Abstract
Background
Anatomical connectivity between the thalamus and cortex, including the prefrontal cortex (PFC), is abnormal in schizophrenia. Overlapping phenotypes, including deficits in executive cognitive abilities dependent on PFC-thalamic circuitry, suggest dysrupted thalamocortical anatomical connectivity may extend to psychotic bipolar disorder. We tested this hypothesis and examined the impact of illness stage to inform when in the illness course thalamocortical dysconnectivity emerges.
Methods
Diffusion-weighted imaging data were collected on 70 healthy individuals and 124 people with a psychotic disorder (schizophrenia spectrum = 75; psychotic bipolar disorder = 49), including 62 individuals in the early stage of psychosis. Anatomical connectivity between major divisions of the cortex and thalamus was quantified using probabilistic tractography and compared between groups. Associations between PFC-thalamic anatomical connectivity and executive cognitive abilities were examined using regression analysis.
Results
Psychosis was associated with lower PFC-thalamic and elevated somatosensory-thalamic anatomical connectivity. Follow-up analyses established that lower PFC-thalamic and elevated somatosensory-thalamic anatomical connectivity were present in both schizophrenia and psychotic bipolar disorder. Lower PFC-thalamic anatomical connectivity was also present in early-stage and chronic psychosis. Contrary to expectations, lower PFC-thalamic anatomical connectivity was not associated with impaired executive cognitive abilities.
Conclusions
Altered thalamocortical anatomical connectivity, especially reduced PFC-thalamic connectivity, is a transdiagnostic feature of psychosis detectable in the early stage of illness. Further work is required to elucidate the functional consequences of the full spectrum of thalamocortical connectivity abnormalities in psychosis.
Keywords: psychosis, thalamus, cortex, anatomical, connectivity, diffusion
Introduction
Characterizing brain abnormalities, establishing phenotypic boundaries between disorders, and uncovering the neural substrates of symptom dimensions are critical challenges in psychiatry. In the case of psychotic disorders, there is considerable evidence that the thalamus is abnormal. Consistent findings include reduced volume, altered activity during cognition, and decreased expression of neurochemical markers of neuronal integrity.1–7 These findings prompted the development of several models of thalamic dysfunction, many of which emphasize dysruption of thalamic interactions with the prefrontal cortex (PFC) in the etiology of psychosis and mechanisms of cognitive impairment.8–14
Thalamocortical models of psychosis are further supported by neuroimaging studies showing that thalamocortical functional connectivity, including functional coupling between the PFC and thalamus, is altered during resting-state and task performance.15–17 Findings from diffusion-weighted imaging (DWI) indicate that functional dysconnectivity is accompanied by dysrupted white matter connectivity between cortex and thalamus.18,19 For instance, we found that anatomical connectivity in schizophrenia is characterized by reduced PFC-thalamic and elevated somatomotor-thalamic connectivity,20 replicating a prior small study by Marenco et al.21 Moreover, reduced PFC-thalamic anatomical connectivity has been linked to cognitive impairment and abnormal PFC activation during working memory, which is consistent with thalamic models of psychosis, and animal and human studies implicating PFC-thalamic connectivity in cognition.20–22
While compelling, critical questions remain unaddressed. First, it is unknown whether thalamic dysfunction extends to other psychotic disorders, primarily bipolar disorder with psychotic features (ie, psychotic bipolar disorder). Shared phenotypes, including overlapping deficits in executive cognitive functions supported by PFC-thalamic circuitry, suggests this may be the case.23,24 Indeed, findings from resting-state functional magnetic resonance imaging (fMRI) studies indicate that dysrupted thalamocortical functional connectivity is also present in bipolar disorder,25,26 including psychotic bipolar disorder.27 However, the thalamus is understudied in psychotic bipolar disorder and anatomical connectivity between cortex and thalamus has not been comprehensively assessed.
It is also unclear when thalamocortical dysconnectivity emerges and how it evolves over the course of illness. Most studies included individuals that had been ill for several years and/or did not explicitly examine the effect of illness stage. Resting-state fMRI studies suggest thalamocortical dysconnectivity is present early in the course of illness, possibly even before the onset of frank psychosis.27–29 However, this has only been demonstrated in schizophrenia spectrum disorders. Studying both early and chronic stages will further inform our understanding of the etiology and course of thalamocortical anatomical dysconnectivity.
Building on prior work by our group and others, the present study sought to (1) confirm thalamocortical anatomical connectivity abnormalities observed in prior studies are present in a relatively large (n > 120) cohort of individuals with a psychotic illness; (2) test the hypothesis that schizophrenia and psychotic bipolar disorder demonstrate similar thalamocortical abnormalities; (3) determine whether abnormalities in thalamocortical anatomical connectivity are present in both the early and chronic stages of psychosis; and (4) verify that associations between PFC-thalamic connectivity and impaired executive cognitive abilities observed in prior smaller studies hold up in a larger cohort of individuals with psychosis.
