Supplemental Digital Content is available in the text.
Keywords: animals; femoral artery; ischemia; lower extremity; muscle, skeletal
Abstract
Objective:
There has been little success in translating preclinical studies of mouse hind limb ischemia into benefit for patients with peripheral artery disease. Using systematic strategies, we sought to define the injury and angiogenesis landscapes in mice subjected to hind limb ischemia and ascertain whether published studies to date have used an analysis strategy concordant with these data.
Approach and Results:
Maps of ischemic injury were generated from 22 different hind limb muscles and 33 muscle territories in 12-week-old C57BL/6 mice, based on loss or centralization of myofiber nuclei. Angiogenesis was similarly mapped based on CD (cluster of differentiation) 31–positive capillary content. Only 10 of 33 muscle territories displayed consistent muscle injury, with the distal anterior hind limb muscles most reliably injured. Angiogenesis was patchy and exclusively associated with zones of regenerated muscle (central nuclei). Angiogenesis was not observed in normal appearing muscle, necrotic muscle, or injury border zones. Systematic review of mouse hind limb angiogenesis studies identified 5147 unique publications, of which 509 met eligibility criteria for analysis. Only 7% of these analyzed manuscripts evaluated angiogenesis in distal anterior hind limb muscles and only 15% consistently examined for angiogenesis in zones of muscle regeneration.
Conclusions:
In 12-week C57BL/6 mice, angiogenesis postfemoral artery excision proceeds exclusively in zones of muscle regeneration. Only a minority of studies to date have analyzed angiogenesis in regions of demonstrably regenerating muscle or in high-likelihood territories. Quality assurance standards, informed by the atlas and mapping data herein, could augment data reliability and potentially help translate mouse hind limb ischemia studies to patient care.
Highlights.
Despite preclinical successes in the mouse hind limb ischemia model of peripheral vascular disease, no proangiogenesis interventions have proven to benefit patients.
Full hind limb mapping in C57BL/6 mice subjected to femoral artery excision revealed that skeletal muscle injury and angiogenesis were inconsistent and regionally variable, with the anterior distal hind limb showing the most consistent and uniform responses.
Angiogenesis in the hind limb skeletal muscle occurred exclusively in zones of muscle regeneration, not in border zones and not in noninjured muscle.
A systematic review of the current published literature revealed that only a small minority of studies have analyzed angiogenesis in demonstrably regenerating muscle or high-likelihood zones. Quality assurance strategies, based on the principles discerned herein, could augment the reliability of this widely used animal model and its translational utility.
See cover image
Peripheral vascular disease is a worldwide health burden.1,2 Its most serious manifestation, critical limb ischemia, carries high risks of limb amputation and death, despite advances in percutaneous and surgical therapies.3,4 Accordingly, treatment innovations for peripheral artery disease are actively investigated, including strategies to augment angiogenesis.
A common approach to investigating ischemia-induced angiogenesis is a mouse model of hind limb ischemia. In 1998, Couffinhal et al5 reported such a model wherein blood flow down the femoral artery was surgically halted, leading to neovascularization of the hind limb skeletal muscles. This experimental system has since been adopted widely to study ischemia-induced angiogenesis and evaluate proangiogenesis therapies.6,7 Drugs, hormones, growth factors, progenitor cells, and biomaterials have been tested, many with positive findings.8–13 This, in turn, has informed paradigms for patient intervention.6,14,15 However, despite preclinical successes in the mouse, no proangiogenesis interventions have proven to benefit patients with peripheral vascular disease.14,16–20 This model-patient discordance constitutes an example of a translational valley of death.21,22
There are several potential explanations for the discordance between the preclinical and clinical outcomes. These include the older age and coexisting chronic diseases of individuals with peripheral artery disease,23,24 challenges in scaling local delivery strategies for humans,6,25 insufficient inclusion of female mice, and the fact that new microvessels may not acquire the necessary complement of vasomotor functions.26–28
In addition to these considerations, it must also be recognized that the histological evaluation required to assess angiogenesis could, itself, compromise data reliability. Angiogenesis is a microscopic process, yet the skeletal muscle territories in the hind limb are vast.29,30 This challenges accurate data extraction, particularly with regional heterogeneity in angiogenesis, as may arise from different skeletal muscle metabolic subtypes31,32 and variable collateral responses.33 Different mouse strains and different interventions used to induce ischemia can further complicate the sampling-based evaluation strategy.34,35 Appropriately selecting microscopic territories from the immense muscle landscape is critical not only for consistent results but also accurate conclusions. For example, inadvertent mismatching of muscle zones for treatment and control intervention groups could lead to concluding the existence of a treatment effect where none exists. Given these concerns, it is noteworthy that a comprehensive understanding of where angiogenesis proceeds in the ischemic hind limb, and its relationship to skeletal muscle pathobiology, is lacking.
Herein, we have undertaken a hybrid, experimental-evaluative investigation of the vulnerabilities in a widely used preclinical mouse model of limb ischemia, using systematic approaches. First, we generated a detailed atlas of muscle injury zones and angiogenesis zones across 22 hind limb muscles of C57BL/6 mice subjected to femoral artery excision. Second, we undertook an unbiased, systematic review of 509 manuscripts, criteria selected from 5147 nonduplicate publications of mouse hind limb ischemia. From these manuscripts, we ascertained the extent to which published investigations used an analysis approach concordant with the mapping data. The findings uncover substantial data risks for mouse hind limb ischemia studies and provide a quality assurance approach that could strengthen the value of this preclinical animal model.
Materials and Methods
The data and methods that support the findings of this study are available from the corresponding author on reasonable request.
Mouse Model of Hind Limb Ischemia
Experiments were conducted in accordance with the University of Western Ontario Animal Care and Use Subcommittee, which follows the policies set out by the Canadian Council on Animal Care. This study was also conducted in accordance with the Animal Research: Reporting In Vivo Experiments guidelines for transparent reporting of animal research36 and the 2018 consensus guidelines for the use and interpretation of angiogenesis assays.23 C57BL/6J (Jackson Laboratories, Bar Harbor, ME) and C57BL/6N (Charles River Laboratories, Wilmington, MA) mice 12 weeks of age were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally. Male mice were studied to reflect the currently used methodologies in the literature and recognizing their robust angiogenic response.37 Hind limb ischemia was induced by ligating the right femoral artery above and below the profunda femoris branch, recently retermed the proximal caudal femoral artery,30 using 6-0 silk sutures and excising the intervening 2- to 3-mm portion of artery.27,35 All hind limb muscles were harvested after 10 days, a time point in which capillary regeneration is known to be robust, and after 28 days, when the process has stabilized.26 Mice were housed in clear, plastic cages containing standard bedding and maintained on a 12-hour light and dark cycle at 23°C and 50% relative humidity. Mice were fed water and normal mouse chow diet (Diet 2018; Harlan Teklad, Madison, WI), which were available ad libitum.
Laser Speckle Contrast Imaging Flow Analysis
Bulk blood flow to the distal hind limb was assessed using laser speckle contrast imaging (moorFLPI-2; Moore Instruments, United Kingdom). Relative limb perfusion was measured pre- and post-ischemia using regions of interest confined to the plantar soles, as reported previously.35 Mice were lightly anesthetized using isofluorane (1.3%–1.5% mixed with 100% oxygen at a rate of 0.8 L/min) and placed on a heating pad, covered by a black mat provided by the manufacturer, to maintain a body temperature of 36.5±0.5°C. The mice were acclimatized to the anesthetic and heating pad for 5 to 7 minutes before imaging and flow data collected according to manufacturer instructions. Image settings were set to Temporal Measure Mode with a Temporal Filter of 250 frames. Postprocessing of images and data extraction were performed using moorFLPI Review V5.0 software (Moore Instruments). Results are expressed as the ratio of perfusion in the ischemic (right) limb versus the nonischemic (left) limb. Mice with postoperative day 0 perfusion ratios that did not decline to 30% of baseline or lower were excluded, in line with currently accepted standards35 and allowing for the higher spatial resolution of laser speckle contrast imaging compared with laser Doppler imaging.
