Abstract
Objective
Disruptions in the dopamine system have been observed in psychiatric disorders. Since dopamine is mainly produced in the ventral tegmental area (VTA), elucidating the differences in the VTA neural network across psychiatric disorders would facilitate a greater understanding of the pathophysiological mechanisms underlying these disorders. However, no study has compared VTA-seed-based functional connectivity across psychiatric disorders. Therefore, we conducted a resting-state functional magnetic resonance imaging (rs-fMRI) study to perform a seed-based fMRI analysis, using the VTA as a seed.
Methods
We included participants with major depressive disorder (MDD; n = 45), schizophrenia (n = 32), and bipolar disorder (BPD; n = 30), along with healthy control participants (n = 46) who were matched for age, gender, and handedness.
Results
The results showed that patients with MDD and BPD had altered VTA-related connectivity in the superior frontal gyrus, frontal pole regions, hippocampus, cerebellum, and posterior cingulate cortex. Some of these differences in connectivity were also found between affective disorders and schizophrenia; however, there were no differences between the schizophrenia and control groups. Connectivity between the VTA and the hippocampus was correlated with positive symptoms in the schizophrenia group. The connectivity was not associated with medication dose, and the results remained significant after controlling for dose.
Conclusions
The results suggest that altered brain functional connectivity related to VTA networks could be associated with the distinctive pathophysiologies of psychiatric disorders, especially affective disorders.
Keywords: psychiatric disorders, the ventral tegmental area, resting-state functional magnetic resonance imaging
Introduction
Dysregulation of the dopamine system is thought to be associated with several psychiatric disorders. Hyperactivity in the mesolimbic dopaminergic pathway is thought to result in positive symptoms in patients with schizophrenia,1 whereas hypoactivity in the mesocortical pathway is associated with negative symptoms of schizophrenia and depressive symptoms of affective disorders, such as major depressive disorder (MDD).2 Similarly, hyperdopaminergia in patients with bipolar disorder (BPD) underlies the development of manic symptoms, whereas hypodopaminergia may cause a depressive episode of the illness.3
Dopamine is mainly produced in the ventral tegmental area (VTA) and the substantia nigra in the midbrain. The VTA is the neural origin of the mesocortical and mesolimbic circuitry, which project via the medial forebrain bundle to the medial prefrontal cortex and subcortical areas, such as the pallidum, hippocampus, and amygdala.4,5 The VTA connections are a central part of the reward network6 and play crucial roles in motivation7 and mood regulation.4,8 Functional and behavioral dysregulation in VTA connections is responsible for the symptoms of several psychiatric disorders.2,9,10 It thus seems plausible that differences in VTA-related neural networks may serve as a specific pathophysiology, especially for affective symptomatologies in psychiatric disorders.
Although not only focusing on the VTA neural networks, recent neuroimaging studies have found differences in neural connections between psychiatric disorders. A review comparing the neural correlates between schizophrenia and BPD showed a consistent association with dysfunctional connectivity, especially related to the prefrontal cortex.11 Compared with patients with MDD, patients with BPD showed elevated recruitment of attentional neural circuits.12 Local functional connectivity was increased in the visual and auditory cortices, including the superior temporal gyrus, motor, and thalami, whereas it was decreased in the orbitofrontal cortex in the following order: controls > MDD > BPD > schizophrenia.13 Likewise, short-range connectivity was localized in the visual cortex, auditory cortex, and thalamus in the following order: controls > MDD > BPD > schizophrenia.14 Exploring shared and distinct functional connectivity in more than 2 psychiatric disorders may help to identify a common and disease-specific pathophysiology among psychiatric disorders.
Additionally, several previous MR imaging studies have examined the alterations in VTA functions or networks in various psychiatric disorders. Patients with schizophrenia were seen to have an abnormal brain response in the VTA in conjunction with the striatum, temporal cortex, parietal lobe, prefrontal cortex, and limbic regions. These responses were seen in saliency, reward-based, decision-making tasks, and memory-based tasks.15–23 A diffusion tensor imaging study showed that the white matter pathway increased between the VTA and amygdala in patients with schizophrenia as compared to healthy controls. This study also showed that the white matter pathway was negatively correlated for patients with schizophrenia when negative symptoms were present.24 The resting-state functional connectivity between the VTA and the striatum, temporal cortex, and prefrontal cortex was seen to be negatively associated with amotivation.25 In patients with schizophrenia who had verbal or auditory hallucinations, resting-state functional connectivity between the VTA and striatum was enhanced as compared to patients without hallucinations.26 Compared to healthy controls, patients with schizophrenia showed reduced resting-state functional connectivity between the VTA and striatum, thalamus, hippocampus, insular cortex, lateral occipital complex, precuneus, cingulate cortex, prefrontal cortex, and cerebellum. In contrast, patients with schizophrenia showed increased resting-state functional connectivity between the VTA and dorsolateral prefrontal cortex.27,28
MDD seems to be associated with a reduction in the resting-state functional connectivity between the VTA and striatum, prefrontal cortex, anterior cingulate cortex, dorsal raphe nucleus, and habenula.29–33 Additionally, MDD has been linked to reduced brain response when reward or motivation based tasks are performed. This occurs in the VTA as well as the striatum, limbic regions, and frontal cortex.17,34–38 BPD was found to be connected to decreased resting-state functional connectivity between the VTA and the striatum.39 BPD was also associated with hypoactivation of the VTA in conjunction with the striatum, frontal and parietal cortices in response to reward-based tasks.18
In summary, schizophrenia would be associated with abnormal VTA activation related to reward, saliency, memory, or decision-making neural networks. Contrastingly, MDD and BPD could be associated with decreased activation of the reward and neural motivation networks, including VTA. Additionally, MDD could be linked to the neural connection between the VTA and the raphe nucleus or habenula.
