Abstract
Patients with asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection give rise to SARS-CoV-2 environmental contamination during childbirth.
INTRODUCTION
Childbirth is the most common reason for hospitalization in the United States (3.9 million/year).1,2 The prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among patients admitted for labor and delivery is between 0% and 15.4%; most patients are asymptomatic.3–5 The extent of SARS-CoV-2 environmental contamination during childbirth is not known. Coughing, speaking, vomiting, and breathing have all been shown to generate aerosols and droplets.6 Expiratory forces are increased during active labor. In addition, the second stage of labor often results in significant fecal contamination, which has been associated with SARS-CoV-2 detection in surface and aerosol samples.7 We aimed to evaluate SARS-CoV-2 environmental contamination in the labor and delivery environment and on personal protective equipment to provide preliminary data as a foundation for future research and guidance for continued protection of health care workers.
METHODS
We conducted this study at a single academic institution from March 2020 to June 2020. The protocol was approved by the Oregon Health & Sciences University Institutional Review Board. Universal SARS-CoV-2 polymerase chain reaction testing occurred for all patients admitted to the labor and delivery unit. Environmental swabs were collected from room surfaces and health care workers' face shields. In addition, passive and active air samples were collected. Reverse transcription polymerase chain reaction targeting the spike gene was used to detect SARS-CoV-2 RNA (see Appendix 1, available online at http://links.lww.com/AOG/C57).
RESULTS
We obtained samples for two vaginal and two cesarean births (four asymptomatic patients who tested positive for SARS-CoV-2). General anesthesia was not used for any of these four deliveries. Patients were unable to consistently wear masks during vaginal birth 2 and cesarean birth 1.
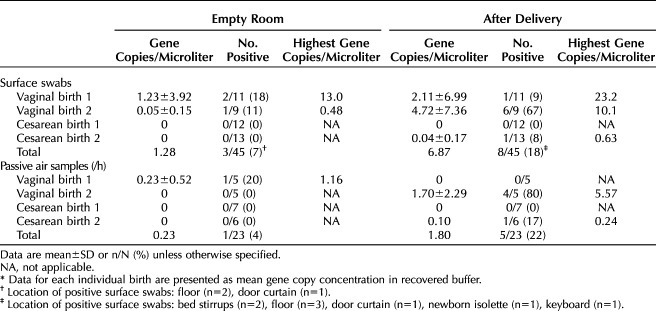
In baseline empty room samples, viral RNA was detected in 3 of 45 (6.7%) surface swabs and 1 of 23 (4.3%) passive air samples. After delivery, the total proportion of positive samples increased for both surface swabs (8/45, 17.8%) and passive air samples (5/23, 21.7%) (Table 1). The greatest change from baseline was after vaginal birth 2 (negative pressure room). After vaginal birth 2, the majority of the passive air settling dishes tested positive (four of five). Three of these positive settling dishes were located more than 6 feet from the patient bed.
Table 1.
Surface Swabs and Passive Air Samples Collected From Unoccupied, Clean Rooms (Empty Room) and After Delivery*
Postdelivery active air sampling results were not consistent by delivery type. No viral RNA was detected by active air sampling before or after cesarean birth 2, whereas it was detected after vaginal birth 2 (14.4 gene copies/L air) and cesarean birth 1 (4.80 gene copies/L air).
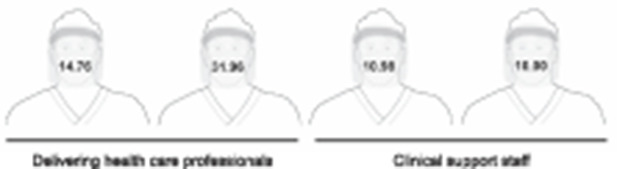
Postdelivery face shield sampling results also varied by delivery type. SARS-CoV-2 RNA was not detected on any face shield (surgeons, anesthesiologist, or nurses) after either cesarean birth. The health care worker personal protective equipment for vaginal birth 1 was not obtainable. All health care worker face shields were positive for SARS-CoV-2 RNA after vaginal birth 2 (Fig. 1).
Fig. 1. Viral copy numbers per face shield swab (gene copies/microliter) from the health care team after vaginal birth 2. Illustration by Dr. Mark Fretz. Used with permission.
Hermesch. SARS-CoV-2 Environmental Contamination and Childbirth. Obstet Gynecol 2020.
DISCUSSION
We found that patients with asymptomatic SARS-CoV-2 infection give rise to SARS-CoV-2 environmental contamination during childbirth. One vaginal birth resulted in viral RNA airborne dispersion at a distance greater than 6 feet, and the care team had significant facial exposure to SARS-CoV-2. Our findings suggest that the risk of SARS-CoV-2 transmission from labor and delivery is higher than previously thought. In contrast, Ong et al8 found no health care worker personal protective equipment contamination among those caring for patients with symptomatic COVID-19 not undergoing aerosol-generating procedures. Speech generates large- and small-particle aerosols. Particle emission rate increases 10-fold with loud as compared with quiet speaking.9 Shouting and heavy breathing during painful active labor likely generates considerable viral-laden particles. We posit that these airborne particles caused personal protective equipment contamination and also provide an explanation for our positive passive air sampling distant from the patient. We suspect that the extent of contamination varies by delivery type and other patient factors, such as mask-wearing.