Tissue Preparation
For tissue harvesting, mice were subjected to isofluorane overdose and perfused sequentially with PBS and 4% paraformaldehyde via left ventricular cannulation at physiological pressure. Whole left and right hind limbs were dissected, immersed in 4% paraformaldehyde overnight, and then decalcified by immersing in 6.5% EDTA solution (pH 7.0) for 10 days.26 Each hind limb was embedded in paraffin and sectioned at 3 defined sites: the proximal hind limb (2 mm beyond the distal suture at the femoral artery excision site), the mid-hind limb (2 mm superior to the mid-knee joint line), and the distal hind limb (the widest muscle portion of the below-knee tissue region).
Histological Analysis—Ischemic Injury Mapping
Five-µm-thick full cross sections of hind limbs were stained for hematoxylin and eosin. Entire cross sections were digitally scanned using a Leica Aperio AT2 Digital Pathology Slide Scanner (Leica Biosystems, Germany) with 40× objective engaged. Skeletal muscle injury areas were identified for all muscles, categorized as either injury-necrosis or injury-regeneration, based on disrupted myofibers devoid of nuclei or regenerated myofibers with centralized nuclei, respectively.38–40 Areas were quantified using ImageJ (National Institutes of Health) and Aperio ImageScope (version 12.3.2.8013; Leica Biosystems). Maps were created based on the locations and the size (area) of the injured territory within a given section (Figure 1). Areas were averaged from 9 C57BL/6J mice at day 10 and 5 C57BL/6J mice at day 28. A validation cohort of 5 C57BL/6N mice harvested at day 10 was similarly analyzed. A second validation cohort of 32 inbred C57BL/6J male mice also subjected femoral artery excision was analyzed, evaluating injury maps specifically in the tibialis anterior and distal gastrocnemius muscles.
Figure 1.
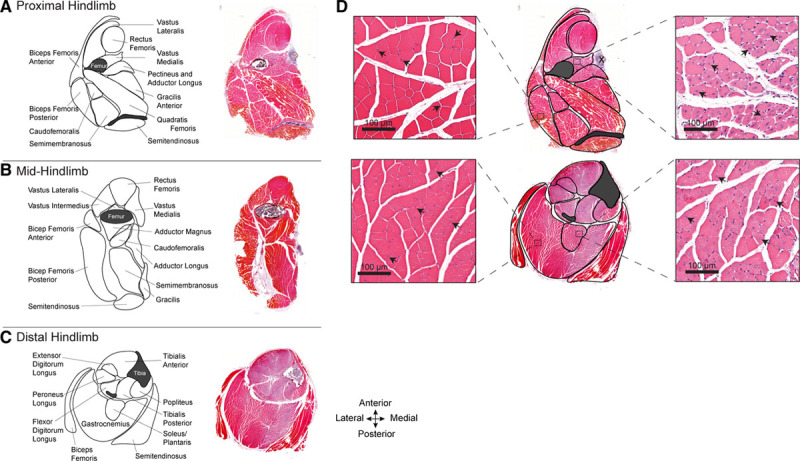
Atlas of the hind limb skeletal muscles of C57BL/6J mice subjected to femoral artery excision. A–C, Maps depicting the skeletal muscles in the proximal (A), mid- (B), and distal mouse hind limb (C). Adjacent to each map is a corresponding hematoxylin and eosin–stained full cross section of the hind limb, 10 d after femoral artery excision. Varying intensities of muscle eosinophilia can be seen; less intense staining is present in territories of ischemic injury. D, High-magnification images, corresponding to the outlined zones within the proximal and distal hind limb, depicting normal (left) and injured (right) regions of the same muscle sections. The uninjured regions have peripheral myofiber nuclei (arrowheads). The injured/regenerating regions have pale myofibers with centralized nuclei (arrowheads).
Histological Analysis—Angiogenesis Mapping
The skeletal muscle vasculature was evaluated in sections near-adjacent to those assessed above by immunostaining for CD (cluster of differentiation) 31–positive endothelial cells. Five-micrometer sections were subjected to antigen retrieval (sodium citrate 0.01 M, pH 6.0 [Sigma], and heating with pressure in a 2100 Retriever device [Prestige Medical]) and immunostained using rat monoclonal anti-mouse CD31 antibody (1:20, Clone SZ31; Dianova) and, in some instances, with rabbit monoclonal anti-mouse Ki-67 antibody (1:400; Abcam). Bound primary antibody was detected using Alexa Fluor-488 conjugated goat anti-rat IgG (1:100; Thermo Fisher) and Alexa Fluor-594 conjugated goat anti-rabbit IgG (1:100; Thermo Fisher; Major Resources Table in the Data Supplement). Nuclei were visualized with DAPI Fluoromount-G (SouthernBiotech; 0100-20). Fluorescence imaging was undertaken by widefield microscopy (Olympus BX-51) using the Northern Eclipse image acquisition and analysis software (EMPIX Imaging, Inc) and the Leica Aperio VERSA Digital Pathology Slide Scanner (Leica Biosystems).
Capillary densities for all muscles were quantified in 5 equally spaced high-powered fields per muscle territory, using ImageJ (National Institutes of Health) or Aperio ImageScope. Capillary densities were quantified within the injury zone, border zone, and uninjured zone. The border zone was defined as a single high-powered field of view (225-µm wide) of myofibers with peripheral nuclei, directly adjacent to the myofiber zone with central nuclei. Angiogenesis was deemed to have occurred if the capillary content was statistically significantly greater than that in the matched contralateral control muscle territory. Angiogenesis maps were generated based on this assessment.
Systematic Publication Review
A search of all publications related to C57BL/6 mouse hind limb ischemia was performed using Medline, Embase, BIOSIS, and Web of Science databases from their respective inceptions (1946, 1947, 1926, and 1900) to July 25, 2019. The initial search strategy was designed to be as inclusive as possible and used the following Medical Subject Headings: mice, ischemia, and hind limb. Subsequently applied exclusion criteria were as follows: abstract-only publications, non-English language publications, review articles, letters and commentaries, studies evaluating collaterogenesis or arteriogenesis rather than capillary-level angiogenesis, mouse models not using the C57BL/6 genetic background, ischemia induced by a surgical technique other than femoral artery excision, and studies that evaluated end points other than skeletal muscle angiogenesis. Non-English articles, review articles, and arteriogenesis articles were identified and excluded based on manuscript title and abstract review. The remaining exclusions were based on a full review of the manuscript and its methods. The final studies included English language peer-reviewed full manuscripts, with histological evaluations of angiogenesis in ischemic skeletal muscle following surgical excision of the femoral artery in C57BL/6 mice. The latter included mice receiving treatment interventions and transgenic mice backcrossed to a C57BL/6 background, to capture the breadth of the relevant studies.
All authors agreed on included and excluded studies. From each of the 509 publications, 3 investigators (J.J.L., H.Y., and J.-M.A.) extracted descriptive and methodologic data. This entailed (1) the year of publication, (2) the age and sex of the mice studied, (3) the vendor from which the mice were obtained and the C57BL/6 substrain, (4) whether flow interruption was confirmed by laser Doppler analyses, (5) the day postfemoral artery excision angiogenesis was histologically analyzed, (6) the specific muscle(s) evaluated for angiogenesis, (7) whether histological images were provided for control (no femoral artery excision) and injured (femoral artery excision) muscles, (8) whether central/internalized myofiber nuclei were evident in images of injured muscle from which angiogenesis was evaluated, and (9) the consistency of central nuclei among all images of injured muscles. There was a 99% agreement (506 of 509 articles) on the extracted information. A fourth investigator (J.G.P.) further evaluated the data, and consensus was achieved.
