google Scholar]
- 39.Harlow SD, Campbell B, Lin X, Raz J. Ethnic differences in the length of the menstrual cycle during the postmenarcheal period. Am J Epidemiol 1997;146:572–80. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Ohnaka M, Shiraki K, Tsujita J, Yoshimura H, Saito N, et al. Comparison of physical characteristics, body temperature and basal metabolism between Thai and Japanese in a neutral temperature zone. Jpn J Physiol 1977;27:525–38. [DOI] [PubMed] [Google Scholar]