Abstract
Catatonia is a psychomotor syndrome defined by a constellation of predominantly motor symptoms. The aim of the present study was to determine whether recently admitted psychiatric patients with catatonia exhibited higher serum C-reactive protein (hs-CRP) levels compared to non-catatonic psychiatric patients and healthy controls (HCs). Recently admitted psychiatric patients were screened and evaluated for the catatonia syndrome using the Bush-Francis Catatonia Rating Scale and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). The study sample was formed by 150 individuals (39 male and 111 female), including 51 catatonic patients, 55 non-catatonic patients, and 44 HCs. Serum hs-CRP levels were processed with the enzyme-linked immunosorbent assay. Serum levels of creatine kinase (CK), adrenocorticotropic hormone (ACTH), immunoglobulin G (IgG), complement component 3 (C3), and complement component 4 (C4) were also determined. There was a significantly higher percentage of patients with high inflammatory levels (hs-CRP > 3000ng/ml) in the catatonic (43.1%) than in the non-catatonic (14.5%) or HCs group (9.1%) (χ 2 =18.9, P < .001). Logistic regression showed that catatonic patients had significantly higher hs-CRP levels compared to non-catatonic patients even after controlling for other clinical and laboratory variables (OR = 3.52, P = .015, 95% CI 1.28–9.79). Multiple linear regression analysis revealed that log-transformed hs-CRP was independently predicted by body mass index and log-transformed C4, ACTH, and Cortisol in catatonic patients. Findings of the present study suggest that catatonia is specifically linked to a higher level of systemic inflammation, not merely attributable to the overall psychopathology, or alterations in the stress level and complement system.
Keywords: psychomotor syndrome, inflammation, serum concentration, psychotic disorders, mood disorders
Introduction
Catatonia is a psychomotor syndrome characterized by motor, affective, and behavioral symptoms, associated with a wide range of physical and mental disorders.1 It has been reported in 4%–67% of patients with schizophrenia and 14%–71% of patients with mood disorders.2 Although catatonia is common, the underlying mechanisms remain largely unclear. Earlier work by Gjessing and colleagues found a periodic nitrogen imbalance corresponding to the periodic rhythm of catatonic relapses.3 They also demonstrated dysregulated norepinephrine and dopamine metabolism in the catatonic state.4 Northoff and colleagues found that plasma homovanillic acid (HVA), a major metabolite of dopamine,5 was higher in acute catatonia and higher levels were associated with positive responses to lorazepam.6 A neuroimaging study showed diffuse cortical sulcal enlargement particularly in the left frontotemporal areas in catatonic schizophrenia patients.7 It has also been shown that catatonic patients exhibited decreased rCBF in the right posterior parietal cortex, and functional connectivity between the medial orbitofrontal and premotor/motor cortex was also altered.8,9 Recently, Walther and colleagues reviewed the cerebral motor network dysfunction, outlined convergent evidence on abnormal hyperactivity in the supplementary and pre-supplementary motor areas, and provided a model of catatonia as a psychomotor syndrome.10 Another important review highlighted the aberrant higher-order frontoparietal networks and emphasized the critical role of γ-aminobutyric-acid (GABA)-ergic dysregulation in the affective domain of catatonia.11
Catatonia was noted in various inflammatory brain diseases12,13 and highly prevalent in psychiatric patients who were positive for N-methyl-d-aspartate (NMDA) receptor antibodies.14,15 A recent review by Rogers and colleagues considered immune activation and downstream glutamatergic or GABA-ergic dysregulation played key roles in the pathogenesis of catatonia, as it occurs in various autoimmune conditions.16 Myelin-producing oligodendrocytes and low-grade neuroinflammation have been implicated in the pathogenesis of catatonia.17 The study by Janova and colleagues demonstrated reduced expression of the structural myelin protein 2′-3′-cyclic nucleotide 3′-phosphodiesterase (CNP) was associated with catatonic signs, both in humans and mice.18 Furthermore, microglial ablation through inhibitor of CSF1 receptor kinase signaling PLX5622 alleviated the catatonia of CNP–/– mice.18
A variant of catatonia known as malignant or lethal catatonia with fever, delirium, and autonomic disturbances clinically resembles neuroleptic malignant syndrome (NMS).19 Low levels of serum iron, a negative acute phase reactant, have been noted in NMS20 and a neuroimmunological hypothesis has been proposed to explain the pathophysiology of NMS.21 Low serum iron has also been found not only in malignant catatonia but also in nonmalignant acute catatonia episodes.22,23 One would hence speculate that the positive acute phase reactant, C-reactive protein (CRP), is likely to be elevated in catatonia.
Accumulating evidence, as reflected by alterations in circulating inflammatory markers,24–28 suggests a transdiagnostic association with low-grade systemic inflammation in schizophrenia, bipolar disorders, depressive, anxiety, and other psychiatric disorders. CRP is directly modulated by IL-6 and IL-1β 29–31 and is commonly used in clinical practice as a reliable biomarker of subclinical and systemic inflammation.32,33 A number of studies have investigated CRP in schizophrenia and mood disorders with inconsistent results. While more than half of the studies reported an elevated blood CRP level,29–31 others reported no difference between patients and healthy controls (HCs).34–36 It remains controversial whether increased CRP in schizophrenia is merely a result of an increased prevalence of risk factors of systemic inflammation, such as stress,37 smoking,38 obesity,39 and physical comorbidities.40,41 Moreover, increased CRP levels may be associated with certain symptom dimensions in schizophrenia, such as negative symptoms,42 aggressive behavior,43 and cognitive symptoms.44,45
To our knowledge, there has been only one study in examining the association of CRP with clinical phenotypes in Arab schizophrenic patients, which found a higher CRP level in those with catatonia.46 However, only chronic schizophrenia patients were included, and only 12 patients had catatonia. Catatonia was not evaluated using a standardized rating scale. Moreover, patients with catatonia (n = 12) were not matched with those without catatonia (n = 87) in terms of body mass index (BMI), smoking, physical comorbidities, and stress level. Catatonic patients often develop various physical complications (eg, pneumonia or deep vein thrombosis) due to symptoms, such as akinesia, stupor, rigidity, swallowing difficulties, or dehydration.47–50 All these variables could contribute to a high systemic inflammatory level, possibly confounding the results.