Statistics
Descriptive data are presented as mean±SD. Comparative data are presented as mean±SEM. Normal distributions were confirmed using D’Agostino and Pearson omnibus normality testing. Comparisons were made by 2 tailed t tests or ANOVA with Bonferonni post hoc test. Multiple t test corrections were performed using the false discovery rate approach with Q=5%.41 The 2-stage step-up method of Benjamini et al42 was used. Associations between the presence of angiogenesis and histological muscle injury status were tested using χ2 analysis.43 Data were analyzed using Prism 8 (version 8.4.2.679; GraphPad Software), and P<0.05 was considered significant unless otherwise stated.
Results
Femoral Artery Excision Produces Heterogeneous Ischemic Injury With Variable Consistency
To investigate the consistency and distribution of skeletal muscle injury following a strong vascular insult, 9 C57BL/6J mice were subjected to unilateral femoral artery ligation and excision. In all mice studied, the postprocedure ischemic to nonischemic hind limb perfusion ratio, determined by laser speckle contrast imaging, fell to at least 0.3 (0.21±0.05; Figure I in the Data Supplement). Decalcified hind limbs were harvested in toto, and full transverse sections across upper, mid, and distal hind limb territories were studied. This encompassed 33 distinct planes of 22 different muscles for a given mouse (Figure 1A through 1C). Myofibers within each cross section were evaluated, and subregions were categorized as (1) uninjured, (2) injured-regenerated (central nuclei), or (3) injured-necrotic (absent nuclei; Figure 1D). This analysis was undertaken on 9 mice harvested 10 days after injury and 5 mice harvested 28 days after injury, corresponding to 297 and 165 muscle territories, respectively.
The cross-sectional areas of uninjured, injured-regenerated, and injured-necrotic muscle zones for each cross-sectional plane for every mouse studied are presented in Tables I and II in the Data Supplement. Remarkably, for almost every mouse, there were more muscle territories with no evidence for muscle injury (normal myofiber morphology, no features of necrosis, regeneration, fibrosis, or inflammation) than with injury. On average, 14 of 33 muscle territories (±4.7) at day 10 displayed injury, and 14 of 33 territories (±7.7) on day 28 had injury zones. Interestingly, the inter-mouse variability in injury (evident in Tables I and II Data Supplement) was not related to differences in perfusion. Below the inclusion threshold perfusion ratio of 0.3, there was no correlation between perfusion ratio and the number of injured muscles (P=0.341; Figure IC in the Data Supplement).
Using the averaged site-specific data, we next generated an atlas of skeletal muscle injury, comprised of injury maps for the full transverse sections across upper, mid, and distal hind limb territories, 10 and 28 days after injury. Both the site and consistency of injury are depicted (Figure 2). In the upper hind limb, 10 days after femoral artery excision, a reproducible injury-regeneration response, defined as present in at least 67% of the mice studied, was found for only the vastus medialis, pectineus, and adductor longus muscles. In the mid-hind limb, muscle injury-regeneration was found but no muscle territory displayed reproducible (≥67% consistency) injury. In the distal hind limb, there was more widespread injury, with reproducible injury-regeneration evident in 8 muscles, namely the tibialis anterior, extensor digitorum longus, peroneus longus, flexor digitorum longus, tibialis posterior, popliteus, soleus, and gastrocnemius (Figure 2A). Foci of injury-necrosis could be seen within injury-regeneration zones, most prominently in the distal hind limb (Figure 2A).
Figure 2.
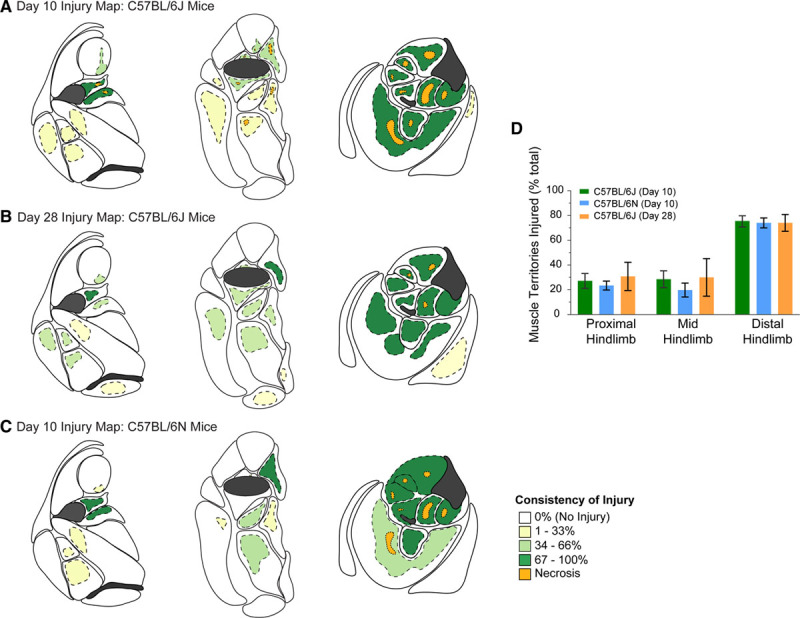
Hind limb injury maps for C57BL/6 mice subjected to femoral artery excision. A, Maps depicting the sites of muscle injury in proximal (left), mid- (middle), and distal (right) hind limb of C57BL/6J mice, 10 d after femoral artery excision. Areas of injury/regeneration are shaded yellow or green, with the respective consistency of injury denoted by the shade. Dark green zones are those with the most consistent injury/regeneration. Areas of injury/necrosis are shaded orange. Map data are based on n=9 mice. B, Maps depicting the sites and consistency of muscle injury in C57BL/6J mice, 28 d after femoral artery excision. Map data are based on n=5 mice. C, Maps depicting the sites and consistency of muscle injury, 10 d after femoral artery excision in the C57BL/6N substrain (Charles River). Map data are based on n=5 mice. D, Graph depicting the extent of muscle injury in each of the proximal, mid, and distal hind limb for the different cohorts of mice analyzed. The proportion of injured distal hind limb muscles is greater than that for the proximal or mid-hind limb (P<0.001) but, for a given hind limb zone, there are no differences among the mouse substrains or time of assessment post-injury (P=0.648). Data are mean±SEM. See Tables I through III in the Data Supplement for individual data values.
The injury maps of tissues harvested 28 days after surgery were similar for those of day 10. For the upper and mid-hind limb, consistent injury-regeneration was only evident for the vastus medialis muscle. In contrast, in the distal hind limb, the 8 muscles consistently showing injury-regeneration on day 10 also consistently displayed injury-regeneration on day 28. Necrotic foci were also seen at day 28, but these zones were less prominent than on day 10.
The injury zones in the distal hind limb occupied all or all but the outer muscle edges of a given muscle, at both 10 and 28 days after femoral artery excision. The exception to this was the gastrocnemius muscle, where much of the gastrocnemius muscle remained uninjured and only deeper regions showed injury-regeneration (Figure 2A and 2B).
Ischemic Injury Profiles in Validation Cohorts
To determine whether the foregoing injury profiles held with C57BL/6 mice from a different vendor, we undertook the same assessment on 5 mice obtained from Charles River Laboratories. These mice are a substrain of C57BL/6 mice (C57BL/6N) that have genomic and phenotype differences with C57BL/6J mice.44 Interestingly, the 10-day injury response to femoral artery excision in C57BL/6N was highly similar to that of C57BL/6J mice, in terms of both the site and consistency of muscle injury (Table III in the Data Supplement; Figure 2C). The distal hind limb muscles were again the most consistently injured, with the distal anterior hind limb muscles, in particular, almost uniformly injured. We also established that, for a given hind limb zone, the overall proportion of muscle territories displaying injury was no different among C57BL/6J mice harvested at 10 days, C57BL/6N mice harvested at 10 days, and C57BL/6J mice harvested at 28 days (P=0.648; Figure 2D).
