Abstract
Differentiation of pluripotent stem cells into functional parathyroid-like cells would accelerate development of important therapeutic options for subjects with parathyroid-related disorders, from the design and screening of novel pharmaceutical agents to the development of durable cellular therapies. We have established a highly reproducible directed differentiation approach leading to PTH-expressing cells from human embryonic stem cells and induced pluripotent stem cells. We accomplished this through the comparison of multiple different basal media, the inclusion of the CDK inhibitor PD0332991 in both definitive endoderm and anterior foregut endoderm stages, and a 2-stage pharyngeal endoderm series. This is the first protocol to reproducibly establish PTH-expressing cells from human pluripotent stem cells and represents a first step toward the development of functional parathyroid cells with broad applicability for medicinal and scientific investigation.
Keywords: hypoparathyroidism, development, stem cells, regenerative medicine, calcium
Regulation of normal bone and mineral metabolism requires a complex interplay between the skeleton, intestine, and kidneys that is controlled in part via the integrated action of PTH and vitamin D. PTH is the principal calciotropic hormone; it is secreted from parathyroid glands in inverse proportion to the concentration of extracellular ionized calcium (Ca2+). Expression of specific calcium-sensing receptors allows parathyroid cells to monitor the extracellular Ca2+ concentration and facilitates rapid changes in the rate of PTH secretion. PTH stimulates the release of calcium from bone and increases the reabsorption of calcium in the kidney. PTH also reduces reabsorption of phosphate in the kidney and increases enzymatic conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, the fully active form of vitamin D that stimulates absorption of calcium from the intestine (1). Genetic or acquired disorders that result in an excess of PTH is embodied in the condition called hyperparathyroidism, which causes pernicious bone density phenotypes in many patients (2). In contrast, disorders that impair synthesis or secretion of PTH lead to hypoparathyroidism, characterized by low serum calcium, increased serum phosphorus, and decreased bone remodeling. Conventional therapy for hypoparathyroidism includes oral calcium and active vitamin D (eg, calcitriol). Although this approach can correct hypocalcemia, it can also lead to or worsen hypercalciuria and is associated with unpredictable episodes of both hypercalcemia and hypocalcemia. Moreover, conventional therapy of hypoparathyroidism does not improve the quality of life in many affected individuals (3). These limitations led to the development of recombinant human PTH (rhPTH[1-84]) as a treatment for hypoparathyroidism in adults (4). Although hormone replacement therapy for hypoparathyroidism with rhPTH(1-84) provides significant benefits over conventional therapy for many individuals (5), daily injections of rhPTH(1-84) are painful and tedious to administer and do not achieve the precise control of serum calcium levels realized by native parathyroid glands, which continuously adjust the PTH secretory rate in response to changes in extracellular Ca2+ concentration. Furthermore, the limited duration of action and short circulating half-life of injected PTH and PTH analogs necessitates careful drug titration and often requires multiple daily injections, thereby complicating the treatment regimen (6). This is analogous to the limitations of current therapy for patients with type 1 diabetes mellitus, for whom insulin injections or infusions produce suboptimal control of serum glucose concentrations because of the multifactorial and dynamic nature of glucose homeostasis. By contrast, improved metabolic control has been achieved by organ transplantation without the need for exogenous hormones for both type 1 diabetes (7) and hypoparathyroidism (8), using whole pancreatic islets and parathyroid tissue, respectively, with variable success. These approaches, however, are severely limited by a shortage of normal viable donor tissue and the requirement for immunosuppression to prevent rejection of transplanted allogenic cells. We therefore sought to develop a protocol to direct the differentiation of pluripotent stem cells to parathyroid-like cells. The development of parathyroid-like cells will not only support the development of novel regenerative treatment options for subjects with parathyroid-related disorders, but will also expand the base of knowledge regarding parathyroid development and function.
Materials and Methods
Human pluripotent cell lines WA09 (H9) human ES cells, as well as Y6-induced pluripotent stem cells (iPSCs) (9), and CHOPWT10.2 iPSC (10) cells were maintained on Growth Factor Reduced Matrigel (Corning #356231) coated plates in mTESR1 (STEMCELL Technologies #85850) medium. Cells maintained as pluripotent cells were used for day 0 samples (undifferentiated RNA) for RT-quantitative PCR. H9 and Y6-iPSCs were incubated in 20% (ambient) O2, 5% CO2 conditions throughout maintenance and differentiation, whereas CHOPWT10.2 iPSCs were incubated in 5% O2, 5% CO2.