In the present study, we systematically screened and evaluated patients admitted to the psychiatric inpatient units in a large teaching hospital in Beijing using a standardized catatonia rating scale. The aims of the present study were (1) to determine whether recently admitted patients with catatonia exhibited higher serum C-reactive protein (hs-CRP) levels compared to non-catatonic patients as well as HCs; (2) to clarify whether the increased serum hs-CRP level in catatonic patients was a primary or just secondary to more severe symptomatology or comorbidities; and (3) to identify the independent predictors of high inflammatory levels in catatonic and non-catatonic patients. The first hypothesis was that serum CRP levels would be higher in catatonic patients than in non-catatonic patients and HCs. The second hypothesis was that a higher serum CRP level would be a distinctive feature of catatonia regardless of the diagnosis, psychopathology, and stress level. The third hypothesis was that higher CRP levels can be independently predicted by a host of clinical and laboratory variables, including the psychopathology, stress hormones, and innate immune variables (complement C3 and/or C4).
Methods and Materials
Participants
Participants were consecutively recruited from in-patient departments at Beijing Anding Hospital between January 2018 and October 2018.
Inclusion criteria for the catatonic patients were as follows: (1) first episode or acute exacerbations of any type of psychiatric disorders according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)51; (2) recently admitted patients with acute catatonia, according to DSM-5 and confirmed in a clinical interview by two attending psychiatrists.
Inclusion criteria for the non-catatonic psychotic patients were: (1) first episode or acute exacerbations of any type of psychiatric disorders according to DSM-5; (2) recently admitted patients without catatonia (fulfilling neither DSM-5 nor Bush-Francis Catatonia Screening Instrument criteria for catatonia),52 which was confirmed by two attending psychiatrists in a clinical interview; (3) matched with catatonic patients in age and gender.
HCs were recruited from the community through advertisements and matched with catatonic patients in age and gender. They also had to go through a structured clinical interview for DSM-IV (The Structured Clinical Interview for DSM-5 is not available in Chinese version) to ensure they were not suffering from any psychiatric disorders or mood disorders.
Exclusion criteria for the three group were as follows: (1) history of or current substance abuse; (2) history of or current major physical conditions, including any symptoms or signs of infections; or any positive laboratory or imaging examinations indicating infections, determined by a thorough chart review, physical examination, routine blood tests, computed tomography (CT), or magnetic resonance imaging (MRI) scan; and (3) pregnancy or breastfeeding.
The study protocol was approved by the Clinical Research Ethics Committee of Beijing Anding Hospital. Written informed consent was obtained from all participants or their guardians (for those who were in catatonic state and not capable of giving a consent).
Clinical Assessments
Catatonia was screened and evaluated with Bush-Francis Catatonia Rating Scale (BFCRS).52 BFCRS was a 23-item scale involving three factors: negative/withdrawn, automatic, repetitive/echo, and agitated/resistive phenomena.53The scale was developed based on the classical description of catatonia from previous literatures.52,54,55 Item 1–14 were selected to form a standardized screening tool for catatonia (BFCSI). If 2 or more of the 14 symptoms were present for more than 24 hours, catatonia features should be considered. Each item was scored from 0 to 3, and the total score ranges from 0 to 69. The treating psychiatrists performed the BFCRS on the day of admission. The inter-rater reliability reached a high level among the treating psychiatrists (Cronbach’s alpha value of 0.89).
Patients’ psychopathology was assessed with the Chinese version of the Positive and Negative Symptom Scale (PANSS).56,57 PANSS was performed on the day of admission or when the patients were able to cooperate with a clinical interview.
Blood Sample Collection and Assessment
Blood samples were drawn from the antecubital vein at 8 am in the morning after an overnight fast of at least 8 hours, and then collected into pre-chilled 5-ml vacutainer tubes with clot activator (serum tube). Serum was isolated from the whole blood by centrifugation (3000g for 10 min), and transferred and aliquoted into Eppendorf tubes and stored in a −70°C freezer. Hs-CRP levels were processed with a commercially available (R&D) enzyme-linked immunosorbent assay (ELISA) at Beijing Key Laboratory of Mental Disorders. Samples were assayed in duplicate; the average concentration of the samples was taken as the final value. Other serum analytes were tested in Clinical Laboratory of Beijing Anding Hospital. Serum IgG and complement C3 and C4 were determined using nephelometry; serum creatine kinase (CK) was determined using enzyme-coupled assay; serum adrenocorticotropic hormone (ACTH) and cortisol were detected by immunoassay.
Data Analysis
All the data analyses were conducted by using SPSS 23.0 for Windows. Comparisons between catatonic patients, non-catatonic patients, and HCs with regard to sociodemographic, clinical, and laboratory variables were performed using independent sample t-tests, Mann-Whitney U tests, Kruskal-Wallis H tests, Fisher’s exact test, and chi-square tests, where appropriate. One-sample Kolmogorov-Smirnov tests were used to check the normality of distributions for the continuous variables. Associations of hs-CRP with sociodemographic, clinical, and laboratory variables in catatonic and non-catatonic patients were analyzed using Pearson correlation analysis if the data followed a normal distribution; otherwise, log transformation was performed to the skewed variables before conduction analyses. Receiver operating characteristic (ROC) curves with the area under the curve (AUC) values were calculated for high inflammatory status (hs-CRP > 3000 ng/ml), showing the predicted probabilities from the final model of logistic regression analysis. Multivariate stepwise linear regression analyses were used to identify predictors of serum hs-CRP level in catatonic patients.