We also assessed a second validation cohort of 32 inbred C57BL/6J male mice, examining the responses specifically in the tibialis anterior muscle and the gastrocnemius muscle, 14 (n=21) and 28 (n=11) days after femoral artery excision. Both muscles displayed injury in 30 of 32 mice. However, the average relative muscle area occupied by injury-regenerated myofibers in the tibialis anterior muscle was 93.8±4.3%, whereas in the gastrocnemius muscle, this was only 66.0±5.9% (P<0.0001).
Together, these quantitative mapping data reveal that in C57BL/6 mice subjected to femoral artery excision, most muscles display either no features of injury or are partially and inconsistently injured. Consistent and relatively widespread injury can, however, be found in the distal anterior hind limb.
Angiogenesis Is Regional and Observed in Zones of Injured-Regenerating Muscle
We next quantified angiogenesis in the hind limb muscles of the mice subjected to femoral artery excision. Angiogenesis was defined as a statistically significant increase in capillary density, based on CD31 immunostaining, relative to the matched site of the contralateral hind limb. This assessment was made in regions of uninjured muscle, injured-regenerated muscle, and injured-necrotic muscle.
As depicted in Figure 3, angiogenesis was evident within injured-regenerating skeletal muscle territories. On day 10, all but 1 of 25 territories that displayed injury, across the 3 hind limb planes, showed an angiogenesis response. On day 28, 20 of 26 injury zones had statistical evidence for angiogenesis (Figure 3B). The average 28-day capillary density in the injured-regenerating zones was found to be less than that on day 10 (1362/mm2 versus 1034/mm2; P<0.0001), suggesting pruning of the neocapillaries (Figure 3D). Notably, there was no significant increase in capillary density in any of the noninjured muscle territories on either day 10 or 28. Also, in most of the injured-necrotic territories, there was a statistically significant decrease in capillary density, relative to the contralateral muscle territory.
Figure 3.
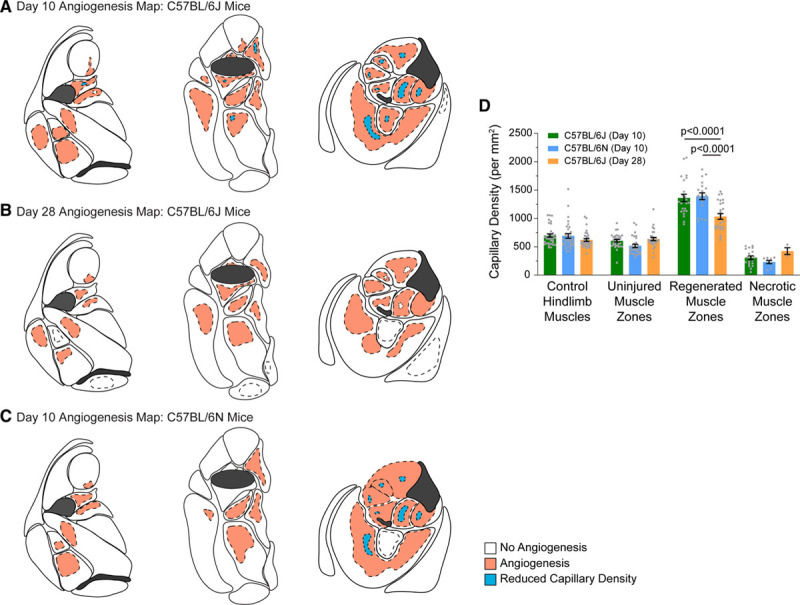
Hind limb angiogenesis maps for C57BL/6 mice subjected to femoral artery excision. A, Maps depicting the sites of angiogenesis and capillary loss in proximal (left), mid- (middle), and distal (right) hind limb of C57BL/6J mice, 10 d after femoral artery excision. Angiogenesis and capillary loss were determined based on a statistically significant increase or decrease in capillary density, relative to the corresponding region in the contralateral control muscle, as determined by immunostaining for CD (cluster of differentiation) 31. Injured/regenerating and injured/necrotic zones are overlaid in dashed lines. Dashed lines surrounding unshaded areas correspond to territories of muscle injury in which a statistically significant increase in capillary density was not found. Map data are based on n=9 mice. B, Maps depicting the sites of angiogenesis and capillary loss in the hind limb in C57BL/6J mice, 28 d after femoral artery excision. n=5 mice. C, Maps depicting the sites of angiogenesis and capillary loss in the hind limb in C57BL/6N mice, 10 d after femoral artery excision. Map data are based on n=5 mice. D, Graph of capillary densities in histologically defined muscle zones for the different cohorts of mice analyzed (mean±SEM).
The finding of angiogenesis in injured-regenerating zones but not in uninjured zones or injured-necrotic zones was also observed in the C57BL/6N validation cohort (Figure 3C).
Angiogenesis Is Exclusive to Injured-Regenerating Muscle Zones
Because much of the hind limb was not injured after femoral artery excision, we considered the possibility that increases in capillaries in at least parts of the uninjured territories could be missed. For example, angiogenesis might proceed in an injury border zone, recognizing that such a site could be ischemic but without overt muscle injury. To test this possibility, we quantified the capillary density in a 225-µm zone directly adjacent to those injury-regenerated regions around which a border zone could be delineated. As depicted in Figure 4, none of 14 border zones were found to have quantitative evidence for angiogenesis. When analyzed together with injured-regenerated zones, injured-necrotic zones, and uninjured zones, contingency analysis revealed an unequivocal and exclusive relationship between angiogenesis and injured-regenerated muscle zones (P=0.0001; Figure 4A).
Figure 4.
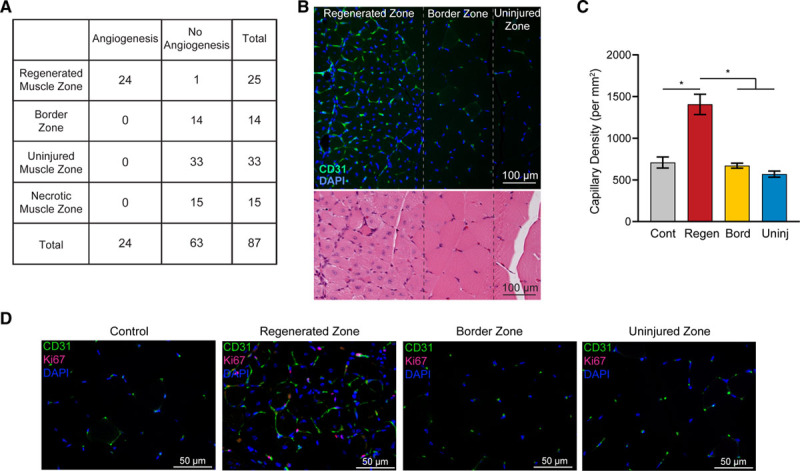
Angiogenesis following femoral artery excision occurs exclusively in injured/regenerating (Regen) muscle zones. A, Contingency table relating the presence of angiogenesis with histologically defined muscle zones. Data from a total of 87 zones from 9 C57BL/6J mice subjected to femoral artery excision and harvested 10 d later are depicted. P<0.0001. B, Micrographs of gastrocnemius muscle sections from a C57BL/6J mouse subjected to hind limb ischemia and harvested 10 d later, depicting the transition from injured to noninjured muscle. Top, Capillary content, based on immunostaining for CD (cluster of differentiation) 31 (green) with 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (blue). Bottom, Near-adjacent section stained with hematoxylin and eosin, illustrating the abrupt transition from centralized to peripheral myofiber nuclei. C, Capillary densities in defined muscle zones of the injured gastrocnemius muscle. *P<0.0001. D, Fluorescence micrographs of regions in the gastrocnemius muscle 10 d after surgery, immunostained for CD31 (green) and Ki-67 (pink). Nuclei are visualized with DAPI. Bord indicates border zone; Cont, control; and Uninj, uninjured zone.