To test this hypothesis, we designed a voxel-based morphometric (VBM) analysis to examine the morphological differences across patients with MDD, schizophrenia, and BPD as well as a healthy control group. An rs-fMRI study was then conducted to analyze seed-based functional connectivity at rest, using the VTA as a seed to compare the VTA networks across the 4 groups. Although rs-fMRI cannot directly measure dopaminergic neural activity, pharmacological neuroimaging studies40,41 and a positron emission tomography study42 suggest that rs-fMRI connectivity reflects dopaminergic neural activity. We thus assumed that the VTA map of rs-fMRI connectivity could represent a portion of the dopaminergic neural network of the VTA. In addition, we used the Asian VTA template because the VTA, which is a small region in the midbrain, shows differences in size and location between Asians and Caucasians.43
Materials and Methods
Participants
We recruited participants with MDD (n = 45), schizophrenia (n = 32), and BPD (n = 30) from the University of Tokyo Hospital, and recruited control participants (n = 46) who were matched for age, gender, and handedness44 (table 1). Four patients with schizophrenia were in the stage of recent onset, within 1 year after emergence of their psychotic symptoms, at the time of the MRI measurements, while 28 were in the chronic stage.
Table 1.
Demographic Characteristics in This Study
| MDD (n = 45) | Schizophrenia (n = 32) | BPD (n = 30) | Healthy controls (n = 46) | P value | Effect size (Partial Eta Squared) | |
|---|---|---|---|---|---|---|
| Age (mean ± SD years) | 37 ± 10.1 | 31 ± 9.5 | 35 ± 10.0 | 36 ± 8.6 | .10 | 0.041 |
| Sex (male/female) | 27 / 18 | 22 / 10 | 19 / 11 | 27 / 19 | .74 | 0.078a |
| Handedness (mean ± SD) | 16.8 ± 7.1 | 15.9 ± 5.0 | 16.6 ± 5.8 | 15.2 ± 3.4 | .56 | 0.014 |
| Estimated IQ (mean ± SD) | 110.2 ± 8.1 | 101.8 ± 11.6 | 107.7 ± 8.5 | 107.0 ± 9.5 | .003 | 0.092 |
| Duration of illness (year) | 18.9 ± 32.9 | 11.0 ± 8.6 | 7.7 ± 7.2 | .052 | 0.044 | |
| Medication equivalent doses (mg/d) | ||||||
| Chlorpromazine | 52.4 ± 114.1 | 606.2 ± 514.1 | 120.3 ± 218.4 | .001 | 0.383 | |
| Biperiden | 0.1 ± 0.4 | 1.4 ± 2.2 | 0.2 ± 0.6 | .001 | 0.174 | |
| Imipramine | 143.5 ± 146.9 | 0.1 ± 0.4 | 81.3 ± 112.5 | .001 | 0.226 | |
| Diazepam | 11.4 ± 11.7 | 8.7 ± 12.0 | 10.6 ± 13.4 | .631 | 0.009 | |
| Lithium | 296.6 ± 344.8 | |||||
| The severity of psychiatric symptoms | ||||||
| HAMD | 11.9 ± 6.4 | 11.0 ± 6.5 | .930 | 0.005 | ||
| mGAF-S | 44.6 ± 10.6 | 43.6 ± 14.1 | 44.9 ± 9.7 | .952 | 0.002 | |
| mGAF-F | 45.3 ± 9.6 | 46.3 ± 11.6 | 45.2 ± 8.6 | .918 | 0.002 | |
| mGAF | 43.8 ± 10.7 | 43.9 ± 13.5 | 43.1 ± 8.3 | .911 | 0.001 | |
| PANSS positive symptom | 16.8 ± 5.1 | |||||
| PANSS negative symptom | 19.3 ± 6.7 | |||||
| PANSS general psychopathology | 34.8 ± 8.5 | |||||
| PANSS total | 72.2 ± 17.0 | |||||
| YMRS | 2.0 ± 3.3 |
Note: MDD, major depressive disorder; BPD, bipolar disorder; HAMD, the GRID-Hamilton rating scale for depression; mGAF-S, the modified Global Assessment of Functioning symptom subscale; mGAF-F, the modified Global Assessment of Functioning function subscale; mGAF, the modified Global Assessment of Functioning; PANSS, the Positive and Negative Syndrome Scale; YMRS, the Young mania rating scale. Statistical differences were tested using analysis of variance or Chi-square test for 3 or more groups and t-test for 2 groups.
aPhi coefficient.
All participants provided written informed consent, and the study was approved by the Ethics Committee at the School of Medicine, the University of Tokyo (No. 3150-20). Psychiatric diagnoses were based on the guidelines given in the Structured Clinical Interview for the Diagnostic and Statistical Manual-IV. Patients with a history of neuropsychological disease other than MDD, schizophrenia, or BPD, alcohol or drug abuse, head trauma with accompanying loss of consciousness, or signal abnormalities on conventional diagnostic MRI were not included. Trained psychiatrists or psychologists screened all control participants using the Japanese version of the Mini International Neuropsychiatric Interview45 to exclude any psychiatric disorders.