Two-tailed tests were used. With regard to hs-CRP levels and the percentage of high inflammatory individuals, the significance level was set at .05 for the comparisons between three groups, and Bonferroni adjustment was applied to the post hoc comparisons (critical α of P < .017). The Bonferroni adjustment was also applied to the exploratory comparisons between groups and correlation analyses, resulting in a critical α of P < .0024 for 21 comparisons (table 1) and a critical α of P < .0038 for correlation analyses between hs-CRP level and 13 variables (table 2).
Table 1.
Comparison of Catatonic Patients, Non-Catatonic Patients (HCs) With Respect to Demographic and Clinical Characteristics
| Catatonic (n= 51) | Non-catatonic (n = 55) | Statistics* | ||||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | χ 2 | P-value | |
| Men | 13 | 25.5 | 15 | 27.3 | 0.04 | .835 |
| Diagnosis of schizophrenia | 16 | 31.4 | 19 | 33.9 | 0.79 | .778 |
| Currently smoking | 2 | 3.9 | 5 | 8.9 | 1.14 | .287 |
| On medication | 48 | 94.1 | 53 | 96.4 | 0.30 | .586 |
| On antipsychotic | 34 | 66.7 | 47 | 85.5 | 5.18 | .023 |
| FGAs | 11 | 21.6 | 19 | 34.5 | 2.20 | .138 |
| SGAs | 23 | 46.0 | 28 | 50.9 | 0.25 | .615 |
| Mean | SD | Mean | SD | t | P-value | |
| Age | 32.8 | 8.7 | 33.9 | 10.2 | −0.60 | .553 |
| Body Mass Index | 22.4 | 3.1 | 23.1 | 3.4 | −1.00 | .321 |
| PANSStot | 81.4 | 2.4 | 57.0 | 18.6 | 7.02 | <.001 |
| PANSSpos | 18.6 | 6.0 | 15.8 | 7.4 | 2.19 | .031 |
| 25th percentile | 75th percentile | 25th percentile | 75th percentile | Z | P-value | |
| Duration of illness | 3.0 | 146.0 | 27.0 | 157.0 | −1.51 | .132 |
| OLZeq | 0 | 6.3 | 2.0 | 10.0 | −2.55 | .011 |
| CK(U/l) | 43.0 | 686.0 | 39.0 | 118.0 | −3.09 | .002 |
| Cortisol (pg/ml) | 14.6 | 20.6 | 13.7 | 20.8 | −0.10 | .922 |
| ACTH (pg/ml) | 20.4 | 41.4 | 24.7 | 64.0 | −2.58 | .010 |
| IgG (g/l) | 9.0 | 12.7 | 9.2 | 12.3 | −0.79 | .427 |
| C3 (g/l) | 0.7 | 0.9 | 0.7 | 1.0 | −1.50 | .133 |
| C4 (g/l) | 0.2 | 0.3 | 0.2 | 0.2 | −0.61 | .539 |
| BFCRS | 14.0 | 22.0 | 0 | 1.0 | −9.05 | <.001 |
| PANSSneg | 14.0 | 26.0 | 7.0 | 15.0 | −6.11 | <.001 |
Note: FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics; PANSSpos, Positive subscale score of the Positive and Negative Syndrome Scale; PANSSneg, Negative subscale score of the Positive and Negative Syndrome Scale; PANSStot, total score of the Positive and Negative Syndrome Scale; BFCRS, Bush-Francis catatonia rating scale; CK, creatine kinase; ACTH, adrenocorticotropic hormone; IgG, immunoglobulin G; C3, complement component 3; C4, complement component 4; OLZeq, olanzapine equivalent dose.
*Bonferroni adjustment for the 21 comparisons to a critical α of P < .0024.
Table 2.
Correlations Between Log10-Transformed Serum Hs-CRP and Demographic, Clinical, and Laboratory Variables in Catatonic and Non-Catatonic Patients.
| Lg (hs-CRP) | Catatonic (n = 51) | Non-catatonic (n = 55) | ||
|---|---|---|---|---|
| r | P-value* | r | P-value* | |
| Men | .181 | .203 | .057 | .680 |
| Diagnosis of schizophrenia | .039 | .783 | −.086 | .534 |
| Currently smoking | .024 | .867 | −.019 | .892 |
| Age | −.008 | .954 | −.152 | .269 |
| Body mass index | .350 | .012 | −.044 | .752 |
| BFCRSa | .276 | .0497 | −.251 | .065 |
| Lg (DOI) | .197 | .283 | −.157 | .254 |
| Lg (CK) | .414 | .003 | .028 | .837 |
| Lg (Cortisol) | .326 | .020 | .045 | .743 |
| Lg (ACTH) | −.308 | .028 | .006 | .965 |
| Lg (IgG) | −.115 | .423 | .002 | .989 |
| Lg (C3) | .077 | .591 | .109 | .429 |
| Lg (C4) | .341 | .014 | .040 | .769 |
Note: Lg, log10 transformed; DOI, duration of illness; BFCRS, Bush-Francis catatonia rating scale; CK, creatine kinase; ACTH, adrenocorticotropic hormone; IgG, immunoglobulin G; C3, complement component 3; C4, complement component 4; OLZeq, olanzapine equivalent dose; hs-CRP, high sensitivity C-reactive protein.
aAll skewed data were log-transformed except for BFCRS in non-catatonic group. In non-catatonic group, 39 individuals were scored 0 on BFCRS, which made log-transformation impossible. In such case, Spearman’s rank correlation was used.
*Bonferroni adjustment to a critical α of P < .0038 for the correlation analyses between lg (hs-CRP) and the 13 variables.