This exclusivity was particularly apparent in relatively large muscles where both injured and uninjured territories were present in similar proportions, such as the gastrocnemius muscle. As depicted in Figure 4B, there is a clear demarcation of the edge of angiogenesis zone, and this edge is found precisely at the edge of injury-regeneration zone, that is, the transition from central to peripheral myofiber nuclei. This abrupt spatial transition in angiogenesis was confirmed by quantifying capillary densities of the respective adjacent zones (Figure 4C). As well, colabeling with CD31 and Ki-67 showed no evidence of proliferating endothelial cells in the border zone, despite its abundance in the injury-regenerated zone (Figure 4D).
Systematic Review: Widely Diverse Muscle Regions Selected for Angiogenesis Assessment
The foregoing data revealed that (1) there is a tight and exclusive linkage between skeletal muscle injury-regeneration and skeletal muscle angiogenesis and (2) only a subset of hind limb muscles consistently undergo injury and regeneration after femoral artery excision. Given these results, we next determined the extent to which published mouse hind limb angiogenesis studies undertook a strategy concordant with the findings. Multidatabase structured searching yielded 5147 unique articles from a total of 11 886 article titles generated from the Medical Subject Headings key word search of 4 databases (Figure 5). Application of exclusion criteria identified 509 articles, all of which studied angiogenesis in hind limb skeletal muscle following femoral artery excision in C57BL/6 mice (Table IV in the Data Supplement).
Figure 5.
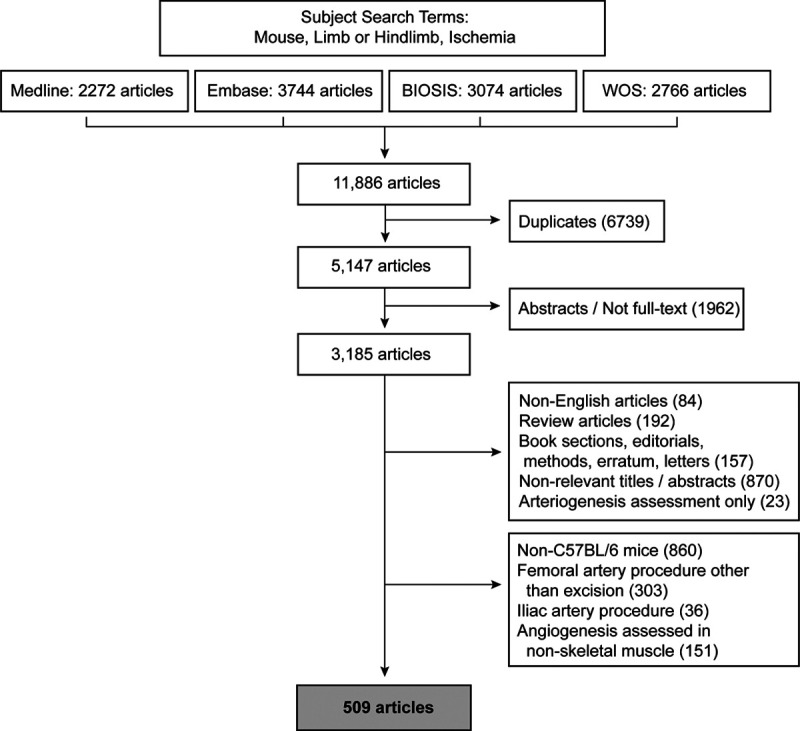
Systematic literature search strategy and manuscript yield. Flowchart indicating Medical Subject Headings search terms, exclusions, and resulting yield of studies included for analysis. WOS indicates Web of Science.
The earliest of these 509 studies was the 1998 report by Couffinhal et al5 that introduced the mouse hind limb femoral artery occlusion model, providing a measure of internal validity to the search strategy. As noted, all studies undertook a femoral artery excision procedure, and most studies used male mice (Figure 6A). Most (88%) studies performed perfusion analyses using a laser Doppler technique. As per the selection criteria, all studies used C57BL/6 mice, and 35% were reported as being obtained from either The Jackson Laboratory or Charles River Laboratories (Figure 6B). The median age of mice was 10 weeks (interquartile range, 8–12 weeks). Most mice were histologically analyzed for angiogenesis between 20 and 29 days after femoral artery excision (Figure 6C).
Figure 6.
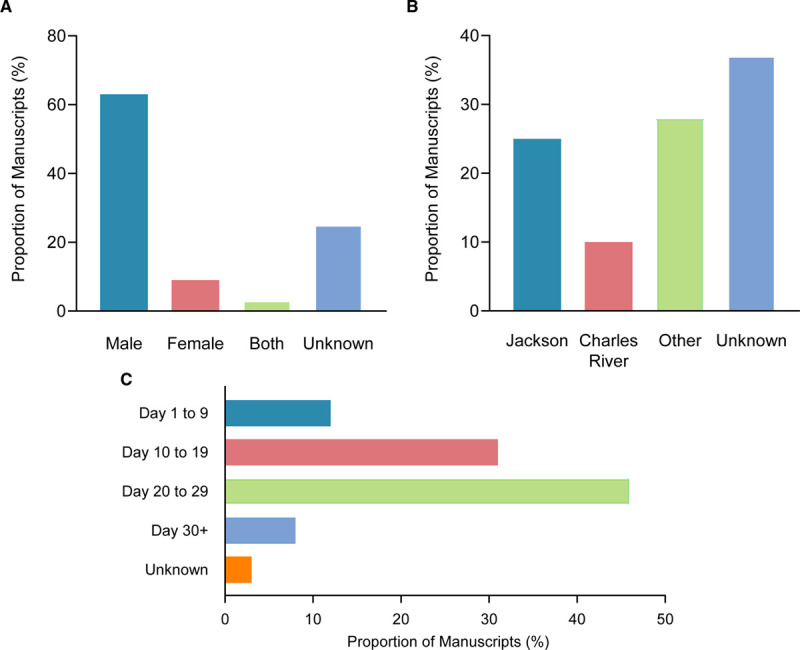
Methodological metrics in published literature evaluating angiogenesis following hind limb ischemia. A, Graph showing the distribution of manuscripts based on the sex of mice used for angiogenesis assessment. B, Graph showing the distribution of manuscripts based on the vendor from which mice were procured. C, Distribution of manuscripts according to the time points at which angiogenesis analysis following femoral artery excision was undertaken.
The specific skeletal muscles histologically analyzed for angiogenesis are depicted in Figure 7. Over half of all manuscripts evaluated angiogenesis in the gastrocnemius muscle (194 of 509, 38%) or the adductor muscle bundle (29%). In the current study, these muscles were inconsistently injured and, when injured, the damage was localized. In contrast, the tibialis anterior and extensor digitorum longus muscles, found in the mapping data to be consistently injured and angiogenic, were an analysis site in only 7% of manuscripts. Also notable was that 20% of studies did not specify which muscles were analyzed for angiogenesis.
Figure 7.
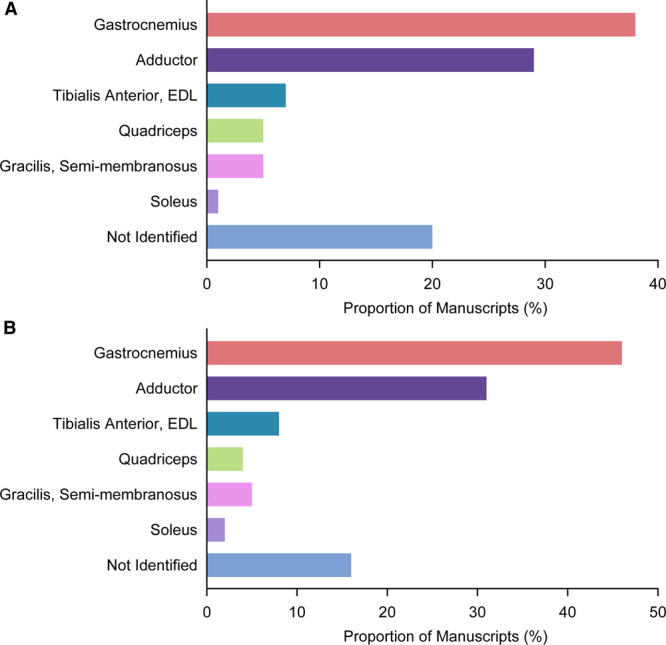
Breakdown of muscles analyzed in the published literature evaluating postischemia angiogenesis. A, Graph showing the prevalence of the specific muscles used for histological angiogenesis evaluation and quantitation, among all manuscripts analyzed (n=509 manuscripts). B, Graph showing the prevalence of the specific muscles used for histological angiogenesis evaluation, after author-level adjustment to account for multiple manuscripts from the same research group (n=283 manuscripts). EDL indicates extensor digitorum longus.