Definitive endoderm (DE) differentiation was performed as previously described (11), with minor modifications. Briefly, 1 well of a 6-well culture plate containing pluripotent cells at 60% to 80% confluence, was harvested as fractionated colonies after Dispase (STEMCELL Technologies #07913) treatment, then transferred to either 6 wells of a 6-well plate or 12 wells of a 12-well plate; all plates were coated with Matrigel (2 mg protein per plate, based on the certificate of analysis for each lot number). Cells were then differentiated to DE as described in Fig. 1, days 0 through 5. The day after transferring cells (day 0), another layer of Matrigel was added (Matrigel overlay) in cold mTESR medium. Once this Matrigel overlay was added, the cells were no longer considered pluripotent. On day 1, cells were allowed to rest with no medium changes. On day 2, a third application of Matrigel was added in cold advanced RPMI 1640 (ARPMI; Life Technologies #35050061) along with Glutamax (Life Technologies #3500061), penicillin/streptomycin (Life Technologies #15140-122), 100 ng/mL Activin A (R&D Systems #338-AC-050), 25 ng/mL WNT3a (R&D Systems #5036-WN-010/CF), and PD0332991 reconstituted in dimethyl sulfoxide (Tocris #4786) and used at 2.25 µM. On days 3 and 4, ARPMI was supplemented with 0.2% Knockout Serum Replacement (KSR; Life Technologies #10828010), Glutamax, penicillin/streptomycin, 100 ng/mL Activin A, 2.25 µM PD0332991. Day 5 medium consisted of ARPMI, 5% KSR, 100 ng/mL Activin A, 2.25 µM PD0332991, all-trans retinoic acid (ATRA; Sigma #R2625) reconstituted in dimethyl sulfoxide and used at 0.25 µM. CHOPWT10.2 iPSC cultures were initially seeded in 10 μm ROCK inhibitor with mTESR1, and CHIR 99021 (Tocris #4423) was used at 2 µM in place of WNT3a during DE. H9 cells were seeded without ROCK inhibitor.
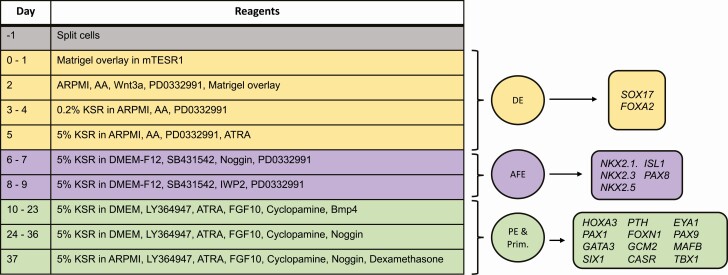
Figure 1.
Gene expression analysis was conducted throughout the protocol. The first column of the table shows the day of the protocol when changes to media were made and the components of the medium are provided in the second column. Color coding of medium changes are matched with the color code for the stage of differentiation (circles) and the genes analyzed for expression (rectangles). AFE, anterior foregut endoderm; DE, definitive endoderm; PE, pharyngeal endoderm; Prim, thymus/parathyroid primordium.
Anterior foregut endoderm (AFE) differentiation was performed over the course of 4 days (Fig. 1, days 6-9) in DMEM-F12 (Life Technologies #11330-032), 5% KSR, Glutamax, and penicillin/streptomycin. On days 6 and 7, medium was supplemented with 20 µM SB431542 (InvivoGen #inh-sb43) and 100 ng/mL NOG (R&D Systems #6057-NG-025/CF). On days 8 and 9, medium was supplemented with 2.25 µM PD0332991 and 1 µM IWP2 (Tocris #3533).
Pharyngeal endoderm (PE) differentiation occurred in 2 phases, with each phase comprising 14 days (Fig. 1). For days 10 through 23, high glucose DMEM (Life Technologies #119650-092) was supplemented with 5% KSR, penicillin/streptomycin, Glutamax, 1 ng/mL BMP4 (R&D Systems #314-BP/CF), 5 µM LY364947 (Cayman Chemicals #13341), 0.1 μM ATRA, 50 ng/mL FGF10 (R&D Systems #345-FG-025/CF), and 0.5 µM cyclopamine (Calbiochem #239807). Medium for days 24 through 36 was the same except BMP4 was removed and replaced with its inhibitor NOG at 400 ng/mL. On day 37, the basal medium was replaced with ARPMI (which is low in calcium and high in phosphate) to stimulate PTH secretion, and dexamethasone (Sigma #D2915) at 10 nM to enhance preproPTH transcription (12).
To isolate RNA, cells were harvested with Accutase (Millipore #SCR005) and processed using either the Qiagen RNeasy (Qiagen # 74104) or RNeasy Plus (#74134) kit. The High Capacity cDNA Reverse Transcription Kit (Applied Biosystems #4368814) was used for first-strand cDNA synthesis. Gene expression analysis for H9 cultures was performed by RT-quantitative PCR with either a C1000 or C1000 Touch thermal cycler (BioRad), using BioRad reagent SsoFast EvaGreen (#1725201) and CAPN10 as the reference gene (13). Primers and all other supplementary figures and data are located in a digital research materials repository (14). For CHOPWT10.2 cells, Taqman assays Hs00757710_g1 (PTH) and Hs00899403_m1 (GCM2) were performed using a QuantStudio 12K Flex platform (Applied Biosystems) with GAPDH (Hs00757710_g1) as the reference gene. We used the Delta-Delta-Ct algorithm to determine relative gene expression in the RT-quantitative PCR experiments, and expressed data as fold change in expression relative to undifferentiated cells after reference gene normalization (15).