Results
Comparison of Mean Serum hs-CRP Levels Between Groups
One hundred and fifty subjects were recruited in this study, including 51 catatonic patients, 55 non-catatonic patients, and 44 HCs. The mean age of the HCs was 31.4 ± 8.0 years old and 11 HCs were male (25.0%). There were no significant differences between the three groups in terms of age (F(147, 2) = 0.94, P = .40) and sex composition (χ 2 = 0.08, P = .96). All the catatonic patients had been in an acute catatonic state for less than a week. In the non-catatonic group, only one patient took benzodiazepine (lorazepam, 1.5 mg daily) before the day of blood test. In the catatonic group, 11 patients took lorazepam (ranging from 1.5 mg to 3 mg daily), and 10 patients took oxazepam (ranging from 15 mg to 90 mg daily) before the day of blood test.
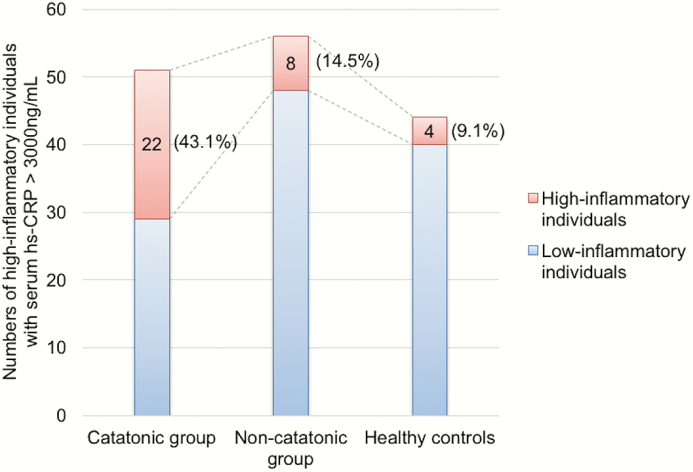
Data of hs-CRP concentration were highly skewed as indicated in one-sample Kolmogorov-Smirnov tests (P < .001). Using hs-CRP > 3000 ng/ml as cutoff value for high inflammation, there was a significantly higher percentage of high inflammatory individuals in the catatonic group (43.1%) than in non-catatonic group (14.5%) or in HC group (9.1%) (χ 2 = 18.9, P < .001; post hoc comparison between catatonic and non-catatonic groups: χ 2 = 10.7, P = .001) (figure 1). Serum hs-CRP levels were compared between catatonic patients, non-catatonic patients, and HCs. Kruskal-Wallis test showed that hs-CRP level was significantly higher in catatonic patients (median = 1797.6 ng/ml) compared with non-catatonic (median = 891.7 ng/ml) and HCs (median = 1144.9 ng/ml) (P = .023; post hoc comparison between catatonic and non-catatonic groups: P = .016). The psychiatric diagnoses of individuals in catatonic as well as non-catatonic groups were shown in the supplementary table S4. Ten patients had extremely high hs-CRP levels (>10 mg/l), but none of them presented any signs or symptoms of infectious diseases (supplementary table S5).
Fig. 1.
Numbers and percentages of high inflammatory individuals (with a serum hs-CRP > 3000 ng/ml) differ by group. There was a significantly higher percentage of patients with high inflammatory levels in the catatonic (n = 22, 43.1%) than in the non-catatonic (n = 8, 14.5%) or healthy controls group (n = 4, 9.1%) (χ 2 = 18.9, P < .001).
The comparisons between catatonic and non-catatonic patients regarding sociodemographic, clinical, and laboratory variables are shown in table 1. With Bonferroni adjustment, catatonic patients exhibited a higher PANSS total score, PANSS positive score, and PANSS negative score compared with non-catatonic patients. However, after controlling for BFCRS total scores and the diagnosis of schizophrenia, Analysis of Covariance revealed no significant difference in PANSS total scores (F(102, 3) = 0.320, P = .573), PANSS positive scores (F(102, 3) = 0.467, P = .496), or PANSS negative scores (F(102, 3) = 1.713, P = 0.194). In the whole patients’ sample, PANSS negative scores were significantly correlated with BFCRS scores (r = .61, P < .001). However, no significant association was found between BFCRS scores and PANSS negative scores in catatonic patients (r = .18, P = .198) or in non-catatonic patients (r = .257, P = 0059). Although not significant with Bonferroni adjustment, catatonic patients were more likely to have a higher serum level of hs-CRP, CK, and ACTH, and to be more unlikely to receive antipsychotics and to be on a lower OLZeq dose (nominal significance, P < .05).
To determine whether catatonia is independently associated with high inflammatory status, we conducted a multivariate logistic regression with backward Wald method. In the regression analyses, high inflammation was entered as the dependent variable and catatonia diagnosis and all the variables indicating stress level and symptomatology were entered as independent variables (ACTH, Cortisol, CK, Diagnosis of schizophrenia, OLZeq, PANSS total score, PANSS negative score, and PANSS positive score). Log-transformation was performed for all skewed variables before conducting regression analyses. The results suggested the independent predictors of high inflammation included catatonia diagnosis (OR = 3.52, P = .015, 95% CI 1.28–9.79), lg (CK) (OR = 2.29, P = .024, 95% CI 1.11–4.71), and lg (Cortisol) (OR = 21.47, P = .071, 95% CI 0.77–601.21). ROC curve for the predictive model showed the AUC was estimated to be 0.766 (P < .001, 95% CI 0.663–0.870), indicating that the overall accuracy of the final model to predict patients’ remission (with a predicted probability of 0.5 or greater) was acceptable.