Our study limitations include a small sample size and the inability to determine airborne particle size and virus viability or infectivity. It is also important to acknowledge that a small number of baseline samples detected SARS-CoV-2 RNA. No environment is sterile, and SARS-CoV-2 RNA may remain detectable in the environment for at least 17 days.10 Prior room occupants tested “nondetectable” for SARS-CoV-2, but asymptomatic visitors and health care workers are not routinely tested. Our results reinforce the importance of universal masking, excellent hand hygiene, environmental cleaning, and visitor-limitation policies that minimize exposure and contamination risk.
These findings support the American College of Obstetricians and Gynecologists' personal protective equipment recommendations for obstetrician–gynecologists.11 Our current practice is to use airborne isolation precautions, including N95 respirators and face shields for all deliveries of patients who test positive for SARS-CoV-2 or are of unknown status. These precautions appear particularly important given the difficulty of patient mask-wearing during active labor. Although preliminary in nature, our data demonstrate unique risk for SARS-CoV-2 exposure during labor through prolonged, close contact and a droplet- and aerosol-generating process.
As personal protective equipment is depleted,12,13 risk stratification of patient interactions is used to ensure availability for the highest-risk situations. To our knowledge (based on a PubMed search using the terms “aerosol-generating procedure” with “vaginal birth” or “cesarean birth” and using the timeframe “all dates”), a direct comparison between childbirth and other aerosol-generating procedures has not been done to support the current Centers for Disease Control and Prevention statement that, “Forceful exhalation during the second stage of labor is not considered an aerosol-generating procedure for respirator prioritization during shortages over procedures more likely to generate higher concentrations of infectious respiratory aerosols.”14 This lack of data highlights the critical need to further evaluate interventions that mitigate health care worker risk while caring for patients during birth, the most frequent hospitalization encounter in the United States.
Footnotes
Financial Disclosure Alison Edelman disclosed receiving royalties from UpToDate. Leslie Dietz disclosed receiving funds from Oregon Health and Science University for working on C. difficile, Viewglass, to evaluate glass as a microbial reservoir. She received a SARE grant to determine effects of mixed species rotational grazing in soil, and a USDA/USFS grant to study microbial presence and persistence on wood in a health care setting. Mark Fretz disclosed previous employment with ZGF Architects that ended in November 2017, and receiving an NSF travel scholarship for the WAFES conference and honorarium with the Oregon Department of Environmental Quality. Kevin Van Den Wymelenberg disclosed that other than his employment with UO, he is currently contemplating a financial relationship with Enviral Tech, a local environmental testing company, as a consultant to their company, but it has not been determined if he will do this or sign any documents. He has received honorariums for travel accommodations as in-kind contributions from conferences and industry events in the last 36 months, including from: Banbury Event Center, Alfred P Sloan Foundation, LightFair International, Illuminating Engineering Society of North America, and the Velux Foundation. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print August 19, 2020.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C58.
REFERENCES
- 1.Pfuntner A, Wier LM, Stocks C. Most frequent conditions in U.S. hospitals, 2011: statistical brief #162. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 2.Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. hospitals, 2011: statistical brief #165. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 3.Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020;382:2163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller ES, Grobman WA, Sakowicz A, Rosati J, Peaceman AM. Clinical implications of universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in pregnancy. Obstet Gynecol 2020;136:232–4. [DOI] [PubMed] [Google Scholar]
- 5.Naqvi M, Burwick RM, Ozimek JA, Greene NH, Kilpatrick SJ, Wong MS. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) universal testing experience on a Los Angeles labor and delivery unit. Obstet Gynecol 2020;136:235–6. [DOI] [PubMed] [Google Scholar]
- 6.Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2006;16:335–47. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020;582:557–60. [DOI] [PubMed] [Google Scholar]
- 8.Ong SWX, Tan YK, Sutjipto S, Chia PY, Young BE, Gum M, et al. Absence of contamination of personal protective equipment (PPE) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Infect Control Hosp Epidemiol 2020;41:614–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep 2019;9:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriarty LF, Plucinski MM, Marston BJ, Kurbatova EV, Knust B, Murray EL, et al. Public health responses to COVID-19 outbreaks on cruise ships—worldwide, February–March 2020. MMWR Morb Mortal Wkly Rep 2020;69:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019. Retrieved June 30, 2020. [Google Scholar]
- 12.Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the covid-19 pandemic. N Engl J Med 2020;382:e41. [DOI] [PubMed] [Google Scholar]
- 13.Monahan R. Even as Gov. Kate Brown reopens Oregon, a shortage of masks and other PPE at hospitals suggests a state still in crisis. Available at: https://www.wweek.com/news/state/2020/05/20/even-as-gov-kate-brown-reopens-oregon-a-shortage-of-masks-and-other-personal-protective-equipment-at-hospitals-suggest-a-state-still-in-crisis/. Retrieved June 30, 2020. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Clinical questions about COVID-19: questions and answers. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html. Retrieved June 30, 2020.