To control for any bias that might be introduced by there being multiple studies from the same research group, we repeated the analysis using only the most recently published article from those with the same senior author. This entailed 283 author-unique articles. The distribution of specific muscles analyzed was not demonstrably altered by this author-level adjustment (Figure 7B).
Systematic Review: Dissociation Between Zones of Injury and Zones of Angiogenesis Analysis
We next ascertained whether angiogenesis quantification was undertaken at sites of injured-regenerated muscle zones. For this, we evaluated the manuscript figures and ascertained whether central nuclei were evident in the histology images of postfemoral artery excision muscle. Most of the 509 studies (94%) displayed hind limb histology images in the main article or the supplemental material. However, unequivocal evidence for central nuclei in at least one of the postinjury hind limb images was present in only 40% of manuscripts and in 43% of those showing muscle histology. The remaining majority of images showed myofibers with exclusively peripheral nuclei or images where the nuclear location could not be interpreted, either because of suboptimal image quality or the lack of a nuclear stain. Furthermore, in only 15% of all articles, was there a consistent depiction of central nuclei in all of the postinjury hind limb images shown (Figure 8A). Author-level adjustment did not impact these findings (Figure 8A).
Figure 8.
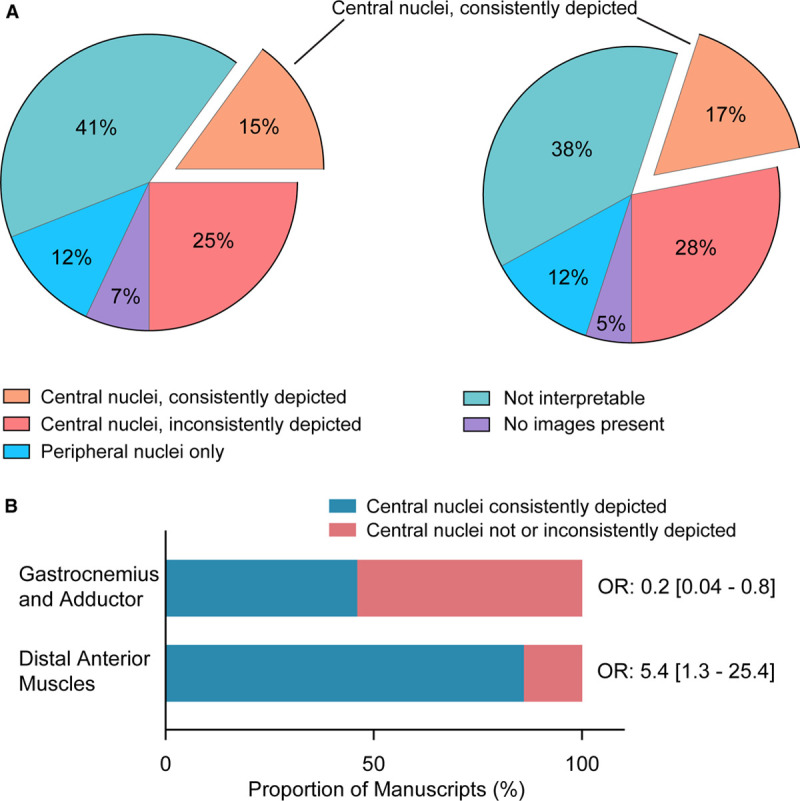
Analysis of injured muscle zones depicted in the published literature evaluating postischemia angiogenesis. A, Pie charts showing the distribution of manuscripts based on the state of the skeletal myofibers evaluated for postinjury angiogenesis, as depicted in the representative histology images. Chart on the left is for all 509 manuscripts; chart on the right is for the 283 unique senior author manuscripts. B, Depiction of the probability of there being centralized nuclei in histological images of tissues from mice subjected to femoral artery excision, depending on the specific muscles analyzed. Data on right indicate the odds ratio (OR) and 95% CIs for there being central nuclei present in the images.
We also ascertained whether the specific muscle analyzed for angiogenesis was related to the likelihood of there being centralized nuclei in images. This proved to be the case. In those minority of manuscripts that studied distal anterior hind limb muscles, the probability of there being centralized nuclei was high (odds ratio, 5.6 [95% CI, 1.3–25.4]; Figure 8B). In contrast, those manuscripts that studied angiogenesis in either gastrocnemius and adductor muscles were likely not to show centralized nuclei (odds ratio, 0.2 [95% CI, 0.04–0.8]; Figure 8B).
Collectively, this body of curated manuscript data points to a common discordance between the site of angiogenesis analysis and the probable site of angiogenesis.
Discussion
We have undertaken a unique, hybrid investigation of the widely used mouse model of ischemia-induced hind limb angiogenesis. This entailed generating a comprehensive atlas of muscle injury and angiogenesis and combining this with a systematic review of the published literature. We used the mapping data to inform a methodological analysis of this large body of manuscripts. In so doing, we have identified elemental vulnerabilities in the mouse hind limb ischemia model that could hinder data reliability and, potentially, clinical translation.
Systematic mapping of the muscle injury and angiogenesis landscapes was enabled by evaluating full transverse sections across the decalcified hind limb at upper, mid, and distal levels. This assessment revealed that, despite pronounced loss of perfusion induced by femoral artery excision, skeletal muscle injury was often inconsistent and always regionalized. Regional variability was evident among different muscles, but also within a given muscle, including within the same cross-sectional plane. We also established that all regions of muscle throughout the injured hind limb could be classified as being in 1 of 3 states—uninjured, necrotic, or regenerating. This proved to be critically important for the study of angiogenesis. Uninjured muscle, which constituted most of the hind limb, showed no angiogenesis whatsoever. Necrotic muscle, found as small internal zones in select muscles, displayed a reduction in capillary count. Postinjury regenerated muscle, and only those zones, displayed angiogenesis. These conclusions were based on the assessment of 691 hind limb muscle territories from 51 C57BL/6 mice. Moreover, the angiogenesis-regenerated muscle relationship existed for tissues harvested both 10 and 28 days after femoral artery excision and in mice obtained from 2 different vendors.
Our finding of an irrevocable linkage between angiogenesis and postischemic regenerating muscle has important implications for the assessment of angiogenesis. Because the majority of the hind limb muscle was not regenerating, there is a risk of harvesting tissue and evaluating angiogenesis in a territory of muscle that will not have it. As well, given the inconsistency and spatial heterogeneity of angiogenesis, there is a risk of inadvertently mismatching tissue regions harvested for control and treatment interventions. Importantly, our mapping data provide 2 strategies with which to mitigate these risks. First, central myofiber nuclei, which denote regenerating skeletal muscle in injured mice,38 provide a natural strategy for identifying a suitable zone for angiogenesis analysis. Second, the hind limb atlas established that the distal anterior muscles of C57BL/6 mice have a high likelihood of injury and angiogenesis, affording a strategy for site reliability.