Exploring the Independent Predictors of High Inflammatory Level
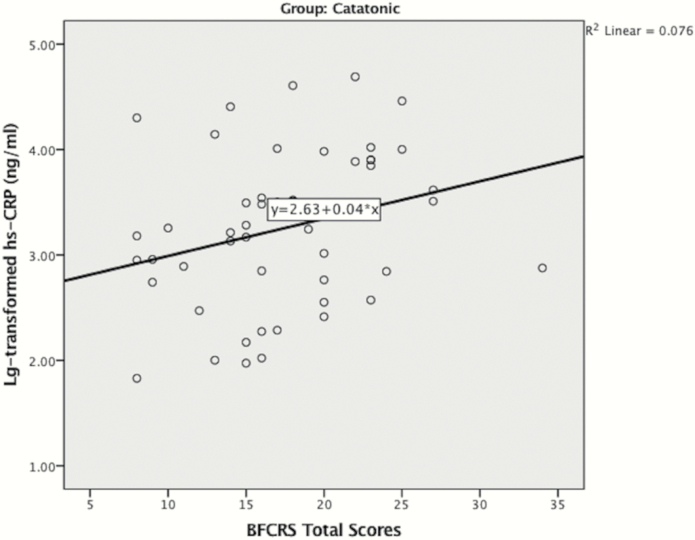
Table 2 presents the relationships between hs-CRP and sociodemographic variables, clinical and laboratory variables. Log-transformation was performed for all skewed variables before conducting correlation analyses. With Bonferroni adjustment, lg (CK) was the only variable significantly associated with lg (hs-CRP) in the catatonic group. Although not significant with Bonferroni adjustment, higher lg (hs-CRP) was likely to be associated with higher BFCRS total scores (figure 2), BMI, lg (Cortisol), lg (C4), and lower lg (ACTH) in the catatonic group (P < .05). No demographic, clinical, or laboratory variable was associated with hs-CRP level in non-catatonic psychotic patients (table 2).
Fig. 2.
Scatterplot for log-transformed hs-CRP vs BFCRS total scores in catatonic group (n = 55). Pearson’s correlation revealed a nominally significant association between lg (hs-CRP) and BFCRS total scores (r = .276, P = .0497).
Table 3.
Results of the Stepwise Multiple Regression Analysis (Catatonic Patients; n = 51)
| Predictor | Beta | P-value | 95% CI | |
|---|---|---|---|---|
| Lg (hs-CRP) Adjusted R2= 0.43; F(5, 45) = 8.5; P < .001 | Lg (C4) | .26 | .02 | 0.20–2.23 |
| Lg (ACTH) | −.31 | .008 | −1.28 to −0.21 | |
| Lg (Cotisol) | .31 | .009 | 0.35–2.34 | |
| BMI | .33 | .006 | 0.02–0.13 |
Note: Lg, log10 transformed; BMI, body mass index; hs-CRP, high sensitivity C-reactive protein; C4, complement component 4; ACTH, adrenocorticotropic hormone.
In the stepwise linear regression analyses in catatonic patients, hs-CRP level was entered as the dependent variable, BMI, lg (CK), lg (Cortisol), lg (C4), and lg (ACTH) were entered as independent variables. The results suggested that higher serum level was significantly predicted by lg (C4) (Beta = .26, P = .020, 95% CI 0.20–2.23), lg (ACTH) (Beta = −.31, P = .008, 95% CI −1.28 to −0.21), lg (Cortisol) (Beta = .31, P = .009, 95% CI 0.35–2.34), and BMI (Beta = .33, P = .006, 95% CI 0.02–0.13) in catatonic patients (table 3).
Discussion
To the best of our knowledge, this is the first study to examine serum hs-CRP levels in catatonia using a standardized rating scale for catatonia. Results of the study partly confirmed the three hypotheses proposed. The hs-CRP level was significantly higher in catatonic patients compared with non-catatonic and HCs. A significantly higher percentage of individuals in the catatonic group (43.1%) had a clinically high hs-CRP level (>3000 ng/ml) than in the non-catatonic group (14.5%) or in HCs group (9.1%). For the second hypothesis, logistic regression analysis suggested that catatonia was significantly associated with a high inflammatory level even after controlling for stress, psychopathology, and other confounding variables. The third hypothesis, however, was proved to be true only in catatonic patients. Only in catatonic patients were hs-CRP levels independently predicted by C4, ACTH, Cortisol, and BMI, as revealed in the multiple linear regression analyses (adjusted R2 = 0.43).
Inflammatory processes have been implicated as a key pathophysiological mechanism in schizophrenia,58–60 and CRP, along with cytokines, has been considered as a potential inflammatory biomarker in schizophrenia.61 Elevated hs-CRP levels have been observed in patients with schizophrenia, although the actual plasma concentration varied among different studies.33,59 Elevated CRP levels suggesting low-grade systemic inflammation have also been shown in other psychiatric disorders, including psychotic disorders (32%), mood disorders (21%), neurotic disorders (22%), and personality disorders (42%),62 indicating that CRP may be a transdiagnostic inflammatory biomarker across major psychiatric disorders.