Methods and Materials
Study Participants
Two hundred and thirty-five individuals that completed a neuroimaging session that included a T1- and diffusion-weighted scan as part of their participation in a now completed study of thalamocortical networks in psychosis (1R01 MH102266) were initially included in this investigation. Data were collected between August 2014 and April 2019. Neuroimaging data from 41 individuals did not pass our quality assurance (QA) procedures described later. Therefore, the final study cohort comprised 194 participants: 70 healthy individuals and 124 individuals with a psychotic illness (schizophrenia spectrum: n = 75; psychotic bipolar disorder: n = 49). A subset of the healthy participants (n = 41) and individuals with schizophrenia (n = 53) were included in an earlier study by our group.20 Consistent with prior studies from our group23 and the critical period of psychosis hypothesis,30 62 individuals within the psychosis group were classified as early stage, defined as duration of psychotic illness ≤ 2 years (schizophrenia spectrum: n = 37; psychotic bipolar disorder: n = 25). The remaining 62 were considered chronic (schizophrenia spectrum: n = 38; psychotic bipolar disorder: n = 24). Demographic data are presented in table 1 and supplementary material, which also contains additional details of the study procedures. This study was approved by the Vanderbilt University Institutional Review Board. All subjects provided written informed consent prior to participating.
Table 1.
Sample Characteristics
| Statistics | |||||||
|---|---|---|---|---|---|---|---|
| Healthy Individuals | Psychosis | t/x2 | df | P | |||
| Sample size | 70 | 124 | |||||
| Gender (M/F) | 44/26 | 85/39 | 0.65 | 1 | .420 | ||
| Race (W/AA/O) | 51/14/5 | 91/28/5 | 0.98 | 2 | .613 | ||
| Age, mean (SD) | 28.2 | 9.0 | 27.3 | 8.6 | 0.66 | 192 | .508 |
| Education, mean (SD) | 16.0 | 1.9 | 13.9 | 2.1 | 7.06 | 192 | <.001 |
| Parental education, mean (SD) | 14.9 | 2.3 | 15.0 | 2.6 | 0.21 | 191 | .837 |
| Premorbid IQ, mean (SD) | 108.0 | 14.6 | 104.0 | 9.8 | 2.29 | 192 | .023 |
| Neuropsychological functioning, mean (SD) | |||||||
| AX-CPT d-prime | 3.5 | 0.7 | 2.7 | 1.1 | 5.61 | 192 | <.001 |
| WCST total errors standard score | 107.4 | 12.5 | 95.8 | 13.9 | 5.43 | 165 | <.001 |
| WMS-III Working Memory Index | 106.6 | 12.6 | 97.5 | 13.2 | 4.69 | 191 | <.001 |
| SCIP Composite Z-score | 0.28 | 0.59 | −0.69 | 0.84 | 8.46 | 192 | <.001 |
| Clinical characteristics | |||||||
| Psychotic disorder (BP/SCZ) | 49/75 | — | — | — | |||
| Early stage/chronic | 62/62 | — | — | — | |||
| APD (yes/no) | 95/29 | — | — | — | |||
| Mood stabilizer (yes/no) | 51/73 | — | — | — | |||
| Age at illness onset, mean (SD) | 22.0 | 5.9 | — | — | — | ||
| Duration of illness (years), mean (SD) | 5.8 | 7.4 | — | — | — | ||
| PANSS positive, mean (SD) | 15.3 | 9.0 | — | — | — | ||
| PANSS negative, mean (SD) | 12.5 | 5.0 | — | — | — | ||
| PANSS general, mean (SD) | 27.9 | 7.9 | — | — | — | ||
| Depression (HAMD), mean (SD) | 10.0 | 9.2 | — | — | — | ||
| Mania (YMRS), mean (SD) | 6.8 | 12.2 | — | — | — | ||
| APD dose (CPZ equiv), mean (SD) | 336.2 | 217.7 | — | — | — | ||
Abbreviations: AA = African American; APD = Antipsychotic Drug; BP = Psychotic Bipolar Disorder; C = Caucasian; CPT = Continuous Performance Test; CPZ = Chlorpromazine; F = female; HAMD = Hamilton Depression Scale; HI = Healthy Individuals; M = male; O = Other; PANSS = Positive and Negative Syndrome Scale; SCIP = Screen for Cognitive Impairment in Psychiatry; SCZ = Schizophrenia; WCST = Wisconsin Card Sorting Test; WMS = Wechsler Memory Scale; YMRS = Young Mania Rating Scale.
Neuropsychological Testing
Study participants completed a brief neuropsychological assessment. Given our a priori interest in PFC-thalamic circuitry, testing concentrated on assessing the 3 domains of executive function identified by Miyake et al31: working memory, set-shifting/cognitive flexibility, and inhibition. The Wechsler Memory Scale-III (WMS-III)32 Working Memory Index, Wisconsin Card Sorting Test-64 Card Version (WCST-64),33 and AX-Continuous Performance Test (AX-CPT)34 were administered to quantify working memory, set-shifting, and inhibition, respectively. The WMS-III Working Memory Index, WCST-64 total errors standard score, and d-prime from the AX-CPT served as the dependent variables. The Screen for Cognitive Impairment in Psychiatry (SCIP)35 was also administered to quantify overall neuropsychological functioning.