We assessed handedness using the rating scale of handedness and estimated the premorbid intelligence quotients (IQ) using the 25-item version of the Japanese Adult Reading Test (JART).46
The general functioning and symptoms were assessed using the modified Global Assessment of Functioning (mGAF)47 for all patient groups. The severity of depressive symptoms was assessed using the 17-item version of GRID-Hamilton rating scale for depression (GRID-HAMD-17)48 for MDD and BPD. Psychiatric symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS)49 for schizophrenia. The severity of manic episodes was assessed using the Young Mania Rating Scale (YMRS)50 for BPD.
Medication doses were calculated using chlorpromazine, imipramine, biperiden, and diazepam equivalent doses.51 The distribution of chlorpromazine equivalent dose was assessed using a Kolmogorov-Smirnov test (see supplementary material for detailed information).
Resting-State Functional Magnetic Resonance Imaging Session
All participants underwent a 10-minute resting-state functional magnetic resonance imaging (rs-fMRI) scan. Each participant was instructed to focus on a fixation cross during the scan. Immediately after the scan, each participant rated their sleepiness during the scan using a 7-point scale (1 = not at all, 7 = more than ever).
Image Acquisition
All MR images were collected using a Discovery MR750w 3.0 Tesla scanner equipped with a 24-channel head coil (GE Healthcare). Each participant underwent an rs-fMRI scan (described in the prior subsection) and a high-resolution anatomical MRI scan. Details of MR image sequences have been described in supplementary material.
A VBM Analysis
For the VBM analysis, we created an region of interest (ROI) for each psychiatric disorder based on 14371 studies contained in the Neurosynth (http://neurosynth.org) database. Then, high-resolution anatomical MRI data were analyzed with FSL-VBM52 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), and an optimized VBM protocol53 was carried out with FSL tools.54 Details of the VTA analysis have been described in supplementary material.
ROI as the Seed
We created a 3-mm sphere on the peak voxel of the Asian VTA probabilistic template43 ([x, y, z] = [−2, −20, −18]) as the VTA ROI.
Preprocessing for Functional MR Images
Conventional preprocessing was performed using tools from the FMRIB Software Library (FSL Version 6.0; http://www.fmrib.ox.ac.uk/fsl/) package. fMRI data were preprocessed as follows: (1) head motion correction by realigning the time series to the middle volume, (2) removing non-brain tissues using the brain extraction tool, (3) slice-timing correction using Fourier-space phase shifting, aligning to the middle slice, (4) image smoothing with a 5-mm full-width at half-maximum Gaussian kernel, (5) application of a bandpass filter of 0.005–0.1 Hz as in a previous study,43 (6) linear detrending, and (7) grand-mean intensity normalization using a single multiplicative factor. To remove the effects of time-points that were corrupted by large motion (motion outliers) from the analysis, a confounder matrix was created using FSL’s toolbox (FSL Motion Outliers) to be used at the participant-level analysis. This confounder matrix also included a time series extracted from individual white matter and cerebrospinal fluid regions. Before group analyses, the high-resolution anatomical image was normalized to the MNI avg152 T1-weighted template (2-mm isotropic resolution) using a nonlinear transformation with a 10-mm warp resolution, as implemented by FSL’s fMRI nonlinear registration tool. Details of preprocessing for rs-fMRI data have been described in supplementary material.
Seed-Based Functional Connectivity Analysis
Time-series extraction from the VTA ROI was performed on non-smoothed preprocessed rs-fMRI data. To create an individual functional connectivity map, the extracted time-series from the VTA ROI was included in a regression model (the general linear model; GLM) using FSL’s fMRI Expert Analysis Tool (FEAT). This model also included a confounder matrix as a nuisance covariate.
Group-level analysis for group comparisons was conducted using a 1-way ANOVA and post hoc 2-sample t-tests. For all functional connectivity analyses, we set the cluster-forming threshold as z > 3.1. Clusters were then formed, their P-values calculated, and those P-values that were above the cluster P-threshold (P < 0.05 family-wise error corrected) were disregarded.
In each patient group, based on the results of the seed-based functional connectivity analysis, VTA-related connectivity values from the group-level significant clusters were tested for their associations with symptom severity. For instance, VTA-related connectivity values from the pallidum formed by the [BPD > schizophrenia] contrast, which was tested for its association with PANSS scales in the schizophrenia group and with YMRS in the BPD group. We adjusted the threshold significance level to correct multiple analyses using Bonferroni correction. We controlled for age, sex, and estimated IQ to test the association. If significant correlations with symptom severity for each group were observed, we also tested whether the correlation remained significant after controlling for medication doses.
Results
Participants
One-way ANOVA showed that there were no significant differences in age and handedness among the 4 groups (F(3,149) = 2.12, P = .10 and F(3,149) = 0.69, P = .56, respectively; table 1). The Chi-squared test showed no association between gender and group (χ 2(3) = 1.26, P = .74). Estimated IQ differed among the 4 groups (F(3,149) = 4.87, P = .003). A post hoc test showed that the JART score in MDD was greater than that in schizophrenia (P = .001, Bonferroni-corrected). We thus included JART scores as a covariate-of-no-interest for further use in rs-fMRI analysis.