The systematic manuscript review revealed that the identified data acquisition vulnerabilities in the mouse model are vulnerabilities that can play out in published studies. We reviewed 509 manuscripts that studied hind limb angiogenesis in C57BL/6 mice subjected to femoral artery excision, conditions similar to those for generating the injury and angiogenesis maps. Remarkably, only 15% of manuscripts consistently assessed angiogenesis in regions of muscle that displayed regeneration, as indicated by the presence of central nuclei in the corresponding images. Furthermore, only 7% of the analyzed manuscripts evaluated angiogenesis in muscles within the distal anterior hind limb, that is, those with the greatest likelihood of being injured and undergoing angiogenesis. Although we cannot definitively link the sites analyzed with the capillary content results, the disparities with the mapping data highlight the potential for reproducibility challenges and possibly inaccurate conclusions. For example, if the site used to assess a test intervention was a regenerating zone but the site used to assess the control intervention was not, this could lead to reporting a proangiogenesis effect of the intervention where one actually does not exist (type I error). Conversely, if each territory used to assess the control and test interventions were not in fact subjected to ischemic damage, an angiogenesis effect could be missed (type II error). These are theoretical scenarios that arise from our data analysis but also tangible risks given the observed heterogeneity within the vast muscle landscape and the sampling inherent in the microscopic assessment of angiogenesis.
The systematic review established that the most commonly used muscles for angiogenesis analysis have been the gastrocnemius muscle and adductor muscle groups. This is not surprising as these are 2 large and easily accessible hind limb muscle tissues.35,45,46 It is notable, therefore, that these muscles were also among those that underwent variable and focal injury and angiogenesis. Why the gastrocnemius muscle—a distal posterior hind limb muscle—was less reliably impacted than the distal anterior muscles is unknown but could reflect collateral network differences.30 Evaluating the most distal aspect of the gastrocnemius muscle has been recommended and may lessen the risk.35 We propose that an analysis strategy that is (1) oriented around centralized nuclei and (2) takes into account any residual zones of necrosis would be important to optimize the value of this muscle. In fact, our data imply that this strategy should hold for any muscle studied, including both oxidative and glycolytic.
Approaches to enhancing the reliability of angiogenesis assessments have recently emerged. A seminal methods report on mouse hind limb ischemia has been published,35 as well as technical variations.47 An expert-guided consensus document on the use and interpretation of several angiogenesis assays has also been published.23 To our knowledge, the current study is the first to map all injury and all angiogenesis zones in the entire hind limb and the first to employ a systematic manuscript review algorithm in the basic angiogenesis field. Systematic reviews are uncommon for basic and preclinical research topics, although recognition of their value is emerging.48–50 Our results add to a growing appreciation of the need for quality assurance in preclinical studies, and they provide a precision-based framework for optimizing the analysis of the injured mouse hind limb.
We restricted our de novo mapping study to young and otherwise healthy mice. Older mice can be expected to show more injury, and the angiogenesis responses could be different.23,24,27 We also only mapped injury and angiogenesis in male mice, to align the data with existing literature. Importantly, our systematic manuscript review quantified the experimental sex bias in the field, establishing that only 10% of manuscripts assessed female mice. Reduced flow recovery has been reported in female mice,37 and it will be important to systematically assess for sex differences in the angiogenesis landscapes. We note also that arteriogenesis responses and the state of collateral vessels are important determinants of the response to ischemia that we have not investigated.33,47,51 Like angiogenesis, these vascular responses are also challenging to quantify, and we propose that the current hybrid mapping-evaluative study of angiogenesis could inform a similar evaluation of the complex feeder vessel response to ischemia.
We also limited the injury and angiogenesis mapping to two commonly used C57BL/6 mouse substrains. Other mouse strains can be expected to have different responses, as will mice subjected to superimposed metabolic and inflammatory challenges.34,52 The latter may better reflect the human peripheral artery disease scenario. That said, we propose that intramouse delineation of skeletal muscle territories as either normal, necrotic, or regenerating, and focusing the angiogenesis assessment accordingly, could be a quality assurance tool that holds across mouse strains, vascular risks, the metabolic phenotype of specific muscles, and modes of inducing ischemia.
Finally, it will be important to ascertain whether the identified linkage between the state of the skeletal muscle and angiogenesis also holds for humans with peripheral artery disease. In this regard, we recently identified central skeletal muscle nuclei and evidence for angiogenesis in tibialis anterior muscle samples in patients with critical limb ischemia.53
In summary, the mouse hind limb ischemia model is an investigative mainstay for exploring therapeutic innovations. However, we have established that evaluating angiogenesis in this model carries substantial data risks. Developing quality assurance parameters, based, in part, on the mapping and review data herein, could augment data reliability and potentially help translate mouse hind limb ischemia studies to patient care.
Acknowledgments
We thank Demetri Pananos, Department of Biostatistics and Epidemiology, Western University, for statistical support.
Sources of Funding
This work was supported by the Canadian Institutes of Health Research (FDN-143326) to J.G. Pickering. J.J. Lee is supported by the Canadian Institutes of Health Research Vanier Scholarship and the Clinician Investigator Program at Western University. J.G. Pickering holds the Heart and Stroke Foundation of Ontario/Barnett-Ivey Chair.
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- CD
- cluster of differentiation
For Sources of Funding and Disclosures, see page 2466.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315028.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 3.Farber A. Chronic limb-threatening ischemia. N Engl J Med. 2018;379:171–180. doi: 10.1056/NEJMcp1709326 [DOI] [PubMed] [Google Scholar]
- 4.Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016;151:1070–1077. doi: 10.1001/jamasurg.2016.2018 [DOI] [PubMed] [Google Scholar]
- 5.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679 [PMC free article] [PubMed] [Google Scholar]
- 6.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–396. doi: 10.1038/nrcardio.2013.70 [DOI] [PubMed] [Google Scholar]
- 7.Vale PR, Isner JM, Rosenfield K. Therapeutic angiogenesis in critical limb and myocardial ischemia. J Interv Cardiol. 2001;14:511–528. doi: 10.1111/j.1540-8183.2001.tb00367.x [DOI] [PubMed] [Google Scholar]
- 8.Said SS, O’Neil C, Yin H, Nong Z, Pickering JG, Mequanint K. Concurrent and sustained delivery of FGF2 and FGF9 from electrospun poly(ester amide) Fibrous mats for therapeutic angiogenesis. Tissue Eng Part A. 2016;22:584–596. doi: 10.1089/ten.TEA.2015.0493 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Shibata R, Kito T, Yamamoto T, Ishii M, Nishio N, Ito S, Isobe K, Murohara T. Comparative angiogenic activities of induced pluripotent stem cells derived from young and old mice. PLoS One. 2012;7:e39562 doi: 10.1371/journal.pone.0039562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, Lévy BI. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res. 2003;93:114–123. doi: 10.1161/01.RES.0000081594.21764.44 [DOI] [PubMed] [Google Scholar]
- 11.Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, Nakagawa K, Hou X, Nagai Y, Hasegawa M, et al. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90:966–973. doi: 10.1161/01.res.0000019540.41697.60 [DOI] [PubMed] [Google Scholar]
- 12.Morishita R, Sakaki M, Yamamoto K, Iguchi S, Aoki M, Yamasaki K, Matsumoto K, Nakamura T, Lawn R, Ogihara T, et al. Impairment of collateral formation in lipoprotein(a) transgenic mice: therapeutic angiogenesis induced by human hepatocyte growth factor gene. Circulation. 2002;105:1491–1496. doi: 10.1161/01.cir.0000012146.07240.fd [DOI] [PubMed] [Google Scholar]