Neuroimaging Data
Neuroimaging data acquisition, preprocessing, and quantification of thalamocortical anatomical connectivity using probabilistic tractography are described in detail in supplementary material. Briefly, a T1-weighted structural scan (1-mm isotropic resolution) and high-angular resolution diffusion-weighted imaging (HARDI) scan (60 directions, 2.5-mm isotropic resolution) were collected on each subject during a single imaging session on a 3T Philips Intera Achieva scanner. Image processing was performed on the Vanderbilt University Institute of Imaging Science XNAT platform.36 Each subject’s structural T1-weighted MRI was segmented into 133 cortical and subcortical brain structures.37 Six bilateral cortical regions-of-interest (ROIs), created by combining selected cortical structures, and the thalamus were used as targets and the seed, respectively, in the probabilistic tractography analysis described later. The 6 cortical ROIs included the PFC, motor cortex/supplementary motor area, somatosensory cortex, posterior parietal cortex, temporal cortex, and occipital cortex (see supplementary material for an example segmentation).
All HARDI data underwent QA using an automated pipeline that includes visualization of HARDI data, and extraction of mean head translation and rotation in the X, Y, and Z directions and average number of voxels (in percent) rejected per gradient due to poor diffusion tensor imaging (DTI) model fitting.38 QA included visual inspection of HARDI scans for artifacts and plotting the distributions for the 7 QA metrics (6 head motion parameters, DTI model fitting outliers) using stem and leaf plots to identify outliers (defined as greater than or equal to 3 times the interquartile range). T1-weighted anatomical scans and segmentation results were also visually inspected for imaging artifacts and segmentations failures. As noted above, 41 participants (8 healthy individuals; 33 individuals with psychosis) did not meet our QA standards: 10 scans were excluded due to imaging artifacts/segmentation failures based on visual inspection, and 31 were excluded for having an outlier value on one or more of the 7 HARDI scan QA metrics. Within the psychosis cohort, individuals excluded from the study due to not meeting QA standards were significantly older, had a higher percentage of females, and later age of onset of psychosis compared with individuals with psychosis that were included in the study (see supplementary material).
Following QA, HARDI data preprocessing and probabilistic tractography were performed within FMRIB’s Diffusion Toolbox (FDT) for FSL software package (http://www.fmrib.ox.ac.uk/fsl/). Briefly, seed-to-target tractography analyses were run using probtrackx2,39 separately in each hemisphere, to quantify anatomical connectivity between the thalamus (ie, seed) and each of the 6 cortical ROIs (ie, targets). A distance-adjusted measure of overall connectivity with the thalamus was calculated for each cortical area from the seed-to-target voxel-wise images generated from probtrackx2. These values, expressed as “total connectivity” (in percent), served as the dependent variables for the primary analysis described later. In addition, voxel-wise probability maps were generated and used in the voxel-wise analysis described later.
Statistical Analysis
For the analysis of total thalamic connectivity, left and right hemisphere connectivity values were averaged as we did not have any a priori hypotheses regarding laterality, and to minimize the number of dependent variables. This resulted in six dependent variables per subject, one for each cortical target ROI. We first compared total thalamocortical anatomical values between healthy individuals and our complete psychosis cohort using independent groups t-tests with the critical alpha adjusted using the Bonferroni–Holm method for correcting for multiple comparisons. Subsequent analyses of diagnosis and illness stage/age effects focused on results surviving correction for multiple comparisons. Specifically, one-way analysis of variance (ANOVA) including healthy individuals, schizophrenia, and psychotic bipolar disorder groups was performed to determine whether schizophrenia and psychotic bipolar disorder demonstrate the same abnormalities in thalamocortical anatomical connectivity. Similarly, one-way analysis of variance (ANCOVA) with healthy individuals and illness stage (early stage, chronic) as the independent group variable and age included as a covariate were performed to determine whether early-stage and chronic psychosis demonstrate similar patterns of thalamocortical anatomical dysconnectivity. Associations between neuropsychological functioning and PFC-thalamic anatomical connectivity were examined using regression analyses with age, sex, and diagnostic group (model 1) as well as group × connectivity interaction terms (model 2) entered as predictors of WMS Working Memory Index, WCST total errors standard score, and AX-CPT d-prime scores. The distributions of the cognitive variables were inspected prior to regression analysis using stem–leaf plots and outliers excluded from the regression analyses.