A VBM Analysis
There was no significant morphological difference seen in the ROI across the 4 patient groups.
rs-fMRI Session
One-way ANOVA showed that there was no significant difference in head motion across all groups (F(3, 152) = 1.12, P = .34) and no significant difference in sleepiness across all groups (F(3, 152) =1.23, P = .30). Details of results of rs-fMRI session have been described in supplementary material.
Seed-Based Functional Connectivity Analysis
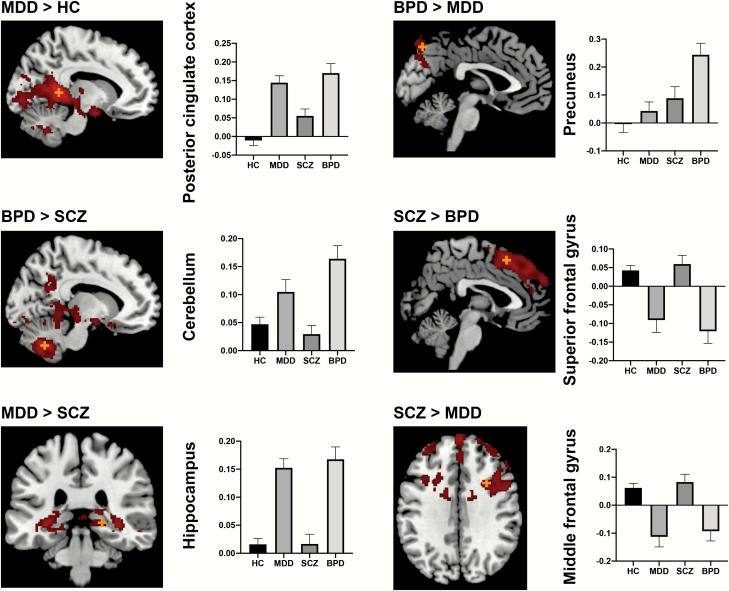
One-way ANOVA showed group differences in the prefrontal cortex, temporal lobe, pre-/postcentral gyrus, occipital lobe, cingulate and paracingulate gyrus, precuneus cortex, limbic system (hippocampus, parahippocampus, and amygdala), basal ganglia (putamen and pallidum), thalamus, and cerebellum. Post hoc t-test analysis showed significant differences in VTA connectivity maps between MDD and controls, BPD and controls, MDD and BPD, schizophrenia and MDD, and schizophrenia and BPD (figure 1, table 2).
Fig. 1.
Group differences in resting-state functional connectivity. The orange cross depicts the peak voxel. The y-axis of the bar graphs depicts the connectivity values (z-value) from the group-level significant clusters. HC, healthy controls; MDD, major depressive disorder; SCZ, schizophrenia; BPD, bipolar disorder.
Table 2.
Differences in VTA-Related Functional Connectivity
| MNI Coordinate | ||||||
|---|---|---|---|---|---|---|
| Region | Cluster Size | z-Value | x | y | z | Effect Size (Cohen’s d) |
| Controls ≥ MDD | ||||||
| Superior Frontal Gyrus | 1588 | 4.95 | 6 | 34 | 50 | 1.047 |
| Middle frontal gyrus | 288 | 4.32 | −36 | 40 | 28 | 0.949 |
| MDD ≥ controls | ||||||
| Posterior cingulate cortex | 22 160 | 5.46 | −12 | −48 | 10 | 1.36 |
| Cerebellum | 1247 | 4.46 | −24 | −68 | −44 | 1.13 |
| Superior temporal gyrus | 676 | 3.76 | 60 | −10 | −2 | 1.27 |
| Controls ≥ BPD | ||||||
| Superior frontal gyrus | 17 585 | 4.80 | 4 | 20 | 52 | 1.16 |
| Middle frontal gyrus | 148 | 3.64 | −56 | 24 | 28 | 0.88 |
| BPD ≥ controls | ||||||
| Posterior cingulate cortex | 40 839 | 6.13 | −8 | −46 | 2 | 1.69 |
| Amygdalaa | 5.59 | 26 | −16 | −8 | 1.28 | |
| Parahippocampusa | 5.55 | 14 | −36 | −2 | 0.88 | |
| MDD ≥ schizophrenia | ||||||
| Hippocampus (right) | 2522 | 4.05 | 34 | −32 | −2 | 1.32 |
| Hippocampus (left) | 556 | 4.19 | −32 | −32 | −2 | 0.89 |
| Cerebellum | 980 | 4.00 | −14 | −66 | −36 | 1.22 |
| Schizophrenia ≥ MDD | ||||||
| Middle frontal gyrus | 4299 | 4.42 | 30 | 10 | 34 | 0.95 |
| Frontal pole | 676 | 3.95 | −34 | 54 | 26 | 0.88 |
| BPD ≥ MDD | ||||||
| Precuneus | 802 | 3.82 | −2 | −76 | 50 | 0.91 |
| Schizophrenia ≥ BPD | ||||||
| Superior frontal gyrus | 5802 | 4.42 | 2 | 20 | 54 | 1.15 |
| Precentral gyrus | 447 | 3.39 | −16 | −18 | 62 | 0.98 |
| BPD ≥ schizophrenia | ||||||
| Cerebellum | 13 853 | 5.04 | −12 | −64 | −38 | 1.21 |
| Palliduma | 4.55 | 22 | −16 | −8 | 1.27 | |
Note: aSubpeak in the clusters.