Previous studies found that systemic inflammation can alter the CNS dopaminergic and glutamatergic neurotransmission and increase the generation of kynurenic acid, an NMDA receptor antagonist.60,63,64 Notably, catatonia is often a prominent manifestation of anti-NMDA encephalitis,12,13 and NMDAR antibodies have been found in patients with psychiatric disorders about three times as high as in HCs.65 The GABA/glutamate functional balance plays a key role in maintaining normal brain functioning, including the modulation of motor functions.66 Based on more recent neuroimaging studies in catatonia,67,68 Hirjak and colleagues proposed catatonia as a paradigmatic model for Research Domain Criteria (RDoC)-based investigation of GABAergic system.69 Benzodiazepines act as positive allosteric modulators of the GABA-A receptor.70 Some benzodiazepines that are effective in resolving catatonia (eg, diazepam) also modulate immune function by binding to translocator protein (TSPO).71,72 By activating GABA-A receptors, benzodiazepines might eventually lead to a reduced level of pro-inflammatory cytokines.71,73
It should be noted that a high inflammatory status also existed in 14.3% non-catatonic patients and 9.1% HCs in the present study. Logistic regression analysis also identified high levels of CK and cortisol (although marginally insignificant, P = .071) as independent predictors of high hs-CRP level in the whole patients’ sample, which could partly explain the high inflammatory individuals in the non-catatonic group. In healthy people, Delongui and colleagues found a serum hs-CRP of >3.0 and ≤10.0 mg/l in 17.6% of adults in Brazil. Authors suggested smoking, lack of physical activity, female, and BMI could be potential contributors to the high hs-CRP level.74 In a Chinese population, Tang and colleagues found that higher serum hs-CRP concentration was associated with older age, male gender, and higher level of serum uric acid but lower level of high-density lipoprotein cholesterol and superoxide dismutase in healthy adults.75
In stepwise linear regression, cortisol and ACTH were both identified as independent predictors of lg (hs-CRP) in catatonic patients, which indicates that stress plays an important role in systemic inflammation. Previous studies suggested that an increased level of stress hormone was linked to a chronic and low-grade inflammation state by alteration of the gene expression of the inflammatory arm of the immune system.37 Elevated cortisol and its metabolites were noted in the first episode and recent onset psychoses and mood disorders.76–79 However, our current study did not find an association between serum cortisol level and PANSS positive or PANSS negative scores, in either catatonic or non-catatonic groups (supplementary table S2). Furthermore, the hs-CRP level was not associated with PANSS positive or PANSS negative scores (supplementary table S1). These results were in line with most of the previous studies76,80,81 but not with that of Murri and colleagues.82 The discrepancies not only reflect the potential methodological heterogeneity among these studies,83 but could also suggest that the abnormal stress response may be a preexisting condition of the illnesses, rather than merely a reaction to psychopathology.84
Stepwise linear regression also revealed that complement C4 was an independent predictor of hs-CRP levels in catatonic patients. Growing evidence indicates that complement is involved in brain development.85,86 A recent genetic study implicated C4 as a susceptibility locus for schizophrenia.87 Dysregulation of the complement system in schizophrenia has been noted in a few small studies.88,89 It has been shown that high CRP levels were associated with high serum levels of complement components in a nonpsychiatric clinical population.90
The strength of the present study includes the stringent diagnostic criteria and standardized rating scale used to assess catatonia, the focus specifically on the inflammatory profile in catatonic patients, the control of confounding variables, such as physical comorbidities, infections, and BMI, and the taking into consideration of stress hormones and innate immune system (complement C3 and C4). The results of the study, however, should be interpreted with caution due to the following methodological limitations: (1) The cross-sectional design of the study does not reveal a causal relationship. We do not know whether the catatonia features are due to a premorbid high inflammatory level, or it is the catatonia syndrome that leads to a high level of inflammatory markers. (2) There has been evidence suggesting overlapping motor symptoms between different motor domains in psychotic disorders, and it is important to assess other motor symptoms, such as extrapyramidal symptoms. However,
in the present study, OLZeq was relatively low in both patients’ groups, particularly in the catatonic group (median: 3.34 mg in catatonic group vs 6.25 mg in non-catatonic group, P = .011), suggesting antipsychotics induced extrapyramidal symptoms unlikely to happen. (3) Correlation analysis revealed a significant correlation between BFCRS total score and PANSS negative scores in the whole patients’ sample, but neither in catatonic nor in the non-catatonic group. This may suggest that the evaluation of negative symptoms could be potentially confounded by catatonic symptoms when assessing a mixed sample with both catatonic and non-catatonic patients. (4) In multivariate analyses, the significant variables only accounted for 43% of the variance in hs-CRP. Although we controlled potential confounding factors, such as BMI, comorbid physical condition, and also took into consideration the stress hormone and innate immune function, there may still be other factors that have a profound effect on systemic inflammation profile; (5) Serum concentration of hs-CRP may not be able to reflect the inflammatory status in the central nervous system (eg, cerebrospinal fluid).
In conclusion, the present study shows that the catatonia could be specifically linked to a high level of systemic inflammation, independent of the primary psychiatric diagnoses. The high level of serum hs-CRP in catatonic patients could not be explained by more severe symptomatology, activation of stress system, or dysregulation of innate immune (complement) system, although the latter two variables are both independent predictors of inflammation. The findings of the present study suggest that catatonia could be a transdiagnostic clinical feature associated with inflammation dysregulation. These findings could have clinical implications when considering potential anti-inflammatory therapeutic interventions. Longitudinal studies with a larger sample size and measurement of inflammatory alteration in the central nervous system are warranted in the future.
Supplementary Material
Acknowledgment
The authors have no conflicts of interest.
Funding
This work was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (grant number: ZYLX201807) and Beijing Municipal Administration of Hospitals’ Youth Programme (grant number: QML20161902).