Results
Thalamocortical Anatomical Connectivity in Psychotic Disorders
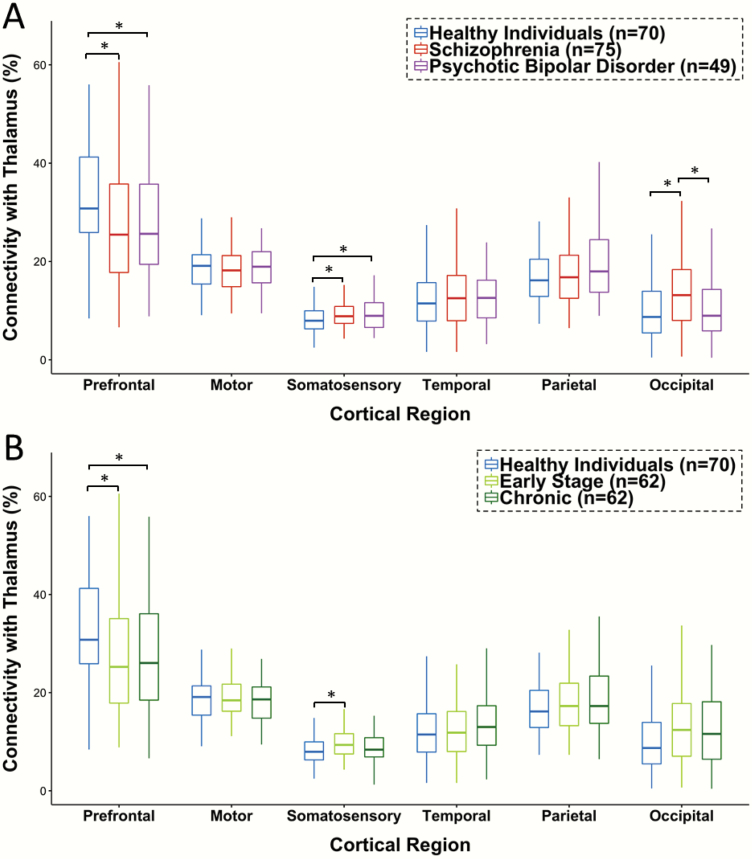
Total connectivity of each cortical ROI with the thalamus in healthy individuals and the combined psychosis cohort are presented in table 2. Psychosis was associated with lower PFC-thalamic connectivity (t(192) = 2.72, P = .007; effect size [Cohen’s d] = −0.41) and higher somatosensory-thalamic connectivity (t(192) = 2.69, P = .008; d = 0.40). As shown in figure 1A, schizophrenia and psychotic bipolar disorder demonstrated similar patterns of thalamocortical dysconnectivity. Follow-up one-way ANOVAs were significant for both PFC (F(2,191) = 3.69, P = .027) and somatosensory (F(2,191) = 3.66, P = .027) anatomical connectivity with the thalamus. Post hoc contrasts indicated that PFC-thalamic connectivity was reduced in both schizophrenia (P = .015; d = −0.40) and psychotic bipolar disorder (P = .032; d = −0.41). Similarly, elevated somatosensory-thalamic anatomical connectivity was present in both schizophrenia (P = .025; d = 0.41) and psychotic bipolar disorder (P = .019; d = 0.43). Although it did not survive correction for multiple comparisons in the initial analysis comparing healthy individuals to the combined psychosis cohort, one-way ANOVA for occipital-thalamic connectivity (F(1,191) = 3.63, P = .028) was significant due to the fact that occipital-thalamic connectivity was higher in schizophrenia compared to both healthy individuals (P =.018, d = 0.40) and psychotic bipolar disorder (P = .031, d = 0.40). Complete statistical results as well as supplemental voxel-wise analyses confirming lower PFC-thalamic and elevated somatosensory-thalamic anatomical connectivity in psychosis are presented in supplementary material.
Table 2.
Thalamocortical Anatomical Connectivity in Psychosis
| Healthy Individuals | Psychosis | Statistics | |||||
|---|---|---|---|---|---|---|---|
| n = 70 | n = 124 | ||||||
| Mean | SD | Mean | SD | t* | P | Effect Size | |
| Prefrontal cortex | 32.39 | 12.46 | 27.43 | 12.00 | 2.72 | .007 | −0.41 |
| Motor cortex | 18.87 | 4.97 | 18.91 | 5.06 | 0.06 | .953 | 0.01 |
| Somatosensory cortex | 8.13 | 2.78 | 9.40 | 3.36 | 2.69 | .008 | 0.40 |
| Temporal cortex | 12.33 | 7.12 | 12.98 | 6.15 | 0.67 | .505 | 0.10 |
| Posterior parietal cortex | 17.59 | 7.52 | 18.83 | 7.76 | 1.09 | .279 | 0.16 |
| Occipital cortex | 10.70 | 7.30 | 12.44 | 7.42 | 1.58 | .114 | 0.24 |
Note: Values for mean and SD indicate percent connectivity with thalamus; effect size = Cohen’s d.
*Degrees of freedom (df) = 192 for all t-statistics.
Figure 1.
Anatomical connectivity between cortex and thalamus in psychosis. (A) Thalamic anatomical connectivity with the prefrontal cortex (PFC) is reduced in both schizophrenia and psychotic bipolar disorder. In contrast, anatomical connectivity between thalamus and somatosensory cortex is increased in schizophrenia and psychotic bipolar disorder. Occipital cortex connectivity with the thalamus is elevated in schizophrenia compared with both healthy individuals and psychotic bipolar disorder. (B) Within the combined psychotic disorders group (ie, schizophrenia plus psychotic bipolar disorder), PFC anatomical connectivity with the thalamus is reduced in both chronic and early-stage psychosis. Elevated somatosensory anatomical connectivity is present only in early-stage psychosis. See text and supplementary material for complete statistical results. *P < .05.