The [MDD < controls] contrast showed several significant clusters in the prefrontal cortex, whereas the [MDD > controls] contrast showed significant clusters in the posterior cingulate cortex, superior temporal gyrus, and cerebellum. The [BPD < controls] contrast showed several significant differences in the prefrontal cortex, whereas the [BPD > controls] contrast showed significant differences in the posterior cingulate cortex and limbic regions. The [MDD > schizophrenia] contrast showed significant differences in the bilateral hippocampus and cerebellum, whereas the [MDD < schizophrenia] contrast showed significant differences in the prefrontal cortex. The [BPD > MDD] contrast showed significant differences in the precuneus. The [schizophrenia > BPD] contrast showed significant differences in the prefrontal cortex and precentral gyrus, whereas the [schizophrenia < BPD] contrast showed significant differences in the cerebellum and pallidum.
The Kolmogorov-Smirnov test showed that the chlorpromazine equivalent dose was normally distributed across the schizophrenia group (P = .111). The Grubbs’ test55 detected one outlier of the chlorpromazine equivalent dose (2150 mg/d). This analysis was then redone after we discarded the outlier from this analysis. We confirmed no significant difference existed from the original analyses conducted.
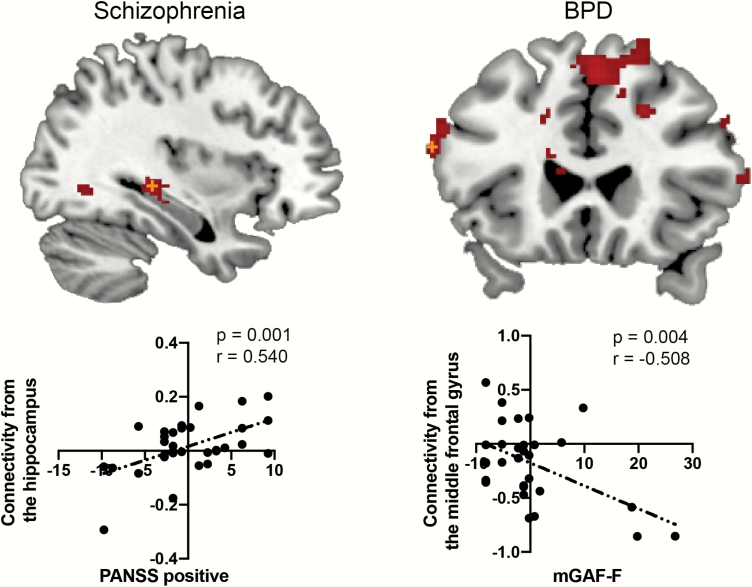
The Association Between Functional Connectivity and Psychiatric Symptoms
In the schizophrenia group, the PANSS positive score was positively correlated with the VTA-hippocampus connectivity (uncorrected P = .001, r = .540; Bonferroni-corrected significant level: P < .005; figure 2). The correlation remained significant after controlling for chlorpromazine (P = .0024), biperiden (P = .0050), and diazepam equivalent doses (P = .0080). In the BPD group, the mGAF-F score was negatively correlated with connectivity in the middle frontal gyrus as seen in the [BPD < controls] contrast (uncorrected P = .004, r = −.508, Bonferroni-corrected significant level: P < .0041; figure 2). The correlation was seen to be at a marginal level after controlling for chlorpromazine (P = .0090), biperiden (P = .005), diazepam (P = .0050), imipramine (P = .0060), and lithium equivalent doses (P = .0060). None of the correlations between connectivity values from the significant clusters in each group and their medication equivalent doses were significant (P > .05).
Fig. 2.
The association between functional connectivity and psychiatric symptoms. The orange cross depicts the peak voxel. The y-axis of the scatter plot depicts connectivity values (z-value) from the group-level significant clusters, while the x-axis depicts demeaned scores of psychiatric symptoms.
Since mGAF, mGAF-F, and mGAF-S data were available for all patient groups, we tested the correlations between connectivity values and each score. However, the connectivities from each significant cluster showed no significant correlation with mGAF or mGAF subscales (P > .05).
Discussion
To our knowledge, this is the first study to investigate the VTA-seed-based functional connectivity among the MDD, schizophrenia, BPD, and control groups in a resting state. Patients with MDD and BPD showed attenuated functional connectivity in the frontal regions and strengthened connectivity in the limbic regions, cerebellum, and posterior cingulate cortex. In these regions, some differences in connectivity were observed between the affective disorders and schizophrenia groups. However, there was no connectivity difference between the schizophrenia and control groups in any region. Within each group, significant differences in some brain regions were also observed in the relationship between VTA-related functional connectivity and symptom severity. None of the connections were significantly correlated with medication equivalent doses, and associations with symptom severity remained after controlling for medication equivalent doses.