For immunofluorescence, H9 cells differentiated to DE were harvested with Accutase, washed with PBS, cytospun as single cells onto slides, and stained with Goat Anti-Human SOX17 (R&D Systems #AF1924; RRID:AB_355060) (16) at 10 µg/mL and Donkey Anti-Goat IgG-FITC (Santa Cruz #sc-2024; RRID:AB_631727) (17) at 1:100 dilution. A Leica DMI6000 microscope was used to capture images at 40× magnification. The number of SOX17+ nuclei was counted and calculated as a percentage of all Dapi+ nuclei for 6 random fields of view for each experimental condition.
Flow cytometry to measure the state of pluripotency of H9 cells after differentiation to DE was performed by staining cells with the PE-conjugated anti-stage-specific embryonic antigen-4 (SSEA4) antibody (clone MC-813-70; Millipore #CS211423; RRID:AB_2861143) (18) at 5 µL per 1 million cells, without cell permeabilization. Briefly, cells were washed and harvested from culture plates using Accutase. Cells were then incubated for 30 minutes on ice in the presence of antibody diluted to a total volume of 100 µL in fluorescence-activated cell sorting buffer comprising PBS, 1% fetal bovine serum, and 2 mM EDTA. Cells were then washed and resuspended in fluorescence-activated cell sorting buffer for analysis. Ten thousand cells were captured/condition on an LSRII cytometer and analyzed with FlowJo software (Becton Dickinson). Gates were set for pre- and post-DE samples based on the inclusion of 1.03% of unstained cells.
Statistical analysis was conducted using the Prism software package (GraphPad Software, Inc.). A paired t test was used to determine whether there was a difference between the number of nuclei observed with and without the use of PD0332991 during DE differentiation (Fig. 2c), and an unpaired t test to assess the significance of differences in gene expression from DE to AFE.
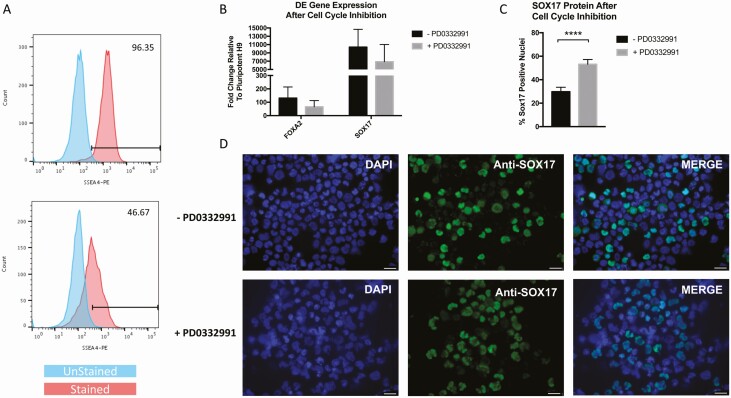
Figure 2.
Definitive endoderm differentiation was improved with the inclusion of the cell cycle inhibitor PD0332991. Cells were stained with SSEA-4 at day 0 of differentiation (A, top plot) and at day 5 (A, bottom plot). Histograms show SSEA-4 expressing cells (red) with gates determined based on unstained cells (blue). (B) The CDK4/6 inhibitor PD0332991 was added to medium as described in Fig. 1 or was absent during DE differentiation. Those cells were then analyzed for RNA expression of FOXA2 and SOX17 at the end of DE differentiation (n = 4 independent experiments). Some cells subjected to ±PD0332991 were reserved for cytospins and immunofluorescently stained with anti-SOX17 and DAPI. Green (SOX17) and blue (DAPI) cells from + or - PD0332991 were counted and graphed in C, with P < 0.0001. Images for SOX17 staining are shown in D with -PD0332991 on the top and +PD0332991 on the bottom. White scalebar represents 25 μm. Error bars on graphs are standard error of the mean (SEM). DE, definitive endoderm; SSEA4, stage-specific embryonic antigen-4.
Results
To develop a protocol for the differentiation of pluripotent cells to parathyroid-like cells, we sought to reproduce the developmental model of parathyroid organogenesis as it is currently understood in mouse models (19). To determine whether we had achieved each developmental milestone, we assessed expression of transcripts corresponding to genes representative of each stage (Fig. 1). By using defined conditions and adjusting the concentration, duration, and combination of cytokines, inhibitors, and basal media, we consistently obtained cultures with PTH-expressing cells. This was accomplished for both human embryonic stem cell (hESC) and iPSC lines in 2 separate laboratories.