References
- 1. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173–1177. [DOI] [PubMed] [Google Scholar]
- 2. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophr Bull. 2018;44(5):1133–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gjessing R, Gjessing L. Some main trends in the clinical aspects of periodic catatonia. Acta Psychiatr Scand. 1961;37(1):1–13. [DOI] [PubMed] [Google Scholar]
- 4. Gjessing LR. A review of periodic catatonia. Biol Psychiatry. 1974;8(1):23–45. [PubMed] [Google Scholar]
- 5. Northoff G, Demisch L, Wenke J, Pflug B. Plasma homovanillic acid concentrations in catatonia. Biol Psychiatry. 1996;39(6):436–443. [DOI] [PubMed] [Google Scholar]
- 6. Northoff G, Wenke J, Demisch L, Eckert J, Gille B, Pflug B. Catatonia: short-term response to lorazepam and dopaminergic metabolism. Psychopharmacology (Berl) 1995;122(2):182–186. [DOI] [PubMed] [Google Scholar]
- 7. Northoff G, Waters H, Mooren I, et al. Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry Res. 1999;91(1):45–54. [DOI] [PubMed] [Google Scholar]
- 8. Northoff G, Pfennig A, Krug M, et al. Delayed onset of late movement-related cortical potentials and abnormal response to lorazepam in catatonia. Schizophr Res. 2000;44(3):193–211. [DOI] [PubMed] [Google Scholar]
- 9. Northoff G, Richter A, Gessner M, et al. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cereb Cortex. 2000;10(1):93–107. [DOI] [PubMed] [Google Scholar]
- 10. Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6(7):610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirjak D, Kubera KM, Wolf RC, Northoff G. Going back to Kahlbaum’s psychomotor (and GABAergic) origins: is catatonia more than just a motor and dopaminergic syndrome? Schizophr Bull. 2020;46(2):272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mythri SV, Mathew V. Catatonic syndrome in anti-NMDA receptor encephalitis. Indian J Psychol Med. 2016;38(2):152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Consoli A, Ronen K, An-Gourfinkel I, et al. Malignant catatonia due to anti-NMDA-receptor encephalitis in a 17-year-old girl: case report. Child Adolesc Psychiatry Ment Health. 2011;5(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsutsui K, Kanbayashi T, Takaki M, et al. N-Methyl-d-aspartate receptor antibody could be a cause of catatonic symptoms in psychiatric patients: case reports and methods for detection. Neuropsychiatr Dis Treat. 2017;13:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kar N, Kumar MT, Barreto S. NMDAR antibody encephalitis and fluctuating catatonia. Prog Neurol Psychiatry. 2017;21(3):11–13. [Google Scholar]
- 16. Rogers JP, Pollak TA, Blackman G, David AS. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hagemeyer N, Goebbels S, Papiol S, et al. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med. 2012;4(6):528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janova H, Arinrad S, Balmuth E, et al. Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest. 2018;128(2):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Philbrick KL, Rummans TA. Malignant catatonia. J Neuropsychiatry Clin Neurosci. 1994;6(1):1–13. [DOI] [PubMed] [Google Scholar]
- 20. Rosebush PI, Mazurek MF. Serum iron and neuroleptic malignant syndrome. Lancet. 1991;338(8760):149–151. [DOI] [PubMed] [Google Scholar]
- 21. Anglin RE, Rosebush PI, Mazurek MF. Neuroleptic malignant syndrome: a neuroimmunologic hypothesis. CMAJ. 2010;182(18):E834–E838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):499–507. [DOI] [PubMed] [Google Scholar]
- 23. Peralta V, Cuesta MJ, Mata I, Serrano JF, Perez-Nievas F, Natividad MC. Serum iron in catatonic and noncatatonic psychotic patients. Biol Psychiatry. 1999;45(6):788–790. [DOI] [PubMed] [Google Scholar]
- 24. Hope S, Ueland T, Steen NE, et al. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr Res. 2013;145(1–3):36–42. [DOI] [PubMed] [Google Scholar]
- 25. Pandey GN, Ren X, Rizavi HS, Zhang H. Proinflammatory cytokines and their membrane-bound receptors are altered in the lymphocytes of schizophrenia patients. Schizophr Res. 2015;164(1–3):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dickerson F, Stallings C, Origoni A, et al. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophr Bull. 2016;42(1):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kegel ME, Bhat M, Skogh E, et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 2014;7:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Windgassen EB, Funtowicz L, Lunsford TN, Harris LA, Mulvagh SL. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad Med. 2011;123(1):114–119. [DOI] [PubMed] [Google Scholar]
- 33. Fernandes BS, Steiner J, Bernstein HG, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21(4):554–564. [DOI] [PubMed] [Google Scholar]
- 34. Sarandol A, Kirli S, Akkaya C, Ocak N, Eroz E, Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J Psychopharmacol. 2007;21(8):857–863. [DOI] [PubMed] [Google Scholar]
- 35. Severance EG, Dickerson FB, Stallings CR, et al. Differentiating nicotine- versus schizophrenia-associated decreases of the alpha7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J Neural Transm (Vienna). 2009;116(2):213–220. [DOI] [PubMed] [Google Scholar]
- 36. Hope S, Dieset I, Agartz I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45(12):1608–1616. [DOI] [PubMed] [Google Scholar]
- 37. Singh B, Chaudhuri TK. Role of C-reactive protein in schizophrenia: an overview. Psychiatry Res. 2014;216(2):277–285. [DOI] [PubMed] [Google Scholar]
- 38. Ohsawa M, Okayama A, Nakamura M, et al. CRP levels are elevated in smokers but unrelated to the number of cigarettes and are decreased by long-term smoking cessation in male smokers. Prev Med. 2005;41(2):651–656. [DOI] [PubMed] [Google Scholar]
- 39. Kao TW, Lu IS, Liao KC, Lai HY, Loh CH, Kuo HK. Associations between body mass index and serum levels of C-reactive protein. S Afr Med J. 2009;99(5):326–330. [PubMed] [Google Scholar]
- 40. Temelkova-Kurktschiev T, Henkel E, Koehler C, Karrei K, Hanefeld M. Subclinical inflammation in newly detected type II diabetes and impaired glucose tolerance. Diabetologia 2002;45(1):151. [DOI] [PubMed] [Google Scholar]
- 41. Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care 1999;22(12):1971–1977. [DOI] [PubMed] [Google Scholar]
- 42. Boozalis T, Teixeira AL, Cho RY, Okusaga O. C-reactive protein correlates with negative symptoms in patients with schizophrenia. Front Public Health. 2017;5:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barzilay R, Lobel T, Krivoy A, Shlosberg D, Weizman A, Katz N. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8–12. [DOI] [PubMed] [Google Scholar]
- 44. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1–3):261–265. [DOI] [PubMed] [Google Scholar]
- 45. Bulzacka E, Boyer L, Schürhoff F, et al. ; FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) Group Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr Bull. 2016;42(5):1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akanji AO, Ohaeri JU, Al-Shammri S, Fatania HR. Association of blood levels of C-reactive protein with clinical phenotypes in Arab schizophrenic patients. Psychiatry Res. 2009;169(1):56–61. [DOI] [PubMed] [Google Scholar]