Thalamocortical Anatomical Connectivity in Early Stage and Chronic Psychosis
One-way ANCOVAs with healthy individuals and psychosis illness stage (early, chronic) entered as group variables and age as a covariate were significant for both PFC (F(2,190) = 3.71, P = .026) and somatosensory (F(2,190) = 4.48, P = .013) connectivity with the thalamus. As shown in figure 1B, post hoc contrasts indicated that PFC-thalamic connectivity was lower in both chronic (P = .014, d = −0.40) and early-stage psychosis at the trend level (P = .054, d = −0.42). In contrast, compared with healthy individuals, somatosensory-thalamic connectivity was elevated in early stage (P = .003, d = 0.63), but not chronic psychosis (P = .152, d = 0.24). Additionally, a 2-factor ANCOVA with illness stage (early, chronic) and diagnosis (schizophrenia, psychotic bipolar disorder) and age and sex as covariates was performed within the psychosis cohort to determine whether the trajectories of thalamocortical anatomical connectivity differ between schizophrenia and psychotic bipolar disorder. There was no evidence of diagnosis by illness stage interactions for either PFC- and somatosensory-thalamic connectivity indicating that illness stage effects did not differ as a function of psychotic disorder diagnosis (see supplementary material for complete statistical results).
Emerging evidence indicates that both functional and anatomical connectivity of cortical networks demonstrate differential aging effects in psychotic disorder. For example, functional connectivity within frontoparietal and cingulo-opercular networks, and the integrity of cortical association white matter tracts show accelerated aging effects in psychosis compared to healthy individuals.40,41 As such, we complemented the primary analysis of illness stage effects presented above with exploratory regression analyses examining the effects of age, group (healthy individuals, psychosis), and age × group interaction on thalamocortical connectivity. Results of these supplementary analysis are summarized in supplementary material. Briefly, the age × group interaction term did not reach significance for any thalamocortical network indicating that age effects were similar in healthy individuals and psychosis.
Thalamic Anatomical Connectivity, Cognition, and Clinical Symptoms
Compared with healthy individuals, WMS-III Working Memory Index, WCST-64 total errors standard score, and AX-CPT d-prime were impaired in psychosis (see table 1). Inspection of the distributions of the neuropsychological test variables identified outlier scores for the WMS Working Memory Index (n = 7), WCST total errors standard score (n = 4), and AX-CPT d-prime (n = 1), which were excluded from the analyses reported below. Preplanned analysis focused on PFC-thalamic connectivity did not find any associations between total PFC-thalamic anatomical connectivity and WMS Working Memory Index, WCST total errors standard score, and AX-CPT d-prime (all t values < 1.72, P > .088). Adding diagnosis by connectivity interaction terms did not significantly improve model fit for any neuropsychological variable and none of the interaction terms reached significance (all t values < 1.41, P > .161).
We also performed exploratory partial correlation analyses examining associations between thalamocortical anatomical connectivity values and symptoms of psychosis (ie, Positive and Negative Syndrome Scale [PANSS] positive, negative, and general scores) controlling for age, sex, and psychotic disorder diagnosis. Total percent connectivity for all thalamocortical networks did not correlate with PANSS positive, negative, and general symptoms (all partial correlations < |.10|, P > .278).
Thalamocortical Anatomical Connectivity, Antipsychotic Medication, and Substance Use
We leveraged our relatively large psychosis cohort to explore the effects of antipsychotic medication and past substance disorder (SUD) on thalamocortical anatomical connectivity. As detailed in supplementary material, thalamocortical anatomical connectivity did not differ between medicated (n = 95) and unmedicated patients (n = 29). Additionally, among patients taking antipsychotic medication, average daily dose of antipsychotic medication (in chlorpromazine equivalents) was unrelated to thalamocortical connectivity (all partial correlations controlling for age and sex < |.07|, P > .507).
Seventy-two of the 124 individuals in our psychosis cohort met DSM-IV criteria for past SUD. To explore the impact of past SUD on thalamocortical anatomical connectivity, we repeated the analyses comparing healthy individuals to psychosis after splitting our psychosis cohort based on past SUD. Age was included as a covariate in this analysis. Briefly, effect of group remained significant for both PFC-thalamic (F(2,190) = 5.10, P = .007) and somatosensory-thalamic (F(1,190) = 4.26, P = .015) anatomical connectivity. Within the psychosis cohort, PFC and somatosensory cortex connectivity with the thalamus did not differ between patients with and without past SUD. However, lower PFC-thalamic anatomical connectivity was more pronounced in psychotic disorder with past SUD (P = .002; d = −0.55) than the subgroup with no history of SUD (P = .235; d = −0.23). In contrast, elevated somatosensory-thalamic anatomical connectivity was present in psychosis individuals without history of SUD (P = .005; d = 0.53) and those with history of SUD, at the trend significance level (P = .076; d = 0.34). Complete results are presented in supplementary material.