Definitive endoderm differentiation
In vivo, DE is generated from the epiblast during gastrulation (20) and is largely driven by Tgfβ/Smad2 signaling (21). To emulate the DE stage, we modified a protocol that included TGFβ/Activin A and Wnt3a (22) by including a Matrigel sandwich and basal medium changes to promote the mesenchymal to epithelial transition and improve cell survival (11). Other published work revealed the propensity of hESCs to initiate endoderm commitment in early G1 phase of mitosis rather than in late G1 when differentiation to neurectoderm is favored (23). Because the CDK4/6 inhibitor PD0332991 increases cells in early G1 and enhances endoderm formation, we tested the use of this inhibitor in the context of our DE protocol. After differentiation, we observed that SSEA4 was downregulated by 49% (Fig. 2a), a greater response than that seen following endoderm differentiation with another popular protocol (24). Although expression of FOXA2 and SOX17 mRNA was not significantly changed as a result of adding PD0332991 (Fig. 2b), the number of SOX17+ nuclei was significantly higher with this cell-cycle inhibitor (Fig. 2c, d).
Anterior foregut endoderm differentiation
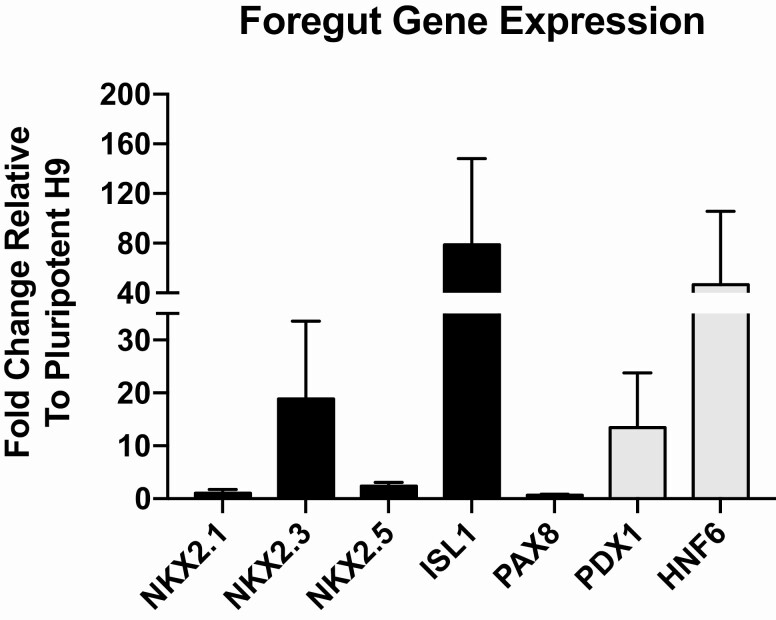
During gastrulation, definitive endoderm cells intercalate with visceral endoderm to generate gut endoderm (25). These cells then form the gut tube from which foregut organs are derived (26), including the liver, pancreas, lung, and parathyroid glands. Anteriorization of the foregut is necessary for parathyroid and lung organogenesis, whereas posteriorization leads to pancreas and liver. We compared differentiation into AFE using a variety of published protocols and found Huang et al (27) to be most effective based on upregulation of foregut genes that are predictive of anterior patterning of the gut tube (28). To induce cells with greater expression of these AFE-associated genes, we extended the use of PD0332991 into the AFE stage (days 6-9) and increased the concentration of the TGFβ inhibitor SB431542. These changes resulted in increases in ISL1 and NKX2.3 expression to 19-fold and 79-fold, respectively (Fig. 3). We were particularly encouraged by the upregulation of NKX2.3, which is important for development of pharyngeal pouch endoderm (29, 30), from which parathyroid progenitors are derived (31). We observed a small increase in NKX2.1 that is less than that previously reported by other investigators (32), and a small increase in NKX2.5, with no change in PAX8 expression levels.
Figure 3.
Foregut gene expression after AFE differentiation. Cells were differentiated to AFE as described in Fig. 1, then RNA was extracted and RT-quantitative PCR conducted to evaluate the expression of both anterior (black bars) and posterior (gray bars) foregut genes. Expression shown is relative to undifferentiated H9 cells, and error bars represent the SEM. n = 4 biological repeats. AFE, anterior foregut endoderm.