- 13.Hisaka Y, Ieda M, Nakamura T, Kosai K, Ogawa S, Fukuda K. Powerful and controllable angiogenesis by using gene-modified cells expressing human hepatocyte growth factor and thymidine kinase. J Am Coll Cardiol. 2004;43:1915–1922. doi: 10.1016/j.jacc.2004.01.034 [DOI] [PubMed] [Google Scholar]
- 14.Iyer SR, Annex BH. Therapeutic angiogenesis for peripheral artery disease: lessons learned in translational science. JACC Basic Transl Sci. 2017;2:503–512. doi: 10.1016/j.jacbts.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120:1326–1340. doi: 10.1161/CIRCRESAHA.116.309045 [DOI] [PubMed] [Google Scholar]
- 16.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E; TAMARIS Committees and Investigators. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. doi: 10.1016/S0140-6736(11)60394-2 [DOI] [PubMed] [Google Scholar]
- 17.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347 [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Trachtenberg J, Mohler E, Olin J, McBride S, Pak R, Rasmussen H, Crystal R. Phase I study of direct administration of a replication deficient adenovirus vector containing the vascular endothelial growth factor cDNA (CI-1023) to patients with claudication. Am J Cardiol. 2002;90:512–516. doi: 10.1016/s0002-9149(02)02524-9 [DOI] [PubMed] [Google Scholar]
- 19.Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled rejuvenating endothelial progenitor cells via transcutaneous intra-arterial supplementation (JUVENTAS) trial. Circulation. 2015;131:851–860. doi: 10.1161/CIRCULATIONAHA.114.012913 [DOI] [PubMed] [Google Scholar]
- 20.Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, Tonn T, Seeger F, Dimmeler S, Lindhoff-Last E, et al. ; PROVASA Investigators. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348 [DOI] [PubMed] [Google Scholar]
- 21.Reis SE, McDonald MC, Byers SJ. Crossing the research valleys of death: the University of Pittsburgh approach. Clin Transl Sci. 2008;1:9–10. doi: 10.1111/j.1752-8062.2008.00021.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a [DOI] [PubMed] [Google Scholar]
- 23.Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RHF, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21:425–532. doi: 10.1007/s10456-018-9613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotfi S, Patel AS, Mattock K, Egginton S, Smith A, Modarai B. Towards a more relevant hind limb model of muscle ischaemia. Atherosclerosis. 2013;227:1–8. doi: 10.1016/j.atherosclerosis.2012.10.060 [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner I, Chronos N, Comerota A, Henry T, Pasquet JP, Finiels F, Caron A, Dedieu JF, Pilsudski R, Delaère P. Local gene transfer and expression following intramuscular administration of FGF-1 plasmid DNA in patients with critical limb ischemia. Mol Ther. 2009;17:914–921. doi: 10.1038/mt.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arpino JM, Nong Z, Li F, Yin H, Ghonaim N, Milkovich S, Balint B, O’Neil C, Fraser GM, Goldman D, et al. Four-dimensional microvascular analysis reveals that regenerative angiogenesis in ischemic muscle produces a flawed microcirculation. Circ Res. 2017;120:1453–1465. doi: 10.1161/CIRCRESAHA.116.310535 [DOI] [PubMed] [Google Scholar]
- 27.Frontini MJ, Nong Z, Gros R, Drangova M, O’Neil C, Rahman MN, Akawi O, Yin H, Ellis CG, Pickering JG. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol. 2011;29:421–427. doi: 10.1038/nbt.1845 [DOI] [PubMed] [Google Scholar]
- 28.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685 [DOI] [PubMed] [Google Scholar]
- 29.Charles JP, Cappellari O, Spence AJ, Hutchinson JR, Wells DJ. Musculoskeletal geometry, muscle architecture and functional specialisations of the mouse hindlimb. PLoS One. 2016;11:e0147669 doi: 10.1371/journal.pone.0147669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochi T, Imai Y, Takeda A, Watanabe Y, Mori S, Tachi M, Kodama T. Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PLoS One. 2013;8:e84047 doi: 10.1371/journal.pone.0084047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherwek DH, Hopkins MB, Thompson MJ, Annex BH, Taylor DA. Fiber type-specific differential expression of angiogenic factors in response to chronic hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2000;279:H932–H938. doi: 10.1152/ajpheart.2000.279.3.H932 [DOI] [PubMed] [Google Scholar]
- 32.Zaccagnini G, Palmisano A, Canu T, Maimone B, Lo Russo FM, Ambrogi F, Gaetano C, De Cobelli F, Del Maschio A, Esposito A, et al. Magnetic resonance imaging allows the evaluation of tissue damage and regeneration in a mouse model of critical limb ischemia. PLoS One. 2015;10:e0142111 doi: 10.1371/journal.pone.0142111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zbinden S, Clavijo LC, Kantor B, Morsli H, Cortes GA, Andrews JA, Jang GJ, Burnett MS, Epstein SE. Interanimal variability in preexisting collaterals is a major factor determining outcome in experimental angiogenesis trials. Am J Physiol Heart Circ Physiol. 2007;292:H1891–H1897. doi: 10.1152/ajpheart.00537.2006 [DOI] [PubMed] [Google Scholar]
- 34.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0 [DOI] [PubMed] [Google Scholar]
- 35.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185 [DOI] [PubMed] [Google Scholar]
- 36.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412 doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng X, Wang J, Lassance-Soares RM, Najafi AH, Sood S, Aghili N, Alderman LO, Panza JA, Faber JE, Wang S, et al. Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2011;300:H2027–H2034. doi: 10.1152/ajpheart.00004.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol. 2013;4:363 doi: 10.3389/fphys.2013.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris JB, Maltin CA. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br J Pharmacol. 1982;76:61–75. doi: 10.1111/j.1476-5381.1982.tb09191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall-Craggs EC. Rapid degeneration and regeneration of a whole skeletal muscle following treatment with bupivacaine (Marcain). Exp Neurol. 1974;43:349–358. doi: 10.1016/0014-4886(74)90176-9 [DOI] [PubMed] [Google Scholar]
- 41.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 42.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507 [Google Scholar]
- 43.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832 [DOI] [PubMed] [Google Scholar]
- 44.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, Wong K, Bedu E, Cartwright EJ, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82 doi: 10.1186/gb-2013-14-7-r82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013 [DOI] [PubMed] [Google Scholar]
- 47.Hellingman AA, Bastiaansen AJ, de Vries MR, Seghers L, Lijkwan MA, Löwik CW, Hamming JF, Quax PH. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 2010;40:796–803. doi: 10.1016/j.ejvs.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 48.Zwetsloot PP, Végh AM, Jansen of Lorkeers SJ, van Hout GP, Currie GL, Sena ES, Gremmels H, Buikema JW, Goumans MJ, Macleod MR, et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ Res. 2016;118:1223–1232. doi: 10.1161/CIRCRESAHA.115.307676 [DOI] [PubMed] [Google Scholar]
- 49.Ramirez FD, Motazedian P, Jung RG, Di Santo P, MacDonald ZD, Moreland R, Simard T, Clancy AA, Russo JJ, Welch VA, et al. Methodological rigor in preclinical cardiovascular studies: targets to enhance reproducibility and promote research translation. Circ Res. 2017;120:1916–1926. doi: 10.1161/CIRCRESAHA.117.310628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suen CM, Stewart DJ, Montroy J, Welsh C, Levac B, Wesch N, Zhai A, Fergusson D, McIntyre L, Lalu MM. Regenerative cell therapy for pulmonary arterial hypertension in animal models: a systematic review. Stem Cell Res Ther. 2019;10:75 doi: 10.1186/s13287-019-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34:1854–1859. doi: 10.1161/ATVBAHA.114.303929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marques SM, Campos PP, Castro PR, Cardoso CC, Ferreira MA, Andrade SP. Genetic background determines mouse strain differences in inflammatory angiogenesis. Microvasc Res. 2011;82:246–252. doi: 10.1016/j.mvr.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 53.Chevalier J, Yin H, Arpino JM, O’Neil C, Nong Z, Gilmore KJ, Lee JJ, Prescott E, Hewak M, Rice CL, et al. Obstruction of small arterioles in patients with critical limb ischemia due to partial endothelial-to-mesenchymal transition. iScience. 2020;23:101251 doi: 10.1016/j.isci.2020.101251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