Unlike the previous report,27 the patients with schizophrenia in this study did not show decreased VTA—hippocampus functional connectivity as compared to that in the healthy control group. Additionally, the functional connectivity positively correlated with the PANSS positive symptom subscores even after the chlorpromazine equivalent dose being regressed out. Increased basal dopamine synthesis is seen in schizophrenia56 and hyperactivity in the mesolimbic dopaminergic pathway is thought to result in the emergence of positive symptoms.1 Also, dysregulation of the hippocampus, which is a crucial part of the dopaminergic circuit and is closely connected, both functionally and anatomically, to the VTA, is thought to be associated with these positive symptoms.57 Positive symptoms in patients with schizophrenia have been reported as associated with volume reduction or deformation in the hippocampus.58–60 Compared to healthy participants, the hippocampal–midbrain loop increased during reward, aversion, and novelty processing in individuals that were considered ultra-high risk for psychosis.61 Together, strengthened functional connectivity between the VTA and hippocampus may be associated with increased dopaminergic connectivity. This could be the underlying psychopathology of the presence of positive symptoms.
We did not observe significant alteration in functional connectivity in the VTA networks between patients with schizophrenia and controls. Previous rs-fMRI studies showed reduced connectivity between the VTA/midbrain and cortical and subcortical regions, including the prefrontal cortex, insular cortex, hippocampus, and cerebellum.27,28 A potential cause may be the different conditions of the patients involved in the studies. Giordano and colleagues examined patients who used lower doses of medication,28 while Hadley and colleagues recruited patients who had been unmedicated for at least 10 days.27 Their results showed decreased connectivity between the VTA and precuneus, posterior cingulate cortex, accumbens, hippocampus, and pallidum27; these findings were similar to those of the present study, with respect to the affective disorder groups. Since most of the patients with schizophrenia in this study were relatively stable and in the chronic stage when MRI measurements were obtained, aberrant dopaminergic activity may have been normalized because of treatment. Indeed, patients with severe positive symptoms in the schizophrenia group had VTA-hippocampus connectivity similar to those in the MDD and BPD groups. Dopaminergic activity is modulated by several subcortical regions, such as the nucleus accumbens, amygdala, and pallidum.62–65 The mechanism underlying the effectiveness of antipsychotics’ D2 receptor blockage for positive symptoms remains unknown. A detailed analysis focused on the dopaminergic system in human fMRI studies with an antipsychotics trial would be a useful future study.
The present study showed that the VTA–prefrontal cortex connectivity was attenuated in patients with MDD and BPD, compared to healthy controls and (for some regions) patients with schizophrenia. These results are consistent with previous findings regarding alteration of functional connectivity in the prefrontal cortex.11,17,33,66 Previous non-human studies exploring the pathway of the dopaminergic system have shown that depressive behaviors are associated with an attenuated mesocortical pathway,67 which may result from decreased reward sensitivity.68 A neuroimaging study has shown that decreased brain response in the VTA, as well as the middle frontal gyrus in response to reward-based tasks, was associated with BPD.18 Therefore, the present findings are consistent with previous human neuroimaging studies and animal studies exploring the dopamine neural pathway. Additionally, the VTA receives serotonergic projections from the dorsal raphe, and the dopaminergic system is modulated by the serotonergic neural system.69 Thus, in order to reveal the neural pathophysiology of depressive symptoms, the role of the mesocortical pathway and serotonergic regions such as the dorsal raphe nucleus and habenula, should be examined in future studies.
Our findings should be interpreted with caution. First, since the participants in the patient groups were on medication, the effects of medication might have influenced our results. As discussed above, the conditions, medication status, and clinical stages of the participants may alter the dopaminergic activity and VTA-related functional connectivity. Although we regressed out the effect of medication to test the correlation between connectivity and symptoms, it would be informative to conduct further studies of unmedicated and/or first-episode patients and assess the effect of medication on connectivity, using repeated measurements. Second, as discussed above, the rs-fMRI connectivity analysis could show a pseudo-relationship provided by a third brain region. Since the dopamine-related neural activity is modified in a complex manner by several interacting brain regions, a causality analysis should be performed in a future study.
In conclusion, this study is the first to compare VTA-related functional connectivity networks among MDD, schizophrenia, BPD, and control groups in the resting state. Patients with MDD and BPD showed similar alterations, when compared with healthy controls and patients with schizophrenia. This result suggests that alterations of the VTA-associated functional network may underlie the common pathophysiology of affective symptoms and disorders. Our results provide new insights into the psychophysiology of psychiatric disorders; however, an analysis of the causal pathway and a clinical trial with functional imaging studies will be needed to clarify the contribution of the dopaminergic system to the etiology of each psychiatric disorder.
Funding
This research is supported by the Japan Agency for Medical Research and Development (AMED). Grant numbers are JP19dm0107120, JP19dm0307001, JP19dm0307004, and JP19dm0207069.
Supplementary Material
Acknowledgments
This study was also supported by UTokyo Center for Integrative Science of Human Behaviour (CiSHuB) and the International Research Center for Neurointelligence (WPI-IRCN) at The University of Tokyo Institutes for Advanced Study (UTIAS). The non-thresholded statistical maps are available at https://neurovault.org/collections/5772/, and the raw MRI data is available at BRAIN/MINDS DATAPORTAL (https://www.brainminds.riken.jp/). The authors have no conflicts of interest to disclose. Y.N. analyzed the data and wrote a draft manuscript. N.O., K.M., and D.K. contributed to performing MRI measurements and obtaining clinical data. S.K. wrote the article. O.A. and A.K. contributed to performing MRI measurements. K.O., K.K., and S.K. supervised the study project and manage funding for the study. All authors reviewed the draft manuscript and approved the final version of the manuscript. The authors declare that they have no conflict of interest.