Pharyngeal endoderm differentiation
Parathyroid-specific Gcm2-positive progenitors first appear in the third pharyngeal pouch at murine E9.5 and coexist with Foxn1-positive thymus progenitors in a common primordium by E11.25 (31). Cells expressing Gcm2 and Foxn1 are compartmentalized within the primordium and later separate and migrate in opposite directions, with parathyroid progenitors moving rostrally to form the parathyroid glands and associate with the thyroid. The signals involved in these events are largely unknown, but some factors such as FGF10, secreted by surrounding neural crest cells and perhaps mesoderm, are important (33). Our first successful upregulation of PTH mRNA expression was accomplished using a protocol that included ARPMI as the basal medium throughout all stages, the CDK4/6 inhibitor only during DE, and inclusion of FGF10, cyclopamine, LY364947, ATRA, and WNT3a during PE. We subsequently tested the addition of chemical modifiers such as dexamethasone, valproic acid, trichostatin A, and 5-azacytidine at various concentrations. Only dexamethasone was found to be beneficial by providing a small increase in GCM2 expression (data not shown). With this new medium formulation containing dexamethasone, most of the PE genes including PTH were upregulated in both H9 hESCs and Y6-iPSCs by day 37 of the protocol (supplementary Fig. 1) (14).
However, because we were not able to consistently obtain PTH upregulation between experiments, we made several adjustments to the protocol. First, basal medium changes were made throughout all the stages. We chose high-glucose DMEM, DMEM-F12, and ARPMI as basal media to test based on the levels of phosphate and calcium present (supplementary Fig. 2) (14). PTH expression and secretion in vivo is dependent on extracellular levels of both calcium and phosphate levels, with expression increased by low calcium and high phosphate conditions (34). We tested the 3 media during the AFE and PE stages. For DE, we started with either ARPMI as used in our previously published work (11), or RPMI 1640, which is the medium used for DE differentiation in another protocol (22). Because ARPMI had the lowest calcium and highest phosphate concentrations of the media we tested, we chose ARPMI to stimulate PTH expression for all cells overnight before RNA extraction. We found clear differences between the different basal media we tested, with ARPMI during DE, DMEM-F12 during AFE, and high-glucose DMEM during PE producing the highest PTH upregulation (supplementary Fig. 2) (14).
In vivo studies suggest an important role for Bmp4 and Nog in the development of the thymus/parathyroid primordium with 1 study showing Nog expression associated with the Gcm2-expressing cells of the thymus/parathyroid primordium, and Bmp4 with the Foxn1-expressing cells in developing mouse embryos (35). A different study shows NOG to be inhibitory for Gcm2 expression in quail/chick chimeras (36). We therefore tested the use of BMP4 during the first half (days 10-22) of the PE stage followed by its inhibition with NOG in the second half (days 23-37), and made changes in DE and AFE (using PD0332991 and SB431542 as described earlier). In comparison to NOG alone, BMP4 increased PTH expression. PTH expression levels were also increased with changes in the DE and AFE stages as illustrated (supplementary Fig. 3) (14). Simultaneous use of both changes as described in Fig. 1 greatly enhanced consistency in induction of PTH-expressing cells and enabled a 23-fold upregulation in PTH expression (Fig. 4a). We found that WNT3a was not required during the PE stage for PTH upregulation and eliminated it from our final formulation (Fig. 1).
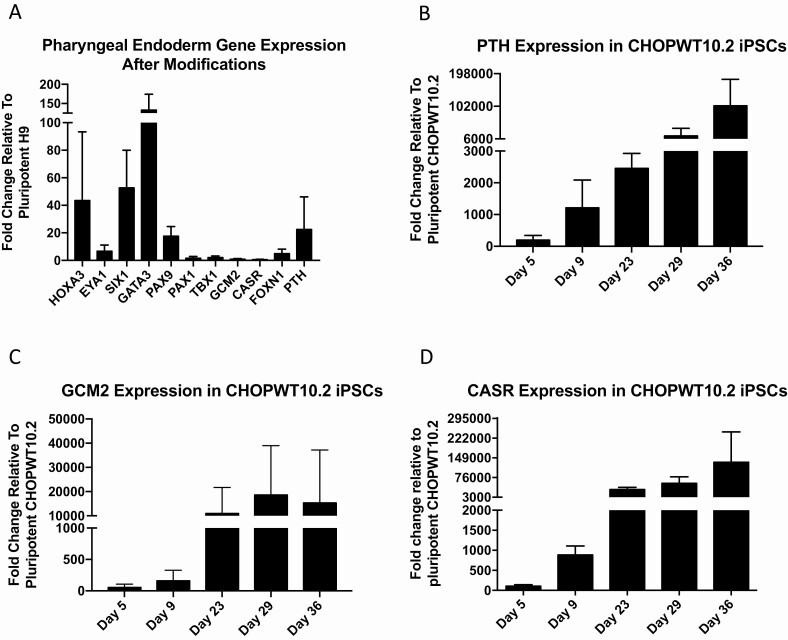
Figure 4.