Discussion
The present study quantified anatomical connectivity between the cortex and thalamus in psychotic disorders using DWI. We confirmed prior reports of reduced PFC-thalamic and elevated somatosensory-thalamic connectivity in a relatively large cohort of individuals with a psychotic disorder. Our hypothesis that abnormal thalamocortical connectivity, particularly anatomical connectivity between the PFC and thalamus, extends to psychotic bipolar disorder was confirmed. Moreover, reduced PFC-thalamic anatomical connectivity was present in both chronic and early stages of psychosis. In contrast, anticipated associations between PFC-thalamic anatomical connectivity and executive cognitive functioning were not detected.
Our investigation was motivated, in part, by the relative paucity of studies examining thalamocortical circuits in bipolar disorder and ongoing debate about whether schizophrenia and psychotic bipolar disorder are distinct disorders with unique neural substrates. The present study makes 2 important contributions to these issues. First, our findings inform the pathophysiology of psychotic bipolar disorder. Although dysrupted thalamocortical anatomical connectivity has been identified previously, existing studies either focused on specific tracts or used methods that are ill-suited for identifying selective dysfunction among thalamocortical networks.42–48 Moreover, no study has examined psychotic bipolar disorder specifically. The current results build on prior studies by localizing dysfunction to specific thalamocortical networks, further supporting models emphasizing PFC and thalamus involvement in bipolar disorder.7
Second, our results add to growing evidence that some brain abnormalities are shared across psychotic disorders. For instance, meta-analyses of structural MRI studies concluded that many abnormalities in brain structure observed in schizophrenia are also present in bipolar disorder, including volume reductions in the PFC and thalamus.49–51 As briefly reviewed earlier, resting-state fMRI studies indicate that psychosis is characterized by reduced PFC-thalamic functional connectivity; although few studies included psychotic bipolar disorder.25,27 The present study extends these findings by demonstrating that reduced PFC-thalamic anatomical connectivity is present in both schizophrenia and psychotic bipolar disorder, thereby further questioning the strict dichotomization of psychotic disorders.
Confirmation that reduced PFC-thalamic anatomical connectivity is present in early-stage psychosis further strengthens the argument that thalamocortical dysconnectivity is involved in the pathophysiology of psychosis. Our results are consistent with prior reports of altered mediodorsal nucleus microstructure and anatomical connectivity in first episode and clinical high-risk individuals,18,22 and dovetail nicely with evidence that thalamic functional dysconnectivity is detectable during the first 2 years of illness and may even precede the onset of a full-blown psychotic disorder.27,28 Our findings may also inform ongoing debates about the trajectory of illness in bipolar disorder. In contrast to schizophrenia, which is typically considered a neurodevelopmental disorder, there is speculation that bipolar disorder is a neuroprogressive illness characterized by relatively normal premorbid brain development and progressive decline following illness onset.52,53 The combination of reduced PFC-thalamic connectivity in early-stage psychosis and the absence of interactions between psychotic disorder diagnosis and illness stage implicate atypical neurodevelopment. Indeed, our group and others have hypothesized that dysrupted thalamocortical connectivity in psychosis is a consequence of atypical brain maturation.20,27,54,55 However, our findings do not rule out the possibility of neuroprogression in nonpsychotic bipolar disorder. Studies in psychotic and nonpsychotic bipolar disorder, ideally longitudinally, as well as mapping the normal developmental trajectories of thalamocortical circuits will further inform the etiology of thalamocortical dysconnectivity in schizophrenia and bipolar disorder.
Contrary to expectations based on a prior report21 and our earlier analyses of a subset of these data,20 PFC-thalamic anatomical connectivity was unrelated to impaired executive cognitive abilities in psychosis. Unfortunately, identifying reliably brain–behavior relationships remains elusive, even in well-characterized circuits, and many associations often fail to replicate.56 Obstacles to identifying brain–behavior associations include small sample sizes, multifactorial nature of many cognitive constructs, and imperfect mapping of functions onto specific brain circuits.57 It is possible that our approach, which quantifies connectivity of the whole PFC, rather than subregions, is too course to reliably identify cognition–connectivity associations. It is also possible that reduced PFC-thalamic anatomical connectivity may be associated with more proximal measures of brain function, including PFC activation and functional connectivity during task performance,21 than behavioral performance.
Although we were especially interested in PFC-thalamic connectivity given theoretical models of psychosis and the importance of PFC-thalamic circuitry to cognitive abilities often impaired in psychosis, our analyses also revealed increased anatomical connectivity between somatosensory cortex and thalamus in psychosis. The combination of reduced PFC and enhanced sensory connectivity is consistent with functional connectivity studies.15,17 The relevance of elevated somatosensory anatomical connectivity to psychosis-related phenotypes is unclear as somatosensory-thalamic circuitry has received considerably less attention than other thalamic networks. Interestingly, abnormal motor behavior, including volitional motor activity, dyskinesia, and catatonia, has been linked to elevated motor-thalamic anatomical and functional connectivity in schizophrenia.58,59 Moreover, increased thalamic functional connectivity with primary motor cortex is associated with psychomotor slowing in clinical high-risk individuals.60 Similar associations may extend to somatic perceptual abnormalities and somatosensory-thalamic dysconnectivity.