References
- 1. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329–336. [DOI] [PubMed] [Google Scholar]
- 2. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashok AH, Marques TR, Jauhar S, et al. . The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18(2):73–85. [DOI] [PubMed] [Google Scholar]
- 6. Haber SN. Neuroanatomy of reward: a view from the ventral striatum. In: Gottfried JA, ed. Neurobiology of Sensation and Reward. Frontiers in Neuroscience. Boca Raton, FL: CRC Press/Taylor & Francis; 2011. http://www.ncbi.nlm.nih.gov/books/NBK92777/. Accessed October 11, 2018. [PubMed] [Google Scholar]
- 7. Saunders BT, Richard JM. Shedding light on the role of ventral tegmental area dopamine in reward. J Neurosci. 2011;31(50):18195–18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nikulina EM, Johnston CE, Wang J, Hammer RP Jr. Neurotrophins in the ventral tegmental area: role in social stress, mood disorders and drug abuse. Neuroscience. 2014;282:122–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deserno L, Schlagenhauf F, Heinz A. Striatal dopamine, reward, and decision making in schizophrenia. Dialogues Clin Neurosci. 2016;18(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frangou S. A systems neuroscience perspective of schizophrenia and bipolar disorder. Schizophr Bull. 2014;40(3):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardoso de Almeida JR, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry. 2013;73(2):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei Y, Chang M, Womer FY, et al. . Local functional connectivity alterations in schizophrenia, bipolar disorder, and major depressive disorder. J Affect Disord. 2018;236:266–273. [DOI] [PubMed] [Google Scholar]
- 14. Xia M, Womer FY, Chang M, et al. . Shared and distinct functional architectures of brain networks across psychiatric disorders. Schizophr Bull. 2019;2011;45(2):450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knolle F, Ermakova AO, Justicia A, et al. . Brain responses to different types of salience in antipsychotic naïve first episode psychosis: an fMRI study. Transl Psychiatry. 2018;8(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dauvermann MR, Moorhead TW, Watson AR, et al. . Verbal working memory and functional large-scale networks in schizophrenia. Psychiatry Res Neuroimaging. 2017;270:86–96. [DOI] [PubMed] [Google Scholar]
- 17. Park IH, Lee BC, Kim JJ, Kim JI, Koo MS. Effort-based reinforcement processing and functional connectivity underlying amotivation in medicated patients with depression and schizophrenia. J Neurosci. 2017;37(16):4370–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trost S, Diekhof EK, Mohr H, et al. . Investigating the impact of a genome-wide supported bipolar risk variant of MAD1L1 on the human reward system. Neuropsychopharmacology. 2016;41(11):2679–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richter A, Petrovic A, Diekhof EK, Trost S, Wolter S, Gruber O. Hyperresponsivity and impaired prefrontal control of the mesolimbic reward system in schizophrenia. J Psychiatr Res. 2015;71:8–15. [DOI] [PubMed] [Google Scholar]
- 20. Rausch F, Mier D, Eifler S, et al. . Reduced activation in ventral striatum and ventral tegmental area during probabilistic decision-making in schizophrenia. Schizophr Res. 2014;156(2-3):143–149. [DOI] [PubMed] [Google Scholar]
- 21. Cuervo-Lombard C, Lemogne C, Gierski F, et al. . Neural basis of autobiographical memory retrieval in schizophrenia. Br J Psychiatry. 2012;201(6):473–480. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen MØ, Rostrup E, Wulff S, et al. . Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71(10):898–905. [DOI] [PubMed] [Google Scholar]
- 23. Murray GK, Corlett PR, Clark L, et al. . Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13(3):239, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bracht T, Horn H, Strik W, et al. . White matter pathway organization of the reward system is related to positive and negative symptoms in schizophrenia. Schizophr Res. 2014;153(1–3):136–142. [DOI] [PubMed] [Google Scholar]
- 25. Xu P, Klaasen NG, Opmeer EM, et al. . Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophr Res. 2019;208:353–359. [DOI] [PubMed] [Google Scholar]
- 26. Rolland B, Amad A, Poulet E, et al. . Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull. 2015;41(1):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadley JA, Nenert R, Kraguljac NV, et al. . Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39(4):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giordano GM, Stanziano M, Papa M, et al. . Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: a resting state functional MRI study. Eur Neuropsychopharmacol. 2018;28(5):589–602. [DOI] [PubMed] [Google Scholar]
- 29. Morris LS, Kundu P, Costi S, et al. . Ultra-high field MRI reveals mood-related circuit disturbances in depression: a comparison between 3-Tesla and 7-Tesla. Transl Psychiatry. 2019;9(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gosnell SN, Curtis KN, Velasquez K, et al. . Habenular connectivity may predict treatment response in depressed psychiatric inpatients. J Affect Disord. 2019;242:211–219. [DOI] [PubMed] [Google Scholar]
- 31. Wohlschläger A, Karne H, Jordan D, Lowe MJ, Jones SE, Anand A. Spectral dynamics of resting state fMRI Within the ventral tegmental area and dorsal raphe nuclei in medication-free major depressive disorder in young adults. Front Psychiatry. 2018;9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagner G, de la Cruz F, Köhler S, Bär KJ. Treatment associated changes of functional connectivity of midbrain/brainstem nuclei in major depressive disorder. Sci Rep. 2017;7(1):8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Downar J, Geraci J, Salomons TV, et al. . Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76(3):176–185. [DOI] [PubMed] [Google Scholar]