Pharyngeal endoderm genes are upregulated from anterior foregut. RT-quantitative PCR was performed from cells after differentiation to PE over 37 days as in Fig. 1 using the H9 cell line. (A) Expression of genes known to be important for PE development relative to undifferentiated (day 0) H9 cells (n = 3 biological repeats). (B-D) Differentiation was also conducted using CHOPWT10.2 iPSCs. Cells were removed at various times throughout the protocol and evaluated for expression of PTH, GCM2, and CASR. Fold change is plotted relative to undifferentiated iPSCs (day 0). These data represent 2 differentiation samples. Error bars represent SEM. iPSC, induced pluripotent stem cell; PE, pharyngeal endoderm.
Gcm2 is the earliest parathyroid-specific gene reported to be expressed after gastrulation in vivo in mice and is required both for parathyroid differentiation from progenitors and for maintaining survival of parathyroid cells after maturation (31). When we began these studies, we initially focused on inducing expression of GCM2, anticipating the propagation of progenitors that we could then expand as PTH-positive, terminally differentiated cells. However, we found that expression of GCM2 was low in pluripotent cells, and did not change significantly in H9 cells throughout the full 37-day protocol (supplementary Fig. 4) (14), whereas we were able to achieve significant and consistent increases in PTH expression (Fig. 4a). This suggests that the low levels of GCM2 expression in pluripotent cells are sufficient to establish and maintain PTH expression under the right conditions.
Time course of PTH expression
An iPSC line derived from human peripheral blood mononuclear cells (CHOPWT10.2) showed early and robust upregulation by 196-fold for PTH with the same protocol (Fig. 4b). The presence of PTH at the end of the DE stage suggests that this iPSC line may be primed for PTH expression and may be enriched for early progenitors. This cell line may therefore provide a unique opportunity to study early parathyroid development by identifying transcription factors and epigenetic states permitting expression. GCM2 expression was upregulated during DE by 55-fold then increased further to 10 900-fold later in the protocol after NOG was added at day 22 (Fig. 4c). Similarly, CASR expression increased from 104-fold at day 5 to 31 458-fold by day 22 (Fig. 4c). The 2 phases in GCM2 induction support its role as both an inducer of PTH transcription and as a factor for the survival and maintenance of PTH-positive cells.
Discussion
An understanding of parathyroid biology is critical to the study of calcium homeostasis. Nevertheless, comprehensive analyses of parathyroid gland biology have been challenged by a number of difficulties, including the small size of the glands, a lack of available healthy human tissue, the lack of suitable cell lines, and the inability to maintain calcium-responsive primary cells in culture for long periods without overgrowth of nonparathyroid cells. As a consequence, pharmaceutical and cell therapeutic treatment options are limited for many patients with parathyroid-related conditions. The development of a protocol that reliably generates parathyroid-like cells from pluripotent stem cells would allow for the production of an unlimited number of parathyroid cells for biological studies, pharmaceutical drug screening, and other cell therapeutic options. One group reported increased expression of PTH from pluripotent stem cells (37, 38); however, this protocol was not reproducible in our hands, likely because of different cell lines used as well as the use of fetal bovine serum, which can vary from lot to lot. Guided by these disappointing experiments, our goal was to develop a reproducible protocol using a serum replacement with defined medium that would enable the differentiation of parathyroid-like cells from pluripotent stem cells from multiple cell lines.
We successfully improved upon our previously published protocol for the derivation of DE, and showed strong upregulation of some AFE-related genes, particularly NKX2.3. The parathyroid foregut progenitor that differentiates into pharyngeal endoderm and later becomes part of the thymus/parathyroid primordium has not been defined. Given that mice null for Nkx2.1 exhibit normal thymus and parathyroid development (39), Nkx2.1 positive progenitors known to be necessary for lung and thyroid development are not required for parathyroid formation. Further, while Nkx2.5 is transiently expressed in PE in vivo and can be induced to form thymic epithelial cells from mouse ES cells in 1 report (40), the Nkx2.5 fraction in mES cells did not express Gcm2, and Pth expression was not tested. There is evidence that NKX2.3-positive foregut progenitors give rise to epithelia of the pharyngeal pouches (29), suggesting a potential role for this gene in thymus/parathyroid primordium formation.
We achieved reproducible upregulation of PTH and other PE genes that could be replicated across cell lines. Although the degree of upregulation may vary between cell lines, close adherence to the protocol should enable PTH expression from pluripotent stem cells regardless of the source. In CHOPWT10.2 iPSCs, the greatest degree of upregulation of GCM2 was noted following day 23 of the protocol, after BMP4 was removed and NOG was introduced. The study of Patel et al (35) shows an association between Bmp4 and FoxN1, and between Nog and Gcm2 in the developing murine thymus/parathyroid primordium. However, because expression of Gcm2 precedes that of Nog in vivo in mice, and because expression did not increase further after day 23 in culture, it is unlikely that NOG was the inducer of GCM2 upregulation during PE. Rather, the increase in PTH may have necessitated an increased need for GCM2 for survival and maintenance.