Our study has several limitations that should be considered when interpreted the results. Exploratory analyses revealed that, although there were no differences between psychosis patients with and without past SUD, only the subgroup of psychosis participants with past SUD exhibited reduced PFC-thalamic anatomical connectivity compared with healthy individuals. Although the findings suggest that substance use might exacerbate PFC-thalamic dysconnectivity, the results should be interpreted very cautiously as there are numerous caveats to the analysis. Patients with past SUD were combined into a single group regardless of the type(s) of drug abused. Moreover, the groups differed on several demographic and clinical variables. Thalamocortical circuitry has been implicated in both the etiology61 and consequences62 of substance abuse. Therefore, it is unclear if thalamocortical dysconnectivity is a cause or consequence of substance abuse. Regardless, our findings underscore the need for additional research on the effects of SUD on thalamocortical connectivity.
Additionally, approximately 20% (n = 33/157) of our original psychosis cohort did not meet our QA criteria and were excluded from the analysis. Demographic differences between included and excluded participants may affect generalization of our findings to the broader population of individuals with a psychotic disorder. Relatedly, the relatively low level of psychosis symptoms may have limited our ability to detect associations between thalamocortical anatomical connectivity and psychotic symptoms. Variability in the size of the cortical seeds may also affect sensitivity of probabilistic tractography to detecting group differences. Finally, our study may have been underpowered to detect subtle differences between schizophrenia and psychotic bipolar disorder. For instance, post hoc analyses revealed increased occipital-thalamic connectivity in schizophrenia compared with healthy individuals and psychotic bipolar disorder. This finding warrants further investigation given evidence that visual hallucinations are more common in schizophrenia than affective psychoses.63
In sum, the current findings identify common thalamocortical anatomical dysconnectivity in schizophrenia and psychotic bipolar disorder that emerges early in the illness course. These results converge with established functional connectivity abnormalities in psychotic disorders. Further work is required to elucidate the functional consequences of thalamocortical anatomical dysconnectivity, including both compromised PFC-thalamic connectivity and exaggerated somatosensory-thalamic connectivity, and clarify the potential effects of substance abuse on thalamic circuitry.
Supplementary Material
Acknowledgments
This work was conducted in part using the resources of the Center for Computational Imaging at Vanderbilt University Institute of Imaging Science and Advanced Computing Center for Research and Education at Vanderbilt University. The authors thank the individuals who participated in the study. The authors also thank Kristan Armstrong, Molly Boyce, Erin Brosey, Victoria Fox, and Yasmeen Iqbal Neal for their assistance recruiting study participants. No commercial support was received for the preparation of this manuscript and the authors have no conflicts of interest to report.
Funding
This work was supported by National Institute of Mental Health grant R01 MH102266 (awarded to NDW), the Charlotte and Donald Test Fund, the Jack Martin MD Research Professorship in Psychopharmacology (JUB), and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/National Institute of Health).
References
- 1. Shenton ME, Dickey CC, Frumin M, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2006;21(4), 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minzenberg MJ, Laird AR, Thelen S, et al. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ragland JD, Laird AR, Ranganath C, et al. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166(8):863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kraguljac NV, Reid M, White D, et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203(2–3):111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerullo MA, Adler CM, Delbello MP, et al. The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry. 2009;21(4):314–322. [DOI] [PubMed] [Google Scholar]
- 8. Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56(9):781–787. [DOI] [PubMed] [Google Scholar]
- 9. Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42(1):27–33. [DOI] [PubMed] [Google Scholar]
- 10. Jones EG. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 1997;23(3):483–501. [DOI] [PubMed] [Google Scholar]
- 11. Swerdlow NR. Integrative circuit models and their implications for the pathophysiologies and treatments of the schizophrenias. Curr Top Behav Neurosci. 2010;4:555–583. [DOI] [PubMed] [Google Scholar]
- 12. Scheibel AB. The thalamus and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 1997;9(3):342–353. [DOI] [PubMed] [Google Scholar]
- 13. Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. [DOI] [PubMed] [Google Scholar]
- 14. Kupferschmidt DA, Gordon JA. The dynamics of disordered dialogue: prefrontal, hippocampal and thalamic miscommunication underlying working memory deficits in schizophrenia. Brain Neurosci Adv. 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang AS, Rogers BP, Woodward ND. Disrupted modulation of thalamus activation and thalamocortical connectivity during dual task performance in schizophrenia. Schizophr Res. 2019;210:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramsay IS. An activation likelihood estimate meta-analysis of thalamocortical dysconnectivity in psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(10):859–869. [DOI] [PubMed] [Google Scholar]
- 18. Cho KI, Shenton ME, Kubicki M, et al. Altered thalamo-cortical white matter connectivity: probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull. 2016;42(3):723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubota M, Miyata J, Sasamoto A, et al. Thalamocortical disconnection in the orbitofrontal region associated with cortical thinning in schizophrenia. JAMA Psychiatry. 2013;70(1):12–21. [DOI] [PubMed] [Google Scholar]