- 47. Medda P, Fornaro M, Fratta S, et al. A case of deep venous thrombosis following protracted catatonic immobility recovered with electroconvulsive therapy: the relevance for an early intervention. Gen Hosp Psychiatry 2012;34(2):209.e205–207. [DOI] [PubMed] [Google Scholar]
- 48. Worku B, Fekadu A. Symptom profile and short term outcome of catatonia: an exploratory clinical study. BMC Psychiatry. 2015;15:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clinebell K, Azzam PN, Gopalan P, Haskett R. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644–651. [DOI] [PubMed] [Google Scholar]
- 50. Levenson JL. Medical aspects of catatonia. Prim Psychiatry. 2009;16(3):23–26. [Google Scholar]
- 51. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) Arlington, VA: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 52. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. [DOI] [PubMed] [Google Scholar]
- 53. Ungvari GS, Goggins W, Leung SK, Gerevich J. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’). II. Factor analysis of the catatonic syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):462–468. [DOI] [PubMed] [Google Scholar]
- 54. Bush G, Petrides G, Francis A. Catatonia and other motor syndromes in a chronically hospitalized psychiatric population. Schizophr Res. 1997;27(1):83–92. [DOI] [PubMed] [Google Scholar]
- 55. Fink M, Taylor MA.. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. New York, NY: Cambridge University Press; 2006. [Google Scholar]
- 56. He Y, Zhang M. The Positive and Negative Syndrome Scale (PANSS) and its application. J Clin Psychiatry (In Chinese) 1997;7(6):353–355. [Google Scholar]
- 57. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 58. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–286. [DOI] [PubMed] [Google Scholar]
- 59. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223–230. [DOI] [PubMed] [Google Scholar]
- 60. Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychopharmacol. 2011;14(7):997–1012. [DOI] [PubMed] [Google Scholar]
- 61. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39(6):1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Osimo EF, Cardinal RN, Jones PB, Khandaker GM. Prevalence and correlates of low-grade systemic inflammation in adult psychiatric inpatients: an electronic health record-based study. Psychoneuroendocrinology 2018;91:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017;112(Pt B):297–306. [DOI] [PubMed] [Google Scholar]
- 64. Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. [DOI] [PubMed] [Google Scholar]
- 65. Pearlman DM, Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res. 2014;157(1–3):249–258. [DOI] [PubMed] [Google Scholar]
- 66. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555–577; discussion 578–604. [DOI] [PubMed] [Google Scholar]
- 67. Hirjak D, Kubera KM, Northoff G, et al. Cortical contributions to distinct symptom dimensions of catatonia. Schizophr Bull. 2019;45(6):1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walther S, Schäppi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43(5):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hirjak D, Wolf RC, Northoff G. GABA and negative affect-catatonia as model of RDoC-based investigation in psychiatry. Schizophr Bull. 2019;45(6):1168–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–223. [PMC free article] [PubMed] [Google Scholar]
- 71. Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fernández Hurst N, Zanetti SR, Báez NS, Bibolini MJ, Bouzat C, Roth GA. Diazepam treatment reduces inflammatory cells and mediators in the central nervous system of rats with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2017;313:145–151. [DOI] [PubMed] [Google Scholar]
- 73. Prud’homme GJ, Glinka Y, Wang Q. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun Rev. 2015;14(11): 1048–1056. [DOI] [PubMed] [Google Scholar]
- 74. Delongui F, Kallaur AP, Oliveira SR, et al. Serum levels of high sensitive C reactive protein in healthy adults from southern Brazil. J Clin Lab Anal. 2013;27(3):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang Y, Liang P, Chen J, et al. The baseline levels and risk factors for high-sensitive C-reactive protein in Chinese healthy population. Immun Ageing. 2018;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mondelli V, Dazzan P, Hepgul N, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116(2–3):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guest PC, Schwarz E, Krishnamurthy D, et al. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology 2011;36(7):1092–1096. [DOI] [PubMed] [Google Scholar]
- 78. Steen NE, Tesli M, Kähler AK, et al. SRD5A2 is associated with increased cortisol metabolism in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1500–1506. [DOI] [PubMed] [Google Scholar]
- 79. Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73(2):114–126. [DOI] [PubMed] [Google Scholar]
- 80. Girshkin L, O’Reilly N, Quidé Y, et al. Diurnal cortisol variation and cortisol response to an MRI stressor in schizophrenia and bipolar disorder. Psychoneuroendocrinology 2016;67:61–69. [DOI] [PubMed] [Google Scholar]
- 81. Nedic Erjavec G, Uzun S, Nikolac Perkovic M, et al. Cortisol in schizophrenia: no association with tobacco smoking, clinical symptoms or antipsychotic medication. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:228–235. [DOI] [PubMed] [Google Scholar]
- 82. Belvederi Murri M, Pariante CM, Dazzan P, et al. Hypothalamic-pituitary-adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology 2012;37(5):629–644. [DOI] [PubMed] [Google Scholar]
- 83. Girshkin L, Matheson SL, Shepherd AM, Green MJ. Morning cortisol levels in schizophrenia and bipolar disorder: a meta-analysis. Psychoneuroendocrinology 2014;49:187–206. [DOI] [PubMed] [Google Scholar]
- 84. Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24(4 Suppl):91–118. [DOI] [PubMed] [Google Scholar]
- 85. Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. [DOI] [PubMed] [Google Scholar]
- 86. Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007;131(6):1164–1178. [DOI] [PubMed] [Google Scholar]
- 87. Sekar A, Bialas AR, de Rivera H, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium Schizophrenia risk from complex variation of complement component 4. Nature 2016;530(7589):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hakobyan S, Boyajyan A, Sim RB. Classical pathway complement activity in schizophrenia. Neurosci Lett. 2005;374(1):35–37. [DOI] [PubMed] [Google Scholar]
- 89. Mayilyan KR, Weinberger DR, Sim RB. The complement system in schizophrenia. Drug News Perspect. 2008;21(4):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dernellis J, Panaretou M. Effects of C-reactive protein and the third and fourth components of complement (C3 and C4) on incidence of atrial fibrillation. Am J Cardiol. 2006;97(2):245–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.