We noted an upregulation of posterior foregut markers PDX1 and HNF6 during AFE differentiation of H9 cells (Fig. 2, supplementary Fig. 5) (14). This suggests that further optimization of the protocol is needed at this stage, as high expression of posterior foregut genes may be inhibitory for expression of genes for anterior foregut-derived tissues such as the parathyroid glands. In the absence of an AFE reporter cell line, this potentially could be accomplished by sorting CD56+CD271+ cells, which is reported to enrich for cells with NKX2.1 expression (41). The CD56–CD271– fraction in that study contained cells positive for posterior foregut markers such as PDX1, whereas the double positive fraction did not. It is possible that the double positive fraction of cells may express other anterior foregut genes and may be more efficient at PTH induction in the absence of cells expressing PDX1.
We anticipate that this protocol will facilitate further identification of the biological determinants of PTH expression and secretion and will serve as a useful resource for development of additional treatment options for patients with parathyroid-related conditions.
Acknowledgments
The authors thank the Yale Stem Cell Core and Yale Stem Cell Center for material and intellectual support of this project.
Financial Support: Funding sources include the Connecticut Stem Cell Research Fund (12-SCA-YALE-23), Connecticut Regenerative Medicine Research Fund (15-RMB-YALE-18), Women’s Health Research at Yale, the CHOP Research Institute, the Aileen K. and Brian L. Roberts Foundation, Peter Berman Fund, Burton Block Fund, Robert Epstein, and the National Cancer Institute (R21 CA240660).
Glossary
Abbreviations
- AFE
anterior foregut endoderm
- ARPMI
advanced RPMI
- ATRA
all-trans retinoic acid
- Ca2+
ionized calcium
- DE
definitive endoderm
- hESC
human embryonic stem cell
- iPSC
induced pluripotent stem cell
- KSR
Knockout Serum Replacement
- PE
pharyngeal endoderm
- rhPTH(1-84)
recombinant human PTH
- SSEA4
stage-specific embryonic antigen-4
Additional Information
Disclosure Summary: J.A.S. is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca, and Eli Lilly; she also receives institutional research funding from Exelixis and Eli Lilly. M.A.L. is a member of the PARADIGHM Global Scientific Steering Committee and a PARADIGHM Investigator for a natural history registry of hypoparathyroidism sponsored by Takeda/Shire. The authors declare no conflicts of interest.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on request.
References and Notes
- 1. Conigrave AD. The calcium-sensing receptor and the parathyroid: past, present, future. Front Physiol. 2016;7:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeLellis RA. Parathyroid tumors and related disorders. Mod Pathol. 2011;24 Suppl 2:S78-S93. [DOI] [PubMed] [Google Scholar]
- 3. Clarke BL. Epidemiology and complications of hypoparathyroidism. Endocrinol Metab Clin North Am. 2018;47(4):771-782. [DOI] [PubMed] [Google Scholar]
- 4. Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1(4):275-283. [DOI] [PubMed] [Google Scholar]
- 5. Mannstadt M, Clarke BL, Bilezikian JP, et al. Safety and efficacy of 5 years of treatment with recombinant human parathyroid hormone in adults with hypoparathyroidism. J Clin Endocrinol Metab. 2019;104(11):5136-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramakrishnan Y, Cocks HC. Impact of recombinant PTH on management of hypoparathyroidism: a systematic review. Eur Arch Otorhinolaryngol. 2016;273(4):827-835. [DOI] [PubMed] [Google Scholar]
- 7. Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev. 2019;40(2):631-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen MS, Dilley WG, Wells SA Jr, et al. Long-term functionality of cryopreserved parathyroid autografts: a 13-year prospective analysis. Surgery. 2005;138(6):1033-1040; discussion 1040. [DOI] [PubMed] [Google Scholar]
- 9. Dash BC, Levi K, Schwan J, et al. Tissue-engineered vascular rings from human iPSC-derived smooth muscle cells. Stem Cell Reports. 2016;7(1):19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maguire JA, Gagne AL, Jobaliya CD, Gandre-Babbe S, Gadue P, French DL. Generation of human control iPS cell line CHOPWT10 from healthy adult peripheral blood mononuclear cells. Stem Cell Res. 2016;16(2):338-341. [DOI] [PubMed] [Google Scholar]
- 11. Lawton BR, Sosa JA, Roman S, Krause DS. Effect of a matrigel sandwich on endodermal differentiation of human embryonic stem cells. Stem Cell Rev Rep. 2013;9(5):578-585. [DOI] [PubMed] [Google Scholar]
- 12. Peraldi MN, Rondeau E, Jousset V, et al. Dexamethasone increases preproparathyroid hormone messenger RNA in human hyperplastic parathyroid cells in vitro. Eur J Clin Invest. 1990;20(4):392-397. [DOI] [PubMed] [Google Scholar]