- 20. Giraldo-Chica M, Rogers BP, Damon SM, et al. Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry. 2018;83(6):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marenco S, Stein JL, Savostyanova AA, et al. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37(2):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho KIK, Kwak YB, Hwang WJ, et al. Microstructural changes in higher-order nuclei of the thalamus in patients with first-episode psychosis. Biol Psychiatry. 2019;85(1):70–78. [DOI] [PubMed] [Google Scholar]
- 23. Menkes MW, Armstrong K, Blackford JU, et al. Neuropsychological functioning in early and chronic stages of schizophrenia and psychotic bipolar disorder. Schizophr Res. 2019;206:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195(6):475–482. [DOI] [PubMed] [Google Scholar]
- 25. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tu PC, Bai YM, Li CT, et al. Identification of common thalamocortical dysconnectivity in four major psychiatric disorders. Schizophr Bull. 2018;12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79(12):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anticevic A, Haut K, Murray JD, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72(9):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen MH, Chang WC, Bai YM, et al. Cortico-thalamic dysconnection in early-stage schizophrenia: a functional connectivity magnetic resonance imaging study. Eur Arch Psychiatry Clin Neurosci. 2019:4-5. [DOI] [PubMed] [Google Scholar]
- 30. Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33):53–59. [PubMed] [Google Scholar]
- 31. Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. [DOI] [PubMed] [Google Scholar]
- 32. Wechsler D. Wechsler Memory Scale, 3rd ed San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 33. Kongs sk, Thompson LL, Iverson GL, et al. Wisconsin Card Sorting Test-64 Card Version. Odessa, FL, Psychological Assessment Resources; 2000. [Google Scholar]
- 34. Carter CS, Minzenberg M, West R, et al. CNTRICS imaging biomarker selections: executive control paradigms. Schizophr Bull. 2012;38(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Purdon SE. The Screen for Cognitive Impairment in Psychiatry (SCIP): Administration Manual and Normative Data. 2005. Edmonton, Alberta, PNL Inc. [Google Scholar]
- 36. Harrigan RL, Yvernault BC, Boyd BD, et al. Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT: a multimodal data archive and processing environment. Neuroimage. 2016;124(Pt B):1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asman AJ, Landman BA. Hierarchical performance estimation in the statistical label fusion framework. Med Image Anal. 2014;18(7):1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauzon CB, Asman AJ, Esparza ML, et al. Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS One. 2013;8(4):e61737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheffield JM, Rogers BP, Blackford JU, et al. Accelerated aging of functional brain networks supporting cognitive function in psychotic disorders. Biol Psychiatry. 2019;86(3):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cetin-Karayumak S, Di Biase MA, et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2019:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Safadi Z, Grisot G, Jbabdi S, et al. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J Neurosci. 2018;38(8):2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Donoghue S, Kilmartin L, O’Hora D, et al. Anatomical integration and rich-club connectivity in euthymic bipolar disorder. Psychol Med. 2017;47(9):1609–1623. [DOI] [PubMed] [Google Scholar]
- 44. Lu LH, Zhou XJ, Fitzgerald J, et al. Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar Disord. 2012;14(6):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan WY, Yang GL, Chia MY, et al. Cortical and subcortical white matter abnormalities in adults with remitted first-episode mania revealed by Tract-Based Spatial Statistics. Bipolar Disord. 2010;12(4):383–389. [DOI] [PubMed] [Google Scholar]
- 46. Liu JX, Chen YS, Hsieh JC, et al. Differences in white matter abnormalities between bipolar I and II disorders. J Affect Disord. 2010;127(1–3):309–315. [DOI] [PubMed] [Google Scholar]
- 47. Barnea-Goraly N, Chang KD, Karchemskiy A, et al. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66(3):238–244. [DOI] [PubMed] [Google Scholar]
- 48. Haznedar MM, Roversi F, Pallanti S, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57(7):733–742. [DOI] [PubMed] [Google Scholar]
- 49. Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117(1):1–12. [DOI] [PubMed] [Google Scholar]
- 50. Yu K, Cheung C, Leung M, et al. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci. 2010;4:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haukvik UK, Tamnes CK, Söderman E, et al. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–226. [DOI] [PubMed] [Google Scholar]
- 52. Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41(2):225–241. [DOI] [PubMed] [Google Scholar]
- 53. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804–817. [DOI] [PubMed] [Google Scholar]
- 54. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 2015;30:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kharabian MS, Eickhoff SB, Hoffstaedter F, et al. Empirical examination of the replicability of associations between brain structure and psychological variables. Elife. 2019;8 3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mathalon DH, Ford JM. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Front Hum Neurosci. 2012;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bracht T, Schnell S, Federspiel A, et al. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res. 2013;143(2–3):269–276. [DOI] [PubMed] [Google Scholar]
- 59. Walther S, Stegmayer K, Federspiel A, et al. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dean DJ, Walther S, Bernard JA, et al. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5): 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Waters AB, Sawyer KS, Gansler DA. White matter connectometry among individuals with self-reported family history of drug and alcohol use disorders. Drug Alcohol Depend. 2020;206:107710. [DOI] [PubMed] [Google Scholar]
- 62. Klein N, Huang AS, Mitchell JA, et al. The thalamus in drug addiction: from rodents to humans. Philos Trans R Soc Lond B Biol Sci. 2018;373(1742):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(suppl 4):S233–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.