- 34. Geugies H, Mocking RJT, Figueroa CA, et al. . Impaired reward-related learning signals in remitted unmedicated patients with recurrent depression. Brain. 2019;142(8):2510–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterpenich V, Vidal S, Hofmeister J, et al. . Increased reactivity of the mesolimbic reward system after ketamine injection in patients with treatment-resistant major depressive disorder. Anesthesiology. 2019;130(6):923–935. [DOI] [PubMed] [Google Scholar]
- 36. Kumar P, Goer F, Murray L, et al. . Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology. 2018;43(7):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry. 2016;6:e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goya-Maldonado R, Weber K, Trost S, et al. . Dissociating pathomechanisms of depression with fMRI: bottom-up or top-down dysfunctions of the reward system. Eur Arch Psychiatry Clin Neurosci. 2015;265(1):57–66. [DOI] [PubMed] [Google Scholar]
- 39. Shi J, Geng J, Yan R, et al. . Differentiation of transformed bipolar disorder from unipolar depression by resting-state functional connectivity within reward circuit. Front Psychol. 2018;9:2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang W, Liu B, Huang B, et al. . Altered resting-state functional connectivity of the striatum in Parkinson’s disease after levodopa administration. PLoS One. 2016;11(9):e0161935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vytlacil J, Kayser A, Miyakawa A, D’Esposito M. An approach for identifying brainstem dopaminergic pathways using resting state functional MRI. PLoS One. 2014;9(1):e87109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohno M, Okita K, Morales AM, et al. . Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: link to impulsivity in methamphetamine users. Mol Psychiatry. 2016;21(11):1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura Y, Okada N, Kunimatsu A, Kasai K, Koike S. Anatomical templates of the midbrain ventral tegmental area and substantia nigra for Asian populations. Front Psychiatry. 2018;9:1–11. doi:10.3389/fpsyt.2018.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okada N, Kasai K, Takahashi T, et al. . Rating scale of handedness for biological psychiatry research among Japanese people (in Japanese). Jpn. J. Biol. Psychiatry. 2014;25(2):118–119. [Google Scholar]
- 45. Otsubo T, Tanaka K, Koda R, et al. . Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59(5):517–526. [DOI] [PubMed] [Google Scholar]
- 46. Fukue T, Fukue M, Ishizuka Y. Relationship between Japanese Adult Reading Test (JART) and cognitive dysfunction. Jpn J Gen Hosp Psychiatry. 2013;25(1):55–62. [Google Scholar]
- 47. Eguchi S, Koike S, Suga M, Takizawa R, Kasai K. Psychological symptom and social functioning subscales of the modified Global Assessment of Functioning scale: reliability and validity of the Japanese version. Psychiatry Clin Neurosci. 2015;69(2):126–127. [DOI] [PubMed] [Google Scholar]
- 48. Williams JB, Kobak KA, Bech P, et al. . The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol. 2008;23(3):120–129. [DOI] [PubMed] [Google Scholar]
- 49. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 50. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 51. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440–447. [DOI] [PubMed] [Google Scholar]
- 52. Douaud G, Smith S, Jenkinson M, et al. . Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(Pt 9):2375–2386. [DOI] [PubMed] [Google Scholar]
- 53. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 54. Smith SM, Jenkinson M, Woolrich MW, et al. . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 55. Grubbs F. Sample criteria for testing outlying observations. Annals of Mathematical Statistics. 1950;21(1):27–58. [Google Scholar]
- 56. Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry. 2018;8(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Edelmann E, Lessmann V. Dopaminergic innervation and modulation of hippocampal networks. Cell Tissue Res. 2018;373(3):711–727. [DOI] [PubMed] [Google Scholar]
- 58. Kühn S, Musso F, Mobascher A, Warbrick T, Winterer G, Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl Psychiatry. 2012;2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136(Pt 3):804–814. [DOI] [PubMed] [Google Scholar]
- 60. Mancini V, Sandini C, Padula MC, et al. . Positive psychotic symptoms are associated with divergent developmental trajectories of hippocampal volume during late adolescence in patients with 22q11DS. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0443-z [DOI] [PubMed] [Google Scholar]
- 61. Winton-Brown T, Schmidt A, Roiser JP, et al. . Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry. 2017;7(10):e1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50(2):371–386. [DOI] [PubMed] [Google Scholar]
- 63. Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 2014;76(3):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90(2):236–249. [DOI] [PubMed] [Google Scholar]
- 66. Ambrosi E, Arciniegas DB, Madan A, et al. . Insula and amygdala resting-state functional connectivity differentiate bipolar from unipolar depression. Acta Psychiatr Scand. 2017;136(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. In: Di Giovann G, Di Matteo V, Esposito E, eds. Progress in Brain Research. Vol 172. Serotonin–Dopamine Interaction: Experimental Evidence and Therapeutic Relevance. Amsterdam, Netherlands: Elsevier; 2008:265–286. [DOI] [PubMed] [Google Scholar]
- 68. Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ogawa SK, Watabe-Uchida M. Organization of dopamine and serotonin system: anatomical and functional mapping of monosynaptic inputs using rabies virus. Pharmacol Biochem Behav. 2018;174:9–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.