- 13. Holmgren G, Ghosheh N, Zeng X, Bogestål Y, Sartipy P, Synnergren J. Identification of stable reference genes in differentiating human pluripotent stem cells. Physiol Genomics. 2015;47(6):232-239. [DOI] [PubMed] [Google Scholar]
- 14. Krause DS Data for: supplemental data and info_Lawton et al., Yale, Dataset. Dryad Digital Repository 2019. Deposited 27 July 2020. 10.5061/dryad.djh9w0vx5. [DOI] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 16. RRID:AB_355060, https://scicrunch.org/resolver/RRID:AB_355060 [Google Scholar]
- 17. RRID:AB_631727, https://scicrunch.org/resolver/RRID:AB_631727 [Google Scholar]
- 18. RRID:AB_2861143, https://antibodyregistry.org/search?q=RRID:AB_2861143 [Google Scholar]
- 19. Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4(4): 278-289. [DOI] [PubMed] [Google Scholar]
- 20. Nowotschin S, Hadjantonakis AK. Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr Opin Genet Dev. 2010;20(4):420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tremblay KD, Hoodless PA, Bikoff EK, Robertson EJ. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development. 2000;127(14):3079-3090. [DOI] [PubMed] [Google Scholar]
- 22. D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392-1401. [DOI] [PubMed] [Google Scholar]
- 23. Pauklin S, Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155(1):135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramirez JM, Gerbal-Chaloin S, Milhavet O, et al. Brief report: benchmarking human pluripotent stem cell markers during differentiation into the three germ layers unveils a striking heterogeneity: all markers are not equal. Stem Cells. 2011;29(9):1469-1474. [DOI] [PubMed] [Google Scholar]
- 25. Viotti M, Nowotschin S, Hadjantonakis AK. SOX17 links gut endoderm morphogenesis and germ layer segregation. Nat Cell Biol. 2014;16(12):1146-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang SX, Green MD, de Carvalho AT, et al. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc. 2015;10(3):413-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowotschin S, Setty M, Kuo YY, et al. The emergent landscape of the mouse gut endoderm at single-cell resolution. Nature. 2019;569(7756):361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li L, Ning G, Yang S, Yan Y, Cao Y, Wang Q. BMP signaling is required for nkx2.3-positive pharyngeal pouch progenitor specification in zebrafish. PLoS Genet. 2019;15(2):e1007996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biben C, Wang CC, Harvey RP. NK-2 class homeobox genes and pharyngeal/oral patterning: Nkx2-3 is required for salivary gland and tooth morphogenesis. Int J Dev Biol. 2002;46(4):415-422. [PubMed] [Google Scholar]
- 31. Gordon J, Bennett AR, Blackburn CC, Manley NR. Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech Dev. 2001;103(1-2):141-143. [DOI] [PubMed] [Google Scholar]
- 32. Huang SX, Islam MN, O’Neill J, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32(1):84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teshima TH, Lourenco SV, Tucker AS. Multiple cranial organ defects after conditionally knocking out Fgf10 in the neural crest. Front Physiol. 2016;7:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naveh-Many T, Nechama M. Regulation of parathyroid hormone mRNA stability by calcium, phosphate and uremia. Curr Opin Nephrol Hypertens. 2007;16(4):305-310. [DOI] [PubMed] [Google Scholar]
- 35. Patel SR, Gordon J, Mahbub F, Blackburn CC, Manley NR. Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr Patterns. 2006;6(8): 794-799. [DOI] [PubMed] [Google Scholar]
- 36. Neves H, Dupin E, Parreira L, Le Douarin NM. Modulation of Bmp4 signalling in the epithelial-mesenchymal interactions that take place in early thymus and parathyroid development in avian embryos. Dev Biol. 2012;361(2):208-219. [DOI] [PubMed] [Google Scholar]
- 37. Bingham EL, Cheng SP, Woods Ignatoski KM, Doherty GM. Differentiation of human embryonic stem cells to a parathyroid-like phenotype. Stem Cells Dev. 2009;18(7):1071-1080. [DOI] [PubMed] [Google Scholar]
- 38. Woods Ignatoski KM, Bingham EL, Frome LK, Doherty GM. Differentiation of precursors into parathyroid-like cells for treatment of hypoparathyroidism. Surgery. 2010;148(6):1186-1189; discussion 1189. [DOI] [PubMed] [Google Scholar]
- 39. Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10(1):60-69. [DOI] [PubMed] [Google Scholar]
- 40. Hidaka K, Nitta T, Sugawa R, et al. Differentiation of pharyngeal endoderm from mouse embryonic stem cell. Stem Cells Dev. 2010;19(11):1735-1743. [DOI] [PubMed] [Google Scholar]
- 41. Brafman DA, Moya N, Allen-Soltero S, et al. Analysis of SOX2-expressing cell populations derived from human pluripotent stem cells. Stem Cell Reports. 2013;1(5):464-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on request.